Abstract
The Bacillus subtilis clpC operon is regulated by two stress induction pathways relying on either ςB or a class III stress induction mechanism acting at a ςA-like promoter. When the clpC operon was placed under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible Pspac promoter, dramatic repression of the natural clpC promoters fused to a lacZ reporter gene was noticed after IPTG induction. This result strongly indicated negative regulation of the clpC operon by one of its gene products. Indeed, the negative regulator could be identified which is encoded by the first gene of the clpC operon, ctsR, containing a predicted helix-turn-helix DNA-binding motif. Deletion of ctsR abolished the negative regulation and resulted in high expression of both the clpC operon and the clpP gene under nonstressed conditions. Nevertheless, a further increase in clpC and clpP mRNA levels was observed after heat shock, even in the absence of ςB, suggesting a second induction mechanism at the vegetative promoter. Two-dimensional gel analysis and mRNA studies showed that the expression of other class III stress genes was at least partially influenced by the ctsR deletion. Studies with different clpC promoter fragments either fused to the reporter gene bgaB or used in gel mobility shift experiments with the purified CtsR protein revealed a possible target region where the repressor seemed to bind in vivo and in vitro. Our data demonstrate that the CtsR protein acts as a global repressor of the clpC operon, as well as other class III heat shock genes, by preventing unstressed transcription from either the ςB- or ςA-dependent promoter and might be inactivated or dissociate under inducing stress conditions.
In response to changes in their natural environment, bacteria undergo a complex program of differential gene expression. External signals are recognized by complex signal transduction pathways via two-component systems, are transmitted by transcriptional regulators and alternative ς factors, and elicit dramatic changes in bacterial gene expression.
Previous investigations have demonstrated that the heat shock response of Bacillus subtilis is regulated by at least three different mechanisms at the level of transcription (for a review, see reference 11). Induction of class I heat shock genes, such as the classical chaperone genes dnaK and groESL, is triggered strongly after exposure to either heat shock or stresses that generate nonnative proteins in the cell (27). Both operons were shown to be negatively controlled by the HrcA repressor interacting with its operator site, CIRCE (35, 49–51). Recently, activation of the HrcA repressor by the GroE chaperonin machine was demonstrated, pointing out a function of GroE as a modulator of the class I heat shock response in B. subtilis (26).
In addition to heat shock, class II genes are induced in response to various other stresses, as well as energy starvation. This large group of general stress genes requires the alternative sigma factor ςB for induction (for reviews, see references 9, 11, and 12). ςB activity is controlled by a complex signal transduction network including at least seven other gene products encoded by the sigB operon (1, 9, 45, 47).
Class III heat shock genes of B. subtilis were defined as general stress genes that remain inducible at vegetative promoters in response to several stresses in a sigB mutant background and in the absence of CIRCE and HrcA. These general stress genes might comprise a heterogeneous group encoding predominantly ATP-dependent proteases and their regulatory ATPase components. Besides the protease/chaperone genes, lon, ftsH, clpP, clpC, clpX, and htpG, thioredoxin gene trxA, and alkylhydroperoxide reductase operon ahpCF were also grouped into this class (2, 5, 7, 8, 21, 31, 34, 36). With respect to their regulation, a subgroup of class III stress genes could be determined, namely, the clpC operon and the clpP and trxA genes. Genes constituting this subgroup are regulated by two stress induction pathways relying on either ςB or a novel stress induction mechanism acting at a vegetative ςA-like promoter (7, 19, 34). On the basis of earlier studies, we suggested that initiation of transcription seems to be the predominant target of regulation, involving a repression mechanism, as well as positive regulation at the ςA-like promoter (19).
The ClpC ATPase of B. subtilis, involved in controlling competence gene expression, degradative enzyme production, sporulation, cell division, and survival under stress conditions (18, 21, 28, 29, 42), is encoded by the fourth gene of a six-gene operon (19). To study the functions of the other five, unknown, gene products encoded by the clpC operon, database analyses were performed and phenotypes of mutants were investigated. The second and third genes encode proteins with similarities to zinc finger proteins (orf2) and arginine kinases (orf3), respectively (20). By phenotypic studies of mutants with changes in the fifth (sms) and sixth (comY) genes, evidence for the involvement of both proteins in DNA repair and competence was obtained (20).
For the product of the first gene, orf1 (yacG or ctsR) (20, 23), a predicted helix-turn-helix DNA-binding motif was detected by the method of Dodd and Egan (6), suggesting a regulatory role for this protein (20). Recently, this regulatory role was confirmed independently by two groups (4, 17). Derré and Msadek (4) renamed the protein CtsR for class three stress gene repressor. In this communication, the regulation of clpC operon expression by CtsR was analyzed as a model of the molecular basis of the class III stress induction mechanism.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli was routinely grown in Luria-Bertani (LB) medium. B. subtilis was cultivated under vigorous agitation at 37°C in LB medium or in a synthetic medium previously described (40). Since clpC mutant cells showed impaired growth in minimal medium (21, 22), the culture was supplemented with 0.05% (wt/vol) yeast extract. The different stress conditions were produced as described earlier (44). Briefly, the culture was divided during exponential growth, and one half of the culture was grown at 37°C (control), whereas the other half was exposed to heat shock at 48 or 50°C. Glucose starvation was accomplished by cultivating the bacteria in synthetic medium with limiting amounts of glucose (0.05% [wt/vol]). Samples were taken during exponential growth immediately prior to the shift or after the shift at the time indicated. The time of the shift was defined as time zero.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| E. coli | ||
| RR1 | F−mcrB mrr hsdS20(rB− mB−) ara-14 proA2 lacY1 leuB2 galK2 rpsL20 (Smr) xyl-5 mtl-1 supE44 | 3 |
| DH5α | F− φ80dlacZΔ M15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) supE44 thi-1 gyrA96 | 10 |
| BL21(DE3)/pLys5 | F−lon hsdSB(rB− mB−) with DE3, a λ prophage carrying the T7 RNA polymerase gene, and plasmid pLysS containing the T7 phage lysozyme gene | 39 |
| B. subtilis | ||
| IS58 | trpC2 lys-3 | 37 |
| QB4756 | trpC2ΔclpC::spc | 28 |
| BEO1 | trpC2 lys-3 sms::pDH88 | 20 |
| BEO2 | trpC2 lys-3 5′orf1::pHV501 | pHORF1→IS58 |
| BGH1 | trpC2 lys-3 sigB::Δ(HindIII-EcoRV)::cat | 25 |
| BEK4 | trpC2 lys-3 ΔclpC::spc | QB4756→IS58 |
| BEK7 | trpC2 lys-3 orf3::pHT181 | pMEC55→IS58 |
| BEK21 | trpC2 lys-3 orf2::pHT181 | pHTORF2→IS58 |
| BEK38 | trpC2 lys-3 sigB::Δ(HindIII-EcoRV)::cat::neo | pCm::Nm→BGH1 |
| BEK49 | trpC2 lys-3 amyE::cat (ctsR-A-′bgaB) | pDL56→IS58 |
| BEK51 | trpC2 lys-3 amyE::cat (ctsR-A-′bgaB) sigB::Δ(HindIII-EcoRV)::cat::neo | BEK38→BEK49 |
| BEK60 | trpC2 lys-3 orf6::pHT181 | 20 |
| BEK61 | trpC2 lys-3 amyE::cat (ctsR-B-′bgaB) | pDL61→IS58 |
| BEK62 | trpC2 lys-3 amyE::cat (ctsR-B-′bgaB) sigB::Δ(HindIII-EcoRV)::cat::neo | BEK38→BEK61 |
| BEK67 | trpC2 lys-3 amyE::cat::er (ctsR-A-′bgaB) | pCm::Er→BEK49 |
| BEK68 | trpC2 lys-3 amyE::cat::er (ctsR-A-′bgaB) sigB::Δ(HindIII-EcoRV)::cat | BEK67→BGH1 |
| BEK84 | trpC2 lys-3 amyE::cat (ctsR-C-′bgaB) | pDL84→IS58 |
| BEK85 | trpC2 lys-3 amyE::cat (ctsR-′bgaB) | pDL85→IS58 |
| BEK86 | trpC2 lys-3 amyE::cat (ctsR-A-′bgaB) ΩaphA3 5′ ctsR::Δ | pDORF1→BEK67 |
| BEK87 | trpC2 lys-3 amyE::cat::er (ctsR-A-′bgaB) ΩaphA3 5′ ctsR::Δ sigB::Δ(HindIII-EcoRV)::cat | BEK86→BGH1 |
| Plasmids | ||
| pBluescriptSKII | Cloning vector; Apr | Stratagene |
| pRSETA | Cloning vector; Apr; allows in-frame cloning for N-terminal 6×His tag and overproduction of protein by T7 transcription | Invitrogen |
| pHV501 | Integration vector; Apr ErrlacZ | 43 |
| pDL | Vector for construction of transcriptional bgaB fusions at amyE site, Apr Cmr | 49 |
| pMEC56 | pAC7 with translational lacZ fusion of entire regulatory region of clpC operon | 19 |
| pCm::Nm | Vector for replacing preexisting Cmr with Nmr Apr Cm::Nmr | 38 |
| pCm::Er | Vector for replacing preexisting Cmr with Err Apr Cm::Err | 38 |
| pDORF1 | pBluescriptSKII carrying 911-bp KpnI/blunt and 409-bp blunt/BamHI PCR fragment of flanking regions with Kmr cassette | This study |
| pRORF1 | pRSETA carrying entire ctsR gene | This study |
| pHORF1 | pHV501 carrying 268-bp HindIII/BamHI PCR fragment of N-terminal region of ctsR gene | This study |
| pDL56 | pDL carrying 271-bp EcoRI/BamHI fragment from pMEC56 | This study |
| pDL61 | pDL carrying 218-bp EcoRI/BamHI fragment amplified with primers EK15 and TM-111 | This study |
| pDL84 | pDL carrying 99-bp EcoRI/BamHI fragment amplified with primers EK16 and TM-110 | This study |
| pDL85 | pDL carrying 46-bp EcoRI/BamHI fragment amplified with primers EK15 and EK16 | This study |
Analysis of mRNA.
Total RNA of B. subtilis IS58 was isolated by the acid phenol method from exponentially growing (control) and stressed cells as previously described (19). Serial dilutions of total RNA were transferred onto a positively charged nylon membrane by slot blotting and hybridized with digoxigenin-labeled RNA probes in accordance with the manufacturer’s (Boehringer Mannheim) instructions. All filters were exposed to Fuji RX films, and the volumes (i.e., the sum of the pixel values within an object) were quantitated with a Personal Densitometer from Molecular Dynamics. Digoxigenin-labeled RNA probes were synthesized in vitro with T7 or T3 RNA polymerase specific for clpC, clpP, clpX, lonA, trxA, dnaK, and ctc as described earlier (7, 8, 21, 31, 34, 44).
The transcription initiation sites of the operon were identified by the primer extension method by means of reverse transcriptase (Stratagene) and [γ-32P]ATP 5′-labelled primer PE1 (CCATTTTGATCTAACACTCG). The corresponding sequence was obtained from plasmid pUP1 (19). Primer extension experiments were performed as described by Wetzstein et al. (46).
β-Galactosidase assay.
For assay of both LacZ and BgaB activities, B. subtilis cells were grown in LB or synthetic medium as indicated in Results. Samples (1 ml) were taken during exponential growth, after entry into stationary phase, and 30 min after heat shock at 50°C. β-Galactosidase activities were determined after permeabilizing the cells with chloroform and sodium dodecyl sulfate in accordance with the method of Kenney and Moran (15). For measurement of BgaB activity, the assay buffer was modified as described by Yuan and Wong (49) and an incubation temperature of 60°C was used.
Construction of mutant strains and reporter gene fusions.
Strain BEO2 was constructed by cloning of the 268-bp HindIII/BamHI fragment obtained with primers TM-120 (AAGGAAGCTTAAAGGAGGGGGTTGAGTGGGAC) and TM-103 (GGAGGATCCTTATTGATCAGGACAACTTCATTG) into plasmid pHV501 (43) and transforming competent cells with the resulting plasmid, pHORF1.
Transcriptional fusions of ctsR with bgaB encoding a heat-stable β-galactosidase were constructed by using plasmid pDL (49). The 271-bp EcoRI/BamHI fragment of pMEC56 (19) was cloned into pDL, giving plasmid pDL56. Several deletions in the promoter region were generated by PCR using primers EK15 (GAAGAATTCCGAGAAAGTTGAAATTCTCG) and TM-111 (GGAGGATCCGAAATATTATGTCCCACTCAACC), primers TM-110 (GAAGAATTCAGGACGCCGCCAAGCAAGCTTAAACCC) and EK16 (GGAGGATCCGACTTTAATCTTACTATAAGCCG), and primers EK15 and EK16. All PCR products were cloned as EcoRI/BamHI fragments into pDL, resulting in plasmids pDL61, pDL84, and pDL85, respectively. Linearized pDL derivatives were integrated into the amyE site of the chromosome by a double-crossover recombination event, giving strains BEK49, BEK61, BEK84, and BEK85. The corresponding strains bearing the sigB deletion were obtained by transforming BEK49, BEK61, BEK84, and BEK85 with chromosomal DNA of sigB mutant BEK38. Transformants carrying chromosomal lacZ fusions in amyE were screened for expression of β-galactosidase activity and deficiency of α-amylase activity. Transformants were picked on LB agar plates containing 1% (wt/vol) starch, and starch degradation was detected by sublimating iodine onto the plates. LacZ or BgaB activity was visualized on plates by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 100 μg/ml) hydrolysis.
To alter antibiotic resistance in the amyE or sigB gene, plasmids pCm::Nm and pCm::Er (38) were used as indicated in Table 1. Transformants were tested for chloramphenicol sensitivity and neomycin or erythromycin resistance, respectively.
A nonpolar in-frame deletion of the ctsR gene was generated by using plasmid pDORF1. Construction of plasmid pDORF1 was performed by cloning of a 911-bp KpnI/blunt PCR fragment (primers PORF1DELUPF [GGTGGTACCATCGCTCAACGGATAAAAGC] and PORF1DELUPR [AGAAATATTATGTCCCACTCAACC]) covering the region upstream of ctsR and the first six codons of the ctsR gene into pBluescriptSK. Behind this fragment, a 409-bp blunt/BamHI PCR fragment (primers PORF1DELDF [CGTGATGAATTAAGAGCGAG] and TM-115 [GGAGGATCCATGCAGGAACTCAAATGTTCA]) was inserted covering the last 19 codons of ctsR and part of the downstream orf2 gene. For selection in B. subtilis, a kanamycin resistance determinant, aphAIII (41), was cloned into the unique HindIII site upstream of the two promoters. ctsR deletion strain BEK86 was obtained after linearizing plasmid pDORF1 with ScaI and transforming B. subtilis BEK49 cells bearing the wild-type promoter-bgaB fusion. Positive candidates were selected for kanamycin resistance and blue colonies at 37°C and verified by PCR. A double-crossover recombination event at the correct sites resulted in the following chromosomal situation for the ctsR deletion strain. A kanamycin resistance cassette upstream of the deletion was followed by the entire regulatory region of the clpC operon and the truncated ctsR gene containing the first six codons, as well as the last 19 codons. The genetic structure and expression of the complete downstream part of the operon were not influenced by this deletion, as confirmed by PCR and mRNA slot blot analysis with a clpC probe.
orf2 was disrupted by integration of plasmid pDORF2, a derivative of pHT181 (24) carrying a 136-bp internal BamHI fragment of orf2 amplified by PCR with primers TM-114 (GGAGGATCCATGCAGGAACTCAAATGTTCA) and TM-115. The mutant strain obtained was BEK21. A 263-bp internal fragment of orf3 was amplified by PCR using primers TM-104 (GGAGGATCCACACCTTTAGAAAAGCGTGT) and TM-105 (GGAGGATCCGGACAGCTGGTTAAGTATCCTCTT) and cloned into the BamHI site of plasmid pHT181 (24) to give plasmid pMEC55. Transformation of B. subtilis resulted in a Campbell-type integration event generating BEK8 containing two copies of orf3, one lacking the last 195 codons and one missing the first 80 codons. Both disruptions were confirmed by Southern blot analysis. Disruptions of orf2, orf3, sms, and comY and the clpC deletion were also introduced into strain BEK49.
2D PAGE.
B. subtilis wild-type and ΔctsR mutant cells were grown at 37°C in LB medium to an optical density at 540 nm of 0.5 before (control) and 30 min after exposure to heat stress (50°C). Disruption of the cells by a French press, two-dimensional (2D) polyacrylamide gel electrophoresis (PAGE) of the protein extracts, and silver staining of the resulting 2D PAGE gels were carried out as described earlier (7, 34). Scanned gels were matched by using the MELANIE II program (Bio-Rad Laboratories, Inc.), and the protein spots were allocated in accordance with the Sub2D database (http://pc13mi.biologie.uni-greifswald.de/sub2D/ sub2d.htm).
Purification of the CtsR protein.
For overproduction and purification of CtsR in E. coli, the entire ctsR gene was amplified by PCR using primers PRORF1F (GGAGGATCCGTGGGACATAATATTTTCTG) and PRORF1R (GAAGAATTCCACCCGCTTATTTTAATTTTAA) and cloned as a BamHI/EcoRI fragment into pRSETA (InVitrogen, Inc.). This plasmid allowed in-frame fusion of the ctsR gene to six histidine codons at the N terminus and transcription from a T7 promoter. Competent cells of E. coli BL21(DE3) (39) containing plasmid pLysS (encoding the phage lysozyme) and the T7 RNA polymerase gene in the chromosome under the control of the lac promoter were transformed with the resulting plasmid, pRORF1. For overproduction, the recombinant strain obtained was grown in LB medium supplemented with ampicillin and chloramphenicol for selection of plasmid-containing cells. At an optical density of 0.3 at 540 nm, the T7 phage RNA polymerase was induced by adding 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) to the culture and incubating it for 2 h. After induction, cells were harvested by centrifugation, washed once in disruption buffer, and disrupted by a French press. Cellular debris were removed by centrifugation, and the crude extract was incubated with Ni-nitrilotriacetic acid-agarose for 2 h at 4°C. Afterwards, the protein-Ni-agarose mixture was loaded onto columns and the 6×His-tagged CtsR protein was purified under native conditions via Ni-nitrilotriacetic acid affinity chromatography as recommended by the manufacturer (Qiagen, Inc.). The 6×His-CtsR protein was substantially pure as judged by sodium dodecyl sulfate-PAGE analysis.
Gel retardation experiments.
Gel retardation experiments were performed by using DNA fragments PCR amplified by means of primer pairs TM-110 and TM-111, EK15 and TM-111, EK15 and EK16, TM-110 and EK16, and TM-103 and TM-120 (for fragments, see above). Purified DNA fragments (0.5 μg) were incubated in 30-μl reaction mixtures containing 20 mM Tris-HCl (pH 8.0), 50 mM KCl, 3% (wt/vol) Ficoll, 20 mM EDTA, 0.5 mM dithiothreitol, and 1 μg of competitor DNA (sonicated herring sperm DNA). After 20 min of incubation at 30°C, the samples were loaded onto 5% polyacrylamide gels with 1× TBE buffer (90 mM Tris/HCl, 90 mM boric acid, 1 mM EDTA) and the gels were stained with ethidium bromide.
General methods.
Plasmid isolation, restriction enzyme analysis, transformation of E. coli, ligation of DNA fragments, and filling in of the recessed 3′ termini by using the Klenow fragment of DNA polymerase I were performed in accordance with standard protocols (32). Recombinant plasmids were sequenced by the dideoxy-chain termination method of Sanger et al. (33). DNA fragments were amplified by PCR as described earlier (21). Some oligonucleotides used for PCR included mismatches allowing the creation of EcoRI, BamHI, KpnI, and HindIII restriction sites. Chromosomal DNA from B. subtilis was isolated by using a Wizard genomic DNA purification kit (Promega, Inc.). Transformation of B. subtilis was carried out by using a two-step protocol (14).
RESULTS
The clpC operon is negatively autoregulated.
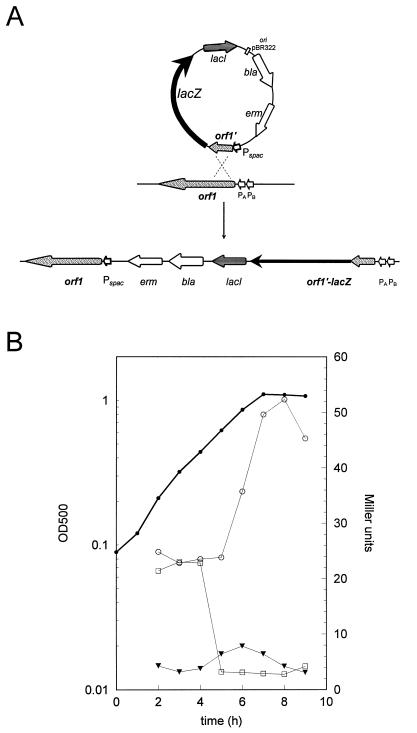
The clpC operon is controlled by stress-inducible ςB- and ςA-dependent promoters. Initiation of transcription seems to be the predominant target of regulation (19). In order to investigate whether any of the genes in the operon might be involved in this regulation, strain BEO2 was constructed with the clpC operon placed under the control of the IPTG-inducible Pspac promoter (13, 48) (see Materials and Methods). In this strain, expression of the clpC operon was dependent on IPTG addition, but the natural promoters were fused to the reporter gene lacZ (Fig. 1A). A plate assay with agar plates containing X-Gal revealed pale blue colonies after growth in the presence of 1 mM IPTG, a condition under which the operon was highly expressed. However, without IPTG, the colonies became dark blue, indicating that one of the genes may encode a negative regulator. This phenomenon was verified by measuring β-galactosidase activity in minimal medium during exponential growth and after entry into stationary phase triggered by glucose starvation. Cell growth without IPTG resulted in a high basal level (approximately 25 Miller units in contrast to 5 U) and in approximately fivefold induction after entry into stationary phase, probably due to increased transcription at the ςB-dependent promoter after glucose starvation (19). By contrast, addition of IPTG to the culture at the beginning of growth caused very low LacZ activity without the typical stationary-phase induction, indicating that the ςB promoter stayed repressed. After addition of IPTG to exponentially growing cells at an optical density of 0.5, β-galactosidase activity decreased very rapidly from 25 to 7 U (Fig. 1B). High-level expression of the clpC operon in the presence of IPTG seems to block the glucose starvation-inducible transcription at the ςB promoter (19), whereas low-level expression of the operon resulted in clear induction after entry into stationary phase (Fig. 1B). These results strongly suggested negative regulation of the clpC operon by one of its gene products.
FIG. 1.
(A) Schematic representation of the Campbell-type integration of plasmid pHORF1 into the chromosome of B. subtilis IS58 and the resulting order of genes in strain BEO2: ςB-dependent promoter PB and vegetative promoter PA fused to lacZ, lacI, bla for the β-lactamase gene, erm for the erythromycin resistance gene, the Pspac promoter, and orf1 ctsR. (B) β-Galactosidase activities, in Miller units per unit of optical density (OD), caused by a ctsR-lacZ fusion in strain BEO2 in the presence or absence of IPTG in the medium. The solid, thick line portrays the growth curve of strain BEO2 in minimal medium with limiting amounts of glucose. Symbols: ○, β-galactosidase activity in the absence of IPTG, ▾, β-galactosidase activity in the presence of IPTG; □, β-galactosidase activity after addition of IPTG during midexponential phase at an optical density of 0.5 at 540 nm.
Phenotype of a ctsR mutant with respect to regulation of the clpC operon.
To study regulation at both promoters of the clpC operon, a reporter gene fusion with the bgaB gene encoding a thermostable β-galactosidase was constructed. The transcriptional bgaB-ctsR fusion was inserted into the amyE site of the chromosome in the wild-type background. In order to determine which gene of the clpC operon might encode its putative negative regulator, it was necessary to test mutant forms of each individual gene of the clpC operon for bgaB expression under nonstressed conditions. A strain with a nonpolar in-frame deletion of the ctsR gene was constructed in which most of the coding region was removed and a kanamycin resistance cassette was placed near the deletion upstream of both promoters (see Materials and Methods). Firstly, the mutant strains were screened on agar plates containing X-Gal for increased expression of the bgaB reporter gene at low temperatures. The wild-type strain gave white colonies under nonstressed growth conditions and dark blue colonies after heat shock. By contrast, deletion of ctsR resulted in dark blue colonies not only after heat shock at 50°C but already at 37°C under nonstressed conditions. With respect to BgaB expression, sms (orf5) and comY (orf6) mutants showed no striking difference from the wild-type reference strain in the plate assay. The orf2, orf3, and clpC mutations produced pale blue colonies at 37°C and dark blue colonies after heat shock (data not shown).
Results similar to those of the plate assay for the ctsR-encoded phenotype were obtained by determination of the BgaB activity of ctsR deletion mutant strain BEK86 before (control) and 30 min after 50°C heat shock in comparison with that of isogenic strain BEK67 (wild type) (Table 2). In the ctsR mutant, the basal level of the operon was increased approximately eightfold in comparison with that in the wild type, but approximately threefold remaining induction by heat shock was noticed. Heat shock induction of the clpC operon occurs predominantly at the ςB-dependent promoter (19). Therefore, a sigB deletion was introduced into reference strain BEK67 and ctsR mutant BEK86 to avoid interference by ςB-dependent transcription. However, BgaB activities similar to those obtained in the wild-type and ctsR deletion backgrounds were obtained with the ctsR sigB double mutant (BEK87) in comparison with the isogenic sigB mutant (BEK68) (Table 2). BgaB activities indeed suggested that the first gene encodes a negative regulator of clpC operon expression. Nevertheless, an induction by heat stress observed for all strains was evident even in the ctsR sigB double mutant (Table 2).
TABLE 2.
Influence of a ctsR mutation on expression of the clpC operona
| Strain (genotype) | β-Galactosidase activity (Miller units)
|
Fold induction | Fold derepression of basal level | |
|---|---|---|---|---|
| Exponential growth (control) | 30-min heat shock at 50°C | |||
| BEK67 (wild type) | 7.7 | 178 | 23 | 1 |
| BEK68 (ΔsigB) | 5.1 | 128 | 25 | 1 |
| BEK86 (ΔctsR) | 59 | 188 | 3.2 | 7.7 |
| BEK87 (ΔctsR ΔsigB) | 49 | 128 | 2.6 | 9.6 |
Cells were grown in LB medium at 37°C to the mid-exponential phase and then exposed to heat shock at 50°C for 30 min. Samples were taken immediately before and after exposure to heat shock, and β-galactosidase activities, induction ratios, and derepression levels were determined. The results are averages of at least three experiments.
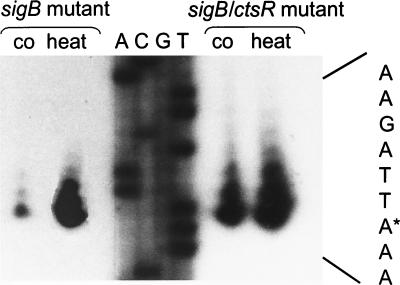
These data were also confirmed by primer extension analysis. Primer extension experiments were performed by using RNA isolated before and 6 min after exposure to 50°C heat shock from sigB mutant strain BGH1 and also from ctsR sigB double mutant strain BEK87. Comparison of the primer extension results of both strains revealed that increased transcription initiation occurred in the ctsR deletion background at the vegetative promoter under nonstressed conditions (Fig. 2). However, the transcription rate from this vegetative promoter was still increased after heat shock, indicating the requirement for an additional regulator element.
FIG. 2.
Initiation of transcription at the ςA-dependent promoter in sigB mutant and sigB ctsR double mutant cells. An autoradiograph of a primer extension experiment mapping the 5′ end of ctsR mRNA is shown. Equal amounts of RNA isolated from sigB mutant and sigB ctsR double mutant cells before (co) and 6 min after (heat) heat shock were used as templates for reverse transcription. The corresponding sequence of plasmid pUP1 was obtained with the appropriate primer. The complement of the DNA sequence illustrated in the sequencing gel is shown on the right. The transcriptional start site is indicated by the asterisk.
Influence of the ctsR deletion on the expression of other stress genes.
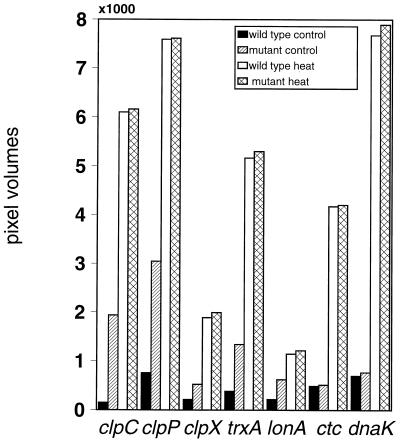
The similar regulation patterns of at least the double-controlled class III heat shock genes, namely, clpC, clpP, and trxA, led to the suggestion that they might be subjected to the same induction mechanism (7, 19, 34). In order to investigate whether other class III stress genes are also influenced by a ctsR deletion, mRNA levels of clpC, clpP, clpX, lon, and trxA were investigated by slot blot analysis. Digoxigenin-labeled RNA probes of these genes were hybridized with RNAs isolated from the wild type and from the ctsR mutant before and 6 min after heat shock at 50°C. Slot blot analysis using the same RNA preparation with probes of the chaperone gene dnaK (class I) and the ςB-dependent gene ctc (class II) served as a control (44). Indeed, no difference in induction levels between the wild type and the ctsR deletion strain was observed for the dnaK and ctc genes. In the wild type and the ctsR deletion strain, both genes showed similar basal levels and were induced after heat shock. By contrast, a clear increase in the basal-level expression of the clpC and clpP genes was observed in the ctsR mutant in comparison with the wild type. In case of the clpX, lon, and trxA genes, this effect was also detected but to a lesser extent (Fig. 3). However, in the ctsR deletion strain, an additional induction after exposure to heat shock was also determined for the other genes as observed for the clpC operon.
FIG. 3.
Comparison of mRNA levels in the wild type and the ctsR deletion strain by slot blot hybridization with probes specific for the clpC, clpP, clpX, trxA, lonA, dnaK, and ctc genes. The schematic representation shows the mRNA levels of the genes using RNA of wild-type cells before (wild-type control; black bars) and 6 min after (wild-type heat; white bars) heat shock at 50°C, as well as the respective signals of the isogenic ctsR deletion mutant before (mutant control; hatched bars) and after (mutant heat; cross-hatched bars) exposure to heat shock. The signal intensity of each slot is given in pixel volumes quantitated by densitometry as described in Materials and Methods. The results are averages of at least five experiments; standard deviations were less than 10%.
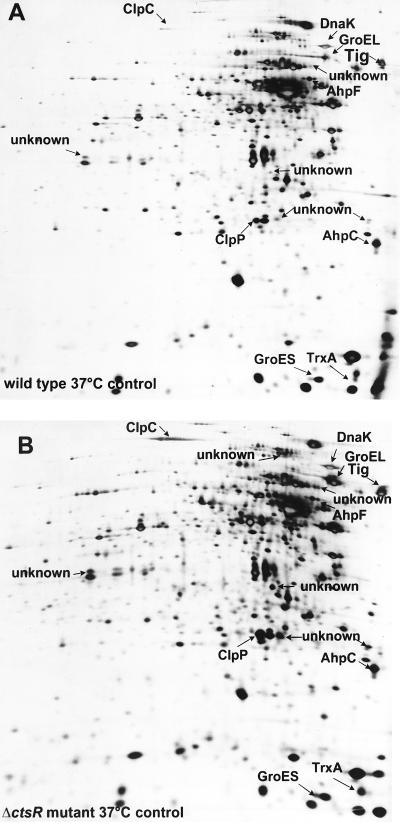
Using the 2D PAGE approach, we tried to confirm our data on the protein level. Protein extracts of wild-type and ctsR mutant cells grown exponentially under nonstressed conditions were separated by 2D PAGE and the 2D protein patterns of the silver-stained gels were compared. The protein patterns of wild-type and ctsR mutant cells differed, suggesting that the CtsR regulator might have a strong influence on the expression of several genes (Fig. 4). Striking differences were found in the ClpP and ClpC proteins, which were present in significantly larger amounts in the ctsR mutant than in the wild-type strain (Fig. 4A and B). A larger amount of the thioredoxin spot was also found, but here the differences between the wild type and the ctsR mutant were less pronounced. Several unidentified protein spots on 2D PAGE gels (marked unknown) were also increased in the ctsR mutant (Fig. 4B), suggesting the existence of a regulon controlled by CtsR. Furthermore, a few protein spots were decreased in the mutant strain. Proteins grouped into class III that are more or less specifically induced by oxidative stress as the alkylhydroperoxide reductase AhpC/AhpF (2) do not seem to be influenced by the ctsR deletion.
FIG. 4.
Influence of the ctsR deletion on the protein pattern of exponentially growing B. subtilis cells. Equal amounts of crude protein extracts (100 μg) of wild-type (A) and ctsR mutant (B) cells were separated by 2D PAGE. Silver-stained gels were scanned and matched as described in Materials and Methods. The identified protein spots are indicated, and the unknown spots are marked as such.
Identification of the CtsR binding site in front of the clpC operon.
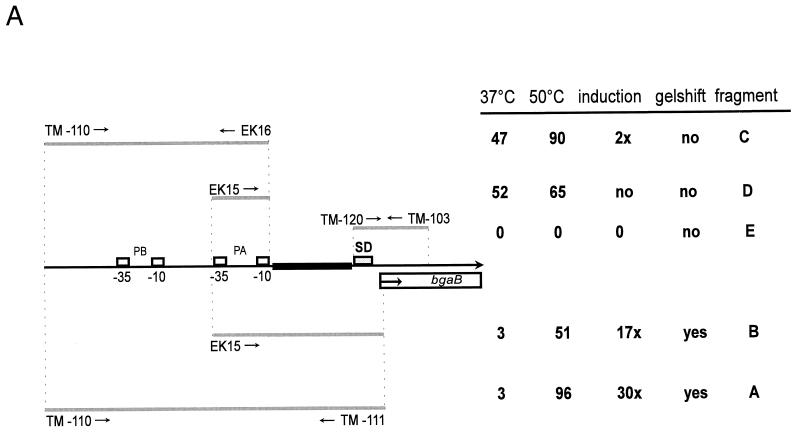
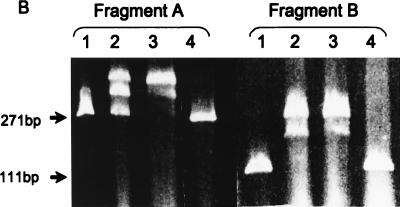
The high probability of a helix-turn-helix DNA-binding motif proposes that CtsR might act directly as a repressor of clpC operon expression. However, no classical repressor-binding sites like inverted repeats could be identified within the regulatory region of the clpC operon (19). From our previous data, it was tempting to speculate that the repression mechanism may act downstream of both promoters, because CtsR appeared to prevent transcription from both the ςB- and ςA-dependent promoters (19). Therefore, we started to define the possible binding region of CtsR in vivo by deletion analysis using different bgaB fusions, as well as in vitro by using purified CtsR and different PCR-generated promoter fragments in gel mobility shift experiments. All bgaB reporter gene fusions and their resulting BgaB activities are schematically represented in Fig. 5A. The corresponding gel shift experiments are summarized in Fig. 5B and C. The strain containing the fusion with fragment A comprising the entire regulatory region of the clpC operon including both promoters served as a control. Exposure to 50°C resulted in about 30-fold induction of BgaB activity. Fragment B, containing the vegetative promoter and its downstream region, gave considerably lower induction, indicating that the sequences upstream of the ςA-dependent promoter may stimulate transcription initiation at this promoter. However, incubation of both fragments A and B with 0.05 or 0.1 nM purified CtsR protein resulted in efficient retardation of the fragment in gel electrophoresis with two retardation signals. Increasing amounts of purified CtsR protein appeared to show increased intensity of the second retardation band (Fig. 5B). Deleting the part downstream of the vegetative promoter, as done for fragments C and D, caused a significant 16-fold increase in expression under nonstressed conditions. Nevertheless, there was still approximately twofold induction for fragment C after heat shock, whereas more or less constitutive expression was observed for fragment D missing the sequences upstream and downstream of the ςA-dependent promoter. Both fragments C and D, as well as fragment E, were not retarded in gel electrophoresis after incubation with purified CtsR protein (Fig. 5C; data not shown for fragment D). These results suggest a localization of the target region for CtsR binding between the −10 region of the vegetative promoter and the ribosome-binding site.
FIG. 5.
(A) Schematic representation of the 5′ upstream region of the clpC operon including simplified maps of ctsR′-bgaB fusions or fragments used in gel mobility shift experiments. The −10 and −35 boxes of the ςB-dependent promoter (PB), the ςA-dependent promoter (PA), the PCR primers (arrows), the Shine-Dalgarno region (SD), and the bgaB reporter gene are indicated. DNA fragments either fused to the bgaB reporter gene or used in gel mobility shift experiments are represented by gray lines, and a possible target region for the repressor is in bold and black. The corresponding results of the BgaB assays before (37°C) and after heat shock at 50°C, induction of expression, and behavior in gel mobility shift experiments are shown. (B) Gel mobility shift experiments with fragments A and B. Gel retardation experiments were performed as described in Materials and Methods. Fragment length (A, 271 bp; B, 111 bp) is indicated on the left. The lanes were loaded as follows: 1, free fragment; 2 and 3, fragments incubated with 0.05 and 0.1 nM purified CtsR protein; 4, fragment incubated with bovine serum albumin. (C) Gel mobility shift experiments with fragments C and E. Fragment length (C, 220 bp; E, 268 bp) is indicated on the left. The lanes were loaded as described above.
DISCUSSION
In order to study possible autoregulation of the clpC operon, the phenotypes produced by mutations within this operon were investigated with respect to its expression. The strongest effect was observed in strains carrying the nonpolar ctsR deletion. These mutants exhibit significant derepression of the basal level, strongly indicating negative control of the clpC operon by CtsR. Mutations in orf2 and orf3, as well as clpC, caused slight upregulation of the expression of the operon, but polar effects on clpC cannot be excluded in these mutants. However, our preliminary data suggest that Orf2 and Orf3, showing similarities to zinc finger proteins or kinases, respectively (20), and the ClpC-ATPase itself might play a role in the control of CtsR activity. Despite this derepression in the ctsR mutant, reproducible induction was evident even in the ctsR sigB double mutant, suggesting a repression mechanism involving CtsR together with additional positive regulation by a different mechanism. This approximately twofold induction by heat shock failed in bgaB fusion strain BEK85 missing the AT-rich sequence upstream of the vegetative promoter (fragment E in Fig. 5A) (16). Such AT-rich regions may cause DNA bending and thereby function as promoter up elements (for a review, see reference 30).
In the case of the clpC operon, the negative effect of the CtsR repressor was dominant and could not be overcome by stationary-phase induction at the ςB promoter. A high CtsR level prevented transcription from both promoters, whereas a low level of the repressor resulted in clear induction after entry into stationary phase (Fig. 1B). CtsR binding seems to block the glucose starvation-inducible transcription at the ςB promoter (19), explaining the relatively low induction after entry into stationary phase for the clpC operon and the clpP gene (7, 19).
Deletion of ctsR led to global changes in the gene expression profile of B. subtilis cells. For the clpP and clpC genes, a clear dependency on the CtsR regulator was determined on the RNA and protein levels (Fig. 3 and 4). An at least partial effect of CtsR on the regulation of thioredoxin gene trxA and the lon and clpX genes was also observed. It is tempting to speculate that these stress-inducible genes are regulated by more than one mechanism. Additionally, synthesis of further proteins appeared to be influenced by the ctsR deletion as observed by 2D PAGE analysis (Fig. 4). Possibly, the induction mechanism of those genes acts directly via CtsR but an indirect effect via repressors which become unstable by protease overproduction in the ctsR mutant might also be considered. Oxidative stress proteins AhpC and AhpF were previously classified as class III heat shock genes because of their weak induction after heat shock and ethanol stress (2). However, no dependency on the CtsR regulator could be shown for those proteins induced particularly in response to oxidative stress. These results suggest a CtsR regulon comprising proteins implicated in protein renaturation, protein repair, or ATP-dependent proteolysis such as ClpC, ClpP, ClpX, and Lon, as well as thioredoxin.
The determination of the CtsR binding region by both in vivo gene fusion studies and in vitro binding of CtsR in gel retardation experiments revealed a target region located in front of the clpC operon between the −10 box of the vegetative promoter and the ribosome-binding site. No striking similarities were observed by comparison of the regulatory regions of those genes influenced by a ctsR deletion. Recently, the target region of a putative repressor of the clpP gene was found to overlap the vegetative promoter by using reporter gene fusions (7). Derré and Msadek (4) determined a target sequence for the CtsR repressor of the clpP gene by site-directed mutagenesis and DNase I footprinting assays. This putative binding site consists of the directly repeated sequence YGTCAAW, which is present in the promoter region of the clpP gene, as well the clpC operon. A similar sequence was found between the −10 box of the vegetative promoter and the ribosome-binding site in front of the lon gene (31).
The induction pattern of the ςA-dependent promoter indicates that the CtsR repressor is inactivated by several stresses, such as heat shock, puromycin stress, ethanol treatment, or oxidative stress, but not by energy starvation or salt stress, which specifically induce ςB-dependent transcription (7, 19). However, the elucidation of the signal transduction pathway leading from the stress signal to the nonfunctional state of the repressor deserves further work.
ACKNOWLEDGMENTS
We thank Steffen Krüger and Gerhard Mittenhuber for critical reading of the manuscript. We acknowledge Elke Witt for support in mutant analysis, Steffen Ohlmeier for construction of strain BEO2, and Uwe Völker and Knut Büttner for help in 2D PAGE analysis. Furthermore, we are grateful to Tarek Msadek for sharing unpublished results and useful discussions. We thank Renate Gloger and Anita Harang for excellent technical assistance.
This work was supported by grants from the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie to M.H.
REFERENCES
- 1.Alper L, Dufour A, Garsin D A, Duncan M L, Losick R. Role of adenosine nucleotide in the regulation of a stress response transcription factor in Bacillus subtilis. J Mol Biol. 1996;260:165–177. doi: 10.1006/jmbi.1996.0390. [DOI] [PubMed] [Google Scholar]
- 2.Antelmann H, Engelmann S, Schmid R, Hecker M. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J Bacteriol. 1996;178:6571–6578. doi: 10.1128/jb.178.22.6571-6578.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolivar F, Rodrigues R L, Greener P J, Betlach M C, Heyneker H L, Boyer H W, Crosa J H, Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–133. [PubMed] [Google Scholar]
- 4.Derré I, Msadek T. Abstracts of posters. 9th International Conference on Bacilli. 1997. CtsR is a DNA-binding protein controlling class III heat shock gene expression in Bacillus subtilis, abstr. G121. [Google Scholar]
- 5.Deuerling E, Paeslack B, Schumann W. The ftsH gene of Bacillus subtilis is transiently induced after osmotic and temperature upshift. J Bacteriol. 1995;177:4105–4112. doi: 10.1128/jb.177.14.4105-4112.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodd I B, Egan J B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990;18:5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerth U, Krüger E, Derré I, Msadek T, Hecker M. Stress induction of the Bacillus subtilis clpP gene encoding the proteolytic component of the Clp protease and involvement of ClpP and ClpX in stress tolerance. Mol Microbiol. 1998;28:787–802. doi: 10.1046/j.1365-2958.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- 8.Gerth U, Wipat A, Harwood C, Carter N, Emmerson P T, Hecker M. Sequence and transcriptional analysis of clpX—a class III heat shock gene of Bacillus subtilis. Gene. 1996;181:77–83. doi: 10.1016/s0378-1119(96)00467-2. [DOI] [PubMed] [Google Scholar]
- 9.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D. Techniques for transformation of Escherichia coli. In: Glover D M, editor. DNA cloning: a practical approach. Vol. 1. Oxford, England: IRL Press; 1985. pp. 109–135. [Google Scholar]
- 11.Hecker M, Schumann W, Völker U. Heat shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 12.Hecker M, Völker U. Non-specific, general and multiple stress resistance of growth restricted Bacillus subtilis cells by the expression of the ςB-regulon. Mol Microbiol. 1998;29:1129–1136. doi: 10.1046/j.1365-2958.1998.00977.x. [DOI] [PubMed] [Google Scholar]
- 13.Henner D J. Inducible expression of regulatory genes in Bacillus subtilis. Methods Enzymol. 1990;185:223–228. doi: 10.1016/0076-6879(90)85022-g. [DOI] [PubMed] [Google Scholar]
- 14.Hoch J A. Genetic analysis in Bacillus subtilis. Methods Enzymol. 1991;204:305–320. doi: 10.1016/0076-6879(91)04015-g. [DOI] [PubMed] [Google Scholar]
- 15.Kenney T J, Moran C P., Jr Organization and regulation of an operon that encodes a sporulation-essential sigma factor in Bacillus subtilis. J Bacteriol. 1987;169:3329–3339. doi: 10.1128/jb.169.7.3329-3339.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krüger, E., and J. Bandow. Unpublished data.
- 17.Krüger E, Gerth U, Hecker M. Abstracts of posters. 9th International Conference on Bacilli. 1997. Stress inducible Clp-proteins: regulation and function, abstr. G120. [Google Scholar]
- 18.Krüger E, Hecker M. Bacillus subtilis ClpC. In: Gething M J, editor. Guidebook to molecular chaperones and protein-folding catalysts. Oxford, United Kingdom: Oxford University Press; 1997. pp. 243–245. [Google Scholar]
- 19.Krüger E, Msadek T, Hecker M. Alternate promoters direct stress induced transcription of the Bacillus subtilis clpC operon. Mol Microbiol. 1996;20:713–723. doi: 10.1111/j.1365-2958.1996.tb02511.x. [DOI] [PubMed] [Google Scholar]
- 20.Krüger E, Msadek T, Ohlmeier S, Hecker M. The Bacillus subtilis clpC operon encodes DNA repair and competence proteins. Microbiology. 1997;143:1309–1316. doi: 10.1099/00221287-143-4-1309. [DOI] [PubMed] [Google Scholar]
- 21.Krüger E, Völker U, Hecker M. Stress induction of clpC in Bacillus subtilis and its involvement in stress tolerance. J Bacteriol. 1994;176:3360–3367. doi: 10.1128/jb.176.11.3360-3367.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunst F, Msadek T, Rapoport G. Signal transduction network controlling degradative enzyme synthesis and competence in Bacillus subtilis. In: Piggot P J, Moran C P Jr, Youngman P, editors. Regulation of bacterial differentiation. Washington, D.C: American Society for Microbiology; 1994. pp. 1–20. [Google Scholar]
- 23.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 24.Lereclus D, Arantes O. spbA locus ensures the segregational stability of pHT1030, a novel type of Gram-positive replicon. Mol Microbiol. 1992;6:35–46. doi: 10.1111/j.1365-2958.1992.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 25.Maul B, Völker U, Riethdorf S, Engelmann S, Hecker M. ςB-dependent induction of gsiB by multiple stimuli in Bacillus subtilis. Mol Gen Genet. 1995;248:114–120. doi: 10.1007/BF02456620. [DOI] [PubMed] [Google Scholar]
- 26.Mogk A, Homuth G, Scholz C, Kim L, Schmid F X, Schumann W. The GroE chaperone machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J. 1997;16:4579–4590. doi: 10.1093/emboj/16.15.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mogk A, Völker A, Engelmann S, Hecker M, Schumann W, Völker U. Nonnative proteins induce expression of the Bacillus subtilis CIRCE regulon. J Bacteriol. 1998;180:2895–2900. doi: 10.1128/jb.180.11.2895-2900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Msadek T, Kunst F, Rapoport G. MecB of Bacillus subtilis, a member of the ClpC ATPase family, is a pleiotropic regulator controlling competence gene expression and survival at high temperature. Proc Natl Acad Sci USA. 1994;91:5788–5792. doi: 10.1073/pnas.91.13.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nanamiya H, Ohashi Y, Asai K, Moriya S, Ogasawara N, Fujita M, Sadiae Y, Kawamura F. ClpC regulates the fate of a sporulation initiation sigma factor, ςH protein, in Bacillus subtilis at elevated temperatures. Mol Microbiol. 1998;29:505–513. doi: 10.1046/j.1365-2958.1998.00943.x. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Martin J, Fernando R, de Lorenzo V. Promoters responsive to DNA bending: a common theme in procaryotic gene expression. Microbiol Rev. 1994;58:268–290. doi: 10.1128/mr.58.2.268-290.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riethdorf S, Völker U, Gerth U, Winkler A, Engelmann S, Hecker M. Cloning, nucleotide sequence, and expression of the Bacillus subtilis lon gene. J Bacteriol. 1994;176:6518–6527. doi: 10.1128/jb.176.21.6518-6527.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scharf C, Riethdorf S, Ernst H, Engelmann S, Völker U, Hecker M. Thioredoxin is as essential protein induced by multiple stresses in Bacillus subtilis. J Bacteriol. 1998;180:1869–1877. doi: 10.1128/jb.180.7.1869-1877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz A, Schumann W. hcrA, the first gene of the of Bacillus subtilis dnaK operon, encodes a negative regulator of class I heat shock genes. J Bacteriol. 1996;178:1088–1093. doi: 10.1128/jb.178.4.1088-1093.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulz A, Schwab S, Homuth G, Versteeg S, Schumann W. The htpG gene of Bacillus subtilis belongs to class III heat shock genes and is under negative control. J Bacteriol. 1997;179:3103–3109. doi: 10.1128/jb.179.10.3103-3109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith I, Paress P, Cabane K, Dubnau E. Genetics and physiology of the rel system of Bacillus subtilis. Mol Gen Genet. 1980;178:271–279. doi: 10.1007/BF00270472. [DOI] [PubMed] [Google Scholar]
- 38.Steinmetz M, Richter R. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene. 1994;142:79–83. doi: 10.1016/0378-1119(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 39.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 40.Stülke J, Hanschke R, Hecker M. Temporal activation of β-glucanase synthesis in Bacillus subtilis is mediated by the GTP pool. J Gen Microbiol. 1993;139:2041–2045. doi: 10.1099/00221287-139-9-2041. [DOI] [PubMed] [Google Scholar]
- 41.Trieu-Cout P, Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3′-5"-aminoglycoside phosphotransferase type III. Gene. 1983;23:331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- 42.Turgay K, Hamoen L, Venema G, Dubnau D. Biochemical characterization of a molecular switch involving the heat shock protein ClpC, which controls the activity of ComK, the competence transcription factor of Bacillus subtilis. Genes Dev. 1997;11:119–128. doi: 10.1101/gad.11.1.119. [DOI] [PubMed] [Google Scholar]
- 43.Vagner V, Dervyn E, Ehrlich S D. Proceedings of the 8th Conference on Bacilli. 1995. A vector for systematic analysis of unknown genes, abstr. T 14; p. 74. [Google Scholar]
- 44.Völker U, Engelmann S, Maul B, Riethdorf S, Völker A, Schmid R, Mach H, Hecker M. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology. 1994;140:741–752. doi: 10.1099/00221287-140-4-741. [DOI] [PubMed] [Google Scholar]
- 45.Völker U, Völker A, Haldenwang W G. Reactivation of the Bacillus subtilis anti-ςB antagonist, RsbV, by stress- or starvation-induced phosphatase activities. J Bacteriol. 1996;178:5456–5463. doi: 10.1128/jb.178.18.5456-5463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wetzstein M, Völker U, Dedio J, Löbau S, Zuber U, Schiesswohl M, Herget C, Hecker M, Schumann W. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J Bacteriol. 1992;174:3300–3310. doi: 10.1128/jb.174.10.3300-3310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X, Kang C M, Brody M S, Price C W. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 1996;10:2265–2275. doi: 10.1101/gad.10.18.2265. [DOI] [PubMed] [Google Scholar]
- 48.Yansura D G, Henner D J. Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci USA. 1984;81:439–443. doi: 10.1073/pnas.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan G, Wong S-L. Regulation of groE expression in Bacillus subtilis: the involvement of the ςA-like promoter of the inverted repeat sequence (CIRCE) J Bacteriol. 1995;177:5427–5433. doi: 10.1128/jb.177.19.5427-5433.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan G, Wong S-L. Isolation and characterization of Bacillus subtilis groE regulatory mutants: evidence for orf39 in the dnaK operon as a repressor gene in regulation of both groE and dnaK. J Bacteriol. 1995;177:6462–6468. doi: 10.1128/jb.177.22.6462-6468.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuber U, Schumann W. CIRCE, a novel heat shock element involved in regulation of heat shock operon dnaK of Bacillus subtilis. J Bacteriol. 1994;176:1359–1363. doi: 10.1128/jb.176.5.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]