Abstract
The ligand-gated outer membrane porin FepA serves Escherichia coli as the receptor for the siderophore ferric enterobactin. We characterized the ability of seven analogs of enterobactin to supply iron via FepA by quantitatively measuring the binding and transport of their 59Fe complexes. The experiments refuted the idea that chirality of the iron complex affects its recognition by FepA and demonstrated the necessity of an unsubstituted catecholate coordination center for binding to the outer membrane protein. Among the compounds we tested, only ferric enantioenterobactin, the synthetic, left-handed isomer of natural enterobactin, and ferric TRENCAM, which substitutes a tertiary amine for the macrocyclic lactone ring of ferric enterobactin but maintains an unsubstituted catecholate iron complex, were recognized by FepA (Kd ≈ 20 nM). Ferric complexes of other analogs (TRENCAM-3,2-HOPO; TREN-Me-3,2-HOPO; MeMEEtTAM; MeME-Me-3,2-HOPO; K3MECAMS; agrobactin A) with alterations to the chelating groups and different net charge on the iron center neither adsorbed to nor transported through FepA. We also compared the binding and uptake of ferric enterobactin by homologs of FepA from Bordetella bronchisepticus, Pseudomonas aeruginosa, and Salmonella typhimurium in the native organisms and as plasmid-mediated clones expressed in E. coli. All the transport proteins bound ferric enterobactin with high affinity (Kd ≤ 100 nM) and transported it at comparable rates (≥50 pmol/min/109 cells) in their own particular membrane environments. However, the FepA and IroN proteins of S. typhimurium failed to efficiently function in E. coli. For E. coli, S. typhimurium, and P. aeruginosa, the rate of ferric enterobactin uptake was a sigmoidal function of its concentration, indicating a cooperative transport reaction involving multiple interacting binding sites on FepA.
Pathogenic and commensal bacteria alike obtain iron from human and animal hosts by competing for the metal with eucaryotic proteins, like transferrin, lactoferrin, and ferritin (11, 25, 49, 54). In the wild, the low solubility of iron in aqueous, aerobic conditions further complicates its acquisition: the concentration of available Fe3+ at neutrality is 10−18 M (34), whereas bacteria require a minimum of 10−8 M for growth and 10−6 M for iron sufficiency (24). A wide variety of microbes synthesize specialized, low-molecular-mass (500 to 1,000 Da) organic chelators called siderophores (5, 16, 17, 18, 33, 37, 38, 40, 41, 46, 52, 66) that solve these problems. Siderophores (32) liberate iron from the sequestering proteins of eucaryotic hosts or solubilize it from precipitates of ferric oxyhydroxide, rendering the metal available for microbial consumption.
Enterobactin, the native siderophore of Escherichia coli and the most avid microbial iron chelator (Ka = 1052 [10]) contains three dihydroxybenzoyl serine groups linked in a macrocyclic lactone ring (Fig. 1). Its three identical catechol groups chelate Fe3+ in a Δ-cis complex, creating a hexadentate iron center with a net charge of −3. Several species of Enterobacteriaceae, including E. coli (37, 46), Salmonella typhimurium, (42) and Klebsiella pneumoniae (40), produce enterobactin in response to iron stress. Many pathogenic bacteria that do not make enterobactin produce membrane transport systems that recognize and transport ferric enterobactin (FeEnt) (48), so the determinants of siderophore uptake may influence the pathogenicity of enteric bacteria.
FIG. 1.
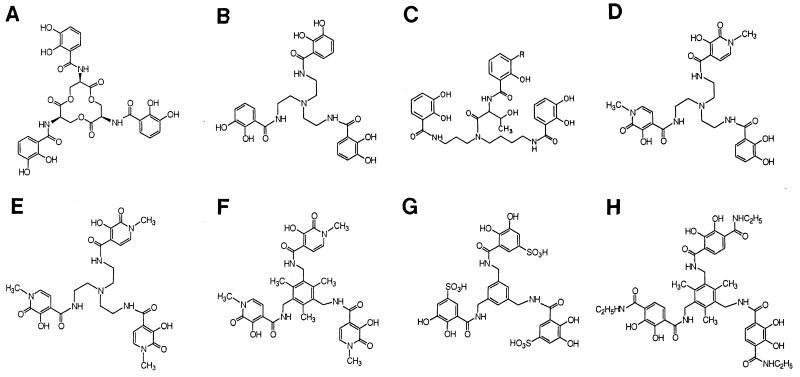
Structures of natural and synthetic catecholate siderophores. (A) Enterobactin; (B) TRENCAM; (C) agrobactin A; (D) TRENCAM-3,2-HOPO; (E) TREN-Me-3,2-HOPO; (F) MeME-Me-3,2-HOPO; (G) K3MECAMS; (H) MeMEEtTAM.
FeEnt binds to FepA, an 81-kDa outer membrane protein that also serves as a receptor for two protein toxins, colicins B and D (19, 28, 39, 44, 62, 69). All three ligands interact with two arginine residues within proposed loop 5 (36) in the central region of FepA. FeEnt transport across the outer membrane requires energy and the participation of TonB (19, 58–60); a complex of proteins (9, 13, 51) mediates uptake through the inner membrane.
Synthetic analogs that mimic enterobactin but change certain aspects of its chemistry were previously used to determine the structural features of the siderophore that are important to its transport. For example, the catechol groups of FeEnt form a right-handed propeller around iron, and experiments with its left-handed analog showed the importance of this chirality: ferric enantioenterobactin (FeEnEnt), the mirror image of the natural siderophore, does not provide iron to E. coli (35). Studies of other analogs with modifications to either the chelating groups or the organic platform from which they arise showed that the iron center contains the primary determinants of the uptake reaction. Replacement of the natural macrocyclic ring had little effect on FeEnt transport (15, 21).
We further analyzed the siderophore transport process by determining the principal features of ferric enterobactin that affect its binding to FepA. Experiments with synthetic siderophores and the natural compound agrobactin A reaffirmed the importance of the iron center in binding: high-affinity adsorption of ferric siderophores to FepA required unadulterated catechol moieties around the central iron atom. Unexpectedly, the chirality of the iron complex did not affect the receptor-ligand interaction: FeEnEnt bound to FepA with affinity comparable to that of FeEnt, indicating that the recognition reaction is not stereospecific. We also studied the biochemical properties of FepA homologs of Bordetella, Pseudomonas, and Salmonella species with regard to FeEnt binding and transport. Although the affinities of the various proteins for the siderophore were comparable, we found differences in their transport rates, especially when they were all expressed and compared in E. coli.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains, plasmids, and sources are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype | Reference |
|---|---|---|
| Strains | ||
| E. coli BN1071 | F− thi entA pro trp rpsL | 24 |
| E. coli KDF541 | BN1071 recA fepA tonA cir | 47 |
| S. typhimurium Enb7 | entA fepA+ | 42 |
| P. aeruginosa K201 | pfeA+ | 43 |
| P. aeruginosa K407 | K201 pfeA::Tn501 | 43 |
| B. bronchisepticus 19385 | bfeA+ | 4 |
| B. bronchisepticus 19387 | 19385 bfeΩpKS3 | 4 |
| Plasmids | ||
| pITS449 | E. coli fepA+ | 2 |
| pENB5 | Salmonella enterica fepA+ | 57 |
| pTY994 | Salmonella enterica iroN+ | 3 |
| pCD | P. aeruginosa pfeA+ | 14 |
| pKP1 | B. pertussis bfeA+ | 4 |
Siderophore preparation.
Enterobactin was purified from E. coli AN102 (24). Iron complexes of enterobactin and its analogs (Fig. 1) were formed by mixing equimolar amounts of the siderophores and FeCl3 dissolved in 0.45 ml of methanol and 0.45 ml of 0.001 M HCl, respectively. For radioisotope studies, 0.05 mCi of 59FeCl3 (Amersham) was added to the FeCl3 solution. The ferric siderophore solution was incubated for 1 to 2 h at room temperature, 100 μl of 0.5 M sodium phosphate was added, and the iron complexes were chromatographically purified on Sephadex LH20 (61). The concentrations of ferric complexes of enterobactin and its analogs were spectrophotometrically determined by using their millimolar extinction coefficients: enterobactin (42), ɛ495 = 5.6; K3MECAMS (64), ɛ488 = 0.81; TRENCAM (67), ɛ486 = 4.27; TRENCAM-3,2-HOPO (68), ɛ538 = 3.1; TREN-Me-3,2-HOPO (67), ɛ530 = 3.6; MeME-Me-3,2-HOPO (68), ɛ548 = 3.78; MeMEEtTAM (68), ɛ524 = 3.2; agrobactin A (38), ɛ513 = 3.5.
Siderophore binding and transport experiments.
Bacteria harboring fepA+ or mutant fepA alleles on pUC plasmids were grown overnight in Luria-Bertani broth (29) containing ampicillin (100 μg/ml), subcultured into MOPS (morpholinepropanesulfonic acid) minimal medium (31) with ampicillin (10 μg/ml), and grown for 5.5 h at 37°C. In some experiments, enterobactin was added to the culture medium at 2 μM. For binding studies, six 10-ml aliquots were collected and incubated on ice for 1 h, and 59Fe siderophore at six different concentrations was added (36). One and 6 min after addition of the siderophore, 5-ml aliquots were collected and filtered through glass fiber filters that then were washed with 10 ml of 0.9% LiCl and counted. Transport experiments (15, 48, 36) were performed quantitatively in MOPS medium and qualitatively as siderophore nutrition assays (62). Binding and transport data were analyzed and plotted using Grafit (versions 3 and 4; Erithacus Software Ltd., Middlesex, England).
Outer membranes and gel electrophoresis.
A total of 1010 cells were resuspended in 10 ml of Tris-buffered saline (TBS) with trace amounts of DNase and RNase and lysed by passage through a French pressure cell at 14,000 lb/in2. Unbroken cells were removed by centrifugation at 3,000 × g for 15 min, and the supernatant containing the inner and the outer membranes was collected by centrifugation at 100,000 × g for 1 h. The pellet was resuspended in 1 ml of TBS containing 0.5% sodium sarcosinate and incubated for 30 min at room temperature (48). The extract was spun for 45 min at 20,000 × g, and the pellet containing the outer membranes was resuspended in sample buffer, boiled for 5 min, briefly centrifuged to remove insoluble material, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (1, 20).
Western blot analysis.
Outer membrane proteins separated on polyacrylamide gels were transferred to nitrocellulose membranes by electrophoresis at 10 V overnight (55). The nitrocellulose was blocked with 1% gelatin, incubated with anti-FepA monoclonal antibodies 2, 27, and 45 (30) followed by goat anti-mouse immunoglobulin G-alkaline phosphatase, and developed with a mixture of nitroblue tetrazolium-bromochloroindolyl phosphate (7).
RESULTS
Binding and transport of FeEnt analogs.
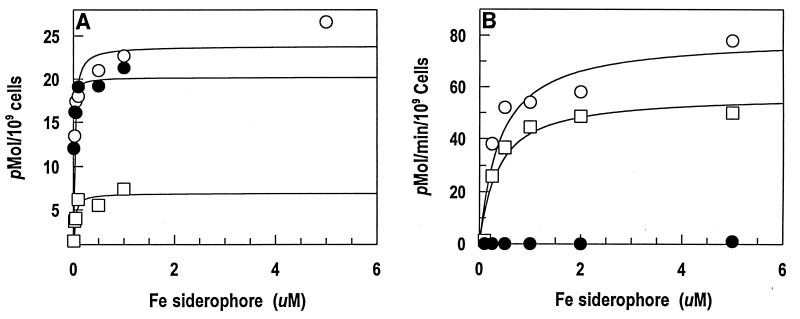
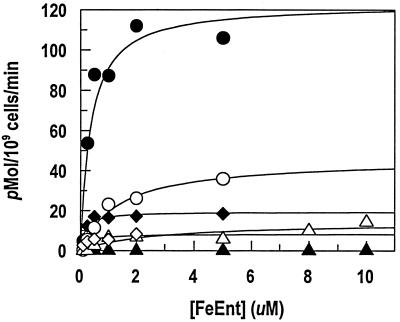
We measured the binding equilibria between enterobactin analogs (Fig. 1) and E. coli FepA by using strain BN1071 (entA) and its derivative KDF541 (entA fepA cir). FeEnEnt, the Λ analog of natural Δ FeEnt, bound to FepA with high affinity (Kd = 21 nM) (Table 2). This result was unexpected because it was previously reported (35), and we confirmed, that FeEnEnt does not promote siderophore nutrition activity for E. coli (Table 2). Similarly, FeTRENCAM, a synthetic analog that mainly differs from enterobactin in the substitution of a tertiary amine for the macrocyclic ester ring of the natural product (Fig. 1), avidly bound to FepA (Fig. 2). The FeTRENCAM-FepA interaction had a Kd of 27 nM, experimentally indistinguishable from that of the FeEnt-FepA interaction (17 nM). However, FeTRENCAM adsorbed to FepA at only one-third the capacity of the native E. coli siderophore. In siderophore nutrition tests, FeTRENCAM did not supply iron to KDF541, but did feed BN1071 and KDF541/pITS449. The growth halo it induced was slightly smaller (15 mm) and noticeably fainter than that generated by FeEnt (18 mm). Quantitative transport assays revealed a Km for FeTRENCAM (Fig. 2) uptake of 0.37 μM, again experimentally indistinguishable from that of FeEnt (Km = 0.41 μM). The Vmax of FeTRENCAM transport was about half that of FeEnt, consistent with the lower binding capacity for the synthetic siderophore and the faint halos it generated in siderophore nutrition assays.
TABLE 2.
Binding and transport characteristics of FeEnt uptake systems in E. coli, Salmonella, Pseudomonas, and Bordetella
| Strain (growth medium supplement) | Protein | Siderophore | Binding resulta
|
Transport resultb
|
|||
|---|---|---|---|---|---|---|---|
| Kd (nM) | Capacity (pmol/109 cells) | Km (nM) | Vmax (pmol/min/109 cells) | Nutrition (mm) | |||
| E. coli BN1071 | EcoFepA | FeTRENCAM | 27 | 7 | 370 | 56 | 15 |
| E. coli BN1071 | EcoFepA | FeEnEnt | 21 | 20 | |||
| E. coli BN1071 | EcoFepA | FeEnt | 17 | 24 | 394 | 101 | 18 |
| S. typhimurium Enb7 | StyFepA | FeEnt | 43 | 38 | 424 | 101 | 18 |
| P. aeruginosa K201 | PaeFepA | FeEnt | NA | ||||
| P. aeruginosa K201 (enterobactin) | PaeFepA | FeEnt | 11 | 15 | 384 | 53 | NA |
| B. bronchisepticus 19385 | BbrFepA | FeEnt | 1,394 | 6 | 2,300 | 25 | NA |
| B. bronchisepticus 19385 (enterobactin) | BbrFepA | FeEnt | 91 | 12 | 1,500 | 65 | NA |
| KDF541/pITS449 | EcoFepA | FeEnt | 13 | 94 | 299 | 82 | 18 |
| KDF541/pENB5 | StyFepA | FeEnt | 19 | 106 | 2,100c | 14c | 14 |
| KDF541/pTY994 | StyIroN | FeEnt | 15 | 78 | 165 | 20 | 16 |
| KD541/pCD | PaeFepA | FeEnt | 18 | 73 | 312 | 70 | 20 |
| KDF541/pKP1 | BpeFepA | FeEnt | 120 | 3 | |||
The mean values for Kd and capacity were derived from three or four experiments and plotted with GRAFIT (Erithacus) using the Bound versus Total equation. The mean standard errors for the values of Kd and capacity were 24 and 9%, respectively.
Data from transport experiments were plotted with Grafit to yield the kinetic parameters Km and Vmax. The mean standard errors for the values of Km and Vmax were 32 and 11%, respectively. Siderophore nutrition assays were conducted twice, and little variation was observed among the trials. NA, not applicable.
Values were calculated from transport experiments using an extended, 1-h uptake period. All other transport data derived from experiments with a 5-min uptake period.
FIG. 2.
Binding (A) and transport (B) of FeEnt (○), FeEnEnt (•), and FeTRENCAM (□) by E. coli BN1071. Bacteria were cultured in iron-deficient MOPS medium and exposed to 59Fe siderophores at the indicated concentrations.
We also measured the binding and transport of several other catecholate siderophores as a means of identifying structural features that affect recognition reaction by FepA. Iron complexes of TRENCAM derivatives that substitute an N-methyl-3-3-hydroxy-2-(1H)-pyridine unit for either two (TRENCAM-3,2-HOPO) or all three (TREN-Me-3,2-HOPO) catechols did not bind to FepA, nor did they supply iron in siderophore nutrition tests. Similarly, the related compounds FeMeMEEtTAM and FeMeME-Me-3,2-HOPO, which contain N-ethyl amide or methyl groups in the para position of the chelating aromatic rings (Fig. 1), did not interact with FepA. E. coli recognizes and utilizes another synthetic analog, MECAM, that replaces the central ester ring of enterobactin with a substituted benzene ring and, like TRENCAM, maintains pure catechol chelation moieties (21). However, FeK3MECAMS, which contains sulfonyl groups in the meta position of the catecholates, did not bind to FepA. In summary, essentially any changes in the face of the iron center, introducing either polar or nonpolar atoms, abrogated the receptor-ligand interaction. Most of the molecules that failed to specifically bind to FepA did nonspecifically adsorb to the bacterial cell surface without saturation. Only TRENCAM-3,2-HOPO and TREN-Me-3,2-HOPO showed saturation binding to another, unknown outer membrane protein, at relatively low capacities (data not shown). The nonproductive nature of these adsorptions was confirmed by the failure of the compounds in siderophore nutrition tests.
Agrobactin A (38) is a spermidine-based siderophore that, like FeEnt, carries three catechol groups (Fig. 1). However, in agrobactin A, only two of the catechols fully chelate ferric ion, and the fifth and sixth ligands to the metal derive from the nitrogen of an oxazoline ring and from the proximal hydroxyl of the third catechol group, leaving the majority of the third aromatic ring displaced away from the iron center (34). In spite of its catecholate chemistry, ferric agrobactin A (FeAgroA) neither bound to FepA nor supported the growth of wild type E. coli BN1071, indicating that even a slight change in the nature of the iron center may debilitate binding.
FeEnt binding and transport by Salmonella, Pseudomonas, and Bordetella.
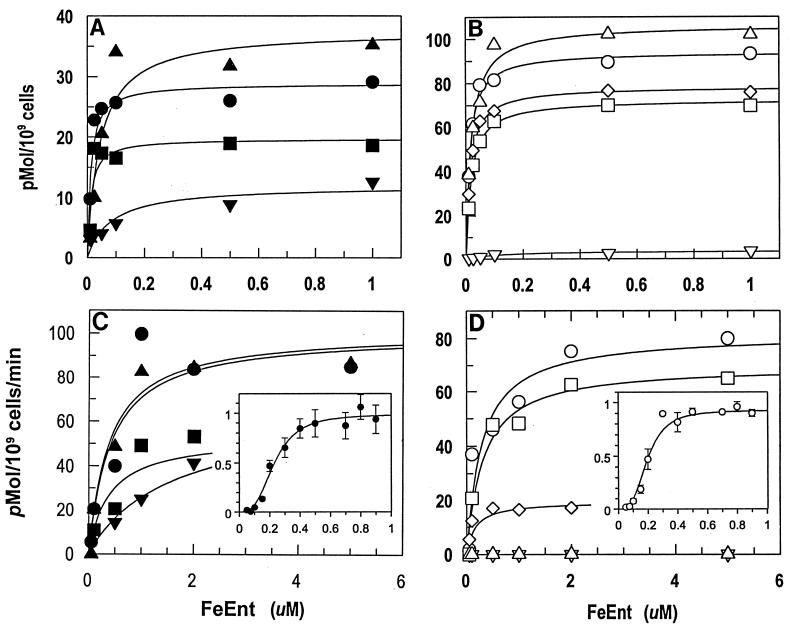
We compared the outer membrane FeEnt transporters of E. coli (EcoFepA), S. typhimurium (StyFepA, StyIroN), Pseudomonas aeruginosa (PaeFepA), Bordetella bronchisepticus (BbrFepA), and Bordetella pertussis (BpeFepA) with regard to 59FeEnt binding and uptake (Table 2). As an initial experiment, we tested four of the bacterial species in vivo in iron-deficient MOPS medium. Under these conditions, E. coli and S. typhimurium bound the siderophore with high affinity (Kd < 50 nM). These two strains transported FeEnt at about the same rate, 100 pmol/min/109 cells. In iron-deficient media, P. aeruginosa did not bind or transport FeEnt, while B. bronchisepticus adsorbed and transported it at very low levels. Neither of the latter two strains synthesizes enterobactin, and therefore they do not normally express their FeEnt transport systems at readily detectable levels, even when subjected to iron stress. However, if they were cultured beforehand with enterobactin, then the binding capabilities of Pseudomonas and Bordetella were similar to those of the Enterobacteriaceae (Fig. 3) (Table 2). When induced in this manner, P. aeruginosa and B. bronchisepticus showed a maximal rate of FeEnt uptake of about half that of E. coli and Salmonella, commensurate with the variations in FepA expression seen in immunoblots (Fig. 4) and inferred from FeEnt binding capacities (Table 2). Precise measurements at concentrations near the transport Km produced sigmoidal (allosteric) uptake curves for E. coli (Fig. 3), S. typhimurium, and P. aeruginosa (data not shown); these data did not fit a hyperbolic (Michaelis-Menten) function. Kinetic analyses of B. bronchisepticus transport data were inconclusive in this respect.
FIG. 3.
Binding and transport of FeEnt by FepA homologs. The concentration dependence of FeEnt binding (A) and transport (C) was measured for chromosomally expressed FepA of E. coli (•) and S. typhimurium (▴) grown in MOPS medium, and P. aeruginosa (■) and B. bronchisepticus (▾) cultured in MOPS medium with enterobactin (2 μM). Binding (B) and transport (D) of FeEnt was also measured for the individual proteins EcoFepA (○), StyFepA (▵), StyIroN (◊), PaeFepA (□), and BpeFepA (▿), expressed from plasmids in E. coli. Insets show transport of FeEnt by E. coli FepA at concentrations near the Km. The plotted data represent mean values (with standard deviations) normalized to Vmax from seven experiments. These curves gave Hill coefficients of 2.98 and 3.19 for chromosome- and plasmid-expressed FepA, respectively, with standard errors less than 5%.
FIG. 4.
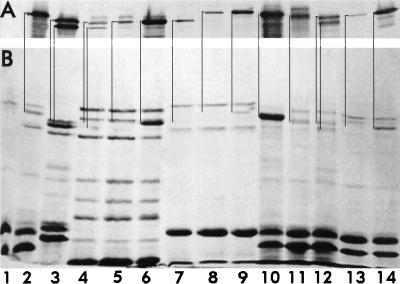
Chromosome- and plasmid-expression of FepA and its homologs. Outer membranes were prepared by Sarkosyl extraction of cell envelopes from bacteria grown in MOPS minimal medium, subjected to SDS-PAGE, and either stained with Coomassie blue (B) or transferred to nitrocellulose and stained with anti-FepA monoclonal antibodies 2, 27, 45, (30), goat anti-mouse immunoglobulin-alkaline phosphatase, and nitroblue tetrazolium-bromochloroindolyl phosphate (7) (A). E. coli KDF541 (lane 1) and BN1071 (lane 2), S. typhimurium Enb7 (lane 3), P. aeruginosa K407 (lane 4) and K201 (lane 5), and B. bronchisepticus 19387 (lane 7) and 19385 (lane 8) were cultured in iron-deficient MOPS medium. Strains K201 and 19385 were also grown in MOPS medium containing enterobactin (2 μM; lanes 6 and 9, respectively). Outer membranes from KDF541, grown in MOPS medium and harboring either pITS449 (EcoFepA; lane 10), pENB5 (StyFepA; lane 11), pTY994 (StyIroN; lane 12), pKP1 (BpeFepA; lane 13), or pCD3 (PaeFepA; lane 14) were also analyzed. Immunoreactive bands in the immunoblot correspond to the indicated stained bands in the SDS-PAGE gel.
FeEnt binding by Salmonella, Pseudomonas, and Bordetella FepA proteins, expressed in E. coli.
The differences in the outer membranes of the species under study suggested that expression of all the FeEnt transporters in a common background might provide a more stringent test of the effects of sequence variation on functionality. Thus, we expressed cloned BpeFepA, PaeFepA, StyFepA, StyIroN, and EcoFepA in KDF541. SDS-PAGE and Western immunoblots with crossreactive anti-(E. coli)FepA monoclonal antibodies verified expression of the five proteins (Fig. 4): each foreign gene directed the synthesis of an immunoreactive, approximately 80-kDa outer membrane protein, albeit weakly in the case of BpeFepA.
Experiments on FeEnt adsorption to the cloned transport proteins in E. coli showed that all the FepA homologs bound the siderophore with affinities comparable to those observed in their native membrane environments. BpeFepA showed about a 10-fold lower avidity for FeEnt than the other proteins, but this result concurred with data collected from B. bronchisepticus in vivo. Comparison of binding data from the laboratory E. coli strain and the pathogenic gram-negative organisms mainly showed disparities in capacities that paralleled FepA expression levels. The affinity of plasmid-mediated EcoFepA for FeEnt was indistinguishable from that of the chromosomally expressed protein (Table 2), but KDF541/pITS449 bound FeEnt with a capacity approximately three times higher than BN1071 (Fig. 3). The increased capacity was expected, because the plasmid system expresses FepA at a higher level (Fig. 4). Conversely, KDF541 expressed BpeFepA from plasmid pKP1 at only very low levels (Fig. 4). The Bordetella protein showed a reasonable affinity for FeEnt (Kd = 120 nM [Fig. 3]), but adsorbed it to a much lower capacity (3 pmol/109 cells) than the other FepA homologs. E. coli expressed PaeFepA, StyFepA, and StyIroN to levels similar to that of EcoFepA (Fig. 4 and data not shown), and under these conditions the three FepA homologs manifested comparable affinity and capacity for FeEnt (Fig. 3) (Table 2).
FeEnt uptake by Salmonella, Pseudomonas, and Bordetella FepA proteins, expressed in E. coli.
Uptake assays in E. coli confirmed the differences seen in binding assays of the five FeEnt transporters, with several nuances and exceptions. First, in spite of their avid binding, StyFepA and StyIroN did not efficiently transport FeEnt. Standard uptake assays failed to detect any transport of 59FeEnt by StyFepA and detected only a small amount of transport by StyIroN. However, StyFepA in KDF541 consistently produced positive siderophore nutrition assays (Fig. 5; Table 2), which led us to reevaluate the transport assay conditions. An increase in the uptake period from 5 to 60 min revealed the transport reaction (Fig. 6) (Vmax = 14 pmol/min/109 cells). StyIroN showed about the same rate in both conditions (Vmax = 10 to 20 pmol/min/109 cells), considerably slower than transport through E. coli FepA (Vmax = 46 pmol/min/109 cells). Thus in E. coli, both Salmonella outer membrane proteins recognized FeEnt but transported it with reduced efficiency, about one-fifth the maximum rate observed for the native strain.
FIG. 5.
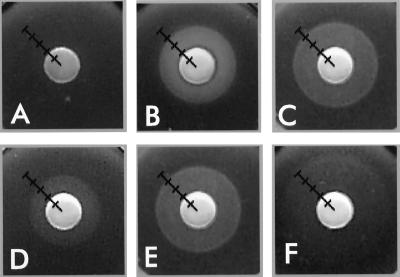
Siderophore nutrition tests. KDF541 (A) grown in Luria-Bertani broth and harboring plasmids pITS449 (EcoFepA) (C), pENB5 (StyFepA) (D), pTY994 (StyIroN) (E), or pCD3 (PaeFepA) (F) was plated in nutrient agar containing ampicillin (10 μg/ml), and 10 μl of 50 μM FeEnt was applied to a sterile paper disc on the agar surface. BN1071 (chromosomal EcoFepA) (B) was tested under the same conditions. A 1-cm ruler was embedded in the photograph.
FIG. 6.
Transport of FeEnt by Salmonella proteins expressed in E. coli. Uptake of 59FeEnt by EcoFepA (○), StyFepA (▵), and StyIroN (◊) was compared by two procedures, 5-min assays (filled symbols) and 60-min assays (open symbols).
The transport characteristics of BpeFepA and PaeFepA in E. coli were consistent with what occurred in Bordetella and Pseudomonas. The pseudomonad protein bound and transported FeEnt much like EcoFepA, while BpeFepA bound the siderophore, but only at a low capacity, and did not transport it at measurable levels. The poor expression of the BpeFepA protein in E. coli (Fig. 4) probably accounts for these results.
Comparisons between chromosome- and plasmid-encoded EcoFepA in E. coli (Fig. 3) showed that the expression system does not affect the affinity of the protein for FeEnt: the binding Kd and transport Km did not vary under the two conditions. Furthermore, as in the chromosomal system, plasmid-encoded FepA catalyzed allosteric uptake reactions (Fig. 3), but at an ostensibly slower rate. That is, the threefold-higher FepA levels created by plasmid-mediated expression did not change Vmax. The maximum uptake rates of the two systems were the same, indicating that chromosomal expression allows faster ligand internalization per FepA monomer, i.e., an ostensibly threefold-higher monomer turnover number.
DISCUSSION
Recognition of FeEnt by FepA involves noncovalent bonds between atoms of the iron chelate and surface-exposed residues of the outer membrane protein. Binding reactions between analogs of FeEnt and E. coli FepA, or between FeEnt and homologs of FepA from other bacteria, may identify the critical aspects of ligand-receptor specificity. Our results from such experiments lead to the following three conclusions about the FeEnt-FepA binding reaction: (i) it is not stereospecific; (ii) it is intolerant of modifications to the catechol groups surrounding the metal; and (iii) its affinity is relatively invariant among diverse bacterial species.
Siderophore nutrition by the Δ-cis-FeEnt complex is stereospecific in E. coli (35, 45, Table 2), but preference for right-handed chirality does not originate at the stage of binding between the ferric siderophore and FepA. Λ-cis-FeEnEnt bound to FepA with equivalent affinity and capacity, showing that the chiral specificity resides in a subsequent stage of the uptake process, likely after transport through FepA (36a). The preference for Δ chirality was not absolute: although 59Fe-EnEnt transport was not observed, a very faint halo developed around the Λ iron complex in siderophore nutrition assays after an extended incubation period (36 h).
FepA recognizes the iron center of FeEnt, three catechol groups complexed to Fe+++, in the initial step of transport through the outer membrane. The efficacy of MECAM in supplying iron to E. coli initially demonstrated the importance of the metal center (15, 21), and the similar ability of TRENCAM underscores this conclusion: both compounds replace the macrocyclic ester ring of FeEnt without changing its catecholate coordination complex and both are recognized and transported by FepA. Experiments that measured the affinity of the cell surface ligand binding reaction confirmed the importance of unsubstituted catecholate moieties around iron in FeEnt. FeTRENCAM bound to FepA with equal affinity and specificity, while analogs that introduced any other substituents on the rings did not bind to FepA. Although these results suggest that the size and shape of the iron center are crucial to the binding reaction, charge, which was implicated by another approach (36), may also play a role in the failure of the synthetic iron chelates to bind. In most of the compounds that did not specifically adsorb, the net charge of the iron center was different.
Nevertheless, the failure of FeAgroA to bind or to supply iron confirms the importance of the shape of the iron center in recognition by the receptor. Like FeEnt, FeAgroA is negatively charged (−2.5 [34]) and contains three catechol groups in a right-handed complex. However, one of these, which provides only a single ligand to iron, projects off the iron center and distorts its size and symmetry (34). Its spermidine backbone also distinguishes FeAgroA, but MECAM and TRENCAM established the relative unimportance of this part of the siderophore in binding, intimating that the different shape of the FeAgroA iron center prevents its adsorption to FepA. The face of the FeEnt iron complex is relatively flat (23), and this feature may be requisite for binding. The lack of siderophore nutrition by FeAgroA concurs with the results of Ong et al. (38), who reported that iron complexes of agrobactin and agrobactin A at concentrations as high as 50 μM did not promote the growth of E. coli.
The different binding capacities of FeEnt and FeTRENCAM must stem from structural variations in the two siderophores that center on the presence of a tertiary amine in the synthetic siderophore. This basic group carries a positive charge at neutrality, while the macrocyclic ring of FeEnt is uncharged. Thus the reduced capacity of FepA for FeTRENCAM may derive from an ionic interaction that reduces its saturation level threefold. One explanation of these data is that, like all the structurally characterized porins (12, 26, 50, 63), FepA exists in vivo as a trimer that accommodates three molecules of FeEnt but only a single molecule of FeTRENCAM. The receptor protein binds the iron center of FeTRENCAM and, although the positive charge on the back of the molecule did not affect the affinity of its adsorption, it may create a charge repulsion barrier that prevents subsequent adsorption of another positively charged chelate, thus reducing binding capacity. An analytical comparison of FeEnt and colicins binding to FepA (39) showed similar results: colicin D bound to FepA at one-third the capacity of colicin B and FeEnt. Furthermore, nondenaturing SDS-PAGE revealed a high-molecular-weight oligomer of FepA with the mass of a trimer (27). Although another ligand-gated porin, the ferrichrome receptor, was purified in monomeric form (8), several independent lines of evidence now support the trimeric structure of FepA in vivo. Relevant to this point, our transport data demonstrate, for the first time, sigmoidal uptake kinetics for FeEnt. When analyzed as allosteric reactions, the transport data yielded Hill coefficients of approximately 3, consistent with a native FepA protein that contains three interacting, cooperative binding sites. These may be equivalent sites on the monomers of a trimer, or three distinct sites on a monomeric protein. In either case, our data indicate that multiple binding sites within FepA function allosterically during FeEnt transport.
The comparable affinities of the five gram-negative bacterial outer membrane transport proteins for FeEnt was unexpected. Against a background of broad overall genetic diversity, the four species manifested a remarkable preservation of avidity for FeEnt. While the structural genes, regulatory systems, and transport components of these organisms adapted to their individual biological needs, the specificity of their outer membrane receptors persevered essentially unchanged. The overall variation in the proteins themselves, from E. coli to B. pertussis, is dramatic, and yet the surface that accepts FeEnt remains relatively unaltered: only a 10-fold drop in affinity occurred in the most distant FepA homolog, B. pertussis. The evolution of bacterial outer membrane proteins occurs most rapidly in their surface loops (22, 48), and the FeEnt binding site illustrates conservation of a functional domain within an overall framework of high sequence variability.
The retention of a FeEnt transport system in Pseudomonas and Bordetella, with modifications of its regulation, illustrates the importance of iron acquisition in bacterial pathogenesis. In both of these disease-causing organisms, the regulatory system has changed from negative and repressible to positive and inducible. That is, the presence of enterobactin induces synthesis of the receptor for its ferric complex, an advantageous strategy for organisms that infect tissues cohabited by enterobactin-secreting bacteria. Transport of FeEnt into P. aeruginosa and B. bronchisepticus occurred at rates similar to those of the enterobactin producers S. typhimurium and E. coli, about 50 pmol/min/109 cells. Although prior work with a Salmonella model system questioned the relationship between FeEnt-mediated iron acquisition and bacterial pathogenesis (6), an overwhelming amount of data now links iron and virulence. The connection appears in studies of Neisseria (Tbp1/transferrin [11]), Vibrio (IrgA [53]), Yersinia (Psn/yersiniabactin [5]), and Escherichia and Salmonella (IutA/aerobactin [65]; TonB [56]) species. In the context of these examples relating iron to infection, the inducible FeEnt transport systems of Bordetella and Pseudomonas emphasize the value of siderophores to bacteria: FeEnt is such a ubiquitous and potent iron complex that these pathogenic bacteria have evolved to steal it from their competitors.
Although plasmid effects are a potential explanation for the poor function of foreign proteins in E. coli, the reduced efficacy of the Salmonella FeEnt transporters StyFepA and StyIroN did not derive from poor expression or targeting to the E. coli outer membrane. Both Salmonella proteins bound FeEnt with high affinity, providing evidence of properly folded, biologically active conformations. The likely explanation for their inferiority is that additional components of the FeEnt uptake systems of Salmonella and Escherichia are sufficiently different to impair the transport reaction. On the other hand, E. coli FepA itself manifested a lower transport rate when expressed from pUC. The multicopy plasmid effected a two- to threefold higher expression level expression than the chromosomal system, but the increase did not cause a higher rate of FeEnt uptake. In both cases we observed an overall rate of 100 pmol/min/109 cells, which translates to a monomer transport rate for chromosomally produced FepA of 1 mol/20 s and for plasmid-expressed FepA of 1 mol/min. These calculations suggest that the higher expression levels encoded by the plasmid exceeded the overall capabilities of the transport complex. The deficiencies in the plasmid system may ensue from inadequate amounts of one or more of the other required components of the cell envelope, including FepB, TonB, or another as-yet-unidentified molecule. At present, however, we cannot fully explain the lower activity of the plasmid-based FepA proteins.
ACKNOWLEDGMENTS
This work was supported by NIH grants 1R01GM53836 and 1P20RR11822 to P.E.K., NIH grant AI11744 to K.N.R., and NSF grant MCB9709418 to P.E.K.
We thank Keith Poole, Bernard Beale, Andreas Baumler, and Rolf Reissbrodt for providing bacterial strains and Paul Cook for helpful discussions.
REFERENCES
- 1.Ames G F-L. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974;249:633–644. [PubMed] [Google Scholar]
- 2.Armstrong S A, Francis C A, McIntosh M A. Molecular analysis of the Escherichia coli ferric enterobactin in receptor FepA. J Biol Chem. 1990;265:14536–14543. [PubMed] [Google Scholar]
- 3.Bäumler A J, Norris T L, Lasco T, Voigt W, Reissbrodt R, Rabsch W, Heffron F. IroN, a novel outer membrane siderophore receptor characteristic of Salmonella enterica. J Bacteriol. 1997;180:1446–1453. doi: 10.1128/jb.180.6.1446-1453.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beall B, Sanden G N. A Bordetella pertussis fepA homologue required for utilization of exogenous ferric enterobactin. Microbiology. 1995;141:3193–3205. doi: 10.1099/13500872-141-12-3193. [DOI] [PubMed] [Google Scholar]
- 5.Bearden S W, Fetherston J D, Perry R D. Genetic organization of the yersiniabactin biosynthetic region and construction of avirulent mutants in Yersinia pestis. Infect Immun. 1997;65:1659–1668. doi: 10.1128/iai.65.5.1659-1668.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin W H, Turnbough C L, Posey B S, Briles D E. The ability of Salmonella typhimurium to produce the siderophore enterobactin is not a virulence factor in mouse typhoid. Infect Immun. 1985;50:392–397. doi: 10.1128/iai.50.2.392-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bentley A T, Klebba P E. Effect of lipopolysaccharide structure on reactivity of antiporin monoclonal antibodies with the bacterial cell surface. J Bacteriol. 1988;170:1063–1068. doi: 10.1128/jb.170.3.1063-1068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulanger P, Le Maire M, Bonhivers M, Dubois S, Desmadril M, Letellier L. Purification and structural and functional characterization of FhuA, a transporter of the Escherichia coli outer membrane. Biochemistry. 1996;35:14216–14224. doi: 10.1021/bi9608673. [DOI] [PubMed] [Google Scholar]
- 9.Brickman T J, McIntosh M A. Overexpression and purification of ferric enterobactin esterase from Escherichia coli. Demonstration of enzymatic hydrolysis of enterobactin and its iron complex. J Biol Chem. 1992;267:12350–12355. [PubMed] [Google Scholar]
- 10.Carrano C J, Raymond K N. Ferric ion sequestering agents. 2. Kinetics and mechanism of iron removal from transferrin by enterobactin and synthetic tricatechols. J Am Chem Soc. 1979;101:5401–5404. [Google Scholar]
- 11.Cornelissen C N, Kelley M, Hobbs M M, Anderson J E, Cannon J G, Cohen M S, Sparling P F. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1997;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 12.Cowan S W, Schirmer T, Rummel G, Steiert M, Ghosh R, Pauptit A, Jansonius J N, Rosenbusch J P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992;358:727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- 13.Cox G B, Gibson F, Luke R K J, Newton N A, O’Brien I G, Rosenberg H. Mutations affecting iron transport in Escherichia coli. J Bacteriol. 1970;104:219–226. doi: 10.1128/jb.104.1.219-226.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean C R, Poole K. Cloning and characterization of ferric enterobactin receptor gene (pfeA) of Pseudomonas aeruginosa. J Bacteriol. 1993;175:317–324. doi: 10.1128/jb.175.2.317-324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ecker D, Matazanke B, Raymond K N. Recognition and transport of ferric enterobactin in Escherichia coli. J Bacteriol. 1986;167:666–673. doi: 10.1128/jb.167.2.666-673.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emery T. Hydroxamic acids of natural origin. Adv Enzymol. 1971;35:135–185. doi: 10.1002/9780470122808.ch4. [DOI] [PubMed] [Google Scholar]
- 17.Gibson F, McGrath D I. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes. Biochim Biophys Acta. 1969;192:175–184. doi: 10.1016/0304-4165(69)90353-5. [DOI] [PubMed] [Google Scholar]
- 18.Gorringe A R, Woods G, Robinson A. Growth and siderophore production by Bordetella pertussis under iron-restricted conditions. FEMS Microbiol Lett. 1990;66:101–106. doi: 10.1016/0378-1097(90)90265-r. [DOI] [PubMed] [Google Scholar]
- 19.Guterman S. Inhibition of colicin B by enterochelin. Biochem Biophys Res Commun. 1971;44:1149–1155. doi: 10.1016/s0006-291x(71)80206-1. [DOI] [PubMed] [Google Scholar]
- 20.Hancock R E W, Hantke K, Braun V. Iron transport of Escherichia coli K-12: involvement of the colicin B receptor and of a citrate-inducible protein. J Bacteriol. 1976;127:1370–1375. doi: 10.1128/jb.127.3.1370-1375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidinger S, Braun V, Pecorara V, Raymond K N. Iron supply to Escherichia coli by synthetic analogs of enterobactin. J Bacteriol. 1983;153:109–115. doi: 10.1128/jb.153.1.109-115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeanteur D, Lakey J H, Pattus F. The bacterial porin superfamily: sequence alignment and structure prediction. Mol Microbiol. 1991;5:2153–2164. doi: 10.1111/j.1365-2958.1991.tb02145.x. [DOI] [PubMed] [Google Scholar]
- 23.Karpishin T B, Raymond K N. The first structural characterization of a metal-enterobactin complex [V(enterobactin)]2. Angew Chem Int Ed Engl. 1992;31:466–468. [Google Scholar]
- 24.Klebba P E, McIntosh M A, Neilands J B. Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J Bacteriol. 1982;149:880–888. doi: 10.1128/jb.149.3.880-888.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konopka K, Bindereif A, Neilands J B. Aerobactin-mediated utilization of transferrin iron. Biochemistry. 1982;21:6503–6508. doi: 10.1021/bi00268a028. [DOI] [PubMed] [Google Scholar]
- 26.Kreusch A, Neubuser A, Schiltz E, Weckesser J, Schulz G E. Structure of the membrane channel porin from Rhodopseudomonas blastica at 2.0 A resolution. Protein Sci. 1994;3:58–63. doi: 10.1002/pro.5560030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Rutz J, Feix J B, Klebba P E. Permeability properties of the channel domain within the ferric enterobactin receptor, FepA. Proc Natl Acad Sci USA. 1993;90:10653–10657. doi: 10.1073/pnas.90.22.10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McIntosh M A, Earhart C F. Coordinate regulation by iron of the synthesis of phenolate compounds and three outer membrane proteins in Escherichia coli. J Bacteriol. 1977;131:331–339. doi: 10.1128/jb.131.1.331-339.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 30.Murphy C K, Kalue V I, Klebba P E. Surface topology of Escherichia coli K-12 ferric enterobactin receptor. J Bacteriol. 1990;172:2736–2746. doi: 10.1128/jb.172.5.2736-2746.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neidhardt F C, Bloch P L, Smith D F. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neilands J B. Evolution of biological iron binding centers. Struct Bonding. 1972;11:145–170. [Google Scholar]
- 33.Neilands J B. Siderophores: structure and function of microbial iron transport compounds. J Biol Chem. 1995;270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- 34.Neilands J B, Peterson T, Leong S A. High affinity iron transport in microorganisms. ACS Symp Ser. 1980;140:264–278. [Google Scholar]
- 35.Neilands J B, Erikson T J, Rastetter W H. Stereospecificity of the ferric enterobactin receptor of Escherichia coli K-12. J Biol Chem. 1981;256:3831–3832. [PubMed] [Google Scholar]
- 36.Newton S M C, Allen J S, Cao Z, Qi Z, Jiang X, Sprencel C, Igo J D, Foster S B, Payne M A, Klebba P E. Double mutagenesis of a positive charge cluster in the ligand-binding site of the ferric enterobactin receptor, FepA. Proc Natl Acad Sci USA. 1997;94:4560–4565. doi: 10.1073/pnas.94.9.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Nishio, T., B. L. Bryan, and K. N. Raymond. Unpublished results.
- 37.O’Brien I G, Gibson F. The structure of enterochelin and related 2,3-dihydroxy-N-benzoyl-serine conjugates from Escherichia coli. Biochim Biophys Acta. 1970;215:393–402. doi: 10.1016/0304-4165(70)90038-3. [DOI] [PubMed] [Google Scholar]
- 38.Ong S A, Peterson T, Neilands J B. Agrobactin, a siderophore from Agrobacterium tumefaciens. J Biol Chem. 1979;254:1860–1865. [PubMed] [Google Scholar]
- 39.Payne M A, Igo J D, Cao Z, Foster S B, Newton S M C, Klebba P E. Biphasic binding kinetics between FepA and its ligands. J Biol Chem. 1997;272:21950–21955. doi: 10.1074/jbc.272.35.21950. [DOI] [PubMed] [Google Scholar]
- 40.Perry, R. D., and C. L. San Clemente. Siderophore synthesis in Klebsiella pneumoniae and Shigella sonnei during iron deficiency. J. Bacteriol. 140:1128–1132. [DOI] [PMC free article] [PubMed]
- 41.Pollack J R, Neilands J B. Enterobactin, an iron transport compound from Salmonella typhimurium. Biochem Biophys Res Commun. 1970;38:989–992. doi: 10.1016/0006-291x(70)90819-3. [DOI] [PubMed] [Google Scholar]
- 42.Pollack J R, Ames B N, Neilands J B. Iron transport in Salmonella typhimurium: mutants blocked in the biosynthesis of enterobactin. J Bacteriol. 1970;104:635–639. doi: 10.1128/jb.104.2.635-639.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poole K, Young L, Neshat S. Enterobactin-mediated iron transport in Pseudomonas aeruginosa. J Bacteriol. 1990;172:6991–6996. doi: 10.1128/jb.172.12.6991-6996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pugsley A P, Reeves P. Characterization of group B colicin-resistant mutants of Escherichia coli K-12: colicin resistance and the role of enterochelin. J Bacteriol. 1976;127:218–228. doi: 10.1128/jb.127.1.218-228.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raymond, K. N. Recognition and transport of natural synthetic siderophores by microbes. Pure Appl. Chem. 66:773–781.
- 46.Rogers H J, Synge C, Kimber B, Bayley P M. Product of enterochelin by Escherichia coli O111. Biochim Biophys Acta. 1977;497:548–557. doi: 10.1016/0304-4165(77)90211-2. [DOI] [PubMed] [Google Scholar]
- 47.Rutz J M, Liu J, Lyons J A, Gorason S K, McIntosh M A, Feix J B, Klebba P E. Formation of a gatted channel by a ligand-specific transport protein in the bacterial outer membrane. Science. 1992;258:471–475. doi: 10.1126/science.1411544. [DOI] [PubMed] [Google Scholar]
- 48.Rutz J M, Abdullah T, Kalve V I, Singh S P, Klebba P E. Evolution of the ferric enterobactin receptor in gram-negative bacteria. J Bacteriol. 1991;173:5964–5974. doi: 10.1128/jb.173.19.5964-5974.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawatzi G. The role of iron-binding proteins in bacterial infection. In: Winkelmann G, Van der Helm D, Neilands J B, editors. Iron transport in microbes, plants, and animals. Weinheim, Federal Republic of Germany: VCH Press; 1987. pp. 477–489. [Google Scholar]
- 50.Schirmer T, Keller T A, Wang Y F, Rosenbusch J P. Structural basis for sugar translocation through maltoporin channels at 3.1 A resolution. Science. 1995;267:512–514. doi: 10.1126/science.7824948. [DOI] [PubMed] [Google Scholar]
- 51.Shea C M, McIntosh M A. Nucleotide sequence and genetic organization of the ferric enterobactin transport system: homology to other periplasmic binding protein-dependent systems in Escherichia coli. Mol Microbiol. 1991;5:1415–1428. doi: 10.1111/j.1365-2958.1991.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 52.Snow G A. Mycobactins: iron-chelating growth factors from mycobacteria. Bacteriol Rev. 1970;34:99–125. doi: 10.1128/br.34.2.99-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tashima K T, Carroll P A, Rogers M B, Calderwood S B. Relative importance of three iron-regulated outer membrane proteins for in vivo growth of Vibrio cholerae. Infect Immun. 1996;64:1756–1761. doi: 10.1128/iai.64.5.1756-1761.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tidmarsh G F, Klebba P E, Rosenberg L T. Rapid release of iron from ferritin by siderophores. J Inorg Biochem. 1983;18:161–168. doi: 10.1016/0162-0134(83)80019-1. [DOI] [PubMed] [Google Scholar]
- 55.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsolis R M, Bäumler A J, Heffron F, Stojiljkovic I. Contribution of TonB- and Feo-mediated iron uptake to growth of Salmonella typhimurium in the mouse. Infect Immun. 1996;64:4549–4556. doi: 10.1128/iai.64.11.4549-4556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tumumuru M K R, Armstrong S K, McIntosh M A. Abstracts of the 90th Annual Meeting of the American Society for Microbiology 1990. Washington, D.C: American Society for Microbiology; 1990. Isolation and characterization of the ferric enterobactin receptor gene (fepA) of Salmonella typhimurium, abstr. K-91; p. 234. [Google Scholar]
- 58.Wang C C, Newton A. Iron transport in Escherichia coli: relationship between chromium sensitivity and high iron requirement in mutants of Escherichia coli. J Bacteriol. 1969;98:1135–1141. doi: 10.1128/jb.98.3.1135-1141.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang C C, Newton A. Iron transport in Escherichia coli: roles of energy-dependent uptake and 2,3-dihydroxybenzoylserine. J Bacteriol. 1969;98:1142–1150. doi: 10.1128/jb.98.3.1142-1150.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang C C, Newton A. An additional step in the transport of iron defined by the tonB locus of Escherichia coli. J Biol Chem. 1971;246:2147–2151. [PubMed] [Google Scholar]
- 61.Wayne R R, Neilands J B. Evidence for common binding sites for ferrichrome compounds and bacteriophage φ80 in the cell envelope of Escherichia coli. J Bacteriol. 1975;121:497–503. doi: 10.1128/jb.121.2.497-503.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wayne R R, Frick K, Neilands J B. Siderophore protection against colicins M, B, V, and Ia in Escherichia coli. J Bacteriol. 1976;126:7–12. doi: 10.1128/jb.126.1.7-12.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss M S, Wacker T, Weckesser J, Welte W, Schulz G E. The three-dimensional structure of porin from Rhodobacter capsulatus at 3 A resolution. FEBS Lett. 1990;267:268–272. doi: 10.1016/0014-5793(90)80942-c. [DOI] [PubMed] [Google Scholar]
- 64.Weitl F L, Harris W R, Raymond K N. Sulfonated catecholamide analogues of enterobactin as iron-sequestering agents. J Am Chem Soc. 1979;22:1281–1283. doi: 10.1021/jm00197a001. [DOI] [PubMed] [Google Scholar]
- 65.Williams P H, Carbonetti N H. Iron, siderophores, and the pursuit of virulence: independence of the aerobactin and enterochelin iron uptake systems in Escherichia coli. Infect Immun. 1986;51:942–947. doi: 10.1128/iai.51.3.942-947.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winkelmann G, Van der Helm D, Neilands J B. Iron transport in microbes, plants, and animals. In: Winkelmann G, Van der Helm D, Neilands J B, editors. Iron transport in microbes, plants, and animals. Weinheim, Federal Republic of Germany: VCH Press; 1987. pp. 73–75. [Google Scholar]
- 67.Xu J, Kullren B, Durbin P W, Raymond K N. Specific sequestering agents for the actinides 28. Synthesis and initial evaluation of multidentate 4-carbamoyl-3-hydroxy-1-methyl-2(1H)-pyridone ligands for in vivo plutonium (IV) chelation. J Med Chem. 1995;38:2606–2614. doi: 10.1021/jm00014a013. [DOI] [PubMed] [Google Scholar]
- 68.Xu, J., and K. N. Raymond. Unpublished results.
- 69.Zhou X H, Van der Helm D, Adjimani J. Purification of outer membrane iron transport receptors from Escherichia coli by fast protein liquid chromatography: FepA and FecA. Biometals. 1993;6:25–35. doi: 10.1007/BF00154229. [DOI] [PubMed] [Google Scholar]