Electrochemical synthesis can provide more sustainable routes to industrial chemicals1–3. Electrosynthetic oxidations often may be performed “reagent-free”, generating H2 derived from the substrate as the sole byproduct at the counter electrode. Electrosynthetic reductions, however, require an external source of electrons. Sacrificial metal anodes are commonly used for small-scale applications4, but more sustainable options are needed at large scale. Anodic water oxidation is an especially appealing option15,6, but many reductions require anhydrous, air-free reaction conditions. This constraint motivates the growing interest in the electrochemical hydrogen oxidation reaction (HOR) under non-aqueous conditions7–12. Here, we report a mediated H2 anode that achieves indirect electrochemical oxidation of H2 by pairing thermal catalytic hydrogenation of an anthraquinone mediator with electrochemical oxidation of the anthrahydroquinone. This quinone-mediated H2 anode is used to support nickel-catalyzed cross-electrophile coupling (XEC), a reaction class gaining widespread adoption within the pharmaceutical industry13–15. Initial validation of this method in small-scale batch reactions is followed by adaptation to a recirculating flow reactor that enables hectogram-scale synthesis of a pharmaceutical intermediate. The mediated H2 anode technology disclosed here offers a general strategy to support H2-driven electrosynthetic reductions.
Electrochemical HOR is well established in fuel cells (Fig. 1a). Humidified H2 gas is delivered to the anode within a membrane-electrode assembly (MEA) that incorporates gas-diffusion, catalyst, and proton-exchange-membrane (PEM) layers. This approach is not readily adapted to organic electrosynthesis in non-aqueous media because protons derived from HOR migrate to the cathode through the PEM layer accompanied by significant quantities of water. For typical electrosynthetic reductions, water would accumulate in concentrations of 1–5 M in the cathode compartment16, significantly changing the reaction medium and complicating moisture-sensitive reduction reactions. Gas-diffusion electrodes (GDEs) offer an alternative approach to achieve non-aqueous electrochemical HOR, and they have been used recently to support lithium-mediated N2 reduction9,11. Contemporary efforts are directed toward mechanistic studies and catalyst design efforts to address the kinetic limitations of electrocatalytic HOR in organic solvent7,8,11. The present study was initiated to explore another strategy, whereby thermal catalytic hydrogenation of a redox-active molecule is used to support indirect electrochemical HOR. Quinone hydrogenation is well established in organic solvent and is featured in the industrial anthraquinone process for hydrogen peroxide synthesis17. Separately, good electrochemical properties of quinones are evident from their recent use in organic redox flow batteries18,19 and mediated fuel cells20. The reversible chemical/electrochemical interconversion of quinone and hydroquinone (Fig. 1b) provides the basis for a quinone-mediated H2 anode to support electrosynthetic reduction reactions. Ni-catalyzed XEC reactions were selected as the cathode reaction for this study (Fig. 1c,d). The widespread use of stoichiometric metal reductants (Zn, Mn) in these reactions complicates large-scale applications, due to the non-uniform reactivity and particle properties of these metals, difficulty in suspending dense metal powders in batch reactors, and formation of stoichiometric Zn or Mn salts as waste21. These issues have motivated considerable efforts to develop electrochemical variants of these reactions22–31, and H2 offers the most atom-economical and sustainable source of electrons for these reactions.
Fig. 1. Strategy to enable the use of H2 as a source of electrons for Ni-catalyzed cross-electrophile coupling (XEC) in organic solvent.
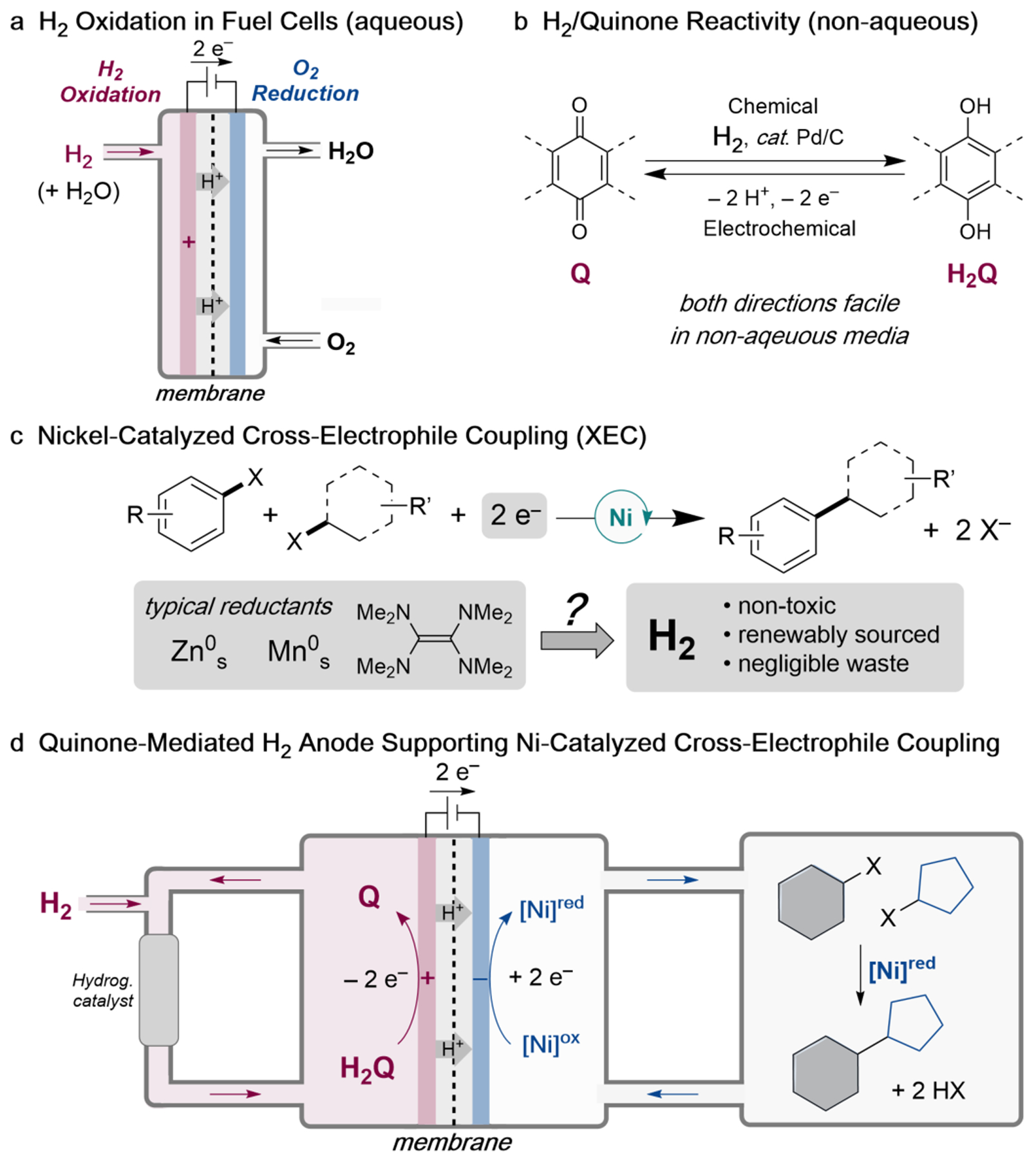
(a) The electrochemical H2 oxidation reaction (HOR) is a key feature of fuel cells. The essential role of water in supporting HOR and transport of water through the membrane limits use of this method in non-aqueous electrochemistry. (b) Catalytic hydrogenation quinones and electrochemical oxidation of hydroquinones are facile in organic solvents. (c) Ni-catalyzed cross-electrophile coupling (XEC) is an important and growing class of reduction reactions that would benefit from the ability to use H2 as a reductant. (d) Quinone-mediated H2 anode concept, designed to support electrochemical Ni-catalyzed XEC.
Ni-catalyzed coupling of aryl and alkyl halides to form C(sp2)–C(sp3) bonds is among the most thoroughly developed class of XEC reactions (see Fig. 1c)32. For the present study, we selected a Ni catalyst composed of NiBr2 and a dual dtbbpy/ttbtpy ligand system, adapted from recent reports26,29 (dtbbpy = 4,4’-di-tert-butyl-2,2’-bipyridine, ttbtpy = 4,4’,4”-tri-tert-butyl-2,2’:6’,2”-terpyridine). Selection of components for the mediated H2 anode was inspired by recent reports using anthraquinones and other substituted quinones under aqueous conditions for redox flow batteries18,19 and mediated fuel cells33,34. Sodium anthraquinone-2-sulfonate (AQS) exhibits good solubility in polar organic solvents, such as N,N-dimethylformamide (DMF) and N-methyl-2-pyrrolidone (NMP). Cyclic voltammetry (CV) analysis of AQS in NMP revealed a redox potential of −0.59 V versus a ferrocenium/ferrocene (Fc+/Fc) reference potential (Fig. 2a and Fig. S3). This value is 200 mV higher than the H+/H2 potential (−0.79 V), measured under the same conditions using CV and open-circuit-potential methods (Fig. 2a and Fig. S2). CV analysis was also used to probe the redox potentials of nickel bromide complexes bearing the dtbbpy and ttbtpy ligands. The measured two- and one-electron reduction potentials of these complexes range from −1.3 to −2.1 V vs Fc+/Fc (Fig. 2a and Fig. S4). The 0.5–1.3 V difference between the redox potentials of H2 and catalytically relevant Ni complexes confirms that H2 lacks the driving force necessary to serve as a chemical reductant for thermal Ni-catalyzed XEC. Application of an external voltage, however, allows the potential of electrons from H2 to be raised to the potential needed to support the reactions under electrochemical conditions.
Fig. 2. Voltammetric analysis and electrochemical Ni XEC using a mediated H2 anode in an H–cell.
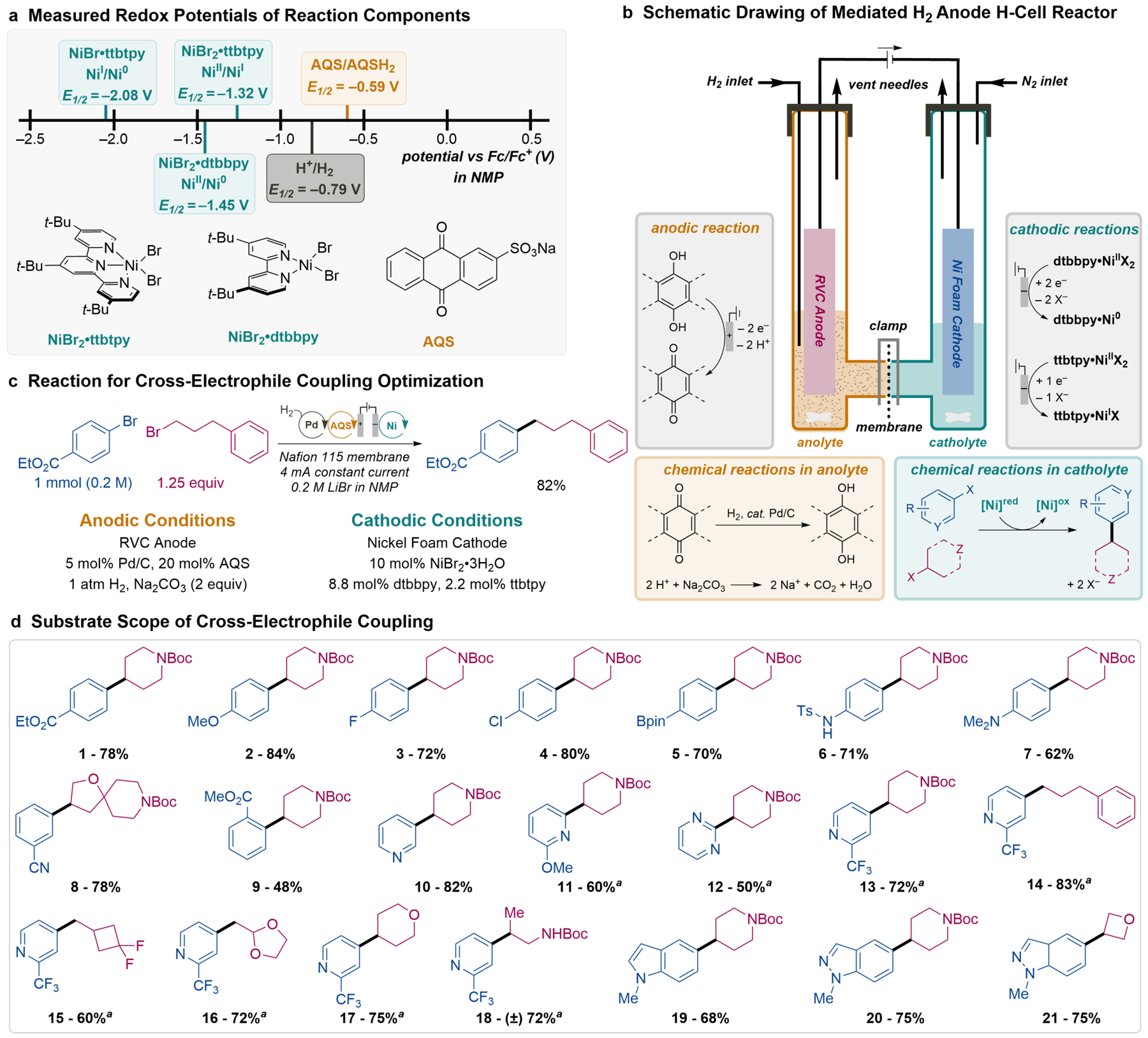
(a) Redox potentials measured for H2, the anthraquinone mediator, and Ni catalyst species show that the potential of H2 is insufficient to drive Ni XEC in the absence of an electrochemical bias. (b) H–cell schematic illustrating the electrochemical and chemical processes in the anolyte and catholyte compartments. (c) XEC substrates used for reaction testing and optimized reaction conditions. (d) Ni XEC products obtained using the H–cell with a mediated H2 anode, shown in b, under conditions identical or similar to those in c (see section 10 of the Supplementary Information for details). aHeteroaryl chloride used instead of the bromide.
The relative redox potentials of H2 and AQS indicate that AQS hydrogenation is thermodynamically favorable, and experimental tests further show that this reaction is kinetically facile when using a heterogeneous Pd/C catalyst with 1 atm of H2 at room temperature (Fig. S6). These results provided a starting point for H–cell batch electrolysis experiments to test a mediated H2 anode system in combination with cathodic Ni-catalyzed XEC (Fig. 2b). Both half-cells feature indirect electrochemical processes with a combination of electrochemical and off-electrode chemical reactions (Fig. 2b, gray and colored boxes, respectively). Optimization of the H2-coupled Ni-catalyzed XEC reaction conditions employed ethyl 4-bromobenzoate and (3-bromopropyl)benzene as the aryl and alkyl electrophiles (Fig. 2c). Individual reaction parameters were varied (see section 5 of the Supplementary Information for details) to identify the most effective conditions for this substrate. The following conditions led to formation of the desired C(sp2)–C(sp3) XEC product in 82% yield: 0.2 M aryl bromide as the limiting reagent, 1.25 equiv of the alkyl bromide, a 10 mol% catalyst composed of NiBr2·3H2O with a 4:1 ratio of dtbbpy/ttbtpy ligands, and 0.2 M LiBr as the supporting electrolyte in NMP. The anode chamber contained 5 wt% Pd/C (with a Pd loading of 5 mol% relative to the Ar–Br), 20 mol% AQS, 2 equiv Na2CO3, and 0.2 M LiBr in NMP. These or closely related conditions proved to be effective with a broad range of pharmaceutically relevant coupling partners (Fig. 2d). For example, both electron-rich and electron-poor aryl bromides undergo coupling in high yields (1–4 and 9), and the method proceeds well with a pendant arylboronic ester group (5), a standard reactive group in cross-coupling reactions. This Ni/dtbbpy/ttbtpy catalyst system tolerates a range of Lewis basic nitrogen heterocycles, which are prevalent in drug candidates. Examples include pyridines substituted at the 2-, 3-, and 4-position (10, 11, 13–18), pyrimidine (12), indole (19), and indazole (20, 21). This catalyst system is also effective with different primary and secondary alkyl bromides (14–18, 21), including those with strained rings (15, 21) and tert-butoxycarbonyl (Boc)-protected primary and secondary amines (1–13, 19, 20). These results show excellent compatibility between the mediated H2 anode and Ni-catalyzed XEC reactions, and they often exceed those obtained in previous studies using the same catalyst system with a tertiary amine reductant26,29.
The primary merits of the mediated H2 anode will be experienced at larger scale. Therefore, subsequent efforts focused on development of a flow cell that could be integrated with a parallel-plate electrochemical reactor. To facilitate development and testing of the system, the mediated anode was paired with a simple cathodic electron-transfer reaction, involving reduction of Bobbitt’s salt, or ACT+, an oxoammonium compound derived from 4-acetamido-2,2,6,6-tetramethylpiperidine-N-oxyl (ACT, Fig. 3a). This reaction was selected for anode characterization studies because one-electron reduction of ACT+ to the aminoxyl ACT exhibits good electrochemical kinetics and thus will not limit anodic performance. The mediated anode was designed with two separate flow loops sharing a common liquid reservoir. The hydrogenation loop includes a packed-bed reactor containing Pd/C and is used to support continuous hydrogenation of AQS to AQSH2. The electrolysis loop circulates the mediator/electrolyte solution over the anode, resulting in electrochemical oxidation of AQSH2 to AQS. This mediated anode design provide a unique opportunity to manage protons generated during electrochemical oxidation of AQSH2. The protons are retained in flowing solution, rather than passing through the membrane, and they are transported back into the anolyte reservoir where they undergo neutralization by Li2CO3. This neutralization exchanges H+ with Li+ ions in solution and results in the transport of lithium ions rather the protons through the Nafion membrane during electrolysis. Inductively coupled plasma - optical emission spectrometry (ICP-OES) analysis of the catholyte solution during operation of the mediated H2 anode showed a linear increase in [Li+], with a magnitude that directly correlates with the charge passed during electrolysis (Fig. S12). This feature contrasts conventional H2 anode configurations, which are designed to ensure efficient proton transport through the membrane to support O2 reduction or other cathodic reactions. In the present system, proton migration would have a deleterious effect, resulting in parasitic H2 evolution at the cathode and/or generating undesirable byproducts via protonolysis of organometallic Ni intermediates (see Section 1 of the Supplementary Information). Thus, the mediated H2 anode enables the use of H2 as a source of electrons – without protons – for the cathodic reduction reaction.
Fig. 3. Quinone-mediated H2 anode flow cell.

(a) Schematic diagram of the mediated H2 anode flow system, integrating a hydrogenation loop with a packed-bed reactor for AQS hydrogenation and an electrolysis loop interfaced with a parallel-plate reactor for anodic oxidation of AQSH2. (b) Analysis of redox states of the quinone mediator in the anolyte reservoir, using in situ UV-visible spectroscopy, while circulating only through the hydrogenation loop, only through the electrolysis loop, and through both the hydrogenation and electrolysis loops. (c) Operation of the mediated hydrogen anode shows stable cell voltage at current densities well beyond that needed to support Ni-catalyzed cross-electrophile coupling (XEC). (d) Assessment of Ni XEC product selectivity at different current densities. Optimal yield and selectivity are obtained at 4 mA/cm2.
To monitor the interplay between the hydrogenation and electrolysis loops, in situ UV-visible spectroscopy was used to follow redox speciation of the quinone mediator in the anolyte reservoir. Operation of the hydrogenation loop without electrolysis leads to full reduction of the AQS to AQSH2 (Fig. 3b, hydrogenation loop only), with an isosbestic point at 355 nm. Ceasing flow in the hydrogenation loop and initiating the electrolysis loop while applying 5 mA current at the anode shows regeneration of AQS, while maintaining the isosbestic point (Fig. 3b, electrolysis loop only). When both loops are operating, the anolyte reservoir reaches a steady-state AQS/AQSH2 ratio that reflects the balanced rates of the chemical and electrochemical reactions (85% AQSH2 state of charge for the conditions shown in Fig. 3b, hydrogenation + electrolysis loops). Stable cell voltages were observed at current densities of 2–16 mA cm−2 (Fig. 3c), extending beyond the current densities determined to be necessary to support optimal performance of Ni-catalyzed XEC at the cathode (Fig. 3d). Analysis of the yields and selectivities of the Ni-catalyzed XEC reaction showed that optimal results with the indicated substrate were obtained at a current density of ~4 mA/cm2.
The mediated H2 anode was then evaluated in larger-scale applications of Ni-catalyzed XEC using flow electrolysis methods. A 5 cm2 parallel-plate flow reactor was used to synthesize several compounds on gram scale (Fig. 4a). The core structure of the antidepressant medication rolipram35 was obtained in 94% isolated yield (1.7 g) using this method. In addition, several candidates from batch screening were invetigated, prioritizing examples previously shown to be challenging with a Ni-catalyzed XEC process using sacrificial amine reductants.29 These gram-scale reactions proceeded in 15–30% higher yields than those obtained previously (for context, see section 6 in the Supplementary Information). The origin of the improvement is not yet understood; however, the positive results highlight the merits of this H2-driven electrolysis method.
Fig. 4. Scalable demonstration of the mediated H2 anode to prepare molecules of pharmaceutical interest.
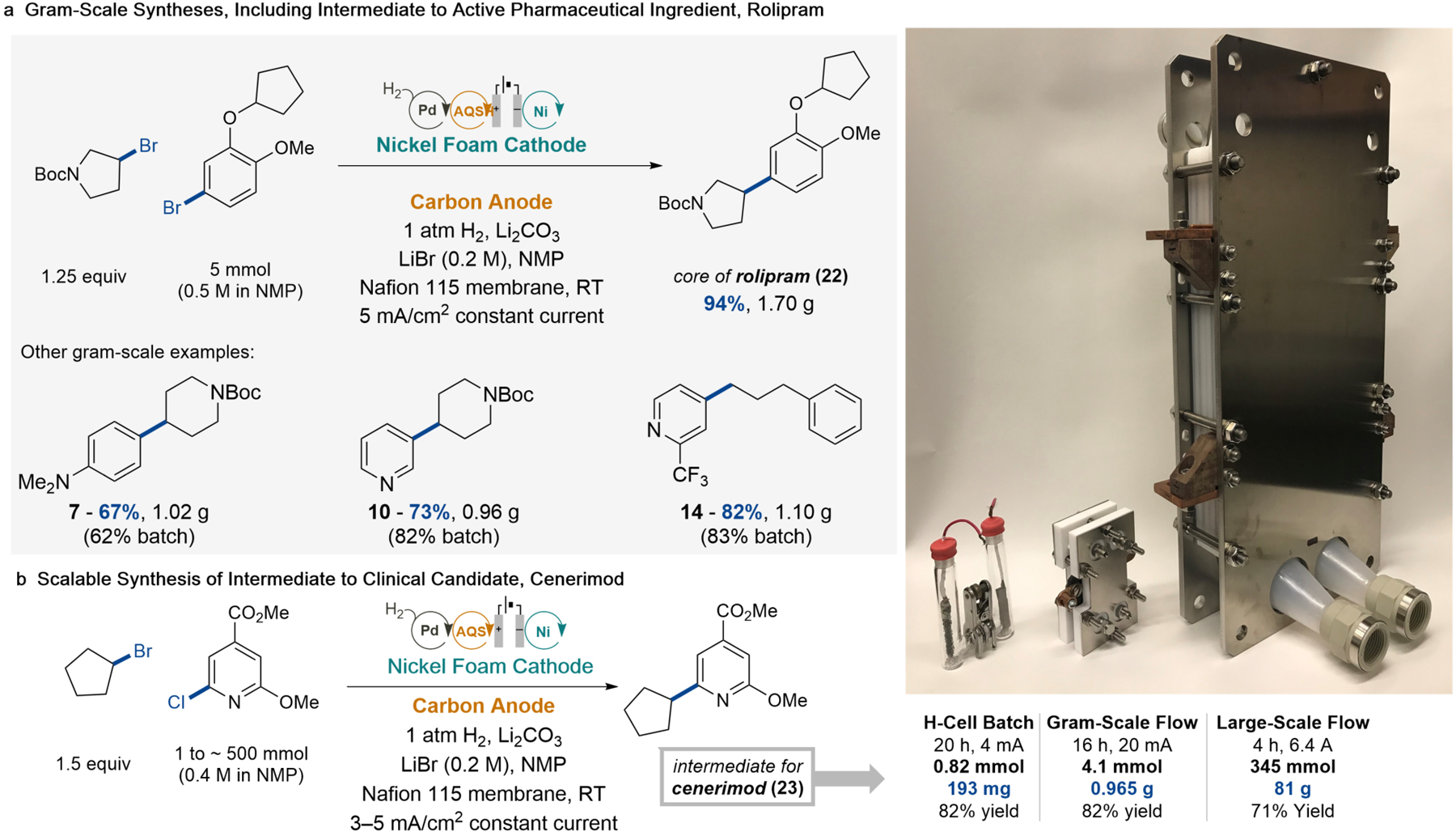
(a) Gram-scale Ni-catalyzed XEC using a small parallel-plate flow reactor (5 cm2 electrode surface area) to synthesize molecules including an intermediate to the drug, rolipram. (b) Synthesis of an intermediate to the drug, cenerimod, in three different formats: an H–cell batch reactor, a small parallel-plate flow reactor (5 cm2 electrode surface area), and a large parallel-plate flow reactor (1600 cm2 electrode surface area). See sections 8 and 9 in the Supplementary Information for full reaction conditions and experimental details.
Gram-scale synthesis of an intermediate to the Phase III clinical candidate, cenerimod36, was similarly effective (Fig. 4b). An 82% product yield was obtained in a batch H–cell, and no loss of yield was observed upon transition to the gram-scale flow reactor. The flow conditions were then translated into a larger-scale commercial parallel-plate reactor that featured a stack of four membrane-electrode units with a total electrode surface area of 1600 cm2 (see section 9 in the Supplementary Information). A current density of 4 mA/cm2 was retained in the larger reactor, and the reaction proceeded 71% yield (without optimization). The flow reactor applications show the stable operation of the H2 anode and Ni XEC flow cell over 4–16 h of operation, and the total current of 6.4 A applied in the large-scale demonstration would be sufficient to deliver approximately 0.5 kg/day of the cross-coupled product.
Hydrogen represents an ideal, sustainable alternative to stoichiometric metal-based reductants, and the quinone-mediated H2 anode system outlined herein establishes a unique strategy to achieve this goal for Ni XEC reactions. This mediated H2 anode concept, however, should be applicable to many other applications. Electrochemistry provides a means to amplify the reducing power of H2, allowing H2 to serve as a source of electrons even when it lacks the intrinsic chemical potential needed to generate highly reduced catalytic intermediates. The quinone mediator serves as a versatile hydrogen carrier, and pairing catalytic hydrogenation of the quinone mediator with electrochemical oxidation of the hydroquinone enables efficient net electrochemical oxidation of hydrogen under non-aqueous conditions. Finally, the recirculating-flow mediated anode offers unique flexibility in proton management that differs from other H2 anodes, such as fuel cell MEAs or GDEs. Protons derived from hydroquinone oxidation are readily retained in the anode compartment, where they can be neutralized and replaced with lithium ions, thereby allowing H2 to serve as a proton-free source of electrons for the cathodic reaction. Each of these features has important implications for future development H2-driven electrosynthetic reduction reactions.
Supplementary Material
Acknowledgments:
The authors thank Brittany Armstrong (EPSE) and Chris Nietupski (HPL) of Merck & Co., Inc., Rahway, NJ, USA for valuable discussions, feedback, and assistance in performing the large-scale implementation of this chemistry. The authors also thank Chase Salazar for assistance with gas uptake experiments. Financial support for development of the mediate H2 anode was provided by the Center for Molecular Electrocatalysis, an Energy Frontier Research Center, funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The development of Ni-catalyzed XEC reactions and their integration with the mediated H2 anode was supported by the NSF (PFI-RP 2122596). Spectroscopic instrumentation was partially supported by the NIH (1S10 OD020022-1) and the NSF (CHE-1048642).
Footnotes
Competing interests: A patent application describing a mediated H2 anode has been filed.
Supplementary information is available for this paper.
References
- 1.Cardoso DSP, Šljukić B, Santos DMF & Sequeira CAC Organic electrosynthesis: From laboratorial practice to industrial applications. Org. Process Res. Dev 21, 1213–1226 (2017). [Google Scholar]
- 2.Pletcher D & Walsh FC Industrial Electrochemistry, 2nd edition. pp. 298–311 (Kluwer, 1990). [Google Scholar]
- 3.Leech MC, Garcia AD, Petti A, Dobbs AP & Lam K Organic electrosynthesis: from academia to industry. React. Chem. Eng 5, 977–990 (2020). [Google Scholar]
- 4.Klein M & Waldvogel SR Counter electrode reactions-important stumbling blocks on the way to a working electro-organic synthesis. Angew. Chem. Int. Ed 61, e202204140 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherbo RS, Delima RS, Chiykowski VA, MacLeod BP & Berlinguette CP Complete electron economy by pairing electrolysis with hydrogenation. Nat. Catal 1, 501–507 (2018). [Google Scholar]
- 6.Danly DE Development and commercialization of the Monsanto electrochemical adiponitrile process. J. Electrochem. Soc 131, 435C–442C (1984). [Google Scholar]
- 7.Barrette WC, Sawyer DT Determination of dissolved hydrogen and effects of media and electrode materials on the electrochemical oxidation of molecular hydrogen. Anal. Chem 56, 653–657 (1984). [Google Scholar]
- 8.Ledezma-Yanez I, Díaz-Morales O, Figueiredo MC, Koper MTM Hydrogen oxidation and hydrogen evolution on a platinum electrode in acetonitrile. ChemElectroChem. 2, 1612–1622 (2015). [Google Scholar]
- 9.Lazouski N, Chung M, Williams K, Gala ML, Manthiram K Non-aqueous gas diffusion electrodes for rapid ammonia synthesis from nitrogen and water-splitting-derived hydrogen. Nat. Catal 3, 463–469 (2020). [Google Scholar]
- 10.Suryanto BHR et al. Nitrogen reduction to ammonia at high efficiency and rates based on a phosphonium proton shuttle. Science 372, 1187–1191 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Hodgetts RY, Du H-L, Nguyen TD, MacFarlane D, Simonov AN Electrocatalytic oxidation of hydrogen as an anode reaction for the Li-mediated N2 reduction to ammonia. ACS Catal. 12, 5231–5246 (2022). [Google Scholar]
- 12.Fu X et al. Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation. Science 379, 707–712 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Goldfogel MJ, Huang L, Weix DJ Cross-electrophile coupling: Principles and new reactions. Nickel Catalysis in Organic Synthesis. pp. 183–222 (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2020). [Google Scholar]
- 14.Nimmagadda SK et al. Development and execution of an Ni(II)-catalyzed reductive cross-coupling of substituted 2-chloropyridine and ethyl 3-chloropropanoate. Org. Process Res. Dev 24, 1141–1148 (2020). [Google Scholar]
- 15.Beutner GL et al. A process chemistry benchmark for sp2–sp3 cross couplings. J. Org. Chem 86, 10380–10396 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Moulik S, Vaishnavi BA, Nagar H & Sridhar S Water Competitive Diffusion. Encyclopedia of Membranes, pp. 1973–1983 (Springer Berlin, Heidelberg, 2016). [Google Scholar]
- 17.Goor G, Glenneberg J, Jacobi S, Dadabhoy J, Candido E, Hydrogen Peroxide. Ullmann’s Encyclopedia of Industrial Chemistry (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2019). [Google Scholar]
- 18.Huskinson B et al. A metal-free organic-inorganic aqueous flow battery. Nature. 505, 195–198 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Kwabi DG, Ji Y, Aziz MJ Electrolyte lifetime in aqueous organic redox flow batteries: A critical review. Chem. Rev 120, 6467–6489 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Anson CW, Stahl SS Mediated fuel cells: Soluble redox mediators and their applications to electrochemical reduction of O2 and oxidation of H2, alcohols, biomass, and complex fuels. Chem. Rev 120, 3749–3786 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acemoglu M, Baenziger M, Krell CM, Marterer W Experiences with Negishi couplings on technical scale in early development. In Transition Metal-Catalyzed Couplings in Process Chemistry. pp 15–23 (Wiley-VCH: Weinheim, 2013). [Google Scholar]
- 22.Jennings PW, Pillsbury DG, Hall JL, Brice VT Carbon-carbon bond formation via organometallic electrochemistry. J. Org. Chem 41, 719–722 (1976). [Google Scholar]
- 23.Conan A, Sibille S, d’Incan E, Périchon J Nickel-catalysed electroreductive coupling of α-halogenoesters with aryl or vinyl halides. J. Chem. Soc. Chem. Commun, 48–49 (1990). [Google Scholar]
- 24.Perkins RJ, Pedro DJ, Hansen EC Electrochemical nickel catalysis for Sp2-Sp3 cross-electrophile coupling reactions of unactivated alkyl halides. Org. Lett 19, 3755–3758 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Li H et al. Ni-catalyzed electrochemical decarboxylative C-C couplings in batch and continuous flow. Org. Lett 20, 1338–1341 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkins RJ, Hughes AJ, Weix DJ, Hansen EC Metal-reductant-free electrochemical nickel-catalyzed couplings of aryl and alkyl bromides in acetonitrile. Org. Process Res. Dev 23, 1746–1751 (2019). [Google Scholar]
- 27.DeLano TJ, Reisman SE Enantioselective electroreductive coupling of alkenyl and benzyl halides via nickel catalysis. ACS Catal. 9, 6751–6754 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiao K-J et al. Nickel-catalyzed electrochemical reductive relay cross-coupling of alkyl halides to aryl halides. Angew. Chem. Int. Ed 132, 6520–6524 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Franke MC et al. Zinc-free, scalable reductive cross-electrophile coupling driven by electrochemistry in an undivided cell. ACS Catal. 12, 12617–12626 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harwood SJ et al. Modular Terpene Synthesis Enabled by Mild Electrochemical Couplings. Science 375, 745–752 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamby TB, LaLama MJ, Sevov CS Controlling Ni redox states by dynamic ligand exchange for electroreductive Csp3-Csp2 coupling. Science. 376, 410–416 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Everson DA, Shrestha R, Weix DJ Nickel-catalyzed reductive cross-coupling of aryl halides with alkyl halides. J. Am. Chem. Soc 132, 920–921 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Preger Y et al. Quinone-mediated electrochemical O2 reduction accessing high power density with an off-electrode Co-N/C catalyst. Joule. 2, 2722–2731 (2018). [Google Scholar]
- 34.Preger Y et al. Anthraquinone-mediated fuel cell anode with an off-electrode heterogeneous catalyst accessing high power density when paired with a mediated cathode. ACS Energy Lett. 5, 1407–1412 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J, Mix E, Winblad B The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS Drug Rev. 7, 387–398 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piali L et al. Cenerimod, a novel selective S1P1 receptor modulator with unique signaling properties. Pharmacol. Res. Perspect 5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


