Abstract
Background
Routine childhood immunisation is one of the most important life-saving public health interventions. However, many children still have inadequate access to these vaccines and millions remain (partially) unvaccinated globally. As the COVID-19 pandemic disrupted health systems worldwide, its effects on immunisation have become apparent. This study aimed to estimate routine immunisation coverage among children under two in Sierra Leone and to identify factors associated with incomplete immunisation during the COVID-19 pandemic.
Methods
A cross-sectional household survey was conducted in three districts in Sierra Leone: Bombali, Tonkolili and Port Loko. A three-stage cluster sampling method was followed to enrol children aged 10–23 months. Information regarding immunisation status was based on vaccination cards or caretaker’s recall. Using WHO’s definition, a fully immunised child received one BCG dose, three oral polio vaccine doses, three pentavalent vaccine doses and one measles-containing vaccine dose. Following the national schedule, full immunisation status can be achieved at 9 months of age. Data were weighted to reflect the survey’s sampling design. Associations between incomplete immunisation and sociodemographic characteristics were assessed through multivariable logistic regression.
Results
A total of 720 children were enrolled between November and December 2021. Full vaccination coverage was estimated at 65.8% (95% CI 60.3%-71.0%). Coverage estimates were highest for vaccines administered at birth and decreased with doses administered subsequently. Adjusting for age, the lowest estimated coverage was 40.7% (95% CI 34.5%-47.2%) for the second dose of the measles-containing vaccine. Factors found to be associated with incomplete immunisation status were: living in Port Loko district (aOR = 3.47, 95% CI = 2.00-6.06; p-value < 0.001), the interviewed caretaker being Muslim (aOR = 1.94, 95% CI = 1.25–3.02; p-value = 0.015) and the interviewed caretaker being male (aOR = 1.93, 95% CI = 1.03–3.59, p-value = 0.039).
Conclusion
Though full immunisation coverage at district level improved compared with pre-pandemic district estimates from 2019, around one in three surveyed children had missed at least one basic routine vaccination and over half of eligible children had not received the recommended two doses of a measles-containing vaccine. These findings highlight the need to strengthen health systems to improve vaccination uptake in Sierra Leone, and to further explore barriers that may jeopardise equitable access to these life-saving interventions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-023-17534-2.
Keywords: Childhood immunisation, Routine immunisation coverage, Household survey, Sierra Leone
Introduction
Immunisation is one of the most important and successful public health interventions to date [1]. It remains crucial in protecting children from the morbidity and mortality caused by vaccine-preventable diseases (VPDs). Through routine vaccination, an estimated 5.1 million lives per year will be saved between 2021 and 2030 [2]. Yet, especially in low-income settings many children have insufficient access to vaccines, leaving almost one in five children (partially) unvaccinated and exposed to preventable diseases, disability and premature death [1, 3].
To ensure equal access to recommended vaccines for all children worldwide, the World Health Organization (WHO) established the Expanded Programme on Immunisation (EPI) in 1974 [4]. Initially, the EPI targeted six major infectious diseases: diphtheria, pertussis, poliomyelitis, tetanus, tuberculosis and measles [4]. In Sierra Leone, since EPI implementation in 1978, the targeted VPDs have been expanded by adding vaccines against yellow fever, hepatitis B, Haemophilus influenzae type b, rotavirus and pneumococcal disease [5, 6]. Improvements in immunisation coverage have been made following extensive efforts to rebuild the country’s health system after a decade-long civil war and after the 2014–2015 Ebola virus disease outbreak [7]. According to Sierra Leone’s Demographic and Health Surveys (DHS), the proportion of children aged 12–23 months that had received all vaccinations appropriate for their age rose from 31% in 2008 to 58% in 2013, and subsequently decreased to 49% in 2019 [8, 9]. In the latter year, 2% had not received any vaccinations. Suboptimal immunisation coverage therefore remains a major public health challenge in the country. [9] After the 2019 DHS was conducted, the COVID-19 pandemic led to another disruption of essential health services. The impact of the pandemic on EPI coverage is unclear since vaccination coverage surveys have not been conducted during this period.
As the COVID-19 pandemic put a strain on health systems around the world, its effect on global infant immunisation coverage became apparent [10, 11]. In 2021, approximately 25 million children missed out on essential vaccines, an estimated six million more than in 2019 [11]. Of these, 18.2 million had not received any vaccinations at all [3]. Consequently, the world has been confronted with a surge in outbreaks of VPDs, including polio, yellow fever and measles [12].
Given the urgency to improve immunisation coverage, WHO launched a global strategy, the Immunisation Agenda 2030, that strives towards equal access to life-saving vaccines for everyone, everywhere, in the coming decade [1]. As part of this strategy, WHO promotes vaccination coverage monitoring at subnational levels. Achieving equity in vaccination uptake requires an understanding of cultural, geographic, socioeconomic and systemic barriers to immunisation. Reporting and analysing vaccination coverage at a local or regional level facilitates the identification of disparities in uptake. Such findings provide critical information needed to prioritise and tailor strategies to close immunisation gaps.
The objective of this study was to determine routine immunisation coverage among children aged 10–23 months living in selected areas of Sierra Leone during the COVID-19 pandemic, and to identify risk factors associated with incomplete immunisation status in this age group.
Methods
Study area
The study was conducted in three districts of Sierra Leone’s Northern and North West Provinces: Bombali, Tonkolili and Port Loko. The three districts were chosen based on malaria prevalence data according to the 2016 Malaria Indicator Survey [13]. Although prevalence in Bombali was slightly lower, the district was selected for logistical reasons.
Previous estimates of full immunisation coverage among children aged 12–23 months were 63% in Bombali, 49% in Tonkolili and 44% in Port Loko, according to Sierra Leone’s 2019 DHS (Fig. 1) [9]. Notably, Port Loko held the lowest immunisation coverage at district level in the country. According to the 2015 national census, Bombali had a total population of around 607k, of whom 105k were children under the age of 5. For Tonkolili and Port Loko, these population figures are respectively 531k (103k) and 615k (118k) [14].
Fig. 1.
Map of HHS districts in Sierra Leone
Study design and population
As part of the MULTIPLY-project (MULTIple doses of Intermittent Preventive Treatment during Infancy –IPTi– Proposal: a Lifesaving high Yield intervention), a cross-sectional community-based household survey (HHS) was designed to estimate baseline malaria prevalence and Perennial Malaria Chemoprevention (PMC; formerly named IPTi) coverage among children living in the selected project areas [15]. This implementation research project is currently ongoing and aims to maximise the delivery and uptake of PMC [16]. Inclusion criteria for the HHS were: (i) age 10–23 months and (ii) residence in a project district at the time of the survey. Caretakers of eligible children were interviewed after they had agreed to participate in the survey by signing an informed consent form.
Sample size
The primary objective of the HHS was to determine the coverage of three doses of PMC. A minimum sample size of 710 children was deemed to be needed, considering the latest available estimate of 32.2% PMC coverage, precision of 5%, significance level of 5%, a 10% increase to account for non-response and a design effect of 2 to adjust for the effect of cluster sampling [17, 18].
Sampling procedures
A three-stage cluster sampling method was followed [19]. In the first stage, clusters were randomly selected using probability proportional to size. The population data used for this survey was an estimated projection based on 2015 census data [14]. After cluster mapping, households were visited subsequently following a list of enumerated households, the first being selected using a random number table. If there were two or more eligible children living in a selected household, the table was used to select one child in the third sampling stage.
Data collection
Data was collected on electronic devices using REDCap software and a pre-tested questionnaire, as recounted in more detail by Fombah et al. [19, 20]. The vaccines that surveyed children received were copied from immunisation cards. If this card was unavailable, vaccine history was based on caretakers’ recall.
Outcomes, definitions and data analysis
Children were categorised as fully immunised, partially immunised or unimmunised (zero-dose). Similar to Sierra Leone’s DHS, the WHO definition of a fully immunised child was used: an infant who has received one dose of bacille Calmette-Guerin (BCG) vaccine, three doses of oral polio vaccine (OPV; excluding the dose given at birth), three doses of pentavalent vaccine and one dose of measles-containing vaccine (MCV) [9, 21]. In this study, these are referred to as basic vaccinations. Full immunisation status can be achieved at 9 months of age in Sierra Leone [5]. Unimmunised, i.e., zero-dose, children will have received none of the previously listed vaccines, whereas partially immunised children will have missed at least one but not all of these doses. Immunisation coverage was defined as the proportion of children who were fully immunised by the time of the interview. Incomplete immunisation status was defined by grouping zero-dose and partially immunised children. Coverage rates of individual vaccines were defined as the proportion of children to whom this vaccine had been administered at any given time before the survey. Secondly, full immunisation coverage was determined considering all age-appropriate vaccinations from birth until 9 months of age following Sierra Leone’s EPI schedule. In addition to the above-mentioned vaccines, these children received a birth dose of OPV, three doses of pneumococcal vaccine, two doses of rotavirus vaccine, one dose of inactivated poliovirus vaccine (IPV) and one dose of yellow fever vaccine. Vitamin A, PMC, deworming and the second MCV dose (MCV2) were not included in this definition. Individual coverage rates of these doses are reported. As deworming and the second vitamin A supplement are administered at 12 months of age following Sierra Leone’s EPI schedule, coverage of these doses is determined amongst the subgroup of children aged 13 months or older, allowing for one month of delay in uptake. Similarly, because MCV2 is administered at 15 months, its coverage is determined considering enrolled children aged 16 months or older.
Survey weights were applied to account for the survey’s multi-stage cluster sampling design (Appendix 1). Weights for all three sampling stages were calculated using data collected by Statistics Sierra Leone in the 2015 national census [14].
Standard descriptive statistics were used to summarise baseline child, caretaker and household characteristics. Categorical variables were reported in frequencies and weighted percentages. Continuous variables were described using the weighted mean and standard deviation (SD). Vaccination card availability was reported. Full, partial and zero-dose immunisation coverage rates and coverage rates of individual vaccines were determined per survey area and at district level. Regarding type of locality, this study followed the definition of Sierra Leone’s national statistics office, where a locality with ≥ 2000 inhabitants is classified as urban and a locality of < 2000 inhabitants is classified as rural.
Univariate and multivariable logistic regression analyses were performed to assess the association between sociodemographic factors and incomplete immunisation status. Based on previous literature, the following predictive factors were included in the models: district of residence, locality (rural versus urban), child’s age and sex, caretaker’s age, sex, highest level of education, literacy level, main type of income, marital status and religion, and the sex of the household head [22, 23]. Two multivariable logistic regression models were constructed, with incomplete immunisation status based on the WHO definition and on Sierra Leone’s EPI schedule. Crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were reported. P-values of less than 0.05 were considered statistically significant.
A subgroup analysis was performed to enable comparisons with other surveys by conforming to the eligibility criteria proposed by the WHO reference manual on vaccination coverage surveys [24]. Coverage rates were determined and logistic regression analyses were conducted amongst children aged 12–23 months.
Statistical analyses were performed using Stata/BE 17.0 [25].
Results
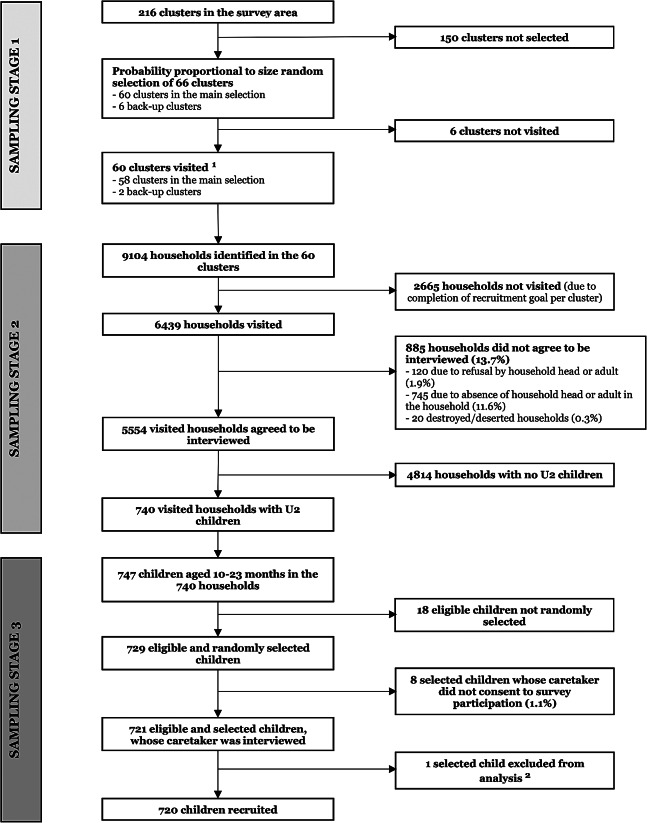
A total of 720 children aged 10–23 months were recruited from November to December 2021 and included in the analysis: 288 in Bombali, 168 in Tonkolili and 264 in Port Loko (Fig. 2). In 86.3% of the 6,439 visited households, an adult consented to provide information on the children living there. Eight out of 729 selected children’s caretakers did not consent to survey participation. The survey’s non-response rate was, therefore, 13.7% at household level and 1.1% at participant level.
Fig. 2.
Flowchart of the survey
U2 = under two years of age
1 To provide the list of clusters within project areas and the maps of the randomly selected clusters, Statistics Sierra Leone used information collected during the national census in 2015. Due to the year difference, two randomly selected clusters were no longer in the project areas and had to be substituted by back up clusters
2 One initially recruited child was later excluded from the analysis, due to their recent relocation from Liberia to a survey cluster, which was not an exclusion criterion at the time of data collection. They had not followed the EPI schedule of Sierra Leone but instead had been vaccinated according to the slightly different program in Liberia, and therefore did not meet the study objectives
Sociodemographic characteristics
Baseline characteristics of enrolled children are described in Table 1. The mean (SD) age of children was 17.2 (4.1) months and 50.4% were female. 40% resided in Bombali, 36.7% in Port Loko, and 23.3% in Tonkolili. The location of their households was classified as rural, as opposed to urban, in 75.0% of cases. Of all interviewed caretakers, 87.0% were female and 77.6% were the mother of the enrolled child.
Table 1.
Baseline characteristics of study children
| n or Mean ± SD | Proportion (%) | ||
|---|---|---|---|
| District (N = 720) | Bombali | 288 | 40.0 |
| Port Loko | 264 | 36.7 | |
| Tonkolili | 168 | 23.3 | |
| Locality (N = 720) | Rural | 540 | 75.0 |
| Urban | 180 | 25.0 | |
| Child’s age in months (N = 720) | 16.68 ± 4.41 | - | |
| Child’s sex (N = 720) | Female | 363 | 50.4 |
| Male | 357 | 49.6 | |
| Caretaker’s age in years 1 (N = 718) | 32.4 ± 12.5 | - | |
| Caretaker’s sex 1 (N = 717) | Female | 624 | 87.0 |
| Male | 93 | 13.0 | |
| Caretaker’s relationship with the child (N = 720) | Mother | 556 | 77.6 |
| Father | 70 | 9.8 | |
| Grandparent | 71 | 9.9 | |
| Aunt or Uncle | 19 | 2.7 | |
| Other | 1 | 1.4 | |
| Caretaker’s highest level of education 1 (N = 718) | Never attended school | 395 | 55.0 |
| Primary | 78 | 10.9 | |
| JSS (Middle School) | 103 | 14.4 | |
| SSS (High School) | 106 | 14.8 | |
| Tertiary | 36 | 5.0 | |
| Caretaker’s main type of income 1 (N = 717) | No salary | 79 | 11.0 |
| Self-employment | 611 | 85.2 | |
| Paid employment | 27 | 3.8 | |
| Is the caretaker able to read and write? (N = 720) | Fully literate | 96 | 13.3 |
| Partially literate | 105 | 14.6 | |
| Illiterate | 519 | 72.1 | |
| Caretaker’s marital status 1 (N = 715) | Married or in union | 578 | 80.8 |
| Single (never married) | 82 | 11.5 | |
| Separated or divorced | 24 | 3.4 | |
| Widowed | 31 | 4.3 | |
| Caretaker’s religion 2 (N = 720) | Muslim | 508 | 70.6 |
| Christian | 210 | 29.2 | |
| None | 2 | 0.3 | |
| Caretaker’s ethnic group (N = 720) | Temne | 449 | 62.6 |
| Limba | 120 | 16.7 | |
| Soso | 17 | 2.4 | |
| Fula | 11 | 1.5 | |
| Mende | 11 | 1.5 | |
| Loko | 73 | 10.1 | |
| Madingo | 14 | 1.9 | |
| Krio | 2 | 0.3 | |
| Sherbro | 2 | 0.3 | |
| Bullom | 1 | 0.1 | |
| Other | 20 | 2.8 | |
| Is the caretaker the household head? (N = 720) | Yes | 365 | 50.7 |
| No | 355 | 49.3 | |
| Household head’s sex 1 (N = 719) | Female | 392 | 54.5 |
| Male | 327 | 45.5 | |
1 Missing values: caretaker’s age = 2; caretaker’s sex = 3; caretaker’s highest level of education = 2; caretaker’s main type of income = 3; caretaker’s marital status = 5, household head’s sex = 1
2 Other religion 0/720
Vaccination coverage
Following the WHO definition of a fully immunised child, 65.8% (95% CI 60.3%-71.0%) of children were fully vaccinated (Table 2). More than half (54.4%; 95% CI 49.1%-59.7%) had received all age-appropriate vaccinations from birth until nine months of age following Sierra Leone’s EPI schedule. Of the 720 enrolled children, 14 (1.9%; 95% CI 1.0%-3.7%) had not received any routine childhood vaccinations.
Table 2.
Immunisation status of children aged 10–23 months in project districts in Sierra Leone
| Survey districts | ||||
|---|---|---|---|---|
| Bombali n/N (%); [95% CI] |
Tonkolili n/N (%); [95% CI] |
Port Loko n/N (%); [95% CI] |
Survey area n/N (%); [95% CI] |
|
| Fully immunised (according to WHO definition)1 |
228/288 (79.2); [71.6–85.2] |
118/168 (70.2); [59.8–78.9] |
128/264 (48.5); [41.2–55.8] |
474/720 (65.8); [60.3–71.0] |
| Fully immunised (all age-appropriate vaccines from birth to 9 months following Sierra Leone’s EPI schedule)2 |
193/288 (67.0); [59.4–73.8] |
87/168 (51.8); [40.6–62.8] |
112/264 (42.4); [35.2–50.0] |
392/720 (54.4); [49.1–59.7] |
| Partially immunised (according to WHO definition)1 |
59/288 (20.5); [14.5–28.1] |
50/168 (29.8); [21.1–40.2] |
123/264 (46.6); [39.5–53.8] |
232/720 (32.2); [27.4–37.5] |
| Partially immunised (some but not all age-appropriate vaccines from birth to 9 months following Sierra Leone’s EPI schedule)2 |
94/288 (32.6); [25.8–40.3] |
81/168 (48.2); [37.2–59.4] |
139/264 (52.7); [45.5–59.7] |
314/720 (43.6); [38.5–48.8] |
| Unimmunised (zero-dose) |
1/288 (0.4); [0.05–2.4] |
0/168 (0.0) |
13/264 (4.9); [2.7-9.0] |
14/720 (1.9); [1.0-3.7] |
1 WHO definition of a fully immunised child: a child who received 1 dose of bacille de Calmette-Guérin (BCG) vaccine for tuberculosis, 3 doses of oral polio vaccine (OPV), 3 doses of pentavalent vaccine and 1 dose of measles-containing vaccine (MCV). Partially immunised: defined as a child who received at least one but not all the previously listed vaccines
2 Following Sierra Leone’s national EPI schedule, a fully immunised child received all vaccines scheduled from birth to 9 months. In addition to the previously listed vaccines, this child received a birth dose of OPV, three doses of pneumococcal vaccine, two doses of rotavirus vaccine, one dose of inactivated poliovirus vaccine and one dose of yellow fever vaccine. Partially immunised: defined as a child who received at least one but not all recommend vaccines following this schedule
At district level, full vaccination coverage was found to be highest in Bombali: 79.2% (95% CI 71.6%-85.2%) of participants had received all basic vaccinations and 67.0% (95% CI 59.4%-73.8%) had received all age-appropriate vaccinations. In Tonkolili, all basic vaccinations were administered to 70.2% (95% CI 59.8%-78.9%) of children and all age-appropriate vaccinations to 51.8% (95% CI 40.6%-62.8%) of children. Port Loko had the lowest immunisation coverage, with 48.5% (95% CI 41.2%-55.8%) of children having received all basic vaccinations and 42.4% (95% CI 35.2%-50.0%) of children all age-appropriate vaccinations. In this district, close to one in 20 children were unimmunised (4.9%; 95% CI 1.0%-3.7%).
Coverage of individual vaccines was the highest for BCG (97.4%; 95% CI 95.6%-98.4%), administered at birth. For the five vaccines that require multidose administration, the first dose was administered most frequently (Table 3; Fig. 3). The vaccine with the lowest coverage was the second MCV dose administered around 15 months of age. Among children aged 16–23 months, 40.7% (95% CI 34.5%-47.2%) had received the vaccine, whereas the first dose was administered to 69.6% (95% CI 64.1%-74.5%) of children around 9 months.
Table 3.
Coverage per EPI contact by source of information
| Recommended timing of EPI contact | Vaccine | Children aged 10–23 months vaccinated at any time before the HHS, according to: | ||
|---|---|---|---|---|
| Vaccination card n/N (%) |
Caretaker’s report n/N (%) |
Total n/N (%); [95% CI] |
||
| At birth | BCG | 564/720 (78.3) | 137/720 (19.0) | 701/720 (97.4); [95.6–98.4] |
| OPV 0 | 553/720 (74.0) | 137/720 (19.0) | 690/720 (95.8); [93.4–97.4] | |
| At 6 weeks | OPV 1 | 548/720 (76.8) | 127/720 (17.6) | 675/720 (93.8); [90.4–96.0] |
| DTP-HepB-Hib (Pentavalent) 1 | 549/720 (76.3) | 130/720 (18.1) | 679/720 (94.3); [91.1–96.4] | |
| Pneumococcal 1 | 545/720 (75.7) | 128/720 (17.8) | 673/720 (93.5); [90.1–95.7] | |
| Rotavirus 1 | 529/720 (73.5) | 129/720 (17.9) | 658/720 (91.4); [87.7–94.1] | |
| At 10 weeks | OPV 2 | 527/720 (73.2) | 126/720 (17.5) | 653/720 (90.7); [87.0-93.4] |
| DTP-HepB-Hib (Pentavalent) 2 | 531/720 (73.8) | 126/720 (17.5) | 657/720 (91.3); [87.6–93.9] | |
| Pneumococcal 2 | 522/720 (72.5) | 126/720 (17.5) | 648/720 (90.0); [86.3–92.8] | |
| Rotavirus 2 | 498/720 (69.2) | 127/720 (17.6) | 625/720 (86.8); [83.0-89.9] | |
| PMC 1 | 421/720 (58.5) | 153/720 (21.3) | 574/720 (79.7); [74.7–83.9] | |
| At 14 weeks | OPV 3 | 503/720 (69.9) | 122/720 (16.9) | 625/720 (86.8); [82.8–90.0] |
| DTP-HepB-Hib (Pentavalent) 3 | 499/720 (69.3) | 122/720 (16.9) | 621/720 (86.3); [82.4–89.4] | |
| Pneumococcal 3 | 498/720 (69.2) | 120/720 (16.7) | 618/720 (85.8); [81.9–89.0] | |
| IPV | 432/720 (60.0) | 130/720 (18.1) | 562/720 (78.1); [73.6–81.9] | |
| PMC 2 | 452/720 (62.8) | 125/720 (17.4) | 577/720 (80.1); [75.7–83.9] | |
| At 6 months | Vitamin A | 466/720 (64.7) | 119/720 (16.5) | 585/720 (81.3); [76.4–85.3] |
| At 9 months | Yellow fever | 407/720 (56.5) | 96/720 (13.3) | 503/720 (69.9); [64.4–74.8] |
| MCV 1 | 400/720 (55.6) | 101/720 (14.0) | 501/720 (69.6); [64.1–74.5] | |
| PMC 3 | 328/720 (45.6) | 92/720 (12.8) | 420/720 (58.3); [53.0-63.5] | |
| At 12 months | De-worming1 | 259/571 (45.4) | 72/571 (12.6) | 331/571 (58.0); [52.1–63.6] |
| Vitamin A1 | 260/571 (45.5) | 74/571 (13.0) | 334/571 (58.5) [52.7–64.0] | |
| At 15 months | MCV 22 | 128/442 (29.0) | 52/442 (11.8) | 180/442 (40.7) [34.5–47.2] |
BCG = bacille Calmette-Guérin; DTP = diphtheria-tetanus-pertussis; HepB = hepatitis B; Hib = Haemophilus influenzae type b; PMC = perennial malaria chemoprevention; IPV = inactivated polio vaccine; MCV = measles-containing vaccine; OPV = oral polio vaccine
1 Among participants aged 13 months or older, allowing one month of delay
2 Among participants aged 16 months or older, allowing one month of delay
Fig. 3.
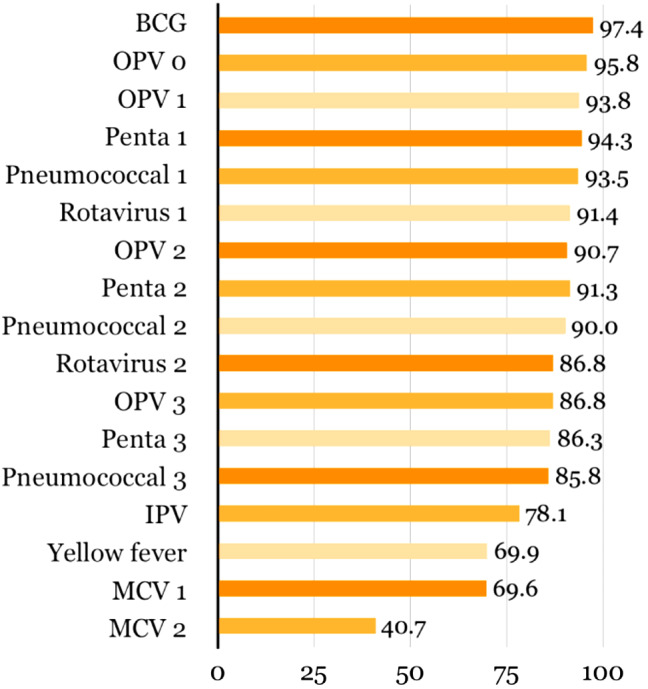
Proportion of children aged 10–23 months vaccinated at any time before the survey1,2
BCG = bacille Calmette-Guerin; Penta = pentavalent vaccine; IPV = inactivated polio vaccine; MCV = measles containing vaccine; OPV = oral polio vaccine
1 Ordered following Sierra Leone’s EPI schedule
2 MCV2: coverage among participants aged 16 months or older, allowing one month of delay
Vaccination card availability
Reported immunisation coverage rates were based on presented vaccination cards or, if absent, on caretakers’ recall. A vaccination card was presented during 80.0% of interviews (Table 4). Reported reasons for unavailability were: the card was lost or damaged in 33.9% of cases, 4.0% of caretakers was not given a card for their child, and someone other than the interviewed caretaker kept the card in 47.6% of cases. Reasons other than the three previously mentioned accounted for 14.5% of unavailable vaccination cards. Of the latter, the caretaker forgot or purposely left the card elsewhere in 10 out of 18 cases, three of which were due to relocation.
Table 4.
Vaccination card availability
| Survey districts | ||||
|---|---|---|---|---|
| Bombali n/N (%); [95% CI] |
Tonkolili n/N (%); [95% CI] |
Port Loko n/N (%); [95% CI] |
Survey area n/N (%); [95% CI] |
|
| Vaccination card available and seen during interview |
245/288 (85.1); [79.9–89.1] |
147/168 (87.5); [81.6–91.7] |
184/264 (69.7); [62.9–75.7] |
576/720 (80.0); [76.1–83.4] |
| Vaccination card not seen during interview |
43/288(14.9); [10.9–20.1] |
21/168 (12.5); [8.3–18.4] |
80/264 (30.3); [24.3–37.1] |
144/720 (20.00); [16.6–23.9] |
| Reasons for unavailability vaccination card 1 | ||||
| The card is lost/damaged | 13/41 (31.7) | 6/20 (30.0) | 23/63 (36.5) | 42/124 (33.9) |
| The caretaker was not given one | 3/41 (7.3) | 2/20 (10.0) | 0/63 (0.0) | 5/124 (4.0) |
| Someone else keeps it | 21/41 (51.2) | 10/20 (50.0) | 28/63 (44.4) | 59/124 (47.6) |
| Other | 4/41 (9.8) | 2/20 (10.0) | 12/63 (19.1) | 18/124 (14.5) |
1 No data for 20 cases: 9 children never attended EPI services and in 11 cases a vaccination card was available but not seen during the interview (2 cases in Bombali, 1 case in Tonkolili and 8 cases in Port Loko)
Factors associated with incomplete immunisation status
Table 5 shows univariate and multivariable analyses with incomplete immunisation status according to the WHO definition as dependent variable. Following multiple logistic regression, three factors were found to be independently associated with incomplete immunisation status. Compared with the district of Bombali, children residing in Port Loko were more likely to have missed at least one basic vaccination (aOR = 3.47, 95% CI = 2.00-6.06; p-value < 0.001). Children whose interviewed caretaker was male were also more likely to be incompletely immunised (aOR = 1.93, 95% CI = 1.03–3.59, p-value = 0.039). Compared to children whose caretaker was Christian, those with a Muslim caretaker were more likely to have missed one or more basic vaccinations as well (aOR = 1.94, 95% CI = 1.25–3.02; p-value = 0.015). Partly due to categories with few observations, the other factors included in the multivariable analysis showed imprecise estimates with wide 95% CIs, prompting the possibility of no association between these factors and incomplete immunisation status.
Table 5.
Logistic regression models (univariate and multivariable), output: Incomplete vaccination status (WHO-definition)
| Variable 1 | Univariate models | Multivariable model 2 | |||
|---|---|---|---|---|---|
| Crude OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | ||
| Child’s age in months (n = 720) | 0.96 (0.92-1.00) | 0.047 | 0.96 (0.92-1.00) | 0.064 | |
| Child’s sex | Female (n = 363) | 1 | 0.997 | 1 | 0.588 |
| Male (n = 357) | 1.00 (0.74–1.36) | 1.10 (0.77–1.59) | |||
| Caretaker’s age in years (n = 718) | 0.99 (0.98-1.00) | 0.112 | 1.00 (0.98–1.01) | 0.417 | |
| Caretaker’s sex | Female (n = 624) | 1 | 0.963 | 1 | 0.039 |
| Male (n = 93) | 1.01 (0.61–1.70) | 1.93 (1.03–3.59) | |||
| Caretaker’s highest level of education | Never attended school (n = 395) | 1 | 0.575 | 1 | 0.928 |
| Primary (n = 78) | 0.84 (0.46–1.52) | 0.89 (0.47–1.68) | |||
| Secondary or higher (n = 245) | 0.83 (0.58–1.19) | 0.93 (0.51–1.67) | |||
| Is the caretaker able to read and write? | Illiterate (n = 519) | 1 | 0.333 | 1 | 0.292 |
| Partially literate (n = 105) | 0.78 (0.47–1.28) | 0.81 (0.40–1.61) | |||
| Fully literate (n = 96) | 0.69 (0.41–1.17) | 0.54 (0.25–1.19) | |||
| Caretaker’s main type of income | No salary (n = 79) | 1 | 0.097 | 1 | 0.131 |
| Paid employment (n = 27) | 0.28 (0.09–0.89) | 0.32 (0.10–0.98) | |||
| Self-employment (n = 614) | 0.86 (0.52–1.40) | 0.79 (0.45–1.41) | |||
| Caretaker’s marital status | Married or in union (n = 578) | 1 | 0.108 | 1 | 0.280 |
| Single (never married) (n = 82) | 0.93 (0.52–1.65) | 0.82 (0.42–1.58) | |||
| Separated or divorced (n = 24) | 0.47 (0.16–1.37) | 0.58 (0.17–1.96) | |||
| Widowed (n = 31) | 0.34 (0.12–0.96) | 0.32 (0.10–1.06) | |||
| Caretaker’s religion | Christian (n = 210) | 1 | < 0.001 | 1 | 0.015 |
| Muslim (n = 508) | 2.77 (1.83–4.19) | 1.94 (1.25–3.02) | |||
| None (n = 2) | 4.12 (0.25–68.09) | 1.91 (0.21–17.05) | |||
| Household head’s sex | Female (n = 392) | 1 | 0.755 | 1 | 0.9193 |
| Male (n = 327) | 1.05 (0.75–1.48) | 0.98 (0.67–1.45) | |||
| District | Bombali (n = 288) | 1 | < 0.001 | 1 | < 0.001 |
| Tonkolili (n = 168) | 1.61 (0.87–2.99) | 1.27 (0.63–2.56) | |||
| Port Loko (n = 264) | 4.04 (2.43–6.70) | 3.47 (2.00-6.06) | |||
| Locality | Rural (n = 540) | 1 | 0.086 | 1 | 0.677 |
| Urban (n = 180) | 1.54 (0.94–2.53) | 1.12 (0.65–1.91) | |||
CI, confidence interval; OR, odds ratio
1 The first listed category of each variable was taken as reference value
2 Following multiple logistic regression, variables significantly associated with incomplete immunisation status are presented in bold
Appendix 2 includes the results of the logistic regression analyses with incomplete immunisation status following Sierra Leone’s EPI schedule as dependent variable.
Subgroup analysis: children aged 12–23 months
Results of the subgroup analysis for the 625 enrolled children aged 12–23 months are presented in Appendix 3. Adjusted findings on coverage of individual doses and immunisation status are similar to the previously presented results.
Discussion
This community-based study was the first survey to assess routine immunisation coverage during the COVID-19 pandemic in Sierra Leone. A low percentage of zero-dose children was reported, yet approximately one in three children had missed at least one basic vaccination and almost half had missed at least one age-appropriate vaccination following the country’s EPI schedule. These findings indicate that too many children are only partially vaccinated and, thereby, exposed to VPDs, disability and premature death.
Coverage of the third DTP (DTP3) dose is often used as an indicator for overall routine immunisation system performance [26]. In our 2021 survey, 86.3% of children had received DTP3. This is lower than the WHO-reported national estimate of 92% that year, but higher than the global DTP3 coverage of 81% and estimates in the neighbouring countries of Guinea (47%) and Liberia (66%) [11, 27]. However, the proportion of vaccinated children in this survey still falls short of the 90% target [28]. Survey findings show that individual vaccine coverage rates were highest for those given at birth and decreased with consecutive EPI appointments at later ages. Accordingly, in the case of multi-dose vaccines including DTP, coverage is highest for the first dose and recedes in subsequent doses. A similar pattern was described by the Sierra Leone DHS in 2019 [9].
The lowest reported coverage was MCV2, administered at the final EPI contact at 15 months. MCV2 coverage is used to evaluate the EPI’s ability to deliver vaccines beyond the first year of life [26]. This survey estimated MCV2 coverage at 40.7% among children aged 16–23 months, which is far below the global coverage of 71% at the end of 2021 [11]. It is also far from the protective threshold of 95% needed to achieve herd immunity and prevent measles outbreaks [29]. Over half of eligible surveyed children had not received the two recommended MCV doses, thus, vaccination catch-up campaigns are warranted. Through the addition of PMC and the recently recommended RTS,S/AS01 malaria vaccine to this final visit at 15 months, renewed emphasis is placed on the need to improve EPI attendance in the second year of life [15, 30]. Strengthening immunisation beyond the first year of life requires implementation of other known strategies as well, including interventions related to community engagement and capacity-building for health workers [31, 32].
Reporting recent vaccination coverage rates is especially relevant in light of the COVID-19 pandemic, which destabilised health systems worldwide [11]. The indirect effects of the pandemic on health service delivery and child health are substantial due to, among others, vaccination campaign interruptions, health resources diversion, supply-chain disruptions, mobility restriction and fear of contagion at health facilities [33–35]. When comparing the coverage rates among a subset of surveyed children aged 12–23 months with the Sierra Leone 2019 DHS report, we found that full immunisation coverage improved in all three districts and that there was no increase in the proportion of zero-dose children [9]. However, it remains unclear whether such differences are due to actual differences in population coverage, or due to differences in survey design and implementation.
This study also found that full immunisation coverage was lower Port Loko than in Bombali and Tonkolili, and around 5% of children in this district were zero-dose. Similarly, Port Loko had the lowest immunisation coverage at district level according to the 2019 DHS in Sierra Leone [9]. Together, these findings suggest possible structural difficulties in this district in reaching all children with immunisation efforts. Underlying challenges on both the demand- and the supply-side should be investigated and addressed in Port Loko.
Children whose interviewed caretaker was male were more likely to have missed at least one routine vaccination. Previous studies conducted in Sub-Saharan Africa identified low male involvement in and poor knowledge of routine childhood immunisation activities as barriers to vaccination uptake [23, 36, 37]. Educating and involving men in matters related to their child’s health can help improve childhood immunisation coverage [38].
In this study, we observed that children with Muslim caretakers were less likely to have received all routine vaccinations. This is in agreement with a previous report showing a lower full immunisation coverage among Muslims than Christians in Sierra Leone [39]. Religion is a complex, multidimensional construct that has long been considered a social determinant of health [40–42]. Understanding religious influences on health-seeking behaviour is crucial to explore possible roots of health inequities [43]. In Sierra Leone, where 77% of the population is Muslim and 22% is Christian, religious leaders have long been involved in mobilising communities and raising awareness on childhood immunisation [44, 45]. This approach has been supported by findings from a previous study in Sierra Leone that observed a higher immunisation confidence and uptake among those who received related information from faith leaders [46]. The findings of the present survey highlight the continued call for involving religious and other influential community leaders in health interventions to promote immunisation uptake. Moreover, tailored interventions may be informed by additional socio-anthropological research that explores religion as a determinant of vaccination uptake in the surveyed area.
It is important to note that although some well-documented determinants of vaccination uptake, such as education and income levels of the caretaker, were not significantly associated with incomplete immunisation status in this survey, they should continue to shape context-specific intervention strategies that strive to close immunisation gaps [22, 23].
Due to frequent incompleteness and inaccuracy of official estimates, vaccination coverage surveys are considered a reliable complement to routine health facility data [47–49]. In this study, we tried to minimise known challenges of coverage surveys [50]. A multi-stage cluster sampling approach was used to select a representative sample. Field workers were trained beforehand and supervised during data collection to maximise data consistency. Furthermore, the use of electronic devices and strict data quality checks during data collection improved the validity of our findings. Lastly, the 1.1% non-response rate among eligible children was much lower than the estimated 10% used to calculate the survey’s sample size, minimising the potential for non-response bias at this level.
A possible study limitation is the fact that some potentially relevant determinants of immunisation uptake were not collected in the survey, such as a wealth and assets index, place of child birth and number of children in the household [22, 23, 51, 52]. Secondly, our estimate of MCV2 coverage does not conform to the target population aged 24–35 months as recommended by the WHO reference manual on vaccination coverage surveys [24]. Instead, our estimate amongst children aged 16–23 months considered a possible delay of one month. Moreover, even though previous studies support the accuracy of caretaker recollection and the majority of caretakers had their child’s vaccination card at hand, a potential recall bias may be present [53–56]. Considering a total of eight EPI visits, our estimates could have been biased by the survey’s reliance on caretakers’ memory in one out of every five interviews. This study has therefore presented individual vaccination coverage rates according to source of information. Lastly, clusters were selected using the 2015 census. Two selected clusters were outside project areas and therefore replaced by backup clusters, leading to potential underrepresentation of part of the area, and thus to potential non-response bias.
Conclusion
Around one in three surveyed children in selected districts of Sierra Leone had missed at least one basic vaccination and more than half of eligible children had not received the recommended two doses of a measles-containing vaccine. Although full immunisation coverage at district level did not drop below pre-pandemic coverage, too many under-vaccinated children remain at risk of preventable morbidity and mortality. These findings highlight the need for supplementary immunisation activities to ensure improved and equitable vaccination uptake. Identified geographic and cultural barriers to immunisation should be investigated and addressed through tailored strategies to reach under-immunised children. Potential approaches to reduce coverage gaps include additional immunisation efforts in Port Loko, promoting male involvement in matters related to child health, and involving religious leaders to advocate for equal access to life-saving vaccines for all.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 2: Logistic regression models (univariate and multivariable), output: Incomplete vaccination status (following Sierra Leone’s EPI schedule)
Appendix 3: Subgroup analysis: children aged 12 - 23 months
Acknowledgements
We would like to extend our warm gratitude to those directly involved with the survey’s data collection in Sierra Leone: to all enumerators, nurses, team supervisors and epidemiologists (specifically Cesc Bertran-Cobo) for their admirable hard work, to all participating children and their caretakers, and to Statistics Sierra Leone for providing valuable support and information. Additionally, we would like to thank Marc Bañuls for facilitating this analysis through his expertise on data management and curation, Andrea Egan and Cristina Raya for their efforts regarding project administration and logistics, Aina Casellas for sharing her statistical expertise.
Abbreviations
- COVID-19
Coronavirus disease 2019
- BCG
Bacille Calmette-Guérin
- DHS
Demographic and Health Survey
- DTP
Diphtheria-pertussis-tetanus
- EPI
Expanded Programme on Immunisation
- HepB
Hepatitis B
- HHS
Household survey
- Hib
Haemophilus influenzae type b
- IPTi
Intermittent Preventive Treatment in infants
- IPV
Inactivated polio vaccine
- MCV
Measles-containing vaccine
- MULTIPLY
MULTIple doses of IPTi Proposal: a Lifesaving high Yield intervention
- OPV
Oral polio vaccine
- PMC
Perennial malaria chemoprevention
- U2
Under two years of age
- UNICEF
United Nations Children’s Fund
- VPD
Vaccine-preventable disease
- WHO
World Health Organization
Author contributions
MW: survey design, methodology, training, supervision of data collection, data analysis and interpretation, visualisation, writing – original draft, review and editing. AEF: MULTIPLY study conceptualisation, survey design and coordination, training, supervision of data collection, writing – review and editing. HC: survey design, methodology, training, supervision of data collection, writing – review and editing. KOK: MULTIPLY study conceptualisation, survey design and coordination, training, supervision of data collection, writing – review and editing. JW: training, methodology, data management and curation, writing – review and editing.JS: training, methodology, data management and curation, writing – review and editing. ML: survey design, methodology, training, supervision of data collection, writing – review and editing. LQ: MULTIPLY study conceptualisation, survey design, methodology, data analysis, writing – review and editing. TS: MULTIPLY study conceptualisation, survey design, resources, supervision, writing – review and editing. MS: MULTIPLY study conceptualisation, survey design, resources, supervision, writing – review and editing. CM: MULTIPLY study conceptualisation, survey design, resources, supervision, data interpretation, writing – review and editing. RG: MULTIPLY study conceptualisation, survey design, supervision, data interpretation, writing – review and editing.
Funding
This research article was produced by the MULTIPLY-project, which is part of the EDCTP2 program supported by the European Union (grant number RIA2020S-3272-MULTIPLY). The funder had no role in the design of the study, the collection, analysis and interpretation of data, nor in writing this article. The views and opinions of the author expressed herein do not necessarily state or reflect those of the EDCTP.
Data Availability
The datasets generated during the current household survey and analysed in this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study is part of the MULTIPLY-project, which was approved by national and institutional ethics committees and national pharmacy boards in Spain, Sierra Leone, Togo and Mozambique. Informed consent was obtained for all enrolled participants. Caretakers of eligible children were interviewed after they had agreed to participate in the survey by signing an informed consent form. In case of illiteracy, an impartial witness who spoke the language of the caretaker was present during the complete informed consent process and the form was signed by the caretaker using their fingerprint. Data was anonymised and confidentiality of the respondents was maintained throughout and after the survey.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Immunization agenda 2030: a global strategy to leave no one behind. 2020. https://www.who.int/publications/m/item/immunization-agenda-2030-a-global-strategy-to-leave-no-one-behind. Accessed 18 May 2022.
- 2.Carter A, Msemburi W, Sim SY, Gaythorpe KAM, Lindstrand A, Hutubessy RCW. Modeling the impact of vaccination for the immunization agenda 2030: deaths averted due to Vaccination against 14 pathogens in 194 countries from 2021–2030. SSRN Electron J. 2021 doi: 10.2139/ssrn.3830781. [DOI] [PubMed] [Google Scholar]
- 3.Rachlin A, Danovaro-Holliday MC, Murphy P, Sodha SV, Wallace AS. Routine vaccination Coverage — Worldwide, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:1396–400. doi: 10.15585/mmwr.mm7144a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardon A, Blume S. Shifts in global immunisation goals (1984–2004): unfinished agendas and mixed results. Soc Sci Med. 2005;60:345–56. doi: 10.1016/j.socscimed.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Vaccination schedule for Sierra Leone. https://immunizationdata.who.int/pages/schedule-by-country/sle.html?DISEASECODE=&TARGETPOP_GENERAL=. Accessed 18 May 2022.
- 6.Government of Sierra Leone M of H and, Comprehensive S. EPI multi-year plan: 2012–2016. 2014. https://extranet.who.int/countryplanningcycles/sites/default/files/country_docs/Sierra%20Leone/cmyp-_2012-2016_narrative_for_sierra_leone_final_updated_on_the_23_jan_2014.pdf. Accessed 18 May 2022.
- 7.Senessie C, Gage GN, von Elm E. Delays in childhood immunization in a conflict area: a study from Sierra Leone during civil War. Confl Health. 2007;1:14. doi: 10.1186/1752-1505-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Statistics Sierra Leone (SSL) and ICF International . Sierra Leone Demographic and Health Survey 2013. Maryland, USA: Freetown, Sierra Leone and Rockville; 2014. [Google Scholar]
- 9.Statistics Sierra Leone (Stats SL) and ICF . Sierra Leone Demographic and Health Survey 2019. Maryland, USA: Freetown, Sierra Leone, and Rockville; 2020. [Google Scholar]
- 10.Saxena S, Skirrow H, Bedford H. Routine vaccination during covid-19 pandemic response. BMJ. 2020;369:m2392. doi: 10.1136/bmj.m2392. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization, UNICEF. Progresses and Challenges with Sustaining and Advancing Immunization Coverage During the COVID-19 Pandemic. 2021. https://www.who.int/publications/i/item/progresses-and-challenges-with-sustaining-and-advancing-immunization-coverage-during-the-covid-19-pandemic. Accessed 18 May 2022.
- 12.World Health Organization. Vaccine-preventable disease outbreaks on the rise in Africa. 2022. https://www.afro.who.int/news/vaccine-preventable-disease-outbreaks-rise-africa. Accessed 18 May 2022.
- 13.National Malaria Control Programme - NMCP/Sierra Leone. Statistics Sierra Leone - SSL, University of Sierra Leone - USL, Catholic Relief Services - CRS, ICF. Sierra Leone Malaria Indicator Survey 2016. Freetown, Sierra Leone;; 2016.
- 14.Statistics Sierra Leone. Sierra Leone 2015 Population and Housing Census. https://www.statistics.sl/index.php/census/census-2015.html. Accessed 18 May 2022.
- 15.National Library of Medicine (U.S.). MULTIple Doses of IPTi Proposal: a Lifesaving High Yield Intervention (MULTIPLY). ClinicalTrials.gov Identifier: NCT05085340. 2021.
- 16.World Health Organization. WHO Guidelines for Malaria. Geneva; 2022.
- 17.Suresh K, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012;5:7–13. doi: 10.4103/0974-1208.97779. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Lahuerta M, Sutton R, Mansaray A, Eleeza O, Gleason B, Akinjeji A, et al. Evaluation of health system readiness and coverage of intermittent preventive treatment of Malaria in infants (IPTi) in Kambia district to inform national scale-up in Sierra Leone. Malar J. 2021;20:74. doi: 10.1186/s12936-021-03615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fombah AE, Chen H, Owusu-Kyei K, Quinto L, Gonzalez R, Williams J, et al. Coverage of intermittent preventive treatment of Malaria in infants after four years of implementation in Sierra Leone. Malar J. 2023;22:145. doi: 10.1186/s12936-023-04575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.REDCap [Internet]. https://www.project-redcap.org/. Accessed 23 May 2022.
- 21.World Health Organization. Fully immunized child rate. The Global Health Observatory. 2020. https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3376. Accessed 18 May 2022.
- 22.World Health Organization. Explorations of inequality: Childhood immunization. 2018. https://www.who.int/data/health-equity/report_2018_immunization. Accessed 13 Jul 2022.
- 23.Bangura JB, Xiao S, Qiu D, Ouyang F, Chen L. Barriers to childhood immunization in sub-saharan Africa: a systematic review. BMC Public Health. 2020;20:1108. doi: 10.1186/s12889-020-09169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. World Health Organization Vaccination Coverage Cluster Surveys: Reference Manual. 2018. https://apps.who.int/iris/handle/10665/272820. Accessed 18 May 2022.
- 25.StataCorp College Station TX. StataCorp LLC. Stata Statistical Software: Release 17. 2021.
- 26.United Nations Statistics Division. SDG Metadata Indicator 3.b.1: Proportion of the target population covered by all vaccines included in their national programme. 2020. https://unstats.un.org/sdgs/metadata/files/Metadata-03-0b-01.pdf. Accessed 1 Jul 2022.
- 27.World Health Organization. The Global Health Observatory: Diphtheria tetanus toxoid and pertussis (DTP3) immunization coverage among 1-year-olds (%). 2022. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/diphtheria-tetanus-toxoid-and-pertussis-(dtp3)-immunization-coverage-among-1-year-olds-(). Accessed 5 Aug 2022.
- 28.World Health Organization. Implementing the Immunization Agenda 2030: A Framework for Action through Coordinated Planning, Monitoring & Evaluation, Ownership & Accountability, and Communications & Advocacy. 2021. https://www.who.int/publications/m/item/implementing-the-immunization-agenda-2030. Accessed 3 May 2023. [DOI] [PMC free article] [PubMed]
- 29.Hübschen JM, Gouandjika-Vasilache I, Dina J. Measles the Lancet. 2022;399:678–90. doi: 10.1016/S0140-6736(21)02004-3. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. WHO recommends groundbreaking malaria vaccine for children at risk. 2021. https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk. Accessed 24 Apr 2023.
- 31.World Health Organization. Establishing and strengthening immunization in the second year of life: practices for vaccination beyond infancy. 2018.
- 32.Tchoualeu DD, Harvey B, Nyaku M, Opare J, Traicoff D, Bonsu G, et al. Evaluation of the impact of immunization Second Year of Life Training interventions on Health Care workers in Ghana. Glob Health Sci Pract. 2021;9:498–507. doi: 10.9745/GHSP-D-21-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shet A, Carr K, Danovaro-Holliday MC, Sodha S, v, Prosperi C, Wunderlich J, et al. Impact of the SARS-CoV-2 pandemic on routine immunisation services: evidence of disruption and recovery from 170 countries and territories. Lancet Glob Health. 2022;10:e186–94. doi: 10.1016/S2214-109X(21)00512-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lassi ZS, Naseem R, Salam RA, Siddiqui F, Das JK. The impact of the COVID-19 Pandemic on Immunization campaigns and programs: a systematic review. Int J Environ Res Public Health. 2021;18:988. doi: 10.3390/ijerph18030988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho LL, Gurung S, Mirza I, Nicolas HD, Steulet C, Burman AL, et al. Impact of the SARS-CoV-2 pandemic on vaccine-preventable Disease campaigns. Int J Infect Dis. 2022;119:201–9. doi: 10.1016/j.ijid.2022.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raji M, Sani A, Ibrahim L, Muhammad H, Oladigbolu R, Kaoje A. Assessment of the knowledge of fathers, uptake of routine immunization, and its associated factors in a rural community of North West Nigeria. Ann Afr Med. 2019;18:97. doi: 10.4103/aam.aam_41_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baguma C, Babirye JN, Oryema P, Wasswa P, Atuyambe L. Reasons for the low male involvement in routine child immunization in Hoima District Uganda using the attitude, Social Influence and Self Efficacy Model. J Immun. 2016;1:9–21. [Google Scholar]
- 38.Gavi the Vaccine Alliance, Unilever, Lifebuoy. From provider to protector: rethinking the father’s role in child health in India. 2022. https://www.gavi.org/sites/default/files/investing/partnering/Gavi-Unilever-Gender-Case-Study.pdf. Accessed 23 Apr 2023.
- 39.Costa JC, Weber AM, Darmstadt GL, Abdalla S, Victora CG. Religious affiliation and immunization coverage in 15 countries in Sub-saharan Africa. Vaccine. 2020;38:1160–9. doi: 10.1016/j.vaccine.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellison CG, Levin JS. The Religion-Health connection: evidence, theory, and future directions. Health Educ Behav. 1998;25:700–20. doi: 10.1177/109019819802500603. [DOI] [PubMed] [Google Scholar]
- 41.Ransome Y. Religion, spirituality, and Health: New considerations for Epidemiology. Am J Epidemiol. 2020;189:755–8. doi: 10.1093/aje/kwaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, VanderWeele TJ. Chen and VanderWeele respond to Religion, spirituality, and Health. Am J Epidemiol. 2020;189:759–60. doi: 10.1093/aje/kwaa021. [DOI] [PubMed] [Google Scholar]
- 43.Watson R, McLaverty R, Sinibaldi S, Faber R. Engaging with local faith actors and communities: a toolkit. 2020. https://www.preventionweb.net/publication/engaging-local-faith-actors-and-communities-toolkit. Accessed 17 Jun 2022.
- 44.Jalloh MF, Wilhelm E, Abad N, Prybylski D. Mobilize to vaccinate: lessons learned from social mobilization for immunization in low and middle-income countries. Hum Vaccin Immunother. 2020;16:1208–14. doi: 10.1080/21645515.2019.1661206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.United States Department of State O of IRF. Sierra Leone 2021 International Religious Freedom Report. 2021. https://www.state.gov/wp-content/uploads/2022/05/SIERRA-LEONE-2021-INTERNATIONAL-RELIGIOUS-FREEDOM-REPORT.pdf.
- 46.Kulkarni S, Sengeh P, Eboh V, Jalloh MB, Conteh L, Sesay T, et al. Role of information sources in Vaccination Uptake: insights from a cross-sectional Household Survey in Sierra Leone, 2019. Glob Health Sci Pract. 2022;10:e2100237. doi: 10.9745/GHSP-D-21-00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cutts FT, Claquin P, Danovaro-Holliday MC, Rhoda DA. Monitoring vaccination coverage: defining the role of surveys. Vaccine. 2016;34:4103–9. doi: 10.1016/j.vaccine.2016.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray CJ, Shengelia B, Gupta N, Moussavi S, Tandon A, Thieren M. Validity of reported vaccination coverage in 45 countries. The Lancet. 2003;362:1022–7. doi: 10.1016/S0140-6736(03)14411-X. [DOI] [PubMed] [Google Scholar]
- 49.Bosch-Capblanch X, Ronveaux O, Doyle V, Remedios V, Bchir A. Accuracy and quality of immunization information systems in forty-one low income countries. Tropical Med Int Health. 2009;14:2–10. doi: 10.1111/j.1365-3156.2008.02181.x. [DOI] [PubMed] [Google Scholar]
- 50.Cutts FT, Izurieta HS, Rhoda DA. Measuring Coverage in MNCH: design, implementation, and Interpretation Challenges Associated with Tracking Vaccination Coverage using Household surveys. PLoS Med. 2013;10:e1001404. doi: 10.1371/journal.pmed.1001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaufman J, Tuckerman J, Bonner C, Durrheim DN, Costa D, Trevena L, et al. Parent-level barriers to uptake of childhood vaccination: a global overview of systematic reviews. BMJ Glob Health. 2021;6:e006860. doi: 10.1136/bmjgh-2021-006860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fenta SM, Biresaw HB, Fentaw KD, Gebremichael SG. Determinants of full childhood immunization among children aged 12–23 months in sub-saharan Africa: a multilevel analysis using demographic and Health Survey Data. Trop Med Health. 2021;49:29. doi: 10.1186/s41182-021-00319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seror V, Cortaredona S, Ly EY, Ndiaye S, Gaye I, Fall M, et al. Vaccination card availability and childhood immunization in Senegal. BMC Public Health. 2020;20:658. doi: 10.1186/s12889-020-08792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Porth JM, Wagner AL, Tefera YA, Boulton ML. Childhood immunization in Ethiopia: accuracy of maternal Recall compared to vaccination cards. Vaccines (Basel) 2019;7:48. doi: 10.3390/vaccines7020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Binyaruka P, Borghi J. Validity of parental recalls to estimate vaccination coverage: evidence from Tanzania. BMC Health Serv Res. 2018;18:440. doi: 10.1186/s12913-018-3270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eze P, Aniebo CL, Agu UJ, Agu SA, Acharya Y. Validity of maternal recall for estimating childhood vaccination coverage – evidence from Nigeria. Vaccine. 2022;40:28–36. doi: 10.1016/j.vaccine.2021.11.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 2: Logistic regression models (univariate and multivariable), output: Incomplete vaccination status (following Sierra Leone’s EPI schedule)
Appendix 3: Subgroup analysis: children aged 12 - 23 months
Data Availability Statement
The datasets generated during the current household survey and analysed in this study are available from the corresponding author on reasonable request.