Abstract
Objective:
The goal of this scoping review was to summarize the literature on facilitators and barriers to surgical practice change. This information can inform research to implement best practices and evaluate new surgical innovations.
Background:
In an era of accelerated innovations, surgeons face the difficult decision to either acknowledge and implement or forgo new advances. Although changing surgical practice to align with evidence is an imperative of health systems, evidence-based guidelines have not translated into consistent change. The literature on practice change is limited and has largely focused on synthesizing information on methods and trials to evaluate innovative surgical interventions. No reviews to date have grounded their analysis within an implementation science framework.
Methods:
A systematic review of the literature on surgical practice change was performed. Abstracts and full-text articles were reviewed for relevance using inclusion and exclusion criteria and data were extracted from each article. Cited facilitators and barriers were then mapped across domains within the implementation science Theoretical Domains Framework and expanded to the Capability, Opportunity, Motivation, and Behavior model.
Results:
Components of the Capability, Opportunity, Motivation, and Behavior model were represented across the Theoretical Domains Framework domains and acted as both facilitators and barriers to practice change depending on the circumstances. Domains that most affected surgical practice change, in order, were: opportunity (environmental context and resources and social influences), capability (knowledge and skills), and motivation (beliefs about consequences and reinforcement).
Conclusions:
Practice change is predicated on a conducive environment with adequate resources, but once that is established, the surgeon’s individual characteristics, including skills, motivation, and reinforcement determine the likelihood of successful change. Deficiencies in the literature underscore the need for further study of resource interventions and the role of surgical team dynamics in the adoption of innovation. A better understanding of these areas is needed to optimize our ability to disseminate and implement best practices in surgery.
Keywords: innovation, practice change, scoping review, surgeon behavior
Recent innovations in surgery have outpaced the study of their efficacy in everyday practice, including the introduction of robotic surgery, advances in image-guidance, the use of new devices, and perioperative protocols. Randomized controlled trials of surgical procedures are scarce and are frequently the target of criticism about generalizability owed to differences in surgeon skill and patient heterogeneity.1,2 Moreover, the well-established volume-outcome relationship in surgery and related learning curve are unique barriers to both trial design and implementation of new procedures.3,4 In an era of accelerated innovations, the tension between consistency and change is constant; that is, surgeons face the difficult decision to either acknowledge and implement or forgo new advances throughout their careers. Understanding the facilitators and barriers influencing a surgeon’s decision regarding a practice change is critical to implementing best practices and de-implementing less effective or harmful surgical practices.
Although changing surgical practice to align with evidence is an imperative of health systems, new evidence and practice guidelines have not consistently translated into change. Studies examining the impact of guidelines on clinical practice patterns attribute this discrepancy to individual, environmental, and social factors, such as unawareness of guidelines, variable strength of evidence underlying guideline recommendations, local or financial barriers to implementation, anecdotal preferences, and perceived lack of contextual and patient relevance.5–12 This research suggests that the implementation of clinical practice guidelines alone may be insufficient and a comprehensive, systematic approach is needed to facilitate sustainable practice change. Moreover, the literature on practice change is limited and has largely focused on synthesizing information on methods and trials to evaluate innovative surgical interventions. The reviews and editorials in this area have focused on (1) ethical considerations surrounding the adoption of innovations,13 (2) promoting learning organizational models and paradigms for innovation development,14,15 and (3) surgeon-level barriers to the uptake of innovations (eg, technical and interpersonal skills).15,16 No reviews to date have grounded their analysis within an implementation science framework.
Implementation science, the study of “strategies to adopt and integrate evidence-based interventions and change practice patterns within specific settings,” has particular relevance to surgical practice.17–19 As surgical outcomes research increasingly involves the dissemination and implementation of evidence-based practice, understanding the extant literature through the lens of implementation science has the potential to inform the adoption of innovations and effectiveness studies moving forward. In this review, we employ the Theoretical Domains Framework (TDF), an implementation science conceptual framework, to identify and understand facilitators and barriers to change.20–22 The TDF includes 14 domains that derive from 3 determinants of behavior (summarized in Table 1): Capability, Opportunity, and Motivation (COM-B model).21,23 These concepts are highly applicable within the context of surgical practice change. Capability refers to the individual’s psychological and physical capacity to engage in an activity (eg, TDF domains: knowledge and skills); Opportunity includes extrinsic factors outside the individual that make the behavior possible or prompt it (eg, TDF domains: environmental context and resources and social influences); Motivation involves brain processes that energize and direct behavior, including habitual processes, emotional responses, and analytical decision-making (eg, TDF domains: reinforcement and beliefs about consequences).24,25
TABLE 1.
Overlap of Theoretical Domains Framework (TDF) and Capability, Opportunity, Motivation, Behavior (COM-B) Model*
| TDF Domain | Definition in Context of Surgical Practice Change | Capability | Opportunity | Motivation |
|---|---|---|---|---|
| Knowledge | Awareness that procedure exists and/or having sufficient knowledge about it to make change | x | ||
| Skills | Physically possessing skills and expertise to make change | x | ||
| Behavioral regulation | Actions taken to directly change behavior | x | ||
| Memory, attention, and decision processes | Recalling the processes to make change and the decision making surrounding it | x | ||
| Social/professional role and identity | Individual behaviors and qualities that impact change | x | ||
| Beliefs about capabilities | Believing they have the skills and capacity to make change | x | ||
| Environmental context and resources | Possessing appropriate resources and/or being in environment or situation conducive to change | x | ||
| Social influences | Interactions between colleagues, mentors, thought leaders, and patients that impact their thoughts and actions surrounding the change | x | ||
| Optimism | Confidence that the change will improve outcomes | x | ||
| Beliefs about consequences | What they think will happen if they make the change | x | ||
| Reinforcement | Being more likely to repeat/carry out change if they experience a positive result | x | ||
| Intentions | Why they made the change | x | ||
| Goals | What they want the change to happen | x | ||
| Emotion | Reactions or feelings surrounding change | x |
Using the TDF framework, this scoping review summarizes the literature on surgical practice change. We identify facilitators and barriers that influence practice change to inform further research focused on implementing and evaluating new surgical practices. Improved understanding of this topic has the potential to promote evidence-based practice and to help de-implement less effective or harmful surgical practices.
METHODS
Systematic Review and Study Selection
We searched the MEDLINE database via Pubmed, Scopus, Web of Science, ERIC, CINAHL, PsycINFO (ProQuest), and Education Research Complete. The literature search was completed in May 2018 to reflect practices over the previous 5 years, using a combination of controlled vocabulary and key terms related to innovation adoption, behavior change, surgery, and surgeons (see Document, Supplemental Digital Content 1, Search Strategy, http://links.lww.com/SLA/C502). Reference lists of articles were hand-searched and recent reviews addressing surgeon practice change to identify additional relevant articles.
Inclusion criteria were developed in consultation with an expert panel of surgeons, health services researchers, and a reference librarian. Included were original studies of all designs involving surgical practice change published in English. Excluded were studies related to medical education or residency training. Three independent investigators (NAA, TG, and MT) reviewed abstracts to determine candidacy for full-text review. Similarly, 3 independent investigators (NAA, TG, and MT) reviewed the full text of candidate studies to determine whether they met inclusion criteria for data extraction. Senior investigators (DOF and SFT) resolved discrepancies. The review was completed using DistillerSR software (Evidence Partners, Ottawa, Canada).
Data Extraction and Analysis
One investigator (TG) extracted data regarding study design, descriptions of study population and intervention and comparison groups (if applicable), and baseline and outcome data using a standardized form. A second investigator independently (NAA) verified the accuracy of the extraction and revised as needed. Principal measures of interest were the facilitators of, barriers to, and neutral factors for change. Barriers, facilitators, and neutral factors were abstracted from study results and were defined as: (a) barrier: factor that impedes the implementation outcomes; (b) facilitator: factor that enables the implementation outcomes; and (c) neutral factor: factor that does not impact the implementation outcomes.26 Any disputes about coding and classification as a facilitator, barrier, or neutral factor involved 2 senior investigators (DOF and SFT) rereviewing data, discussing the dispute, and making a consensus determination. Study setting refers to the scope and environment in which the data for the study was collected (academic or community hospitals, regional/national, or global). We also identified whether a targeted intervention was studied (eg, introduction of clinical practice guidelines) and examined which barriers and facilitators within the TDF framework impacted its effectiveness at changing practice.
Qualitative Synthesis
Significant heterogeneity in outcomes reported and study design precluded meta-analysis; thus, we synthesized the data qualitatively using the TDF. Two independent reviewers (NAA and TG) mapped results from included studies to categorize facilitators and barriers within TDF domains and their derivative COM-B components based on the published conceptual framework.21,23 Senior investigators (DOF and SFT) were consulted to reconcile disagreements. Recurring concepts within the dominant TDF domains and COM-B components further characterized the literature.
RESULTS
Characteristics of the Studies
Our search identified 2427 citations of which 104 met inclusion criteria (Fig. 1). All studies included and data extracted are listed in a supplementary table (see Table, Supplemental Digital Content 1, Characteristics of Selected Surgical Practice Change Articles, http://links.lww.com/SLA/C502). Included studies were predominantly from the United States (n = 47, 45.2%) and Europe (n = 28, 26.9%) and conducted in regional/national (n = 50, 48.1%) or hospital settings (academic only: n = 14; 13.5%; community only: n = 2, 1.9%; both: n = 14; 13.5%; hospital type not specified: n = 18, 26.9%; Table 2). Subspecialties with the most publications on surgical practice change were urology (n = 12, 11.5%), obstetrics and gynecology (n = 11, 10.6%), and orthopedics (n = 11, 10.6%).
FIGURE 1.
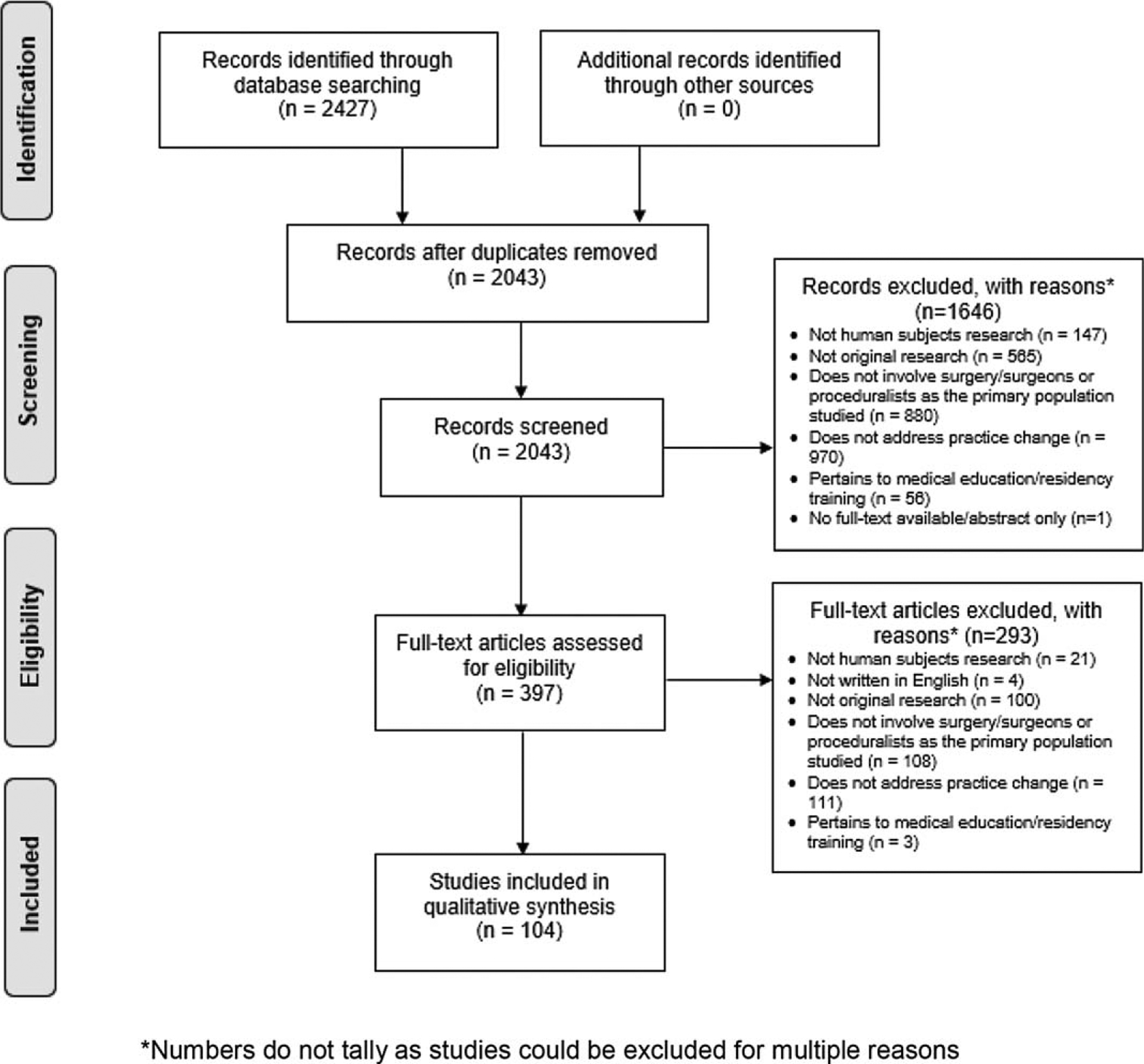
PRISMA diagram.
TABLE 2.
Descriptive Statistics of Full-text Articles Included
| Geographic Region | n | % |
|---|---|---|
| USA | 47 | 45.2 |
| Europe | 28 | 26.9 |
| Canada | 10 | 9.6 |
| Asia | 9 | 8.7 |
| Australia | 4 | 3.9 |
| Africa | 1 | 1.0 |
| 2 or more regions | 5 | 4.8 |
| Setting of study* | ||
| Regional/national | 50 | 48.1 |
| Hospital(s), nonspecific† | 18 | 17.3 |
| Mix of academic and community hospitals | 14 | 13.5 |
| Academic center(s) only | 14 | 13.5 |
| Global | 6 | 5.8 |
| Community hospital(s) only | 2 | 1.9 |
| Included a targeted intervention | ||
| No | 79 | 76.0 |
| Yes | 25 | 24.0 |
| Field of surgery | ||
| Urology | 12 | 11.5 |
| Obstetrics and Gynecology | 11 | 10.6 |
| Orthopedics | 11 | 10.6 |
| Surgery, nonspecific‡ | 11 | 10.6 |
| Cardiac | 8 | 7.7 |
| Breast | 5 | 4.8 |
| General surgery | 5 | 4.8 |
| Pediatric | 5 | 4.8 |
| Bariatric | 4 | 3.9 |
| Colorectal | 4 | 3.9 |
| 2 or more fields | 7 | 6.7 |
| Other | 21 | 20.2 |
Settings are mutually exclusive; mixed settings indicate that the study took place in a combination of these settings.
Hospital(s), nonspecific indicates that the study did not state hospital type.
Surgery, nonspecific indicates that the study did not state field of surgery.
Facilitators and Barriers to Surgeon Practice Change
Capability, Opportunity, and Motivation were represented across TDF domains and, depending on circumstances, acted as facilitators and barriers. The most frequently cited behavioral determinants (TDF domains), in order, were: Opportunity (environmental context and resources and social influences), Capability (knowledge and skills), and Motivation (beliefs about consequences and reinforcement). Overlap of COM-Bcomponents and TDF domains are shown in Figure 2 and described below in order of prevalence. How specific TDF domains affected practice change is best described by a spectrum; that is, depending on the circumstances, they could act as facilitators, barriers, or as neutral factors (ie, no effect on outcome; Fig. 3).
FIGURE 2.
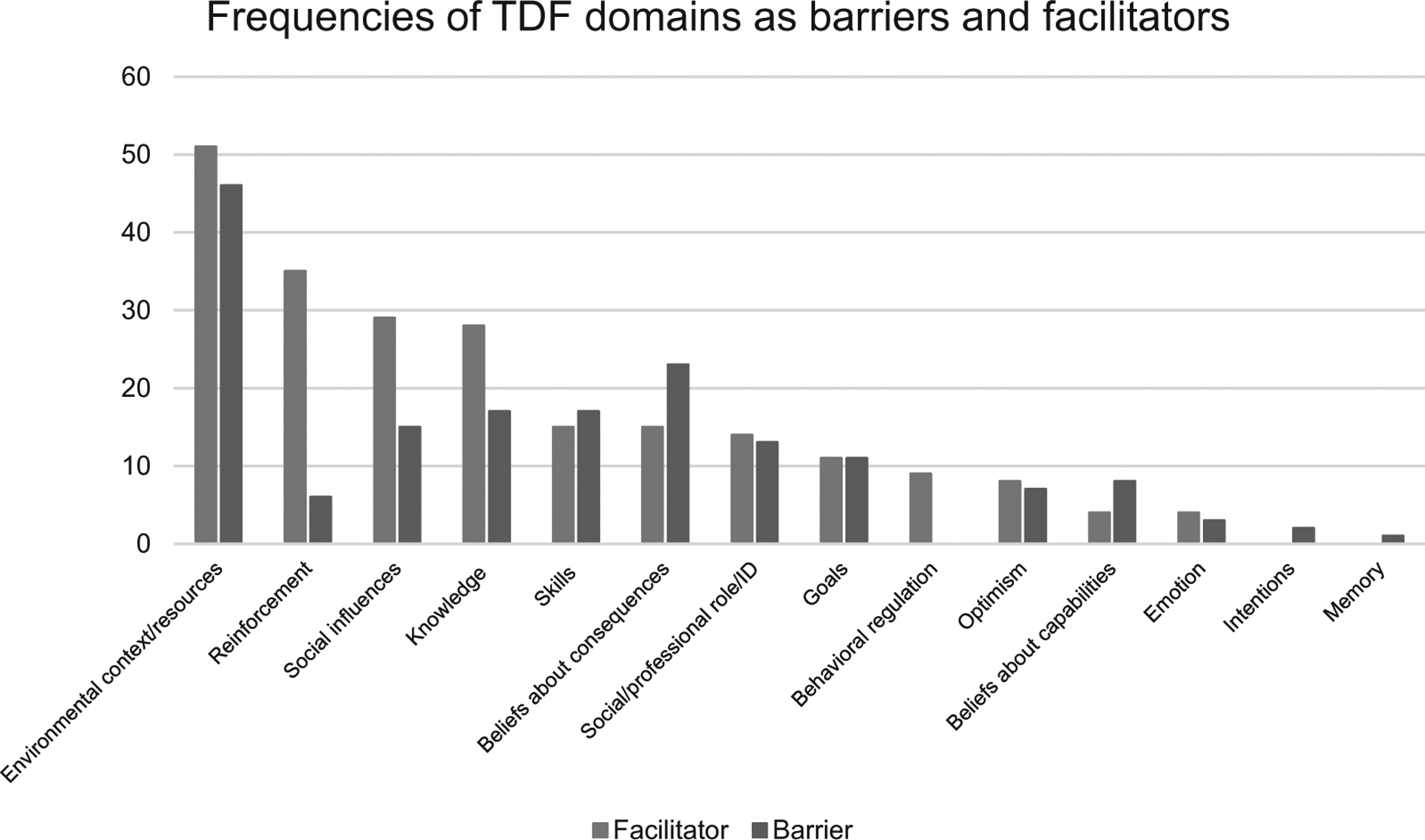
Frequencies of TDF domains as barriers and facilitators.
FIGURE 3.
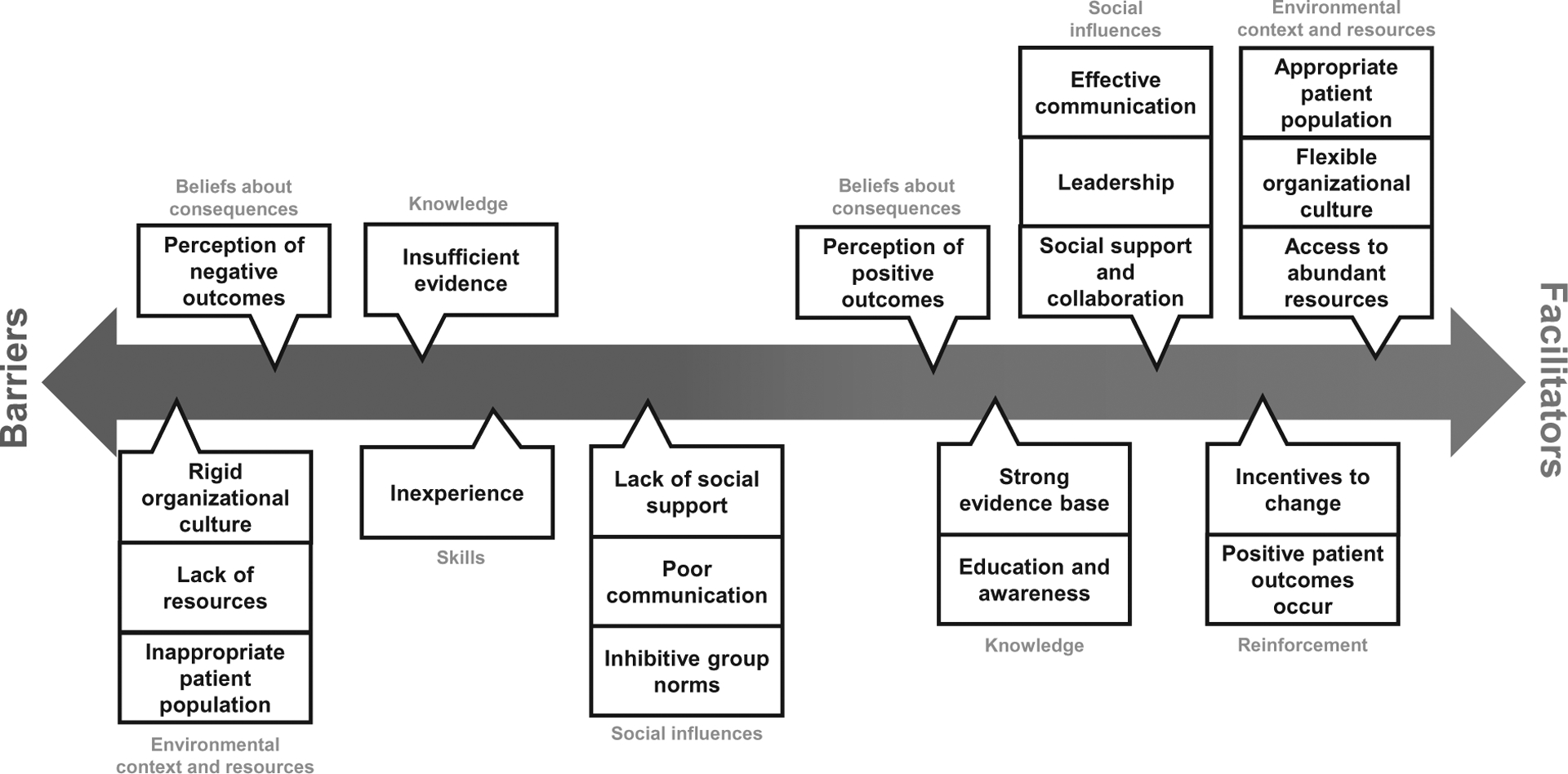
Recurring factors affecting surgical practice change derived from the literature are shown on a spectrum. The spectrum illustrates the bidirectionality of TDF domains (denoted in blue) and examples within these domains (denoted in black). Position on the spectrum derives from the frequency that domains/examples were documented in this literature.
Opportunity
Opportunity is an extrinsic determinant of behavior change that linked to the most frequently cited TDF domains affecting surgeon change: environmental context and resources and social influences. Both domains acted as both as facilitators or barriers to change depending on circumstances.
Environmental context and resources represented the greatest facilitator of and barrier to practice change (n = 51; 49% and n = 46; 44.2%; respectively). Within environment and resources, 5 categories affecting change recurred: (1) physical (eg, facility type/capacity/geographic location); (2) infrastructure (eg, available resources); (3) cultural (eg, culture and climate), (4) extrinsic factors (eg, local competition), and (5) patient population (eg, insurance, demographics).
Facilitators:
Practice change was fostered in well-resourced regional referral centers27–59 that embraced innovation and were amenable to changes in surgical guidelines.36,50,51,60–65 In 1 study, robot-assisted radical prostatectomy was more likely to be adopted by high-volume surgeons and was more common at teaching, larger-sized (200 or more beds), or urban hospitals.34 Change was also accelerated in settings with high patient demand and when competing facilities offered the innovation.36,43,54,56,65–70 For example, Horwitz et al found that hospitals were 79% more likely to adopt percutaneous coronary intervention if neighboring hospitals offered the service.67 In addition, specific patient factors and conditions facilitated practice change.35,38,42,47,49,56,58,68,71–76 For instance, areas with patient populations of higher socioeconomic status were associated with earlier uptake of bariatric surgery in the United Kingdom.
Barriers:
Surgical practice change was less likely to occur in poorly resourced centers30–33,36–39,42,44,46,47,50,57,58,60,61,67,68,70,72,75–98 with rigid, nonconforming organizational structures or practice patterns.29,32,44,50,67,68,76,79,81,84–86 For example, Schootman et al found a more rapid uptake of robot-assisted colectomy in teaching versus nonteaching hospitals between 2010 and 2012 (35.7% vs 9.1%).31 Certain patient factors also acted as barriers to practice change, including sex, age, race, and socioeconomic status, and affected their candidacy for a new practice.32,38,39,42,47,58,65,72,80,82,88,91 One study found that diffusion of bariatric surgery was slower in older (50–59 year old) obese populations due to the higher risk of complications and in geographic areas with fewer morbidly obese patients.33
Social influences were another common facilitator (n = 29; 27.9%) and barrier (n = 15; 14.5%) to practice change. This domain captures professional interactions between colleagues, mentors, and thought leaders, educational interactions with instructors, students, and residents, and clinical interactions with patients.
Facilitators:
Positive interpersonal interactions, such as support and encouragement among colleagues,50,63,70,96,99 strong senior leadership and mentorship,27,29,36,43,44,46,53,55,60,61,70,99–104 and effective communication36,46,51,54,62,87,94,99,104 led to faster and more widespread uptake of a new practice. Encouraged collaboration (eg, opportunities for interdisciplinary partnership)29,43,44,50,61,62,64,94, 104–106 and constructive pressure to promote growth (eg, competition among colleagues or potential disruption of referral base)98 were also observed to influence the likelihood of change. In a qualitative study examining attitudes toward adopting accelerated partial breast radiotherapy, encouragement from peers and positive social pressure from patients influenced breast surgeons’ and radiation oncologists’ adoption of thetechnology.70 Similarly, feeling a strong sense of community in local obstetrics and gynecology groups, effective exposure and dissemination, and strong thought-leader support facilitated surgeons’ adoption of salpingectomies in Canada.99
Barriers:
Prominent social influence barriers to adoption of a new practice were negative interpersonal interactions, such as adherence to group norms,29,44,50,85,105 social pressure to conform,61,77,101 lack of social support or collaboration,50,60,70,101,105 power dynamics between senior and junior surgeons,70,79,90 and poor communication.44,49,60,85,90,97 For example, Brindle, et al found that the successful implementation of a new surgical debriefing program was hindered by loss of leadership support, poor communication, and lack of meaningful and early feedback.60 In addition, the implementation of the innovative Da Vinci minimally invasive surgical system was less likely in diverse professional social networks (eg, age, specialty) and under conditions of less collaboration with other providers.105
Capability
A surgeon’s or organization’s capability to engage in behavior change was also an essential feature across dominant domains. Knowledge was a frequently observed facilitator (n = 28; 26.9%) or barrier (n = 17; 16.3%). Surgeon awareness of the procedure or guidelines was directly related to their capability to carry out the change.
Facilitators:
Surgeons, practice groups, and organizations were more likely to change if they were aware of a procedure, had sufficient knowledge to implement the practice (eg, published literature or evidence-based guidelines),44,57,61,70,82,85,87,88,93,107–111 had knowledge of data showing positive outcomes,46,81,93 and had opportunities for procedural education and awareness.29,43,44,51, 81,90,93,94,99,103,104,106,112 For example, Gramlich et al observed a 25% increase in adherence to Enhanced Recovery after Surgery (ERAS) protocol guidelines after implementing a system-wide campaign of educational interventions.29 Giusti et al, found that guidelines on surgical antibiotic prophylaxis in children were well-received and implemented if they had a strong evidence base, were developed by respected clinical leaders, and were disseminated appropriately.87
Barriers:
Change was less likely to occur if individual or group of surgeons were unaware of the procedure or did not have enough supporting information to make the change, such as insufficient or unreliable evidence or poor-quality guidelines.61,64,75,81,87,90,94,113,114 For example, gaps in knowledge about the timing of umbilical cord clamping, including unclear criteria for patient selection (35%), unfamiliarity (31.6%), and poor understanding of the benefits (29.9%), impeded provider compliance with the evidence-based protocol.82 Furthermore, insufficient knowledge about potential patient burden (eg, risks, complications, negative long-term outcomes) deterred surgeons from changing.36,41,77,81,92,94,96,101,106,115 For instance, barriers to implementing guidelines for adjuvant radiotherapy after prostatectomy included urologists’ perceived lack of evidence and underrepresentation of patient burden, such as side effects and unnecessary treatment.106
Skills also appeared as a facilitator (n = 15; 14.4%) but more prominently as a barrier (n = 17; 15.4%) to practice change. Lacking the skills (eg, inexperience, lack of training) to adopt the new procedure negatively affected the surgeon’s likelihood of making the change.36,37,59,61,74,75,79,84,89,90,92,95,96,100,101,109,113 For instance, some cardiac surgeons were deterred from adopting transcatheter aortic valve implantation (TAVI) because of the complex and intensive training, and multidisciplinary expertise required.102 Although frequently cited, possessing the appropriate skillset (eg, proficiency, training) was represented as a promotor of change in a smaller proportion of studies.
Motivation
Individual’s motivation to pursue or reject behavior change categorized the remaining dominant domains. Surgeons’ beliefs about consequences acted as a facilitator of (n = 15; 14.4%) and a barrier to change (n = 23; 22.1%). These beliefs, in many cases, are experiential and affected the surgeons’ motivation to change.
Facilitators:
Surgeons were more likely to adopt new practices if they believed that patient outcomes would be improved,47,57,70,77,96,101,111,113 personal and organizational benefits outweigh the risks,78,93,116–118 the change would improve safety and efficiency,63,74,77 and there would be consequences if they did not change practice.72 For example, orthopedic surgeons would be more likely to incorporate robotic technology into knee arthroscopy if they believed it had the potential to improve efficiency and decrease iatrogenic cartilage damage in patients.117 In addition, the perception of usefulness was an integral facilitator in the orthopedic surgeons’ decision to continue using computer-assisted surgical approaches.63
Barriers:
Surgeons resisted change if they believed patient outcomes would be negative,74,86,91,113 the financial, logistical, or staff burden outweighed the benefits,36,49,60,70,74,77,84,90,95,96,106,114–116,118 or the practice was not useful.47,64,82,114,119 For example, orthopedic surgeons would not change their total knee replacement technique if they believed that risks outweigh the benefits or the learning curve would negatively affect patient outcomes.84 Another study found that surgeons would not implement the use of hysterectomy clinical pathways if they believed it was a waste of time or inappropriate for their patient population.82
Reinforcement (ie, surgeon’s likelihood to repeat or carry out a change increased if they experienced a positive result) appeared as one of the most frequent facilitators (n = 35; 33.7%) and was rarely a cited barrier in the studies reviewed. Studies with a targeted intervention were also largely affected by positive reinforcement. Practice change was more likely to be repeated and fully implemented if the procedure was successful and there was positive feedback (eg, no unexpected events, positive patient outcomes, or reimbursement).28–30,36,44,46,51,56,60,64,67,69,70,77,80–82,85,87,90,91,97,100,101,103,104,108,111–114,117,120–122 For instance, a telementoring program for sleeve gastrectomy was implemented without intraoperative complications and was well-received by the participating surgeons. After successful completion, surgical mentees were more likely to implement new techniques learned into their practice and be more confident in their technical skill.102
DISCUSSION
Identifying the facilitators and barriers that affect a surgeon’s decision to change clinical behavior helps to inform implementation of best practices. In the present scoping review, we used the TDF implementation science framework to characterize what is known about changing surgical practice. Many aspects of the framework are reflected in our analysis of the literature on how surgeons change practice. However, our results emphasize the importance of environmental context and resources in both facilitating and inhibiting surgical practice change. In other words, successful implementation of new surgical practices is driven by a conducive environmental context with adequate resources. Furthermore, evidence suggests that co-occurring facilitators of change (eg, knowledge, social influences, and beliefs about consequences) are most effective within a conducive environment.
Theoretical Domains Framework and Behavior Change
The environmental context and resources domain falls within the COM-B model component of opportunity.21,23 Environment and resources are foundational to change and often intransigent. In contrast, TDF domains related to individual capability and motivation (knowledge, skills, beliefs about consequences, and reinforcement) are popular targets of behavior change interventions. Specifically, our review found that most barriers to change were deficiencies in opportunity (environmental context and resources, social influences) and capability (knowledge, skills). Similarly, opportunity was the most cited facilitator of change (environmental context and resources, social influences) followed by motivation (beliefs about consequences, reinforcement). Together, the findings suggest environment, circumstances, and resources largely determine the adoption of new surgical practices. In the context of supportive infrastructure, it is individual knowledge, technical skill, and personal beliefs that encourage the initiation and sustainment of practice change.
In contrast, personal responses or reflection (eg, emotion, optimism or pessimism, and beliefs about capabilities) were infrequently observed as facilitators or barriers to change across all studies (range: 2.9%–7.7%). Such reflective responses are integral to a deeper understanding of how surgeons individually process practice change; however, they are difficult to measure. It is likely that these domains play a major role in surgeon behavior change, but they are not measured in hospital-level, population-level, or interventional studies. Qualitative research focused on the implementation of new techniques, devices, or behaviors is needed to better understand how surgeons process and reflect on clinical experiences to initiate and sustain practice change.
Practice Change Interventions: What’s Been Tried and What Works?
Although environmental context and resources were paramount to practice change, these factors were rarely targeted by interventions to promote the adoption of new practices. It is possible that these implementations are not published. However, it is more likely that developing interventions to alter infrastructure is difficult and expensive. Instead, most effective change interventions focused on individual factors related to surgeons’ knowledge, feedback loops with positive reinforcement, and perceived advantage of the new practice.
Despite great enthusiasm for evidence-based guidelines, studies show that their existence alone has limited effect on surgical practice.123–125 However, the adoption of evidence-based practice is greatly increased when implemented in conjunction with educational, interactive, systematic, and regulatory components. For example, multifaceted implementation of an evidence-based protocol for prevention of orthopedic fractures achieved 90% compliance among US surgeons. This intervention included guideline dissemination that was integrated with surgeon- and patient-level education, mentorship, collaboration, and reinforcing feedback.104 Furthermore, implementation and dissemination of guidelines and provision of best practice has been directly related to the strength of the evidence based underpinning the guideline recommendations.9–11 Our study highlights that guideline implementation requires targeted provider-level and patient-level education reinforced by reliable evidence and adapted for the unique practice environment.
Contextual Factors in Adoption of Innovations
Similar to other types of practice change, surgeons’ likelihood of adopting new technologies or innovations is influenced by clinical environment (eg, financial capacity and need to change). The significance of such contextual factors in adopting innovation is recognized in existing frameworks.126–131 These frameworks, Rogers’diffusion of innovation theory being the most prominent, characterize context by modeling the complex interactions between leadership, infrastructure, culture, the meaningful use of evidence, and individual characteristics in implementing innovation and fostering sustainable change.132 The dominant TDF domains discussed herein (eg, environmental context and resources, knowledge, social influences) mirror these concepts, emphasizing the role of influential thought leaders within well-resourced and well-informed institutions as contextual facilitators of change.
Limitations
This was a scoping review which differs from a systematic review or meta-analysis in its purpose. Specifically, a scoping review provides an overview of the available research evidence without producing a summary answer to discrete a priori research questions. The multiple exclusion criteria and the heterogeneity of reporting limit the ability to produce definitive conclusions. Publication bias is inherent to any systematic review of the literature (eg, nonsignificant findings are often not published) and null findings may be under-reported. However, most studies evaluated multiple implementation outcomes simultaneously and therefore, allowed for the identification of null change factors. Furthermore, our study was not designed for quantitative summative assessment of change interventions and consequently, any quantitative findings reported could be subject to selection and/or misclassification bias. It does, however, provide a broad overview of the concepts and general roadmap for what affects behavior/practice change among surgeons. At the same time, its breadth underscores the heterogeneity that prevents direct recommendations about approaches to surgeon behavior change in specific situations and in particular surgical subspecialties.
CONCLUSIONS
To ensure best practice in a rapidly changing healthcare environment, it is critical to promote understanding of facilitators and barriers influencing surgeons’ likelihood of changing behavior. Our review shows that practice change is predicated on a conducive environment with adequate resources, but once that is established, the surgeons’ individual characteristics, including skills, motivation, and reinforcement determine the likelihood of successful change. Enriching our understanding the factors that impact surgical practice change has major implications. Deficiencies in the literature underscore the need for further study of resource interventions and the role of surgical team dynamics in the adoption of innovation. A better understanding of these areas is needed to optimize our ability to disseminate and implement best practices in surgery.
Supplementary Material
Acknowledgments
This paper is supported by National Institutes of Health grants 5K23DC013559-07 and 1R21DC016724-01.
Footnotes
The authors declare no conflict of interests.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Farrokhyar F, Karanicolas PJ, Thoma A, et al. Randomized controlled trials of surgical interventions. Ann Surg. 2010;251:409–416. [DOI] [PubMed] [Google Scholar]
- 2.Kuehn BM. Surgeons continue to debate place of randomized trials of surgical procedures. JAMA. 2009;302:1513–1514. 1519. [DOI] [PubMed] [Google Scholar]
- 3.Boudourakis LD, Wang TS, Roman SA, et al. Evolution of the surgeon-volume, patient-outcome relationship. Ann Surg. 2009;250:159–165. [DOI] [PubMed] [Google Scholar]
- 4.Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39(8 Suppl 2):Ii46–Ii54. [DOI] [PubMed] [Google Scholar]
- 6.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362:1225–1230. [DOI] [PubMed] [Google Scholar]
- 7.Grol R, Wensing M. What drives change? Barriers to and incentives for achieving evidence-based practice. Med J Aust. 2004;180(S6):S57–60. [DOI] [PubMed] [Google Scholar]
- 8.Kessel L, Erngaard D, Flesner P, et al. Do evidence-based guidelines change clinical practice patterns? Acta Ophthalmol. 2017;95:337–343. [DOI] [PubMed] [Google Scholar]
- 9.Grove A, Clarke A, Currie G. How are evidence and knowledge used in orthopaedic decision-making? Three comparative case studies of different approaches to implementation of clinical guidance in practice. Implement Sci. 2018;13:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.In H, Greenberg CC. Lack of uniformity in levels of evidence and recommendation grades in surgical oncology guidelines. World J Surg. 2012; 36:2273–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg CC, Lipsitz SR, Neville B, et al. Receipt of appropriate surgical care for Medicare beneficiaries with cancer. Arch Surg. 2011;146:1128–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.In H, Neville BA, Lipsitz SR, et al. The role of National Cancer Institute-designated cancer center status: observed variation in surgical care depends on the level of evidence. Ann Surg. 2012;255:890–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broekman ML, Carriere ME, Bredenoord AL. Surgical innovation: the ethical agenda: a systematic review. Medicine (Baltimore). 2016;95: e3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meakins JL. Innovation in surgery: the rules of evidence. Am J Surg. 2002;183:399–405. [DOI] [PubMed] [Google Scholar]
- 15.Barkun JS, Aronson JK, Feldman LS, et al. Evaluation and stages of surgical innovations. Lancet. 2009;374:1089–1096. [DOI] [PubMed] [Google Scholar]
- 16.Ergina PL, Cook JA, Blazeby JM, et al. Challenges in evaluating surgical innovation. Lancet. 2009;374:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzales R, Handley MA, Ackerman S, et al. A framework for training health professionals in implementation and dissemination science. Acad Med. 2012;87:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher ES, Shortell SM, Savitz LA. Implementation science: a potential catalyst for delivery system reform. JAMA. 2016;315:339–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Telem DA, Dimick J, Skolarus TA. Dissecting surgeon behavior: leveraging the theoretical domains framework to facilitate evidence-based surgical practice. Ann Surg. 2018;267:432–434. [DOI] [PubMed] [Google Scholar]
- 20.Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci. 2012;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atkins L, Francis J, Islam R, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci. 2017;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michie S, Johnston M, Abraham C, et al. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care. 2005;14:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson M, Khouja CL, Sutcliffe K, et al. Using the theoretical domains framework and the behavioural change wheel in an overarching synthesis of systematic reviews. BMJ Open. 2019;9:e024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michie S, Atkinns L, West R. The Behavior Change Wheel: A Guide to Designing Interventions. Great Britain: Silverback Publishing; 2014. [Google Scholar]
- 26.Nilsen P Making sense of implementation theories, models and frameworks. Implement Sci. 2015;10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung H, Wang Y, Chang SL, et al. Adoption of robot-assisted partial nephrectomies: a population-based analysis of U.S. surgeons from 2004 to 2013. J Endourol. 2017;31:886–892. [DOI] [PubMed] [Google Scholar]
- 28.Ament SMC, Gillissen F, Moser A, et al. Factors associated with sustainability of 2 quality improvement programs after achieving early implementation success. A qualitative case study. J Eval Clin Pract. 2017;23:1135–1143. [DOI] [PubMed] [Google Scholar]
- 29.Gramlich LM, Sheppard CE, Wasylak T, et al. Implementation of enhanced recovery after surgery: a strategy to transform surgical care across a health system. Implement Sci. 2017;12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leow JJ, Weissman JS, Kimsey L, et al. Radical prostatectomy innovation and outcomes at military and civilian institutions. Am J Manag Care. 2017; 23:342–347. [PubMed] [Google Scholar]
- 31.Schootman M, Hendren S, Ratnapradipa K, et al. Adoption of robotic technology for treating colorectal cancer. Dis Colon Rectum. 2016;59: 1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson EE, Simpson AN, Harvey JB, et al. Bariatric surgery implementation trends in the USA from 2002 to 2012. Implement Sci. 2016;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu TH, Huang YT, Lee JC, et al. Characteristics of early and late adopting hospitals providing percutaneous coronary intervention in Taiwan. J Am Heart Assoc. 2015;4:e002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang SL, Kibel AS, Brooks JD, et al. The impact of robotic surgery on the surgical management of prostate cancer in the USA. BJU Int. 2015;115:929–936. [DOI] [PubMed] [Google Scholar]
- 35.Tan HJ, Meyer AM, Kuo TM, et al. Provider-based research networks and diffusion of surgical technologies among patients with early-stage kidney cancer. Cancer. 2015;121:836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Compagni A, Mele V, Ravasi D. How Early Implementations Influence Later Adoptions of Innovation: Social Positioning and Skill Reproduction in the Diffusion of Robotic Surgery. Acad Management J. 2014; 58:24–278. [Google Scholar]
- 37.Hibi T, Cherqui D, Geller DA, et al. International survey on technical aspects of laparoscopic liver resection: a web-based study on the global diffusion of laparoscopic liver surgery prior to the 2nd international consensus conference on laparoscopic liver resection in Iwate, Japan. J Hepatobiliary Pancreat Sci. 2014;21:737–744. [DOI] [PubMed] [Google Scholar]
- 38.Parsons JK, Messer K, Palazzi K, et al. Diffusion of surgical innovations, patient safety, and minimally invasive radical prostatectomy. JAMA Surg. 2014;149:845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer AM, Reeder-Hayes KE, Liu H, et al. Differential receipt of sentinel lymph node biopsy within practice-based research networks. Med Care. 2013;51:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goutte N, Bendersky N, Barbier L, et al. Laparoscopic left lateral sectionectomy: a population based study. Hpb. 2017;19:118–125. [DOI] [PubMed] [Google Scholar]
- 41.Haider AH, Piper LC, Zogg CK, et al. Military-to-civilian translation of battlefield innovations in operative trauma care. Surgery. 2015;158:1686–1695. [DOI] [PubMed] [Google Scholar]
- 42.Farges O, Goutte N, Dokmak S, et al. How surgical technology translates into practice: the model of laparoscopic liver resections performed in France. Ann Surg. 2014;260:916–921. discussion 921–912. [DOI] [PubMed] [Google Scholar]
- 43.Slusher J, Bates CA, Johnson C, et al. Standardization and improvement of care for pediatric patients with perforated appendicitis. J Pediatr Surg. 2014;49:1020–1024. discussion 1024–1025. [DOI] [PubMed] [Google Scholar]
- 44.Herbert G, Sutton E, Burden S, et al. Healthcare professionals’ views of the enhanced recovery after surgery programme: a qualitative investigation. BMC Health Serv Res. 2017;17:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wasterlain AS, Bello RJ, Vigdorchik J, et al. Surgeons’ perspectives on premium implants in total joint arthroplasty. Orthopedics. 2017;40:e825–e830. [DOI] [PubMed] [Google Scholar]
- 46.Callea G, Cavallo MC, Tarricone R, et al. Learning effect and diffusion of innovative medical devices: the case of transcatheter aortic valve implantation in Italy. J Comp Eff Res. 2017;6:279–292. [DOI] [PubMed] [Google Scholar]
- 47.Boveda S, Lenarczyk R, Haugaa K, et al. Implantation of subcutaneous implantable cardioverter defibrillators in Europe: results of the European Heart Rhythm Association survey. Europace. 2016;18:1434–1439. [DOI] [PubMed] [Google Scholar]
- 48.Schulman AM, Mirrielees JA, Leverson G, et al. Reexcision surgery for breast cancer: an analysis of the American Society of Breast Surgeons (ASBrS) Mastery (SM) database following the SSO-ASTRO “No Ink on Tumor” guidelines. Ann Surg Oncol. 2017;24:52–58. [DOI] [PubMed] [Google Scholar]
- 49.Arakawa T, Kumasaka L, Nakanishi M, et al. Regional clinical alliance path and cardiac rehabilitation after hospital discharge for acute myocardial infarction patients in Japan- a nationwide survey. Circ J. 2016;80:1750–1755. [DOI] [PubMed] [Google Scholar]
- 50.Urquhart R, Jackson L, Sargeant J, et al. Health system-level factors influence the implementation of complex innovations in cancer care. Healthc Policy. 2015;11:102–118. [PMC free article] [PubMed] [Google Scholar]
- 51.Ament SM, Gillissen F, Moser A, et al. Identification of promising strategies to sustain improvements in hospital practice: a qualitative case study. BMC Health Serv Res. 2014;14:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sivarajan G, Taksler GB, Walter D, et al. The effect of the diffusion of the surgical robot on the hospital-level utilization of partial nephrectomy. Med Care. 2015;53:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shinn L The impact of star physicians on diffusion of a medical technology: the case of laparoscopic gastric bypass surgery. J Health Care Finance. 2014;40:67–85. [PubMed] [Google Scholar]
- 54.Abrishami P, Boer A, Horstman K. Understanding the adoption dynamics of medical innovations: affordances of the da Vinci robot in the Netherlands. Soc Sci Med. 2014;117:125–133. [DOI] [PubMed] [Google Scholar]
- 55.Gershengorn HB, Wunsch H. Understanding changes in established practice: pulmonary artery catheter use in critically ill patients. Crit Care Med. 2013;41:2667–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barbash GI, Friedman B, Glied SA, et al. Factors associated with adoption of robotic surgical technology in US hospitals and relationship to radical prostatectomy procedure volume. Ann Surg. 2014;259:1–6. [DOI] [PubMed] [Google Scholar]
- 57.Gallego G, van Gool K, Casey R, et al. Surgeons’ views of health technology assessment in Australia: online pilot survey. Int J Technol Assess Health Care. 2013;29:309–314. [DOI] [PubMed] [Google Scholar]
- 58.Woldrich JM, Palazzi K, Stroup SP, et al. Trends in the surgical management of localized renal masses: thermal ablation, partial and radical nephrectomy in the USA, 1998–2008. BJU Int. 2013;111:1261–1268. [DOI] [PubMed] [Google Scholar]
- 59.Poon SA, Silberstein JL, Chen LY, et al. Trends in partial and radical nephrectomy: an analysis of case logs from certifying urologists. J Urol. 2013;190:464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brindle ME, Henrich N, Foster A, et al. Implementation of surgical debriefing programs in large health systems: an exploratory qualitative analysis. BMC Health Serv Res. 2018;18:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan KJ, Wayne C, Patey AM, et al. Barriers and facilitators to the implementation of evidence-based practice by pediatric surgeons. J Pediatr Surg. 2017;52:1666–1673. [DOI] [PubMed] [Google Scholar]
- 62.Sartelli M, Labricciosa FM, Barbadoro P, et al. The Global Alliance for Infections in Surgery: defining a model for antimicrobial stewardship-results from an international cross-sectional survey. World J Emerg Surg. 2017;12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu HM, Chang IC, Lai TW. Physicians’ perspectives of adopting computer-assisted navigation in orthopedic surgery. Int J Med Inform. 2016;94:207–214. [DOI] [PubMed] [Google Scholar]
- 64.de Groot JJ, van Es LE, Maessen JM, et al. Diffusion of enhanced recovery principles in gynecologic oncology surgery: is active implementation still necessary? Gynecol Oncol. 2014;134:570–575. [DOI] [PubMed] [Google Scholar]
- 65.Schroeck FR, Kaufman SR, Jacobs BL, et al. The impact of technology diffusion on treatment for prostate cancer. Medical Care. 2013;51:1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sethi RKV, Henry AJ, Hevelone ND, et al. Impact of hospital market competition on endovascular aneurysm repair adoption and outcomes. J Vasc. 2013;58:596–606. [DOI] [PubMed] [Google Scholar]
- 67.Horwitz JR, Nichols A, Nallamothu BK, et al. Expansion of invasive cardiac services in the United States. Circulation. 2013;128:803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wright JD, Tergas AI, Hou JY, et al. Effect of regional hospital competition and hospital financial status on the use of robotic-assisted surgery. JAMA Surg. 2016;151:612–620. [DOI] [PubMed] [Google Scholar]
- 69.Sacks W, Wong RM, Bresee C, et al. Use of evidence-based guidelines reduces radioactive iodine treatment in patients with low-risk differentiated thyroid cancer. Thyroid. 2015;25:377–385. [DOI] [PubMed] [Google Scholar]
- 70.Gold HT, Pitrelli K, Hayes MK, et al. Decision to adopt medical technology: case study of breast cancer radiotherapy techniques. Med Decis Making. 2014;34:1006–1015. [DOI] [PubMed] [Google Scholar]
- 71.Alam M, Bhanderi S, Metthews J, et al. Regional diffusion of bariatric surgery in the UK - a 10 year longitudinal comparative study. Paper presented at: 20th World Congress of the International Federation for the Surgery of Obesity & Metabolic Disorders (IFSO); 2015; Vienna, Austria. [Google Scholar]
- 72.Sears ED, Lu YT, Wood SM, et al. Diagnostic testing requested before surgical evaluation for carpal tunnel syndrome. J Hand Surg Am. 2017;42:623–629. e621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Torbica A, Banks H, Valzania C, et al. Investigating regional variation of cardiac implantable electrical device implant rates in European healthcare systems: what drives differences? Health Econ. 2017;26(Suppl 1):30–45. [DOI] [PubMed] [Google Scholar]
- 74.Trinh AT, Roberts CL, Ampt AJ. Knowledge, attitude and experience of episiotomy use among obstetricians and midwives in Viet Nam. BMC Pregnancy Childbirth. 2015;15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roberts JI, Hrazdil C, Wiebe S, et al. Neurologists’ knowledge of and attitudes toward epilepsy surgery: a national survey. Neurology. 2015; 84:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y, Hollenbeck BK, Schroeck FR, et al. Managed care and the dissemination of robotic prostatectomy. Surgical Innovation. 2014;21: 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dharampal N, Cameron C, Dixon E, et al. Attitudes and beliefs about the surgical safety checklist: Just another tick box? Can J Surg. 2016;59:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cundy TP, Marcus HJ, Hughes-Hallett A, et al. International attitudes of early adopters to current and future robotic technologies in pediatric surgery. J Pediatr Surg. 2014;49:1522–1526. [DOI] [PubMed] [Google Scholar]
- 79.Choy I, Kitto S, Adu-Aryee N, et al. Barriers to the uptake of laparoscopic surgery in a lower-middle-income country. Surg Endosc. 2013;27:4009–4015. [DOI] [PubMed] [Google Scholar]
- 80.Boveda S, Lenarczyk R, Haugaa KH, et al. Use of leadless pacemakers in Europe: results of the European Heart Rhythm Association survey. Europace. 2018;20:555–559. [DOI] [PubMed] [Google Scholar]
- 81.Gams RL, Popp KK, Cramer J, et al. How to engage your team to implement delayed cord clamping. Nurs Womens Health. 2017;21:489–498. [DOI] [PubMed] [Google Scholar]
- 82.Sanei-Moghaddam A, Goughnour S, Edwards R, et al. Hysterectomy pathway as the global engine of practice change: implications for value in care. Cent Asian J Glob Health. 2017;6:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bekelis K, Skinner J, Gottlieb D, et al. De-adoption and exnovation in the use of carotid revascularization: retrospective cohort study. BMJ. 2017; 359:j4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vertullo CJ, Grimbeek PM, Graves SE, et al. Surgeon’s preference in total knee replacement: a quantitative examination of attributes, reasons for alteration, and barriers to change. J Arthroplasty. 2017;32:2980–2989. [DOI] [PubMed] [Google Scholar]
- 85.Rongen JJ, van Tienen TG, Buma P, et al. Meniscus surgery is still widely performed in the treatment of degenerative meniscus tears in The Netherlands. Knee Surg Sports Traumatol Arthrosc. 2018;26:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nezhat F, Apostol R, Greene AD, et al. To morcellate or not to morcellate: a cross-sectional survey of gynecologic surgeons. JSLS. 2017;21:e2016.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giusti A, Spila Alegiani S, Ciofi Degli Atti ML, et al. Surgical antibiotic prophylaxis in children: a mixed method study on healthcare professionals attitudes. BMC Pediatr. 2016;16:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bailin S, Noiseux N, Pottinger JM, et al. Screening patients undergoing total hip or knee arthroplasty with perioperative urinalysis and the effect of a practice change on antimicrobial use. Infect Control Hosp Epidemiol. 2017;38:281–286. [DOI] [PubMed] [Google Scholar]
- 89.Abdelsattar ZM, Allen MS, Shen KR, et al. Variation in hospital adoption rates of video-assisted thoracoscopic lobectomy for lung cancer and the effect on outcomes. Ann Thorac Surg. 2017;103:454–460. [DOI] [PubMed] [Google Scholar]
- 90.Cook S, de Kok B, Odland ML. ‘It’s a very complicated issue here’: understanding the limited and declining use of manual vacuum aspiration for postabortion care in Malawi: a qualitative study. Health Policy Plan. 2017;32:305–313. [DOI] [PubMed] [Google Scholar]
- 91.Hart RA, DePasse JM, Daniels AH. Failure to launch: what the rejection of lumbar total disk replacement tells us about American spine surgery. Clin Spine Surg. 2017;30:E759–e764. [DOI] [PubMed] [Google Scholar]
- 92.Sinha A, Chandhiok N, Sahay S, et al. Male circumcision for HIV prevention in India: emerging viewpoints and practices of health care providers. AIDS Care. 2015;27:1196–1198. [DOI] [PubMed] [Google Scholar]
- 93.Bousleiman SZ, Rice MM, Moss J, et al. Use and attitudes of obstetricians toward 3 high-risk interventions in MFMU Network hospitals. Am J Obstet Gynecol. 2015;213:398.e1–398.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arts-de Jong M, Harmsen MG, Hoogerbrugge N, et al. Risk-reducing salpingectomy with delayed oophorectomy in BRCA1/2 mutation carriers: patients’ and professionals’ perspectives. Gynecol Oncol. 2015;136:305–310. [DOI] [PubMed] [Google Scholar]
- 95.Trevisonno M, Kaneva P, Watanabe Y, et al. A survey of general surgeons regarding laparoscopic inguinal hernia repair: practice patterns, barriers, and educational needs. Hernia. 2015;19:719–724. [DOI] [PubMed] [Google Scholar]
- 96.Bergholm A, Ostberg AL, Gabre P. Implementation of laser technology and treatment at county level in the Swedish Public Dental Service. Swed Dent J. 2014;38:111–120. [PubMed] [Google Scholar]
- 97.Spellman E, Sulayman N, Eggly S, et al. Conveying genomic recurrence risk estimates to patients with early-stage breast cancer: oncologist perspectives. Psychooncology. 2013;22:2110–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bekelis K, Missios S, Labropoulos N. Regional intensity of neurosurgical care and integration of aneurysm coiling in the United States. Int Angiol. 2014;33:446–454. [PubMed] [Google Scholar]
- 99.Lander B, Wilcox E, McAlpine JN, et al. Changing clinical practice: evaluation of implementing recommendations for opportunistic salpingectomy in British Columbia and Ontario. Int J Gynecol Cancer. 2018;28:1101–1107. [DOI] [PubMed] [Google Scholar]
- 100.Leggott KT, Martin M, Sklar D, et al. Transformation of anesthesia for ambulatory orthopedic surgery: a mixed-methods study of a diffusion of innovation in healthcare. Healthc (Amst). 2016;4:181–187. [DOI] [PubMed] [Google Scholar]
- 101.Merkel S, Eikermann M, Neugebauer EA, et al. The transcatheter aortic valve implementation (TAVI)-a qualitative approach to the implementation and diffusion of a minimally invasive surgical procedure. Implement Sci. 2015;10:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pollack CE, Soulos PR, Gross CP. Physician’s peer exposure and the adoption of a new cancer treatment modality. Cancer. 2015;121:2799–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nguyen NT, Okrainec A, Anvari M, et al. Sleeve gastrectomy telementoring: a SAGES multi-institutional quality improvement initiative. Surg Endosc. 2018;32:682–687. [DOI] [PubMed] [Google Scholar]
- 104.Bunta AD, Edwards BJ, Macaulay WB Jr, et al. Own the bone, a system-based intervention, improves osteoporosis care after fragility fractures. J Bone Joint Surg Am. 2016;98:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iacopino V, Mascia D, Cicchetti A. Professional networks and the alignment of individual perceptions about medical innovation. Health Care Manage Rev. 2018;43:92–103. [DOI] [PubMed] [Google Scholar]
- 106.Brown B, Young J, Kneebone AB, et al. Knowledge, attitudes and beliefs towards management of men with locally advanced prostate cancer following radical prostatectomy: an Australian survey of urologists. BJU Int. 2016;117(Suppl 4):35–44. [DOI] [PubMed] [Google Scholar]
- 107.Hirshoren N, Kaganov K, Weinberger JM, et al. Thyroidectomy practice after implementation of the 2015 American Thyroid association guidelines on surgical options for patients with well-differentiated thyroid carcinoma. JAMA Otolaryngol Head Neck Surg. 2018;144:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shigeta S, Nagase S, Mikami M, et al. Assessing the effect of guideline introduction on clinical practice and outcome in patients with endometrial cancer in Japan: a project of the Japan Society of Gynecologic Oncology (JSGO) guideline evaluation committee. J Gynecol Oncol. 2017;28:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Savin DD, Zamfirova I, Iannotti J, et al. Survey study suggests that reverse total shoulder arthroplasty is becoming the treatment of choice for four-part fractures of the humeral head in the elderly. Int Orthop. 2016;40:1919–1925. [DOI] [PubMed] [Google Scholar]
- 110.Costa ML, Jameson SS, Reed MR. Do large pragmatic randomised trials change clinical practice?: Assesing the impact of the Distal Radius Acute Fracture Fixation Trial (DRAFFT). Bone Joint J. 2016;98B:410–413. [DOI] [PubMed] [Google Scholar]
- 111.Aiken AM, Haddow JB, Symons NR, et al. Use of antibiotic prophylaxis in elective inguinal hernia repair in adults in London and south-east England: a cross-sectional survey. Hernia. 2013;17:657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nolan HR, Fitzgerald M, Howard B, et al. The trauma time-out: evaluating the effectiveness of protocol-based information dissemination in the traumatically injured patient. J Trauma Nurs. 2017;24:170–173. [DOI] [PubMed] [Google Scholar]
- 113.Chopra S, Hachach-Haram N, Baird DL, et al. Integrated Patient Coordination System (IntPaCS): a bespoke tool for surgical patient management. Postgrad Med J. 1086;92:208–216. [DOI] [PubMed] [Google Scholar]
- 114.O’Brien MA, Charles C, Lovrics P, et al. Enablers and barriers to using patient decision aids in early stage breast cancer consultations: a qualitative study of surgeons’ views. Implement Sci. 2014;9:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brown B, Egger S, Young J, et al. Changing attitudes towards management of men with locally advanced prostate cancer following radical prostatectomy: a follow-up survey of Australia-based urologists. J Med Imaging Radiat Oncol. 2016;60:744–755. [DOI] [PubMed] [Google Scholar]
- 116.Simunovic M, Coates A, Smith A, et al. Uptake of an innovation in surgery: observations from the cluster-randomized Quality Initiative in Rectal Cancer trial. Can J Surg. 2013;56:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jaiprakash A, O’Callaghan WB, Whitehouse SL, et al. Orthopaedic surgeon attitudes towards current limitations and the potential for robotic and technological innovation in arthroscopic surgery. J Orthop Surg (Hong Kong). 2017;25:2309499016684993. [DOI] [PubMed] [Google Scholar]
- 118.Apramian T, Watling C, Lingard L, et al. Adaptation and innovation: a grounded theory study of procedural variation in the academic surgical workplace. J Eval Clin Pract. 2015;21:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang EH, Gross CP, Tilburt JC, et al. Shared decision making and use of decision AIDS for localized prostate cancer: perceptions from radiation oncologists and urologists. JAMA Intern Med. 2015;175:792–799. [DOI] [PubMed] [Google Scholar]
- 120.Gillissen F, Ament SM, Maessen JM, et al. Sustainability of an enhanced recovery after surgery program (ERAS) in colonic surgery. World J Surg. 2015;39:526–533. [DOI] [PubMed] [Google Scholar]
- 121.Yu X, Huang Y, Guo Q, et al. Clinical motivation and the surgical safety checklist. Br J Surg. 2017;104:472–479. [DOI] [PubMed] [Google Scholar]
- 122.Farias-Kovac M, Szubski CR, Hebeish M, et al. Effect of price capitation on implant selection for primary total hip and knee arthroplasty. J Arthroplasty. 2014;29:1345–1349. [DOI] [PubMed] [Google Scholar]
- 123.Lamartina L, Durante C, Lucisano G, et al. Are evidence-based guidelines reflected in clinical practice? An analysis of prospectively collected data of the Italian thyroid cancer observatory. Thyroid. 2017;27:1490–1497. [DOI] [PubMed] [Google Scholar]
- 124.Ligier K, Maynou C, Leroy X, et al. Improvement of the initial management of sarcomas after the dissemination of evidence-based guidelines depends on the primary sarcoma location: a population-based study. BMC Cancer. 2015;15:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Padia R, Olsen G, Henrichsen J, et al. Hospital and surgeon adherence to pediatric tonsillectomy guidelines regarding perioperative dexamethasone and antibiotic administration. Otolaryngol Head Neck Surg. 2015;153:275–280. [DOI] [PubMed] [Google Scholar]
- 126.Rogers EM. Diffusion of Innovations. 5th ed: New York: Simon and Schuster; 2003. [Google Scholar]
- 127.Squires JE, Estabrooks CA, O’Rourke HM, et al. A systematic review of the psychometric properties of self-report research utilization measures used in healthcare. Implement Sci. 2011;6:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kitson AL, Rycroft-Malone J, Harvey G, et al. Evaluating the successful implementation of evidence into practice using the PARiHS framework: theoretical and practical challenges. Implement Sci. 2008;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kitson A, Harvey G, McCormack B. Enabling the implementation of evidence based practice: a conceptual framework. Qual Health Care. 1998;7:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26:13–24. [DOI] [PubMed] [Google Scholar]
- 131.Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Squires JE, Graham ID, Hutchinson AM, et al. Identifying the domains of context important to implementation science: a study protocol. Implement Sci. 2015;10:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


