Abstract
BACKGROUND
RET mutations occur in 70% of medullary thyroid cancers and RET fusions rarely in other thyroid cancers. We evaluated the efficacy and safety of selpercatinib, a selective RET inhibitor, in patients with advanced RET-altered thyroid cancers.
METHODS
Patients with RET-mutant medullary thyroid cancer with and without prior vandetanib or cabozantinib treatment, as well as previously treated RET fusion-positive thyroid cancers, were enrolled in a first-in-human, phase 1/2 trial of selpercatinib. The primary endpoint was objective response rate by independent review committee. Secondary endpoints included duration of response, progression-free survival, and safety.
RESULTS
In the first 55 consecutively enrolled RET-mutant medullary thyroid cancer patients previously treated with vandetanib and/or cabozantinib, the response rate was 69% (95% CI,55 to 81) and 1-year progression-free survival rate 82% (95% CI: 69–90). In 88 patients with RET-mutant vandetanib and cabozantinib-naïve medullary thyroid cancer, the response rate was 73% (95% CI, 62–82) and 1-year progression-free survival rate 92% (95% CI: 82–97). In 19 patients with previously-treated RET fusion-positive thyroid cancer, the response rate was 79% (95% CI, 54–94). The most common grade ≥3 adverse events were hypertension (21%), increased alanine aminotransferase (11%), increased aspartate aminotransferase (9%), hyponatremia (8%), and diarrhea (6%). Four patients (3%) discontinued selpercatinib due to drug-related adverse events.
CONCLUSIONS
In this phase 1/2 trial, selpercatinib demonstrated durable efficacy with mainly low-grade toxicity in medullary thyroid cancer patients with and without prior vandetanib or cabozantinib treatment, and promising activity in RET fusion-positive thyroid cancer.
INTRODUCTION
The RET proto-oncogene encodes a transmembrane receptor tyrosine kinase that is constitutively activated through two distinct mechanisms: mutations involving the cysteine-rich or kinase domains, and structural rearrangements leading to the fusion of the RET gene to a 5′ upstream partner. Collectively, these alterations result in ligand-independent signaling and oncogenesis.
Germline RET mutations result in hereditary multiple endocrine neoplasia (MEN) 2A (MEN2A) and MEN2B, resulting in nearly complete penetrance of medullary thyroid cancer in gene carriers. Collectively, these hereditary syndromes account for 25% of all medullary thyroid cancer cases at diagnosis. Of the remaining 75% of sporadic medullary thyroid cancer cases, approximately 60% harbor somatic RET mutations.1 RET mutations are associated with more aggressive disease in medullary thyroid cancer and thus a majority of patients who develop metastatic medullary thyroid cancer harbor RET mutations. 1
In non-medullary thyroid cancers that arise from follicular cells of the gland, including papillary, poorly differentiated, anaplastic, and Hürthle thyroid cancer, RET fusions are found in fewer than 10% of differentiated thyroid cancers, and are even more uncommon in anaplastic carcinomas. 2–5 The incidence of RET fusions is higher in thyroid cancers arising in children and young adults, as well as in cases linked to environmental radiation exposure.6–11
Multitargeted kinase inhibitors are approved for medullary thyroid cancer (vandetanib and cabozantinib) as well as radioiodine-refractory, differentiated thyroid cancer (sorafenib and lenvatinib). 12–15 These multitargeted kinase inhibitors include RET coverage in addition to many other kinases simultaneously. While response rates achieved with these agents range from 12% to 65%, the safety and durability of responses to these agents is at least partially limited by toxicities. These adverse events are primarily attributed to the their more potent inhibition of non-RET kinases, especially VEGFR2.16,17
Currently, no selective RET inhibitors are approved for treating patients with RET-altered cancers. Selpercatinib (LOXO-292) is a novel, ATP-competitive, highly selective small molecule RET kinase inhibitor. In experimental models, selpercatinib has nanomolar potency against diverse RET alterations including the acquired gatekeeper resistance mutation at residue V804, as well as antitumor activity in the brain.18 We evaluated the safety and efficacy of selpercatinib in LIBRETTO-001, a phase 1/2 clinical trial in adolescent and adult patients with any solid tumor type harboring an activating RET gene alteration. Here we report the safety and efficacy of selpercatinib in RET-mutant medullary thyroid cancer and RET fusion-positive thyroid cancer.
METHODS
PATIENTS
Full eligibility criteria for the LIBRETTO-001 trial are detailed in the protocol, available at NEJM.org. Eligible patients were aged 12 years or older (where allowed by regulatory authorities, otherwise 18 years of age or older) and had a diagnosis of an advanced or metastatic solid tumor. Radiographic tumor progression (either by local or central determination) was not an explicit inclusion criterion, though patients were required to be in need of systemic therapy for their disease. After dose level 2 (20mg twice daily), where steady state selpercatinib pharmacokinetic exposures reached a trough exceeding the predicted RET IC50, a prospectively identified RET alteration (fusion or mutation) was required. RET alteration status was determined by locally obtained molecular testing performed in a certified laboratory, using either next-generation sequencing, fluorescence in situ hybridization, or polymerase chain reaction. Central confirmation of the locally identified RET alteration was not required. Other inclusion criteria included an ECOG performance status score of 0 to 2 (on a 5-point scale in which higher scores reflect greater disability), adequate organ function, and a QTc of ≤470 msec. Any number of prior therapies, including multitargeted kinase inhibitors, were allowed. For inclusion in the present analyses, patients were required to have either RET-mutant medullary thyroid cancer or RET fusion-positive thyroid cancer of any histological type.
This trial was conducted in accordance with the standard of Good Clinical Practice, the principles expressed in the Declaration of Helsinki, and all applicable country and local regulations. The protocol was approved by the institutional review board at each site. All patients or guardians provided written informed consent before the initiation of any trial-related procedure.
TRIAL DESIGN AND TREATMENT
Patients were enrolled in an open label, multi-country (n=12), multicenter (n=65) phase 1/2 trial (Table S1). Selpercatinib was administered orally (capsule or liquid), continuously, in 28-day cycles, until disease progression, death, unacceptable toxicity, or withdrawal of consent. Patients enrolled in the phase 1 dose escalation portion of the study received selpercatinib at doses ranging from 20 mg once daily to 240 mg twice daily. Intrapatient dose escalation to higher doses determined to be safe was permitted after a minimum of 1 cycle of treatment. All patients enrolled to the phase 2 portion received the recommended dose of 160 mg twice daily. Patients with documented disease progression could continue selpercatinib if they were deriving clinical benefit.
The primary endpoint was objective response rate, determined by blinded independent review committee comprised of expert radiologists, according to RECIST version 1.1.19 Secondary endpoints included progression-free survival, duration of response and safety. All responses required confirmation to be reported. Biochemical response rate was an exploratory endpoint.
TRIAL ASSESSMENTS
Radiological tumor assessments were conducted at baseline, every 8 weeks for 1 year, and every 12 weeks thereafter. Image guidelines for liver metastasis response assessment in medullary thyroid cancer patients were (in order of preference): MRI with IV contrast, triple phase CT (non-enhanced, arterial phase, and portal venous phase), or routine single phase CT (portal venous phase). For medullary thyroid cancer patients, serum calcitonin and carcinoembryonic antigen (CEA) levels were followed longitudinally. Best biochemical response for serum calcitonin and CEA was defined as follows: complete response, normalization of serum levels; partial response, ≥ 50% decrease from baseline levels; stable disease, if <50% decrease or increase from baseline; and progression if ≥50% increase from baseline (each maintained for at least 4 weeks).12 Adverse events were assessed from the first dose (or from the date that informed consent was obtained for serious adverse events) until 28 days after the last dose of selpercatinib was administered and graded according to the CTCAE, version 4.03.
TRIAL OVERSIGHT
This trial was designed jointly by the sponsor, Loxo Oncology and Eli Lilly, and investigators. The sponsor collected, analyzed and interpreted the trial data in collaboration with the authors. The first draft of the manuscript was written in collaboration by the first author, senior authors and the sponsor. All authors provided critical input for the manuscript and approved the final version. A medical writer funded by the sponsor provided writing assistance. All authors vouch for the completeness and accuracy of clinical data, as well as adherence to the trial protocol.
STATISTICAL CONSIDERATIONS
All analyses were conducted in accordance with the statistical analysis plan. Fig. S1 provides an illustration of all patients dosed with selpercatinib. The primary analysis set, defined with US Food and Drug Administration (FDA) input, comprised the first 55 consecutively treated patients across both the phase 1 and 2 portions of LIBRETTO-001 with medullary thyroid cancer harboring a prospectively-identified activating RET mutation, who had measurable disease by RECIST v1.1, and had received prior vandetanib and/or cabozantinib therapy. By agreement with health authorities, patients with non-measurable disease enrolled in the phase 1 dose escalation part of the trial were also included in the primary analysis set. Efficacy was also investigated separately in patients with RET-mutant vandetanib and cabozantinib-naïve medullary thyroid cancer and RET fusion-positive thyroid cancer. RET fusion-positive thyroid cancer patients were additionally required to have radioiodine-refractory disease (or have a histologic subtype in which radioiodine is not utilized, such as anaplastic thyroid cancer) and have received at least one prior systemic therapy other than radioiodine.
Efficacy analyses were performed according to the intention-to-treat principle. A true objective response rate of at least 40% in the RET-mutant vandetanib and/or cabozantinib-treated medullary thyroid cancer primary analysis set cohort was hypothesized, and a sample of 55 patients would provide the study with 89% power to establish a lower boundary on the response rate of 20% for a two-sided 95% exact binomial confidence interval. Ruling out a lower limit of 20% for the objective response rate was considered clinically meaningful for patients whose tumors had progressed on standard first-line therapy and had limited remaining treatment options. Confidence intervals for objective response rates were calculated using the Clopper–Pearson method. Progression-free survival and duration of response were estimated by the Kaplan–Meier method. Efficacy analyses for RET-mutant vandetanib and cabozantinib-naïve medullary thyroid cancer and RET fusion-positive thyroid cancer patients were considered exploratory. Safety was analyzed in both the RET-altered medullary and non-medullary thyroid cancer patients defined above as well as in the overall cohort of 531 selpercatinib treated patients treated by June 17, 2019 on LIBRETTO-001. The data cut was taken on December 16, 2019.
RESULTS
PATIENTS
From May 2017 to June 2019, 162 patients were treated across the three efficacy analysis cohorts (55 RET-mutant vandetanib and/or cabozantinib-treated medullary thyroid cancer, 88 RET-mutant vandetanib and cabozantinib-naïve medullary thyroid cancer, and 19 RET fusion-positive previously treated thyroid cancer). Demographic characteristics for each group are summarized in Table 1. Among RET-mutant vandetanib and/or cabozantinib-treated medullary thyroid cancer patients, the median number of prior therapies was 2 (range: 1–8), 18 (33%) patients had received vandetanib, 13 (24%) cabozantinib, and 24 (44%) both. Among patients in the RET-mutant vandetanib and cabozantinib-naïve medullary thyroid cancer, 7 (8%) patients had received other prior multitargeted kinase inhibitor therapy, and 9 (10%) had received other prior systemic therapy. Among RET fusion-positive thyroid cancer, subtypes enrolled included 13 (68%) papillary, 3 (16%) poorly differentiated, 2 (11%) anaplastic, and 1 (5%) Hürthle cell. Overall 15 (79%) of RET fusion-positive thyroid cancer patients had received a multitargeted kinase inhibitor, with the remainder receiving other systemic agents. RET M918T mutation and CCDC6-RET fusion were the most common RET alterations in the medullary thyroid cancer and RET fusion-positive thyroid cancer groups, respectively. In total, 66 unique locally obtained assays were utilized to enroll patients across all cohorts (Table S2). After accounting for intrapatient dose escalation permitted during phase 1 portion of the trial, 82% of the vandetanib- and/or cabozantinib-treated cohort received the selpercatinib at recommended dose of 160 mg twice daily. Almost all patients in the RET-mutant vandetanib- and cabozantinib treatment naïve and RET fusion-positive thyroid groups received selpercatinib 160 mg twice daily (98 and 95%, respectively).
Table 1.
Baseline Characteristics
| RET-mutant MTC | RET Fusion-Positive Thyroid Cancer | ||
|---|---|---|---|
| Characteristic | Vandetanib and/or Cabozantinib pre-treated | Vandetanib/Cabozantinib Naïve | |
| (N=55) | (N=88) | (N=19) | |
| Age, Median (range) – yr | 57 (17–84) | 58 (15–82) | 54 (25–88) |
| Sex – no. (%) | |||
| Male | 36 (66) | 58 (66) | 9 (47) |
| Female | 19 (35) | 30 (34) | 10 (53) |
| Race – no. (%) * | |||
| White | 49 (89) | 75 (85) | 14 (74) |
| Asian | 0 | 4 (5) | 2 (11) |
| Black or African American | 1 (2) | 1 (1) | 1 (5) |
| Other | 5 (9) | 8 (9) | 2 (11) |
| ECOG performance status – no. (%) | |||
| 0 | 11 (20) | 43 (49) | 5 (26) |
| 1 | 41 (75) | 42 (48) | 12 (63) |
| 2 | 3 (6) | 3 (3) | 2 (11) |
| Thyroid cancer tumor type | |||
| Medullary | 55 (100) | 88 (100) | |
| Papillary | 13 (68) | ||
| Poorly differentiated | 3 (16) | ||
| Hürthle cell | 1 (5) | ||
| Anaplastic | 2 (11) | ||
| Median prior systemic regimens (range) – no. | 2 (1–8) | 0 (0–2) | 4 (1–7) |
| Prior cabozantinib and/or vandetanib | 55 (100) | 0 | |
| Vandetanib only | 18 (33) | 0 | |
| Cabozantinib only | 13 (24) | 0 | |
| Cabozantinib and vandetanib | 24 (44) | 0 | |
| Prior radioactive iodine (RAI) | 16 (84) | ||
| Prior sorafenib and/or lenvatinib | 13 (68) | ||
| Prior multikinase inhibitor therapy – no. (%) | 55 (100) | 7 (8) | 15 (79) |
| 1 | 26 (47) | 6 (7) | 7 (37) |
| ≥2 | 29 (53) | 1 (1) | 8 (42) |
| Prior non-multikinase inhibitor therapy– no. (%) | 17 (31) | 9 (10) | 14 (74) |
| Brain metastases – no. (%) | 4 (7) | 2 (2) | 6 (32) |
| RET alteration – no. (%) | |||
| RET M918T mutation | 33 (60) | 49 (56) | - |
| RET V804M/L mutation | 5 (9) | 6 (7) | - |
| RET extracellular cysteine mutation** | 7 (13) | 20 (23) | - |
| Other mutations† | 10 (18) | 13 (15) | - |
| CCDC6-RET fusion | - | - | 9 (47) |
| NCOA4-RET fusion | - | - | 6 (32) |
| Other RET fusion‡ | - | - | 4 (21) |
Includes 1 RET fusion-positive patient with missing race
Extracellular cysteine mutation defined as mutation including at least 1 of the following cysteine residues: 609, 611, 618, 620, 630, and 634.
Other includes: D631-L633delinsE, E632-L633del, A883F, D631-L633delinsV, L790F, D898-E901del, D898_E901del + D903_S904delinsEP, K666N, T636-V637insCRT, D378-G385delinsE.
Fusions identified in single tumors included CCDC186-RET, ERC1-RET, KTN1-RET, and RUFY3-RET.
Eastern Cooperative Oncology Group (ECOG) performance status scores range from 0 to 5, with higher scores indicating greater disability. Total % may be different than the sum of the individual components due to rounding.
EFFICACY
RET-mutant Vandetanib- and/or Cabozantinib-Treated Medullary Thyroid Cancer
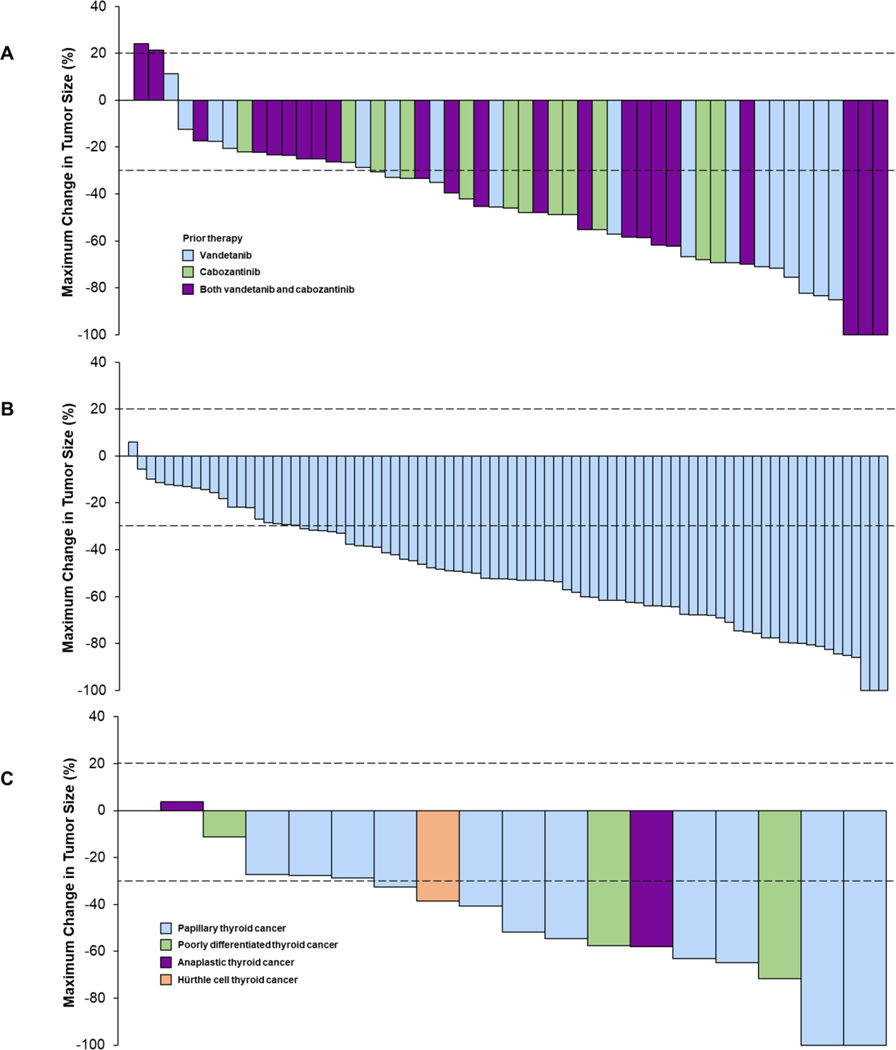
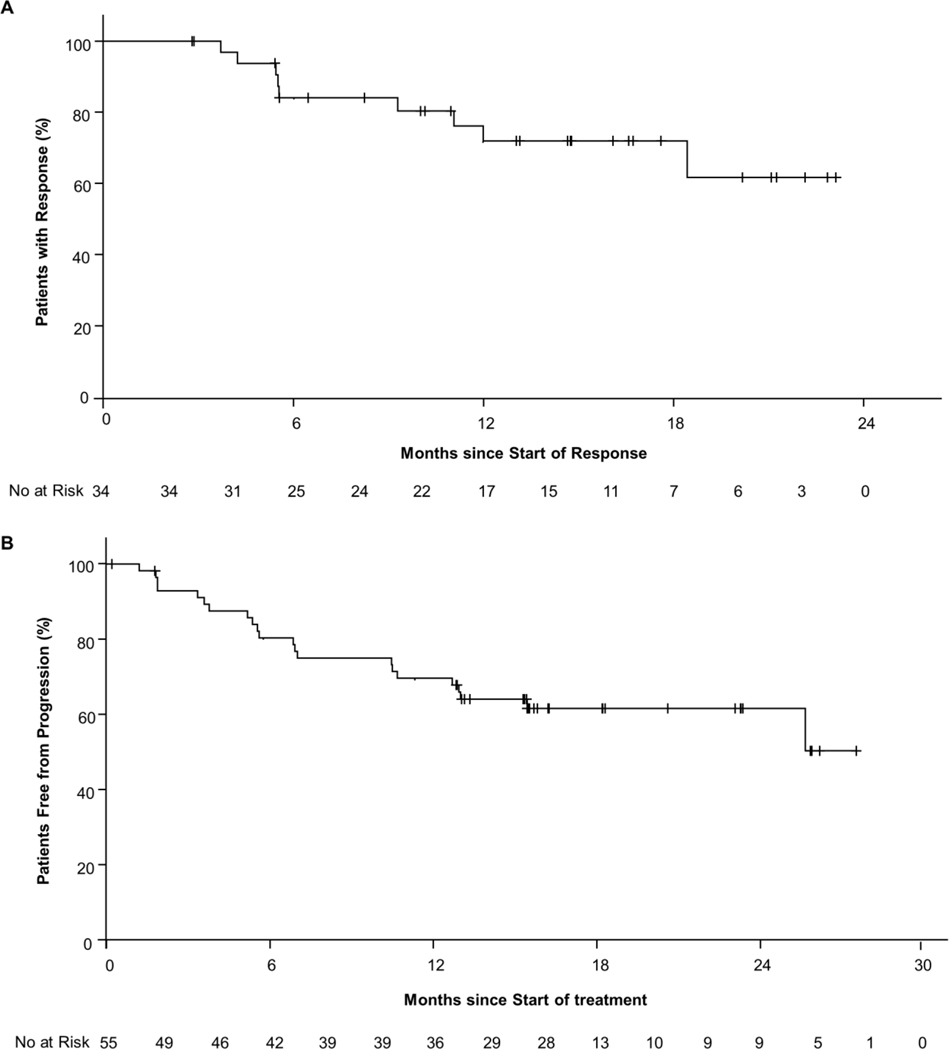
Efficacy in the 55 RET-mutant vandetanib- and/or cabozantinib-treated medullary thyroid cancer patients per investigator assessments and independent review committee are summarized in Table 2, Fig. 1 and Fig. 2. The objective response rate was 69% (95% CI, 55 to 81) by independent review committee. Overall 5 patients (9%) had a complete response and 33 (60%) a partial response. Efficacy was observed regardless of the number of lines of prior multitargeted kinase inhibitor therapy received (vandetanib only, response rate 67%, cabozantinib only, 69%, both vandetanib and cabozantinib, 71%, Fig S2A). Similarly, responses were observed across all qualifying RET mutations, including 3 patients harboring the acquired resistance mutation RET V804 (Fig. S2B). At 1-year 86% (95% CI, 67–95) of responses were ongoing (Fig. S3A) and 82% (95 % CI, 69–90) of all patients remained progression-free (Fig S3B). Outcomes in this cohort per investigator assessments are shown in Fig S4. The biochemical response rates were 91% (95% CI, 80–97) by calcitonin in 54 evaluable patients and 66% (95% CI, 52–79) by CEA in 53 evaluable patients (Fig S5, S6, Table S3). The median time to calcitonin response was 0.5 months (range: 0.4, 1.9), and to CEA response 1.8 months (range: 0.4, 18.8).
Table 2:
Efficacy
| Vandetanib and/or Cabozantinib pretreated | Vandetanib and Cabozantinib naive | RET fusion-positive Thyroid Cancer | ||||
|---|---|---|---|---|---|---|
| RET-mutant MTC | RET-mutant MTC | |||||
|
| ||||||
| Response | Independent Review | Investigator Assessment | Independent Review | Investigator Assessment | Independent Review | Investigator Assessment |
| (n=55) | (n=55) | (n=88) | (n=88) | (n=19) | (n=19) | |
|
| ||||||
| Objective response rate – % (95% CI) | 69 (55–81) | 62 (48–75) | 73 (62–82) | 71 (60–80) | 79 (54–94) | 58 (34–80) |
| Best response – no. % | ||||||
| Complete response | 5 (9) | 3 (6) | 10 (11) | 3 (3) | 1 (5) | 0 |
| Partial response | 33 (60) | 31 (56) | 54 (61) | 59* (67) | 14 (74) | 11 (58) |
| Stable disease | 14 (26) | 16 (29) | 20 (23) | 24 (27) | 4 (21) | 7 (37) |
| Progressive disease | 1 (2) | 3 (6) | 2 (2) | 0 | 0 | 0 |
| Not evaluable | 2 (4) † | 2 (4) † | 2 (2) | 2 (2) | 0 | 1 (5) |
| Duration of Response | ||||||
| Responders | 38 | 34 | 64 | 59# | 15 | 11 |
| Censored, n (%) | 32 (84.2) | 25 (73.5) | 60 (93.8) | 56 (94.9) | 9 (60.0) | 8 (72.7) |
| Median, months (95% CI) | NE (19.1-NE) | NE (18.4-NE) | 22.0^ (NE-NE) | 22.0^ (NE-NE) | 18.4 (7.6-NE) | NE (9.5-NE) |
| Median follow-up, months | 14.1 | 14.8 | 7.8 | 8.0 | 17.5 | 17.5 |
| Progression-Free Survival | ||||||
| Censored, n (%) | 42 (76) | 33 (60) | 80 (91) | 82 (93) | 11 (58) | 12 (63) |
| Median, months (95% CI) | NE (24.4-NE) | 27.4 (13.7-NE) | 23.6 (NE-NE) | 23.6 (23.6-NE) | NE (10-NE) | 20.1 (9.4-NE) |
| Median follow-up, months | 16.7 | 16.7 | 11.1 | 11.1 | 13.7 | 19.3 |
| 1-year PFS rate, % (95% CI) | 82.3 (68.7–90.4) | 67.9 (53.6–78.7) | 92.4 (82.1–96.8) | 95.4 ((85.9–98.5) | 64.4 (33.0–80.6) | 61.4 (37.0–82.3) |
Includes 1 patient who died prior to their first response assessment
Includes 3 patients with unconfirmed partial responses pending confirmation.
Includes only confirmed responses.
Unstable median, based on fewer than 10% of total number of events. Total % may be different than the sum of the individual components due to rounding. NE, not estimable; PFS, progression-free survival.
Figure 1. Waterfall plots of the maximum change in tumor size in (A) vandetanib and/or cabozantinib pretreated RET-mutant MTC patients, (B) vandetanib/cabozantinib naïve RET-mutant MTC patients, and (C) RET fusion-positive thyroid cancer patients per investigator assessments.
For each patient, the best (minimum) percent change from baseline in the sum of diameters for all target lesions is represented by a vertical bar. Data for 4 patients not shown in vandetanib and/or cabozantinib pretreated RET-mutant MTC group: 2 discontinued prior to any post-baseline imaging assessments, and 2 did not have measurable disease at baseline. Data for 4 patients not shown in vandetanib/cabozantinib naïve RET-mutant MTC group: 2 patients discontinued prior to any post-baseline imaging assessments and 2 did not have measurable disease at baseline. RET fusion-positive thyroid cancer patients; Data for 2 patients not shown in RET fusion-positive thyroid cancer group: 1 did not have measurable disease at baseline, and 1 deemed not evaluable on study by the investigator.
Figure 2:
Kaplan-Meier plots of (A) duration of response among 34 patients with a response and (B) progression-free survival in all 55 patients in the vandetanib and/or cabozantinib pretreated RET-mutant MTC group according to investigator assessments.
RET-mutant Vandetanib- and Cabozantinib-Naïve Medullary Thyroid Cancer
Among 88 RET-mutant vandetanib- and cabozantinib-naïve MTC patients, the objective response rate was 73% (95% CI, 62–82), by independent review committee (Table 2). Overall 10 patients (11%) had a complete response and 54 (61%) a partial response. Responses were observed across all RET mutations (Fig. S7). At a median duration of follow-up of 8.0 months, 59 of 64 responses were ongoing (range: 1.8–21.9). At 1-year, 91% (95% CI, 72–97) of responses were ongoing, and 92% (95% CI, 82–97) were progression-free (Fig S8).
RET Fusion-Positive Previously Treated Thyroid Cancer
Among 19 patients with RET fusion-positive previously treated thyroid cancer, the objective response rate was 79% (95% CI, 54 to 94) by independent review committee. Activity was seen across multiple thyroid cancer histologies including papillary, poorly differentiated, Hürthle cell and anaplastic carcinomas and across different fusion partners (Fig 1C, Fig S9). Among 2 anaplastic thyroid cancer patients treated, 1 responded for 18 months with response ongoing. At 1-year, 71% (95% CI, 39–88) of responses were ongoing, and 64% (95% CI, 37–82) were progression-free.
ADVERSE EVENTS
Table 3 shows treatment-emergent adverse events of any attribution and the subset of treatment-emergent adverse events judged by the investigator as related to selpercatinib treatment, for the population of thyroid cancer patients whose efficacy is reported here. The most common grade 3 or 4 treatment-emergent adverse events were hypertension (21%), alanine aminotransferase increase (11%), aspartate aminotransferase increase (9%) and hyponatremia (8%), and diarrhea (6%). Five (3%) grade 5 adverse events (hemoptysis, post-procedure hemorrhage, sepsis, cardiac arrest, and cardiac failure) were observed, all deemed unrelated to selpercatinib. The adverse event profile of selpercatinib in RET-altered thyroid cancers was broadly similar to overall safety profile for all 531 patients dosed with selpercatinib (Table S4). Of note, one patient across the entire safety cohort with medullary thyroid cancer developed grade 3 tumor lysis within one week of initiating selpercatinib, requiring hydration, supportive care, and ultimately permanent discontinuation. Across all 531 selpercatinib-treated patients, 160 (30%) required dose reduction due to treatment-related adverse events, and 12 (2%) patients discontinued selpercatinib due to treatment-related adverse events, the most common of which were alanine aminotransferase increase (2 patients) and drug hypersensitivity (2 patients).
Table 3:
Adverse Events in Selpercatinib Treated Patients with RET-mutant MTC and RET fusion-positive thyroid cancer (N=162)
| Adverse Event | Adverse Events, Regardless of Attribution | Treatment-Related Adverse Events | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. (percent of patients with event) | ||||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Any Grade | Grade 3 | Grade 4 | Any Grade | |
| Any adverse event | 9 (6) | 42 (26) | 95 (59) | 11 (7) | 162 (100) | 45 (28) | 3 (2) | 153 (94) |
| Dry mouth | 69 (43) | 5 (3) | 0 | 0 | 74 (46) | 0 | 0 | 63 (39) |
| Hypertension | 10 (6) | 25 (15) | 34 (21) | 0 | 69 (43) | 19 (12) | 0 | 49 (30) |
| Diarrhea | 44 (27) | 8 (5) | 9 (6) | 0 | 61 (38) | 4 (3) | 0 | 27 (17) |
| Fatigue | 35 (22) | 24 (15) | 2 (1) | 0 | 61 (38) | 1 (1) | 0 | 41 (25) |
| Aspartate aminotransferase increased | 37 (23) | 6 (4) | 13 (8) | 1 (1) | 57 (35) | 12 (7) | 1 (1) | 45 (28) |
| Nausea | 44 (27) | 13 (8) | 0 | 0 | 57 (35) | 0 | 0 | 25 (15) |
| Constipation | 44 (27) | 11 (7) | 1 (1) | 0 | 56 (35) | 0 | 0 | 26 (16) |
| Alanine aminotransferase increased | 26 (16) | 7 (4) | 17 (11) | 1 (1) | 51 (32) | 16 (10) | 1 (1) | 42 (26) |
| Headache | 36 (22) | 11 (7) | 4 (3) | 0 | 51 (32) | 1 (1) | 0 | 21 (13) |
| Edema peripheral | 42 (26) | 5 (3) | 1 (1) | 0 | 48 (30) | 0 | 0 | 29 (18) |
| Blood creatinine increased | 27 (17) | 12 (7) | 0 | 0 | 39 (24) | 0 | 0 | 22 (14) |
| Abdominal pain | 25 (15) | 8 (5) | 5 (3) | 0 | 38 (24) | 0 | 0 | 6 (4) |
| Arthralgia | 25 (15) | 10 (6) | 0 | 0 | 35 (22) | 0 | 0 | 8 (5) |
| Vomiting | 26 (16) | 8 (5) | 1 (1) | 0 | 35 (22) | 0 | 0 | 12 (7) |
| Hypocalcemia | 14 (9) | 13 (8) | 6 (4) | 1 (1) | 34 (21) | 0 | 0 | 5 (3) |
| Back pain | 19 (12) | 10 (6) | 2 (1) | 0 | 31 (19) | 0 | 0 | 1 (1) |
| Electrocardiogram QT prolonged | 11 (7) | 16 (10) | 4 (3) | 0 | 31 (19) | 3 (2) | 0 | 21 (13) |
| Cough | 25 (15) | 4 (3) | 0 | 0 | 29 (18) | 0 | 0 | 2 (1) |
| Rash | 25 (15) | 3 (2) | 0 | 0 | 28 (17) | 0 | 0 | 13 (8) |
| Dizziness | 25 (15) | 2 (1) | 0 | 0 | 27 (17) | 0 | 0 | 9 (6) |
| Abdominal distension | 18 (11) | 7 (4) | 0 | 0 | 25 (15) | 0 | 0 | 12 (7) |
| Hypothyroidism | 14 (9) | 11 (7) | 0 | 0 | 25 (15) | 0 | 0 | 12 (7) |
| Weight increased | 11 (7) | 9 (6) | 5 (3) | 0 | 25 (15) | 1 (1) | 0 | 8 (5) |
The adverse events listed here are those that occurred at any grade in at least 15% of patients, regardless of attribution. The relatedness of adverse events to treatment was determined by the investigators. Total % for any given AE may be different than the sum of the individual grades, due to rounding.
In total, 5 patients experienced grade 5 adverse events including hemoptysis, post-procedure hemorrhage, sepsis, cardiac arrest, and cardiac failure (one each), all deemed unrelated to selpercatinib.
DISCUSSION
In this Phase 1/2 trial, selpercatinib showed marked and durable anti-tumor activity in patients with RET-mutant medullary thyroid cancer with and without previous vandetanib or cabozantinib treatment. In addition, promising activity was observed in a smaller group of patients with RET fusion-positive previously treated thyroid cancers of various histologies.
The objective response rate of selpercatinib was broadly consistent across medullary thyroid cancer groups at approximately 70%, regardless of prior treatment with vandetanib and/or cabozantinib. Tumor regressions were seen in nearly all patients, and activity was observed regardless of the individual RET mutation. Activity also appeared durable with 86% and 91% of responses ongoing at 1-year in the vandetanib- or cabozantinib-treated and treatment-naïve medullary thyroid cancer groups, respectively. Continued follow-up will be needed to define the ultimate durability of selpercatinib efficacy across all cohorts.
Both vandetanib and cabozantinib were previously approved for the treatment of metastatic medullary thyroid cancer on the basis of two randomized placebo-controlled phase 3 trials.12,13 In the cabozantinib trial, the objective response rate was 28%, and median progression-free survival was 11.2 months. In the vandetanib trial, the objective response rate was 45%, and median progression-free survival was 30.5 months; however, the latter trial’s eligibility criteria resulted in entry of a more indolent patient population to the study, potentially accounting for some difference in outcomes. While both agents meaningfully improve progression-free survival compared to placebo in medullary thyroid cancer, adverse events necessitated dose reduction in 35% of patients on vandetanib and 79% of patients on cabozantinib, and permanent discontinuation of therapy in 12% and 16% of patients, respectively. Inhibition of VEGFR2 in particular has been implicated in many of the dose-limiting toxicities of these agents, and some patients are simply not eligible for these therapies on the basis of unacceptable risk for bleeding or concerns over wound-healing.20 By comparison, consistent with selpercatinib’s selectivity for RET, most related adverse events were grade 1 or 2, dose reductions were relatively uncommon, and treatment discontinuation due to treatment-related adverse events was necessary in only 3% of patients. The objective response rate observed here with selpercatinib in previously-treated RET-mutant medullary thyroid cancer appears to exceed that of currently approved first-line agents, while potentially offering improved tolerability. Caution is necessary interpreting results because comparisons between studies may not take into account important factors that influence response rates.
In conclusion, selpercatinib demonstrated a high response rate and durable antitumor activity given the length of follow up in patients with RET-mutant medullary thyroid cancer, whether or not patients had received previous vandetanib and/or cabozantinib. Activity was also seen in a small series of patients with RET fusion-positive previously treated thyroid cancer. Activity was seen across all RET alterations and thyroid cancer histologies enrolled. Selpercatinib was associated mainly with grade 1–2 toxicity that appears to allow for long-term administration in most patients. The implementation of effective RET molecular screening strategies for patients with medullary thyroid cancer will be essential in identifying patients who may benefit from RET inhibition.
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients and their caregivers and the study teams at the participating centers as well as the International Thyroid Oncology Group. This study was funded by Loxo Oncology, Inc., a wholly owned subsidiary of Eli Lilly and Company. We would like to acknowledge Sandya Govinda Raju of Loxo Oncology for her contribution to drafting and revising the manuscript. Medical writing support was provided by Dr Jim Heighway of Cancer Communications and Consultancy Ltd. (Knutsford, UK), which was funded by Loxo Oncology, Inc.
(Funded by Loxo Oncology; ClinicalTrials.gov, NCT03157128).
REFERENCES
- 1.Ciampi R, Romei C, Ramone T, et al. Genetic Landscape of Somatic Mutations in a Large Cohort of Sporadic Medullary Thyroid Carcinomas Studied by Next-Generation Targeted Sequencing. iScience 2019;20:324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research N. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014;159:676–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Hou P, Ji M, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. The Journal of clinical endocrinology and metabolism 2008;93:3106–16. [DOI] [PubMed] [Google Scholar]
- 4.Landa I, Ibrahimpasic T, Boucai L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. The Journal of clinical investigation 2016;126:1052–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pozdeyev N, Gay LM, Sokol ES, et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clinical cancer research : an official journal of the American Association for Cancer Research 2018;24:3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoro M, Papotti M, Chiappetta G, et al. RET activation and clinicopathologic features in poorly differentiated thyroid tumors. The Journal of clinical endocrinology and metabolism 2002;87:370–9. [DOI] [PubMed] [Google Scholar]
- 7.Duan H, Li Y, Hu P, et al. Mutational profiling of poorly differentiated and anaplastic thyroid carcinoma by the use of targeted next-generation sequencing. Histopathology 2019;75:890–9. [DOI] [PubMed] [Google Scholar]
- 8.Nikiforov YE, Rowland JM, Bove KE, Monforte-Munoz H, Fagin JA. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res 1997;57:1690–4. [PubMed] [Google Scholar]
- 9.Ciampi R, Giordano TJ, Wikenheiser-Brokamp K, Koenig RJ, Nikiforov YE. HOOK3-RET: a novel type of RET/PTC rearrangement in papillary thyroid carcinoma. Endocrine-related cancer 2007;14:445–52. [DOI] [PubMed] [Google Scholar]
- 10.Vanden Borre P, Schrock AB, Anderson PM, et al. Pediatric, Adolescent, and Young Adult Thyroid Carcinoma Harbors Frequent and Diverse Targetable Genomic Alterations, Including Kinase Fusions. The oncologist 2017;22:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su X, Li Z, He C, Chen W, Fu X, Yang A. Radiation exposure, young age, and female gender are associated with high prevalence of RET/PTC1 and RET/PTC3 in papillary thyroid cancer: a meta-analysis. Oncotarget 2016;7:16716–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells SA Jr., Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2012;30:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2013;31:3639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 2014;384:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621–30. [DOI] [PubMed] [Google Scholar]
- 16.Brose MS, Bible KC, Chow LQM, et al. Management of treatment-related toxicities in advanced medullary thyroid cancer. Cancer Treat Rev 2018;66:64–73. [DOI] [PubMed] [Google Scholar]
- 17.Cabanillas ME, Habra MA. Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer Treat Rev 2016;42:47–55. [DOI] [PubMed] [Google Scholar]
- 18.Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2018;29:1869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Shao Y, Wang K. Incidence and risk of hypertension associated with cabozantinib in cancer patients: a systematic review and meta-analysis. Expert review of clinical pharmacology 2016;9:1109–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.