Abstract
Background
Poor oral health has been identified as a prognostic factor potentially affecting the survival of patients with head and neck squamous cell carcinoma. However, evidence to date supporting this association has emanated from studies based on single cohorts with small-to-modest sample sizes.
Methods
Pooled analysis of 2449 head and neck squamous cell carcinoma participants from 4 studies of the International Head and Neck Cancer Epidemiology Consortium included data on periodontal disease, tooth brushing frequency, mouthwash use, numbers of natural teeth, and dental visits over the 10 years prior to diagnosis. Multivariable generalized linear regression models were used and adjusted for age, sex, race, geographic region, tumor site, tumor-node-metastasis stage, treatment modality, education, and smoking to estimate risk ratios (RR) of associations between measures of oral health and overall survival.
Results
Remaining natural teeth (10-19 teeth: RR = 0.81, 95% confidence interval [CI] = 0.69 to 0.95; ≥20 teeth: RR = 0.88, 95% CI = 0.78 to 0.99) and frequent dental visits (>5 visits: RR = 0.77, 95% CI = 0.66 to 0.91) were associated with better overall survival. The inverse association with natural teeth was most pronounced among patients with hypopharyngeal and/or laryngeal, and not otherwise specified head and neck squamous cell carcinoma. The association with dental visits was most pronounced among patients with oropharyngeal head and neck squamous cell carcinoma. Patient-reported gingival bleeding, tooth brushing, and report of ever use of mouthwash were not associated with overall survival.
Conclusions
Good oral health as defined by maintenance of the natural dentition and frequent dental visits appears to be associated with improved overall survival among head and neck squamous cell carcinoma patients.
Head and neck squamous cell carcinoma is the sixth most common malignancy worldwide, with 878 348 newly diagnosed patient cases in 2020 (1). Although survival has improved over the past decades, head and neck squamous cell carcinoma remains one of the most lethal malignancies worldwide, with 444 347 reported deaths in 2020 (1). Variation in global head and neck squamous cell carcinoma incidence (2) reflects differences in the distribution of known risk factors including smoking and tobacco exposure (3), alcohol (4), human papillomavirus (HPV) (5), and low socioeconomic status (6,7). Importantly, these risk factors have also been associated with survival differences of head and neck squamous cell carcinoma patients (8-11).
Poor oral health has been reported as an independent risk factor for head and neck squamous cell carcinoma (12). Specifically, measures of poor oral health including tooth loss, periodontal disease, infrequent tooth brushing, and lack of dental visits have been associated with weak to moderate increases in head and neck squamous cell carcinoma risk (12,13). Although the mechanisms underlying these associations remain unclear, chronic trauma (14), oral inflammation (15), and alterations in the oral microbiome (16) have been proposed. For example, oxidative stress is found in periodontal inflammation (17) and epithelial mutagenesis (18) and could link oral inflammation with cancer initiation and progression. Also, Fusobacterium species known to be increased in oral squamous cell carcinoma (19-21) were recently reported to induce the upregulation of programmed cell death ligand 1 and extracellular signal-regulated kinase 1 (ERK1) pathway signaling to the MYC proto-oncogene in head and neck squamous cell carcinoma and thus potentially affect tumor biology and treatment responses (22).
Notably, data on the impact of oral health on head and neck squamous cell carcinoma survival are currently limited and emanate from single cohorts with relatively small to moderate sample sizes (23-25). The definition of oral hygiene varies by study, and its association between poor oral hygiene and survival is inconsistent (23,25). In this study, we sought to add to the evidence base of oral health and determinants of overall survival in head and neck squamous cell carcinoma patients by analyzing demographic, clinicopathologic, oral health, treatment, and survival data from epidemiologic studies participating in the International Head and Neck Cancer Epidemiology (INHANCE) Consortium. This study reports the results of the largest pooled analysis of oral health and head and neck squamous cell carcinoma patient overall survival performed to date.
Methods
Participants and data
The pooled cohort of the INHANCE Consortium studies comprised 10 042 participants with head and neck cancer from 10 INHANCE case-control studies conducted in North America (Seattle, Washington, USA; Los Angeles, California, USA; HOTSPOT, CHANCE/North Carolina, USA), South America (Sao Paulo 1, Sao Paulo 2, Latin America), and Europe (Central Europe, Western Europe, Head and Neck 5000 [HN5000]). Data were obtained via self-reported questionnaires and harmonized as previously described (12,26,27). Study participants provided written informed consent, and studies were approved by the institutional review board at each institution involved (28).
We used available information for participants’ age, sex, race, smoking status, education level, year of diagnosis, tumor-node-metastasis (TNM) stage (American Joint Committee on Cancer 7th edition), tumor site, and treatment modality. Age was measured as a continuous value (range = 21-92 years), sex was binary (male, female), race was categorical (Asian and Pacific Islanders, Black, Brazilian, Others, White), geographic region was categorical (North America, South America, Europe), tumor site was categorical (oral cavity, oropharynx, hypopharynx and/or larynx, head and neck squamous cell carcinoma not otherwise specified [NOS]), TNM stage was categorical (I-IV), treatment was categorical, and tumors located in overlapping regions within the head and neck were termed as head and neck squamous cell carcinoma NOS, education was categorical (less than junior high school, some high school, high school graduate, technical school, college graduate or above), and smoking was categorical (current, former, never smokers).
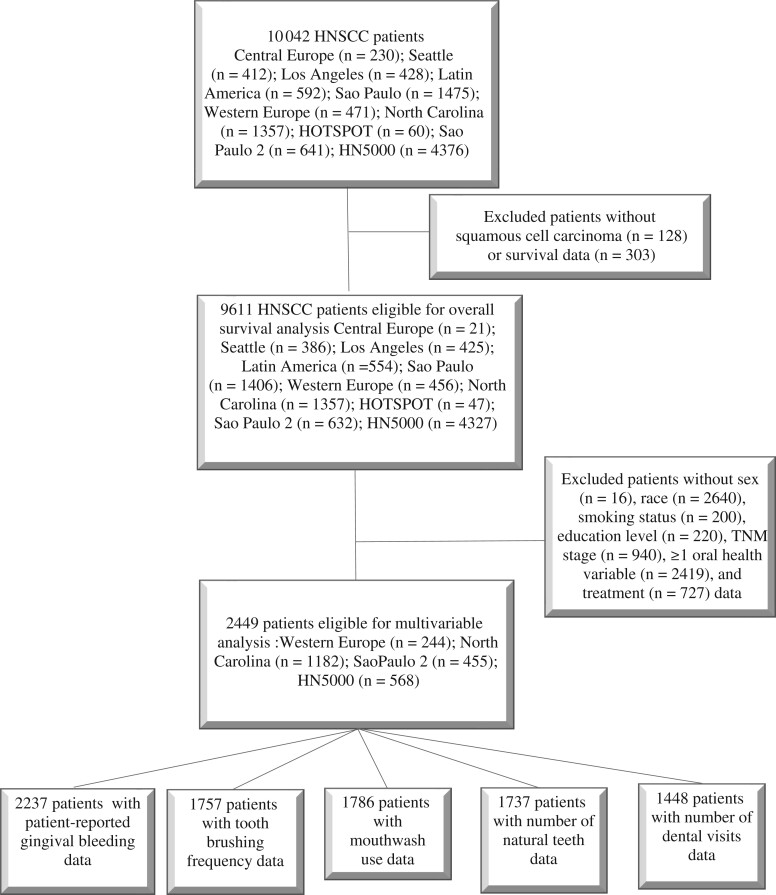
Information on HPV status was available only for 1 study and thus was not considered in this pooled analysis. Alcohol consumption information was available only for a subset (568 of 2449; 23%) of participants. Educational level was used as surrogate for socioeconomic status. Participants diagnosed with a histologic type other than head and neck squamous cell carcinoma (n = 128), as well as those with missing data on race (n = 2640), TNM stage (n = 940), treatment modality (n = 727), survival (n = 303), education level (n = 220), smoking status (n = 200), sex (n = 16), and availability of at least 1 health variable (ie, self-reported gingival bleeding, toothbrushing frequency, mouthwash use, number of remaining natural teeth, dental visits during the past 10 years; n = 2419) were excluded (see Figure 1). A total of 2449 eligible head and neck squamous cell carcinoma patients defined the analytical sample for survival analyses.
Figure 1.
Flowchart of head and neck squamous cell carcinoma patient selection from the International Head and Neck Cancer Epidemiology Consortium for inclusion in the survival analysis. HN5000 = Head and Neck 5000; HNSCC = head and neck squamous cell carcinoma; HOTSPOT = Human Papillomavirus (HPV) Oral Transmission Study in Partners Over Time.
Information on patients’ oral health was available for self-reported gingival bleeding, tooth brushing frequency, mouthwash use, number of natural teeth, and number of dental visits during the past 10 years. Oral health measures were categorized according to definitions employed in the contributing studies. Whenever there was an incompatibility between individual study definitions, we used the definition that allowed the inclusion of the maximum number of participants. Specifically, self-reported gingival bleeding (yes, no), toothbrushing frequency (brushing <1 per day or brushing ≥1 times per day), and mouthwash use (yes, no) were dichotomized. The numbers of remaining natural teeth (≥20 teeth, 10-19 teeth, 1-9 teeth and no natural teeth) and dental visits during the past 10 years (no dental visits, 1-5 visits, and >5 visits) were treated as categorical variables. All measures referred to a time frame prior to cancer diagnosis, as previously described (10,12,29).
Statistical analysis
Summary statistics for demographic characteristics were compared using frequencies and means. χ2 tests were used to compare dental visits during the past 10 years and early (stages I and II) vs late (stages III and IV) head and neck squamous cell carcinoma diagnosis. Survival time in years was compared using medians and Kaplan–Meier curves. We attempted to estimate hazard ratios (HRs) for various demographic and clinical predictors using Cox proportional hazards regression and thereafter tested for the proportionality assumption and discovered the assumption was violated. Therefore, we estimated the 5-year and 10-year survival functions using the Kaplan–Meir curves and examined between-group differences using the Wilcoxon–Breslow–Gehan test. Crude 5- and 10-year survival rates were estimated to compare survival based on key oral health variables including gingival bleeding, tooth brushing frequency, mouthwash use, missing teeth, and dental visits during the past 10 years. A generalized linear regression model using a log-link function with Poisson family and robust variance was used to estimate associations between measures of oral health and survival. The model included age, sex, race, smoking status, education level, TNM stage, tumor site, and treatment modality. Incidence rate ratios (rate ratio [RR]) and corresponding 95% confidence intervals (CI) of measures of oral health with overall survival were estimated. Age, sex, race, TNM stage, tumor site, and treatment modality were selected a priori for the models based on prior knowledge in the literature (12,21,24), whereas smoking and education were included as plausible confounders of the association between oral health survival. Analyses were carried out using Stata 16.1 (StataCorp LP, College Station, TX, USA).
Results
The analytical sample included 2449 patients from the North Carolina (48%), HN5000 (23%), Sao Paulo 2 (19%), and Western Europe (10%) studies (Table 1). Participants were predominantly male (78%), had a mean age of 59.8 years, were diagnosed between 2002 and 2014, and were subsequently followed for a median 4.2 years (patient reported gingival bleeding, 4 years; toothbrushing frequency, 4 years; mouthwash use, 3.6 years; number of natural teeth, 3.4; number of dental visits, 4.8 years) (Table 2). Most participants were current (52%) or former (33%) smokers and of low educational attainment (53% attended some high school or less).
Table 1.
Samples of patients with oral health measures in International Head and Neck Cancer Epidemiology contributing studies, overall and according to tumor sitea
| Entire sample | Oral cavity | Oropharynx | Hypopharynx and/or Larynx | Head and neck squamous cell carcinoma NOS | |
|---|---|---|---|---|---|
| No. (col. %) | No. (row %) | No. (row %) | No. (row %) | No. (row %) | |
| All participants | 2449 (100) | 621 (25) | 744 (30) | 865 (35) | 219 (9) |
|
| |||||
| North Carolina | 1182 (48) | 167 (14) | 324 (27) | 476 (40) | 215 (18) |
| Sao Paulo 2 | 455 (19) | 202 (44) | 110 (24) | 143 (31) | 0 (0) |
| Western Europe | 244 (10) | 84 (34) | 62 (25) | 94 (39) | 4 (2) |
| Head and Neck 5000 | 568 (23) | 168 (30) | 248 (44) | 152 (27) | 0 (0) |
Col = column; NOS = not otherwise specified.
Table 2.
Demographic characteristics, smoking status, and oral health measures of head and neck squamous cell carcinoma patients from the International Head and Neck Cancer Epidemiology Consortium studies in the entire sample and according to tumor site
| Entire sample | Oral cavity | Oropharynx | Hypopharynx and/or Larynx | Head and neck squamous cell carcinoma NOS | |
|---|---|---|---|---|---|
| Characteristics | No. (col. %) | No. (col. %) | No. (col. %) | No. (col. %) | No. (col. %) |
| Sex | |||||
| Male | 1921 (78) | 433 (70) | 606 (81) | 726 (84) | 156 (71) |
| Female | 528 (22) | 188 (30) | 138 (19) | 139 (16) | 63 (29) |
| Age, mean (SD), y | 59.8 (10.5) | 60.3 (11.4) | 57.3 (9.4) | 61.9 (10.0) | 58.2 (11.2) |
| Race | |||||
| Asian and Pacific Islanders | 16 (1) | 9 (1) | 3 (0) | 4 (0) | 0 (0) |
| Black | 320 (13) | 58 (9) | 81 (11) | 133 (15) | 48 (22) |
| Braziliana | 2 (9) | 0 (0) | 1 (0) | 1 (0) | 0 (0) |
| Othersb | 138 (6) | 47 (8) | 41 (6) | 46 (5) | 4 (2) |
| White | 1973 (81) | 507 (82) | 618 (83) | 681 (79) | 167 (76) |
| Education level | |||||
| No more than junior high school | 333 (14) | 117 (19) | 79 (11) | 125 (14) | 12 (5) |
| Some high school | 945 (39) | 262 (42) | 272 (37) | 362 (42) | 49 (22) |
| High school graduate | 466 (19) | 107 (17) | 122 (16) | 177 (20) | 60 (27) |
| Technical school | 431 (18) | 78 (13) | 154 (21) | 138 (16) | 61 (28) |
| At least college graduate | 274 (11) | 57 (9) | 117 (16) | 63 (7) | 37 (17) |
| Smoking status | |||||
| Never smoker | 355 (14) | 105 (17) | 156 (21) | 43 (5) | 51 (26) |
| Former smoker | 816 (33) | 190 (31) | 285 (38) | 290 (34) | 51 (26) |
| Current smoker | 1278 (52) | 326 (52) | 303 (41) | 532 (62) | 117 (53) |
| Gum bleeding | |||||
| Yes | 647 (29) | 159 (29) | 175 (25) | 246 (31) | 67 (31) |
| No | 1590 (71) | 386 (71) | 512 (75) | 541 (69) | 151 (69) |
| Missing | 212 | 76 | 57 | 78 | 1 |
| Tooth brushing | |||||
| <1 time/day | 1224 (70) | 200 (50) | 330 (70) | 486 (73) | 208 (95) |
| ≥1 time/day | 533 (30) | 202 (50) | 141 (30) | 180 (27) | 10 (5) |
| Missing | 692 | 219 | 273 | 199 | 1 |
| Mouthwash use | |||||
| Yes | 824 (46) | 169 (40) | 211 (45) | 313 (46) | 131 (60) |
| No | 962 (54) | 249 (60) | 263 (55) | 364 (54) | 86 (40) |
| Missing | 663 | 203 | 270 | 188 | 2 |
| Dental visits in last 10 y | |||||
| 0 | 305 (21) | 86 (22) | 68 (17) | 124 (25) | 27 (18) |
| 1-5 | 661 (46) | 225 (57) | 168 (42) | 231 (46) | 37 (24) |
| >5 | 482 (33) | 81 (21) | 162 (41) | 151 (30) | 88 (58) |
| Missing | 1001 | 229 | 346 | 359 | 67 |
| Natural teeth | |||||
| 0 | 395 (23) | 83 (25) | 90 (16) | 180 (29) | 42 (20) |
| 1-9 | 166 (10) | 28 (8) | 46 (8) | 77 (12) | 15 (7) |
| 10-19 | 312 (18) | 64 (19) | 119 (21) | 106 (17) | 23 (11) |
| ≥20 | 864 (50) | 157 (47) | 313 (55) | 261 (42) | 133 (62) |
| Missing | 712 | 289 | 176 | 241 | 6 |
| TNM stage | |||||
| I | 458 (19) | 140 (23) | 35 (5) | 233 (27) | 50 (23) |
| II | 407 (17) | 118 (19) | 64 (9) | 178 (21) | 47 (21) |
| III | 391 (16) | 74 (12) | 134 (18) | 149 (17) | 34 (16) |
| IV | 1193 (49) | 289 (47) | 511 (69) | 305 (35) | 88 (40) |
| Treatment | |||||
| Surgery | 484 (20) | 232 (37) | 55 (7) | 110 (13) | 87 (40) |
| Surgery plus aRT | 444 (18) | 146 (24) | 97 (13) | 162 (19) | 39 (18) |
| Surgery plus CRT | 307 (13) | 89 (14) | 135 (18) | 60 (7) | 23 (11) |
| Surgery plus chemotherapy | 9 (0) | 2 (0) | 1 (0) | 6 (1) | 0 (0) |
| Chemo only | 82 (3) | 25 (4) | 25 (3) | 30 (3) | 2 (1) |
| Radiation only | 366 (15) | 24 (4) | 72 (10) | 251 (29) | 19 (9) |
| CRT, no surgery | 656 (27) | 69 (11) | 329 (44) | 213 (25) | 45 (21) |
| No treatment | 101 (4) | 34 (5) | 30 (4) | 33 (4) | 4 (2) |
Brazilian: nationality, due lack of race/ethnicity information for the Sao Paulo study. aRT = adjuvant radiotherapy; Col = column; CRT = chemoradiotherapy; NOS = not otherwise specified.
Others: American Indian or Alaska Native.
Most (35%) tumors were hypopharyngeal and/or laryngeal, followed by oropharyngeal (30%) and oral (25%). Approximately two-thirds (65%) of patient cases were late-stage (III and IV), and 51% of reported treatment modalities were surgery based (ie, surgery alone, surgery plus adjuvant radiotherapy, surgery plus chemoradiotherapy), and 45% were chemo- and/or radiotherapy based (ie, chemotherapy alone, radiotherapy alone, chemoradiotherapy). Surgery-based regimes were reported for 75% of oral, 39% of hypopharyngeal or laryngeal, and 38% of oropharyngeal squamous cell carcinoma patients, and most included adjuvant treatment (ie, chemotherapy, radiotherapy, or both) (Supplementary Tables 1 and 2, available online).
In terms of oral health, most patients had more than 20 natural teeth, brushed less than once daily, used mouthwash, and visited their dentist 1-5 times over the past decade (Table 2). Among patients with available data, gingival bleeding was reported by approximately one-third of patients. The number of natural teeth was associated with tumor location; patients diagnosed with oral, hypopharyngeal, and laryngeal squamous cell carcinoma had fewer natural teeth than those with tumors in other sites. Smoking status was also statistically significantly associated with the number of natural teeth; reports of at least 20 natural teeth were 44% among current and 50% among former smokers, compared with 65% among nonsmokers.
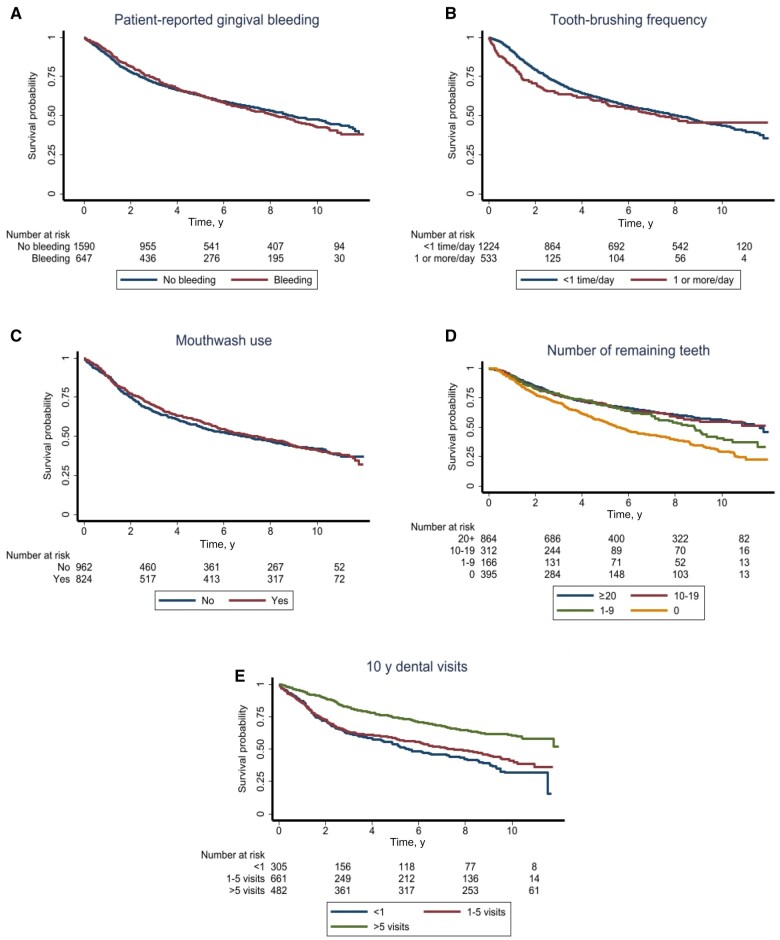
Comparisons of crude survival curves between strata of oral health measures revealed statistically significant associations with edentulism, maintenance of natural dentition, tooth brushing, and dental visits (Figure 2, A-E). For example, a survival benefit was found among participants who reported more than 5 dental visits during the past 10 years (5-year overall survival = 74% and 10-year overall survival = 60%) compared with those with no dental visits (5-year overall survival = 54% and 10-year overall survival = 32%) (Figure 2, D). Of note, dental visits during the past 10 years prior to diagnosis were also associated with early vs late-stage diagnosis, with the percentage of early stage patient cases diagnosed increasing from patients reporting no dental visits (21%) to patients reporting 1-5 dental visits (37%) and patients reporting more than 5 dental visits (42%) (P < .0005). When stratifying by site, these differences persisted only among patients with oral or hypopharyngeal and/or laryngeal head and neck squamous cell carcinoma (Table 3). Moreover, having no natural remaining teeth was associated with 15% lower 5-year overall survival compared with at least 20 natural teeth (Figure 2, E). Smaller survival differences (ie, <5%) were found for patient reported gingival bleeding, tooth brushing, and mouthwash use.
Figure 2.
Kaplan–Meier curves for self-reported gingival bleeding (A), tooth brushing (B), mouthwash use (C), number of natural teeth (D), and number of dental visits during the past 10 years (E).
Table 3.
Associations between dental visits during the past 10 years and early (stages I and II) vs late (stages III and IV) head and neck squamous cell carcinoma diagnosisa
| Dental visits during the past 10 years |
|||||||
|---|---|---|---|---|---|---|---|
| <1 |
1-5 |
>5 |
|||||
| Site | Early stage (row %) | Late stage (row %) | Early stage (row %) | Late stage (row %) | Early stage (row %) | Late stage (row %) | P |
| All sites | 101 (7) | 204 (14) | 173 (12) | 488 (34) | 198 (14) | 284 (20) | <.0005 |
| Oral cavity | 28 (7) | 58 (15) | 56 (14) | 169 (43) | 45 (11) | 36 (9) | <.0005 |
| Oropharynx | 12 (3) | 56 (14) | 19 (5) | 149 (37) | 18 (5) | 144 (36) | .339 |
| Hypopharynx and/or Larynx | 50 (10) | 74 (15) | 84 (17) | 147 (29) | 87 (17) | 64 (13) | <.0005 |
| Head and neck squamous cell carcinoma NOS | 11 (7) | 16 (11) | 14 (9) | 23 (15) | 48 (32) | 40 (26) | .164 |
NOS = not otherwise specified.
Frequent dental visits and presence of natural teeth were associated with better survival in fully adjusted multivariable analyses (Table 4). Specifically, remaining natural teeth were associated with improved survival compared with no natural teeth (eg, RR = 0.81, 95% CI = 0.69 to 0.95). Dental visits were also associated with better survival (eg, >5 visits during the last 10 years compared with none: RR = 0.77, 95% CI = 0.66 to 0.91). These associations persisted after excluding edentulous patients. Associations between natural teeth and survival were more pronounced among hypopharyngeal/laryngeal (RR = 0.75, 95% CI = 0.59 to 0.96) and NOS squamous cell carcinoma patient cases (RR = 0.66, 95% CI = 0.46 to 0.94), whereas those for dental visits were more pronounced among oropharyngeal and NOS squamous cell carcinoma (Table 4). Patient-reported gingival bleeding, tooth brushing, and mouthwash use did not show any important associations. Patients located in South America or Europe had statistically significantly decreased risk of dying compared with patients located in North America, while associations between oral health and survival were mostly similar (Supplementary Table 3, available online).
Table 4.
Association of measures of oral health measures with overall survival estimated with a generalized linear regression model using log-link function and Poisson family regression in head and neck squamous cell carcinoma patients from the International Head and Neck Cancer Epidemiology Consortium studies
| All sitesa | Oral cavityb | Oropharynxb | Hypopharynx and/or Larynxb | Head and neck squamous cell carcinoma NOSb | |
|---|---|---|---|---|---|
| Risk ratio (95% CI) |
Risk ratio (95% CI) |
Risk ratio (95% CI) |
Risk ratio (95% CI) |
Risk ratio (95% CI) |
|
| Patient reported gingival bleeding | |||||
| No | Referent | Referent | Referent | Referent | Referent |
| Yes | 1.04 (0.95 to 1.14) | 0.96 (0.78 to 1.18) | 1.04 (0.85 to 1.28) | 1.04 (0.90 to 1.20) | 1.13 (0.89 to 1.45) |
| Tooth brushing | |||||
| <1 time daily | Referent | Referent | Referent | Referent | Referent |
| ≥1 time(s) daily | 0.90 (0.74 to 1.09) | 0.88 (0.60 to 1.28) | 1.05 (0.76 to 1.47) | 0.80 (0.56 to 1.14) | 0.88 (0.45 to 1.71) |
| Mouthwash | |||||
| No | Referent | Referent | Referent | Referent | Referent |
| Yes | 1.07 (0.98 to 1.18) | 1.09 (0.87 to 1.36) | 1.26 (1.01 to 1.57) | 1.06 (0.91 to 1.22) | 0.93 (0.76 to 1.15) |
| Natural teeth | |||||
| 0 | Referent | Referent | Referent | Referent | Referent |
| 1-9 | 0.90 (0.76 to 1.06) | 0.95 (0.57 to 1.61) | 0.83 (0.54 to 1.27) | 0.82 (0.65 to 1.03) | 1.29 (0.94 to 1.76) |
| 10-19 | 0.81 (0.69 to 0.95) | 0.93 (0.66 to 1.32) | 0.80 (0.57 to 1.11) | 0.75 (0.59 to 0.96) | 0.66 (0.46 to 0.94) |
| ≥20 | 0.88 (0.78 to 0.99) | 1.11 (0.85 to 1.43) | 0.83 (0.64 to 1.08) | 0.87 (0.73 to 1.04) | 0.66 (0.50 to 0.88) |
| Dental visits during the last 10 y | |||||
| 0 | Referent | Referent | Referent | Referent | Referent |
| 1-5 | 0.95 (0.82 to 1.09) | 1.10 (0.82 to 1.48) | 0.77 (0.57 to 1.03) | 1.01 (0.81 to 1.25) | 0.71 (0.46 to 1.10) |
| >5 | 0.77 (0.66 to 0.91) | 1.15 (0.78 to 1.68) | 0.67 (0.49 to 0.93) | 0.82 (0.64 to 1.05) | 0.57 (0.38 to 0.85) |
All sites column is adjusted for age, sex, race, geographic region, tumor site, TNM stage, treatment, education, and smoking. CI = confidence intervals; NOS = not otherwise specified; TNM = tumor-node-metastasis.
Oral cavity, Oropharynx, Hypopharynx and/or Larynx, and head and neck squamous cell carcinoma NOS columns are stratified by tumor site and adjusted for age, sex, race, TNM stage, treatment, education, and smoking.
Discussion
In this report, we present the results of a comprehensive analysis of the largest cohort of head and neck squamous cell carcinoma patients used to investigate the impact of oral health on survival. We found that head and neck squamous cell carcinoma patients with more than 10 natural teeth had better survival compared with those with no teeth, while those with a history of more than 5 dental visits during the past 10 years had better survival compared with those with no dental visits. These associations persisted after adjustment for potential confounders, including age, sex, race, geographic region, tumor site, smoking status, and education level with or without additional adjustments for TNM stage and treatment modality. These results identify 2 important measures of oral health—natural dentition and dental visits—as independent prognostic factors in head and neck squamous cell carcinoma. The important benefits of dental visits are further illustrated by the finding of an association between frequent dental visits and early stage head and neck squamous cell carcinoma diagnosis. Collectively, these results identify a previously overlooked role of oral health in the survival of head and neck cancer patients.
Although oral health and oral health proxies have been previously identified as risk factors in the pooled cohort of INHANCE Consortium patients (12), data regarding the role of oral health measures in head and neck squamous cell carcinoma patient survival are limited. Farquhar et al. (24) analyzed data from the CHANCE study (n = 1381 patient cases, also included in this pooled analysis) and identified strong associations between dental visits (>10 over the past decade) and overall survival (HR = 0.63, 95% CI = 0.46 to 0.89), with the association being more pronounced among patients with oral squamous cell carcinoma (HR = 0.40, 95% CI = 0.17 to 0.93). Notably, factors associated with an elevated risk, including periodontal disease, tooth mobility, and no tooth brushing were not associated with survival in this cohort. This, combined with the more pronounced effect of dental visits among patients with oral squamous cell carcinoma, indicates that dental visits are associated with survival in the context of their role as an oral health proxy and not as a health-maintenance proxy. This was further supported by the fact that this study evaluated other routine screening exams (ie, physical exams, eye exam, and colonoscopies), none of which was associated with survival. Chang et al. (23) analyzed a cohort of 740 head and neck squamous cell carcinoma patients and identified an association between a binary variable of regular dental visits and a poor oral hygiene score, defined as brushing less than twice a day, no use of dental floss, and no regular dental visits to be associated with survival. Contrary, a study of 263 head and neck squamous cell carcinoma cases by Friemel et al. (25) failed to find an association between oral hygiene score or mouthwash use and overall, disease-specific, or progression-free survival.
The small sample sizes of previous studies, the limited number of oral health measures examined, and the inconsistent associations among different studies have limited the ability to draw firm conclusions about the influence of oral health on patient survival. This is also reflected in the recent guidelines of the European Society of Medical Oncology–European Head and Neck Society–European Society for Radiotherapy and Oncology published in 2020 (30), which recommend dental screening only in the context of staging and/or prevention of radiotherapy-related adverse oral health outcomes [eg, osteonecrosis (31)]. On the other hand, the American Society for Clinical Oncology 2017 guidelines define dental care as the “diagnosis and treatment of dental caries, periodontal disease, and other intraoral conditions” and endorse this practice along with smoking cessation for patients diagnosed with head and neck squamous cell carcinoma in the context of health promotion (32). The findings of our study support this American Society for Clinical Oncology guideline by highlighting the importance of dental care, not only for the prevention of treatment-related adverse outcomes and improved quality of life but also for tertiary prevention of head and neck squamous cell carcinoma, and thus aiming to improve patient survival and ameliorate disease impact. Although the American Dental Association is endorsing screening for oral cancer and premalignant lesions on all patients (33), the current US Preventive Service Task Force recommendation regarding oral cancer screening states that the data on dental visits are insufficient to endorse it as a secondary or tertiary preventive measure (34). However, our results, and especially the associations between frequency of dental visits prior to diagnosis and early stage diagnosis with improved survival, indicate that oral examination may provide early disease detection and thus improve survival. Recent evidence from a population-representative study (35) further supports the links between oral health and cancer mortality, wherein each 10 missing permanent teeth were associated with 19% higher risk for cancer mortality among US adults.
Potential explanations for the associations between oral health and overall survival include either a mechanistic link between oral health and head and neck squamous cell carcinoma or an indirect association where oral health is a proxy for overall wellness (eg, adherence to follow-up, more frequent screening appointments). The retrospective nature of this study prohibits it from answering this, but accumulating evidence supports a link between inflammation and cancer (36). Our results also indicate an association of geographic region with survival, with patients living in South America or Europe having better survival than patients living in North America, after adjusting for other demographic, clinicopathological, treatment, and oral health variables. These associations could be attributed to several factors including racial differences, different proportions of patients from rural vs urban vs metropolitan context (37), access to health-care differences (38), different proportion of HPV-positive head and neck squamous cell carcinoma patient cases (39), and different times that each study took place (the North American cohort was established much earlier than some of the European [UK5000] and all South American cohorts).
Although our study has several strengths, there are some limitations. Not all INHANCE studies included information on all oral health measures, and definitions and measurements of these oral health parameters frequently varied. We addressed this by opting for the definition that allowed the inclusion of the largest number of patient cases. Also, most patients were missing posttreatment oral hygiene data, and thus our estimates could not be adjusted for this. In addition, alcohol consumption was available for only a small subset of the entire cohort, and thus, the variable was excluded from further analysis. The retrospective nature of all studies introduces the possibilities of recall bias and risk of exposure misclassification for some participants. However, given that any association between oral health and head and neck squamous cell carcinoma survival was probably not widely known among study participants, any potential recall bias should be minimal. Also, the evaluation of periodontal tissue status was not done clinically but rather was based on patient-reported bleeding in most studies. The absence of gingival bleeding when evaluated with a periodontal probe by a trained dentist is an indicator with very high (98%) negative predictive value for periodontal health. Although there are no data to compare patient-reported bleeding and bleeding on probing, it is reasonable to assume that a reduction on sensitivity with the former. HPV status and p16 expression were not available for most of the studies included and thus were not part of the analysis. However, most oropharyngeal squamous cell carcinoma are HPV positive, therefore it is possible that associations detected between measures of oral health and oropharyngeal squamous cell carcinoma could also be associated with HPV status. To address this limitation and avoid the potential confounding effect of not adjusting for HPV status, we performed supplementary analyses after excluding all oropharyngeal patient cases. The effect magnitude and precision were similar between analytical samples including and excluding oropharyngeal cases. Residual confounding by smoking and socioeconomic status, which is represented by educational level, remains a possibility. Also, the lack of performance status, comorbidity, and cancer treatment adherence variables could confound the results.
In conclusion, we present an analysis of the largest cohort of head and neck squamous cell carcinoma patients with oral health measures, wherein we identify strong associations between retention of natural teeth and dental visits with better survival. These results emphasize the role of oral health maintenance not only to avoid treatment-related adverse outcomes like osteoradionecrosis but also as a potentially independent prognostic parameter for head and neck squamous cell carcinoma patients. Additional prospective studies are needed to replicate and extend our findings and help elucidate mechanistic pathways at play.
Supplementary Material
Acknowledgements
The authors would like to thank all patients who participated in the INHANCE Consortium contributing studies. None of the funding sources listed in the Funding section were involved in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Contributor Information
Jason Tasoulas, Lineberger Comprehensive Cancer Center, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Department of Otolaryngology/Head and Neck Surgery, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Division of Oral and Craniofacial Health Sciences, Adams School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Douglas R Farquhar, Department of Otolaryngology/Head and Neck Surgery, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Siddharth Sheth, Division of Hematology/Oncology, Department of Medicine, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Trevor Hackman, Department of Otolaryngology/Head and Neck Surgery, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Wendell G Yarbrough, Lineberger Comprehensive Cancer Center, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Department of Otolaryngology/Head and Neck Surgery, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Department of Pathology and Laboratory Medicine, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Chris B Agala, Department of Surgery, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Alzina Koric, Division of Public Health, Department of Family and Preventive Medicine and Huntsman Cancer Institute, University of Utah School of Medicine, Salt Lake City, UT, USA.
Luca Giraldi, Section of Hygiene, University Department of Life Sciences and Public Health, Università Cattolica del Sacro Cuore, Rome, Italy.
Eleonora Fabianova, Regional Authority of Public Health, Banska Bystrica, Slovakia.
Jolanta Lissowska, Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland.
Beata Świątkowska, Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland.
Marta Vilensky, Institute of Oncology Angel H. Roffo, University of Buenos Aires, Buenos Aires, Argentina.
Victor Wünsch-Filho, Epidemiology Department, School of Public Health, University of São Paulo, São Paulo, Brazil; Oncocentro Foundation of São Paulo, São Paulo, Brazil.
Marcos Brasilino de Carvalho, Department of Head and Neck, Heliopolis Hospital, São Paulo, Brazil.
Rossana Verónica Mendoza López, Cancer Institute of the State of São Paulo (ICESP), São Paulo, Brazil.
Ivana Holcátová, Institute of Hygiene and Epidemiology, Charles University in Prague, Prague, Czech Republic.
Diego Serraino, Unit of Cancer Epidemiology, Centro di Riferimento Oncologico di Aviano (CRO) IRCCS, Aviano, Italy.
Jerry Polesel, Unit of Cancer Epidemiology, Centro di Riferimento Oncologico di Aviano (CRO) IRCCS, Aviano, Italy.
Cristina Canova, University of Padua, Padova, Italy.
Lorenzo Richiardi, Department of Medical Sciences, University of Turin, Turin, Italy.
Jose P Zevallos, Department of Otolaryngology/Head and Neck Surgery, University of Pittsburgh, PA, USA.
Andy Ness, Bristol Dental School, University of Bristol, Bristol, UK.
Miranda Pring, Bristol Dental School, University of Bristol, Bristol, UK.
Steve J Thomas, Bristol Dental School, University of Bristol, Bristol, UK.
Tom Dudding, Bristol Dental School, University of Bristol, Bristol, UK.
Yuan-Chin Amy Lee, Division of Public Health, Department of Family and Preventive Medicine and Huntsman Cancer Institute, University of Utah School of Medicine, Salt Lake City, UT, USA.
Mia Hashibe, Division of Public Health, Department of Family and Preventive Medicine and Huntsman Cancer Institute, University of Utah School of Medicine, Salt Lake City, UT, USA.
Paolo Boffetta, Stony Brook Cancer Center, Department of Family, Population and Preventive Medicine, Stony Brook University, Stony Brook, NY, USA; Department of Medical and Surgical Sciences, University of Bologna, Italy.
Andrew F Olshan, Department of Otolaryngology/Head and Neck Surgery, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Department of Epidemiology, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Kimon Divaris, Department of Epidemiology, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Division of Pediatric and Public Health, Adams School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Antonio L Amelio, Lineberger Comprehensive Cancer Center, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Department of Otolaryngology/Head and Neck Surgery, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Division of Oral and Craniofacial Health Sciences, Adams School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Department of Tumor Biology, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, USA; Department of Head and Neck-Endocrine Oncology, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, USA.
Data availability
Description of the studies in the INHANCE Consortium can be found in the consortium webpage (https://medicine.utah.edu/dfpm/inhance/members/studies). Data are available from the corresponding author upon reasonable request for scientific purposes, with the permission of the INHANCE Consortium.
Author contributions
Jason Tasoulas, MD, DMD (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing—original draft; Writing—review & editing), Andrew F Olshan, PhD (Conceptualization; Data curation; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing—original draft; Writing—review & editing), Paolo Boffetta, MD, MPH (Data curation; Methodology; Project administration; Resources; Validation; Writing—review & editing), Mia Hashibe, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Yuan-Chin Lee, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Tom Dudding, BDS, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Steve Thomas, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Miranda Pring, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Andy Ness, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Jose P Zevallos, MD, MPH (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Lorenzo Richiardi, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Cristina Canova, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Jerry Polesel, MD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Diego Serraino, MD (Methodology; Project administration; Resources; Writing—review & editing), Kimon Divaris, DDS, PhD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing—original draft), Ivana Holcátová, MD, PhD (Methodology; Project administration; Resources; Writing—review & editing), Marcos Brasilino de Carvalho, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Victor Wünsch-Filho, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Marta Vilensky, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Beata Świątkowska, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Jolanta Lissowska, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Eleonora Fabianova, PhD, MPH (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Luca Giraldi, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Alzina Koric, PhD (Data curation; Methodology; Project administration; Resources; Writing—review & editing), Chris Agala, PhD (Formal analysis; Methodology; Writing—review & editing), Wendell Yarbrough, MD (Funding acquisition; Resources; Supervision; Writing—review & editing), Trevor Hackman, MD (Funding acquisition; Resources; Supervision; Writing—review & editing), Siddharth Sheth, DO, MPH (Formal analysis; Methodology; Writing—review & editing), Doug Farquhar, MD (Formal analysis; Methodology; Validation; Writing—review & editing), Rossana Verónica Mendoza López, PhD (Investigation; Methodology; Project administration; Writing—review & editing), and Antonio L Amelio, PhD (Conceptualization; Funding acquisition; Project administration; Resources; Supervision; Writing—original draft; Writing—review & editing)
Funding
The INHANCE Consortium was supported by NIH grants NCI R03CA113157 and NIDCR R03DE016611. Studies participating in the pooled analysis were supported by: Latin America: Fondo para la Investigacion Cientifica y Tecnologica (FONCYT) Argentina, IMIM (Barcelona), Fundação de Amparo à Pesquisa do Estado de Sao Paulo (FAPESP) (No. 01/01768-2), and European Commission (IC18-CT97-0222). North Carolina (1994 to 1997): NIH (R01CA061188), and in part by a grant from the National Institute of Environmental Health Sciences (P30ES010126). Central Europe study: World Cancer Research Fund and the European Commission INCO-COPERNICUS Program (Contract No. IC15-CT98-0332). The Sao Paulo & Sao Paulo 2 studies were supported by the Sao Paulo Research Foundation—FAPESP (GENCAPO 04/12054-9, 10/51168-0. The Western Europe study was supported by European Community (5th Framework Programme) (grant no QLK1-CT-2001-00182). The HN5000 study was funded by the National Institute for Health Research (NIHR) under its Program Grants for Applied Research scheme (RP-PG-0707-10034). This work was also supported in part by the NCI Center Core Support Grant 5P30CA016080-42 to the UNC Lineberger Comprehensive Cancer Center, University Cancer Research Fund (UCRF; to AL Amelio), a UNC Lineberger Tier 3 Developmental Award (to A Olshan and AL Amelio), and NIH/NIDCR R01DE030123 (to AL Amelio), and by monies from the State of Florida to the H. Lee Moffitt Cancer Center & Research Institute.
Conflicts of interest
ALA is a Global Advisory Board member and paid consultant for LG Chem Life Sciences Innovation Center. SS receives honoraria from Naveris, Medscape; Speakers’ Bure: Exelixis; research funding from AstraZeneca/MedImmune, Merck, Inovio Pharmaceuticals; Exelixis; Regeneron; WGY is a consultant for Olympus Medical Systems. All other authors declare no potential conflicts of interest.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 2. Patterson RH, Fischman VG, Wasserman I, et al. Global burden of head and neck cancer: economic consequences, health, and the role of surgery. Otolaryngol Head Neck Surg. 2020;162(3):296-303. [DOI] [PubMed] [Google Scholar]
- 3. Wyss A, Hashibe M, Chuang SC, et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Am J Epidemiol. 2013;178(5):679-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di Credico G, Polesel J, Dal Maso L, et al. Alcohol drinking and head and neck cancer risk: the joint effect of intensity and duration. Br J Cancer. 2020;123(9):1456-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hobbs CG, Sterne JA, Bailey M, et al. Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol. 2006;31(4):259-266. [DOI] [PubMed] [Google Scholar]
- 6. Stanford-Moore G, Bradshaw PT, Weissler MC, et al. Interaction between known risk factors for head and neck cancer and socioeconomic status: the Carolina Head and Neck Cancer Study. Cancer Causes Control. 2018;29(9):863-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conway DI, McKinney PA, McMahon AD, et al. Socioeconomic factors associated with risk of upper aerodigestive tract cancer in Europe. Eur J Cancer. 2010;46(3):588-598. [DOI] [PubMed] [Google Scholar]
- 8. O’Rorke MA, Ellison MV, Murray LJ, et al. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48(12):1191-1201. [DOI] [PubMed] [Google Scholar]
- 9. Osazuwa-Peters N, Adjei Boakye E, Chen BY, et al. Association between head and neck squamous cell carcinoma survival, smoking at diagnosis, and marital status. JAMA Otolaryngol Head Neck Surg. 2018;144(1):43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giraldi L, Leoncini E, Pastorino R, et al. Alcohol and cigarette consumption predict mortality in patients with head and neck cancer: a pooled analysis within the International Head and Neck Cancer Epidemiology (INHANCE) Consortium. Ann Oncol. 2017;28(11):2843-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D’Souza G, Anantharaman D, Gheit T, et al. Effect of HPV on head and neck cancer patient survival, by region and tumor site: a comparison of 1362 cases across three continents. Oral Oncol. 2016;62:20-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hashim D, Sartori S, Brennan P, et al. The role of oral hygiene in head and neck cancer: results from International Head and Neck Cancer Epidemiology (INHANCE) consortium. Ann Oncol. 2016;27(8):1619-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Divaris K, Olshan AF, Smith J, et al. Oral health and risk for head and neck squamous cell carcinoma: the Carolina Head and Neck Cancer Study. Cancer. Causes & Control. 2010;21(4):567-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perry BJ, Zammit AP, Lewandowski AW, et al. Sites of origin of oral cavity cancer in nonsmokers vs smokers: possible evidence of dental trauma carcinogenesis and its importance compared with human papillomavirus. JAMA Otolaryngol Head Neck Surg. 2015;141(1):5-11. [DOI] [PubMed] [Google Scholar]
- 15. Feller L, Altini M, Lemmer J.. Inflammation in the context of oral cancer. Oral Oncol. 2013;49(9):887-892. [DOI] [PubMed] [Google Scholar]
- 16. Hayes RB, Ahn J, Fan X, et al. Association of oral microbiome with risk for incident head and neck squamous cell cancer. JAMA Oncol. 2018;4(3):358-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sczepanik FSC, Grossi ML, Casati M, et al. Periodontitis is an inflammatory disease of oxidative stress: we should treat it that way. Periodontol 2000. 2020;84(1):45-68. [DOI] [PubMed] [Google Scholar]
- 18. Canli O, Nicolas AM, Gupta J, et al. Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell. 2017;32(6):869-883 e5. [DOI] [PubMed] [Google Scholar]
- 19. Al-Hebshi NN, Nasher AT, Maryoud MY, et al. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci Rep. 2017;7(1):1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao H, Chu M, Huang Z, et al. Variations in oral microbiota associated with oral cancer. Sci Rep. 2017;7(1):11773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hashimoto K, Shimizu D, Ueda S, et al. Feasibility of oral microbiome profiles associated with oral squamous cell carcinoma. J Oral Microbiol. 2022;14(1):2105574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michikawa C, Gopalakrishnan V, Harrandah AM, et al. Fusobacterium is enriched in oral cancer and promotes induction of programmed death-ligand 1 (PD-L1). Neoplasia. 2022;31:100813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chang CC, Lee WT, Hsiao JR, et al. Oral hygiene and the overall survival of head and neck cancer patients. Cancer Med. 2019;8(4):1854-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farquhar DR, Divaris K, Mazul AL, et al. Poor oral health affects survival in head and neck cancer. Oral Oncol. 2017;73:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friemel J, Foraita R, Gunther K, et al. Pretreatment oral hygiene habits and survival of head and neck squamous cell carcinoma (HNSCC) patients. BMC Oral Health. 2016;16:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conway DI, Hashibe M, Boffetta P, et al. ; for the INHANCE Consortium. Enhancing epidemiologic research on head and neck cancer: INHANCE–the international head and neck cancer epidemiology consortium. Oral Oncol. 2009;45(9):743-746. [DOI] [PubMed] [Google Scholar]
- 27. Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99(10):777-789. [DOI] [PubMed] [Google Scholar]
- 28. Toporcov TN, Znaor A, Zhang ZF, et al. Risk factors for head and neck cancer in young adults: a pooled analysis in the INHANCE consortium. Int J Epidemiol. 2015;44(1):169-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ness AR, Waylen A, Hurley K, et al. ; for the Head and Neck 5000 Study Team. Establishing a large prospective clinical cohort in people with head and neck cancer as a biomedical resource: head and neck 5000. BMC Cancer. 2014;14:973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Machiels JP, Rene Leemans C, Golusinski W, et al. ; for the ESTRO Executive Board. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1462-1475. [DOI] [PubMed] [Google Scholar]
- 31. Owosho AA, Kadempour A, Yom SK, et al. Radiographic osteoradionecrosis of the jaw with intact mucosa: proposal of clinical guidelines for early identification of this condition. Oral Oncol. 2015;51(12):e93-6-e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nekhlyudov L, Lacchetti C, Davis NB, et al. Head and neck cancer survivorship care guideline: American Society of Clinical Oncology clinical practice guideline endorsement of the American Cancer Society guideline. J Clin Oncol. 2017;35(14):1606-1621. [DOI] [PubMed] [Google Scholar]
- 33. Lingen MW, Abt E, Agrawal N, et al. Evidence-based clinical practice guideline for the evaluation of potentially malignant disorders in the oral cavity: a report of the American Dental Association. J Am Dent Assoc. 2017;148(10):712-727 e10. [DOI] [PubMed] [Google Scholar]
- 34. Moyer VA; for the U.S. Preventive Services Task Force. Screening for oral cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(1):55-60. [DOI] [PubMed] [Google Scholar]
- 35. Liu J, Zong X, Vogtmann E, et al. Tooth count, untreated caries and mortality in US adults: a population-based cohort study. Int J Epidemiol. 2022;51(4):1291-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greten FR, Grivennikov SI.. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clarke JA, Despotis AM, Ramirez RJ, et al. Head and neck cancer survival disparities by race and rural-urban context. Cancer Epidemiol Biomarkers Prev. 2020;29(10):1955-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kwok J, Langevin SM, Argiris A, et al. The impact of health insurance status on the survival of patients with head and neck cancer. Cancer. 2010;116(2):476-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Louie KS, Mehanna H, Sasieni P.. Trends in head and neck cancers in England from 1995 to 2011 and projections up to 2025. Oral Oncol. 2015;51(4):341-348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Description of the studies in the INHANCE Consortium can be found in the consortium webpage (https://medicine.utah.edu/dfpm/inhance/members/studies). Data are available from the corresponding author upon reasonable request for scientific purposes, with the permission of the INHANCE Consortium.