Abstract
Background
Anal intraepithelial neoplasia grade III is a precursor to squamous cell carcinoma of the anus for which rates are nearly 20-fold higher in people with HIV than in the general population in the United States. We describe trends in anal intraepithelial neoplasia grade III diagnosis and risk of squamous cell carcinoma of the anus following anal intraepithelial neoplasia grade III by HIV status and sex.
Methods
We used data from a population-based linkage between cancer and HIV registries in 11 US states; Puerto Rico; and Washington, DC, during 1996-2019. We identified all individuals with a diagnosis of anal intraepithelial neoplasia grade III and determined their HIV status. We estimated the average annual percentage change of anal intraepithelial neoplasia grade III using Poisson regression stratified by HIV status and sex. We estimated the 5-year cumulative incidence of squamous cell carcinoma of the anus following an anal intraepithelial neoplasia grade III diagnosis stratified by sex, HIV status, and prior AIDS diagnosis.
Results
Among people with HIV, average annual percentage changes for anal intraepithelial neoplasia grade III were 15% (95% confidence interval [CI] = 12% to 17%) per year among females and 12% (95% CI = 11% to 14%) among males. Average annual percentage changes for those without HIV were 8% (95% CI = 7% to 8%) for females and 8% (95% CI = 6% to 9%) for males. Among people with HIV, a prior AIDS diagnosis was associated with a 2.7-fold (95% CI = 2.23 to 3.40) and 1.9-fold (95% CI = 1.72 to 2.02) increased risk of anal intraepithelial neoplasia grade III diagnosis for females and males, respectively. Five-year cumulative incidence of squamous cell carcinoma of the anus following anal intraepithelial neoplasia grade III for people with HIV with a prior AIDS diagnosis were 3.4% and 3.7% for females and males, respectively.
Conclusions
Rates of anal intraepithelial neoplasia grade III diagnoses have increased since 1996, particularly for people with HIV, likely influenced by increased screening. A prior AIDS diagnosis was strongly associated with risk of anal intraepithelial neoplasia grade III diagnosis.
Squamous cell carcinoma of the anus is predominantly caused by infection with high-risk human papillomavirus. Though anal cancer is relatively rare in the United States, incidence and mortality rates increased 2.7% and 3.1% per year during 2001-2015, respectively (1). Rates of anal cancer are elevated in immunosuppressed populations, particularly among people with HIV, where rates are nearly 20-fold higher compared with the US general population (2). Males with HIV constituted 35% of squamous cell carcinoma of the anus diagnoses among all males, while females with HIV constituted 2% of squamous cell carcinoma of the anus diagnoses among females in the United States during 2013-2015 (3). Among people with HIV, anal cancer rates are notably higher among men who have sex with men (MSM), and risk increases with age and a history of AIDS diagnosis (2). Low CD4 cell counts and high HIV RNA levels are also associated with anal cancer risk in this population (4).
Anal intraepithelial neoplasia grade III, also known as severe dysplasia of the anus, is a precursor of squamous cell carcinoma of the anus and, along with p16-positive anal intraepithelial neoplasia grade II, constitutes high-grade squamous intraepithelial lesions (HSIL). Although the majority of anal intraepithelial neoplasia grade III does not progress to squamous cell carcinoma of the anus and some regress completely, an estimated 1%-5% will progress to cancer within 5 years of detection (5-7). The vast majority of anal intraepithelial neoplasia grade III are asymptomatic and therefore require screening for detection and diagnostic confirmation, which varies substantially by region and HIV status. The gold standard approach for detecting anal intraepithelial neoplasia grade III is high-resolution anoscopy and high-resolution anoscopy–guided biopsy (8-10). In some settings, anal cytology is used as an initial screening test to identify patients who are at risk for anal precancer and require high-resolution anoscopy referral (9,11,12). Importantly, recent results from the Anal Cancer–HSIL Outcomes Research (ANCHOR) trial have shown that among people with HIV, treatment of HSIL can reduce the 4-year cumulative incidence of anal cancer by 57% (13).
Because of the rarity of anal cancer, screening efforts are focused on groups with the highest risk for anal intraepithelial neoplasia grade III and anal cancer, such as people with HIV and those who engage in receptive anal intercourse (14). However, consensus recommendations are lacking, and clinical practice varies widely (12). In part because of the infrequency of anal intraepithelial neoplasia grade III screening and the rarity of anal cancer in the general population, no study to date has had sufficient sample sizes to compare the risk of squamous cell carcinoma of the anus following a diagnosis of anal intraepithelial neoplasia grade III by HIV status. And finally, identifying populations at greater risk of anal cancer following anal precancer may be informative for public health strategies to prevent and detect anal cancer.
Methods
Study population and disease classifications
In this population-based registry linkage study, we used data from all participating regions in the HIV/AIDS Cancer Match study, a data linkage study of HIV and cancer registries, from 1996 through 2019. Participating regions included 11 US states (Colorado, Connecticut, Georgia, Louisiana, Massachusetts, Maryland, Michigan, New Jersey, New York, North Carolina, and Texas); Washington, DC; and Puerto Rico. We included individuals aged 20-85 years in our study population. For individuals with HIV, follow-up began at the latter of HIV report date, beginning of registry coverage, or at age 20 years and ended at the first of death, end of registry coverage, or at age 85 years.
Cancer registry data include cancer diagnosis date, tumor site, morphology, and stage. Cases of anal intraepithelial neoplasia grade III are reportable to cancer registries, meaning that they are cases that the registry is required to collect and report (15), and were identified using International Classification of Diseases for Oncology (ICD-O-3) site codes C21.0 (anus, not otherwise specified, excludes skin of anus and perianal skin), C21.1 (anal canal), C21.2 cloacogenic zone), and C21.8 (overlapping lesion in rectum, anus, and anal canal) with behavior code 2 for in situ (16). Cases of anal intraepithelial neoplasia grade III and squamous cell carcinoma of the anus were restricted to histology codes 8010-8084. To classify stage at diagnosis, we used the Surveillance, Epidemiology, and End Results (SEER) Summary Stage 2000: localized (confined to anus), regional (spread outside the anal area to nearby structures or lymph nodes), distant (spread to distant parts of the body), and unknown stage. For anal intraepithelial neoplasia grade III, we restricted to those with a diagnostic confirmation based on positive histology (131 diagnoses were based on positive cytology and excluded). HIV status of cancer cases was determined based on linkage to the HIV registries.
The HIV/AIDS Cancer Match study was approved by institutional review boards at participating registries and was exempt from review at the US National Institutes of Health. Consent of participants was not required for the use of data collected through public health surveillance.
Statistical approach
We described the patient characteristics of individuals with a diagnosis of anal intraepithelial neoplasia grade III by sex and HIV status. The number of cases of anal intraepithelial neoplasia grade III and total person-years in people with HIV were subtracted from general population estimates within sex, age, race and ethnicity, year, and registry-specific strata to estimate associations for risk of anal intraepithelial neoplasia grade III in males and females with and without HIV. We compared the incidence of anal intraepithelial neoplasia grade III in people with HIV with that in the general population using standardized incidence ratios and 95% confidence intervals (CIs). Standardized incidence ratios were derived by dividing the observed number of cases in people with HIV by the expected number based on incidence rates in the general population within strata defined by sex, age, race and ethnicity, calendar year, and registry.
We used Poisson regression to estimate the average annual percent change in anal intraepithelial neoplasia grade III rates with an offset of the natural log of person-years, adjusted for age group, race and ethnicity, and region within sex and HIV status–specific strata. We conducted additional sensitivity analyses to investigate the linearity assumption for a trend over time by visually inspecting the adjusted incidence rate ratios for each year within strata. Based on visual inspection, we compared the average annual percent changes for years 1996-2004 with those of 2005-2019 to identify potential differences in trends during those 2 periods.
We estimated 5-year cumulative incidence of squamous cell carcinoma of the anus following anal intraepithelial neoplasia grade III diagnosis stratified by HIV status using the Fine and Gray (17) cumulative incidence regression model to account for the competing risk of death. We estimated the subdistribution hazard ratios (HRs) for subsequent risk of squamous cell carcinoma of the anus following anal intraepithelial neoplasia grade III according to sex, HIV status, and AIDS diagnosis, adjusted for age at anal intraepithelial neoplasia grade III diagnosis. We used females without HIV as the reference group because they had the largest number of anal intraepithelial neoplasia grade III and we hypothesized that their risk would be lowest.
Analyses were conducted in R using the cmprsk and mstate packages (18).
Results
In more than 1 billion person-years of observation during 1996-2019, we observed 2815 and 1714 cases of anal intraepithelial neoplasia grade III in females and males, respectively, without HIV. Among people with HIV, we observed 4.5 million person-years and 774 and 3958 cases of anal intraepithelial neoplasia grade III in females and males, respectively (Table 1). For males with and without HIV, anal intraepithelial neoplasia grade III tended to be diagnosed at younger ages than in females. Among people with HIV with anal intraepithelial neoplasia grade III, 78% of females and 65% of males had a prior diagnosis of AIDS.
Table 1.
Characteristics of individuals with a diagnosis of anal intraepithelial neoplasia grade III from 1996 through 2019 according to HIV status and sex in 11 US states; Puerto Rico; and Washington, DCa
| Variable | HIV- female (n = 2815) | HIV+ female (n = 774) | HIV- male (n = 1714) | HIV+ male (n = 3958) |
|---|---|---|---|---|
| Race and ethnicity | ||||
| Hispanic | 307 (11%) | 328 (42%) | 151 (8.8%) | 1094 (28%) |
| Non-Hispanic Black | 387 (14%) | 335 (43%) | 238 (14%) | 1146 (29%) |
| Non-Hispanic White | 2030 (72%) | 68 (8.8%) | 1135 (66%) | 1377 (35%) |
| Another race or missing | 91 (3.2%) | 43 (5.6%) | 190 (11%) | 341 (8.6%) |
| Age at anal intraepithelial neoplasia grade III diagnosis, y | ||||
| 20-29 | 69 (2.5%) | 20 (2.6%) | 140 (8.2%) | 446 (11%) |
| 30-39 | 289 (10%) | 106 (14%) | 264 (15%) | 936 (24%) |
| 40-49 | 569 (20%) | 269 (35%) | 392 (23%) | 1298 (33%) |
| 50-59 | 837 (30%) | 296 (38%) | 462 (27%) | 979 (25%) |
| 60 and older | 1049 (37%) | 83 (11%) | 448 (26%) | 297 (7.5%) |
| Unknownb | 2 | 0 | 8 | 2 |
| Time since HIV diagnosis, y | ||||
| <1 | — | 5 (0.7%) | — | 307 (8.5%) |
| 1-5 | — | 39 (5.5%) | — | 680 (19%) |
| 5-10 | — | 90 (13%) | — | 742 (21%) |
| >10 | — | 570 (81%) | — | 1880 (52%) |
| Unknownb | 70 | 349 | ||
| Prior AIDS diagnosis | ||||
| No | — | 168 (21.7%) | — | 1390 (35.1%) |
| Yes | — | 606 (78.3%) | — | 2568 (64.9%) |
HIV+ = with HIV; HIV- = without HIV.
Conducted as sensitivity analyses.
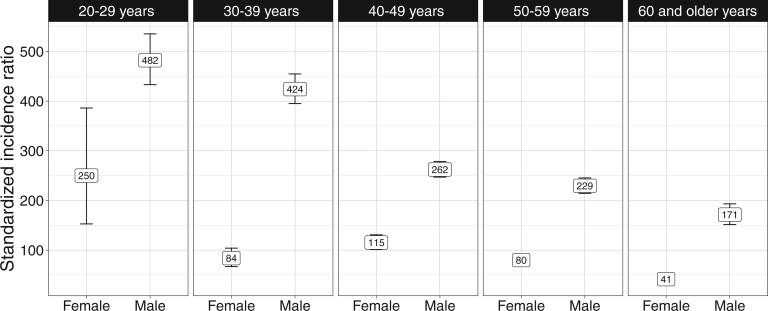
The overall standardized incidence ratios for anal intraepithelial neoplasia grade III in females and males with HIV were 82.0 (95% CI = 76.0 to 88.4) and 278.6 (95% CI = 269.2 to 288.1), respectively, and were greatest in the youngest age groups. Among individuals aged 20-29 years, standardized incidence ratios showed that anal intraepithelial neoplasia grade III rates in people with HIV were elevated 482.2-fold (95% CI = 433.1 to 535.3) in males and 250.0-fold (95% CI = 152.7 to 386.1) in females (Figure 1).
Figure 1.
Standardized incidence ratio of anal intraepithelial neoplasia grade III diagnosis in females and males with HIV compared with the general population within age group in 11 US states; Puerto Rico; and Washington, DC, 1996-2019.
Incidence rates and annual percentage changes
Based on Poisson regression estimates, we observed large average annual percentage changes for males and females with and without HIV. Rates of anal intraepithelial neoplasia grade III increased 8% (95% CI = 7% to 8%) per year in females without HIV and 15% (95% CI = 12% to 17%) per year in females with HIV during 1996-2019. For males during 1996-2019, rates of anal intraepithelial neoplasia grade III increased by 6% (95% CI = 5% to 7%) per year in males without HIV and 14% (95% CI = 13% to 14%) per year in males with HIV. We also examined trends separately for 1996-2004 and 2005-2019. We found no evidence of a difference in average annual percentage changes for females between calendar periods 1996-2004 and 2005-2019. However, in males with and without HIV during 1996-2004, there was no evidence of a trend that was statistically different from zero for males without HIV (average annual percentage change = 1%, 95% CI = -3% to 6%) or males with HIV (average annual percentage change = 1%, 95% CI = -5% to 8%). During 2005-2019, rates of anal intraepithelial neoplasia grade III increased 8% (95% CI = 6% to 9%) in males without HIV and 12% (95% CI = 11% to 14%) for males with HIV.
We present incidence rate ratios based on Poisson regression estimates, which account for the underlying demographic distributions in these populations. Among females without HIV, incidence rates of anal intraepithelial neoplasia grade III were greatest among non-Hispanic Black and non-Hispanic White females compared with Hispanic females or those with another race or ethnicity. Anal intraepithelial neoplasia grade III diagnoses also increased with age. For males without HIV, incidence rates were greatest in non-Hispanic Black males (incidence rate ratio = 1.62, 95% CI = 1.45 to 1.81 compared with non-Hispanic White males) and in males aged 40-59 years (Table 2).
Table 2.
Risk factors associated with a diagnosis of anal intraepithelial neoplasia grade III from 1996 through 2019 according to HIV status and sex in 11 US states; Puerto Rico; and Washington, DCa
| Variable | HIV- female Incidence rate ratios (95% CI) (n = 2815) |
HIV+ female Incidence rate ratios (95% CI) (n = 774) |
HIV- male Incidence rate ratios (95% CI) (n = 1714) |
HIV+ male Incidence rate ratios (95% CI) (n = 3958) |
|---|---|---|---|---|
| Race and ethnicity | ||||
| Hispanic | 0.81 (0.71 to 0.92) | 1.72 (1.29 to 2.34) | 0.88 (0.75 to 1.02) | 0.77 (0.70 to 0.84) |
| Non-Hispanic Black | 0.90 (0.80 to 1.00) | 1.24 (0.93 to 1.68) | 1.22 (1.07 to 1.38) | 0.66 (0.60 to 0.72) |
| Non-Hispanic White | Referent | Referent | Referent | Referent |
| Another race or missing | 0.20 (0.14 to 0.29) | 0.38 (0.02 to 1.74) | 0.30 (0.21 to 0.42) | 0.70 (0.49 to 0.97) |
| Age, y | ||||
| 20-29 | 0.13 (0.10 to 0.16) | 0.57 (0.35 to 0.87) | 0.36 (0.30 to 0.43) | 1.32 (1.15 to 1.51) |
| 30-39 | 0.58 (0.50 to 0.67) | 0.75 (0.57 to 0.97) | 0.68 (0.58 to 0.79) | 1.29 (1.17 to 1.42) |
| 40-49 | Referent | Referent | Referent | Referent |
| 50-59 | 1.56 (1.40 to 1.75) | 0.91 (0.76 to 1.10) | 1.07 (0.93 to 1.22) | 0.75 (0.67 to 0.81) |
| 60-85 | 1.36 (1.22 to 1.51) | 0.51 (0.38 to 0.66) | 0.86 (0.75 to 0.98) | 0.43 (0.37 to 0.50) |
| Time since HIV diagnosis, y | ||||
| <1 | Referent | 0.29 (0.07 to 0.76) | Referent | 2.05 (1.73 to 2.42) |
| 1-5 | Referent | 0.49 (0.34 to 0.69) | Referent | 1.21 (1.09 to 1.35) |
| 5-10 | Referent | 0.58 (0.45 to 0.74) | Referent | 0.94 (0.86 to 1.04) |
| >10 | Referent | Referent | Referent | Referent |
| Prior AIDS diagnosis | ||||
| No | Referent | Referent | Referent | Referent |
| Yes | Referent | 2.74 (2.23 to 3.40) | Referent | 1.86 (1.72 to 2.02) |
Poisson regression models were adjusted for calendar year, race and ethnicity, categorical age, and registry. Analyses for males and females with HIV were additionally adjusted for time since HIV diagnosis and prior AIDS diagnosis. CI = confidence interval; HIV+ = with HIV; HIV- = without HIV.
Hispanic females with HIV were 72% (incidence rate ratios = 1.72, 95% CI = 1.29 to 2.34) more likely to have an anal intraepithelial neoplasia grade III diagnosis compared with non-Hispanic White and non-Hispanic Black females with HIV (Table 2). We observed similar rates of anal intraepithelial neoplasia grade III diagnoses in females with HIV aged 50-59 years as those aged 40-49 years, whereas incidence rates were lower for age groups younger than 40 years as well as for those aged 60-85 years. For females with HIV, incidence rates of anal intraepithelial neoplasia grade III increased with increasing time since HIV diagnosis. Females with a prior diagnosis of AIDS were 2.74 (95% CI = 2.23 to 3.40) times more likely to have a diagnosis of anal intraepithelial neoplasia grade III than females with HIV but no diagnosis of AIDS.
Among males with HIV, non-Hispanic Black males, Hispanic males, and males with another race or with missing race and ethnicity were 34% (incidence rate ratio = 1.34, 95% CI = 0.60 to 0.72), 23% (incidence rate ratio = 1.23, 95% CI = 0.70 to 0.84), and 30% (incidence rate ratio = 1.3, 95% CI = 0.49 to 0.97) less likely to have received an anal intraepithelial neoplasia grade III diagnosis compared with non-Hispanic White males with HIV, respectively (Table 2). Compared with males with HIV with more than 10 years since HIV diagnosis, those with less than 1 year were over twice as likely to have an anal intraepithelial neoplasia grade III diagnosis (incidence rate ratios = 2.07, 95% CI = 1.75 to 2.43), and incidence rate ratios declined with increasing time since HIV diagnosis. Males with a prior AIDS diagnosis were 86% (incidence rate ratio, 95% CI = 1.72 to 2.02) more likely to have an anal intraepithelial neoplasia grade III diagnosis compared with those with HIV and no prior AIDS diagnosis.
Cumulative incidence of squamous cell carcinoma of the anus following anal intraepithelial neoplasia grade III diagnosis
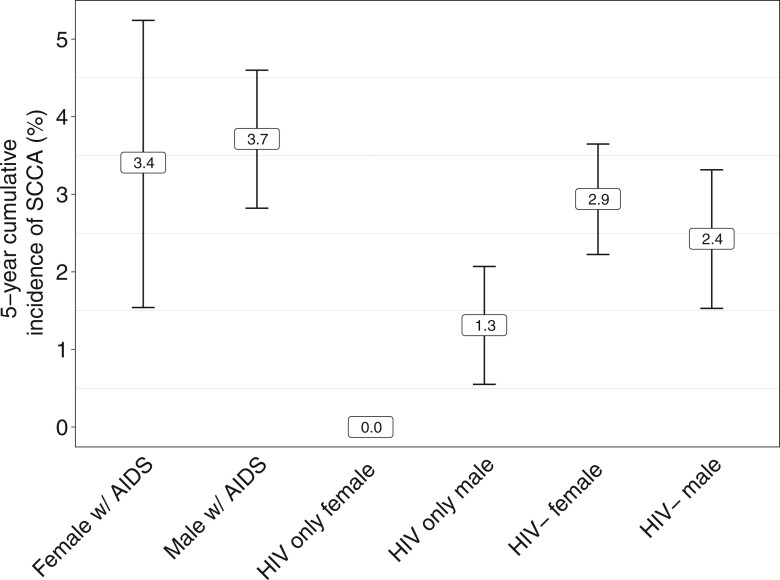
We compared the 5-year cumulative incidence of squamous cell carcinoma of the anus following anal intraepithelial neoplasia grade III diagnosis among males and females 1) without HIV, 2) with HIV and without a prior AIDS diagnosis, and 3) with HIV and a prior AIDS diagnosis. For those without HIV, the 5-year cumulative incidence following anal intraepithelial neoplasia grade III diagnosis was 2.4% (95% CI = 1.5% to 3.3%) for males and 2.9% (95% CI = 2.2% to 3.6%) for females. For those with HIV and who had not been diagnosed with AIDS prior to anal intraepithelial neoplasia grade III, the cumulative incidence was 1.3% (95% CI = 0.5% to 2.1%) in males, and no females in this group had a subsequent squamous cell carcinoma of the anus diagnosis within 5 years (95% CI = 0% to 1.6%), although 2 cases of squamous cell carcinoma of the anus occurred at 5.3 years and 9.3 years from anal intraepithelial neoplasia grade III diagnosis (Figure 2). The 5-year cumulative incidence of squamous cell carcinoma of the anus was highest in those with a prior AIDS diagnosis at 3.7% (95% CI = 2.8% to 4.6%) in males and 3.4% (95% CI = 1.5% to 5.2%) and females.
Figure 2.
Five-year cumulative incidence of squamous cell carcinoma of the anus (SCCA) following a diagnosis of anal intraepithelial neoplasia grade III among persons with AIDS diagnosis prior to anal intraepithelial neoplasia grade III, those with HIV without a prior AIDS diagnosis, and those without HIV or AIDS by sex. No observed cases were reported within strata where cumulative incidence estimates are missing. w/ = with.
Compared with those diagnosed with anal intraepithelial neoplasia grade III at ages 40-49 years, those diagnosed with anal intraepithelial neoplasia grade III at ages 60-85 years had a hazard ratio of 1.43 (95% CI = 1.02 to 2.01) for risk of squamous cell carcinoma of the anus, and those aged 20-29 years had a hazard ratio of 0.35 (95% CI = 0.14 to 0.87) (Table 3). Compared with females without HIV, males with a prior AIDS diagnosis had a greater risk of squamous cell carcinoma of the anus following anal intraepithelial neoplasia grade III diagnosis with a hazard ratio of 1.57 (95% CI = 1.15 to 2.14), while males with HIV but no AIDS diagnosis prior to anal intraepithelial neoplasia grade III had the lowest risk (HR = 0.43, 95% CI = 0.24 to 0.79).
Table 3.
Risk factors associated with a diagnosis of squamous cell carcinoma of the anus following anal intraepithelial neoplasia grade 3 in 11 US states; Puerto Rico; and Washington, DC, 1996-2019a
| Variable | Counts (%) | Events | Cox proportional hazards for risk of death (95% CI) |
P | Subdistribution HR (95% CI) (Squamous cell carcinoma of the anus competing risk regression) |
P |
|---|---|---|---|---|---|---|
| Age at anal intraepithelial neoplasia grade III diagnosis, y | ||||||
| 20-29 | 674 (7.3) | 5 | 0.49 (0.32 to 0.75) | .001 | 0.35 (0.14 to 0.87) | .024 |
| 30-39 | 1595 (17.2) | 42 | 0.94 (0.77 to 1.16) | .586 | 0.96 (0.66 to 1.40) | .830 |
| 40-49 | 2528 (27.4) | 50 | Referent | Referent | ||
| 50-59 | 2574 (27.8) | 53 | 1.21 (1.02 to 1.45) | .033 | 0.77 (0.55 to 1.10) | .150 |
| 60-85 | 1877 (20.3) | 68 | 2.98 (2.51 to 3.53) | < .001 | 1.43 (1.02 to 2.01) | .036 |
| AIDS, HIV status, and sex | ||||||
| Female without HIV | 2813 (30.4) | 84 | Referent | Referent | ||
| Female HIV only | 182 (2.0) | 2 | 0.72 (0.37 to 1.39) | .323 | 0.53 (0.13 to 2.17) | .380 |
| Female with AIDS | 592 (6.4) | 19 | 1.40 (1.07 to 1.84) | .015 | 1.40 (0.85 to 2.33) | .190 |
| Male without HIV | 1706 (18.4) | 40 | 1.22 (1.03 to 1.44) | .021 | 0.91 (0.63 to 1.33) | .640 |
| Male HIV only | 1556 (16.8) | 13 | 0.71 (0.54 to 0.93) | .012 | 0.43 (0.24 to 0.79) | .006 |
| Male with AIDS | 2399 (25.9) | 90 | 1.59 (1.35 to 1.87) | < .001 | 1.57 (1.15 to 2.14) | .005 |
CI = confidence interval; HR = hazard ratio.
Discussion
Using a large population-based registry linkage between HIV and cancer registries in the United States, we showed substantial increases in incidence rates of anal intraepithelial neoplasia grade III diagnoses across all groups of males and females with and without HIV. For males with and without HIV, we did not observe any increasing trend prior to 2005. Although larger increases were observed for those with HIV, this increase likely is strongly driven by screening strategies for prevention of squamous cell carcinoma of the anus in the populations known to be at greatest risk (19). Importantly, men with HIV without a prior diagnosis of AIDS appeared to have the lowest risk among all groups for subsequent squamous cell carcinoma of the anus diagnosis among people with anal intraepithelial neoplasia grade III diagnoses.
Using data from the California cancer registry from 2000 through 2009, Simard et al. (20) found that 87% of anal intraepithelial neoplasia grade III was seen among males and that annual incidence of anal intraepithelial neoplasia grade III in females did not change over the period of observation. In our large, population-based study, we found that annual incidence did not change for males with and without HIV prior to 2005. Mix et al. (21) showed statistically increases in anal intraepithelial neoplasia grade III in males and females aged 30-39 years but were not able to look at HIV status. It is important to note that people with HIV constitute more than 40% of anal intraepithelial neoplasia grade III diagnoses, likely because of screening practices as most are asymptomatic. Some of our registries overlap with sites included in the ANCHOR study, which may have contributed to the increasing trends for people with HIV but would not directly impact trends for those without HIV or greater rates in males with HIV aged younger than 35 years as the inclusion criteria required individuals to be older than 35 years (13). State-level variation in squamous cell carcinoma of the anus incidence has been described and shown to be correlated with state-level prevalence of AIDS for males and smoking for females (22). Although we adjusted for registry in our analyses of anal intraepithelial neoplasia grade III and believe the general comparisons are valid, data are based on a limited number of registries.
A previous time-to-event analysis that used data from cancer registries in the SEER program of 2074 individuals with a diagnosis of anal intraepithelial neoplasia grade III reported a 5-year cumulative incidence of squamous cell carcinoma of the anus following anal intraepithelial neoplasia grade III as 9.5% but was unable to identify individuals with HIV (16). In an analysis of SEER–Medicare among older people with HIV, Arens et al. (23) estimated a 5-year cumulative incidence of 5.7% for squamous cell carcinoma of the anus following anal intraepithelial neoplasia grade III, which they recognized as higher than previously reported estimates. They also found greater risk for squamous cell carcinoma of the anus for Black people with HIV and those with a history of anogenital condylomata but no difference in risk by anal intraepithelial neoplasia grade III treatment status (23). Arens et al. (23) did not investigate the potential impact of an AIDS diagnosis on subsequent squamous cell carcinoma of the anus following anal intraepithelial neoplasia grade III, which may have explained the elevated risk in Black people with HIV as their risk of AIDS is greater because of inequities in HIV care. Neither study accounted for competing risk of death, which biases comparisons. We report higher cumulative incidence rates of anal squamous cell carcinoma of the anus among people with HIV and anal intraepithelial neoplasia grade III than shown in either arm of the ANCHOR study, which only had 0.2% of participants receive a diagnosis of anal cancer (13). The ANCHOR study included anal intraepithelial neoplasia grade II diagnoses that were p16 positive, which may explain the lower incidence of invasive cancers compared with our study of anal intraepithelial neoplasia grade III exclusively, as lower grade precancers are less likely to become invasive and take longer to do so. Additionally, our study may have included some instances of anal intraepithelial neoplasia grade III, which were symptomatic and could indicate more aggressive disease.
For females without HIV, recommendations have suggested referral to anal cancer screening for females with a history of cervical dysplasia (14). Importantly, females without HIV bear the greatest burden of anal cancer in terms of absolute numbers (3). These observed increases in anal intraepithelial neoplasia grade III are even greater than the observed increases in rates of squamous cell carcinoma of the anus in females, including rates of later stage cancers (1). However, we observed a consistent increase in rates of anal intraepithelial neoplasia grade III diagnosis for females, which suggests that screening practices may not have changed in this group over the time period although uptake of screening may still be improving. In contrast, we observed a statistically change in incidence for males without HIV in 2005. It is likely that MSM constitute the majority of these anal intraepithelial neoplasia grade III diagnoses, as anal human papillomavirus infection is more common in this population and associated with receptive anal intercourse (24). However, the lack of ascertainment of sexual orientation by cancer registries limits confirmation of this assumption. Similarly, we report on males and females based on cancer registry information for sex assigned at birth, and thus we were unable to provide estimates for gender diverse and/or expansive individuals. Among people with HIV, transgender individuals were shown to have higher prevalence of HSIL compared with men and women in the ANCHOR study and are likely at greater risk for squamous cell carcinoma of the anus, but more research is needed (13).
Among people with HIV, those with a prior AIDS diagnosis were approximately twice as likely to have a diagnosis of anal intraepithelial neoplasia grade III compared with those without an AIDS diagnosis. This observation may be driven by differential patterns of referral to high-resolution anoscopy, which resulted in higher rates of screening for individuals with AIDS or greater prevalence of anal intraepithelial neoplasia grade III detected at high-resolution anoscopy in people with a prior AIDS diagnosis. The ANCHOR study found that among those screened, 55.1% of males and 47.2% of females with HIV had prevalent biopsy-confirmed anal HSIL, which includes moderate dysplasia (ie, p16-positive anal intraepithelial neoplasia grade II) (13). However, differences in prevalence of HSIL according to history of AIDS diagnosis have not yet been reported by ANCHOR and will be informative to understand whether our observations are a result of differences in screening patterns (ie, people with a history of AIDS are more likely to be screened) or higher prevalence of HSIL. The incorporation of a prior diagnosis of AIDS should be considered for screening, as these individuals have been shown to have the highest cumulative incidence of squamous cell carcinoma of the anus across all age groups (25).
We note that we refer to the risk of anal intraepithelial neoplasia grade III diagnosis or detection, as these estimates reflect possible biases in screening and not necessarily risk for anal intraepithelial neoplasia grade III itself. Additionally, we have not referred to this risk of squamous cell carcinoma of the anus following anal intraepithelial neoplasia grade III as progression because of the limited information of the precancer. In this analysis, we had limited information regarding treatment and were concerned that this information had informative missingness (eg, 5% of females were missing information regarding surgery of anal intraepithelial neoplasia grade III compared with 16% missing for males with or without HIV) for which we could not account through statistical methodology. We had limited information regarding treatment of anal intraepithelial neoplasia grade III and could not reliably estimate whether receipt of ablative therapy impacted cumulative incidence estimates. It is possible that the lower cumulative incidence among those with HIV but no prior AIDS diagnosis was because of greater access to care, including better follow-up after anal intraepithelial neoplasia grade III diagnosis and monitoring for new or missed neoplasia. We were also limited by the small number of anal intraepithelial neoplasia grade III cases in earlier years, as well as among females with HIV and no prior AIDS diagnosis. As a result, cumulative incidence estimates could not be calculated for some groups. We used diagnosis of AIDS as reported to HIV registries as our primary measure of advanced HIV in lieu of longitudinal laboratory measures of CD4 count and/or viral load, which have not been consistently captured through surveillance programs but may provide additional information to identify individuals at greater risk of squamous cell carcinoma of the anus following anal intraepithelial neoplasia grade III (26). The use of these data sources also limits our ability to look at a number of factors that likely impact access to high-resolution anoscopy, such as income, rurality, and health insurance. It is important to note that the linkage between cancer and HIV registries is imperfect and results in differential misclassification of HIV status, as a missing HIV diagnosis is more likely than a misattributed HIV diagnosis (3,27).
No national anal cancer screening guidelines have been adopted to date. The New York State Department of Health AIDS Institute has recommended anal cytology for males with HIV, particularly MSM, and females with HIV since 2007. The US Preventative Services Task Force is currently drafting recommendations. As discussions proceed regarding anal cancer screening recommendations, our study provides insight into trends over the last 2 decades in anal intraepithelial neoplasia grade III diagnoses in the United States. Overall, anal intraepithelial neoplasia grade III incidence has increased, however, the greatest increases have been for people with HIV, likely as a result of more frequent screening in this population. Trends in squamous cell carcinoma of the anus for people with HIV have begun to decline in recent years, possibly suggesting an impact of anal cancer screening on preventing cancer risk. Continued surveillance of trends in anal intraepithelial neoplasia grade III and squamous cell carcinoma of the anus may help elucidate the potential impact of cancer screening at the population level. Screening guidelines should consider incorporating information regarding a history of AIDS diagnosis for people with HIV, as these individuals were at greater risk of anal intraepithelial neoplasia grade III diagnosis and show some evidence for greater subsequent risk of squamous cell carcinoma of the anus.
Acknowledgements
The funder had no role in the design of the study; the collection, analysis, or interpretation of the data; or the writing of the manuscript but did provide approval for publication.
The authors gratefully acknowledge the support and assistance provided by individuals at the following state HIV/AIDS and cancer registries: Colorado, Connecticut, District of Columbia, Georgia, Louisiana, Maryland, Massachusetts, Michigan, New Jersey, New York, North Carolina, Puerto Rico, and Texas. We also thank Timothy McNeel at Information Management Services for programming support.
Contributor Information
Cameron B Haas, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Eric A Engels, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Joel M Palefsky, Department of Medicine, University of California, San Francisco, CA, USA.
Megan A Clarke, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Aimée R Kreimer, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Qianlai Luo, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Ruth M Pfeiffer, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Baozhen Qiao, New York State Department of Health, Albany, NY, USA.
Karen S Pawlish, New Jersey Department of Health, Trenton, NJ, USA.
Analise Monterosso, Texas Department of State Health Services, Austin, TX, USA.
Meredith S Shiels, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Data availability
Interested investigators can access data from individual state (or region) linkages by obtaining approval directly from the HIV and cancer registries in those states. In addition, collaboration between outside investigators and the NCI is possible. Data analyses can be done by NCI staff or, in some circumstances, NCI may be able to release tabulated data. Contact Qialai Luo (qianlai.luo@nih.gov) for data requests.
Author contributions
Cameron Brady Haas, PhD, MPH (Conceptualization; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing—original draft; Writing—review & editing), Eric A. Engels, MD (Conceptualization; Methodology; Supervision; Writing—review & editing), Joel M. Palefsky, MD (Investigation; Writing—review & editing), Megan A. Clarke, PhD (Investigation; Writing—review & editing), Aimée R. Kreimer, PhD (Investigation; Writing—review & editing), Qianlai Luo, PhD (Project administration; Writing—review & editing), Ruth M. Pfeiffer, PhD (Methodology; Writing—review & editing), Baozhen Qiao, PhD (Data curation; Writing—review & editing), Karen S. Pawlish, PhD (Data curation; Writing—review & editing), Analise Monterosso, PhD (Data curation; Writing—review & editing), and Meredith S. Shiels, PhD (Conceptualization; Data curation; Project administration; Supervision; Writing—review & editing).
Funding
The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or official policies of the National Cancer Institute, Centers for Disease Control and Prevention or the Department of Health and Human Services, HIV/AIDS or cancer registries, or their contractors, nor does the mention of trade names, commercial practices, or organizations imply endorsement by the US government. This research was supported in part by the Intramural Research Program of the National Cancer Institute.
The following cancer registries were supported by the cooperative agreement funded by the Centers for Disease Control and Prevention, National Program of Cancer Registries: Colorado (NU58DP006347-01), District of Columbia (NU62PS924565), Georgia (5U58DP003875-01), Louisiana (NU58DP006332-03-00), Maryland (NU58DP006333), Massachusetts (NU58DP006271-04-00), Michigan (17NU58DP006334), New Jersey (NU58/DP003931-05-00), New York (6NU58/DP006309), North Carolina (1NU58DP006281), Texas (5 NU58DP006308-04-00). District of Columbia is supported by the Centers for Disease Control and Prevention cooperative agreement DP006302.
The following cancer registries were supported by the SEER Program of the National Cancer Institute: Connecticut (HHSN261201300019I), Louisiana (HHSN261201800007I/HHSN26100002), Massachusetts (HHSN261201800008l), New Jersey (HHSN261201300021I, N01-PC-2013-00021), and New York (HHSN261201800009I). The New Jersey State Cancer Registry was also supported by the state of New Jersey, the Maryland Cancer Registry was supported by the State of Maryland and the Maryland Cigarette Restitution Fund, the Louisiana Tumor Registry was also supported by the state of Louisiana (0587200015), and the New York State Cancer Registry was also supported by the state of New York.
The following HIV registries were supported by HIV Incidence and Case Surveillance Branch of the Centers for Disease Control and Prevention, National HIV Surveillance Systems: Colorado (NU62PS003960), Connecticut (5U62PS001005-05), Louisiana (NU62PS924522-02-00), Michigan (U62PS004011-02), New Jersey (U62PS004001-2), New York (NU62PS924546-02-00; PS18-1802: Integrated HIV Surveillance and Prevention Programs for Health Departments, National Center for HIV, Viral Hepatitis, STD, and TB Prevention ([NCHHSTP]).
Conflicts of interest
Authors do not have any disclosures to report.
References
- 1. Deshmukh AA, Suk R, Shiels MS, et al. Recent trends in squamous cell carcinoma of the anus incidence and mortality in the United States, 2001-2015. J Natl Cancer Inst. 2020;112(8):829-838. doi: 10.1093/jnci/djz219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colon-Lopez V, Shiels MS, Machin M, et al. Anal cancer risk among people with HIV infection in the United States. J Clin Oncol. 2018;36(1):68-75. doi: 10.1200/JClinOncol.2017.74.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang ER, Pfeiffer RM, Austin A, et al. Impact of HIV on anal squamous cell carcinoma rates in the United States, 2001-2015. J Natl Cancer Inst. 2022;114(9):1246-1252., doi: 10.1093/jnci/djac103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hernandez AL, Efird JT, Holly EA, Berry JM, Jay N, Palefsky JM.. Incidence of and risk factors for type-specific anal human papillomavirus infection among HIV-positive MSM. AIDS. 2014;28(9):1341-1349. doi: 10.1097/QAD.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berry JM, Jay N, Cranston RD, et al. Progression of anal high-grade squamous intraepithelial lesions to invasive anal cancer among HIV-infected men who have sex with men. Int J Cancer. 2014;134(5):1147-1155. doi: 10.1002/ijc.28431. [DOI] [PubMed] [Google Scholar]
- 6. Ye Y, Burkholder GA, Mukherjee A, et al. A 12-year retrospective evaluation of anal pre-cancerous lesions and cancer in people living with HIV-1 infection in the Southeastern U.S. Infect Agent Cancer. 2021;16(1):14. doi: 10.1186/s13027-021-00354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cachay E, Agmas W, Mathews C.. Five-year cumulative incidence of invasive anal cancer among HIV-infected patients according to baseline anal cytology results: n inception cohort analysis. HIV Med. 2015;16(3):191-195. doi: 10.1111/hiv.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palefsky JM. Anal cancer prevention in HIV-positive men and women. Curr Opin Oncol. 2009;21(5):433-438. doi: 10.1097/CCO.0b013e32832f511a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun J, Wiley D, Barrett BW, et al. Comparison of anal pre-cancer screening strategies among men who have sex with men. Int J STD & AIDS. 2023;34(2):87-97. doi: 10.1177/09564624221137974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moscicki A-B, Darragh TM, Berry-Lawhorn JM, et al. Screening for anal cancer in women. J Low Genit Tract Dis. 2015;19(3 suppl 1):S27-S42. doi: 10.1097/LGT.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clarke MA, Wentzensen N.. Strategies for screening and early detection of anal cancers: a narrative and systematic review and meta-analysis of cytology, HPV testing, and other biomarkers. Cancer Cytopathol. 2018;126(7):447-460. doi: 10.1002/cncy.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leeds IL, Fang SH.. Anal cancer and intraepithelial neoplasia screening: a review. World J Gastrointest Surg. 2016;8(1):41-51. doi: 10.4240/wjgs.v8.i1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palefsky JM, Lee JY, Jay N, et al. ; ANCHOR Investigators Group. Treatment of anal high-grade squamous intraepithelial lesions to prevent anal cancer. N Engl J Med. 2022;386(24):2273-2282. doi: 10.1056/NEJMoa2201048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. New York State Department of Health AIDS Institute (NYSDoH AI). Screening for Anal Dysplasia and Cancer in Adults With HIV. 2022. https://cdn.hivguidelines.org/wp-content/uploads/20220809150151/NYSDOH-AI-Screening-for-Anal-Dysplasia-and-Cancer-in-Adults-With-HIV-pocket-guide_8-9-2022_HG.pdf. Accessed June 10, 2023.
- 15. Adamo M, Groves C, Dickie L, Ruhl J.. SEER Program Coding and Staging Manual 2021. National Cancer Institute. 2021. https://seer.cancer.gov/manuals/2021/SPCSM_2021_MainDoc.pdf. Accessed June 10, 2023.
- 16. Lee GC, Kunitake H, Milch H, et al. What is the risk of anal carcinoma in patients with anal intraepithelial neoplasia III? Dis Colon Rectum. 2018;61(12):1350-1356. doi: 10.1097/DCR.0000000000001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fine JP, Gray RJ.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 18. Gray B. cmprsk. R package version 2.2-11; 2022. https://cran.r-project.org/web/packages/cmprsk/cmprsk.pdf. Accessed June 10, 2023.
- 19. Clifford GM, Georges D, Shiels MS, et al. A meta‐analysis of anal cancer incidence by risk group: toward a unified anal cancer risk scale. Int J Cancer. 2021;148(1):38-47. doi: 10.1002/ijc.33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simard EP, Watson M, Saraiya M, Clarke CA, Palefsky JM, Jemal A.. Trends in the occurrence of high-grade anal intraepithelial neoplasia in San Francisco: 2000-2009. Cancer. 2013;119(19):3539-3545. doi: 10.1002/cncr.28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mix JM, Saraiya M, Senkomago V, Unger ER.. High-grade vulvar, vaginal, and anal precancers among U.S. adolescents and young adults after human papillomavirus vaccine introduction. Am J Prev Med. 2022;62(1):95-99. doi: 10.1016/j.amepre.2021.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Damgacioglu H, Lin YY, Ortiz AP, et al. State variation in squamous cell carcinoma of the anus incidence and mortality, and association with HIV/AIDS and smoking in the United States. J Clin Oncol. 2023;41(6):1228-1238. doi: 10.1200/JClinOncol.22.01390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arens Y, Gaisa M, Goldstone SE, et al. Risk of invasive anal cancer in HIV-infected patients with high-grade anal dysplasia: a population-based cohort study. Dis Colon Rectum. 2019;62(8):934-940. doi: 10.1097/DCR.0000000000001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meites E, Wilkin TJ, Markowitz LE.. Review of human papillomavirus (HPV) burden and HPV vaccination for gay, bisexual, and other men who have sex with men and transgender women in the United States. Hum Vaccin Immunother. 2022;18(1):2016007. doi: 10.1080/21645515.2021.2016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haas CB, Engels EA, Horner MJ, et al. Cumulative incidence of anal cancer since HIV or AIDS diagnosis in the United States. J Natl Cancer Inst. 2023. doi: 10.1093/jnci/djad128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karim A, Freeman MJ, Yang Q, et al. Duration of time CD4/CD8 ratio is below 0.5 is associated with progression to anal cancer in patients with HIV and high-grade dysplasia. Ann Surg Oncol. 2023;30(8):4746-4747. doi: 10.1245/s10434-023-13213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shiels MS, Pfeiffer RM, Hall HI, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980-2007. JAMA. 2011;305(14):1450-1459. doi: 10.1001/jama.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Interested investigators can access data from individual state (or region) linkages by obtaining approval directly from the HIV and cancer registries in those states. In addition, collaboration between outside investigators and the NCI is possible. Data analyses can be done by NCI staff or, in some circumstances, NCI may be able to release tabulated data. Contact Qialai Luo (qianlai.luo@nih.gov) for data requests.