Abstract
Context
Nonalcoholic fatty liver disease (NAFLD) is prevalent in 25–30% of British and European populations, representing a potential global public health crisis. Marine omega-3 (n-3) polyunsaturated fatty acids offer well-evidenced benefits to NAFLD biomarkers; however, the effect of plant-based n-3 has not been evaluated with a systematic review and meta-analysis.
Objective
The review aimed to systematically evaluate the effect of plant-based n-3 supplementation on NAFLD surrogate biomarkers and parameters.
Data Sources
Medline (EBSCO), PubMed, CINAHL (EBSCO), Cochrane Central Register of Controlled Trials, the International Clinical Trials Registry Platform, and Google Scholar databases were searched to identify randomized controlled trials published between January 1970 and March 2022 evaluating the impact of plant-based n-3 interventions on diagnosed NAFLD. The review followed the PRISMA checklist and is PROSPERO registered (CRD42021251980).
Data Extraction
A random-effects model and generic inverse variance methods synthesized quantitative data, followed by a leave-one-out method for sensitivity analysis. We identified 986 articles; after the application of selection criteria, six studies remained with 362 patients with NAFLD.
Results
The meta-analysis showed that plant-based n-3 fatty acid supplementation significantly reduced alanine aminotransferase (ALT) (mean difference: 8.04 IU/L; 95% confidence interval: 14.70, 1.38; I2 = 48.61%) and plasma/serum triglycerides (44.51 mg/dL; 95% confidence interval: –76.93, –12.08; I2 = 69.93%), alongside body-composition markers in patients with NAFLD (P < 0.05).
Conclusion
Plant-based n-3 fatty acid supplementation improves ALT enzyme biomarkers, triglycerides, body mass index, waist circumference, and weight loss when combined with lifestyle interventions to increase physical activity and a calorie-controlled diet. Further research is needed to identify the most effective plant-based n-3 sources in larger numbers of patients with NAFLD over longer study durations.
Systematic Review Registration
PROSPERO registration no. CRD42021251980.
Keywords: nonalcoholic fatty liver disease, omega-3, plant-based, supplementation
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is an important noncommunicable disease (NCD) that poses a major challenge to public health.1,2 The global prevalence of NAFLD is approximately 25% in the world population, although there is wide geographical variation, and reaching 30% in Middle Eastern and South American countries.2,3 The disease is recognized as a spectrum of conditions, ranging from ectopic fat accumulation in the liver4 or simple steatosis (accumulation of lipid droplets within >5% of hepatocytes)5 to nonalcoholic steatohepatitis (NASH), and ultimately cirrhosis.6 The estimated prevalence of NAFLD is 25–30% in British and European populations, with younger generations increasingly affected.5,7
With increasingly high prevalence in the United Kingdom and Western Europe, obesity is one of the main contributary factors for NAFLD. The latest statistics for England published in 2020 show that approximately two-thirds of adults are above a healthy weight and half are living with obesity,8 of whom 90% will have some degree of NAFLD.2,9 Furthermore, obesity accounted for over 1 000 000 UK hospital admissions in 2019/2020, resulting in annual National Health Service costs in excess of £6 billion.8,10 The European Health Interview Survey shows a similar pattern, with 53% of adults over 18 years living with overweight or obesity in Europe11 and over 4 million people died globally in 2017 due to being overweight or obese.12 Lifestyle changes, usually aimed at weight loss, are a primary aim of recommended therapeutic strategies, while drug approaches have generally yielded disappointing results so far regarding liver histology outcomes.13
Omega-3 (n-3) fatty acids offer well-evidenced benefits to NAFLD biomarkers,14 and diets low in n-3 have been identified as a risk factor for NAFLD mortality using the Global Burden of Disease 2019 data.15,16
Previous systematic reviews and meta-analyses have shown that regular marine-based n-3 consumption predominantly in the form of fish or krill oils through diet and/or supplementation offers significant (P < 0.05) benefits to liver fat, alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), steatosis score, plasma/serum triglycerides (TGs), insulin resistance, and glycemic control biomarkers.17–22 These marine-based n-3 fatty acids (predominantly consisting of long-chain n-3 eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) can contain relatively high concentrations of marine pollutants and pose sustainability issues. Fish oil is thought to be the main vector carrying marine pollutants to human consumers.23 Exposure to heavy metals in adolescence is thought to lead to alterations in energy homeostasis and excessive adiposity in later life.24 In terms of sustainability, there is increasing demand for highly purified EPA and DHA for the preparation of ethyl ester–based pharmaceutical products, which incurs production losses of 90–95%.25 In addition, climate change has resulted in the decline of marine microalgae from which oily fish species obtain their EPA and DHA, with a reduction in DHA content for oily fish of 58% predicted by 2100.26
Marine sources of n-3 by their nature are unsuitable for vegetarians and vegans, and public health organizations are increasingly recommending a plant-based dietary approach for health and sustainability.27,28 Plant-based n-3 sources have been shown to improve markers of NAFLD-related comorbidities, including cardiovascular disease (CVD) and the metabolic syndrome.29–31 Commercial applications have recently successfully developed direct sources of EPA- and DHA-rich oil from microalgal sources, which provide a plant-based bioequivalent source of n-3 to those found in marine sources.32 Algal sources of EPA and DHA are freely available food-based supplements, widely recommended as a suitable supplement for non–fish eaters,25,33 with safe, tolerable, nonclinical dosages of up to 3 g/day of EPA/DHA.34–36 Walnut, flax, chia, canola, hemp, echium, and perilla seed oils are all good plant-based sources of n-3 alpha-linolenic acid (ALA) and offer various health benefits. These include improvements in insulin sensitivity, inflammation, hepatic steatosis, and CVD risk factors, although the health benefits of ALA are not as well established as those attributed to EPA and DHA.34 Although the benefits of marine n-3 consumption on NAFLD are well established, the effects of plant-based n-3 fatty acids are yet to be examined in a systematic review and meta-analysis. Therefore, the aim of this systematic review and meta-analysis was to evaluate the potential benefits of plant-based n-3 supplementation on NAFLD surrogate biomarkers and parameters.
METHODS
The current systematic review and meta-analyses follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines37 and additional nutrition-based systematic review and meta-analysis guidance38 and is registered in the International Prospective Register of Systematic Reviews (PROSPERO CRD42021251980).39
Search strategy
The searches for data and articles for inclusion in this systematic review with meta-analysis were carried out until March 2022. Relevant trials were identified through systematic searches of Medline (EBSCO), CINAHL (EBSCO), PubMed, and Google Scholar databases along with Cochrane Central Register of Controlled Trials and the International Clinical Trials Registry Platform to identify randomized controlled trials (RCTs) published between January 1970 and March 2022. The search used the following keywords: “Flax* oil” OR “Linseed oil” OR “Chia seed oil” OR “Rapeseed oil” OR “Echium seed oil” OR “Hemp seed oil” OR “Perilla seed oil” OR “Alga* oil” in the article title or abstract OR “Omega*3” OR “Alpha-linolenic acid” OR “Stearidonic acid” OR “Eicosapentaenoic acid” OR “Docosahexaenoic acid” in the title or abstract AND “liver” (MeSH) OR “hepat*” OR “steat*” OR “fibrosis” OR “cirrhosis” OR “fatty liver” OR “nonalcoholic” OR “Non-alcoholic fatty liver disease” OR “NAFLD” OR Nonalcoholic fatty liver disease” OR “Non-alcoholic fatty liver” OR “Non-alcoholic steatohepatitis” OR “NASH” OR “non-alcoholic steatohepatitis” in the title, abstract, or key words. The full search strategy can be found in Supplementary Material S1 (please see the Supporting Information online).
To further minimize the effects of publication bias, a snowball method, characterized by manual checking of references from retrieved articles, was applied to ensure complete collection. Identified studies were directly exported into Endnote (version X9; Thomson Reuters) bibliography software for criterion application, whereby a reference lists of eligible studies and review articles were screened for eligibility. Publication alerts were set to identify any studies published after the date of the literature search.
Study selection criteria
Randomized controlled trials evaluating dietary interventions of at least 2 weeks’ duration were included. Studies were excluded on the basis that 1 or more of the following criteria applied: (1) RCTs that only evaluated marine oil n-3 fatty acid sources, (2) RCTs that did not utilize an n-3 fatty acid–rich plant-based source, (3) studies that did not provide data on the levels of the outcomes of interest at baseline and/or at the end of trial, (4) studies that did not indicate the specific n-3 fatty acid or dosage, (5) nonhuman studies, (6) observational studies, (7) cross-sectional studies, (8) case reports, (9) studies not published in the English language, and (10) studies without full access to published findings. Please see the Participant, Interventions, Comparisons, Outcomes and Study Design (PICOS) criteria in Table 1.
Table 1.
PICOS criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
|
|
|
|
|
Outcomes |
|
|
|
|
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, γ-glutamyl transferase; HDL, high density lipoprotein; HOMA-IR, homeostatic model of insulin resistance; Hs-CRP, high-sensitivity C-reactive protein; LDL, low density lipoprotein; MetS, metabolic syndrome; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PICOS, Participant, Interventions, Comparisons, Outcomes and Study Design; TC, total cholesterol; TG, triglyceride; WC, waist circumference.
Participants
Inclusion criteria: Adult, adolescent, and infant populations diagnosed with NAFLD or NASH using any recognized diagnostic criteria.
Exclusion criteria: Participants diagnosed with a serious unrelated pathology other than NAFLD/NASH, metabolic syndrome, type 2 diabetes, overweight, and obesity (such as cancer, kidney disease, cystic fibrosis, myocardial infarction, organ transplant) and participants with altered endocrinological state (such as pregnancy, lactation, endometriosis, or polycystic ovary syndrome).
Intervention: Plant-based n-3 supplementation using good sources of n-3 fatty acids (with “good” defined as typically >23% of total fatty acids).34,40,41
Comparator: Placebo or nonintervention control (treatment as usual/standard-care group, lifestyle modification to lose 7–10% of total body weight by diet and exercise is the first-line standard-care treatment for NAFLD).5 Marine and other oils as a comparator to plant-based n-3 fatty acids. Studies using animals were excluded. Single-arm intervention studies without a control group were excluded.
Primary outcome: A comparison of the effects of plant-based n-3 fatty acid supplementation on recognized NAFLD-related surrogate biomarkers, including liver enzyme biomarkers (ALT, AST, and GGT). Lipid profiles (total cholesterol [TC], high-density-lipoprotein [HDL] cholesterol, and low-density-lipoprotein [LDL] cholesterol), plasma/serum TGs, inflammatory markers (high-sensitivity C-reactive protein [Hs-CRP]), and effects on glycemic-control biomarkers (blood glucose, insulin, homeostatic model of insulin resistance [HOMA-IR]) were also evaluated along with body-composition markers (body mass index [BMI], waist circumference [WC], and weight loss).17–22
Data extraction
Screening was conducted with 2 independent researchers (E.M. and K.E.L.) in duplicate. Differences in opinion were resolved by group consultation (E.M., K.E.L., I.G.D., and I.P.) until consensus was reached. Extracted data included author, study year, country, study design, intervention source/dose, number of participants and intervention allocation, participant health status, intervention time frame, and main findings. Data were captured for baseline, endpoint, and changes in surrogate biomarkers measured in routine NAFLD patient care and monitoring.42 Biomarkers included ALT, AST, GGT, TC, HDL cholesterol, LDL cholesterol, TGs, Hs-CRP, blood glucose, insulin, HOMA-IR alongside BMI, and WC, all of which can be elevated in patients with NAFLD,14,43 and weight loss, the most effective treatment for NAFLD.42 The SI units were included for each outcome measure.
Risk-of-bias assessment
Risk of bias of RCTs was evaluated independently by 2 investigators (E.M. and K.E.L.). The assessment was performed at the study level with the revised Cochrane risk-of-bias tool, which grades the risk of selection, performance, attrition, detection, and reporting biases.44 This tool assesses whether a study has a low risk of bias, some concerns, or a high risk of bias. Differences in opinion were resolved by group consultation (E.M., K.E.L., and I.G.D.) until consensus was reached.
Statistical analysis
All data were collected as means ± SDs. The SD of the unreported mean difference (MD) of the change was calculated by the following formula: SD2 = [(SDbaseline2 + SDfinal2) − (2 × R × SDbaseline × SDfinal)],45 correlation coefficients (R) were computed for each outcome from study data.46,47 Mean differences with 95% confidence intervals (CIs) of changes with forest plots were estimated with the DerSimonian and Laird random-effects model.48 Heterogeneity between studies was assessed using Cochrane’s Q test (P < 0.10) and I2 statistic (low heterogeneity: <25%; moderate heterogeneity: 25–75%; and high heterogeneity: >75%),49 and visual inspection of the Galbraith plot (seeSupplementary Material S2 [Figures 1.1–1.14] in the Supporting Information online). A sensitivity analysis was conducted to evaluate the effect of each study with significant clinical heterogeneity on overall effect size using the “leave-one-out method,”50 by omitting 1 study at a time to ensure findings were not driven by any single study. STATA statistical software (version 17; StataCorp) was used for the whole process of meta-analysis. A 2-tailed P value <0.05 was considered to be statistically significant.
Figure 1.
PRISMA flow diagram. Abbreviations: CCRCT, Cochrane Central Register of Controlled Trials; ICTRP, International Clinical Trials Registry Platform; NAFLD, nonalcoholic fatty liver disease; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
RESULTS
Selection process
Figure 1 presents the study selection process in line with the PRISMA flow chart; the PRISMA checklist can be found in Supplementary Materials S3in the Supporting Information online. The systematic database search collected a total of 986 articles, 566 from Medline, 263 from PubMed, and 153 from CINAHL. A total of 3 articles were located through additional manual searches using Google Scholar and 1 study was registered with the International Clinical Trials Registry Platform. A total of 360 duplicate articles were removed, leaving 626 articles eligible for screening. Once titles and abstracts had been screened, a total of 566 articles were excluded based on the eligibly criteria. A total of 60 articles remained eligible for full-text screening. A total of 52 articles were excluded on the basis that one of the following applied: (1) participants with elevated NAFLD risk markers but not officially diagnosed, (2) marine oil study only, (3) healthy participants, (4) not n-3, (5) n-3 source/dosage unclear, and (6) serious illness/disease. In summary, a total of 8,46,47,51–56 journal articles (showing findings from 6 RCTs) with 362 participants (210 adults, 152 children/adolescents) remained eligible for quantitative synthesis. A full summary table of study design and findings are presented in Table 2,46,47,51–56 including author, study year, country, study design, intervention source/dose, number of participants and intervention allocation, participant health status, intervention time frame, and main findings. All included studies incorporated hypocaloric diets and/or lifestyle interventions aiming to increase physical activity for participants in both the intervention and control/placebo groups.
Table 2.
Summary of study design and findings
| Author, article/s, year, country | Study design | Intervention source and dose | No. of participants and intervention allocation | Participant health status | Time period | Main findings |
|---|---|---|---|---|---|---|
| Adult studies | ||||||
| Yari et al47 2021, Iran | RCT | Ground whole flaxseed powder flaxseed (WF; 30 g/d, 6.9 g/d ALA) | 24 (WF) | NAFLD patients detected by FibroScan (Echosens), recruited if >37% hepatic fat | 12 wk | Hesperidin and flaxseed supplementation improved glucose and lipid metabolism, while reducing inflammation and hepatic steatosis (controlled attenuation parameter) in NAFLD patients. |
| Hesperidin (HS) (1 g/d) | 22 (HS) | |||||
| Flaxseed-hesperidin (HWF) (30 g/d [6.9 g/d ALA] – 1 g/d) | 25 (HWF) | |||||
| Control (LM and HD only) | 21 (C) | |||||
| LM and HD for all groups | ||||||
| Rezaei et al51 2020, Iran | RCT-DB | Flaxseed oil 20 g/d (9.96 g/d ALA) | 34 (flaxseed) | NAFLD patients assessed by ultrasound | 12 wk | In the context of a low-energy diet and moderate PA, flaxseed oil may benefit NAFLD patients to improve fatty liver grade, weight, and IL-6 compared with sunflower oil. |
| Sunflower oil 20 g/d | 34 (control) | |||||
| HD and 30–40 min moderate PA/d both groups | ||||||
| Yari et al47,52 2016, Iran | RCT-OL | Brown milled flaxseeds 30 g/d 6.9 g/d ALA (WF) | 25 | NAFLD patients diagnosed by FibroScan | 12 wk | Flaxseed supplementation plus LM is more effective than LM alone for NAFLD management. |
| Control (C) (LM and HD only) | ||||||
| LM and HD for all groups | 25 | |||||
| Child and adolescent studies | ||||||
| Pacifico et al53 2015, Italy | RCT-DB | Algal oil 250 mg/d DHA (AO) | 25 | NAFLD diagnosed with MRI | 6 mo | DHA supplementation decreases liver and visceral fat and ameliorates metabolic abnormalities in children with NAFLD. |
| Placebo wheat germ oil 290 mg/d LA (WGO) | ||||||
| LM and HD in both groups | 26 | |||||
| Nobili et al54 2013 and Nobili et al55 2011, Italy | RCT-DB | Algal oil 250 mg/d DHA (AO—250) | 20 | NAFLD diagnosed with liver biopsy | 24 mo | DHA supplementation improves liver steatosis in children with NAFLD. Doses of 250 mg/d and 500 mg/d of DHA appear to be equally effective in reducing liver fat content. |
| Algal oil 500 mg/d DHA (AO—500) | ||||||
| Placebo wheat germ oil 290 mg/d LA (WGO) | 20 | |||||
| HD prescribed and PA suggested to both groups | 20 | |||||
| Della Corte et al56 2016, Italy | RCT-DB | Algal oil 500 m/d DHA and 800 IU vitamin D (AO+D) | 18 | NAFLD diagnosed with liver biopsy and decreased serum vitamin D | 24 wk | DHA plus vitamin D treatment improved insulin resistance, lipid profile, ALT, and NAS in obese children. There was also decreased HSC activation and collagen content with treatment. |
| Placebo group (no intervention) | ||||||
| LM and HD in all groups | 23 | |||||
Abbreviations: ALA, alpha-linolenic acid; ALT, alanine aminotransferase; DB, double-blinded; DHA, docosahexaenoic acid; HD, hypocaloric diet; HSC, hepatic stellate cells; IL-6, interleukin-6; LA, linoleic acid; LM, lifestyle modification; MRI, magnetic resonance imaging; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD Activity Score; OL, open label; PA, physical activity; RCT, randomized controlled trial.
Adult study characteristics
A total of 3 included studies recruited adult patients with NAFLD and were carried out in Iran.46,47,51,52 All of these were RCTs, one of which was double-blinded,51 another was open-label,47,52 and the third was not disclosed.46 With regard to intervention, Yari et al46 introduced ground whole flaxseed powder (WF; 30 g/d; 6.9 g/d ALA, 112.5 mg lignans),57 hesperidin (HS; 1 g/d) and flaxseed and hesperidin combined (HWF; 30 g/d; 6.9 g/d ALA) hesperidin; 1 g/d, 112.5 mg lignans).57 Similarly, Yari et al47 introduced a brown milled flaxseed (WF) intervention (30 g/d; 6.9 g/d ALA, 112.5 mg lignans).57 Rezaei et al51 intervened with flaxseed oil (20 g/d; 9.96 g/d ALA), alongside the active control, sunflower oil (20 g/d). All patients consumed a hypocaloric diet and were recommended to perform 30 to 40 minutes of moderate physical activity per day. The mean dosage of ALA for the adult studies was 7.665 g/d and all interventions ran for a period of 12 weeks.46,47,51,52
Child and adolescent studies
The remaining 3 studies recruited children and adolescent patients with NAFLD and were completed in Italy and used algal oil containing 39% DHA.53–56 The Pacifico et al53 study intervened with algal oil dosages of 250 mg/d DHA; Nobili et al54,55 used study arms with dosages of 250 mg/d DHA and 500 mg/d DHA, whereas Della Corte et al56 included 500 mg/d DHA from algal oil and 800 IU vitamin D. A total of 2 studies introduced placebo wheat germ oil (290 mg/d; omega-6 [n-6] linolenic acid),53–55 as an active control; and 1 study included a hypocaloric diet and lifestyle modifications with no other intervention.56 Algal oil intervention durations ranged from 24 weeks53,56 to 24 months.54,55
Risk-of-bias assessment
Details of the quality assessment can be found in Table 346,47,51–56 and Figures 2 and 3.46,47,51–56 The child/adolescent algal oil studies by Nobili et al54 and the adult flaxseed study Pacifico et al53 showed an overall low risk of bias. The remaining RCTs46,47,51,52,56 showed some concerns in at least 1 domain, including randomization process and measurement of outcome, but were not deemed to be at high risk for any domain.44
Table 3.
Cochrane risk-of-bias tool for randomized trials—quality assessment of all studies
| Author, year | Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported result | Overall |
|---|---|---|---|---|---|---|
| Yari et al47 | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Rezaei et al51 | Low risk | Low risk | Low risk | Some concerns | Low risk | Some concerns |
| Yari et al47,52 | Some concerns | Low risk | Low risk | Some concerns | Low risk | Some concerns |
| Pacifico et al53 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Nobili et al54,55 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Della Corte et al56 | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
Figure 2.
Risk-of-bias graph: authors’ judgments for each risk-of-bias item are presented as percentages across all included studies.
Figure 3.

Risk-of-bias summary for the included studies.
META-ANALYSIS
Effects on liver enzyme parameters
Alanine aminotransferase
The pooled estimate from all 6 studies of the effects of plant-based n-3 fatty acids on ALT from interventions including 83 adults and 63 child/adolescent patients showed a significant (P = 0.02) pooled reduction (MD: −8.04 IU/L; 95% CI: −14.7, −1.38; I2 = 48.61%)46,51–55 (Table 446,47,51–56 and Figure 4A4,6,47,51–56).
Table 4.
Liver enzyme baseline and endpoint biomarkers
| Author and citation | Participants (n) and study group | ALT, IU/L |
AST, IU/L |
GGT, IU/L |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Endpoint | Mean difference | P value | Baseline | Endpoint | Mean difference | P value | Baseline | Endpoint | Mean difference | P value | ||
| Adult studies | |||||||||||||
| Yari et al47 | 21 (C) | 27.18 ± 11.63 | 22.31 ± 8.15 | −4.87 ± 13.66 | 0.205 | 20.70 ± 4.85 | 18.66 ± 5.47 | −2.04 ± 7.36 | 0.318 | 32.92 ± 11.96 | 34.48 ± 22.93 | 1.56 ± 13.69 | 0.676 |
| 25 (HS) | 19.97 ± 8.39 | 12.77 ± 5.36 | −7.20 ± 6.55† | 0.001 | 16.88 ± 5.25 | 17.44 ± 4.78 | 0.56 ± 5.81‡ | 0.707 | 25.69 ± 12.99 | 18.64 ± 7.21 | −7.05 ± 8.10 | 0.002 | |
| 22 (WF) | 28.12 ± 16.94 | 17.00 ± 7.05 | −11.12 ± 11.84 | <0.001 | 24.66 ± 8.39 | 19.29 ± 5.83 | −5.37 ± 7.82 | 0.003 | 35.26 ± 22.13 | 23.72 ± 13.87 | −11.54 ± 13.90 | <0.001 | |
| 21 (HWF) | 21.78 ± 9.16 | 15.77 ± 7.43 | −6.00 ± 5.76 | <0.001 | 20.67 ± 9.11 | 16.91 ± 4.28 | −3.76 ± 10.47 | 0.115 | 41.38 ± 23.69 | 23.97 ± 9.88 | −17.40 ± 22.43 | 0.002 | |
| Rezaei et al51 | 34 (FO) | 27.6 ± 20.8 | 23.0 ± 15.8 | 0.009 | 16.6 ± 9.2 | 14.4 ± 7.9 | 0.001 | 28.7 ± 16.5 | 26.3 ± 18.3 | 0.22 | |||
| 34 (SO) | 28.9 ± 16.4 | 23.3 ± 11.3 | 0.003 | 18.5 ± 8.1 | 15.4 ± 4.0 | 0.007 | 30.1 ± 14.2 | 27.2 ± 12.7 | 0.07 | ||||
| Yari et al47,52 | 25 (WF) | 28.12 ± 16.94 | 17 ± 7.05 | −11.12 (−6.12, −16.13) | (P < 0.001) between-group | 27.58 ± 15.88 | 19.29 ± 5.83 | 8.29 (−3.49, −13.09) | (P < 0.001) between groups | 39.42 ± 34.61 | 23.72 ± 13.87 | −15.7 (−3.26, −28.15) | (P < 0.001) |
| 25 (C) | 35.71 ± 4.93 | 32.01 ± 6.03 | −3.7 (−2.38, −5.02) | 34.13 ± 5.1 | 30.13 ± 7.05 | −3.7 (−2.38, −5.02) | 44.45 ± 1.25 | 41.82 ± 1.64 | −2.62 (−1.84, −3.41) | Between groups | |||
| Child and adolescent studies | |||||||||||||
| Pacifico et al53 | 25 (AO) | 57 (20) | 27 (14) | 0.004 | |||||||||
| 26 (WGO) | 56 (19) | 45 (22) | 0.27 | ||||||||||
| Nobili et al54,55 | 20 (AO—250 mg/d) | 70 (25) | NS | ||||||||||
| 20 (AO—500 mg/d) | 57 (27) | NS | |||||||||||
| 20 (WGO) | 78 (37) | NS | |||||||||||
| Della Corte et al56 | 18 (AO+D) | 40.25 (24.59) | 24.5 (16.58) | 0.013* | 28.55 (10.51) | 20 (23.76) | 0.21 | 20.05 (12.92) | 18.5 (18.12) | 0.22 | |||
| 23 Placebo | 33.05 (18.72) | 35.05 (36.98) | 0.47 | 33.05 (18.72) | 35.05 (36.98) | 0.47 | 21.88 (13.45) | 18.78 (14.33) | 0.52 | ||||
| P = 0.0003 | P = 0.05 | ||||||||||||
Abbreviations: ALT, alanine aminotransferase; AO, algal oil; AO+D, algal oil + vitamin D; AST, aspartate aminotransferase; C, control; FO, flaxseed oil; GGT, γ-glutamyl transferase; HS, hesperidin; HWF, hesperidin and whole flaxseed; NS, not significant; WF, whole flaxseed; WGO, wheat germ oil.
*ANOVA (P < 0.05) between baseline and 12 months.
Statistically significant difference (P < 0.008) compared with the Control and Flax group, respectively.
Figure 4A.
Forest plot of differences in alanine aminotransferase between n-3 and placebo arms. Abbreviation: CI, confidence interval.
Aspartate aminotransferase and γ-glutamyl transferase
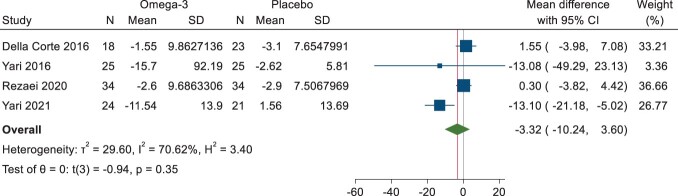
The pooled estimate on the effects of plant-based n-3 fatty acids on AST and GGT from 4 studies showed no significant pooled effect (AST—MD: −2.29 U/L; 95% CI: −6.76, 2.18; I2 = 34.61%; GGT—MD: −3.32 U/L; 95% CI: −10.24, 3.30; I2 = 70.62%). All 3 of the adult flaxseed studies were included (83 patients) in the meta-analysis46,47,51,52 along with the child/adolescent algal oil study (18 patients) by Della Corte et al56 (Table 4, Figure 4B)46,47,51,56 and (Figure 4C).46,47,51,56
Figure 4B.
Forest plot of differences in aspartate aminotransferase between n-3 and placebo arms. Abbreviation: CI, confidence interval.
Figure 4C.
Forest plot of differences in γ-glutamyl transferase between n-3 and placebo arms. Abbreviation: CI, confidence interval.
Effect on glycemic-control biomarkers
Glucose and insulin
The pooled estimate on the effects of plant-based n-3 fatty acids on blood glucose and insulin from 4 studies showed a marginally significant (P = 0.09) pooled effect for glucose (MD: −1.80 mg/dL; 95% CI: −3.85, 0.25; I2 = 75.92%) and was nonsignificant for insulin (MD: −0.50 µU/mL; 95% CI: −1.59, 0.59; I2 = 16.12%) for the included studies, which accounted for 2 adult flaxseed ALA interventions46,51 (58 patients) and 2 child/adolescent (43 patients) DHA trials53,56 (Table 5,46,47,51–56Figure 5A,46,51,53,56 and Figure 5B46,47,51,53,54,56).
Table 5.
Glycemic-control biomarker baseline and endpoint measures
| Author and citation | Participants (n) and study group | Glucose, mg/dL |
Insulin, µU/mL |
HOMA-IR |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Endpoint | Mean difference | P value | Baseline | Endpoint | Mean difference | P value | Baseline | Endpoint | Mean difference | P value | ||
| Adult studies | |||||||||||||
| Yari et al47 | 21 (C) | 101.38 ± 18.31 | 96.62 ± 15.18 | −4.75 ± 8.15 | 0.048 | 15.26 ± 8.13 | 12.41 ± 6.43 | −2.85 ± 3.88 | 0.017 | 3.84 ± 2.17 | 3.03 ± 1.77 | −0.81 ± 1.08 | 0.015 |
| 25 (HS) | 104.44 ± 16.36 | 96.87 ± 11.03 | −7.56 ± 9.84 | 0.008 | 10.42 ± 3.18 | 8.28 ± 2.28 | −2.14 ± 1.84 | <0.001 | 2.74 ± 1.06 | 2.00 ± 0.69 | −0.73 ± 0.57 | <0.001 | |
| 22 (WF) | 104.0 ± 8.88 | 97.87 ± 8.03 | −6.12 ± 5.08 | <0.001 | 11.32 ± 8.61 | 6.88 ± 5.57 | −4.44 ± 4.27 | <0.001 | 2.80 ± 2.17 | 1.58 ± 1.32 | −1.22 ± 1.27 | <0.001 | |
| 21 (HWF) | 119.80 ± 21.14 | 101.00 ± 10.43 | −18.81 ± 16.79† | <0.001 | 13.45 ± 7.14 | 10.27 ± 5.64 | −3.17 ± 3.10 | <0.001 | 4.14 ± 2.67 | 2.63 ± 1.54 | −1.51 ± 1.65† | <0.001 | |
| Rezaei et al51 | 34 (FO) | 5.9 ± 2.4 | 5.4 ± 1.7 | 0.005 | 3.18 ± 1.87 | 2.96 ± 1.82 | 0.46 | 0.92 ± 1.0 | 0.76 ± 0.55 | 0.21 | |||
| 34 (SO) | 5.3 ± 0.6 | 5.2 ± 0.8 | 0.45 | 2.60 ± 1.83 | 2.46 ± 1.61 | 0.63 | 0.68 ± 0.77 | 0.59 ± 0.43 | 0.38 | ||||
| Yari et al47,52 | 25 (WF) | Not measured | 2.8 ± 2.16 | −1.22 (−0.74, −1.69) | <0.001 between groups | ||||||||
| 25 (C) | 2.69 ± 1.06 | −0.31 (−0.20, −0.42) | |||||||||||
| Child and adolescent studies | |||||||||||||
| Pacifico et al53 | 25 (AO) | 83 (7) | 82 (6) | 0.88 | 20 (13–24) | 11 (10–15) | 0.005 | 4.0 (2.4–4.9) | 2.50 (2.0–3.1) | 0.028 | |||
| 26 (WGO) | 84 (7) | 84 (6) | 0.94 | 18 (12–21) | 15 (8–21) | 0.28 | 3.27 (2.0–4.4) | 2.97 (1.7–4.4) | 0.19 | ||||
| Nobili et al54,55 | 20 (AO—250 mg/d) | ||||||||||||
| 20 (AO—500 mg/d) | |||||||||||||
| 20 (WGO) | |||||||||||||
| Della Corte et al56 | 18 (AO+D) | 84.85 (6.44) | 77.82 (8.91) | 0.49 | 25.03 (21.22) | 21.16 (15.46) | 0.33 | 4.59 (4.26) | 3.42 (2.90) | 0.05 | |||
| 23 Placebo | 82.50 (7.36) | 80.80 (6.27) | 0.52 | 22.31 (14.74) | 21.71 (12.23) | 0.96 | 4.56 (3.13) | 4.33 (2.52) | 0.73 | ||||
Abbreviations: AO, algal oil; AO+D, algal oil + vitamin D; C, control; FO, flaxseed oil; HOMA-IR, homeostatic model of insulin resistance; HS, hesperidin; HWF, hesperidin and whole flaxseed; WF, whole flaxseed; WGO, wheat germ oil.
Statistically significant difference (P < 0.008) compared with the Control group.
Figure 5A.
Forest plot of differences in blood glucose between n-3 and placebo arms. Abbreviation: CI, confidence interval.
Figure 5B.
Forest plot of differences in insulin between n-3 and placebo arms. Abbreviation: CI, confidence interval.
HOMA-IR.
The pooled estimate on the effects of plant-based n-3 fatty acids on HOMA-IR for all 6 of the included studies46,51–55 showed a marginally significant (P = 0.07) pooled effect (MD: −0.22; 95% CI: −0.44, 0.01; I2 = 0.00%) at the intervention follow-up for 63 adults and 83 child/adolescent patients (Table 546,47,51–56 and Figure 5C46,47,51,53,54,56).
Figure 5C.
Forest plot of differences in homeostatic model of insulin resistance between n-3 and placebo arms. Abbreviation: CI, confidence interval.
Effect on lipid profiles
Total cholesterol and HDL cholesterol.
The pooled estimate on the effects of plant-based n-3 fatty acids on TC and HDL cholesterol for the 5 included studies46,47,51,53,56 showed no significant pooled effect (TC—MD: −4.91 mg/dL; 95% CI: −18.14, 8.32; I2 = 59.26%; HDL cholesterol—MD: 1.054 mg/dL; 95% CI: −3.20, 5.31; I2 = 89.39%) at the intervention follow-up in 43 child and adolescent patients and 83 adults (Table 6,46,47,51–56Figure 6A,46,47,51,53,56 and Figure 6B46,47,51,56).
Table 6.
Lipid profile biomarkers—total cholesterol, HDL cholesterol, and LDL cholesterol baseline and endpoint measures
| Author and citation | Participants (n) and study group | TC |
HDL cholesterol |
LDL cholesterol |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Endpoint | Mean difference | P value | Baseline | Endpoint | Mean difference | P value | Baseline | Endpoint | Mean difference | P value | ||
| Adult studies | |||||||||||||
| Yari et al47 | 21 (C) | 204.14 ± 36.16 | 197.78 ± 31.10 | −6.36 ± 29.90 | 0.441 | 33.21 ± 7.13 | 33.14 ± 7.56 | −0.06 ± 2.71 | 0.931 | 133.66 ± 37.13 | 127.10 ± 36.41 | −6.56 ± 26.67 | 0.374 |
| 25 (HS) | 186.12 ± 46.61 | 166.12 ± 45.49 | −20.00 ± 21.41 | 0.002 | 35.79 ± 11.98 | 37.71 ± 11.19 | 1.92 ± 11.29 | 0.521 | 125.33 ± 38.53 | 105.61 ± 37.48 | −19.72 ± 26.71 | 0.010 | |
| 22 (WF) | 203.42 ± 35.00 | 171.71 ± 26.49 | −31.71 ± 28.43 | <0.001 | 33.75 ± 7.47 | 36.96 ± 8.66 | 3.20 ± 5.64 | 0.011 | 125.93 ± 27.13 | 103.28 ± 22.63 | −22.64 ± 25.88 | <0.001 | |
| 21 (HWF) | 215.28 ± 35.40 | 188.76 ± 39.96 | −26.53 ± 25.72 | <0.001 | 38.15 ± 9.74 | 41.65 ± 10.40 | 3.50 ± 8.99 | 0.090 | 133.03 ± 27.24 | 106.74 ± 39.24 | −26.29 ± 38.29 | 0.005 | |
| Rezaei et al51 | 34 (FO) | 4.82 ± 1.13 | 4.63 ± 1.18 | 0.15 | 1.03 ± 0.17 | 1.05 ± 0.19 | 0.32 | 2.88 ± 0.93 | 2.69 ± 0.93 | 0.10 | |||
| 34 (SO) | 4.78 ± 0.83 | 4.67 ± 0.74 | 0.52 | 1.08 ± 0.19 | 1.09 ± 0.21 | 0.84 | 2.83 ± 0.78 | 2.72 ± 0.88 | 0.42 | ||||
| Yari et al47,52 | 25 (WF) | 203.42 ± 35.00 | −31.7 (−19.7, −43.71) | 0.368 between groups | 44.5 ± 12.49 | 0.75 (−2.56, 4.06) | 0.848 between groups | 125.93 ± 27.12 | −22.64 (−11.71, −33.58) | 0.979 between groups | |||
| 25 (C) | 166.77 ± 37.09 | −14.22 (−10.65, −17.8) | 44.04 ± 5.27 | 0.09 (−2.11, 2.29) | 86.69 ± 42.68 | −12.80 (−8.14, −17.44) | |||||||
| Child and adolescent studies | |||||||||||||
| Pacifico et al53 | 25 (AO) | 152 (33) | 158 (40) | 0.54 | 41 (10) | 43 (9) | 0.41 | ||||||
| 26 (WGO) | 150 (43) | 147 (30) | 0.46 | 47 (9) | 50 (11) | 0.22 | |||||||
| Nobili et al54,55 | 20 (AO—250 mg/d) | ||||||||||||
| 20 (AO—500 mg/d) | |||||||||||||
| 20 (WGO) | |||||||||||||
| Della Corte et al56 | 18 (AO+D) | 163 (27.28) | 157 (25.77) | 0.23 | 34.5 (8.55) | 43.77 (7.31) | 0.008 | 112.05 (24.28) | 105.5 (22.24) | 0.08 | |||
| 23 Placebo | 154.45 (30.85) | 143.35 (18.41) | 0.59 | 46.55 (8.53) | 47.51(8.55) | 0.87 | 95.36 (32.74) | 95.38 (33.7) | 0.88 | ||||
Abbreviations: AO, algal oil; AO+D, algal oil + vitamin D; C, control; FO, flaxseed oil; HDL, high density lipoprotein; HS, hesperidin; HWF, hesperidin and whole flaxseed; LDL, low density lipoprotein; TC, total cholesterol; WF, whole flaxseed; WGO, wheat germ oil.
Figure 6A.
Forest plot of differences in total cholesterol between n-3 and placebo arms. Abbreviation: CI, confidence interval.
Figure 6B.
Forest plot of differences in high-density-lipoprotein cholesterol between n-3 and placebo arms. Abbreviation: CI, confidence interval.
LDL cholesterol
The pooled estimate on the effects of plant-based n-3 fatty acids on LDL-C from 4 studies showed a nonsignificant pooled reduction effect (MD: −8.75 mg/dL; 95% CI: −17.53, 0.04; I2 = 0.00%). All of the adult flaxseed studies were included (83 adult patients) in the meta-analysis,46,47,51,52 along with the child/adolescent algal oil study (18 patients) by Della Corte et al56 (Table 6 and Figure 6C46,47,53,56).
Figure 6C.
Forest plot of differences in low-density-lipoprotein cholesterol between n-3 and placebo arms. Abbreviation: CI, confidence interval.
Plasma/serum triglycerides
The pooled estimate on the effects of plant-based n-3 fatty acids on plasma/serum TGs from 4 studies showed a significant (P = 0.01) pooled reduction (MD: −44.51 mg/dL; 95% CI: −76.93, −12.08; I2 = 69.93%)46,47,53–56 (Table 746,47,51–56 and Figure 6D46,47,53,54,56). All of the child/adolescent algal oil studies53,54,56 were included (63 patients) in the meta-analysis alongside 2 adult ALA studies (49 patients).46,47
Table 7.
Triglycerides, high-sensitivity C-reactive protein, and tumor necrosis factor baseline and endpoint measures
| Author and citation | Participants (n) and study group | TGs, mg/dL |
Hs-CRP, ng/dL |
TNF, pg/mL |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Endpoint | Mean difference | P value | Baseline | Endpoint | Mean difference | P value | Baseline | Endpoint | Mean difference | P value | ||
| Adult studies | |||||||||||||
| Yari et al47 | 21 (C) | 163.00 ± 29.51 | 165.64 ± 71.36 | −2.64 ± 66.28 | 0.884 | 3810.93 ± 3306.55 | 3040.79 ± 2677.64 | 22.36 ± 5.61 | 20.16 ± 5.93 | −2.20 ± 3.55 | 0.037 | ||
| 25 (HS) | 191.37 ± 88.96 | 135.06 ± 60.79 | −56.31 ± 69.57 | 0.006 | 4177.88 ± 2619.89 | 2985.63 ± 2342.90 | 27.87 ± 12.69 | 21.31 ± 6.95 | −6.56 ± 6.75‡ | 0.001 | |||
| 22 (WF) | 206.17 ± 82.01 | 144.83 ± 59.76 | −61.33 ± 42.62 | <0.001 | 4678.75 ± 2043.92 | 4807.92 ± 6721.06 | 19.46 ± 5.41 | 18.97 ± 5.64 | −0.49 ± 2.71 | 0.383 | |||
| 21 (HWF) | 157.28 ± 35.40 | 112.76 ± 41.43 | −45.19 ± 35.37 | <0.001 | 6194.48 ± 5499.53 | 4621.90 ± 4936.66 | 26.18 ± 6.39 | 19.87 ± 4.66 | −6.30 ± 4.21 | <0.001 | |||
| Rezaei et al51 | 34 (FO) | ||||||||||||
| 34 (SO) | |||||||||||||
| Yari et al47,52 | 25 (WF) | 206.17 ± 82.01 | −61.33 (−43.33, −79.33) | <0.001 between groups | 4.66 ± 2.85 | 3.59 ± 2.12 | −1.30 (−0.40, −2.20) | 0.001 between groups | |||||
| 25 (C) | 225.18 ± 90.37 | −9.50 (−2.36, −16.64) | 4.18 ± 2.82 | 3.03 ± 1.01 | −0.14 (−0.07, −0.21) | ||||||||
| Child and adolescent studies | |||||||||||||
| Pacifico et al53 | 25 (AO) | 0.54 | 92 (58–143) | 75 (49–118) | 2700 (990–5100) | 2800 (800–5100) | |||||||
| 26 (WGO) | 0.46 | 85 (55–126) | 75 (48–109) | 2000 (999–4000) | 1450 (475–3650) | ||||||||
| Nobili et al54,55 | 20 (AO—250 mg/d) | ||||||||||||
| 20 (AO—500 mg/d) | |||||||||||||
| 20 (WGO) | |||||||||||||
| Della Corte et al56 | 18 (AO+D) | 0.23 | 174.5 (75.63) | 102.15 (22.24) | |||||||||
| 23 Placebo | 0.59 | 87.20 (47.40) | 89.44 (44) | ||||||||||
Abbreviations: AO, algal oil; AO+D, algal oil + vitamin D; C, control; FO, flaxseed oil; HS, hesperidin; Hs-CRP, high-sensitivity C-reactive protein; HWF, hesperidin and whole flaxseed; TG, triglyceride; TNF, tumor necrosis factor; WF, whole flaxseed; WGO, wheat germ oil.
Statistically significant difference (P < 0.008) compared with the Flax group.
Figure 6D.
Forest plot of differences in triglycerides between n-3 and placebo arms. Abbreviation: CI, confidence interval.
Inflammatory markers
High-sensitivity C-reactive protein
The pooled estimate on the effects of plant-based n-3 on Hs-CRP showed no significant pooled effect for the 3 included studies (MD: −1.03 ng/dL; 95% CI: −7.20, 5.15; I2 = 0.00%) at the intervention follow-up in 25 child53 and adolescent patients and 49 adults46,47,52 (Table 7 and (Figure 746,47,53).
Figure 7.
Forest plot of differences in high-sensitivity C-reactive protein between n-3 and placebo arms. Abbreviation: CI, confidence interval.
Body composition
Plant-based n-3 fatty acids showed favorable effects for all 3 body-composition markers. With respect to BMI, the pooled estimate on the effects of plant-based n-3 showed a significant (P < 0.001) pooled reduction effect (MD: −1.83 kg/m2; 95% CI: −2.99, 0.68; I2 = 76.37%) for all 6 of the included studies at the intervention follow-up including 83 adult46,47,52 and 63 child/adolescent patients53–56 (Table 846,47,51–56 and Figure 8A46,47,51–56). Similar results we observed for WC, with a significant (P < 0.001) pooled reduction effect (MD: −3.21 cm; 95% CI: −4.92, −1.50; I2 = 20.32%) for 5 studies including 63 children/adolescents53,54 and 45 adult46,47,51 patients (Table 8 and Figure 8B46,47,51,53,56). Finally, weight loss showed a significant (P = 0.01) pooled reduction effect (MD: −4.68 kg; 95% CI: −8.33, −1.04; I2 = 0.00%) for 3 studies including 25 child/adolescent patients53 and 59 adults46,51 (Table 8 and Figure 8C47,51,53).
Table 8.
Body-composition measures—body mass index, waist circumference, and weight baseline and endpoint measures
| Author and citation | Participants (n) and study group | BMI, kg/m2 |
WC, cm |
Weight, kg |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Endpoint | Mean difference | P value | Baseline | Endpoint | Mean difference | P value | Baseline | Endpoint | Mean difference | Within group | ||
| Adult studies | |||||||||||||
| Yari et al47 | 21 (C) | 33.37 ± 5.56 | 32.42 ± 5.98 | 0.015 | 104.53 ± 8.42 | 102.07 ± 11.20 | 0.133 | ||||||
| 25 (HS) | 31.32 ± 4.39 | 28.40 ± 3.20 | 0.029 | 101.75 ± 6.58 | 96.19 ± 5.89 | <0.001 | |||||||
| 22 (WF) | 30.37 ± 4.42 | 28.05 ± 3.89 | <0.001 | 100.08 ± 8.63 | 90.58 ± 19.32 | 0.028 | |||||||
| 21 (HWF) | 33.94 ± 5.95 | 32.02 ± 8.83 | <0.001 | 105.60 ± 6.97 | 98.24 ± 8.83 | <0.001 | |||||||
| Rezaei et al51 | 34 (FO) | 30.1± 4.1 | 28.2± 3.8 | <0.001 | 102.4 ± 11.1 | 93.6 ± 10.1 | <0.001 | 83.2 ± 13.1 | 78.0 ± 12.2 | <0.001 | |||
| 34 (SO) | 29.6± 3.9 | 28.7± 3.9 | <0.001 | 99.7 ± 9.2 | 92.3 ± 9.7 | <0.001 | 80.7 ± 110.8 | 78.4 ± 12.2 | <0.001 | ||||
| Yari et al47,52 | 25 (WF) | 29.96 ± 3.96 | −3.13 (−3.73, −2.53) | <0.001 between groups | 100.08 ± 8.63 | −9.5 (−17.90, −1.11) | 0.119 between groups | 83.58 ± 14.75 | −9.02 (−11.05, −6.98) | <0.001 between groups | |||
| 25 (C) | 31.53 ± 2.23 | −1.18 (−1.41, −0.96) | 102.55 ± 6.06 | −2.86 (−4.15, −1.57) | 81.38 ± 13.89 | −3.04 (−3.63, −2.46) | |||||||
| Child and adolescent studies | |||||||||||||
| Pacifico et al53 | 25 (AO) | 28.9 (4.3) | 27.3 (4.1) | 0.024 | 94 (12) | 91 (11) | 0.056 | 66 (18) | 63 (17) | 0.22 | |||
| 26 (WGO) | 27.5 (5.5) | 27.2 (5.4) | 0.71 | 91 (12) | 92 (14) | 0.68 | 63 (20) | 64 (19) | 0.69 | ||||
| Nobili et al54,55 | 20 (AO—250 mg/d) | ||||||||||||
| 20 (AO—500 mg/d) | |||||||||||||
| 20 (WGO) | |||||||||||||
| Della Corte et al56 | 18 (AO+D) | 28.42 (4.08) | 24.58 (3.61) | 0.002 | 89.47 (9.35) | 85 (7.29) | 0.76 | ||||||
| 23 Placebo | 28.39 (5.42) | 28.02 (5.63) | 0.33 | 89.95 (9.91) | 89.15 (9.74) | 0.85 | |||||||
Abbreviations: AO, algal oil; BMI, body mass index; C, control; D, vitamin D; FO, flaxseed oil; HS, hesperidin; HWF, hesperidin and whole flaxseed; WC, waist circumference; WF, whole flaxseed; WGO, wheat germ oil.
Figure 8A.
Forest plot of differences in body mass index between n-3 and placebo arms. Abbreviation: CI, confidence interval.
Figure 8B.
Forest plot of differences in waist circumference between n-3 and placebo arms. Abbreviation: CI, confidence interval.
Figure 8C.
Forest plot of differences in weight loss between n-3 and placebo arms. Abbreviation: CI, confidence interval.
DISCUSSION
The present systematic review and meta-analysis evaluated RCTs investigating the effect of plant-based n-3 fatty acids on NAFLD-related surrogate biomarkers, including liver enzymes, glycemic-control biomarkers, lipid profiles, inflammatory markers, and body composition. To the authors’ knowledge, this is the first study to evaluate plant-based n-3 fatty acids using NAFLD surrogate markers. The current novel meta-analysis shows that plant-based n-3 fatty acid supplementation has beneficial effects on ALT, TGs, and body-composition markers, including BMI, WC, and total weight. Three studies recruited adult patients with NAFLD and were carried out in Iran where NAFLD prevalence is estimated at 27.88% in men and 30.17% in women.58 The remaining 3 studies recruited children and adolescents in Italy where NAFLD prevalence is 22.5–27.0% in the general population.59 This study aimed to evaluate the benefits of plant-based n-3 fatty acid supplementation and did not make direct comparisons to marine-based n-3 studies.
Liver enzymes
The meta-analysis showed a significant (P = 0.01) pooled reduction of ALT with plant-based n-3; however, there was no effect on AST or GGT. This suggests that plant-based n-3 does not offer improvements to AST and GGT; however, it should be noted that 2 of the child/adolescent studies53–55 did not measure AST and GGT, so the meta-analysis predominantly represents the adult ALA studies for these biomarkers. The adult flaxseed studies had a relatively short 12-week duration, dosages of 6.946,47 or 9.96 g/d51 ALA, and small sample sizes, which may have resulted in null findings for AST and GGT. It should also be noted in some of the study arms, baseline ALT levels were within the upper-normal limits for NAFLD.60,61 Although this is the first systematic review and meta-analysis of plant-based n-3 fatty acid supplementation and NAFLD-related liver enzyme biomarkers, findings on ALT are similar to recent marine oil n-3 meta-analyses. Yan et al18 showed significant (P < 0.05) improvements in ALT in 18 RCTs evaluating fish-oil supplementation in 1424 patients. The study also showed benefits to AST and GGT but with a larger number of studies and patients. Similarly, Lee et al62 showed significant improvements in ALT (P < 0.001) and AST (P < 0.001) in a systematic review and meta-analysis of 22 marine/fish-oil RCTs.
Glycemic-control biomarkers
NAFLD is thought to be a manifestation of metabolic syndrome63 and insulin resistance is a common factor that connects obesity, hypertension, dyslipidemia, type 2 diabetes, and NAFLD progression.63,64 In the present article, the meta-analysis showed that plant-based n-3 supplementation offered no significant improvements in glycemic-control biomarkers and glucose levels were within healthy ranges for some of the study arms.61 The marginal significance obtained for blood glucose (P = 0.09) and HOMA-IR (P = 0.07) could result from a small sample size and short-term study durations and, although not statistically significant, should be interpreted with caution. The findings for this study are in agreement with a systematic review and meta-analyses of 7 RCTs by He et al,21 who showed the efficacy of marine n-3 including fish oil was unclear in relation to glycemic-control markers. The findings of this study agree with the growing consensus in recent literature showing that, despite improvements in insulin sensitivity in rodent studies, the vast majority of human intervention studies fail to demonstrate beneficial effects of n-3 fatty acids in glycemic-control biomarkers in type 2 diabetes or insulin resistance.65,66
Lipid profiles
The meta-analyses showed a significant reduction in plant-based n-3 from flaxseed oil and algal oil on TGs (P = 0.01) and a trend in lowering LDL-C (P = 0.05). Patients with NAFLD have a substantially increased risk of mortality from CVD,67,68 and lipid profiles form an important part of CVD risk management.69 Moderate increases in LDL-C in patients with NAFLD can increase the risk of a cardiovascular event.70 The significant TG reductions are important as TG measurement represents a useful noninvasive biomarker for diagnosis, prognosis, and monitoring of NAFLD progression.71 This study’s findings relating to plant-based n-3 supplementation and changes in lipid profiles partially agree with recently published literature. Meta-analyses of flaxseed oil supplementation studies and CVD risk by Hadi et al29 and Masjedi et al31 showed flaxseed supplementation significantly lowered TGs (P < 0.05). In contrast to the findings of the present review, significant reductions were also shown for TC (P = 0.01). However, both meta-analyses had higher numbers of studies/participants and therefore greater statistical power than the present study. In a meta-analysis of 47 RCTs with 1305 individuals, Yue et al72 showed that dietary intake of ALA significantly (P < 0.05) reduced lipid profile biomarkers. A meta-analysis by Naghshi et al73 showed that a high intake of ALA (defined as ≥3 g/d) was significantly (P < 0.05) associated with a lower risk of death from CVD and coronary heart disease compared with low intake (defined as ≤0.35 g/d).
Body composition
The meta-analyses showed significant improvements in body-composition measures, including BMI (MD: −1.83 kg/m2; 95% CI: −2.99, −0.68; P = 0.00; I2 = 76.37%), WC (MD: −3.21 cm; 95% CI: −4.92, −1.50; P = 0.00; I2 = 20.32%), and weight (MD: −4.68 kg; 95% CI: −8.33, −1.04; P = 0.01; I2 = 0.00%). Weight loss is widely recognized as the most effective treatment for NAFLD,63,74 with current treatment guidelines recommending lifestyle interventions aiming to lose 7–10% of total weight in patients with NAFLD.5,63 Improvements in body composition compare with previously published lifestyle intervention studies. A systematic review and meta-analyses by Katsagoni et al75 showed that diet interventions and exercise decreased BMI and WC from 20 RCTs with 1073 patients with NAFLD. Improvements in body composition also translated to liver enzyme biomarkers, which showed significant decreases in ALT and AST (both P < 0.05).
Potential mechanisms of beneficial effects of n-3 treatment for NAFLD
Long-chain n-3 polyunsaturated fatty acids are categorized by the presence of a double bond at the third carbon atom from the methyl end of the carbon chain.4 Where direct marine-based n-3 sources are scarce or not present in the diet, n-3 ALA (18:3; which was used in the 3 adult RCTs in the present review)46,47,51 must undergo conversion to the more biologically active longer chain highly unsaturated EPA (20:5)76 and DHA (22:6)77 in the n-3 metabolic pathway. High dietary n-6 ratios, which are indicative of low n-3 intakes, can be proinflammatory and limit the conversion of ALA to EPA and DHA in the n-3 metabolic pathway.34,78 Those consuming low intakes of n-3 coupled with a high n-6 to n-3 dietary fatty acid ratio are at increased risk of developing NAFLD.15,79 Incorporating a direct source of DHA, which was used in the child/adolescent RCTs identified in the present review,53,54,56 is the most effective way to obtain DHA in the diet for low/non–fish consumers.34,41 The molecular mechanisms causing the initiation and progression of NAFLD have yet to be fully recognized and understood; however, inflammation and oxidative stress induced by reactive oxygen species are likely to be significant mechanisms that can lead to hepatic cell death and tissue injury.80 NAFLD is usually believed to have a mutual and bidirectional nexus with metabolic syndrome and its individual components and, therefore, to be a noncommunicable disease. However, Helicobacter pylori infection is also believed to play a role in the development of NAFLD, as does the gut microbiota.81–84 Dietary n-3 fatty acids are thought to have beneficial effects in bioactive metabolites involved in inflammatory pathways and liver metabolism, including the reduction in hepatic TG accumulation and regulation of hepatic lipid metabolism and adipose tissue function.4,47 Furthermore, dietary n-3 fatty acids have been shown to regulate various H. pylori–associated gastric diseases.85 However, further studies are required to evaluate the underlying mechanisms involved.4,19,79,85
Strengths and limitations
To the authors’ knowledge this is the first systematic review and meta-analysis to evaluate the effect of plant-based n-3 fatty acid interventions on NAFLD biomarkers and parameters. However, there are limitations that should be considered. The systematic search criteria identified 6 RCTs with 362 patients with NAFLD, representing a small number of participants and short study durations compared with similar systematic reviews and meta-analysis that have evaluated fish/marine-based oil n-3 supplementation. As the search criteria identified that there have only been 6 relevant clinical trials investigating the topic since 1970, this highlights an overall scarcity of plant-based n-3 intervention studies in this area of research. The use of liver enzyme measurements on the whole may be a limitation as up to 78% of patients with NAFLD have liver enzyme biomarkers within normal ranges.2 The relatively short duration of studies may limit inferences on the discordance between results of significance between ALT, AST, and GGT. The AST-to-ALT ratio can be a useful indicator of fibrosis in NAFLD86; however, the study by Rezaei et al51 was the only RCT identified in the present study to use this measure, which showed no significant differences between the active treatment control and intervention groups. Outcomes in this review (biomarkers and parameters) are surrogate endpoints, which might not directly translate to patient benefit or predict improvements in clinical outcomes for patients.5,87 The measurement of surrogate biomarkers in the identified studies with no histological confirmation of the diagnosis and outcomes does not address American Association for the Study of Liver Diseases and European Association for the Study of the Liver NAFLD/NASH accepted endpoints, including changes in liver steatosis, hepatic inflammation, and fibrosis.84,88 A number of publications have used magnetic resonance imaging for the noninvasive measurement of adult and adolescent liver steatosis19,89,90; however, none of the studies identified in this review used magnetic resonance imaging measurements to monitor the effectiveness of interventions.
The study findings concerning body composition represent a key limitation as it was not possible to delimit and separate the beneficial effects of plant-based n-3 and weight loss, the first-line standard-care treatment for NAFLD5 in the meta-analysis. However, it is noteworthy that the control/placebo groups in 3 of the 6 included studies by Pacifico et al53 (active treatment control: wheat germ oil), Yari et al,46 and Della Corte et al56 (inactive treatment control) showed significant reductions to BMI in plant-based n-3 intervention groups (P < 0.05) but not in the control/placebo groups despite the same lifestyle intervention.
Significant heterogeneity was found in the present meta-analyses. The effect of plant-based n-3 supplementation in young and adult populations may be affected by type, duration, dosage, and further effects of weight loss. However, due to the limited number of relatively short-term studies focusing on this area, and translational small patient cohorts included in the meta-analysis, it was not possible to conduct further subgroup analysis to identify potential sources of heterogeneity; hence, the results need to be interpreted with caution. Some of the null findings could be related to the dosages administered or the samples sizes used. The search did not identify any studies that made direct comparisons between marine and plant-based n-3 fatty acid sources in surrogate NAFLD biomarkers.
Further consideration must be given to wheat germ oil,91 which was used as a placebo/control in RCTs by Pacifico et al53 and Nobili et al54 and represents a good source of vitamin E and n-6 (α-tocopherol ∼1179 mg/kg and β-tocopherol ∼398 mg/kg; linolenic acid: 49–60% [n-6]).91 Vitamin E supplementation shows beneficial effects in patients with NAFLD, both with and without weight loss, as evidenced by several systematic reviews and meta-analyses92–94 and the Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis (PIVENS) trial,95 posing a potential limitation for the studies using a wheat germ oil placebo/controls. In the present review, neither of the 2 RCTs53,54 specified the exact quantity of wheat germ oil used, stating a dose of 290 mg/d linolenic acid (n-6), representing 49–60% of fatty acids in wheat germ oil composition.91 A cross-sectional study in 6693 adults in the United States showed that both dietary n-3 and n-6 fatty acids may offer benefits in NAFLD; however, further studies are needed to verify this and to identify the associated mechanisms. The summary findings in Table 2 show that there were no significant improvements between baseline and endpoint for any of the biomarkers included in the meta-analysis for the comparator groups in studies using wheat germ oil as a placebo/control.
A further limitation of this meta-analysis is that the 3 included studies of adult populations were all conducted in 1 country, Iran. As such, these studies may be affected by environmental factors such as local diet that may not be applicable in other countries. This may also limit the generalizability of the results to other, diverse populations and/or ethnicities. The use of whole ground flaxseed by Yari et al46,47 in 2 of the 3 identified adult studies may also represent a confounding effect as fiber intake is known to ameliorate NAFLD96; this may have added to the heterogeneity between studies. Due to the limited number of studies in this area, there is a lack of consensus surrounding effective doses of ALA for the amelioration of NAFLD. The adult RCTs identified in the review used flaxseed interventions and the child/adolescent RCTs used algal oil comprising ALA and DHA, respectively. The child studies used doses of either 250 or 500 mg/d DHA, which is in line with dietary recommendations based on cardiovascular risk considerations for European adults; again, there is a lack of consensus surrounding effective DHA doses for NAFLD in children and adolescents due the shortage of published studies. It was not possible to separate the effect for the 2 different n-3 sources; although the identified studies included study arm demographics for males and females, none of them conducted separate statistical analyses by sex and reproductive status, which can account for differences in prevalence, risk factors, fibrosis, and clinical outcomes in NAFLD.97
Future directions
Research investigating the distinct effects of n-3 supplementation in separate male and female populations is warranted. This highlights a gap in the evidence for DHA-based adult studies and ALA-based child/adolescent studies. Both sources have shown improvements in cardiometabolic risk biomarkers.34,72 However, their effects on NAFLD parameters have yet to be extensively evaluated. Furthermore, none of the RCTs identified in the present review examined plant-based EPA or EPA and DHA combined (mainly found in algal oils),34 which are thought to be the most health advantageous sources of n-3.98 Further RCTs are now needed to investigate the potential benefits of ALA from flaxseed oil and EPA/DHA- and DHA-rich algal oil on NAFLD biomarkers using appropriate placebo/controls, larger numbers of patients with NAFLD, and longer study durations to address these limitations. In addition to the analysis of NAFLD surrogate markers, provision for additional noninvasive markers such as magnetic resonance imaging should be included in future studies.
CONCLUSION
When combined with lifestyle interventions to increase physical activity and a calorie-controlled diet, plant-based n-3 fatty acid supplementation leads to improvements in ALT enzyme biomarkers, TGs, BMI, WC, and weight loss, therefore offering a potential adjunct to the first-line treatment of lifestyle modification (ie, losing 7–10% of weight by diet and exercise). Further well-designed human studies are needed to better understand the beneficial effects offered by plant-based n-3 fatty acids, with and without lifestyle and dietary interventions and with a specific focus on the different effects of ALA, and plant-based EPA and DHA using larger numbers of patients with NAFLD and longer study durations.
Supplementary Material
Acknowledgment
The authors thank Mrs. Jackie Fealey (Librarian, Liverpool John Moores University) for support and assistance with the database searches.
Author contributions. The authors’ contributions were as follows—K.E.L. and I.P. conceptualized the review and formulated the systematic search strategy; I.G.D. assisted with the formulation of search strategies; K.E.L. and E.M. conducted the database searches; K.E.L., E.M., I.G.D., and I.P. assessed article eligibility and risk of bias; K.E.L., E.M., and A.J. performed data extraction; M.M. and A.J. conducted the meta-analysis; K.E.L. and E.M. drafted the manuscript; I.P., I.G.D., R.P.K., and L.N. critically evaluated and amended draft manuscripts. All authors read and approved the final manuscript.
Funding. No external funding was received to support this work.
Declaration of interest. The authors have no relevant interests to declare.
Abbreviations: ALA, alpha-linolenic acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; GGT, γ-glutamyl transferase; HDL, high density lipoprotein; HOMA-IR, homeostatic model of insulin resistance; Hs-CRP, high-sensitivity C-reactive protein; LDL, low density lipoprotein; MD, mean difference; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; RCT, randomized controlled trial; TC, total cholesterol; TG, triglyceride; WC, waist circumference.
Abbreviations:
- ALA
alpha-linolenic acid
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- GGT
γ-glutamyl transferase
- HDL
high density lipoprotein
- HOMA-IR
homeostatic model of insulin resistance
- Hs-CRP
high-sensitivity C-reactive protein
- LDL
low density lipoprotein
- MD
mean difference
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- RCT
randomized controlled trial
- TC
total cholesterol
- TG
triglyceride
- WC
waist circumference
Contributor Information
Ella Moore, Research Institute for Sport and Exercise Sciences, Liverpool John Moores University, Liverpool, United Kingdom.
Imran Patanwala, Manchester Royal Infirmary, Manchester, United Kingdom.
Alireza Jafari, Physiology Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran.
Ian G Davies, Research Institute for Sport and Exercise Sciences, Liverpool John Moores University, Liverpool, United Kingdom.
Richard P Kirwan, Research Institute for Sport and Exercise Sciences, Liverpool John Moores University, Liverpool, United Kingdom.
Lisa Newson, School of Psychology, Liverpool John Moores University, Liverpool, United Kingdom.
Mohsen Mazidi, Medical Research Council Population Health Research Unit, Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom; Department of Twin Research and Genetic Epidemiology, King’s College London, London, United Kingdom.
Katie E Lane, Research Institute for Sport and Exercise Sciences, Liverpool John Moores University, Liverpool, United Kingdom.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Appendix S1 NAFLD plant n-3 search strategy
Figure S2 NAFLD n-3 Galbraith plots
Appendix S3 NAFLD n-3 plant PRISMA 2020 checklist
References
- 1. Lazarus JV, Mark HE, Anstee QM, et al. ; NAFLD Consensus Consortium.Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol. 2022;19:60–78. doi: 10.1038/s41575-021-00523-4. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3. Mitra S, De A, Chowdhury A.. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. 2020;5:16–16. doi: 10.21037/tgh.2019.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scorletti E, Byrne CD.. Omega-3 fatty acids and non-alcoholic fatty liver disease: evidence of efficacy and mechanism of action. Mol Aspects Med. 2018;64:135–146. doi: 10.1016/j.mam.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 5. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 6. Abeysekera KW, Fernandes GS, Hammerton G, et al. Prevalence of steatosis and fibrosis in young adults in the UK: a population-based study. Lancet Gastroenterol Hepatol. 2020;5:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. British Liver Trust. The alarming impact of liver disease in the UK—facts and statistics. 2019. Available at: https://britishlivertrust.org.uk/wp-content/uploads/The-alarming-impact-of-liver-disease-FINAL-June-2019.pdf. Accessed August 5, 2021.
- 8. NHS Digital. Health Survey for England, 2019: adult and child overweight and obesity report. 2020. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2019. Accessed August 5, 2021.
- 9. Houttu V, Csader S, Nieuwdorp M, et al. Dietary interventions in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Nutr. 2021;8:1–15. doi: 10.3389/fnut.2021.716783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. NHS Digital. Statistics on obesity, physical activity and diet, England, 2021. 2021. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet/england-2021#highlights. Accessed May 10, 2022.
- 11. Eurostat. European Health Interview Survey—overweight and obesity. BMI statistics. 2019. Available at: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Overweight_and_obesity_-_BMI_statistics#Obesity_in_the_EU:_gender_differences. Accessed June 8, 2021.
- 12. World Health Organization. Obesity and overweight—key facts. Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed May 20, 2022.
- 13. Ratziu V, Friedman SL.. Why do so many NASH trials fail? Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.05.046. [DOI] [PubMed] [Google Scholar]
- 14. Puri P, Baillie RA, Wiest MM, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763 [DOI] [PubMed] [Google Scholar]
- 15. Paik JM, Mir S, Alqahtani SA, et al. Dietary risks for liver mortality in NAFLD: global burden of disease data. Hepatol Commun. 2022;6:90–100. doi: 10.1002/hep4.1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Golabi P, Paik JM, AlQahtani S, et al. Burden of non-alcoholic fatty liver disease in Asia, the Middle East and North Africa: data from Global Burden of Disease 2009-2019. J Hepatol. 2021;75:795–809. doi: 10.1016/j.jhep.2021.05.022 [DOI] [PubMed] [Google Scholar]
- 17. Yang J, Fernández-Galilea M, Martínez-Fernández L, et al. Oxidative stress and non-alcoholic fatty liver disease: effects of omega-3 fatty acid supplementation. Nutrients. 2019;11:872. doi: 10.3390/nu11040872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yan J-H, Guan B-J, Gao H-Y, et al. Omega-3 polyunsaturated fatty acid supplementation and non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97:e12271. doi: 10.1097/MD.0000000000012271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Musa-Veloso K, Venditti C, Lee HY, et al. Systematic review and meta-analysis of controlled intervention studies on the effectiveness of long-chain omega-3 fatty acids in patients with nonalcoholic fatty liver disease. Nutr Rev. 2018;76:581–602. doi: 10.1093/nutrit/nuy022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu L, Yuan M, Wang L.. The effect of omega-3 unsaturated fatty acids on non-alcoholic fatty liver disease: a systematic review and meta-analysis of RCTs. Pak J Med Sci. 2017;33:1022–1028. doi: 10.12669/pjms.334.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He X-X, Wu X-L, Chen R-P, et al. Effectiveness of omega-3 polyunsaturated fatty acids in non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. PLoS One. 2016;11:e0162368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parker HM, Johnson NA, Burdon CA, et al. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944–951. doi: 10.1016/j.jhep.2011.08.018 [DOI] [PubMed] [Google Scholar]
- 23. Sun S-X, Hua X-M, Deng Y-Y, et al. Tracking pollutants in dietary fish oil: from ocean to tablet. Environ Pollut. 2018;240:733–744. doi: 10.1016/j.envpol.2018.05.027 [DOI] [PubMed] [Google Scholar]
- 24. Betanzos-Robledo L, Téllez-Rojo MM, Lamadrid-Figueroa H, et al. Differential fat accumulation in early adulthood according to adolescent-BMI and heavy metal exposure. New Dir Child Adolesc Dev. 2022;2022:37–51. doi: 10.1002/cad.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kitessa SM, Abeywardena M, Wijesundera C, et al. DHA-containing oilseed: a timely solution for the sustainability issues surrounding fish oil sources of the health-benefitting long-chain omega-3 oils. Nutrients. 2014;6:2035–2058. doi: 10.3390/nu6052035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jovanovic S, Dietrich D, Becker J, et al. Microbial production of polyunsaturated fatty acids—high-value ingredients for aquafeed, superfoods, and pharmaceuticals. Curr Opin Biotechnol. 2021;69:199–211. doi: 10.1016/j.copbio.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 27. Graça J, Godinho CA, Truninger M.. Reducing meat consumption and following plant-based diets: current evidence and future directions to inform integrated transitions. Trends in Food Science & Technology. 2019/09/. 2019;91:380–390. doi: 10.1016/j.tifs.2019.07.046 [DOI] [Google Scholar]
- 28. Fortuna G. EU pressed to address meat reduction, plant-based diet in new food policy. Available at: https://www.euractiv.com/section/agriculture-food/news/eu-pressed-to-address-meat-reduction-plant-based-diet-in-new-food-policy/. Accessed August 5, 2021.
- 29. Hadi A, Askarpour M, Salamat S, et al. Effect of flaxseed supplementation on lipid profile: an updated systematic review and dose-response meta-analysis of sixty-two randomized controlled trials. Pharmacol Res. 2020;152:104622. doi: 10.1016/j.phrs.2019.104622. [DOI] [PubMed] [Google Scholar]
- 30. Bernstein AM, Ding EL, Willett WC, et al. A meta-analysis shows that docosahexaenoic acid from algal oil reduces serum triglycerides and increases HDL-cholesterol and LDL-cholesterol in persons without coronary heart disease. J Nutr. 2012;142:99–104. doi: 10.3945/jn.111.148973 [DOI] [PubMed] [Google Scholar]
- 31. Masjedi MS, Pour PM, Shokoohinia Y, et al. Effects of flaxseed on blood lipids in healthy and dyslipidemic subjects: a systematic review and meta-analysis of randomized controlled trials. Curr Prob Cardiol. 2022;47:100931. doi: 10.1016/j.cpcardiol.2021.100931. [DOI] [PubMed] [Google Scholar]
- 32. Craddock JC, Neale EP, Probst YC, et al. Algal supplementation of vegetarian eating patterns improves plasma and serum docosahexaenoic acid concentrations and omega-3 indices: a systematic literature review. J Hum Nutr Diet. 2017;30:693–699. doi: 10.1111/jhn.12474. [DOI] [PubMed] [Google Scholar]
- 33. Ryan L, Symington AM.. Algal-oil supplements are a viable alternative to fish-oil supplements in terms of docosahexaenoic acid (22:6n-3; DHA). J Funct Foods. 2015;19:852–858. doi: 10.1016/j.jff.2014.06.023 [DOI] [Google Scholar]
- 34. Lane KE, Wilson M, Hellon TG, et al. Bioavailability and conversion of plant based sources of omega-3 fatty acids—a scoping review to update supplementation options for vegetarians and vegans. Crit Rev Food Sci Nutr. 2022;62:4982–4997. doi: 10.1080/10408398.2021.1880364 [DOI] [PubMed] [Google Scholar]
- 35. EFSA Panel on Dietetic Products Nutrition and Allergies. Scientific Opinion on the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. 2012;10:2815. doi: 10.2903/j.efsa.2012.2815. [DOI] [Google Scholar]
- 36. Thoma C, Day CP, Trenell MI.. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255–266. doi: 10.1016/j.jhep.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 37. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kelley GA, Kelley KS.. Systematic reviews and meta-analysis in nutrition research. Br J Nutr. 2019;122:1279–1294. doi: 10.1017/s0007114519002241. [DOI] [PubMed] [Google Scholar]
- 39. Lane KE. The effect of plant-based omega-3 fatty acid supplementation on non-alcoholic fatty liver disease (NAFLD) biomarkers: a systematic review and meta-analysis. Available at: https://www.crd.york.ac.uk/PROSPERO/#searchadvanced. Accessed November 29, 2021.
- 40. Mozaffarian D. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence. Alternat Ther Health Med. 2005;11:24–30. [PubMed] [Google Scholar]
- 41. Fleming JA, Kris-Etherton PM.. The evidence for α-linolenic acid and cardiovascular disease benefits: comparisons with eicosapentaenoic acid and docosahexaenoic acid. Adv Nutr. 2014;5:863S–876S. doi: 10.3945/an.114.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dyson JK, McPherson S, Anstee QM.. Non-alcoholic fatty liver disease: non-invasive investigation and risk stratification. J Clin Pathol. 2013;66:1033–1045. doi: 10.1136/jclinpath-2013-201620. [DOI] [PubMed] [Google Scholar]
- 43. Deprince A, Haas JT, Staels B.. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol Metab. 2020;42:101092. doi: 10.1016/j.molmet.2020.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 45. Borenstein M, Hedges LV, Higgins JP, et al. Introduction to Meta-Analysis. Chichester: Wiley; 2009. [Google Scholar]
- 46. Yari Z, Cheraghpour M, Alavian SM, et al. The efficacy of flaxseed and hesperidin on non-alcoholic fatty liver disease: an open-labeled randomized controlled trial. Eur J Clin Nutr. 2021;75:99–111. doi: 10.1038/s41430-020-0679-3. [DOI] [PubMed] [Google Scholar]
- 47. Yari Z, Rahimlou M, Eslamparast T, et al. Flaxseed supplementation in non-alcoholic fatty liver disease: a pilot randomized, open labeled, controlled study. Int J Food Sci Nutr. 2016;67:461–469. doi: 10.3109/09637486.2016.1161011. [DOI] [PubMed] [Google Scholar]
- 48. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 49. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989x.11.2.193. [DOI] [PubMed] [Google Scholar]
- 50. Willis BH, Riley RD.. Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice. Stat Med. 2017;36:3283–3301. doi: 10.1002/sim.7372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rezaei S, Sasani MR, Akhlaghi M, et al. Flaxseed oil in the context of a weight loss programme ameliorates fatty liver grade in patients with non-alcoholic fatty liver disease: a randomised double-blind controlled trial. Br J Nutr. 2020;123:994–1002. doi: 10.1017/s0007114520000318. [DOI] [PubMed] [Google Scholar]
- 52. Yari Z, Rahimlu M, Poustchi H, et al. Effect of flaxseed supplementation on liver enzymes, hepatic fibrosis and steatosis in nonalcoholic fatty liver disease: a randomized-controlled clinical trial. Iran J Nutr Sci Food Technol. 2016;10:1–12. [Google Scholar]
- 53. Pacifico L, Bonci E, Di Martino M, et al. A double-blind, placebo-controlled randomized trial to evaluate the efficacy of docosahexaenoic acid supplementation on hepatic fat and associated cardiovascular risk factors in overweight children with nonalcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2015;25:734–741. doi: 10.1016/j.numecd.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 54. Nobili V, Alisi A, Della Corte C, et al. Docosahexaenoic acid for the treatment of fatty liver: randomised controlled trial in children. Nutr Metab Cardiovasc Dis. 2013;23:1066–1070. doi: 10.1016/j.numecd.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 55. Nobili V, Bedogni G, Alisi A, et al. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child. 2011;96:350–353. doi: 10.1136/adc.2010.192401. [DOI] [PubMed] [Google Scholar]
- 56. Della Corte C, Carpino G, De Vito R, et al. Docosahexanoic acid plus vitamin D treatment improves features of NAFLD in children with serum vitamin D deficiency: results from a single centre trial. PLoS One. 2016;11:e0168216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Morris DH. Flax: a health and nutrition primer. Flax Council of Canada. 2007. Available at: https://flaxcouncil.ca/resources/nutrition/technical-nutrition-information/flax-a-health-and-nutrition-primer/. Accessed May 10, 2022.
- 58. Motamed N, Khoonsari M, Panahi M, et al. The incidence and risk factors of non-alcoholic fatty liver disease: a cohort study from Iran. Hepat Mon. 2020;20:E98531. doi: 10.5812/hepatmon.98531. [DOI] [Google Scholar]
- 59. Marchesini G, Bugianesi E, Burra P, et al. Non-alcoholic fatty liver disease in adults 2021: a clinical practice guideline of the Italian Association for the Study of the Liver (AISF), the Italian Society of Diabetology (SID) and the Italian Society of Obesity (SIO). Nutr Metabol Cardiovasc Dis. 2022;32:1–16. doi: 10.1016/j.numecd.2021.04.028 [DOI] [PubMed] [Google Scholar]
- 60. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. [DOI] [PubMed] [Google Scholar]
- 61. Mach F, Baigent C, Catapano AL, et al. ; ESC Scientific Document Group.2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 62. Lee C-H, Fu Y, Yang S-J, et al. Effects of omega-3 polyunsaturated fatty acid supplementation on non-alcoholic fatty liver: a systematic review and meta-analysis. Nutrients. 2020;12:2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mundi MS, Velapati S, Patel J, et al. Evolution of NAFLD and its management. Nutr Clin Pract. 2020;35:72–84. doi: 10.1002/ncp.10449. [DOI] [PubMed] [Google Scholar]
- 64. Marchesini G, Marzocchi R, Agostini F, et al. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol. 2005;16:421–427. [DOI] [PubMed] [Google Scholar]
- 65. Lepretti M, Martucciello S, Burgos Aceves MA, et al. Omega-3 fatty acids and insulin resistance: focus on the regulation of mitochondria and endoplasmic reticulum stress. Nutrients. 2018;10:350. doi: 10.3390/nu10030350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lalia AZ, Lanza IR.. Insulin-sensitizing effects of omega-3 fatty acids: lost in translation? Nutrients. 2016;8:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Golabi P, Paik J, Fukui N, et al. Patients with lean nonalcoholic fatty liver disease are metabolically abnormal and have a higher risk for mortality. Clin Diabetes. 2019;37:65–72. doi: 10.2337/cd18-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 69. Kasper P, Martin A, Lang S, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110:921–937. doi: 10.1007/s00392-020-01709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Labenz C, Prochaska JH, Huber Y, et al. Cardiovascular risk categories in patients with nonalcoholic fatty liver disease and the role of low-density lipoprotein cholesterol. Hepatol Commun. 2019;3:1472–1481. doi: 10.1002/hep4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Masoodi M, Gastaldelli A, Hyötyläinen T, et al. Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat Rev Gastroenterol Hepatol. 2021;18:835–856. doi: 10.1038/s41575-021-00502-9. [DOI] [PubMed] [Google Scholar]
- 72. Yue H, Qiu B, Jia M, et al. Effects of α-linolenic acid intake on blood lipid profiles: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2021;61:2894–2910. doi: 10.1080/10408398.2020.1790496. [DOI] [PubMed] [Google Scholar]
- 73. Naghshi S, Aune D, Beyene J, et al. Dietary intake and biomarkers of alpha linolenic acid and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of cohort studies. BMJ. 2021;375:n2213. doi: 10.1136/bmj.n2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. National Institute for Health and Care Excellence. Non-alcoholic fatty liver disease (NAFLD): assessment and management. 2016. Available at: https://www.nice.org.uk/guidance/ng49. Accessed January 25, 2022. [PubMed]
- 75. Katsagoni CN, Georgoulis M, Papatheodoridis GV, et al. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: a meta-analysis. Metabolism. 2017;68:119–132. doi: 10.1016/j.metabol.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 76. Mason RP, Libby P, Bhatt DL.. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2020;40:1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ghasemi Fard S, Wang F, Sinclair AJ, et al. How does high DHA fish oil affect health? A systematic review of evidence. Crit Rev Food Sci Nutr. 2019;59:1684–1727. [DOI] [PubMed] [Google Scholar]
- 78. Mariamenatu AH, Abdu EM.. Overconsumption of omega-6 polyunsaturated fatty acids (PUFAs) versus deficiency of omega-3 PUFAs in modern-day diets: the disturbing factor for their “balanced antagonistic metabolic functions” in the human body. J Lipids. 2021;2021:8848161. doi: 10.1155/2021/8848161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cui J, Li L, Ren L, et al. Dietary n-3 and n-6 fatty acid intakes and NAFLD: a cross-sectional study in the United States. Asia Pac J Clin Nutr. 2021;30:87–98. [DOI] [PubMed] [Google Scholar]
- 80. Farzanegi P, Dana A, Ebrahimpoor Z, et al. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): roles of oxidative stress and inflammation. Eur J Sport Sci. 2019;19:994–1003. doi: 10.1080/17461391.2019.1571114. [DOI] [PubMed] [Google Scholar]
- 81. Li F, Ye J, Shao C, et al. Compositional alterations of gut microbiota in nonalcoholic fatty liver disease patients: a systematic review and meta-analysis. Lipids Health Dis./. 2021;20:22. doi: 10.1186/s12944-021-01440-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mantovani A, Turino T, Altomari A, et al. Association between Helicobacter pylori infection and risk of nonalcoholic fatty liver disease: an updated meta-analysis. Metabolism. 2019;96:56–65. Juldoi: 10.1016/j.metabol.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 83. Milosevic I, Vujovic A, Barac A, et al. Gut-liver axis. Int J Mol Sci. 2019;20:395. doi: 10.3390/ijms20020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rinella ME, Tacke F, Sanyal AJ, et al. ; Participants of the AASLD/EASL Workshop.Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. J Hepatol. 2019;71:823–833. doi: 10.1016/j.jhep.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 85. Park J-M, Jeong M, Kim E-H, et al. Omega-3 polyunsaturated fatty acids intake to regulate Helicobacter pylori-associated gastric diseases as nonantimicrobial dietary approach. Biomed Res Int. 2015;2015:712363. doi: 10.1155/2015/712363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–1269. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 87. Noureddin M, Loomba R.. Nonalcoholic fatty liver disease: indications for liver biopsy and noninvasive biomarkers. Clin Liver Dis (Hoboken). 2012;1:104–107. doi: 10.1002/cld.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Long MT, Gandhi S, Loomba R.. Advances in non-invasive biomarkers for the diagnosis and monitoring of non-alcoholic fatty liver disease. Metabolism. 2020;111s:154259. doi: 10.1016/j.metabol.2020.154259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stine JG, Munaganuru N, Barnard A, et al. Change in MRI-PDFF and histologic response in patients with nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19:2274–2283, e5. doi: 10.1016/j.cgh.2020.08.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Orci LA, Gariani K, Oldani G, et al. Exercise-based interventions for nonalcoholic fatty liver disease: a meta-analysis and meta-regression. Clin Gastroenterol Hepatol. 2016;14:1398–1411. doi: 10.1016/j.cgh.2016.04.036 [DOI] [PubMed] [Google Scholar]
- 91. Ghafoor K, Özcan MM, AL-Juhaımı F, et al. Nutritional composition, extraction, and utilization of wheat germ oil: a review. Eur J Lipid Sci Technol. 2017;119:1600160. doi: 10.1002/ejlt.201600160 [DOI] [Google Scholar]
- 92. Vadarlis A, Antza C, Bakaloudi DR, et al. Systematic review with meta-analysis: the effect of vitamin E supplementation in adult patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2021;36:311–319. doi: 10.1111/jgh.15221 [DOI] [PubMed] [Google Scholar]
- 93. Sato K, Gosho M, Yamamoto T, et al. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Nutrition. 2015;31:923–930. doi: 10.1016/j.nut.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 94. Abdel-Maboud M, Menshawy A, Menshawy E, et al. The efficacy of vitamin E in reducing non-alcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Therap Adv Gastroenterol. 2020;13:1756284820974917. doi: 10.1177/1756284820974917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hoofnagle JH, Van Natta ML, Kleiner DE. et al. ; Non-alcoholic Steatohepatitis Clinical Research Network (NASH CRN).Vitamin E and changes serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38:134–143. Jul doi: 10.1111/apt.12352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhao H, Yang A, Mao L, et al. Association between dietary fiber intake and non-alcoholic fatty liver disease in adults. Front Nutr. 2020;7:593735. doi: 10.3389/fnut.2020.593735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lonardo A, Nascimbeni F, Ballestri S, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70:1457–1469. doi: 10.1002/hep.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kris-Etherton PM, Grieger JA, Etherton TD.. Dietary Reference Intakes for DHA and EPA. Prostaglandins Leukot Essent Fatty Acids. 2009;81:99–104. doi: 10.1016/j.plefa.2009.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.