Abstract
Eleven small, acid-soluble proteins (SASP) which are present in spores but not in growing cells of Bacillus subtilis were identified by sequence analysis of proteins separated by acrylamide gel electrophoresis of acid extracts from spores which lack the three major SASP (α, β, and γ). Six of these proteins are encoded by open reading frames identified previously or by analysis of the complete sequence of the B. subtilis genome, including two minor α/β-type SASP (SspC and SspD) and a putative spore coat protein (CotK). Five proteins are encoded by short open reading frames that were not identified as coding regions in the analysis of the complete B. subtilis genomic sequence. Studies of the regulation of two of the latter genes, termed sspG and sspJ, showed that both are expressed only in sporulation. The sspG gene is transcribed in the mother cell compartment by RNA polymerase with the mother cell-specific sigma factor for RNA polymerase, ςK, and is cotranscribed with a downstream gene, yurS; sspG transcription also requires the DNA binding protein GerE. In contrast, sspJ is transcribed in the forespore compartment by RNA polymerase with the forespore-specific ςG and appears to give a monocistronic transcript. A mutation eliminating SspG had no effect on sporulation or spore properties, while loss of SspJ caused a slight decrease in the rate of spore outgrowth in an otherwise wild-type background.
Dormant spores of Bacillus subtilis contain a number of proteins which are not present in growing cells, including spore coat proteins, components of the spore germination apparatus, a few unique spore enzymes, and a group of small, acid-soluble spore proteins (SASP) (38, 39). Among the latter proteins are the multiple α/β-type SASP and the single γ-type SASP; three of these proteins (SASP α, β, and γ) make up the great majority of all SASP in spores (38, 40). However, B. subtilis spores also contain a number of minor SASP, and similar minor proteins are present in spores of other Bacillus species (16, 37, 38, 46). While one of the minor SASP in B. subtilis is a minor α/β-type SASP termed SspC (46), the identity of the other minor proteins is not known.
Identification and analysis of these additional minor SASP and study of the regulation of their coding genes may be of interest for a number of reasons. First, since the minor SASP are undoubtedly very small, it is possible that their coding regions were not identified as open reading frames (ORFs) in the recently completed B. subtilis genomic sequence (18). Identification of any new ORFs will thus assist in completion of the analysis of the genomic sequence. Second, if the new minor SASP are indeed spore-specific proteins, then their coding genes should exhibit sporulation-specific expression. Study of the regulation of expression of these new genes, and in particular of their dependence on sporulation-specific sigma factors for RNA polymerase and their promoter sequences, would thus expand our knowledge of regulation of sporulation-specific genes. Finally and most importantly, several SASP, particularly the major α/β-type SASP, have major functions in the dormant spore in (i) providing resistance to spore DNA against damage caused by heat and oxidizing agents (6, 40); (ii) altering spore DNA photochemistry, thus providing a major element of spore UV resistance (25, 36, 40); and (iii) generating free amino acids for protein synthesis by their degradation early in spore germination (38). It is certainly possible that the new minor SASP has redundant functions in the spore, and thus loss of only one may have no phenotypic effect or at most a minor one, as is the case for the two major α/β-type SASP (25, 40). However, the essential role of the latter proteins in several of the properties unique to or characteristic of bacterial spores suggests that the new minor SASP might also have some specific function in sporulation, spores, or spore germination. Consequently, mutagenesis of the genes encoding these new minor SASP, alone or in various combinations, might give new insight into mechanisms determining various aspects of sporulation, spore properties, and spore germination. Given these reasons, we have determined the N-terminal amino acid sequences of minor B. subtilis SASP and have identified the genes encoding 11 of these proteins; five of these genes were not identified as ORFs in the B. subtilis genomic sequence. We also report detailed studies on the regulation of expression and function of two of the latter genes, both of which are new sporulation-specific genes.
MATERIALS AND METHODS
Bacterial strains and spore preparation.
Escherichia coli TG1 (33) and DH5α (11) were used for cloning; the B. subtilis strains used in this study are listed in Table 1. B. subtilis PS482 was used for identification of minor SASP, as this strain carries deletions of the sspA, -B, and -E genes, which code for the three major B. subtilis SASP, α, β, and γ, respectively (9). This strain (termed α−β−γ−) was sporulated at 37°C in 2× SG medium (8), and the spores were purified and stored as described previously (29). Growing cells of strain PS482 were prepared in the same medium but harvested in the late log phase of growth (optical density at 600 nm [OD600] ≅ 1) and washed once with 0.15 M NaCl, and the cell pellet fraction was frozen and lyophilized. B. subtilis strains with the PS832 background were used to study sspG and sspJ expression and for analysis of the phenotypes of the sspG and sspJ mutants; B. subtilis strains with a PY79 genetic background were used for studies of the genetic dependence of sspG and sspJ expression. PS832 and PY79 are very similar wild-type strains of B. subtilis, but PS832 sporulates slightly more efficiently, while a number of mutations in genes for sporulation sigma factors are available in the PY79 background. All transformations of B. subtilis strains were carried out as described previously (1).
TABLE 1.
B. subtilis strains used in this study
| Strain | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| PS482a | ΔsspA ΔsspB ΔsspE Cmrb (α−β−γ−) | 9 |
| PS832a | Wild-type derivative of strain 168 | Laboratory stock |
| PY79c | Wild type | 47 |
| IB464a | sspG::sspG-lacZ Cmr | pIB454→PS832 |
| IB465a | sspJ::sspJ-lacZ Cmr | pIB452→PS832 |
| IB488a | ΔsspG-yurS::spc Spr d | This work |
| IB490a | ΔsspJ::spc Spr | This work |
| IB466c | spoIIAC sspG::sspG-lacZ Cmr | IB464→SC1159 |
| IB467c | spoIIGB sspG::sspG-lacZ Cmr Emre | IB464→SC137 |
| IB468c | spoIIIG sspG::sspG-lacZ Cmr | IB464→SC500 |
| IB469c | spoIVCB sspG::sspG-lacZ Cmr Emr | IB464→SC64 |
| IB470c | sspG::sspG-lacZ Cmr | IB464→PY79 |
| IB471c | spoIIAC sspJ::sspJ-lacZ Cmr | IB465→SC1159 |
| IB472c | spoIIGB sspJ::sspJ-lacZ Cmr Emr | IB465→SC137 |
| IB473c | spoIIIG sspJ::sspJ-lacZ Cmr | IB465→SC500 |
| IB474c | spoIVCB sspJ::sspJ-lacZ Cmr Emr | IB465→SC64 |
| IB475c | sspJ::sspJ-lacZ Cmr | IB465→PY79 |
| IB502c | sspG::sspG-lacZ pspac-sigK sigKΔ19 Cmr Spr | IB470→BZ536 |
| IB480c | sspJ::sspJ-lacZ spoIIIG [pSDA4] Cmr Kmrf | pSDA4→IB473 |
| IB481c | sspJ::sspJ-lacZ spoIIIG [pDG298] Cmr Kmr | pDG298→IB473 |
| IB492g | sspG::sspG-lacZ trpC2 Cmr | IB464→SG38 |
| IB494g | gerE36 sspG::sspG-lacZ trpC2 Cmr | IB464→522.2 |
| IB498g | SpβcotA-lacZ trpC2 Cmr Emr | Spβ::cotA-lacZ→SG38 |
| IB511a | ΔsspG-yurS::spcΔ sspA ΔsspB ΔsspE Cmr Spr | IB488→PS482 |
| IB515a | ΔsspJ::spc ΔsspA ΔsspB ΔsspE Cmr Spr | IB490→PS482 |
| SC64c | spoIVCB Emr | S. Cutting |
| SC137c | spoIIGB Emr | S. Cutting |
| SC500c | spoIIIGΔ1 | S. Cutting |
| SC1159c | spoIIAC1 | S. Cutting |
| BZ536c | Pspac-PsigK sigKΔ19 Spr | L. Kroos |
| 522.2g | gerE36 trpC2 | L. Kroos |
| SG38g | trpC2 | J. Errington |
Genetic background is PS832.
Cmr, chloramphenicol (5 μg/ml) resistance.
Genetic background is PY79.
Spr, spectinomycin (100 μg/ml) resistance.
Emr, erythromycin (1 μg/ml) resistance.
Kmr, kanamycin (10 μg/ml) resistance.
Derivatives of B. subtilis strain CU267 (originally obtained from S. A. Zahler).
Identification and analysis of minor SASP.
Lyophilized dormant spores (100 mg [dry weight]) or late-log-phase cells were broken in a dental amalgamator (Wig-L-Bug) with glass beads (100 mg) as the abrasive for either 10 min (spores) or 2 min (growing cells). The dry powder was extracted twice at 4°C for 30 min with 5 ml of 3% acetic acid, and the supernatant fluids were combined and dialyzed against 1 liter of 1% acetic acid in Spectrapor 3 tubing (molecular weight cutoff, 3,500) for 16 h at 6°C with two changes. The pellet from the acetic acid extract was further extracted twice with 5 ml of 0.3 N HCl at 4°C, and the supernatant fluids were pooled and dialyzed as described above. The dialyzed material was lyophilized, and the dry residue was dissolved in 200 μl of 8 M urea plus 100 μl of acid gel diluent (31). Aliquots of the redissolved material were subjected to polyacrylamide gel electrophoresis (PAGE) at low pH (31), proteins were electrophoretically transferred to polyvinylidine difluoride paper (Immobilon) in 10% methanol–0.7% acetic acid for 45 min at 200 mA (24), and the proteins on the paper were stained lightly with Coomassie blue, destained, and air dried. Selected protein bands were cut from the paper with a clean razor blade and subjected to protein sequence analysis as described previously (46).
For analysis of minor SASP in decoated spores, 55 mg (dry weight) of spores of strain PS482 were decoated in 8 M urea–1% sodium dodecyl sulfate (SDS)–50 mM dithiothreitol–10 mM EDTA–50 mM Tris-HCl (pH 8.0) for 90 min at 37°C, and the spores were washed as described previously (29). The decoated spores were lyophilized, and SASP were extracted as described above. In other experiments, wild-type and mutant spores were boiled in SDS-PAGE loading buffer as described previously (13), and low-molecular-weight soluble proteins were analyzed by SDS-PAGE (35). For analysis of minor SASP in germinated spores, 50 mg (dry weight) of spores of strain PS482 in 2 ml of water was heat shocked for 30 min at 70°C and then cooled on ice. The spores were germinated for 45 min in 200 ml of prewarmed (37°C) 2× YT medium ([per liter] tryptone, 16 g; yeast extract, 10 g; NaCl, 5 g) containing 4 mM l-alanine to stimulate the initiation of spore germination, harvested by centrifugation, washed once with 100 ml of 0.15 M NaCl, lyophilized and broken with 8 min of dry rupture, extracted with both acetic acid and HCl, dialyzed, lyophilized, redissolved, and analyzed as described above.
For analysis of minor SASP in strains with mutations in new ssp genes, spores were prepared as described above, and 75 OD600 U of cleaned spores were lyophilized, disrupted, extracted twice with 3% acetic acid (1 ml), dialyzed, lyophilized, and redissolved as described above, and aliquots were analyzed by PAGE at low pH (31).
Construction of B. subtilis strains containing translational sspG- and sspJ-lacZ fusions.
Fragments encompassing 220 bp upstream of the sspG ORF as well as 22 bp of the coding region or 201 bp upstream of the sspJ ORF and 20 bp of the coding region were amplified by PCR. The primers used for sspG were prot2TXN5′ (5′-GGAATTCGAGATGATAAGCCGTCG-3′) and prot2TXN3′ (5′-CGGGATCCTTTTCATGACGATTTTCGCTC-3′). The primers used for sspJ were prot3-5′ (5′-GGAATTCGCGATGCCTCCCATGATG-3′) and prot3-3′ (5′-CGGGATCCTCTTTATTAAAGAAACCCATTC-3′). In all cases, the primers had extra residues at the 5′ end, including an EcoRI or a BamHI site (underlined). The PCR fragments were cut with EcoRI and BamHI and cloned between the EcoRI and BamHI sites of pJF751, a vector for construction of translational lacZ fusions (7). The resulting plasmids, termed pIB454 (sspG) and pIB452 (sspJ), were integrated into the PS832 chromosome by a single crossover event with selection for Cmr. Transformants containing a single copy of the translational sspG- or sspJ-lacZ fusion at the sspG or sspJ locus as shown by Southern blot analysis were called strains IB464 and IB465, respectively. Chromosomal DNA was isolated from these strains and used to transform B. subtilis strains containing different spo mutations in the PY79 background or strain 522.2 to Cmr.
Analysis of β-galactosidase activity in sporulating cells, spores, and vegetative cells.
Sporulation of B. subtilis was induced at 37°C by the resuspension method (43) or by the nutrient exhaustion method in 2× SG medium (8, 20), and samples (1 ml each) were harvested by centrifugation and stored frozen. Strains carrying genes encoding ςF, ςG, or ςK under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible spac promoter (Pspac) were grown at 37°C in 2× YT medium to an OD600 of 0.25. The culture was then divided in half, IPTG was added to 2 mM to one-half, incubation was continued, and samples were taken and stored frozen as described above. β-Galactosidase activity was determined with o-nitrophenyl-β-d-galactopyranoside as the substrate as described previously (29); lysozyme (200 μg/ml) was used for cell permeabilization prior to enzyme assay. To analyze β-galactosidase activity in spores, the spores were first decoated and then treated with lysozyme prior to enzyme assays as described previously (29). All β-galactosidase specific activities are expressed in Miller units (27).
Determination of the sspG-yurS and sspJ transcription start sites.
Total RNA was extracted from cells of strains IB464 and IB465 sporulating in 2× SG medium as described previously (28). The RNA was used in primer extension reactions at 47°C with avian myeloblastosis virus reverse transcriptase (28). The primers used were prot2-55 (5′-CTTTTGCTAATCCGCTGTTTTGG-3′), which anneals to sspG mRNA; yurS-140 (5′-GTTAAGAGGAATGATGTTTTCGTTC-3′), which anneals to yurS mRNA; prot3-50 (5′-TCTTCAAGAGCTCCTTGGATTAC-3′), which anneals to sspJ mRNA; and lacZ-70 (5′-AAGGCGATTAAGTTGGGTAACG-3′), which anneals to the lacZ portion of sspG-lacZ or sspJ-lacZ mRNAs. Size standards for analysis of the primer extension products were produced with the same four primers in DNA sequencing reactions. The prot2-55 and yurS-140 primers were used with plasmid pIB517, which carries a 1,055-bp fragment encompassing the sspG and yurS region (see below); the prot3-50 primer was used with plasmid pIB460, which carries a 1,190-bp fragment encompassing the sspJ region (see below); and the lacZ-70 primer was used with plasmids pIB454 and pIB452, which carry the translational sspG-lacZ and sspJ-lacZ fusions, respectively, in plasmid pJF751.
Cloning of a fragment encompassing the sspG-yurS and sspJ regions.
A fragment encompassing 1,055 bp of the sspG-yurS region was amplified by PCR. The primers used were prot2TXN5′ (see above) and prot2mut4 (5′-GCTCTAGATCAAGACATGGCACTGG-3′). The PCR product was cloned in the TA-cloning vector pCR2.1 (Invitrogen) according to the manufacturer’s instructions, and the resulting plasmid was called pIB517.
A fragment encompassing 1,190 bp of the sspJ region was also amplified by PCR. The primers used were prot3mut5′ (5′-GGAATTCGCGGATCGTGGAAGGG-3′) and prot3mut3′ (5′-CGGGATCCACGGACTCGCAATTGAAGC-3′); the primers had extra residues at the 5′ end, including an EcoRI or a BamHI site (underlined). The PCR product was cut with EcoRI and BamHI and cloned between the EcoRI and BamHI sites of pSGMU2 (30), giving plasmid pIB460.
Construction of an sspG-yurS null mutant.
A 490-bp fragment containing the region directly upstream of the sspG ORF was amplified by PCR. The primers used were prot2mut1 (5′-GGAATTCTTATACGCCCTTTCCTCC-3′) and prot2mut2 (5′-AACTGCAGGTATCATCCTTTCTCTATTG-3′), each containing extra residues, including an EcoRI or PstI site at their 5′ ends (underlined). The PCR fragment was cut with EcoRI and PstI and cloned between the EcoRI and PstI sites of plasmid pJL74 (19), which contains an Spr cassette, giving plasmid pIB458. A 539-bp fragment containing the region starting 149 bp downstream of the sspG ORF and encompassing the second half of the yurS ORF was amplified by PCR. The primers used were prot2mut3 (5′-CGGGATCCGCCGCAAAGCAGATGAC-3′) and prot2mut4new (5′-ATAAGAATGCGGCCGCATCAAGACATGGCACTGG-3′), each containing extra residues, including a BamHI or NotI site at their 5′ ends (underlined). The PCR fragment was cut with BamHI and NotI and cloned between the BamHI and NotI sites of plasmid pIB458. The resulting plasmid (pIB479) contains the sspG flanking regions, with the Spr cassette replacing the sspG ORF and the first half of the yurS ORF. pIB479 was linearized with XhoI and used to transform B. subtilis PS832 to Spr (100 μg/ml). In this transformation the Spr cassette was integrated into the B. subtilis chromosome by a double crossover event removing the sspG ORF and the first half of the yurS ORF. One Spr transformant whose expected chromosomal structure was confirmed by Southern blot analysis (data not shown) was termed IB488. To construct an sspG-yurS null mutant that did not produce SASP α, β, and γ, we transformed strain PS482 to Spr with chromosomal DNA from strain IB488. The resulting Cmr Spr strain was called IB511.
Construction of the sspJ null mutant.
A 521-bp fragment containing the region immediately downstream of the sspJ ORF was amplified by PCR. The primers used were prot3mut1 (5′-GGAATTCGCTCCAAACGGACTCGC-3′) and prot3mut2 (5′-AACTGCAGCCACATGCGGATAGGGC-3′), each containing extra residues, including an EcoRI or PstI site at their 5′ ends (underlined). The PCR fragment was cut with EcoRI and PstI and cloned between the EcoRI and PstI sites of plasmid pJL74 (19), giving plasmid pIB476. A 513-bp fragment containing the first 7 bp of the sspJ ORF and 506 upstream bp was amplified by PCR. The primers used were prot3mut3 (5′-CGGGATCCAACCCATTCGTATCACCTC-3′) and prot3mut4 (5′-TCCCCGCGGTGATTCCGTTCACCGTCC-3′), each containing extra residues, including a BamHI or SacII site at their 5′ ends (underlined). The PCR fragment was cut with BamHI and SacII and cloned between the BamHI and SacII sites of plasmid pIB476. The resulting plasmid (pIB482) contains the sspJ flanking regions, with the Spr cassette replacing the sspJ ORF. pIB482 was linearized with XhoI and used to transform B. subtilis PS832 to Spr. In this transformation the Spr cassette was integrated into the B. subtilis chromosome by a double crossover event, removing the sspJ ORF. One Spr transformant whose chromosomal structure was confirmed by Southern blot analysis (data not shown) was termed IB490. To construct an sspJ null mutant which does not produce SASP α, β, and γ, we transformed strain PS482 to Spr with chromosomal DNA from strain IB490. The resulting Cmr Spr strain was called IB515.
Analysis of resistance, germination, and outgrowth of B. subtilis spores.
Spores were harvested from cultures grown for 48 h at 37°C in 2× SG medium and purified as described previously (25, 29). Spores in water were heat treated (85°C) or UV irradiated with 254-nm light, and survival was measured as described previously (6, 25). For analyses of spore lysozyme and chloroform resistance, spores were diluted to an OD600 of 1 in 10 mM potassium phosphate buffer (pH 7.4) containing 50 mM KCl and 1 mM MgSO4 and treated with lysozyme at 1.5 mg per ml for 30 min at 37°C or with chloroform as described previously (29).
For spore germination and outgrowth, purified spores in water were heat activated for 30 min at 65°C (for spores lacking SASP α, β, and γ) or 70°C, cooled on ice, and then diluted to an OD600 of 0.4 to 0.5 in 2× YT medium containing 4 mM l-alanine or an OD600 of 0.7 to 0.9 in Spizizen’s minimal medium (42) without Casamino Acids but containing 4 mM l-alanine and 50 μg of l-tryptophan/ml. Cultures were incubated at 37°C with good aeration, and the OD600s of the cultures were monitored.
RESULTS
Identification of new SASP and their coding genes.
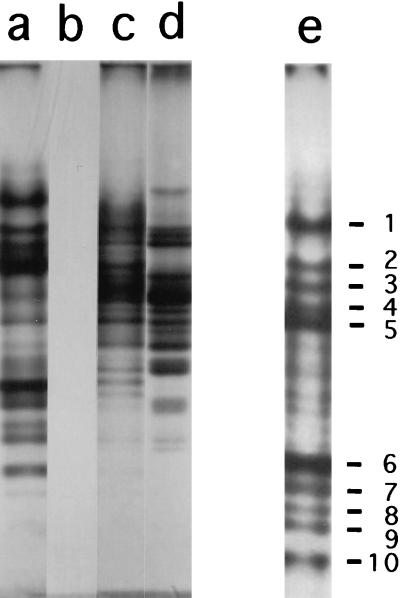
PAGE at low pH of acetic acid extracts of B. subtilis spores (termed α−β−γ−) lacking the three major SASP gave a series of protein bands, none of which were present in acetic acid extracts of growing cells (Fig. 1, lanes a and b). The levels of at least some of these acetic acid-soluble proteins unique to spores were similar in both α−β−γ− and wild-type spores (data not shown); however, the presence of SASP α, β, and γ precluded the observation of several of the minor protein bands in extracts of wild-type spores. Consequently, all routine analysis of these proteins was carried out with α−β−γ− spores. Subsequent extraction of α−β−γ− spores with HCl dissolved more of the proteins that were present in the acetic acid extract as well as a number of additional proteins (Fig. 1, lane c). However, many of these latter additional proteins appeared to be present in the HCl extract of growing cells (Fig. 1, lane d). Consequently, we focused on the acetic acid-soluble proteins from α−β−γ− spores and identified 10 distinct protein bands which were present in significant amounts in acetic acid extracts from spores but not in HCl extracts of growing cells (Fig. 1, lane e). Automated protein sequence analysis showed that band 8 was the product of the cotK gene, while band 5 has been shown previously to be the minor α/β-type SASP SspC (Table 2) (46). Band 4 gave two different protein sequences, one identical to the product of an additional gene, sspD, coding for a minor α/β-type SASP (SspD), with the other sequence derived from an ORF encoded by a gene of unknown function termed ysfA. All other bands gave single unambiguous amino acid sequences. Two were products of additional ORFs of unknown function which had been identified in the B. subtilis genomic sequencing project, while five were products of small ORFs in intergenic regions which had not previously been identified as coding genes (Table 2). None of these five new proteins exhibits any significant sequence similarity to known proteins (data not shown). Since the new proteins identified are small, acid-soluble proteins that appear unique to spores, with two exceptions we have termed them SASP and their coding genes ssp (Table 2). The exceptions are (i) the gene encoding band 2, which had been termed tlp, based on the encoded protein’s homology to thioredoxin; and (ii) the gene encoding band 8, termed cotK, for which there is some evidence that the encoded protein is a spore coat protein (12).
FIG. 1.
Low-pH PAGE analysis of minor SASP from B. subtilis. Acetic acid and HCl extracts from dormant spores and growing cells of strain PS482 were prepared and redissolved as described in Materials and Methods, aliquots were run on PAGE at low pH, either the gel was stained with Coomassie blue (lanes a to d) or proteins were transferred to polyvinylidene difluoride paper, and the paper was stained with Coomassie blue (lane e). The samples (and amounts) of redissolved extract run in the various lanes were as follows: a and e, acetic acid extract of spores (20 μl); b, acetic acid extract of growing cells (20 μl); c, HCl extract of spores (20 μl); and d, HCl extract of growing cells (5 μl). The numbers adjacent to lane e denote protein bands present in spores but not in growing cells and are the numbers of the bands analyzed in Table 2.
TABLE 2.
Characterization of minor SASP in B. subtilis sporesa
| Protein bandb | N-terminal sequence | Gene identified in B. subtilis genomec | No. of amino acid residuesd | New gene designation |
|---|---|---|---|---|
| 1 | SENRHENEENRRDA- | 3353125 to 3353268 | 47 | sspG |
| 2 | TKNQNQYQQPN- | tlpe | 82 | tlp |
| 3 | MNIQRAKEIVES- | yfjU | 59 | sspH |
| 4 | ASRNKLVVPGVE- and MDLNLRHAVIAN- | sspD | 63 | sspD |
| ysfA | 71 | sspI | ||
| 5 | AQQSRSRSNNNN-f | sspC | 71 | sspC |
| 6 | GFFNKDKGKRSE- | 3420667 to 3420530 | 45 | sspJ |
| 7 | VRNKEKGFPYEN- | 927771 to 927622 | 49 | sspK |
| 8 | VKRKANHVINGM- | cotK | 47 | cotK |
| 9 | MKKKDKGRLTGG- | 2310101 to 2310226 | 42 | sspL |
| 10 | MKTRPKKAGQQK- | 2338912 to 2339013 | 34 | sspM |
Protein bands were generated and sequenced as described in Materials and Methods.
As labeled in Fig. 1, lane e.
Gene names are as in the B. subtilis genomic sequence available on the World Wide Web at www.pasteur.fr/Bio/SubtiList.html. Numbers given are the locations in the genome of regions coding for proteins not previously identified as ORFs. The first and second numbers are those of the first nucleotide of the start codon and the last nucleotide of the coding sequence, respectively.
Residues in the intact protein; the N-terminal methionine of a number of the proteins is removed posttranslationally.
This was the major sequence observed; however, there were also significant amounts of the same sequence but with an N-terminal methionine (∼20% of the total) or beginning at the first lysine residue (∼30% of the total).
Sequence from reference 46.
All of the proteins identified in this study are small, 34 to 83 amino acids in length (Table 2), as is expected given their acid solubility. Analysis of the levels of the new minor SASP in spores from which the majority of coat proteins were removed, as described in Materials and Methods, showed that none, including CotK and SspG (data not shown), were removed by the extraction procedure used. However, for a number of the new proteins, the great majority (>80%) disappeared after 45 min of spore germination (see Materials and Methods); the proteins that disappeared included SspC, SspD, SspH, SspI, SspK, SspL, SspM, and Tlp, while CotK, SspG, and SspJ did not (<25%) disappear during spore germination (data not shown).
Properties of sspG and sspJ.
While the new SASP described above appeared unique to the spore, we decided to investigate this in more detail by examining the regulation of expression of two of the genes encoding these proteins. The two we chose for this detailed analysis were sspG and sspJ.
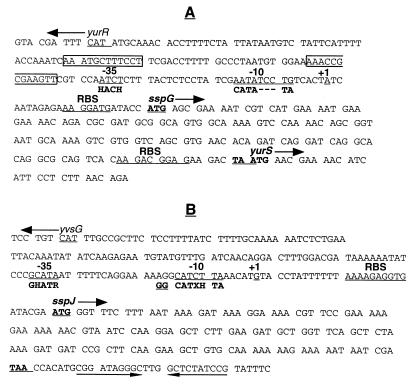
The sspG ORF has 48 codons and is located in the intergenic region between two divergently oriented ORFs encoded by yurR and yurS (Fig. 2A). The sspG gene is preceded by a good ribosome binding site, but there is no obvious transcription terminator after the gene. Since the last nucleotide of the sspG stop codon is the first nucleotide of the yurS start codon and there is a transcription terminator immediately downstream of yurS (18), sspG and yurS may constitute an operon in which the two genes are translationally coupled. There are 158 bp between the sspG start codon and the start codon of the upstream divergently oriented gene yurR, which is more than enough space to accommodate a prokaryotic promoter.
FIG. 2.
Nucleotide sequence of the sspG-yurS (A) and sspJ (B) regions. (A) The sequence shown includes nucleotides 3352955 to 3353300 in the B. subtilis genome. The sspG start and stop codons are in bold and underlined. The start codon for yurR is underlined, and that for yurS is in bold. Sequences corresponding to −10 and −35 promoter elements and the sspG and yurS ribosome binding sites (RBS) as well as those corresponding to the start of transcription of sspG-yurS (+1) are underlined. The consensus −35 and −10 sequences for ςK-dependent promoters (10, 48) are shown in bold below these elements in the sspG sequence; the abbreviation used in the −35 consensus sequence is H for A or C. The boxed residues denote putative GerE binding sites (32); one is from positions 3353061 to 3353050 on the strand transcribed to give sspG, and the other is from positions 3353013 to 3353024 on the nontranscribed strand. (B) The sequence shown includes nucleotides 3420842 to 3420491 in the B. subtilis genome (note that the direction of transcription of sspJ is counterclockwise). The sspJ start and stop codons are in bold and underlined; the start codon for yvsG is underlined. Sequences corresponding to −10 and −35 promoter elements and the sspJ ribosome binding site (RBS) as well as those corresponding to the start of transcription (+1) are underlined. The consensus −10 and −35 sequences for ςG-dependent promoters (10) are shown in bold below these elements in the sspJ sequence. The designations in the consensus sequence are H for A or C, R for A or G, and X for A or T. The two underlined G residues upstream of the −10 consensus sequence show the position of the two G residues in promoters recognized primarily by ςF (44). The apposed arrows denote the putative transcriptional terminator of sspJ.
The sspJ ORF has 46 codons and is located between two divergently oriented ORFs, yvsG upstream and yvsH downstream (Fig. 2B). The coding region of sspJ is preceded by a good ribosome binding site and is followed by a strong potential transcriptional terminator. There are 166 bp between the start codon of sspJ and the upstream divergently oriented yvsG start codon and 369 bp between the sspJ’s terminator and the yvsH start codon. Consequently, sspJ appears likely to give only a monocistronic transcript.
Expression and regulation of sspG and sspJ during sporulation.
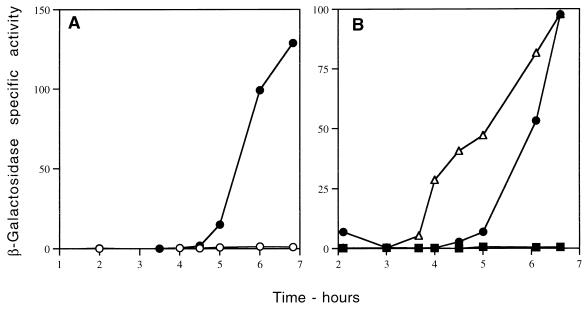
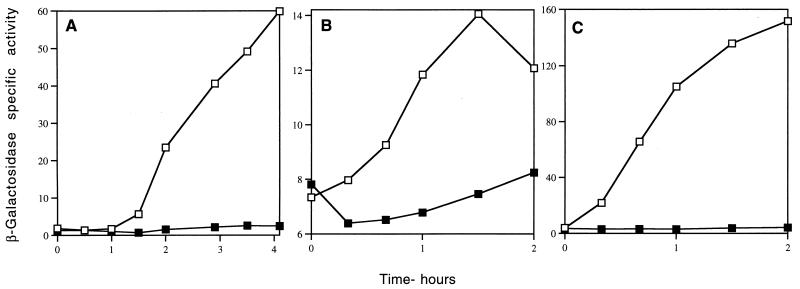
To examine sspG and sspJ expression, we integrated translational sspG- and sspJ-lacZ fusions at the sspG and sspJ loci, respectively, and measured β-galactosidase activity during vegetative growth and sporulation and in dormant spores. No significant expression of either fusion was observed in vegetatively growing cells (data not shown and see below). However, the sspG-lacZ fusion was expressed beginning ∼4 h after the induction of sporulation, with maximum β-galactosidase specific activity attained ∼6 h after induction of sporulation (Fig. 3A and data not shown); the sspJ-lacZ fusion was expressed beginning at ∼2.5 h after induction of sporulation, with maximum specific activity attained ∼4 h after induction of sporulation (Fig. 4A). No detectable β-galactosidase activity was found in purified spores of the sspG-lacZ fusion, but ∼100% of sspJ-driven β-galactosidase was found in purified spores (data not shown). These data indicate that both sspG and sspJ are sporulation-specific genes which are likely expressed in the mother cell and the forespore compartment, respectively, of the sporulating cell.
FIG. 3.
Expression of the translational sspG-lacZ fusion in various spo mutants. Strains with a PY79 background (A) and a CU267 background (B) were sporulated by the resuspension method, and β-galactosidase was assayed as described in Materials and Methods. Time 0 is when sporulation was initiated. The symbols used for the various strains are as follows. (A) •, IB470 (spo+); ○, IB469 (spoIVCB), (B) •, IB492 (spo+); ■, IB494 (gerE36); ▵, IB498 (this strain does not contain an sspG-lacZ fusion but rather has a cotA-lacZ fusion in a spo+ background).
FIG. 4.
Expression of the translational sspJ-lacZ fusion in various spo mutants. Strains with a PY79 background were sporulated by the resuspension method, and β-galactosidase was assayed as described in Materials and Methods. Time 0 is when sporulation was initiated. The symbols used for the various strains are as follows. (A) •, IB475 (spo+); ▵, IB474 (spoIVCB), (B) □, IB471 (spoIIAC); ■, IB472 (spoIIGB); ○, IB473 (spoIIIG).
To analyze the genetic dependence of sspG expression further, we examined the expression of the sspG-lacZ fusion in different spo mutant backgrounds. Mutations in all genes coding for sporulation-specific RNA polymerase sigma factors (spoIIAC [ςF], spoIIGB [ςE], spoIIIG [ςG], and spoIVCB [ςK]) abolished expression of the sspG-lacZ fusion (Fig. 3A and data not shown). These data indicate that sspG expression likely depends on the sigma factor which is last in the sporulation regulatory cascade, the late mother cell-specific sigma factor ςK, encoded in part by spoIVCB. Some ςK-dependent genes require the transcriptional regulator GerE for their transcription (10, 32); consequently, we also introduced the sspG-lacZ fusion into a gerE mutant and again measured β-galactosidase activity during sporulation (Fig. 3B). Since the expression of the sspG-lacZ fusion was abolished in the gerE mutant, this indicates that GerE is necessary to activate sspG expression, thus placing sspG in the last temporal class of mother cell-specific genes, those dependent on both ςK and GerE. Indeed, sspG expression is switched on 1 h later in sporulation than is the expression of cotA, a gene requiring only ςK for its expression (Fig. 3B) (10, 34).
In contrast to the results with sspG, a mutation in spoIVCB did not block sspJ-lacZ expression during sporulation (Fig. 4A). However, a mutation in spoIIIG, which codes for the late forespore-specific sigma factor ςG, decreased the level of sspJ-driven lacZ expression to only ∼5% of that of the wild-type level (Fig. 4B), and a mutation in the spoIIAC gene, which codes for the early forespore-specific sigma factor ςF, essentially abolished sspJ-lacZ expression (Fig. 4B). Since ςF is required for synthesis of ςG (10), these data indicate that sspJ is a forespore-specific gene which is transcribed primarily by EςG and to a very small extent by EςF. The level of expression of the sspJ-lacZ fusion in a spoIIGB mutant lacking the mother cell-specific ςE was higher than in a ςG mutant (Fig. 4B), as was observed previously for some other genes dependent at least in part on ςF (17, 21–23).
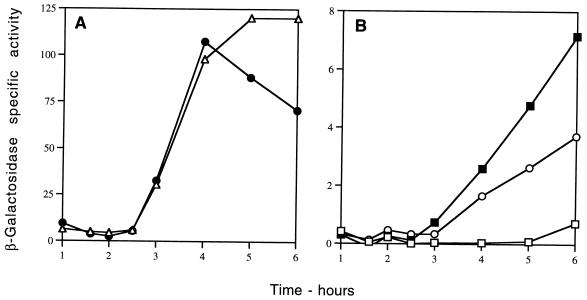
To provide additional evidence that ςK is able to direct the transcription of sspG, we used B. subtilis IB502, which contains the sspG-lacZ fusion and a chromosomal copy of the structural gene for the mature form of ςK under the control of the IPTG-inducible Pspac. While vegetatively growing cells of this strain had no β-galactosidase activity, upon IPTG induction of ςK synthesis, a significant increase in β-galactosidase activity was observed starting ∼1 h after addition of IPTG (Fig. 5A). In a parallel experiment, with a similar strain containing a cotA-lacZ fusion, β-galactosidase activity increased immediately after addition of IPTG (data not shown). This difference is likely due to the requirement for both ςK and GerE for sspG expression, in contrast to cotA, which needs only ςK. The 1-h delay in β-galactosidase synthesis from the sspG-lacZ fusion after the addition of IPTG is presumably the time needed for the ςK-dependent gerE gene to be expressed and the GerE protein to accumulate to a level sufficient to stimulate sspG expression.
FIG. 5.
Induction of expression in vegetative growing cells of sspG-lacZ in cells expressing ςK (A) or sspJ-lacZ in cells expressing ςF (B) or ςG (C). Cells of strains IB502 (sspG-lacZ Pspac-ςK) (A), IB480 (sspJ-lacZ Pspac-ςF) (B), or IB481 (sspJ-lacZ Pspac-ςG) (C) were grown at 37°C in 2× YT medium. An OD600 of 0.25 (time 0 in the figure), the cultures were divided in half, one-half was made 2 mM in IPTG, incubation was continued, and samples were taken from both cultures for assay of β-galactosidase. ■, without IPTG; □, with IPTG. Note the different scales in panels B and C.
To prove conclusively that ςG and, to a lesser extent, ςF are able to direct the transcription of sspJ, we introduced plasmid pSDA4 (41), which contains the structural gene for ςF under Pspac control into the strain containing the sspJ-lacZ fusion as well as a mutation in spoIIIG. While vegetatively growing cells of this strain had no β-galactosidase activity, upon induction of ςF synthesis with IPTG, a significant increase in β-galactosidase activity was observed (Fig. 5B), showing that ςF was able to direct some expression of sspJ-lacZ. However, vegetative cells containing plasmid pDG298 (45) carrying spoIIIG under Pspac control gave more than 10-fold-higher expression of the sspJ-lacZ fusion upon induction of ςG synthesis (Fig. 5C). Therefore, we conclude that sspJ is transcribed primarily by EςG but can also be recognized to a small extent by ςF. However, it is unclear whether this ςF-dependent expression is of any functional significance.
Localization of the sspG-yurS and sspJ promoters.
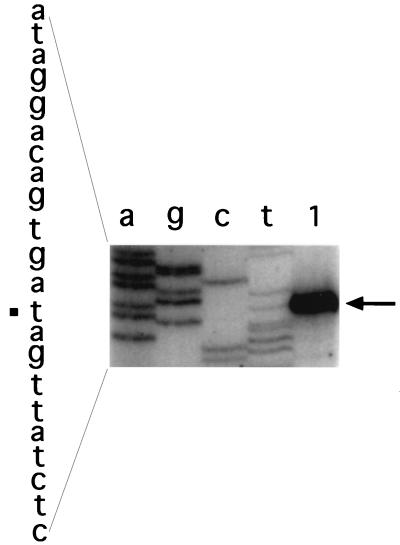
To localize the sspG promoter and to determine if yurS is also transcribed from the same promoter, we carried out primer extension analysis with RNA from sporulating cells of strain IB464 containing the translational sspG-lacZ fusion at the sspG locus. Three different primers were used for this analysis; one was annealed to the lacZ portion of sspG-lacZ mRNA, another annealed only to the sspG mRNA, and the third was complementary to RNA transcribed from yurS. All three primers gave the same transcription start site, indicating that sspG and yurS are indeed transcribed from the same promoter, with transcription initiating 23 nt upstream of the sspG AUG codon, at an A residue (Fig. 2 and 6 and data not shown). Additionally, sequences centered approximately 10 and 35 nt upstream of the transcription start site show good similarity to the −10 and −35 consensus sequences recognized by ςK, and upstream of the −35 element are several putative GerE binding sites (see Discussion).
FIG. 6.
Primer extension analysis of the start site for transcription of sspG and yurS. RNA from cells of strain IB464 was isolated 7.5 h into sporulation, and the primer extension product was obtained and analyzed as described in Materials and Methods. The primer used is yurS-140, which anneals only to yurS. Lanes a, g, c, and t are DNA sequencing reactions with the same primer and plasmid pIB517; lane 1 is a primer extension reaction with sporulating-cell RNA. The primer extension product is marked with an arrow, and the transcription start site on the sspG upstream sequence to the left of the figure is marked with a square. Note that the sequence shown is the complement of the mRNA sequence.
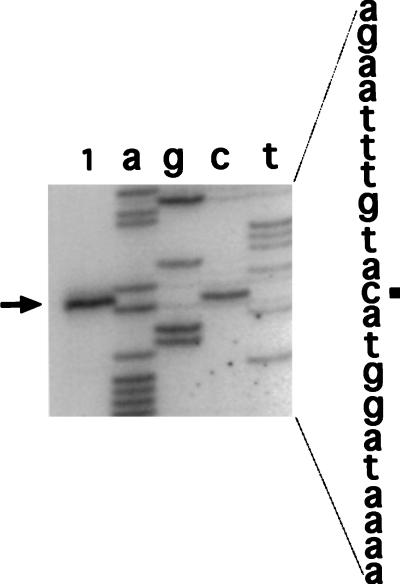
To localize the sspJ promoter by primer extension analysis, we used RNA from sporulating cells of strain IB465 containing the translational sspJ-lacZ fusion at the sspJ locus. Two different primers were used for this analysis; one annealed to the lacZ portion of sspJ-lacZ mRNA, and the other annealed only to the sspJ mRNA. Both primers gave the same start site for transcription, as transcription initiates 29 nt upstream of the sspJ AUG codon, at a G residue (Fig. 2 and 7 and data not shown). Sequences centered approximately 10 and 35 nucleotides upstream of the transcription start site also show good similarity to the −10 and −35 consensus sequences recognized by both ςG and ςF (see Discussion).
FIG. 7.
Primer extension analysis of the sspJ transcription start site. RNA from cells of strain IB465 was isolated 5 h into sporulation, and primer extension products were obtained and analyzed as described in Materials and Methods. The primer used is prot3-50, which anneals only to sspJ mRNA. Lanes a, g, c, and t are DNA sequencing reactions with the same primer and plasmid pIB460; lane 1 is the primer extension reaction with sporulating-cell RNA. The primer extension product is marked with an arrow, and the transcription start site on the sspJ upstream sequence to the right of the figure is marked with a square. Note that the sequence shown is the complement of the mRNA sequence.
Characterization of sspG-yurS and sspJ null mutants.
While it was clear that both SspG and SspJ were spore-specific gene products, it was not clear if these proteins had any function in the spore or any role in sporulation or spore germination. Consequently, we generated and analyzed sspG and sspJ null mutants. In the sspG-yurS mutant, the entire sspG ORF and the first half of the yurS ORF were removed, and a Spr cassette was inserted; in the sspJ mutant the Spr cassette was substituted for the sspJ ORF. To confirm that the mutations eliminated synthesis of SspG and SspJ, we transferred each of the mutations into a strain lacking the three major SASP, purified spores of the resultant strains, and extracted and analyzed SASP from these spores by PAGE at low pH. As expected, the bands corresponding to SspG and SspJ were absent from the extracts of the sspG-yurS and sspJ mutant spores, respectively (data not shown). In an attempt to localize SspG, and possibly YurS, to spore coats, we also extracted coat proteins from wild-type and sspG yurS spores by boiling and analyzed the extracts by SDS-PAGE as described in Materials and Methods. However, the coat proteins revealed by this analysis had molecular weights that did not even approximate those of the proteins that were absent from the mutant spores (data not shown).
Despite the absence of SspG (and presumably YurS) or SspJ, mutant strains lacking these proteins sporulated normally, with the kinetics and yield of phase-bright spores being identical to those of the wild-type strain, and the mutant spores had the same resistance to heat and UV radiation as did the wild-type spores (data not shown). The mutant spores exhibited no defect in the initiation of spore germination, as measured by the initial fall in OD of a spore culture following mixing of spores with germinant (data not shown). However, sspJ spores did have a slight defect in spore outgrowth in a minimal medium, as they returned to vegetative growth slightly more slowly than did wild-type spores (data not shown); this was observed with two different spore preparations (data not shown). However, sspG-yurS spores had no such outgrowth defect (data not shown). We also compared the resistance and germination properties of spores of the sspG-yurS α−β−γ− and sspJ α−β−γ− strains and the parental α−β−γ− strain. Again, there were no differences in the resistance properties or germination properties of spores of these strains (data not shown). In addition, the outgrowth kinetics of sspJ α−β−γ− spores were identical to those of α−β−γ− spores (data not shown), although the rate of outgrowth of the latter spores is significantly slower than that of wild-type spores in a minimal medium (9).
DISCUSSION
The identification of 10 new proteins in spores from their amino-terminal sequences confirms that four ORFs identified by sequence analysis of the B. subtilis genome (tlpA, yfjU, ysfA, and cotK) do indeed code for proteins and also identifies five new coding genes which were not identified as such in the analysis of the genome’s sequence. All five of the latter code for quite small proteins, which is presumably why the genes were not initially identified as coding regions. However, the translational initiation codon for all five is ATG, the preferred initiation codon in B. subtilis, and these are preceded by sequences with good to reasonable homology to the 3′ end of 16s rRNA, which is presumably a ribosome binding site; all coding sequences terminate with TAA, which is also the preferred termination codon in B. subtilis (18). The new proteins, however, appear present in spores at rather low levels. Previous work has shown that the level of SspC, the most prominent minor SASP, is only ∼35% of that of SASP-β, indicating that SspC is ∼0.5% of total spore protein, since SASP-β is ∼1.5% (38, 46). Comparison of the intensities of the other minor SASP bands with those of SspC indicates that each, including the minor α/β-type SASP SspD, comprises only 0.05 to 0.2% of total spore protein, assuming that all bands stain equally. Even though these latter values are rather low, given the small size of these proteins, there are clearly a large number of molecules of each of them per spore.
All SASP analyzed previously in B. subtilis have been shown to be located in the spore core, and the presence of the β-galactosidase expressed from an sspJ-lacZ fusion in the dormant spore, specifically the spore core, suggests that SspJ is also present in the spore core. The situation with SspG, however, is different, as sspG is clearly expressed in the mother cell and β-galactosidase from an sspG-lacZ fusion is not found in the spore. These data are consistent with SspG being a spore coat protein, but we were unable to detect SspG in spore coat extracts. While SspG might be in the spore cortex, it is not obvious why it should move there. Clearly, further work, possibly using direct protein localization techniques such as immunofluorescence or immunoelectron microscopy, will be needed to definitively establish the location of SspG in the spore.
The identification of SspD in spores brings to four the number of α/β-type SASP identified in B. subtilis spores, which is the number of genes encoding α/β-type SASP present in the B. subtilis genome (18). Interestingly, one Bacillus species, B. megaterium, has at least seven genes encoding α/β-type SASP (38, 40), with the additional genes encoding minor proteins. The reason(s) for the loss of multiple genes encoding α/β-type SASP in B. subtilis (or their gain in B. megaterium) is not clear, but it is known that deletion of genes encoding at least one minor α/β-type SASP has no obvious phenotypic effect in B. subtilis (38, 40).
Information available to date indicates that all the minor SASP identified in this work are sporulation-specific proteins. This was shown previously for SspC and SspD, whose coding genes are expressed only in the developing forespore during sporulation under the control of ςG (26). The two genes studied in this work, sspG and sspJ, are also sporulation specific, and preliminary work in our laboratory has shown that expression of tlp, sspH, and sspL is also sporulation specific (4). If, as seems likely, given the absence of obvious vegetative cell protein bands comigrating with CotK, SspI, SspK, and SspM, all of these new genes are indeed sporulation specific, a question to ask is what function, if any, is served by these genes or their products. As shown in this work, deletion of sspG (and also yurS) has no obvious phenotypic effect, while deletion of sspJ has only a minor effect on spore outgrowth in a wild-type genetic background. In previous work, we found that loss of another minor small spore-specific protein (but one which is not acid soluble) termed YhcN also causes a slight spore outgrowth defect (3). However, the reason(s) for these outgrowth defects is not clear. The combination of mutations deleting yhcN, sspJ, and other minor SASP may be responsible for a major phenotype. However, it also seems possible that the situation will be similar to that observed with deletions removing many of the spore coat proteins, as these mutations often have no discernible effect beyond the loss of an individual protein from spores (39). Perhaps these new minor SASP have no function individually, have a function that requires special conditions to become obvious, or have functions that are redundant with one or more other minor SASP. Indeed, the latter is the case for the minor α/β-type SASP SspC and SspF, as loss of these proteins has no noticeable effect on spore resistance (24, 38, 40). However, in a strain lacking the major α/β-type SASP α and β, overexpression of SspC (or SspF) can restore some to much of the resistance to α−β− spores (24, 38, 40). Perhaps overexpression of some of the new minor SASP in spores (wild type or α−β−) may assist in elucidating their function.
The detailed analysis of the regulation of the two new ssp genes analyzed in this work indicates that transcription of the sspG operon is directed by EςK and also requires GerE, while transcription of sspJ depends primarily on EςG. Examination of the sequence upstream of the transcription start site of sspG reveals sequences with good matches to the consensus −10 and −35 sequences recognized by EςK (Fig. 2A). Slightly further upstream but still in the intergenic region between yurR and sspG are also two sequences with a reasonable match to the consensus sequence recognized and bound by GerE (Fig. 2A). Preliminary in vitro transcription of sspG templates with partially purified EςK has also given transcripts of the size expected based on the 5′ end of sspG mRNA determined in vivo, and this in vitro transcription by EςK was strongly stimulated by GerE (14). While the precise location of the GerE binding site(s) in the sspG promoter has not yet been determined, the analysis of sspG transcription has added another promoter sequence to define the ςK consensus recognition sequence. Upstream of the sspJ transcription start site are also −10 and −35 sequences, which match rather well the consensus −10 and −35 sequences recognized by EςG (Fig. 2B). The sspJ promoter was recognized not only by EςG but also to a slight degree by EςF, both during sporulation and upon induction of ςF synthesis in growing cells. The −10 and −35 promoter sequences recognized by ςG and ςF are quite similar, with a major difference being the presence of G residues at both positions −14 and −15 in good ςF promoters (2, 44). The presence of one G residue in these positions in the sspJ promoter is consistent with the poor but significant transcription of sspJ by EςF compared to that of EςG (44).
The transcription of the sspG yurS operon by EςK plus GerE suggests that SspG and YurS may well be spore coat proteins. However, SspG was not removed from spores by solubilization of much of the spore coat, and neither SspG nor YurS was detected in spore coat extracts. Since certainly SspG should be soluble in the solutions used for preparing spore coat extracts, the precise location of this protein in the spore is presently unclear. The product of the cotK gene was also previously suggested to be a coat protein (12), but again CotK was not removed from spores by an extraction procedure removing most spore coat proteins. Consequently, the identity of CotK as a coat protein is also problematic at present. However, neither CotK nor SspG was lost upon spore germination, in contrast to SspC, -D, -H, -I, -K, -L, and -M and Tlp, which were lost, presumably by their degradation. However, with the exception of SspC and -D, none of these latter proteins have sequences similar to the somewhat-loose recognition sequence of the spore-specific protease that initiates degradation of both α/β- and γ-type SASP during spore germination (5, 15, 38). Consequently, the identity of the protease that initiates degradation of Tlp and SspH, -I, -K, -L, and M during spore germination is not evident. Clearly, there is much yet to be learned about the metabolism and function of these minor spore proteins.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health, GM 19698.
We are grateful to John Leszyk for assistance with the protein sequencing, to Adam Driks for strains, and to Lee Kroos for advice and communication of unpublished results.
REFERENCES
- 1.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:741–748. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagyan I, Casillas-Martinez L, Setlow P. The katX gene, which codes for the catalase in spores of Bacillus subtilis, is a forespore-specific gene controlled by ςF, and KatX is essential for hydrogen peroxide resistance of the germinating spore. J Bacteriol. 1998;180:2057–2062. doi: 10.1128/jb.180.8.2057-2062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagyan I, Noback M, Bron S, Paidhungat M, Setlow P. Characterization of yhcN, a new forespore-specific gene of Bacillus subtilis. Gene. 1998;212:179–188. doi: 10.1016/s0378-1119(98)00172-3. [DOI] [PubMed] [Google Scholar]
- 4.Cabrera-Hernandez, A., J.-L. Sanchez-Salas, M. Paidhungat, and P. Setlow. 1998. Unpublished results. [DOI] [PubMed]
- 5.Carillo-Martinez Y, Setlow P. Properties of Bacillus subtilis small, acid-soluble spore proteins with changes in the sequence recognized by their specific protease. J Bacteriol. 1994;176:5357–5363. doi: 10.1128/jb.176.17.5357-5363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fairhead H, Setlow B, Setlow P. Prevention of DNA damage in spores and in vitro by small, acid-soluble proteins from Bacillus species. J Bacteriol. 1993;175:1367–1374. doi: 10.1128/jb.175.5.1367-1374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari E, Howard S M H, Hoch J A. Effect of sporulation mutations on subtilisin expression, assayed using a subtilisin–β-galactosidase gene fusion. In: Hoch J A, Setlow P, editors. Molecular biology of microbial differentiation. Washington, D.C: American Society for Microbiology; 1985. pp. 180–184. [Google Scholar]
- 8.Goldrick S, Setlow P. Expression of a Bacillus megaterium sporulation-specific gene in Bacillus subtilis. J Bacteriol. 1983;155:1459–1462. doi: 10.1128/jb.155.3.1459-1462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hackett R H, Setlow P. Properties of spores of Bacillus subtilis strains which lack the major small, acid-soluble protein. J Bacteriol. 1988;170:1403–1404. doi: 10.1128/jb.170.3.1403-1404.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 12.Henriques A O, Beall B W, Moran C P., Jr CotM of Bacillus subtilis, a member of the α-crystallin family of stress proteins, is induced during development and participates in spore outer coat formation. J Bacteriol. 1997;179:1887–1897. doi: 10.1128/jb.179.6.1887-1897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henriques A O, Beall B W, Roland K, Moran C P., Jr Characterization of cotJ, a ςE-controlled operon affecting the polypeptide composition of the coat of Bacillus subtilis spores. J Bacteriol. 1995;177:3394–3406. doi: 10.1128/jb.177.12.3394-3406.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichikawa, H., and K. Kroos. 1998. Unpublished results.
- 15.Illades-Aguiar B, Setlow P. Studies of the processing of the protease which initiates degradation of small, acid-soluble proteins during germination of spores of Bacillus species. J Bacteriol. 1994;176:2788–2795. doi: 10.1128/jb.176.10.2788-2795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson W C, Tipper D J. Acid-soluble spore proteins of Bacillus subtilis. J Bacteriol. 1981;146:972–982. doi: 10.1128/jb.146.3.972-982.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karow M L, Glaser P, Piggot P J. Identification of a gene, spoIIR, that links the activation of ςE to the transcriptional activity of ςF during sporulation in Bacillus subtilis. Proc Natl Acad Sci USA. 1995;92:2012–2016. doi: 10.1073/pnas.92.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunst F, Ogasawara N, et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 19.LeDeaux J R, Grossman A D. Isolation and characterization of kinC, a gene that encodes a sensor kinase homologous to the sporulation sensor kinases KinA and KinB in Bacillus subtilis. J Bacteriol. 1995;177:166–175. doi: 10.1128/jb.177.1.166-175.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leighton T J, Doi R H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971;246:3189–3195. [PubMed] [Google Scholar]
- 21.Lewis P J, Partridge S R, Errington J. ς factors, asymmetry, and the determination of cell fate in Bacillus subtilis. Proc Natl Acad Sci USA. 1994;91:3849–3853. doi: 10.1073/pnas.91.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Londono-Vallejo J-A, Stragier P. Cell-cell signalling pathway activating a developmental transcription factor in Bacillus subtilis. Genes Dev. 1995;9:503–508. doi: 10.1101/gad.9.4.503. [DOI] [PubMed] [Google Scholar]
- 23.Londono-Vallejo J-A, Frehel C, Stragier P. spoIIQ, a forespore-expressed gene required for engulfment in Bacillus subtilis. Mol Microbiol. 1997;24:29–39. doi: 10.1046/j.1365-2958.1997.3181680.x. [DOI] [PubMed] [Google Scholar]
- 24.Loshon C A, Kraus P, Setlow B, Setlow P. Effect of inactivation or overexpression of the sspF gene on properties of Bacillus subtilis spores. J Bacteriol. 1997;179:272–275. doi: 10.1128/jb.179.1.272-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason J M, Setlow P. Essential role of small, acid-soluble spore proteins in the resistance of Bacillus subtilis spores to UV light. J Bacteriol. 1986;167:174–178. doi: 10.1128/jb.167.1.174-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason J M, Hackett R H, Setlow P. Studies on the regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores using lacZ gene fusions. J Bacteriol. 1988;170:239–244. doi: 10.1128/jb.170.1.239-244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 28.Moran C P. Measuring gene expression in Bacillus. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, England: John Wiley and Sons; 1990. pp. 267–293. [Google Scholar]
- 29.Nicholson W L, Setlow P. Sporulation, germination and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, England: John Wiley and Sons; 1990. pp. 391–450. [Google Scholar]
- 30.Perego M. Integrational vectors for genetic manipulation in Bacillus subtilis. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics, p. 615–624. Washington, D.C: American Society for Microbiology; 1993. pp. 615–624. [Google Scholar]
- 31.Reisfeld R A, Lewis V J, Williams D E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- 32.Roels S, Losick R. Adjacent and divergently oriented operons under the control of the sporulation regulatory protein GerE in Bacillus subtilis. J Bacteriol. 1995;177:6263–6275. doi: 10.1128/jb.177.21.6263-6275.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Sandman K, Kroos L, Cutting S, Youngman P, Losick R. Identification of the promoter for a spore coat protein gene in Bacillus subtilis and studies on the regulation of its induction at a late stage of sporulation. J Mol Biol. 1988;200:461–473. doi: 10.1016/0022-2836(88)90536-0. [DOI] [PubMed] [Google Scholar]
- 35.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 36.Setlow B, Setlow P. Thymine-containing dimers as well as spore photoproducts are found in ultraviolet-irradiated Bacillus subtilis spores that lack small, acid-soluble proteins. Proc Natl Acad Sci USA. 1987;84:421–423. doi: 10.1073/pnas.84.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Setlow P. Purification and characterization of additional low-molecular-weight basic proteins degraded during germination of Bacillus megaterium spores. J Bacteriol. 1978;136:331–340. doi: 10.1128/jb.136.1.331-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Setlow P. Small, acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function and degradation. Annu Rev Microbiol. 1988;42:319–338. doi: 10.1146/annurev.mi.42.100188.001535. [DOI] [PubMed] [Google Scholar]
- 39.Setlow P. Spore structural proteins. In: Hoch J A, Losick R, Sonenshein A L, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 801–809. [Google Scholar]
- 40.Setlow P. Mechanisms for the prevention of damage to the DNA in spores of Bacillus species. Annu Rev Microbiol. 1995;49:29–54. doi: 10.1146/annurev.mi.49.100195.000333. [DOI] [PubMed] [Google Scholar]
- 41.Shazand K, Frandsen N, Stragier P. Cell-type specificity during development in Bacillus subtilis: the molecular and morphological requirements for ςE activation. EMBO J. 1995;14:1439–1445. doi: 10.1002/j.1460-2075.1995.tb07130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spizizen J. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci USA. 1958;44:1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sterlini J M, Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to the development of actinomycin resistance. Biochem J. 1969;113:29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun D, Fajardo-Cavazos P, Sussman M D, Tovar-Rojo F, Cabrera-Martinez R-M, Setlow P. Effect of chromosome location of Bacillus subtilis forespore genes on their spo gene dependence and transcription by EςF: identification of features of good EςF-dependent promoters. J Bacteriol. 1991;173:7867–7874. doi: 10.1128/jb.173.24.7867-7874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun D, Stragier P, Setlow P. Identification of a new ς-factor involved in compartmentalized gene expression during sporulation of Bacillus subtilis. Genes Dev. 1989;3:141–149. doi: 10.1101/gad.3.2.141. [DOI] [PubMed] [Google Scholar]
- 46.Tovar-Rojo F, Setlow P. Analysis of the effects of mutant small, acid-soluble spore proteins from Bacillus subtilis on DNA in vivo and in vitro. J Bacteriol. 1991;173:4827–4835. doi: 10.1128/jb.173.15.4827-4835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Youngman P, Perkins J, Losick R. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid. 1984;12:1–9. doi: 10.1016/0147-619x(84)90061-1. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Ichikawa H, Halberg R, Kroos L, Aronson A. Regulation of the transcription of a cluster of Bacillus subtilis coat genes. J Mol Biol. 1994;240:405–415. doi: 10.1006/jmbi.1994.1456. [DOI] [PubMed] [Google Scholar]