Abstract
Eukaryotic translation initiation factor 4E (eIF4E), which plays a pivotal role in initiating translation in eukaryotic organisms, is often hijacked by the viral genome‐linked protein to facilitate the infection of potyviruses. In this study, we found that the naturally occurring amino acid substitution D71G in eIF4E is widely present in potyvirus‐resistant watermelon accessions and disrupts the interaction between watermelon eIF4E and viral genome‐linked protein of papaya ringspot virus‐watermelon strain, zucchini yellow mosaic virus or watermelon mosaic virus. Multiple sequence alignment and protein modelling showed that the amino acid residue D71 located in the cap‐binding pocket of eIF4E is strictly conserved in many plant species. The mutation D71G in watermelon eIF4E conferred resistance against papaya ringspot virus‐watermelon strain and zucchini yellow mosaic virus, and the equivalent mutation D55G in tobacco eIF4E conferred resistance to potato virus Y. Therefore, our finding provides a potential precise target for breeding plants resistant to multiple potyviruses.
Keywords: eIF4E, Potyvirus, PRSV‐W, PVY, recessive resistance, ZYMV
The amino acid substitution D71G in the cap‐binding pocket of watermelon eIF4E and its equivalent substitution D55G in tobacco eIF4E confers resistance to multiple potyviruses by disrupting the interaction between eIF4E and VPg.

1. INTRODUCTION
Plant viruses cause serious diseases in crops and result in huge economic losses (Varanda et al., 2021). Developing and planting resistant cultivars is the most reliable and cost‐effective measure to control plant viral diseases (Guner et al., 2019). Resistance to plant viruses is mainly divided into two types: dominant resistance and recessive resistance. Dominant resistance genes recognize specific viral virulence genes to trigger immune responses (Truniger & Aranda, 2009), while recessive resistance genes usually encode essential susceptibility factors with mutations (Diaz‐Pendon et al., 2004). Compared with dominant resistance, recessive resistance usually provides more durable and broad‐spectrum protection against plant viruses, and thereby attracts the increasing attention of agronomists (Ahmar et al., 2020; Choudhary & Suresh, 2020; de Ronde et al., 2014; Li et al., 2022; Pavan et al., 2010).
Viruses of the genus Potyvirus in the family Potyviridae constitute the largest group of plant‐infecting RNA viruses and infect a wide range of plants (Gadhave et al., 2020). Most identified recessive resistance genes to potyviruses encode eukaryotic translation initiation factor 4E (eIF4E) family proteins (Kang et al., 2005; Lee et al., 2023; Liu et al., 2017). The viral genome‐linked protein (VPg) of potyviruses is covalently linked to the 5′ end of the viral RNA and interacts with eIF4E family proteins by serving as an m7G cap analogue of the mRNAs (Choudhary & Suresh, 2020; Khan et al., 2008). Previous studies have shown that many eIF4E‐mediated recessive resistance genes against potyviruses result from the eIF4E mutations that disrupt the interaction between VPg and eIF4E (Charron et al., 2008; Naderpour et al., 2010). Thus, eIF4E is considered as a target for resistance engineering. However, as eIF4E also plays a critical role in the initiation of the translation of plant mRNA, knocking out the eIF4E gene in plants usually leads to developmental defects (Bastet et al., 2017, 2018; Pechar et al., 2022). With the development of gene editing technology, we can precisely introduce mutations into eIF4E to develop cultivars resistant to potyviruses.
Watermelon (Citrullus lanatus) is one of the most economically important cucurbitaceous crops worldwide. However, most modern sweet watermelons are susceptible to diseases and pests (Guo et al., 2019). Some wild watermelon species such as C. lanatus var. citroides (C. amarus), C. mucosospermus and C. colocynthis have been used as sources of disease and pest resistance to improve the sweet watermelon cultivars (Levi et al., 2017). For example, C. mucosospermus accession PI 595203 carrying a single recessive gene zym is an important source of resistance for developing watermelon cultivars with resistance to viruses (Guner et al., 2018). C. lanatus var. citroides accessions PI 244019, PI 244017 and PI 485883 are highly resistant to papaya ring spot virus‐watermelon strain (PRSV‐W) (Martín‐Hernández & Picó, 2021; Strange et al., 2002), and this resistance is controlled by the same single recessive gene prv (Guner et al., 2018). Quantitative trait locus (QTL) mapping data have indicated that the eIF4E homologue is located at the QTL peak of the prv locus in PI 244019 (Branham et al., 2020). In the previous studies, PI 244019, PI 244017 and PI 485883 have also been reported to be resistant to zucchini yellow mosaic virus (ZYMV) and watermelon mosaic virus (WMV) (Gillaspie & Wright, 1993; Guner et al., 2019; Martín‐Hernández & Picó, 2021; Strange et al., 2002). Nevertheless, the resistance mechanism of prv in these watermelon accessions is unknown.
In this study, we revealed that the naturally occurring amino acid substitution D71G of eIF4E in watermelon accessions was involved in the resistance to PRSV, ZYMV and WMV. In addition, the amino acid residue D71 is located in the cap‐binding pocket of eIF4E, which is highly conserved in multiple plant species from different families. We also showed the mutation of the equivalent amino acid substitution D55G in tobacco eIF4E conferred resistance to potato virus Y (PVY).
2. RESULTS
2.1. Amino acid substitutions in eIF4E are widely present in potyvirus‐resistant watermelon accessions
To investigate the relationship between eIF4E variation and PRSV resistance, we compared the eIF4E of multiple PRSV resistance accessions in the Cucurbit Genomics Database (CuGenDB) (http://cucurbitgenomics.org/v2) (Guo et al., 2020). Among the 14 watermelon accessions with the highest resistance to PRSV‐W (Strange et al., 2002), 12 resistant accessions were listed in the CuGenDBv2 database. We were surprised to find that, compared with the susceptible accessions, eIF4E of the 12 resistant accessions carried the non‐synonymous substitutions. Among the 12 resistant accessions, nine accessions carried the non‐synonymous nucleotide substitution A212 to G that induced the amino acid change of D71 to G, two accessions carried the nucleotide substitutions G341 to A and A635 to T that led to the amino acid changes of R114 to K and D212 to V (Yu et al., 2022), while the other one carried the nucleotide substitution A241 to C that led to the amino acid substitution of T81 to P (Ling et al., 2009) (Figure 1a). We then compared the eIF4E in ZYMV‐ and WMV‐resistant accessions of other watermelons in the CuGenDBv2 database (Gillaspie & Wright, 1993; Guner et al., 2019; Xu et al., 2004). The result indicated that most ZYMV‐ and WMV‐resistant accessions also carried the amino acid changes of D71G or R114K/D212V in eIF4E (Figure 1a).
FIGURE 1.

Geographic distribution of potyvirus‐resistant watermelon accessions and variation of eukaryotic translation initiation factor 4E (eIF4E). (a) The eIF4E amino acid substitutions and information of potyvirus‐resistant watermelon accessions. (b) Geographic distribution of watermelon accessions with amino acid substitutions D71G, R114K/D212V or T81P in eIF4E. The red colour indicates the accessions with D71G, the orange colour indicates the accessions with R114K/D212V, and the blue colour indicates the accessions with T81P.
It is worth noting that all the resistant accessions carrying the amino acid change D71G or R114K/D212V belonged to C. lanatus var. citroides (Figure 1a). Among the accessions that carried the D71G amino acid substitution, seven were from Zimbabwe, three were from Botswana, and the other three were from South Africa (Figure 1b). Among the accessions that carried R114K/D212V amino acid substitutions, three were from Zimbabwe, and the other three were from South Africa (Figure 1b).
2.2. The eIF4E from potyvirus‐resistant watermelon accessions fails to interact with VPg
To investigate the effect of the eIF4E amino acid substitutions on the interaction between watermelon eIF4E (CleIF4E) and VPg of PRSV, ZYMV or WMV, we performed yeast two‐hybrid (Y2H) assays and bimolecular fluorescence complementation (BiFC) assays. Y2H results showed that the VPg of PRSV, ZYMV and WMV could interact with CleIF4EWT and CleIF4ER114K, but not with CleIF4ED71G, CleIF4ER114K/D212V or CleIF4ED212V (Figures 2a and S1a). This Y2H result was further confirmed by BiFC assay, in which strong fluorescence of yellow fluorescence protein (YFP) was observed in Nicotiana benthamiana leaf cells co‐expressing CleIF4EWT‐NE or CleIF4ER114K‐NE with PRSV VPg‐CE, ZYMV VPg‐CE or WMV VPg‐CE. In contrast, the leaf cells co‐expressing CleIF4ED71G‐NE, CleIF4ER114K/D212V‐NE or CleIF4ED212V‐NE with PRSV VPg‐CE, ZYMV VPg‐CE or WMV VPg‐CE did not show YFP fluorescence (Figures 2b and S1b). These results showed that the amino acid substitution D71G or D212V in CleIF4E disrupted the interaction between CleIF4E and VPg of PRSV, ZYMV or WMV.
FIGURE 2.

The interactions between watermelon eIF4E (CleIF4E) or its mutants and viral genome‐associated protein (VPg) of papaya ringspot virus‐watermelon strain (PRSV‐W) or zucchini yellow mosaic virus (ZYMV) in vitro and in vivo. (a) Yeast two‐hybrid analysis of the interaction between CleIF4E or its mutants and VPg of PRSV or ZYMV. The yeast cells co‐transformed with AD‐CleIF4E or its mutants and BD‐PRSV VPg or BD‐ZYMV VPg were subjected to 10‐fold serial dilutions and plated on the SD/−Trp/−Leu/−His selection medium for 4 days. (b) Bimolecular fluorescence complementation analysis of the interactions between CleIF4E or its mutants and VPg of PRSV‐W or ZYMV in Nicotiana benthamiana leaves. PRSV VPg‐CE or ZYMV VPg‐CE were individually co‐expressed with CleIF4E‐NE or its mutants in N. benthamiana leaves. Confocal imaging was performed at 48 h post‐inoculation (hpi). Scale bars = 20 μm.
2.3. The amino acid residue D71 in the cap‐binding pocket is conserved in the eIF4E of multiple plant species
We then predicted the structure of the CleIF4E protein based on the available crystallographic structures of melon eIF4E and analysed single nucleotide polymorphisms in the eIF4E sequence of 615 watermelon accessions in CuGenDBv2. Besides the non‐synonymous substitutions mentioned above, we identified three other non‐synonymous nucleotide substitutions T535 to C, A629 to G and T684 to G, which led to the amino acid changes of S179 to P, Y210 to C and H228 to Q, respectively.
The cap‐binding pocket in eIF4E is the key region for binding VPg (Charron et al., 2008; Duan et al., 2012; Robaglia & Caranta, 2006). We found that all these amino acid substitutions are located in or around the cap‐binding pocket of the CleIF4E protein. Moreover, the amino acid substitutions D71G, T81P and R114K were clustered in two regions of the cap‐binding pocket (Figure 3a,b). Next, we wanted to explore whether these amino acid sites with changes in different watermelon accessions are conserved in other plant species. Multiple sequence alignment and WebLogo analysis showed that the amino acid residue D71 was the most conserved site among the seven amino acid residues of eIF4E and was strictly conserved in many plants (Figure 3c, Table S2).
FIGURE 3.

The location and conservation analysis of amino acid substitutions in eIF4E proteins of watermelon accessions. (a) Single amino acid substitutions in the eIF4E protein of different watermelon accessions. A schematic representation of the eIF4E secondary structure is placed above the alignment. (b) Amino acid substitutions mapped onto the 3D structure of the watermelon eIF4E protein predicted using the crystal structure of melon eIF4E (5ME7) as the template. The two black arrows point to the cap‐binding pocket and dorsal surface. (c) Multiple amino acid sequence alignment and WebLogo analysis of eIF4E of multiple plant species. The height of the letter shows the conservation of the amino acid. The regions highlighted in yellow and green boxes indicate the two regions involved in eIF4E‐mediated resistance against potyviruses in the cap‐binding pocket. The amino acid substitution site D71G is highlighted in red, the amino acid substitution sites R114K and D212V are highlighted in orange, and the amino acid substitution site T81P is highlighted in blue. Other amino acid substitution sites are highlighted in pink.
2.4. The naturally occurring variation D71G in eIF4E determines the resistance to PRSV‐W in watermelon plants
We individually cloned and sequenced the coding sequence (CDS) of eIF4E of PI 244019 and another watermelon cultivar Fufeng. The amino acid sequence alignment showed that eIF4EPI 244019 only had a D‐to‐G amino acid change at position 71, compared with eIF4EFufeng (Figure S2a). Then, the PRSV‐W infectious clone pCB301‐PRSV‐GFP was individually agroinfiltrated into the cotyledons of PI 244019 and Fufeng cultivars. Results showed that PRSV‐GFP induced mosaic and distortion symptoms in the upper leaves of Fufeng under normal light and strong green fluorescence under ultraviolet illumination (Figure S2b). In contrast, the upper leaves of PI 244019 showed no obvious viral symptoms under normal light and faint green fluorescence near the veins under ultraviolet illumination (Figure S2b). Western blotting analysis showed that the accumulation of PRSV coat protein (CP) in PI 244019 was lower than that in Fufeng (Figure S2b).
To further investigate whether the amino acid change D71G in eIF4E was involved in the resistance against PRSV in PI 244019 plants, we individually overexpressed CleIF4EWT and CleIF4ED71G in Fufeng and PI 244019 plants. As the transformation efficiency of cucurbits is very low (Xin et al., 2022), we adopted an effective and fast approach to overexpress CleIF4Es in watermelon (Agaoua et al., 2022; Andrade et al., 2009). We individually cloned the CDS of CleIF4EWT and CleIF4ED71G into pCB301‐PRSV to produce pCB301‐PRSV‐CleIF4EWT and pCB301‐PRSV‐CleIF4ED71G (Figure 4a). Agrobacterium cells carrying pCB301‐PRSV‐CleIF4EWT, pCB301‐PRSV‐CleIF4ED71G, or pCB301‐PRSV‐GFP were separately infiltrated into the cotyledons of PI 244019 and Fufeng plants.
FIGURE 4.
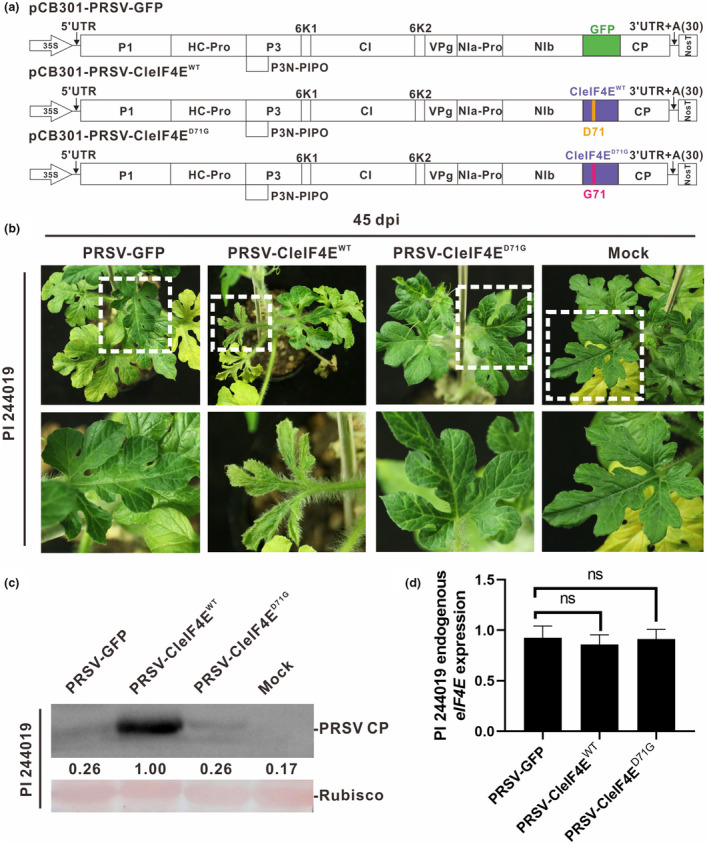
The infection of pCB301‐PRSV‐GFP, pCB301‐PRSV‐CleIF4EWT and pCB301‐PRSV‐CleIF4ED71G in PI 244019 plants. (a) Schematic representation of pCB301‐PRSV‐GFP, pCB301‐PRSV‐CleIF4EWT and pCB301‐PRSV‐CleIF4ED71G. GFP is marked in green, CleIF4EWT and CleIF4ED71G are marked in purple. (b) Phenotypes of PI 244019 plants individually inoculated with pCB301‐PRSV‐GFP, pCB301‐PRSV‐CleIF4EWT and pCB301‐PRSV‐CleIF4ED71G at 45 days post‐inoculation (dpi). Plants in the absence of virus infection (Mock) were used as the control. (c) Western blotting analysis of accumulation levels of PRSV coat protein (CP) in the upper leaves of PI 244019 plants individually inoculated with pCB301‐PRSV‐GFP, pCB301‐PRSV‐CleIF4EWT and pCB301‐PRSV‐CleIF4ED71G at 45 dpi. The Ponceau S staining of ribulose‐1,5‐bisphosphate carboxylase/oxygenase (RuBisCO) shows sample loadings. (d) Endogenous eIF4E expression levels of PI 244019 plants individually inoculated with pCB301‐PRSV‐GFP, pCB301‐PRSV‐CleIF4EWT and pCB301‐PRSV‐CleIF4ED71G. Error bars represented the standard deviations of the means from biological repeats. The t test was used to test the significance of the differences. ‘ns’ represents no significant difference (α = 0.05).
At 45 days post‐inoculation (dpi), PRSV‐CleIF4EWT caused severe mosaic and distortion symptoms in PI 244019, while neither PRSV‐CleIF4ED71G nor PRSV‐GFP caused obvious viral symptoms in PI 244019 (Figure 4b). Western blotting analysis showed that the coat protein (CP) accumulation level of PRSV‐CleIF4EWT was significantly higher than that of PRSV‐GFP and PRSV‐CleIF4ED71G in PI 244019 plants (Figure 4c). In contrast, the infectivity of PRSV‐CleIF4EWT, PRSV‐CleIF4ED71G and PRSV‐GFP did not show significant differences in Fufeng plants (Figure S3a). To investigate whether the overexpression of the exogenous CleIF4E gene caused the silencing of endogenous CleIF4E, we measured the expression level of endogenous CleIF4E using primers flanking the untranslated region of eIF4E (Table S1). The reverse transcription‐quantitative PCR (RT‐qPCR) results showed that the expression level of the endogenous CleIF4E gene did not show a significant difference in the plants separately infected by PRSV‐CleIF4EWT, PRSV‐CleIF4ED71G or PRSV‐GFP (Figures 4d and S3a). We also showed that overexpression of CleIF4EWT could break the resistance to PRSV‐W in another watermelon accession PI 485583 that carries amino acid substitution D71G in eIF4E (Figure S4a,b). Thus, we concluded that the naturally occurring substitution D71G in eIF4E determined the resistance to PRSV‐W in watermelon.
2.5. The amino acid substitution D71G in eIF4E of watermelon plants also confers resistance to ZYMV
Given that the amino acid substitution D71G in CleIF4E also broke the interaction between CleIF4E and VPg of ZYMV or WMV, we wanted to dissect whether the D71G mutation of CleIF4E also confers resistance to ZYMV and WMV. We individually inoculated the infectious clones of ZYMV and WMV to PI 244019 and Fufeng plants. The result showed that ZYMV‐GFP caused mosaic symptoms and strong green fluorescence in Fufeng plants but did not cause any obvious symptoms or green fluorescence in PI 244019 plants (Figure S2b). We also found that WMV‐GFP caused strong green fluorescence in Fufeng plants, but not in PI 244019 plants (Figure S2b). Because neither Fufeng nor PI 244019 plants showed obvious viral symptoms after inoculation with pCB301‐WMV‐GFP (Figure S2b), we focused on ZYMV for further investigation.
We individually inserted the CDS of CleIF4EWT and CleIF4ED71G into pCB301‐ZYMV to produce pCB301‐ZYMV‐CleIF4EWT and pCB301‐ZYMV‐CleIF4ED71G (Figure 5a). Then, pCB301‐ZYMV‐CleIF4EWT and pCB301‐ZYMV‐CleIF4ED71G were separately inoculated into Fufeng and PI 244019 plants. At 35 days post‐inoculation (dpi), the PI 244019 plants inoculated with pCB301‐ZYMV‐CleIF4EWT showed mosaic and distortion symptoms in the upper leaves, while these symptoms were not observed in PI 244019 plants inoculated with pCB301‐ZYMV‐CleIF4ED71G (Figure 5b). Western blotting analysis also showed that the CP accumulation in the PI 244019 plants inoculated with pCB301‐ZYMV‐CleIF4ED71G was significantly lower than the PI 244019 plants inoculated with pCB301‐ZYMV‐CleIF4EWT (Figure 5c). In Fufeng plants, the symptom and CP accumulation had no significant difference after inoculation with pCB301‐ZYMV‐GFP, pCB301‐ZYMV‐CleIF4EWT or pCB301‐ZYMV‐CleIF4ED71G (Figure S3b). Results of RT‐qPCR showed the expression level of the endogenous CleIF4E gene was not affected by the overexpression of the exogenous CleIF4E gene (Figures 5d and S3b). Moreover, the overexpression of CleIF4EWT can also break the resistance to ZYMV in PI 485583 (Figure S4a,b). Therefore, these results indicated that the D71G mutation in eIF4E also resulted in resistance to ZYMV.
FIGURE 5.

The infection of pCB301‐ZYMV‐GFP, pCB301‐ZYMV‐CleIF4EWT and pCB301‐ZYMVV‐CleIF4ED71G in PI 244019 plants. (a) Schematic representation of pCB301‐ZYMV‐GFP, pCB301‐ZYMV‐CleIF4EWT and pCB301‐ZYMV‐CleIF4ED71G. GFP is marked in green, CleIF4EWT and CleIF4ED71G is marked in purple. (b) Phenotype of PI 244019 plants individually inoculated with pCB301‐ZYMV‐GFP, pCB301‐ZYMV‐CleIF4EWT and pCB301‐ZYMV‐CleIF4ED71G at 35 days post‐inoculation (dpi). Plants in the absence of virus infection (Mock) were used as the control. (c) Western blotting analysis of accumulation levels of ZYMV coat protein (CP) in the upper leaves of PI 244019 plants individually inoculated with pCB301‐ZYMV‐GFP, pCB301‐ZYMV‐CleIF4EWT and pCB301‐ZYMV‐CleIF4ED71G at 35 dpi. The Ponceau S staining of RuBisCO shows sample loadings. (d) Endogenous eIF4E expression level in PI 244019 plants individually inoculated with pCB301‐ZYMV‐GFP, pCB301‐ZYMV‐CleIF4EWT and pCB301‐ZYMV‐CleIF4ED71G. Error bars represent the standard deviations of the means from three biological repeats. The t test was used to test the significance of the difference. ‘ns’ represented no significant difference (α = 0.05).
2.6. The equivalent amino acid mutation D55G in the cap‐binding pocket of tobacco eIF4E confers resistance to PVY
Next, we wanted to explore whether the equivalent mutation of D71G in tobacco eIF4E (NteIF4E) confers resistance to PVY. We first predicted the structure of NteIF4E. The equivalent amino acid residue of D55 in NteIF4E was also located in the cap‐binding pocket of NteIF4E (Figure 6a). We further explored whether the mutation of D55 in NteIF4E affects the interaction between PVY VPg and NteIF4E. In the Y2H assay, robust growth was observed in yeast transformed with BD‐PVY VPg and AD‐NteIF4EWT on SD/−Trp/−Leu/−His selection medium but was not observed in yeast co‐transformed with BD‐PVY VPg and AD‐NteIF4ED55G (Figure 6b). The BiFC assay showed that strong YFP fluorescence was observed in N. benthamiana leaf cells co‐expressing with NteIF4EWT‐NE and PVY VPg‐CE, while the leaf cells co‐expressing with NteIF4ED55G‐NE and PVY VPg‐CE did not show YFP fluorescence (Figure 6c). These results suggested that the single amino acid change D55G in NteIF4E abolished its interaction with PVY VPg.
FIGURE 6.

Tobacco eIF4E carrying D55G mutation in cap‐binding pocket conferred resistance to PVY. (a) The location of D55 in the structure of tobacco eIF4E. Two regions involved in resistance against potyviruses in the cap‐binding pocket are individually indicated in yellow and green. The amino acid site D55 is highlighted in red. The two black arrows point to the cap‐binding pocket and dorsal surface. (b) Determination of the interaction between NteIF4EWT or NteIF4ED55G and PVY VPg through the yeast two‐hybrid system. The yeast cells co‐transformed with AD‐NteIF4EWT or AD‐NteIF4ED55G and BD‐PVY VPg were subjected to 10‐fold serial dilutions and plated on the SD/−Trp/−Leu/−His selection medium for 4 days. Yeast cells co‐transformed with AD and BD‐PVY VPg, AD‐NteIF4EWT or NteIF4ED55G and BD were used as negative controls. (c) Bimolecular fluorescence complementation analysis of the interaction between NteIF4EWT or NteIF4ED55G and PVY VPg in Nicotiana benthamiana leaves. NteIF4EWT‐NE or NteIF4ED55G‐NE were co‐expressed with PVY VPg‐CE in N. benthamiana. Confocal imaging was performed at 48 h post‐inoculation (hpi). Scale bars = 20 μm. (d) Schematic representation of pCB301‐PVY‐NteIF4EWT and pCB301‐PVY‐NteIF4ED55G. NteIF4E WT and NteIF4E D55G are marked in blue. (e) Phenotypes of Nicotiana tabacum 'Virgin A Mutant' (VAM) plants individually inoculated with pCB301‐PVY‐NteIF4EWT and pCB301‐PVY‐NteIF4ED55G at 15 days post‐inoculation (dpi). Plants in the absence of virus infection (Mock) were used as the control. (f) Western blot analysis of accumulation levels of coat protein (CP) in the upper leaves of VAM individually inoculated with PVY‐NteIF4EWT and PVY‐NteIF4ED55G at 15 dpi. The Ponceau S staining of RuBisCO shows sample loadings. (g) Endogenous eIF4E expression level of PI 244019 individually inoculated with pCB301‐PVY‐GFP, pCB301‐PVY‐NteIF4EWT and pCB301‐PVY‐NteIF4ED55G. Error bars represent the standard deviation of the means from biological repeats. The t test was used to test the significance of the differences. ‘ns’ represented no significant difference (α = 0.05).
The Nicotiana tabacum 'Virgin A Mutant' (VAM) tobacco line, which carries the va resistance gene, is resistant against PVY (Koelle, 1961). Previous studies have reported that a sequence deletion in the eIF4E gene is responsible for the PVY resistance conferred by the va gene in VAM (Julio et al., 2015). N. tabacum ‘Xanthi’ is a cultivar susceptible to PVY. NteIF4E WT and NteIF4E D55G were individually cloned into pCB301‐PVY to generate pCB301‐PVY‐NteIF4EWT and pCB301‐PVY‐NteIF4ED55G (Figure 6d). Then, pCB301‐PVY‐NteIF4EWT and pCB301‐PVY‐NteIF4ED55G were individually infiltrated into Xanthi and VAM. At 15 dpi, we found that only PVY‐NteIF4EWT caused vein necrosis symptoms in VAM plants, while PVY‐NteIF4ED55G did not cause obvious PVY symptoms in VAM plants (Figure 6e). Western blotting analysis showed that the CP accumulation of PVY‐NteIF4ED55G in VAM was much lower than that in Xanthi (Figure 6f). However, the infectivity of PVY‐NteIF4EWT and PVY‐NteIF4ED55G showed no significant difference in Xanthi (Figure S3c). The result of RT‐qPCR showed that the expression level of the endogenous eIF4E gene was not affected by the overexpression of the exogenous eIF4E gene (Figures 6g and S3c). These results showed that the D55G mutation in tobacco eIF4E conferred resistance to PVY.
3. DISCUSSION
Recessive resistance against potyviruses is usually related to mutations in eIF4E (Kang et al., 2005; Lee et al., 2023; Liu et al., 2017). In this study, we show that the amino acid substitution D71G in watermelon eIF4E, which is present in many of the potyvirus‐resistant watermelon accessions, confers resistance to PRSV‐W and ZYMV in watermelon by disrupting the interaction between eIF4E and VPg. Moreover, the amino acid residue corresponding to D71 is highly conserved in many plant species. The mutation in the equivalent amino acid residue D55 of tobacco eIF4E confers resistance to PVY, suggesting this residue is critical for eIF4E–VPg interactions in different potyvirus–host pathosystems.
Mutations in susceptibility genes usually confer resistance against potyviruses (Zlobin & Taranov, 2023). Recently, variants of some other non‐eIF4E susceptibility factors have also been reported to confer resistance to potyviruses. For example, a single substitution (A120G) in eukaryotic translation initiation factor 2B‐β (eIF2Bβ) confers resistance against turnip mosaic virus (Shopan et al., 2017). However, this amino acid residue is not conserved in eIF2Bβ of different plant species. Another study has shown that amino acid substitutions in vacuolar protein sorting 4 (VPS4) are responsible for the resistance to WMV, ZYMV and bean common mosaic virus (Agaoua et al., 2022; Amano et al., 2013; Soler‐Garzón et al., 2021). However, the resistance to WMV and ZYMV is separately controlled by VPS4 alleles encoding different amino acid substitutions. These changes occur in non‐conserved amino acid residues (Robaglia & Caranta, 2006), which only confer resistance to limited potyviruses. PRSV‐W resistance accessions PI 244017, PI 244019 and PI 485583 have been reported to carry the same recessive resistance allele prv. In this study, we found that the eIF4E of both PI 244019 and PI 485583 carries a D71G amino acid substitution, while the eIF4E of PI 244017 carries the other amino acid substitutions R114K/D212V (Figure 1a). Then, we showed that the eIF4E that carries D71G or D212V fails to interact with PRSV VPg (Figure 2a,b). It is worth noting that the replacement of the hydrophilic amino acid aspartate by the hydrophobic amino acid glycine or valine may individually change the local structure of CleIF4ED71G or CleIF4ED212V (Sang & Barbosa, 1992), and disrupt the eIF4E–VPg interaction. In contrast, the replacement of the arginine by the lysine in CleIF4ER114K is a conservative substitution that does not affect the interaction with VPg (Summers et al., 1989). Our results further showed that the single D71G mutation in eIF4E is responsible for the resistance to PRSV‐W in PI 244019 and PI 485583 (Figures 4b,c and S4a,b). In addition, we found that the amino acid residue D71 in the cap‐binding pocket of eIF4E is highly conserved in plants (Figure 3a–c), and amino acid change of D71 to G results in resistance to ZYMV in watermelon plants (Figure 5b,c). Moreover, the mutation of the corresponding amino acid residue D55 of tobacco eIF4E confers resistance against another potyvirus, PVY (Figure 6e,f). These data indicated that the amino acid substitution D71G in eIF4E may result in broad‐range resistance to potyviruses. Besides eIF4E, eIF(iso)4E also plays important roles in the infection of many potyviruses (Zlobin & Taranov, 2023). We found the amino acid residue D71 also present in eIF(iso)4E. In the future, we hope to explore whether the equivalent amino acid substitution D71G in eIF(iso)4E confers resistance to potyviruses.
Gene editing can accelerate the progress of resistance engineering. eIF4E is a hotspot for conferring crop resistance against potyviruses. For example, genetic knockout or deletion of eIF4E using CRISPR/Cas9 technology confers resistance to cucumber vein yellowing virus, ZYMV, WMV and PRSV‐W in cucumber (Chandrasekaran et al., 2016; Fidan et al., 2023). Another study reported that knockout of eIF4E using CRISPR/Cas9 in melon confers resistance to Moroccan watermelon mosaic virus (Pechar et al., 2022). However, the eIF4E‐knockout melon plants have male sterility and reduced growth phenotype in T0 and F2 homozygous mutants (Pechar et al., 2022). The eIF4E‐knockout Arabidopsis plants were found to display a late‐bolting phenotype (Bastet et al., 2018). Recently, eIF4E was shown to be involved in nitrate regulation in Arabidopsis plants, eIF4E‐knockout Arabidopsis plants display decreased fresh weight, shorter primary root length, and reduced seed size, seed weight, yield per plant and nitrogen use efficiency than wild‐type Arabidopsis plants suggesting that eIF4E plays an important role in plant nutrient regulation (Li et al., 2023). Therefore, knockout or deletion of eIF4E may not be directly used in breeding resistant crop cultivars. In this study, we found that the single amino acid substitution D71G in the cap‐binding pocket of eIF4E is sufficient to confer resistance to PRSV‐W and ZYMV in watermelon or PVY in tobacco. Moreover, the PI 244019 male flower development was normal and could produce fruits through self‐pollination (Figure S5), suggesting the amino acid substitution D71G in eIF4E does not affect plant growth and male sterility.
In conclusion, our study provides a precise target for breeding crop cultivars against potyviruses. With technology development, base editors and prime editors based on CRISPR/Cas9 have been used for precise gene editing (Anzalone et al., 2019; Gaudelli et al., 2017; Komor et al., 2016). In the future, editing of the conserved site D71 or D55 of eIF4E employing these precision gene‐editing methods will generate plants resistant to multiple potyviruses.
4. EXPERIMENTAL PROCEDURES
4.1. RNA extraction, RT‐qPCR and gene data analysis
Total RNA was extracted from plant leaf tissues using the TRIzol reagent (TransGen Biotech) and then treated with the gDNA wiper (Vazyme) to eliminate the genomic DNA. Reverse transcription was conducted with HiScript II Q RT SuperMix kit (Vazyme) following the manufacturer's instructions. The RT‐qPCR was performed using ChamQ SYBR qPCR Master Mix (Vazyme) in a PCR machine (LC96; Roche). Gene‐specific primers were designed to amplify the watermelon EF1α gene or tobacco EF1α gene as the internal reference genes (Table S1). Each RT‐qPCR was performed with three biological replicates. Data were analysed using GraphPad Prism software.
BLAST search of the C. lanatus (97103) v2 genome was performed via the Cucurbit Genomics Database (http://www.cucurbitgenomics.org/) using Arabidopsis thaliana eIF4E (AT4G18040) as the template. Total RNA was individually extracted from leaves of PI 244019, Fufeng, and Xanthi using TRIzol reagent following the manufacturer's instructions. Reverse transcription was performed using 3′ end‐specific primers and SMART M‐MLV reverse transcriptase (TaKaRa) (Table S1). PCR was performed using High‐fidelity Phusion DNA polymerase (Thermo Fisher Scientific) together with the primers designed for the untranslated region of eIF4E (Table S1). The resultant product was sequenced by Sangon Biotech Company. The amino acid sequences of eIF4E from other plant species were retrieved from the NCBI (https://www.ncbi.nlm.nih.gov/). The nucleotide polymorphisms of eIF4E in different watermelon accessions were analysed using the ‘Genotype’ module in CuGenDBv2 (http://cucurbitgenomics.org/v2). Nucleotide and amino acid sequences were analysed using DNAMAN and Bioedit software. The conservation analysis of eIF4E of multiple plants was performed using the WebLogo website (https://weblogo.berkeley.edu/logo.cgi).
4.2. Protein modelling
SWISS‐MODEL was used to generate the 3D protein structures of eIF4Es. First, the amino acid sequence of CleIF4E was loaded into the online server (https://swissmodel.expasy.org/). Then, the melon eIF4E protein (5ME7) with the highest Seq Identity of 96.69% was selected as the template for homology modelling (Miras et al., 2017). The best‐quality model had the highest GMQE (0.73) and QMEAND of CoLocal (0.84 ± 0.6). The 3D view of NteIF4E protein structure was predicted by the same method. The protein models were analysed using PyMOL software.
4.3. Plasmid construction
The CDS of eIF4E was PCR‐amplified using High‐fidelity Phusion DNA polymerase from reverse transcription products from PI 244019, Fufeng and Xanthi plants. The amplification products were separately cloned into pGADT7 and pCam35S‐NE (a vector containing the coding sequence of the N terminal YFP). The VPg genes of PRSV, ZYMV, WMV and PVY were separately PCR‐amplified from the pCB301‐PRSV‐GFP, pCB301‐ZYMV‐GFP, pCB301‐WMV‐GFP and pCB301‐PVY‐GFP plasmids and were separately cloned into pGBKT7 and pCam35S‐CE (a vector containing the coding sequence of the C terminal YFP). The CDS of eIF4E from PI 244019 and Fufeng were individually inserted between NIb and CP to take the place of GFP in pCB301‐PRSV‐GFP or pCB301‐ZYMV‐GFP to produce pCB301‐PRSV‐CleIF4EWT and pCB301‐PRSV‐CleIF4ED71G, or pCB301‐ZYMV‐CleIF4EWT and pCB301‐ZYMV‐CleIF4ED71G, respectively. The CDS of eIF4E from Xanthi plants was inserted NIb and CP to take the place of GFP in pCB301‐PVY‐GFP to produce pCB301‐PRSV‐NteIF4EWT. Site‐directed mutagenesis was performed through a PCR‐based method using specific primers (Liu & Naismith, 2008) (Table S1).
4.4. Plant growth and inoculation
All plants used in this study were grown at 23°C in a greenhouse under a 16 h light/8 h dark cycle with 60% relative humidity. Plasmid vectors were individually transformed into competent cells of Agrobacterium tumefaciens GV3101 by the freeze/thaw method. The transformed A. tumefaciens cultures were grown overnight in Luria Bertani liquid medium with 50 μg/mL kanamycin and 50 μg/mL rifampicin at 28°C, then individually resuspended in an induction buffer containing 10 mM MgCl2, 10 mM MES and 150 μM acetosyringone and incubated for 3 h at 28°C. Agrobacterium cultures were diluted to OD600 = 0.5 for virus inoculation. The diluted Agrobacterium cultures carrying PRSV/ZYMV/PVY‐based constructs were individually infiltrated into the cotyledon of watermelon and tobacco leaves using 1 mL needleless syringes. Agrobacterium cultures were adjusted to OD600 = 1.0 and then inoculated into N. benthamiana leaves for BiFC.
4.5. Y2H analysis
The Y2H assay was performed according to the manufacturer's protocol (Clontech). The Y2H Gold yeast cells were transformed with constructs of interest and then grown on an SD selection medium lacking Leu and Trp for 3 days. The selected colonies were then grown on a highly stringent selection medium lacking Leu, Trp and His for 4 days.
4.6. BiFC analysis
The BiFC assay was performed as described previously (Geng et al., 2015). The YFP interaction signal was detected using a Zeiss LSM800 confocal microscope at 48 hpi. The excitation wavelength for YFP was set at 488 nm and the emission was captured at 560–585 nm. The excitation wavelength for mCherry was set at 561 nm and the emission was captured at 590–620 nm. The captured images were further processed using ZEN blue v. 3.2 software.
4.7. Protein extractions and western blotting
Total proteins were extracted from plant leaves using a protein extraction buffer as described previously (Geng et al., 2015). The crude extracts were centrifuged at 12,000 g for 10 min. The supernatant was collected from each sample and mixed with equal volume 2 × SDS‐PAGE loading buffer (250 mM Tris–HCl pH 6.8, 10% wt/vol SDS, 0.5% bromophenol blue, 50% vol/vol glycerol, 500 mM dithiothreitol) and boiled at 100°C for 10 min. Each sample was separated in 12% SDS‐polyacrylamide gels through electrophoresis and then blotted onto nitrocellulose membranes. The protein was detected by polyclonal antibodies specific for PRSV/ZYMV/PVY CP or GFP. A horseradish peroxidase‐conjugated goat anti‐rabbit IgG (Sigma‐Aldrich) was used as the secondary antibody. The detection signal was visualized using a chemiluminescent imaging SH‐Compact 523 (Shenhua). The RuBisCO/CP or RuBisCO/GFP bands of western blots were quantified using ImageJ software.
Supporting information
FIGURE S1. The interactions between watermelon eIF4E (CleIF4E) or its mutants and viral genome‐associated protein (VPg) of watermelon mosaic virus (WMV) in vitro and in vivo. (a) The interaction between CleIF4E or its mutants and WMV VPg in yeast two‐hybrid (Y2H) system. The yeast cells co‐transformed with AD‐CleIF4E or its mutants and BD‐WMV VPg were subjected to 10‐fold serial dilutions and plated on the SD/‐Trp/‐Leu/‐His selection medium for 4 days. Yeast cells co‐transformed with AD‐T‐ant and BD‐p53 were used as the positive control, while yeast cells individually co‐transformed with AD‐T‐ant and BD‐lam, BD‐PRSV VPg, BD‐ZYMV VPg or BD‐WMV‐VPg and AD, AD‐CleIF4E or its mutants and BD were used as negative controls. (b) Bimolecular fluorescence complementation (BiFC) analysis of the interactions between CleIF4E or its mutants and WMV VPg in Nicotiana benthamiana leaves. WMV VPg‐CE was individually co‐expressed with CleIF4E‐NE or its mutants in N. benthamiana leaves. Leaves individually co‐expressed with GUS‐NE and PRSV VPg‐CE, ZYMV VPg‐CE or WMV VPg‐CE, CleIF4E‐NE or its mutants and GUS‐CE were used as negative controls. Confocal imaging was performed at 48 h post‐inoculation. Scale bars = 20 μm.
FIGURE S2. The watermelon accession PI 244019 plants carrying the naturally occurring amino acid substitution of D71 to G in eIF4E are resistant to PRSV‐W, ZYMV and WMV. (a) Sequence alignment of eIF4E amino acid sequences of PI 244019 and Fufeng plants. The red box indicates amino acid substitution observed between eIF4EPI 244019 and Fufeng plants. (b) Phenotypes and western blotting analysis of viral accumulation levels in Fufeng and PI 244019 plants individually inoculated with pCB301‐PRSV‐GFP, pCB301‐ZYMV‐GFP and pCB301‐WMV‐GFP. The Ponceau S staining of RuBisCO shows sample loadings.
FIGURE S3. Phenotypes, virus accumulation levels and endogenous eIF4E expression levels of Fufeng and Xanthi plants individually inoculated with eIF4E overexpression vectors based on virus infectious clones. (a) Phenotypes, virus accumulation levels and endogenous eIF4E expression levels of Fufeng plants individually inoculated with pCB301‐PRSV‐GFP, pCB301‐PRSV‐CleIF4EWT and pCB301‐PRSV‐CleIF4ED71G at 45 days post‐inoculation (dpi). (b) Phenotypes, virus accumulation levels and endogenous eIF4E expression levels of Fufeng plants individually inoculated with pCB301‐ZYMV‐GFP, pCB301‐ZYMV‐CleIF4EWT and pCB301‐ZYMV‐CleIF4ED71G at 35 dpi. (c) Phenotypes, virus accumulation levels and endogenous eIF4E expression levels of Xanthi plants individually inoculated with pCB301‐PVY‐GFP, pCB301PVY‐CleIF4EWT and pCB301‐PVY‐CleIF4ED71G at 15 dpi. The Ponceau S staining of RuBisCO shows sample loadings. Plants in the absence of virus infection (Mock) were used as the control. Error bars represent the standard deviations of the means from biological repeats. The t test was used to test the significance of the differences. ‘ns’ represents no significant difference (α = 0.05).
FIGURE S4. The infection of eIF4E overexpression vectors based on infectious clones of PRSV and ZYMV in PI 485583. (a) Symptoms of PI 485583 plants individually infected with eIF4E overexpression vectors based on infectious clones of PRSV and ZYMV. Plants in the absence of virus infection (Mock) were used as the control. (b) Western blotting analysis of the accumulation levels of PRSV coat protein (CP) and ZYMV CP in the upper leaves of PI 485583 plants individually infected with eIF4E overexpression vectors based on infectious clones of PRSV and ZYMV.
FIGURE S5. The amino acid substitution D71G in eIF4E did not affect the male sterility of PI 244019 plants. (a) Phenotype of PI 244019 male flowers. (b) Fruits produced from self‐pollinated PI 244019 plants.
TABLE S1. Primers used in this work.
TABLE S2. List of GenBank accession number of the eIF4E amino acid sequences of multiple plants.
ACKNOWLEDGEMENTS
This study was funded by the National Natural Science Foundation of China (NSFC; 31801704 and 31720103912), China Postdoctoral Science Foundation (2021M691973 and 2022T150389), the Research and Development Program in Shandong Province (2021LZGC015), the Youth Innovation Team Plan for Shandong High Education Institution (2022KJ241) and the Taishan Scholar project (TS201712023, 2022‐028). We appreciate the U.S. National Plant Germplasm System for providing the seeds of PI 244019 and PI 285583.
Zhou, L.‐X. , Tian, Y.‐P. , Ren, L.‐L. , Yan, Z.‐Y. , Jiang, J. , Shi, Q.‐H. et al. (2024) A natural substitution of a conserved amino acid in eIF4E confers resistance against multiple potyviruses. Molecular Plant Pathology, 25, e13418. Available from: 10.1111/mpp.13418
Contributor Information
Chao Geng, Email: chaogeng@sdau.edu.cn.
Xiang‐Dong Li, Email: xdongli@sdau.edu.cn.
DATA AVAILABILITY STATEMENT
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
REFERENCES
- Agaoua, A. , Rittener, V. , Troadec, C. , Desbiez, C. , Bendahmane, A. , Moquet, F. et al. (2022) A single substitution in vacuolar protein sorting 4 is responsible for resistance to watermelon mosaic virus in melon. Journal of Experimental Botany, 73, 4008–4021. [DOI] [PubMed] [Google Scholar]
- Ahmar, S. , Saeed, S. , Khan, M.H.U. , Ullah Khan, S. , Mora‐Poblete, F. , Kamran, M. et al. (2020) A revolution toward gene‐editing technology and its application to crop improvement. International Journal of Molecular Sciences, 21, 5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano, M. , Mochizuki, A. , Kawagoe, Y. , Iwahori, K. , Niwa, K. , Svoboda, J. et al. (2013) High‐resolution mapping of zym, a recessive gene for zucchini yellow mosaic virus resistance in cucumber. Theoretical and Applied Genetics, 126, 2983–2993. [DOI] [PubMed] [Google Scholar]
- Andrade, M. , Abe, Y. , Nakahara, K.S. & Uyeda, I. (2009) The cyv‐2 resistance to clover yellow vein virus in pea is controlled by the eukaryotic initiation factor 4E. Journal of General Plant Pathology, 75, 241–249. [Google Scholar]
- Anzalone, A.V. , Randolph, P.B. , Davis, J.R. , Sousa, A.A. , Koblan, L.W. , Levy, J.M. et al. (2019) Search‐and‐replace genome editing without double‐strand breaks or donor DNA. Nature, 576, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastet, A. , Lederer, B. , Giovinazzo, N. , Arnoux, X. , German‐Retana, S. , Reinbold, C. et al. (2018) Trans‐species synthetic gene design allows resistance pyramiding and broad‐spectrum engineering of virus resistance in plants. Plant Biotechnology Journal, 16, 1569–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastet, A. , Robaglia, C. & Gallois, J.‐L. (2017) eIF4E resistance: natural variation should guide gene editing. Trends in Plant Science, 22, 411–419. [DOI] [PubMed] [Google Scholar]
- Branham, S.E. , Patrick Wechter, W. , Ling, K.‐S. , Chanda, B. , Massey, L. , Zhao, G. et al. (2020) QTL mapping of resistance to Fusarium oxysporum f. sp. niveum race 2 and papaya ringspot virus in Citrullus amarus . Theoretical and Applied Genetics, 133, 677–687. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran, J. , Brumin, M. , Wolf, D. , Leibman, D. , Klap, C. , Pearlsman, M. et al. (2016) Development of broad virus resistance in non‐transgenic cucumber using CRISPR/Cas9 technology. Molecular Plant Pathology, 17, 1140–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron, C. , Nicolaï, M. , Gallois, J.‐L. , Robaglia, C. , Moury, B. , Palloix, A. et al. (2008) Natural variation and functional analyses provide evidence for co‐evolution between plant eIF4E and potyviral VPg. The Plant Journal, 54, 56–68. [DOI] [PubMed] [Google Scholar]
- Choudhary, N. & Suresh, S. (2020) Plant translation factors and virus resistance. In: Awasthi, L.P. (Ed.) Applied plant virology. London: Academic Press, pp. 657–669. [Google Scholar]
- de Ronde, D. , Butterbach, P. & Kormelink, R. (2014) Dominant resistance against plant viruses. Frontiers in Plant Science, 5, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz‐Pendon, J.A.T.V. , Nieto, C. , Garcia‐Mas, J. , Bendahmane, A. & Aranda, M.A. (2004) Advances in understanding recessive resistance to plant viruses. Molecular Plant Pathology, 5, 223–233. [DOI] [PubMed] [Google Scholar]
- Duan, H. , Richael, C. & Rommens, C.M. (2012) Overexpression of the wild potato eIF4E‐1 variant Eva1 elicits Potato virus Y resistance in plants silenced for native eIF4E‐1 . Transgenic Research, 21, 929–938. [DOI] [PubMed] [Google Scholar]
- Fidan, H. , Calis, O. , Ari, E. , Atasayar, A. , Sarikaya, P. , Tek, M.I. et al. (2023) Knockout of elF4E using CRISPR/Cas9 for large‐scale production of resistant cucumber cultivar against WMV, ZYMV, and PRSV. Frontiers in Plant Science, 14, 1143813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadhave, K.R. , Gautam, S. , Rasmussen, D.A. & Srinivasan, R. (2020) Aphid transmission of Potyvirus: the largest plant‐infecting RNA virus genus. Viruses, 12, 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudelli, N.M. , Komor, A.C. , Rees, H.A. , Packer, M.S. , Badran, A.H. , Bryson, D.I. et al. (2017) Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature, 551, 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng, C. , Cong, Q.‐Q. , Li, X.‐D. , Mou, A.‐L. , Gao, R. , Liu, J.‐L. et al. (2015) Developmentally regulated plasma membrane protein of Nicotiana benthamiana contributes to potyvirus movement and transports to plasmodesmata via the early secretory pathway and the actomyosin system. Plant Physiology, 167, 394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillaspie, A.G. & Wright, J.M. (1993) Evaluation of Citrullus sp. germplasm for resistance to watermelon mosaic virus 2. Plant Disease, 77, 352–354. [Google Scholar]
- Guner, N. , Pesic‐VanEsbroeck, Z. , Rivera‐Burgos, L.A. & Wehner, T.C. (2018) Inheritance of resistance to papaya ringspot virus‐watermelon strain in watermelon. HortScience, 53, 624–627. [Google Scholar]
- Guner, N. , Pesic‐VanEsbroeck, Z. , Rivera‐Burgos, L.A. & Wehner, T.C. (2019) Screening for resistance to zucchini yellow mosaic virus in the watermelon germplasm. HortScience, 54, 206–211. [Google Scholar]
- Guo, J. , Xu, W. , Hu, Y. , Huang, J. , Zhao, Y. , Zhang, L. et al. (2020) Phylotranscriptomics in Cucurbitaceae reveal multiple whole‐genome duplications and key morphological and molecular innovations. Molecular Plant, 13, 1117–1133. [DOI] [PubMed] [Google Scholar]
- Guo, S. , Zhao, S. , Sun, H. , Wang, X. , Wu, S. , Lin, T. et al. (2019) Resequencing of 414 cultivated and wild watermelon accessions identifies selection for fruit quality traits. Nature Genetics, 51, 1616–1623. [DOI] [PubMed] [Google Scholar]
- Julio, E. , Cotucheau, J. , Decorps, C. , Volpatti, R. , Sentenac, C. , Candresse, T. et al. (2015) A eukaryotic translation initiation factor 4E (eIF4E) is responsible for the “va” tobacco recessive resistance to potyviruses. Plant Molecular Biology Reporter, 33, 609–623. [Google Scholar]
- Kang, B.‐C. , Yeam, I. & Jahn, M.M.J.A.R.P. (2005) Genetics of plant virus resistance. Annual Review of Phytopathology, 43, 581–621. [DOI] [PubMed] [Google Scholar]
- Khan, M.A. , Miyoshi, H. , Gallie, D.R. & Goss, D.J. (2008) Potyvirus genome‐linked protein, VPg, directly affects wheat germ in vitro translation: interactions with translation initiation factors eIF4F and eIFiso4F. Journal of Biological Chemistry, 283, 1340–1349. [DOI] [PubMed] [Google Scholar]
- Koelle, G. (1961) Genetische Analyse einer Y‐Virus‐(Rippenbräune) resistenten Mutante der Tabaksorte Virgin A. Der Züchter, 31, 71–72. [Google Scholar]
- Komor, A.C. , Kim, Y.B. , Packer, M.S. , Zuris, J.A. & Liu, D.R. (2016) Programmable editing of a target base in genomic DNA without double‐stranded DNA cleavage. Nature, 533, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.‐R. , Siddique, M.I. , Kim, D.‐S. , Lee, E.‐S. , Han, K. , Kim, S.‐G. et al. (2023) CRISPR/Cas9‐mediated gene editing to confer turnip mosaic virus (TuMV) resistance in Chinese cabbage (Brassica rapa). Horticulture Research, 10, uhad078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi, A. , Jarret, R. , Kousik, S. , Patrick Wechter, W. , Nimmakayala, P. & Reddy, U.K. (2017) Genetic resources of watermelon. In: Grumet, R. , Katzir, N. & Garcia‐Mas, J. (Eds.) Genetics and genomics of Cucurbitaceae. Cham: Springer International Publishing, pp. 87–110. [Google Scholar]
- Li, N. , Duan, Y. , Ye, Q. , Ma, Y. , Ma, R. , Zhao, L. et al. (2023) The Arabidopsis eIF4E1 regulates NRT1.1‐mediated nitrate signaling at both translational and transcriptional levels. New Phytologist, 240, 338–353. [DOI] [PubMed] [Google Scholar]
- Li, S. , Lin, D. , Zhang, Y. , Deng, M. , Chen, Y. , Lv, B. et al. (2022) Genome‐edited powdery mildew resistance in wheat without growth penalties. Nature, 602, 455–460. [DOI] [PubMed] [Google Scholar]
- Ling, K.‐S. , Harris, K.R. , Meyer, J.D.F. , Levi, A. , Guner, N. , Wehner, T.C. et al. (2009) Non‐synonymous single nucleotide polymorphisms in the watermelon eIF4E gene are closely associated with resistance to zucchini yellow mosaic virus. Theoretical and Applied Genetics, 120, 191–200. [DOI] [PubMed] [Google Scholar]
- Liu, H. & Naismith, J.H. (2008) An efficient one‐step site‐directed deletion, insertion, single and multiple‐site plasmid mutagenesis protocol. BMC Biotechnology, 8, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J.‐Z. , Li, F. & Liu, Y. (2017) Plant immunity against viruses. Frontiers in Microbiology, 8, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín‐Hernández, A.M. & Picó, B. (2021) Natural resistances to viruses in cucurbits. Agronomy, 11, 23. [Google Scholar]
- Miras, M. , Truniger, V. , Silva, C. , Verdaguer, N. , Aranda, M.A. & Querol‐Audí, J. (2017) Structure of eIF4E in complex with an eIF4G peptide supports a universal bipartite binding mode for protein translation. Plant Physiology, 174, 1476–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderpour, M. , Lund, O.S. , Larsen, R. & Johansen, E. (2010) Potyviral resistance derived from cultivars of Phaseolus vulgaris carrying bc‐3 is associated with the homozygotic presence of a mutated eIF4E allele. Molecular Plant Pathology, 11, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan, S. , Jacobsen, E. , Visser, R.G. & Bai, Y. (2010) Loss of susceptibility as a novel breeding strategy for durable and broad‐spectrum resistance. Molecular Breeding, 25, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechar, G.S. , Donaire, L. , Gosalvez, B. , García‐Almodovar, C. , Sánchez‐Pina, M.A. , Truniger, V. et al. (2022) Editing melon eIF4E associates with virus resistance and male sterility. Plant Biotechnology Journal, 20, 2006–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaglia, C. & Caranta, C. (2006) Translation initiation factors: a weak link in plant RNA virus infection. Trends in Plant Science, 11, 40–45. [DOI] [PubMed] [Google Scholar]
- Sang, B.‐C. & Barbosa, M.S. (1992) Single amino acid substitutions in “low‐risk” human papillomavirus (HPV) type 6 E7 protein enhance features characteristic of the “high‐risk” HPV E7 oncoproteins. Proceedings of the National Academy of Sciences of the United States of America, 89, 8063–8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shopan, J. , Mou, H. , Zhang, L. , Zhang, C. , Ma, W. , Walsh, J.A. et al. (2017) Eukaryotic translation initiation factor 2B‐beta (eIF2Bβ), a new class of plant virus resistance gene. The Plant Journal, 90, 929–940. [DOI] [PubMed] [Google Scholar]
- Soler‐Garzón, A. , McClean, P.E. & Miklas, P.N. (2021) Coding mutations in vacuolar protein‐sorting 4 AAA+ ATPase endosomal sorting complexes required for transport protein homologs underlie bc‐2 and new bc‐4 gene conferring resistance to bean common mosaic virus in common bean. Frontiers in Plant Science, 12, 769247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange, E.B. , Guner, N. , Pesic‐VanEsbroeck, Z. & Wehner, T.C. (2002) Screening the watermelon germplasm collection for resistance to papaya ringspot virus type‐W. Crop Science, 42, 1324–1330. [Google Scholar]
- Summers, R.G. , Harris, C.R. & Knowles, J.R. (1989) A conservative amino acid substitution, arginine for lysine, abolishes export of a hybrid protein in Escherichia coli: implications for the mechanism of protein secretion. Journal of Biological Chemistry, 264, 20082–20088. [PubMed] [Google Scholar]
- Truniger, V. & Aranda, M.A. (2009) Recessive resistance to plant viruses. Advances in Virus Research, 75, 119–231. [DOI] [PubMed] [Google Scholar]
- Varanda, C.M. , Félix, M.D.R. , Campos, M.D. , Patanita, M. & Materatski, P. (2021) Plant viruses: from targets to tools for CRISPR. Viruses, 13, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin, T. , Tian, H. , Ma, Y. , Wang, S. , Yang, L. , Li, X. , et al. (2022) Targeted creating new mutants with compact plant architecture using CRISPR/Cas9 genome editing by an optimized genetic transformation procedure in cucurbit plants. Horticulture Research, 9:uhab086. 10.1093/hr/uhab086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y. , Kang, D. , Shi, Z. , Shen, H. & Wehner, T. (2004) Inheritance of resistance to zucchini yellow mosaic virus and watermelon mosaic virus in watermelon. Journal of Heredity, 95, 498–502. [DOI] [PubMed] [Google Scholar]
- Yu, J. , Wu, S. , Sun, H. , Wang, X. , Tang, X. , Guo, S. et al. (2022) CuGenDBv2: an updated database for cucurbit genomics. Nucleic Acids Research, 51, D1457–D1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlobin, N. & Taranov, V. (2023) Plant eIF4E isoforms as factors of susceptibility and resistance to potyviruses. Frontiers in Plant Science, 14, 1041868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1. The interactions between watermelon eIF4E (CleIF4E) or its mutants and viral genome‐associated protein (VPg) of watermelon mosaic virus (WMV) in vitro and in vivo. (a) The interaction between CleIF4E or its mutants and WMV VPg in yeast two‐hybrid (Y2H) system. The yeast cells co‐transformed with AD‐CleIF4E or its mutants and BD‐WMV VPg were subjected to 10‐fold serial dilutions and plated on the SD/‐Trp/‐Leu/‐His selection medium for 4 days. Yeast cells co‐transformed with AD‐T‐ant and BD‐p53 were used as the positive control, while yeast cells individually co‐transformed with AD‐T‐ant and BD‐lam, BD‐PRSV VPg, BD‐ZYMV VPg or BD‐WMV‐VPg and AD, AD‐CleIF4E or its mutants and BD were used as negative controls. (b) Bimolecular fluorescence complementation (BiFC) analysis of the interactions between CleIF4E or its mutants and WMV VPg in Nicotiana benthamiana leaves. WMV VPg‐CE was individually co‐expressed with CleIF4E‐NE or its mutants in N. benthamiana leaves. Leaves individually co‐expressed with GUS‐NE and PRSV VPg‐CE, ZYMV VPg‐CE or WMV VPg‐CE, CleIF4E‐NE or its mutants and GUS‐CE were used as negative controls. Confocal imaging was performed at 48 h post‐inoculation. Scale bars = 20 μm.
FIGURE S2. The watermelon accession PI 244019 plants carrying the naturally occurring amino acid substitution of D71 to G in eIF4E are resistant to PRSV‐W, ZYMV and WMV. (a) Sequence alignment of eIF4E amino acid sequences of PI 244019 and Fufeng plants. The red box indicates amino acid substitution observed between eIF4EPI 244019 and Fufeng plants. (b) Phenotypes and western blotting analysis of viral accumulation levels in Fufeng and PI 244019 plants individually inoculated with pCB301‐PRSV‐GFP, pCB301‐ZYMV‐GFP and pCB301‐WMV‐GFP. The Ponceau S staining of RuBisCO shows sample loadings.
FIGURE S3. Phenotypes, virus accumulation levels and endogenous eIF4E expression levels of Fufeng and Xanthi plants individually inoculated with eIF4E overexpression vectors based on virus infectious clones. (a) Phenotypes, virus accumulation levels and endogenous eIF4E expression levels of Fufeng plants individually inoculated with pCB301‐PRSV‐GFP, pCB301‐PRSV‐CleIF4EWT and pCB301‐PRSV‐CleIF4ED71G at 45 days post‐inoculation (dpi). (b) Phenotypes, virus accumulation levels and endogenous eIF4E expression levels of Fufeng plants individually inoculated with pCB301‐ZYMV‐GFP, pCB301‐ZYMV‐CleIF4EWT and pCB301‐ZYMV‐CleIF4ED71G at 35 dpi. (c) Phenotypes, virus accumulation levels and endogenous eIF4E expression levels of Xanthi plants individually inoculated with pCB301‐PVY‐GFP, pCB301PVY‐CleIF4EWT and pCB301‐PVY‐CleIF4ED71G at 15 dpi. The Ponceau S staining of RuBisCO shows sample loadings. Plants in the absence of virus infection (Mock) were used as the control. Error bars represent the standard deviations of the means from biological repeats. The t test was used to test the significance of the differences. ‘ns’ represents no significant difference (α = 0.05).
FIGURE S4. The infection of eIF4E overexpression vectors based on infectious clones of PRSV and ZYMV in PI 485583. (a) Symptoms of PI 485583 plants individually infected with eIF4E overexpression vectors based on infectious clones of PRSV and ZYMV. Plants in the absence of virus infection (Mock) were used as the control. (b) Western blotting analysis of the accumulation levels of PRSV coat protein (CP) and ZYMV CP in the upper leaves of PI 485583 plants individually infected with eIF4E overexpression vectors based on infectious clones of PRSV and ZYMV.
FIGURE S5. The amino acid substitution D71G in eIF4E did not affect the male sterility of PI 244019 plants. (a) Phenotype of PI 244019 male flowers. (b) Fruits produced from self‐pollinated PI 244019 plants.
TABLE S1. Primers used in this work.
TABLE S2. List of GenBank accession number of the eIF4E amino acid sequences of multiple plants.
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.


