Abstract
To evaluate the efficacy of one‐step acellular dermis combined with autologous split thickness skin grafting in the treatment of burn or trauma wounds by a multicenter controlled study. In patients with extensive burns, it is even difficult to repair the wounds due to the shortage of autologous skin. The traditional skin grafting method has the disadvantages of large damage to the donor site, insufficient skin source and unsatisfactory appearance, wear resistance and elasticity of the wound tissue after skin grafting. One‐step acellular dermis combined with autologous ultra‐thin split thickness skin graft can achieve better healing effect in the treatment of burn and trauma wounds. A total of 1208 patients who underwent single‐layer skin grafting and one‐step composite skin grafting in the First Affiliated Hospital of Wannan Medical College, Wuhan Third People's Hospital and Lu ‘an People's Hospital from 2019 to 2022 were retrospectively analysed. The total hospitalization cost, total operation cost, hospitalization days after surgery, wound healing rate after 1 week of skin grafting and scar follow‐up at 6 months after discharge were compared and studied. The total cost of hospitalization and operation in the composite skin grafting group was significantly higher than those in the single‐layer autologous skin grafting group. The wound healing rate after 1 week of skin grafting and the VSS score of scar in the follow‐up of 6 months after discharge were better than those in the single‐layer skin grafting group. One‐step acellular dermis combined with autologous ultra‐thin split thickness skin graft has high wound healing rate, less scar, smooth appearance and good elasticity in repairing burn and trauma wounds, which can provide an ideal repair method for wounds.
Keywords: acellular dermis, burns, composite skin grafting, trauma
1. INTRODUCTION
Skin defects caused by burns or trauma are generally full‐thickness defects of the skin (including the epidermis layer, dermis layer and subcutaneous tissue), which are more damaging to the body, and this defect can be repaired by skin grafting or flap. The traditional skin grafting method is to cut the patient's own skin for transplantation and repair of the wound, which is divided into ultra‐thin split thickness skin grafting, medium‐thick skin grafting, full‐thickness skin grafting and skin grafting with subdermal vascular network according to the thickness or structure of the patient's own skin. The ultra‐thin split thickness skin sheet has the least damage to the donor area, but because it lacks dermal structure, the appearance, wear resistance and elasticity of the wound after skin grafting are not ideal; medium‐thick skin grafting, full‐thickness skin grafting and skin grafting with subcutaneous vascular network can meet the requirements of tissue appearance, wear resistance, elasticity and other aspects of skin grafting wounds, but there are disadvantages such as different degrees of scarring left in the donor area and limited skin area; patients with extensive burns are even unable to repair the wound due to insufficient autologous cortical sources. 1 Therefore, how to obtain skin tissue that meets the needs of wound structure, reduce the damage of the donor area and even obtain sufficient skin tissue without the limitation of autologous skin source has always been one of the hot spots in the field of wound repair.
Acellular allogeneic dermal matrix (ADM) is the use of biological tissue engineering technology to remove the epidermis of skin tissue, remove cells and retain the structure, morphology and composition of the extracellular matrix; after implantation into the host body, its tissue compatibility is excellent, almost no rejection reaction, good mechanical properties, low absorption rate, strong ability to promote normal tissue regeneration and other advantages. 2 , 3 At present, the technology of acellular dermis combined with autologous ultra‐thin split thickness skin composite transplantation has been applied clinically, which can effectively solve the shortcomings of skin sheet contracture, poor elasticity, scarring and other shortcomings after single‐layer thick skin grafting transplantation, and at the same time alleviate the shortcomings of damage and insufficient skin source in the full‐thickness skin grafting donor area. 2 , 4 , 5 However, current reports on acellular dermis mostly use a two‐step transplantation method, that is, transplantation of acellular dermis on the wound surface is carried out through dressing change or negative pressure wound therapy (NPWT), and then, thick skin sheet transplantation is performed after granulation covers the acellular dermis. The treatment process is cumbersome, and the hospital stay is long. 6 In this paper, the one‐step acellular dermis combined with autologous ultra‐thin split thickness skin composite transplantation and the single‐layer autologous blade thick and medium‐thick skin sheet transplantation technology were compared by multicenter retrospective analysis method, which provided a basis for the safe use of one‐step decellularized dermis in the treatment of burn wounds.
2. METHODS
This is an open, randomized, controlled, multicenter, noninferior experiment of traditional skin graft repair wounds combined with acellular dermis combined with autologous ultra‐thin split thickness skin composite transplantation, which was conducted in three centers in two provinces of China from January 2019 to December 2021. The endpoint of this experiment was 6 months after discharge. The trial was approved by the institutional review boards of the participating hospitals, and all patients provided written informed consent.
Inclusion criteria: (1) the number of days of hospitalization after admission≧ 24 hours; (2) clear consciousness, ablility to communicate and answer questions normally; (3) the medical record data are complete, and there is no missing important information. Exclusion criteria: (1) patients with various benign and malignant tumours; (2) chemical drugs, radioactive burns; (3) the burn area is greater than 20%, or it is necessary to divide skin grafting, dragnet, stamps and meek skin grafting; (4) patients with compound injuries such as fractures, craniocerebral injuries, chest and abdominal injuries; (5) those with mental illnesses who cannot cooperate with long‐term medication; (6) patients with chronic diseases and internal environment disorders that affect wound healing such as diabetes, abnormal kidney and liver function, severe anaemia, severe hypoproteinemia, heart failure, venous ulcers, etc.; (7) unable to cooperate with follow‐up.
From January 2019 to December 2021, a total of 3610 patients with skin grafting were enrolled, including the Department of Burn Plastic Surgery of the First Affiliated Hospital of Wannan Medical College, the Department of Burn Plastic Surgery of Lu'an People's Hospital, and the Department of Burn Plastic Surgery of Wuhan Third People's Hospital. According to the exclusion criteria, 2402 patients were excluded. The remaining 1208 patients were divided into acellular dermal composite skin grafting group and single‐layer autologous skin grafting group according to the skin grafting method according to their own choice after being informed in detail about the surgical plan, advantages and disadvantages before the operation. Because patients with deep burns often include mixed second‐degree wounds that need dressing change to heal, the two groups of patients are divided into trauma group and burn group according to the cause of injury. All study patients ranged in age from 2 to 71 years, with a mean age of 39 years, 997 males and 211 females, including 12 children. There were 448 cases in the trauma group, including 89 cases of foot and ankle, 155 cases of lower leg, 49 cases of thigh, 52 cases of wrist, 64 cases of upper limb, 14 cases of trunk, 7 cases of head, face and neck and 18 cases of multiple injuries. There were 760 cases in the burn group, including 108 cases of foot and ankle, 72 cases of lower leg, 48 cases of thigh, 144 cases of wrist, 86 cases of upper limb, 12 cases of trunk, 12 cases of head, face and neck and 278 cases of multiple injuries (Figure 1).
FIGURE 1.
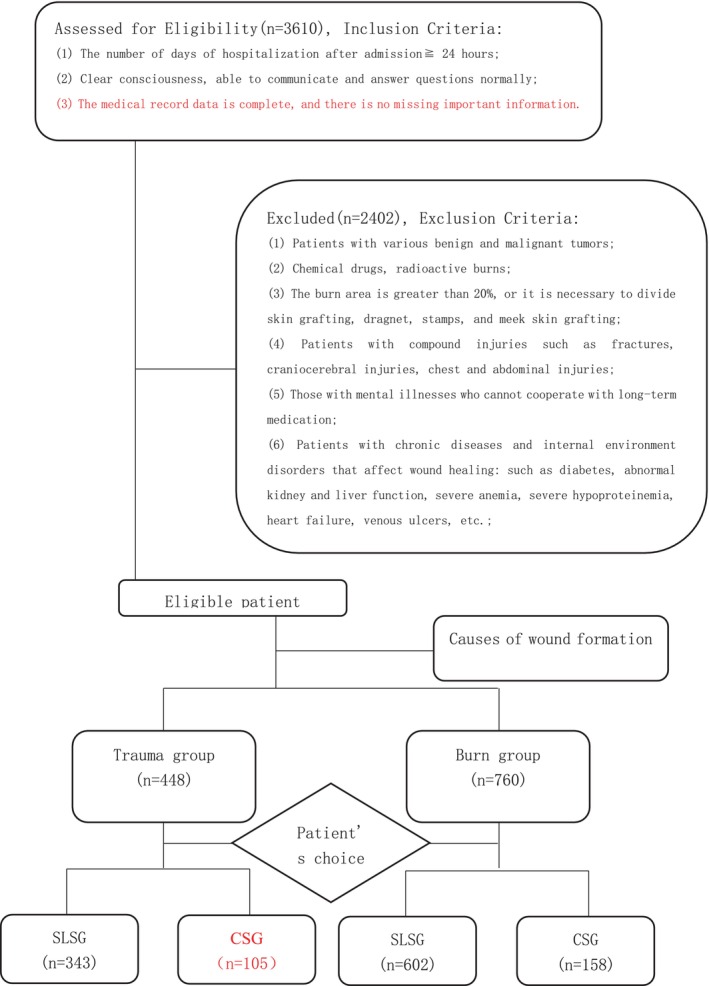
CONSORT flow diagram (SLSG—single‐layer skin grafting; CSG—compound skin grafting).
2.1. Implant material
Acellular dermis was made of J‐1 acellular allogeneic dermis (Beijing Jieya Laifu Biotechnology Co., LTD., Sinophamedo (Zhun) Word 2000 No. 346027).
2.2. Surgery and treatment procedures
All patients should complete preoperative examination after admission, maintain stable internal environment and correct anaemia, hypoproteinemia and other factors affecting wound healing. The wound is debridemented by one or more surgeries, negative pressure aspiration, wound dressing change and other measures until there is no necrotic tissue residue on the wound, the granulation is fresh, the granulation has fully covered the wound, there is no tendon, bone, blood vessel, nerve exposure and the preoperative wound bacterial culture is negative. Ensure that the wound bed meets the conditions for skin grafting.
During the operation, normal saline + chlorhexidine was given to repeatedly rinse the wound, cut the aging granulation tissue and decellulate the dermal group according to the size of the wound: Trim the large decellularized dermal scaffold such as the wound, cover the granulation wound after dragging the net, absorb the thread suture to fix the decellularized dermal stent, use a leather knife to cut the wound equal‐bladed thick skin sheet on the back, thigh or head, cover the decellularized dermal stent and suture fixation. Dry cotton baling and pressurization or negative pressure suction pressure bandaging. Monolayer autologous dermal transplantation group: Depending on the size of the wound, use a leather knife to cut the wound on the back, thigh or head, and other large‐bladed thick skin sheets, medium‐thick skin sheets or full‐thick skin sheets, and transplant medium‐thick or full‐thick skin sheets in joint areas and ultra‐thin split thickness skin sheets in nonjoint parts.Dry cotton baling and pressurization or negative pressure suction pressure bandaging.
After surgery, all patients should be treated with active symptomatic treatment such as skin graft area immobilization and nutritional support. Observe the survival of the skin for 7 days after surgery.
Residual wounds with a survival rate of 80%–100% of the skin slice should be treated with dressing change to promote wound healing, and the survival rate of the skin slice below 60% and some residual wounds of 60%–80% need to undergo skin grafting repair again and continue to change the dressing after surgery until the patient's wounds are all healed and then discharged.
After surgery, strengthen the patient's activity exercise, elastic clothing anti‐scarring and other treatments.
2.3. Observe the metrics
The survival of the wound skin graft, the composite skin grafting group and the monolayer autologous skin transplantation group, on the 7th day after skin grafting, the survival of the skin sheet was observed, and the skin piece was judged to be viable if the graft skin was well colonized, no displacement, no subcutaneous effusion and secretion, no decellularized dermis exposed and the skin sheet was rosy. Skin survival rate = skin slice survival area/total wound area × 100%;
The length of postoperative hospital stay, total length of hospital stay, total hospitalization cost, total operation cost and the number of reoperation cases were obtained from the medical records of each patient, and the reoperation rate was calculated. Reoperation rate = number of reoperation cases/total number of cases in the group ×100%;
Cases were followed up 6 months after discharge and the patient's scarring was scored using the Vancouver Scale.
2.4. Statistical processing
Excel 2017 was used to establish the database, SPSS 20.0 software was used to statistically analyse the data, the counting data were expressed by frequency and percentage, the measurement data were expressed by mean ± standard deviation (x ¯±s), the χ 2 test and t‐test were used for comparison, the total hospitalization cost and the total surgical cost were logarithmic and conformed to the normal distribution, and the p < 0.05 was statistically significant.
3. OUTCOME
3.1. Single‐layer skin grafting and one‐step compound skin grafting in the burn group
Table 1 shows that the total number of patients with monolayer skin grafting and 158 patients with compound skin grafting in the burn group was 32.53 and 19.26 days after surgery, respectively, which were higher than those in monolayer skin grafting, but the differences were not statistically significant. The total cost of hospitalization and surgery in patients with monolayer skin grafting was significantly lower than those in patients with composite skin grafting, and the differences were statistically significant (p < 0.05). The average number of cases requiring reoperation for monolayer skin grafting was higher than that of compound skin grafting, but it was not statistically significant. The VSS score of the single‐layer skin graft group at 6 months after discharge was significantly higher than that in the composite skin grafting group, which was statistically significant (p < 0.05).
TABLE 1.
Comparative analysis of monolayer skin grafting and one‐step compound skin grafting in burn group ().
| Project | Single‐layer skin grafting | Compound skin grafting | t | p‐value |
|---|---|---|---|---|
| Total length of stay | 28.00 ± 16.40 | 32.53 ± 13.20 | 1.720 | 0.089 |
| Total cost of hospitalization | 56537.75 ± 51587.16 | 196805.28 ± 162966.72 | 4.114 | <0.001 |
| Total cost of surgery | 7885.53 ± 8062.26 | 31814.37 ± 39686.54 | 4.168 | <0.001 |
| Number of days in hospital after surgery | 15.46 ± 9.55 | 19.26 ± 10.02 | 1.966 | 0.052 |
| The average number of cases requiring reoperation | 0.049 ± 0.034 | 0.033 ± 0.021 | −1.439 | 0.154 |
| VSS scoring | 5.8 ± 2.41 | 3.9 ± 2.1 | 4.126 | <0.05 |
| Total number of cases | 602 | 158 | ‐ | ‐ |
3.2. Single‐layer skin grafting and one‐step compound skin grafting in the trauma group
Table 2 showed that there were 343 patients with monolayer skin grafting and 105 patients with compound skin grafting in the trauma group, and the total number of hospital days and hospital days after surgery were higher than those in the patients with single skin grafting (30.93 VS 24.74, 16.00 VS 14.10), and the differences were not statistically significant. The total hospitalization cost of composite skin grafting patients was 116185.82 yuan, and the total cost of surgery was 16251.88 yuan, which was statistically significant compared with single‐layer skin grafting patients (p < 0.05). The average number of cases requiring reoperation with compound skin grafting was statistically significant compared with that of monolayer skin grafting (p < 0.05). The VSS score of the single‐layer skin graft group at 6 months after discharge was significantly higher than that in the composite skin grafting group, which was statistically significant (p < 0.05).
TABLE 2.
Comparative analysis of monolayer skin grafting and one‐step compound skin grafting in trauma group ().
| Project | Single‐layer skin grafting | Compound skin grafting | t | p‐value |
|---|---|---|---|---|
| Total length of stay | 24.74 ± 13.00 | 30.93 ± 12.82 | 3.973 | 0.053 |
| Total cost of hospitalization | 39361.06 ± 57554.30 | 116185.82 ± 78187.12 | 5.393 | <0.001 |
| Total cost of surgery | 3512.10 ± 3986.66 | 16251.88 ± 12228.86 | 6.938 | <0.001 |
| Number of days in hospital after surgery | 14.10 ± 10.17 | 16.00 ± 9.07 | 0.931 | 0.354 |
| The average number of cases requiring reoperation | 0.12 ± 0.23 | 0.057 ± 0.90 | 3.096 | 0.037 |
| VSS scoring | 4.6 ± 2.02 | 3.1 ± 1.90 | 3.224 | <0.05 |
| Total number of cases | 343 | 105 | ‐ | ‐ |
3.3. Wound healing
From Table 3, it can be seen that the wound healing rate of 69.62% of burn patients after 7 days of one‐step compound skin grafting was 100%, and the wound healing rate of 83.00% of burn patients after monolayer skin grafting was ≧80%, and the difference was statistically significant by the chi‐square test (p < 0.05). The wound healing rate of 63.27% of patients after monolayer skin grafting in the trauma group was 100%, and the wound healing rate of 70.48% of patients after one‐step compound skin grafting was 100%, and the difference was statistically significant (p < 0.05).
TABLE 3.
Wound healing in burn and trauma patients after skin grafting.
| Project | Number of examples | Skin flake healing rate | χ2 | p‐value | |||
|---|---|---|---|---|---|---|---|
| 100% | ≧80%,<100% | ≧60%,<80% | <60% | ||||
| Burn single‐layer skin grafting | 602 | 394 (65.40) | 106 (17.60) | 78 (12.96) | 24 (3.99) | 5.634 | <0.01 |
| Burn complex skin grafting | 158 | 110 (69.62) | 20 (12.66) | 25 (13.92) | 3 (1.90) | ||
| Trauma monolayer skin grafting | 343 | 217 (63.27) | 61 (17.78) | 37 (10.79) | 28 (8.16) | 4.823 | <0.01 |
| Trauma complex skin grafting | 105 | 74 (70.48) | 18 (17.14) | 7 (6.67) | 6 (5.71) | ||
Note: Values in parentheses represent the percentage of values in each row.
4. STAINING ANALYSIS
In the follow‐up visit, some composite skin grafting cases require fracture internal fixation removal after fracture surgery, and at the same time, the surgical incision of fracture internal fixation removal was located at the implantation site of the decellularized dermis, and after obtaining the consent of the patient, the pathological skin tissue of the patient's skin graft was collected. HE staining showed that acellular dermis was well integrated with skin tissue, with good tissue morphology, complete dermis structure and orderly arrangement. Victoria blue method + Ponceau staining showed that the dermal tissue was filled with a large number of elastic fibres and collagen fibres, and the shape was good (Figure 2).
FIGURE 2.
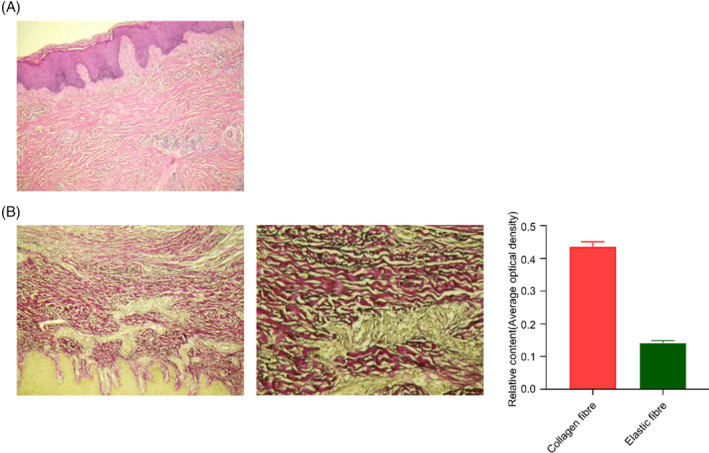
HE and Victoria blue method + Ponceau staining showed that skin tissue changes 6 months after composite skin grafting surgery. (A) HE staining; (B) Victoria blue method + Ponceau staining, collagen fibres red, elastic fibres stain turquoise, background yellow.
5. TYPICAL CASE
A patient with foot skin declusion had good wound granulation growth followed by one‐step compound skin grafting and followed up 6 months after surgery, and the patient's skin graft site had a full appearance, normal colour, good elasticity, no obvious scarring and good joint movement (Figure 3).
FIGURE 3.
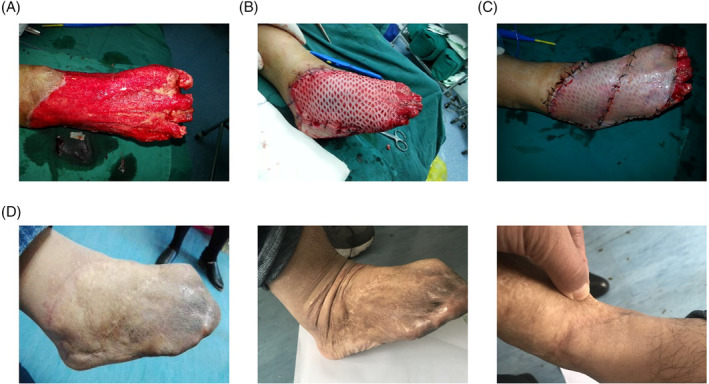
Wound healing and follow‐up of patients with skin decapsulation after compound skin grafting. (A) Granulation wound on the day of operation; (B) Acellular dermis was implanted into the granulation wound on the day of surgery; (C) One‐step autologous ultra‐thin split thickness skin covering acellular dermis on the day of surgery; (D) 6 months after the operation, the wound healed well, with full appearance, good skin elasticity and movement and no scar hyperplasia.
6. DISCUSSION
6.1. Analysis of the efficacy of single‐layer skin grafting and one‐step compound skin grafting
In this study, through a multicenter, retrospective and prospective study, the therapeutic effect of acellular dermis combined with autologous thick skin sheet compound skin grafting technology and traditional single‐layer autologous skin transplantation technology in patients with burn trauma was compared. Through this study, it can be found that: (1) There is no obvious difference between one‐step composite skin grafting technology and single‐layer autologous skin grafting technology in preoperative wound preparation, surgical steps and postoperative wound management, and the technical difficulty is low. (2) In terms of skin graft survival rate, we can find that the survival rate of compound skin grafting in the trauma group and burn group is significantly higher than that in the traditional skin grafting group, and most wounds can be changed several times after surgery to achieve all wound healing. A few wounds were poorly grafted and reoperated to repair the residual wounds. The average number of composite skin grafting reoperations was lower than that in the monolayer skin grafting group, and it was statistically significant in the trauma group. The reason may be that the preoperative wound bed preparation of the composite skin grafting group is more adequate, and the granulation is fresh and the wound is covered before skin grafting. (3) The top two reasons for requiring reoperation are wound infection and poor immobilization of the skin grafting area. The wound infection bacteria are mainly Pseudomonas aeruginosa, and poor immobilization in the skin graft area is mainly due to premature walking of foot skin grafting patients, causing skin sheet displacement and necrosis. (4) In terms of total hospital stay time and wound healing time (days required from surgery to discharge), there was no statistically significant difference in the two skin implant methods in the trauma group, and the vast majority of patients with compound skin grafting and ordinary skin grafting were discharged 12–20 days after surgery. The wound healing time of ordinary skin grafting patients in the burn group was slightly lower than that in the composite skin grafting group, and the family financial burden of the ordinary skin grafting group after the burn injury was heavier, and they chose to be discharged after the wound healed. A small number of burn patients in the composite skin grafting group insisted on hospitalization for rehabilitation exercise after wound healing, resulting in a prolonged total hospital stay. (5) In terms of surgical cost and total treatment cost, the composite skin grafting group of trauma and burn patients was significantly higher than that of ordinary skin grafting group, mainly because the cost of decellularized dermal materials accounted for a higher proportion of the total cost of surgery, which was also a major factor restricting the large use of decellularized dermis. (6) In the postoperative follow‐up, compared with the monolayer autologous skin transplantation group, especially the patients with single‐layer thick skin transplantation, the composite skin grafting area had a full appearance, good elasticity, good mobility, close colour to the surrounding normal skin and no obvious pigmentation. (7) According to the Vancouver scoring table, the composite skin grafting group was significantly better than the ordinary skin grafting group. In a few full‐thickness skin grafting cases, the grafting area is close to the compound grafting area.
6.2. Principle of action of the decellularized dermis
Acellular dermal matrix (ADM) is a biological material obtained by human or animal skin after decellularization treatment that retains the extracellular matrix of the dermal and the structure of the three‐dimensional spatial frame. 7 , 8 , 9 It has the characteristics of deepidermis, decellularization, no bacterial growth, nontoxicity, nonirritation and no immune rejection, and has the characteristics of elasticity, soft texture and not easy to break. And ADM collagen composition and arrangement is very close to autologous skin, with a complete three‐dimensional collagen structure, its biocompatibility is also very high, its extracellular matrix in the dermis has good biological activity, not only to provide mechanical support for tissues but also to promote cell adhesion and regulate cell behavior, 10 , 11 and it is an ideal alternative to human skin. At the same time, ADM, as a natural dermal substitute, has a bifacial structure, namely basement membrane surface and dermal surface, the basement membrane surface is conducive to the colonization of epithelial cells, and the dermal surface is conducive to the formation of vascularization. ADM can be divided into allogeneic ADM (humanoid) and heterogeneous ADM (animal‐born) according to material source. The main source of allogeneic ADM is made from donated allogeneic dermis, which retains the extracellular matrix, collagen and proteoglycans in the dermis, 12 , 13 its biocompatibility is high and the incidence of immune rejection is extremely low; the main source of xenogenic ADM is soft tissue from pigs and cattle, 14 although its structure is similar to that of allogeneic ADM, the inflammatory response after xeno‐ADM transplantation is greater than that of allogeneic ADM due to the large differences in the molecular structure of the histocompatibility complex between donors and receptors and the composition of the basement membrane. Studies have found that scar growth is obvious during the healing process if the wound lacks the dermis layer, and studies have also shown that the lack of elastic fibres and collagen arrangement disorder is the most important histological features of scar tissue. 15 Although medium‐thick skin grafting and full‐thickness skin grafting contain the original dermal structure, their damage to the donor area is large, which restricts their use. The decellularized dermis used in this study can provide a regular arrangement of elastic fibres and collagen bundles for the wound, integrate with the recipient tissue without discrimination, also guide the growth and differentiation of neo‐epithelial cells, induce the orderly growth of autologous fibroblasts, promote the connection between the epidermis and the dermis layer and create good conditions for the survival of the autologous epidermis. 16 Therefore, the composite graft showed less scar growth. The acellular dermis used in this study was all J‐1 acellular dermis from Beijing Jieya Laifu Co., LTD. No rejection reaction was found in clinical application, and it survived for a long time. Burn trauma is often accompanied by full‐thickness skin avulsion and necrosis. Skin grafting is also the preferred method of wound repair. In order to ensure the survival rate of skin sheet transplantation, traditional methods mostly use ultra‐thin split thickness skin sheet transplantation, but ultra‐thin split thickness skin sheet lacks the original dermal structure. In his research, it was found that if the wound lacks the dermis layer, the scar growth is obvious, the skin tissue is thin and the abrasion resistance is poor, and the degree of healing required by the appearance cannot be achieved. In some joint parts, due to scar growth and scar contracture, joint deformity and mobility disorders affect function. At the same time, studies have also shown that the lack of elastic fibres and the disorder of collagen arrangement are the most important histological features of scar tissue. 15 Although medium‐thick skin grafting and full‐thickness skin grafting contain the original dermal structure, their damage to the donor area is large, which restricts their use. The decellularized dermis used in this study can provide regular arrangement of elastic fibres and collagen bundles for the wound, integrate with the recipient tissue without discrimination, also guide the growth and differentiation of neo‐epithelial cells, induce the orderly growth of autologous fibroblasts, promote the connection between the epidermis and the dermis layer and create good conditions for the survival of the autologous epidermis. 16 Therefore, the scar growth of the composite piece transplant is small. And J‐1 type decellularized dermis is removal of skin attachments, epidermal cells and fibroblasts. No rejection reaction was found in clinical applications and long‐term survival.
6.3. Precautions for one‐step compound skin grafting
6.3.1. Timing of surgery
At present, acellular allogeneic dermis is widely used clinically for the repair of burn wounds, traumatic wounds, wounds after scar resection, donor valve wounds, muscle flap surface wounds, diabetic foot wounds and other acute and chronic wounds. 4 Similarly, in terms of surgical timing, all acute and chronic wounds that meet the wound conditions of traditional skin grafting surgery can be repaired by decellularized allogeneic dermal technology. However, the timing of decellularized allogeneic dermal implantation surgery varies depending on the wound formed by different causes.
The use of acellular allogeneic dermal technology for deep burn wounds is currently mainly concentrated in two periods, the wound after scab cutting in the early stage of burn (3–5 days after injury) and the fresh granulation wound in the late burn stage. The author usually gives a shallow incision of the deep wound after the tissue edema of the burn site begins to subside 3–5 days after the injury, and at the same time, the decellularized allogeneic dermis combined with a large autologous ultra‐thin split thickness skin (thin, medium and thick skin) is used to repair the wound in one step, and the long‐term follow‐up skin appearance fullness, softness, elasticity, abrasion resistance, colour and function of the skin after the recovery of composite skin transplantation are satisfactory. For the deep wound treatment of patients with large‐scale burns, on the basis of reducing the burn wound as soon as possible, the author's unit gave the decellularized allogeneic dermis combined with autologous ultra‐thin split thickness skin (thin, medium‐thick skin) one‐step repair wound on the fresh granulation wound formed after cutting/scab cutting or scab as soon as possible, and also obtained satisfactory results. The author unit conducted one‐step follow‐up of the wound repair of acellular allogeneic dermis combined with autologous ultra‐thin split thickness skin (thin medium‐thick skin) at different times (1 year) in burn patients and compared the skin appearance fullness, softness, elasticity, abrasion resistance, colour, function, scarring and other indicators after composite skin transplantation at different stages of burn injury and found that the above indicators of skin after early (superficial) scab simultaneous composite skin grafting were better than those of granulation wound composite skin graft in the late burn stage.
6.3.2. Surgical wound selection
Wounds formed by acute trauma, diabetic foot, various acute and chronic infections of skin and soft tissues, etc., these wounds need to be debridged, dressed changed, wound negative pressure aspiration and other treatments in the early stage, and only when there is no necrotic tissue, foreign body, blood clot and other residues on the wound, fresh granulation tissue of the wound without edema and secretion, and no obvious bone, tendon, internal fixation and other exposed wounds, can decellularized allogeneic dermal implantation be performed. Fresh wounds formed during surgery, such as wounds after scar resection, wounds in the donor valve area that are difficult to suture directly and wounds on the surface of the muscle flap after muscle flap formation, can be implanted with decellularized allogeneic dermis at the same time as surgery.
In summary, the one‐step decellularized allogeneic dermis combined with bladed thick skin sheet transplantation technology is not only used to repair deep burn wounds but also widely used in various acute traumatic wounds, as well as chronic wounds, donor wounds, muscle valve surfaces and other wounds, which can better meet the curative effect of the wound in the affected area and reduce the scarring of the donor area. Compared with the traditional single‐ultra‐thin split thickness skin sheet, medium‐thick skin sheet and full‐thick skin sheet transplantation technology, the wound healing time is the same, and the wound appearance is flat, the elasticity and mobility are good, and the scar is less, which is worthy of further promotion in the clinic.
FUNDING INFORMATION
This study was supported by the 2021 Wuhu Science and Technology Plan Project (2021jc2‐4): the 2022 Wannan Medical College Teaching Quality and Teaching Reform Project (2022jyxm69).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The authors are grateful to the patients and their families who participated in this study, as well as to the care and researchers at each hospital.
Chen L, Yang J, Wang D, et al. Multicenter effect analysis of one‐step acellular dermis combined with autologous ultra‐thin split thickness skin composite transplantation in treating burn and traumatic wounds. Int Wound J. 2024;21(1):e14341. doi: 10.1111/iwj.14341
Lei Chen (chenlei1619@163.com) contributed to this work.
Contributor Information
Zun‐jiang Zhao, Email: zhaozunjiang1974@163.com.
Xu‐Lin Chen, Email: okcxl@126.com.
Da‐lun Lv, Email: ldl0776@126.com.
DATA AVAILABILITY STATEMENT
The data that support the fndings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Łabuś W, Kitala D, Szapski M, Klama‐Baryła A, Kraut M, Smętek W. Tissue engineering in skin substitute. Adv Exp Med Biol. 2021;1345:193‐208. [DOI] [PubMed] [Google Scholar]
- 2. Ge L, Zheng S, Wei H. Comparison of histological structure and biocompatibility between human acellular dermal matrix (ADM) and porcine ADM. Burns. 2009;35(1):46‐50. [DOI] [PubMed] [Google Scholar]
- 3. Gierek M, Łabuś W, Kitala D, et al. Human acellular dermal matrix in reconstructive surgery—a review. Biomedicines. 2022;10(11):2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barootchi S, Tavelli L, Zucchelli G, Giannobile W, Wang H. Gingival phenotype modification therapies on natural teeth: a network meta‐analysis. J Periodontol. 2020;91(11):1386‐1399. [DOI] [PubMed] [Google Scholar]
- 5. Qi Y, Dong Z, Chu H, et al. Denatured acellular dermal matrix seeded with bone marrow mesenchymal stem cells for wound healing in mice. Burns. 2019;45(7):1685‐1694. [DOI] [PubMed] [Google Scholar]
- 6. Guo Z, Qiu L, Gao Y, et al. Use of porcine acellular dermal matrix following early dermabrasion reduces length of stay in extensive deep dermal burns. Burns. 2016;42(3):598‐604. [DOI] [PubMed] [Google Scholar]
- 7. Cazzell S, Vayser D, Pham H, et al. A randomized clinical trial of a human acellular dermal matrix demonstrated superior healing rates for chronic diabetic foot ulcers over conventional care and an active acellular dermal matrix comparator. Wound Repair Regen. 2017;25(3):483‐497. [DOI] [PubMed] [Google Scholar]
- 8. Tavelli L, Barootchi S, Di Gianfilippo R, et al. Acellular dermal matrix and coronally advanced flap or tunnel technique in the treatment of multiple adjacent gingival recessions. A 12‐year follow‐up from a randomized clinical trial. J Clin Periodontol. 2019;46(9):937‐948. [DOI] [PubMed] [Google Scholar]
- 9. Barootchi S, Tavelli L, Gianfilippo R, et al. Acellular dermal matrix for root coverage procedures: 9‐year assessment of treated isolated gingival recessions and their adjacent untreated sites. J Periodontol. 2021;92(2):254‐262. [DOI] [PubMed] [Google Scholar]
- 10. Cramer M, Badylak S. Extracellular matrix‐based biomaterials and their influence upon cell behavior. Ann Biomed Eng. 2020;48(7):2132‐2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Madl CM, Heilshorn SC, Blau HM. Bioengineering strategies to accelerate stem cell therapeutics. Nature. 2018;557(7705):335‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim Y, Hwang K, Kim K, Sung I, Kim S. Application of acellular human dermis and skin grafts for lower extremity reconstruction. J Wound Care. 2019;28:S12‐S17. [DOI] [PubMed] [Google Scholar]
- 13. Almeida I, Gonqalves A, Corrêa F, et al. Evaluation of clinical and biomechanical features of scars resulting from the treatment of burn contractures comparing acellular dermal matrices: a randomized clinical trial. Ann Surg. 2023;277(2):198‐205. [DOI] [PubMed] [Google Scholar]
- 14. Srivastava A, DeSagun E, Jennings L, et al. Use of porcine acellular dermal matrix as a dermal substitute in rats. Ann Surg. 2001;233(3):400‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jabeen S, Clough E, Thomlinson A, Chadwick S, Ferguson M, Shah M. Partial thickness wound: does mechanism of injury influence healing? Burns. 2019;45(3):531‐542. [DOI] [PubMed] [Google Scholar]
- 16. Hur GY, Seo DK, Lee JW. Contracture of skin graft in human burns: effect of artificial dermis. Burns. 2014;40(8):1497‐1503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the fndings of this study are available from the corresponding author upon reasonable request.


