Abstract
This study aims to explore the association between the triglyceride‐glucose (TyG) index and all‐cause mortality in patients with diabetic foot ulcers (DFUs) through an ambispective cohort study. A total of 555 inpatients with DFUs were qualified to participate in the trial study from 2013 to 2022. Throughout a median 63‐month period, all subjects were followed up every 6 months. According to the three quantiles of the TyG index, participants were divided into three groups: low‐level (≤8.75, n = 185), moderate‐level (8.76–9.33, n = 185) and high‐level (≥9.34, n = 185). The association between the TyG index and all‐cause mortality in patients with DFUs was then assessed. During the follow‐up period, out of 555 patients with DFUs, 116 died (20.9%). After adjusting for confounding factors, the TyG index was positively associated with all‐cause mortality in patients with DFUs (HR = 1.733; 95% CI = 1.341–2.241; p < 0.001). Compared with the low‐level TyG index, the moderate‐level TyG index (HR = 1.685; 95% CI = 1.011–2.810; p = 0.045) and the high‐level TyG index (HR = 2.769; 95% CI = 1.678–4.568; p < 0.001) were positively correlated with all‐cause mortality in patients with DFUs. Additionally, in subgroup analysis, both females (HR = 1.905; 95% CI = 1.250–2.904; p = 0.003), males (HR = 1.729; 95% CI = 1.240–2.409; p = 0.001), younger (<65 years old) (HR = 1.467; 95% CI = 1.008–2.135; p = 0.046) and elderly (≥ 65) (HR = 1.933; 95% CI = 1.339–2.791; p < 0.001) showed a positive correlation between TyG index and all‐cause mortality rate in patients with DFUs. Furthermore, in the high‐level TyG index group compared, males (HR = 2.699; 95% CI = 1.457–4.998) and participants aged <65 years (HR = 2.031; 95% CI = 0.972–4.242), with the TyG index level increase by 1.0, the risk for all‐cause mortality increased 3.277‐fold in females (HR = 4.277; 95% CI = 1.645–11.124) and 1.909‐fold in elderly aged ≥65 years (HR = 2.909; 95% CI = 1.486–5.695), respectively. Kaplan–Meier survival curve analysis showed that the higher the TyG index level, the higher risk of all‐cause mortality in patients with DFUs (log‐rank, all p < 0.001). Briefly, this study implies a strong positive correlation between the TyG index and all‐cause mortality in patients with DFUs, especially in older women. Therefore, special attention should be paid to elderly females with DFUs because they have a higher TyG index level and risk of all‐cause mortality than other populations in daily clinical practice.
Keywords: all‐cause mortality, diabetic foot ulcers, triglyceride‐glucose index
1. INTRODUCTION
Deficiencies in insulin secretion, insulin action or both are the hallmarks of the metabolic disorders known as diabetes, characterized by glycotoxicity and lipotoxicity, which is increasingly becoming one of the leading medical issues worldwide. 1 With an 11.2% prevalence incidence among persons over 18, China is one of the nations with the highest prevalence rate of diabetes, of which more than 90% are type 2 diabetes. 2 , 3 As a diabetic complication with increased morbidity, disability and mortality, diabetic foot ulcers (DFUs) profoundly impact the quality of life and put a heavy financial and mental burden on sufferers. 4 , 5 , 6 , 7 , 8 DFUs refer to a foot ulcer, infection or profound tissue destruction related to lower limb peripheral neuropathy and peripheral vascular disease. 9 , 10 Regardless of age, diabetes type and diabetes course, the mortality rate of diabetic foot patients is more than twice that of non‐diabetic foot patients. 11 The 1‐year, 2‐year and 5‐year survival rates of patients with DFUs were 81%, 69% and 29%, respectively. The impact of the diabetic foot on mortality was more significant than that of diagnosed coronary artery disease or stroke. 12 Consequently, the early identification and assessment of risk factors for all‐cause mortality in patients with DFUs is crucial in clinical practice.
The onset and progression of diabetic mellitus are significantly influenced by insulin resistance (IR) and pancreatic islet cell dysfunction. 13 IR can contribute to several metabolic conditions, such as dyslipidemia and poor glucose metabolism. 14 Therefore, functional IR evaluation aids in the identification of diabetic patients at high risk for developing severe complications. Recently, it has been demonstrated that the classical HOMA‐IR approach can be substituted by the triglyceride‐glucose (TyG) index formulated by Ln [fasting triglycerides (mg/dL) × fasting blood glucose (mg/dL)/2] to evaluate insulin resistance. 15 , 16 , 17
The TyG index may also be more accurate than HOMA‐IR at predicting adverse cardiovascular outcomes associated with diabetes, 18 such as diabetic nephropathy 19 , 20 and diabetic retinopathy. 21 TyG index has prospective application prospects owing to being more straightforward to obtain and compute in clinical practice. To date, no study has explored the relationship between the TyG index and all‐cause mortality in DFU patients. Therefore, the purpose of this study was to investigate whether the TyG index could potentially be used to predict all‐cause mortality in patients with DFUs.
2. METHODS
2.1. Enrollment in subjects
Between 2013 and 2022, all subjects with DFUs were randomly selected from the Foot Healthcare Center of Guangxi People's Hospital. The inclusion criteria were as follows: (1) in accordance with the World Health Organization (WHO) guidelines in 1999, type 2 diabetes was identified 22 ; (2) according to the recommendations of the International Working Group on Diabetic Foot (IWGDF), all participants were verified with DFUs. 23 Exclusion criteria were as follows: (1) non‐type 2 diabetes; (2) a non‐diabetic foot ulcer; (3) with tumour and use of glucocorticoids or immunosuppressive medications; (4) age <18 years old; (5) missing hospitalization data, lost to follow‐up and a follow‐up period of less than a year. A total of 555 inpatients with DFUs were finally qualified to participate as study participants. According to the three quantiles of the TyG index, the selected patients were divided into three groups: low‐level (≤8.75, n = 185), moderate‐level (8.76–9.33, n = 185) and high‐level (≥9.34, n = 185). All participants had the same ethic category and all were Zhuang nationals. All subjects were followed up every 6 months for a median of 63 months through outpatient services, the WeChat app and telephone visits. All patients were given standard programmes for controlling blood glucose, blood pressure, blood lipid and anticoagulation according to China Diabetes Guideline, and there was no difference among the three groups. It was evaluated whether the TyG index was related to the all‐cause mortality rate in patients with DFUs.
2.2. Data gathering
Demographic data, anthropometric data, laboratory biochemical index data, inpatient medical records information and follow‐up data were collected anonymously based on information from the management database of the Foot Healthcare Centre of Guangxi People's Hospital.
2.3. Related definitions
The relevant definitions in this study were as follows: (1) the endpoint event referred to the all‐cause mortality acquired through outpatient services, the WeChat app and telephone visits; (2) TyG index = Ln [fasting triglycerides (mg/dL) × fasting blood glucose (mg/dL)/2], TyG index ≤8.75 was defined as a low‐level, moderate‐level TyG index was defined as the range of 8.76–9.33, TyG index ≥9.34 was defined as a high‐level; (3) aged ≥65 are defined as elderly persons.
2.4. Statistical analysis
The mean (±SD) was used to express variables with normal distributions, and the median (interquartile ranges) was used to express variables with non‐normal distributions. Discontinuous variables are represented as frequencies. The normal distribution continuous variable group comparison was tested using analysis of variance. In contrast, a non‐normal distribution was tested using Kruskal–Wallis H test. Categorical variables were compared between groups using the Chi‐squared test. Cox regression analysis was used to assess the risk factors for all‐cause mortality. Cox regression stratified analysis was used in the subgroup. Kaplan–Meier survival curve analysis and log‐rank test were used to evaluate the all‐cause mortality rate in patients with DFUs. The statistical package SPSS 23.0 was used to conduct the data analysis. p < 0.05 was adopted as the statistical significance level.
3. RESULTS
3.1. Baseline characteristics
A total of 555 patients with DFUs were included in this longitudinal cohort analysis. The baseline characteristics data of participants are shown in Table 1. The average age of the study population was 64.5 years, of which 68.3% were males. According to the three quantiles of the TyG index, participants were divided into three groups: low‐level (≤8.75, n = 185), moderate‐level (8.76–9.33, n = 185) and high‐level (≥9.34, n = 185). Among the three groups, gender, age, all‐cause mortality, DBP, WBC, TC, TG, LDL‐C, HDL‐C, FBG, HbA1c and TyG index were statistically significant (all p < 0.05), while hypertension, CHD, CVD, duration of diabetes, duration of ulcer before hospitalization, SBP, Hb, ALB, ALT, AST and Cr were not statistically significant (all p > 0.05). The all‐cause mortality among the three groups is shown in Figure 1. During the follow‐up period, 116 (20.9%) patients with DFUs died. The all‐cause mortality rates were 14.1% for the low‐level group, 20.0% for the moderate‐level group and 28.6% for the high‐level group, respectively.
TABLE 1.
Baseline clinical characteristics of participants.
| Clinical parameters | All participants (n = 555) | Low‐level (≤8.75) (n = 185) | Moderate‐level (8.76–9.33) (n = 185) | High‐level (≥9.34) (n = 185) | p‐value |
|---|---|---|---|---|---|
| Gender, n (%) | 0.048* | ||||
| Female | 176 31.7) | 58 (31.4) | 48 (25.9) | 70 (37.8) | |
| Male | 379 (68.3) | 127 (68.6) | 137 (74.1) | 115 (62.2) | |
| Age, years | 64.49 ± 11.31 | 67.49 ± 11.47 | 63.96 ± 10.81 | 62.02 ± 11.02 | <0.001* |
| Hypertension, n (%) | 0.747 | ||||
| No | 225 (40.5) | 74 (40.0) | 79 (42.7) | 72 (38.9) | |
| Yes | 330 (59.5) | 111 (60.0) | 106 (57.3) | 113 (61.1) | |
| CHD, n (%) | 0.125 | ||||
| No | 487 (87.7) | 155 (83.8) | 165 (89.2) | 167 (90.3) | |
| Yes | 68 (12.3) | 30 (16.2) | 20 (10.8) | 18 (9.7) | |
| CVD, n (%) | 0.406 | ||||
| No | 494 (89.0) | 161 (87.0) | 164 (88.6) | 169 (91.4) | |
| Yes | 61 (11.0) | 24 (13.0) | 21 (11.4) | 16 (8.6) | |
| All‐cause mortality, n (%) | 0.002* | ||||
| No | 159 (85.9) | 148 (80.0) | 132 (71.4) | ||
| Yes | 26 (14.1) | 37 (20.0) | 53 (28.6) | ||
| Duration of diabetes, years | 10 (4, 15) | 10 (3, 15.5) | 10 (4.5, 14) | 10 (5, 15) | 0.855 |
| SBP, mmHg | 142.6 ± 24.95 | 141.26 ± 23.08 | 141.47 ± 24.11 | 145.08 ± 27.39 | 0.256 |
| DBP, mmHg | 73.18 ± 12.09 | 70.81 ± 11.01 | 73.22 ± 12.26 | 75.51 ± 12.56 | 0.001* |
| WBC, 109/L | 8.226 (7, 10.82) | 8.06 (6.38, 10.14) | 8.42 (6.97, 11.27) | 8.15 (6.74, 11.14) | 0.044* |
| Hb, g/L | 112.01 ± 22.54 | 111.45 ± 21.79 | 112.04 ± 23.32 | 112.52 ± 22.60 | 0.901 |
| TC, mmol/L | 4.03 (3.33, 4.94) | 3.72 (3.07, 4.55) | 3.89 (3.24, 4.87) | 4.52 (3.66, 5.40) | <0.001* |
| TG, mmol/L | 1.25 (0.94, 1.73) | 0.91 (0.71, 1.09) | 1.3 (1.03, 1.61) | 1.81 (1.5, 2.56) | <0.001* |
| HDL‐C, mmol/L | 0.93 (0.74, 1.11) | 0.97 (0.77, 1.15) | 0.88 (0.72, 1.07) | 0.92 (0.72, 1.12) | 0.016* |
| LDL‐C, mmol/L | 2.56 (2.01, 3.24) | 2.34 (1.86, 2.92) | 2.52 (1.99, 3.13) | 2.82 (2.34, 3.52) | <0.001* |
| ALB, g/L | 33.8 (29.5, 37.2) | 33.9 (30.3, 36.35) | 33.4 (28.7, 37.5) | 34.3 (29, 37.95) | 0.802 |
| ALT, U/L | 15 (11, 21) | 15 (10, 20) | 15 (10, 22) | 15 (11, 21.5) | 0.851 |
| AST, U/L | 18 (14, 23) | 18 (15, 23) | 18 (14.5, 24) | 17 (14, 23) | 0.202 |
| Cr, μmol/L | 90 (66, 119) | 91 (73, 111.35) | 88 (65.5, 116) | 89 (64, 137) | 0.497 |
| FBG, mmol/L | 8.04 (6.13, 11.12) | 6.04 (4.92, 7.23) | 8.35 (6.68, 10.5) | 11.47 (9.04, 14.43) | <0.001* |
| HbA1c, % | 8.6 (7, 10.7) | 7.5 (6.55, 9.15) | 9 (7.45, 10.95) | 9.7 (7.9, 11.4) | <0.001* |
| TyG index, mg2/dL2 | 9.06 (8.59, 9.48) | 8.42 (8.15, 8.59) | 9.06 (8.92, 9.19) | 9.72 (9.48, 9.97) | <0.001* |
Note: Mean ± standard deviation (SD) and Median (Inter Quartile Range) for continuous variables. Percentage (%) for categorical variables.
Abbreviations: ALB, serum albumin; ALT, alanine transaminase; AST, aspartate alaninetransaminase; CHD, coronary heart disease; Cr, creatinine; CVD, cerebrovascular disease; DBP, diastolic blood pressure; FBG, fasting blood glucose; Hb, haemoglobin; HbA1c, glycosylated haemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; TyG, triglyceride–glucose; WBC, leukocyte.
p < 0.05.
FIGURE 1.
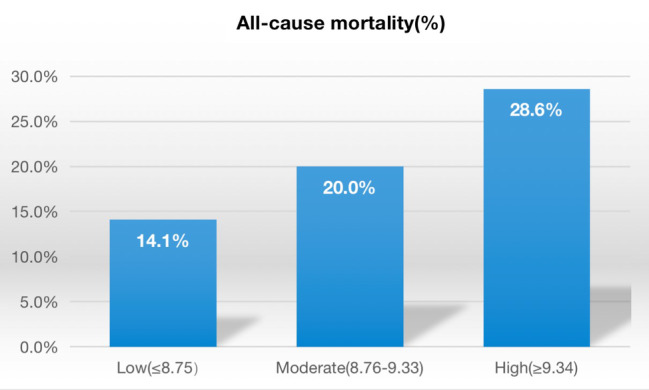
Comparison of the all‐cause mortality in the three groups. The incidence rates of all‐cause mortality were related to the levels of the triglyceride‐glucose (TyG) index in a dose‐response fashion (all‐cause mortality: 14.1%, 20.0% and 28.6% for low, moderate and high levels of the TyG index, respectively; all p for trend <0.01).
3.2. Cox proportional hazard models for all‐cause mortality
The effects of the TyG index on the risk of all‐cause mortality are shown in Table 2. To identify the factors influencing participant all‐cause mortality, a univariate Cox regression analysis was conducted. Next, variables with P < 0.1 in univariate Cox regression analysis were included in multivariate Cox regression. In model I (unadjusted), the TyG index was positively correlated with all‐cause mortality (HR = 1.611; 95% CI = 1.263–2.054; p < 0.001). Model II (adjusted model I with gender and age) (HR = 1.750; 95% CI = 1.363–2.248; p < 0.001) and model III (adjusted model II with hypertension, CHD, CVD, duration of diabetes, SBP, DBP, Hb and Cr) (HR = 1.733; 95% CI = 1.341–2.241; p < 0.001) also demonstrated a positive correlation between the TyG index and all‐cause mortality, respectively. The TyG index was changed into a categorical variable according to the three quantiles, and sensitivity analysis and trend test were used to assess the risk of all‐cause mortality. Similarly, model I (HR = 1.516; 95% CI = 1.203–1.910; p < 0.001), model II (HR = 1.656; 95% CI = 1.306–2.101; p < 0.001) and model III (HR = 1.662; 95% CI = 1.299–2.126; p < 0.001) showed that the higher the level of the TyG index, the higher the all‐cause mortality.
TABLE 2.
Unadjusted and multivariate Cox proportional hazard models for all‐cause mortality in subjects.
| HR, 95% CI and p values | |||
|---|---|---|---|
| Model I | Model II | Model III | |
| TyG index | 1.611 (1.263–2.054) | 1.750 (1.363–2.248) | 1.733 (1.341–2.241) |
| p values | <0.001* | <0.001* | <0.001* |
| Low‐level (≤8.75) | Reference | Reference | Reference |
| Moderate‐level (8.76–9.33) | 1.467 (0.888–2.423) | 1.643 (0.991–2.722) | 1.685 (1.011–2.810) |
| p values | 0.134 | 0.054 | 0.045* |
| High‐level (≥9.34) | 2.282 (1.427–3.649) | 2.739 (1.689–4.441) | 2.769 (1.678–4.568) |
| p values | 0.001* | <0.001* | <0.001* |
| TyG index | 1.516 (1.203–1.910) | 1.656 (1.306–2.101) | 1.662 (1.299–2.126) |
| p for trend | <0.001* | <0.001* | <0.001* |
Note: Model I: adjust for none. Model II: adjust for gender, age. Model III: adjust for Model II, hypertension, CHD, CVD, duration of diabetes, SBP, DBP, Hb, Cr.
Abbreviations: CHD, coronary heart disease; CI, confidence interval; Cr, creatinine; CVD, cerebrovascular disease; DBP, diastolic blood pressure; Hb, haemoglobin; HR, hazard ratio; SBP, systolic blood pressure; TyG, triglyceride‐glucose.
p < 0.05.
3.3. Stratified analysis with subgroups
The stratified analysis by age and gender in subgroups is shown in Table 3. Adjusting variables included gender, age, hypertension, CHD, CVD, duration of diabetes, SBP, DBP, Hb and Cr. The findings demonstrated that the risk of all‐cause mortality was steady across different subgroups. The trend test of the three groups was significant, whether in women, men, young people or older adults. The risk of all‐cause mortality increased with the TyG index (HR >1, all P < 0.05). In subgroup analysis, both females (HR = 1.905; 95% CI = 1.250–2.904; p = 0.003), males (HR = 1.729; 95% CI = 1.240–2.409; p = 0.001), younger (<65 years old) (HR = 1.467; 95% CI = 1.008–2.135; p = 0.046) and elderly (≥ 65) (HR = 1.933; 95% CI = 1.339–2.791; p < 0.001) showed a positive correlation between TyG index and all‐cause mortality rate in patients with DFUs. Additionally, in the high‐level TyG index group compared, males (HR = 2.699; 95% CI = 1.457–4.998) and participants aged <65 years (HR = 2.031; 95% CI = 0.972–4.242), with the TyG index level increase by 1.0, the risk for all‐cause mortality increased 3.277‐fold in females (HR = 4.277; 95% CI = 1.645–11.124) and 1.909‐fold in elderly aged ≥65 years (HR = 2.909; 95% CI = 1.486–5.695), respectively.
TABLE 3.
Cox regression stratified analysis with subgroups.
| Subgroups | Case/total | Serum TyG index tertiles | Serum TyG index as a continuous variable | p for trend | ||
|---|---|---|---|---|---|---|
| Low‐level | Moderate‐level HR (95% CI) | High‐level HR (95% CI) | ||||
| Gender | ||||||
| Female | 45/176 | Ref | 4.673 (1.81–12.068) | 4.277 (1.645–11.124) | 1.905 (1.250–2.904) | 1.797 (1.214–2.662) |
| p values | 0.001* | 0.003* | 0.003* | 0.003* | ||
| Male | 71/379 | Ref | 0.958 (0.496–1.85) | 2.699 (1.457–4.998) | 1.729 (1.240–2.409) | 1.721 (1.240–2.389) |
| p values | 0.898 | 0.002* | 0.001* | 0.001* | ||
| Age, years | ||||||
| <65 | 56/280 | Ref | 1.084 (0.483–2.433) | 2.031 (0.972–4.242) | 1.467 (1.008–2.135) | 1.478 (1.017–2.148) |
| p values | 0.845 | 0.059 | 0.046* | 0.041* | ||
| ≥65 | 60/275 | Ref | 2.102 (1.088–4.06) | 2.909 (1.486–5.695) | 1.933 (1.339–2.791) | 1.685 (1.221–2.327) |
| p values | 0.027* | 0.002* | <0.001* | 0.002* | ||
Note: Models are adjusted for gender, age, hypertension, CHD, CVD, duration of diabetes, SBP, DBP, Hb, Cr.
Abbreviations: CHD, coronary heart disease; CI, confidence interval; Cr, creatinine; CVD, cerebrovascular disease; DBP, diastolic blood pressure; Hb, haemoglobin; HR, hazard ratio; SBP, systolic blood pressure; TyG, triglyceride–glucose.
p < 0.05.
3.4. Triglyceride‐glucose index and endpoint events
The evaluation of all‐cause mortality events in the three groups is shown in Figure 1. There was a dose–response relationship between the levels of TyG index and the incidence rates of all‐cause mortality (all‐cause mortality: 14.1%, 20.0% and 28.6% for low‐level TyG index, moderate‐level TyG index and high‐level TyG index, respectively; all P for trend <0.01). The Kaplan–Meier survival curve analysis revealed that compared to the low‐level TyG index, the moderate‐level and high‐level TyG indexes were associated positively with the high risk of all‐cause mortality in patients with DFUs (log‐rank, all p < 0.001) (Figure 2).
FIGURE 2.
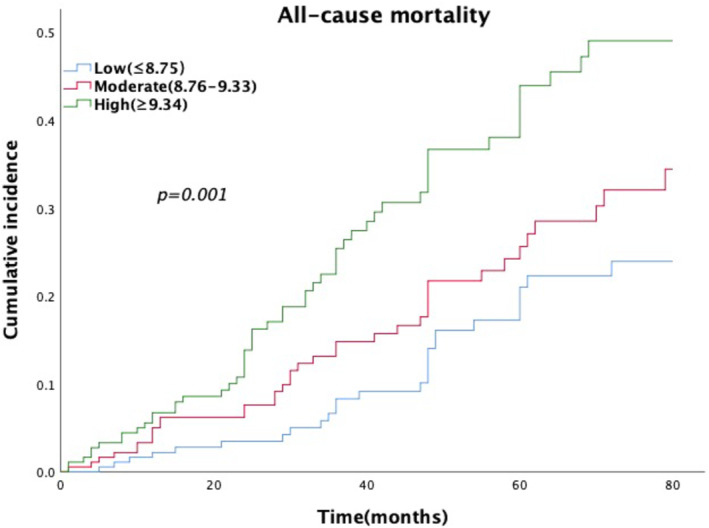
Cumulative incidence of all‐cause mortality by triglyceride‐glucose (TyG) index level. Kaplan–Meier analysis showed a significant positive association between the TyG index and the incidence rates of all‐cause mortality (log‐rank, all p < 0.001).
4. DISCUSSION
Glycotoxicity and lipotoxicity form a vicious cycle in diabetes. TyG index has received much attention recently since it takes into account these two pathological features that influence the progression of diabetes. In this study, the association between the TyG index and all‐cause mortality in patients with DFUs was investigated for the first time.
The retrospective cohort study showed that after adjusting for confounding factors such as age, gender, hypertension, CHD, CVD, duration of diabetes, SBP, DBP, Hb and Cr, the TyG index was positively correlated with all‐cause mortality in patients with DFUs (HR = 1.733; 95% CI = 1.341–2.241; p < 0.001). Compared with the low‐level TyG index, the moderate‐level TyG index and the high‐level TyG index increased the risk of all‐cause mortality in patients with DFUs by 0.685‐fold (HR = 1.685; 95% CI = 1.011–2.810; p = 0.045) and 1.769‐fold (HR = 2.769; 95% CI = 1.678–4.568; p < 0.001), respectively. Despite gender or age disparities, the stratified analysis revealed a substantial positive correlation between the TyG index and all‐cause mortality in DFU patients. Moreover, the all‐cause mortality rate was also observed to be greater in women (HR = 1.905; 95% CI = 1.250–2.904; p = 0.003) and elderly patients with DFUs (HR = 1.933; 95% CI = 1.339–2.791; p < 0.001), respectively. This finding may be accounted for by the greater risk of diabetes in older females. 24 Furthermore, visceral adipose tissue rises with age, which could contribute to insulin resistance. Diabetic foot prognosis is adversely affected by poor blood glucose control. 25 In this study, after adjusting age, diabetes course, hypertension, CHD, CVD and creatinine, the conclusion that the TyG index was positively related to all‐cause mortality remained statistically significant in patients with DFUs. This result redemonstrated in full that the TyG index is a valuable biochemical indicator with potential predictive value for the prognosis of patients with DFUs and has popularization and application value in clinical practice.
Several studies showed that the TyG index aided in early identification of high‐risk diabetic patients and appeared more accurate than fasting blood glucose (FBG) or triglycerides (TG) at predicting the possible onset of diabetes mellitus in patients with normal blood glucose levels. 26 According to reports, several mechanisms could account for the association between diabetes and the TyG index. On the one hand, a rise in TG levels may reduce the effectiveness of insulin, promote the generation of inflammatory cytokines and muscle catabolism and disrupt cell function. 27 On the other hand, increasing the amount of reactive oxygen species could result in cells' harmful effects and raise the concentration of glucose. 28 Therefore, controlling TG and FBG levels may enhance pancreatic islet function and insulin sensitivity based on the above mechanisms. The TyG index serves as a binding indicator for TG and FBG, and a high TyG index reflects reduced insulin secretion and resistance status. The baseline data from the study also revealed an association between the levels of total cholesterol, triglycerides, low‐density lipoprotein, fasting blood glucose and glycated haemoglobin and the TyG index, with higher levels of TyG index being related to higher levels of these variables. This fact demonstrated that the control of blood lipids and blood glucose was worse with a higher TyG index, which was evidently harmful to the prognosis of diabetic foot. Growing body evidence has shown that TyG index is related to diabetic macrovascular and microvascular complications, 29 , 30 , 31 which is directly related to the pathological mechanisms of diabetic foot. 32 , 33 As a result, physically and clinically, the association between the TyG index and the prognosis of the diabetic foot makes sense. Kaplan–Meier survival curve analysis in this study also revealed that all‐cause mortality in patients with DFUs increased with the TyG index (p < 0.001).
The current research has several advantages. First, sensitivity analysis and trend test were used to assess the impact of the TyG index on all‐cause mortality rate in patients with DFUs, based on the TyG index as both a categorical variable and a continuous variable, further improving the credibility of the conclusions. Second, the TyG index has advantages over other indicators concerning convenience and affordable medical costs. Indeed, this study also has some limitations. Firstly, the sample size of this study was small, and the selected risk factors were limited. Secondly, this was a single‐centre retrospective clinical cohort study with little applicability to other populations, and long‐term follow‐up studies and extensive epidemiological research are still needed to confirm the findings.
5. CONCLUSION
In conclusion, this study implied a strong positive correlation between the TyG index and all‐cause mortality in patients with DFUs, particularly in older women. Therefore, special attention should be paid to elderly females with DFUs because they have a higher TyG index level and risk of all‐cause mortality than other populations in daily clinical practice. Meanwhile, better control of the TyG index level is conducive to improving the prognosis and reducing mortality in patients with DFUs.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Huang X, Han J, Nong Y, et al. Triglyceride‐glucose index is strongly associated with all‐cause mortality in elderly females with diabetic foot ulcers: A 9‐year follow‐up study. Int Wound J. 2024;21(1):e14344. doi: 10.1111/iwj.14344
Xiuxian Huang, Jiaxia Han, Yuechou Nong and Jingxia Sun contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67‐S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. doi: 10.1136/bmj.m997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239‐2251. doi: 10.1016/S0140-6736(17)30058-2 [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . 10. Microvascular complications and foot care [published correction appears in diabetes care. 2017 Jul;40(7):986]. Diabetes Care. 2017;40(Suppl 1):S88‐S98. [DOI] [PubMed] [Google Scholar]
- 5. Zhang L, Long J, Jiang W, et al. Trends in chronic kidney disease in China. N Engl J Med. 2016;375(9):905‐906. [DOI] [PubMed] [Google Scholar]
- 6. Pop‐Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position Statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rahman MH, Kamrul‐Hasan AB, Islam MR, et al. Frequency and risk factors of diabetic retinopathy among patients with type 2 diabetes mellitus: a single‐center study from Bangladesh. Mymensingh Med J. 2020;29(4):807‐814. [PubMed] [Google Scholar]
- 8. Hobizal KB, Wukich DK. Diabetic foot infections: current concept review. Diabet Foot Ankle. 2012;3(1):18409. doi: 10.3402/dfa.v3i0.18409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khanolkar MP, Bain SC, Stephens JW. The diabetic foot. QJM. 2008;101(9):685‐695. [DOI] [PubMed] [Google Scholar]
- 10. Zubair M, Malik A, Ahmad J. Clinico‐microbiological study and antimicrobial drug resistance profile of diabetic foot infections in North India [published correction appears in Foot (Edinb). 2011 Dec;21(4):209–10]. Foot (Edinb). 2011;21(1):6‐14. [DOI] [PubMed] [Google Scholar]
- 11. Boyko EJ, Ahroni JH, Smith DG, Davignon D. Increased mortality associated with diabetic foot ulcer. Diabet Med. 1996;13(11):967‐972. [DOI] [PubMed] [Google Scholar]
- 12. Brennan MB, Hess TM, Bartle B, et al. Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes. J Diabetes Complications. 2017;31(3):556‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88‐98. [DOI] [PubMed] [Google Scholar]
- 14. Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126(1):12‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simental‐Mendía LE, Rodríguez‐Morán M, Guerrero‐Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299‐304. [DOI] [PubMed] [Google Scholar]
- 16. Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guerrero‐Romero F, Simental‐Mendía LE, González‐Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic‐hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347‐3351. [DOI] [PubMed] [Google Scholar]
- 18. Wang L, Cong HL, Zhang JX, et al. Triglyceride‐glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu L, Xia R, Song X, et al. Association between the triglyceride‐glucose index and diabetic nephropathy in patients with type 2 diabetes: a cross‐sectional study. J Diabetes Investig. 2021;12(4):557‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lv L, Zhou Y, Chen X, et al. Relationship between the TyG index and diabetic kidney disease in patients with Type‐2 diabetes mellitus. Diabetes Metab Syndr Obes. 2021;14:3299‐3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Srinivasan S, Singh P, Kulothungan V, Sharma T, Raman R. Relationship between triglyceride glucose index, retinopathy and nephropathy in type 2 diabetes. Endocrinol Diabetes Metab. 2020;4(1):e00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alberti KGMM, Zimmet PZ. Diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Med. 1998;15:539‐553. [DOI] [PubMed] [Google Scholar]
- 23. Lipsky BA, Senneville É, Abbas ZG, et al. on behalf of the International Working Group on the Diabetic Foot (IWGDF)Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36:e3280. [DOI] [PubMed] [Google Scholar]
- 24. Greenman Y, Golani N, Gilad S, Yaron M, Limor R, Stern N. Ghrelin secretion is modulated in a nutrient‐ and gender‐specific manner. Clin Endocrinol (Oxf). 2004;60(3):382‐388. [DOI] [PubMed] [Google Scholar]
- 25. Machann J, Thamer C, Schnoedt B, et al. Age and gender related effects on adipose tissue compartments of subjects with increased risk for type 2 diabetes: a whole body MRI/MRS study. Magma. 2005;18(3):128‐137. [DOI] [PubMed] [Google Scholar]
- 26. Navarro‐González D, Sánchez‐Íñigo L, Pastrana‐Delgado J, Fernández‐Montero A, Martinez JA. Triglyceride‐glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the vascular‐metabolic CUN cohort. Prev Med. 2016;86:99‐105. [DOI] [PubMed] [Google Scholar]
- 27. Unger RH. Lipotoxicity in the pathogenesis of obesity‐dependent NIDDM. genetic and clinical implications. Diabetes. 1995;44(8):863‐870. [DOI] [PubMed] [Google Scholar]
- 28. Robertson RP, Harmon J, Tran PO, Poitout V. Beta‐cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl 1):S119‐S124. [DOI] [PubMed] [Google Scholar]
- 29. Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34(31):2436‐2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yao H, Sun Z, Yuan W, et al. Relationship between the triglyceride‐glucose index and type 2 diabetic macroangiopathy: a single‐center retrospective analysis. Diabetes Metab Syndr Obes. 2022;15:3483‐3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neelam K, Aung KCY, Ang K, Tavintharan S, Sum CF, Lim SC. Association of Triglyceride Glucose Index with prevalence and incidence of diabetic retinopathy in a Singaporean population. Clin Ophthalmol. 2023;17:445‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Megallaa MH, Ismail AA, Zeitoun MH, Khalifa MS. Association of diabetic foot ulcers with chronic vascular diabetic complications in patients with type 2 diabetes. Diabetes Metab Synd. 2019;13(2):1287‐1292. [DOI] [PubMed] [Google Scholar]
- 33. Zhang L, Fu G, Deng Y, et al. Risk factors for foot ulcer recurrence in patients with comorbid diabetic foot osteomyelitis and diabetic nephropathy: a 3‐year follow‐up study. Int Wound J. 2023;20(1):173‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


