ABSTRACT
Cefiderocol is a siderophore cephalosporin designed to target multi-drug-resistant Gram-negative bacteria. Previously, the emergence of cefiderocol non-susceptibility has been associated with mutations in the chromosomal cephalosporinase (PDC) along with mutations in the PirA and PiuA/D TonB-dependent receptor pathways. Here, we report a clinical case of cefiderocol-resistant P. aeruginosa that emerged in a patient during treatment. This resistance was associated with mutations not previously reported, suggesting potential novel pathways to cefiderocol resistance.
KEYWORDS: gram-negative bacteria, siderophores, Pseudomonas aeruginosa, cefiderocol
INTRODUCTION
Cefiderocol is a novel siderophore antibiotic designed to target multi-drug-resistant (MDR) Gram-negative bacteria. Cefiderocol overcomes traditional efflux and porin-mediated resistance, by binding to extracellular iron and utilizing TonB-dependent iron-siderophore transporters (TBDT) to gain access to the periplasmic space (1). In vitro studies have established PirA and PiuA/PiuD as the major TBDT pathways responsible for cefiderocol uptake in Pseudomonas aeruginosa, and we have previously reported mutations in these pathways in cefiderocol non-susceptible clinical isolates (2, 3). In addition, the size of the large siderophore moiety decreases cefiderocol’s vulnerability to degradation by beta-lactamases, such as the AmpC cephalosporinase intrinsic to P. aeruginosa (4). These factors have made it a promising last-line agent in the treatment of MDR P. aeruginosa infections. However, there are concerns of poor clinical outcomes and treatment failures with cefiderocol monotherapy despite in vitro susceptibility (5). Heteroresistance, or the survival of a small subpopulation of bacteria at or above the minimum inhibitory concentration (MIC) of an antibiotic, has been postulated as a mechanism for this observation (6). In this report, we identify treatment-emergent cefiderocol resistance in a patient associated with gene mutations not previously reported arising from a heteroresistant isolate.
A man in his 30s with a medical history of type II diabetes mellitus was admitted to an outside facility for acute respiratory distress syndrome (ARDS) due to SARS-CoV-2. He required venovenous extracorporeal membrane oxygenation over a 100-day period with minimal improvement. During this stay, he developed a bronchopleural fistula with associated right-sided empyema, and cultures grew MDR P. aeruginosa. Initial susceptibility testing demonstrated resistance to amikacin, meropenem, ceftazidime-avibactam, and ceftolozane-tazobactam (C/T). The patient underwent treatment with chest tube placement and an 8-week course of cefiderocol therapy; however, there was minimal clinical improvement, and cultures from the sputum and pleural fluid remained positive. He was transferred for lung transplantation evaluation. Repeat sputum culture 2 days after transfer showed persistent MDR P. aeruginosa (isolate BSL5) and at this time a new course of therapy was initiated with cefiderocol (2 g intravenously every 6 hours for augmented renal clearance) plus inhaled colistin (75 mg q12h by nebulization). Cultures of the pleural fluid were obtained after 31 days (BSL9), and 48 days (BSL15) of cefiderocol therapy due to lack of clinical improvement on directed antibiotics. The patient was ultimately deemed ineligible for transplant and was transitioned to comfort care after 81 days of hospitalization.
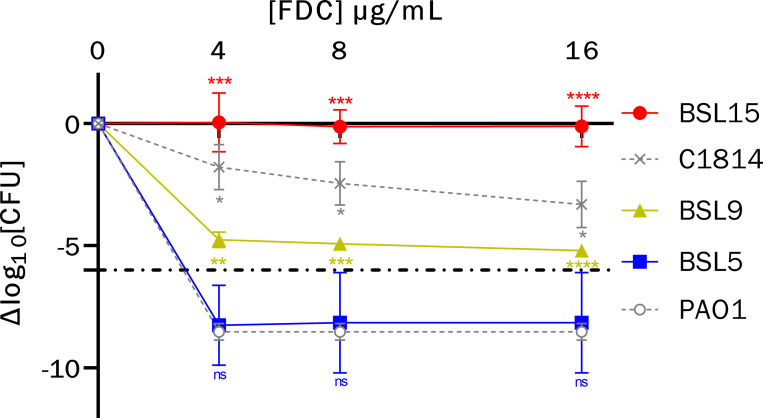
Cefiderocol susceptibilities of the isolates were initially screened by gradient strips, and subsequently confirmed by broth microdilution in iron-depleted Mueller-Hinton (ID-MH) media and Kirby-Bauer disk diffusion testing on standard Mueller-Hinton agar per Clinical Laboratory Standards Institute (CLSI) guidelines. In addition, population analysis profile (PAP) testing was performed via agar dilution using serial cefiderocol concentrations (0, 4, 8, and 16 ug/mL) on Mueller-Hinton agar. The number of surviving colonies at each cefiderocol concentration (read at 24 and 48 hours) was subtracted from the number of colonies on the growth control plate to calculate the change in CFU (ΔCFU). The ΔCFU was plotted across each cefiderocol concentration, and the area under the curve (AUC) was calculated using the trapezoidal method with a baseline of −10 in Prism (GraphPad, Boston, MA, USA). We defined heteroresistance as less than a 6 log10 decrease in bacterial growth at the broth microdilution cefiderocol breakpoint (4 ug/mL) or above, according to the criteria of Band and Weiss (7). The strains PAO1 (susceptible) and C1814 (heteroresistant) were used as controls (8). Genomic DNA was isolated from each strain and sequencing was performed on the MiSeq platform (Illumina, Inc.) with 2 × 300 bp paired-end reads. Variant calling was performed using snippy (https://github.com/tseemann/snippy) using PAO1 as a reference. Genomes were assembled using Spades v.3.15.5 and resistome was determined using AMRFinderPlus v.3.11.14 (9, 10). Sequence data for the isolates are available on the National Center for Biotechnology Information database under the Bioproject accession number PRJNA1034644.
Cefiderocol susceptibility testing by broth microdilution in ID-MH media showed categorical agreement with initial screening (Table 1). Population analysis profiles showed a progressive loss of cefiderocol susceptibility with BSL5 displaying a susceptible phenotype and BSL15 displaying a frankly resistant phenotype (Fig. 1). BSL9 met the criteria for heteroresistance, with a small subpopulation surviving at a cefiderocol concentration of 16 µg/mL. Statistical analyses revealed a significant difference between BSL9 survival versus both PAO1 and BSL5 (P-value = 0.005 and 0.02, respectively).
TABLE 1.
Susceptibilities of the isolates investigated g
| Strain | MEM MIC (µg/mL) a | C/T MIC (µg/mL) b | CZA MIC (µg/mL) a | CST MIC (µg/mL) c | FDC MIC (µg/mL) d | DD (mm) e | PAP-AUC |
|---|---|---|---|---|---|---|---|
| PAO1 | ND | ND | ND | ND | 0.25 | 31 | 40.63 |
| hR control | ND | ND | ND | ND | 1 | 21 | 124.9 |
| BSL5 | >8 | >256 | 48 | 4 | 2 | 26 | 45.35 |
| BSL9 | >8 | >256 | ND f | 4 | 4 | 24 | 90.58 |
| BSL15 | >8 | >256 | ND f | 4 | >32 | 13 | 158.9 |
Clinical microbiology laboratory.
Research laboratory, gradient diffusion strip.
Research laboratory, broth microdilution in cation adjusted Mueller-Hinton.
Research laboratory, broth microdilution in iron-depleted Mueller-Hinton media.
Kirby-Bauer disk diffusion zone diameter, in millimeters.
Presumed resistant.
C/T, ceftolozane-tazobactam; CZA, ceftazidime-avibactam; CST, colistin; FDC, cefiderocol; hR, heteroresistant; MEM, meropenem; PAP-AUC, population analysis profile-area under the curve; ND, not determined.
Fig 1.
Population analysis profiles of sequentially collected multi-drug-resistant P. aeruginosa. Isolate profiles were compared to representative susceptible control (PAO1, open circle) and heteroresistant control (C1814, X) phenotypes. Dash-dot line represents the cutoff for heteroresistance. Error bars represent 95% confidence intervals across triplicate runs with a controlled inoculum of approximately 107–108. P-values for comparison of individual isolate growth relative to PAO1: ns, not significant, * ≤0.05, ** ≤0.01, *** ≤0.001, **** ≤0.0001.
Whole-genome sequencing data revealed all three isolates were related, with 54, 49, and 65 variants present exclusively in BSL5, BSL9, and BSL15, respectively (all non-synonymous changes are listed in Table S1). Each isolate shared the same mutations in the oprD, ampC [encoding the Pseudomonas-derived cephalosporinase (PDC)], ampR, pirA, pirR, pirS, piuA, and piuC genes (Table S2). No mutations were found in ftsI encoding penicillin-binding protein 3. All three isolates carried the bla VIM-4 gene, encoding the Verona integron-encoded metallo-β-lactamase. A version of the modified carbapenem inactivation method using cefiderocol disks did not suggest the VIM-4 enzyme significantly contributed to the cefiderocol resistance phenotype (Table S3) (11). Compared to isolates BSL5 and BSL9, the resistant isolate BSL15 harbored 15 unique non-synonymous mutations in a coding region, three of which led to insertion, deletion, or frameshift mutations (Table 2). The first was in PA5279, predicted to encode a protein of the DUF484 family with a GAF domain, part of a six open reading frame operon and upstream of a site-specific recombinase, implicated in zinc-associated pyoverdine production and pyocin production (12, 13). However, a pyoverdine fluorescence assay showed a higher pyoverdine production in BSL5 as compared to BSL15, suggesting that an increase in pyoverdine synthesis was not related to the decreased cefiderocol susceptibility of FDC15. The second was in the alpE gene (PA0911), part of the AlpBCDE programmed cell death pathway implicated in P. aeruginosa virulence (14). The third gene encodes the CpxS sensor histidine kinase (PA3206), which regulates the cell envelope stress response in E. coli and has been shown to activate the MexAB-OprM efflux pump in P. aeruginosa (15). Interestingly, in silico analysis shows a consensus CpxR binding motif in the intergenic region between piuA and piuC, suggesting the potential for the CpxRS system to regulate piuA expression, although this has not been confirmed experimentally. There was also a reversion to wild type in BSL15 of a disruptive insertion mutation present in BSL5 and BSL9 that led to the insertion of asparagine at AA position 44 of the ClpS protein (PA2621).
TABLE 2.
Nucleotide changes in a coding region unique to BSL15
| POS a | Effect | Nucleotide change b | Amino acid change | Gene loci c |
|---|---|---|---|---|
| 473432 | Missense variant | c.242A > C | p.Glu81Ala | PA0426 |
| 750530 | Missense variant | c.574C > G | p.Leu192Val | PA0690 |
| 750652 | Missense variant | c.696_697delGGinsCA | p.Asp233Asn | PA0690 |
| 994768 | Inframe insertion | c.70_71insAGGCTGCCGGGAGTGTCGTCGAGACGGGGCAAG | p.Gln24_Ala25insAlaAlaGlySerValValGluThrGlyGlnGlu | PA0911 |
| 1566317 | Missense variant | c.2351_2352delCGinsTT | p.Ser784Phe | PA1436 |
| 2262929 | Missense variant | c.154G > A | p.Ala52Thr | PA2064 |
| 2263733 | Missense variant | c.1244_1245delGCinsAT | p.Ser415Asn | PA2065 |
| 2647470 | Missense variant | c.939_941delGTTinsCTG | p.Phe314Cys | PA2392 |
| 2685507 | Missense variant | c.1672G > A | p.Val558Ile | PA2402 |
| 3596196 | Inframe deletion | c.256_294delCGCCTGGTCGACGGCACCTACCTGCCGCGCCCCCACCAC | p.Arg86_His98del | PA3206 |
| 3686843 | Missense variant | c.1808C > A | p.Thr603Lys | PA3294 |
| 4476328 | Missense variant | c.374A > G | p.His125Arg | PA3995 |
| 4732282 | Missense variant | c.4517C > A | p.Pro1506Gln | PA4225 |
| 5726546 | Missense variant | c.690_693delGACTinsAGCC | p.Thr231Ala | PA5088 |
| 5944026 | Frameshift variant | c.422delA | p.His141fs | PA5279 |
Position in reference to P. aeruginosa PAO1 chromosome.
Numbering indicates nucleotide from start codon.
Gene loci references homologous gene in PAO1 chromosome.
The medical community has cited increasing concerns about rapidly emerging cefiderocol non-susceptibility and unanticipated treatment failure (3, 5, 16). Prior reports suggest that the emergence of cefiderocol non-susceptibility in P. aeruginosa occurs in the setting of mutations affecting PBP3, AmpC, or TonB-dependent receptors PirA and PiuA/D. In the present case, we describe the emergence of cefiderocol resistance via a heteroresistant intermediate after prolonged antibiotic exposure. This was associated with new potential pathways for the emergence of cefiderocol non-susceptibility. Further studies will be needed to characterize the specific contributions of these genes to the cefiderocol resistance phenotype.
ACKNOWLEDGMENTS
This research was funded in part by the National Institutes of Health, National Institute of Allergy and Infectious Diseases grant number R01AI140287 to V.H.T., R21AI175821 to W.R.M., and a training fellowship from the Gulf Coast Consortia, Texas Medical Center Training Program in Antimicrobial Resistance (TPAMR) (NIAID) T32 AI141349 to S.L.E.
N.T.: primary manuscript drafting and data acquisition. S.L.E.: manuscript editing, figures, data acquisition, and genomic analysis. K.P.: study conceptualization and design and data acquisition. R.P.B.: primary genomic analyses. V.H.T.: study conceptualization and design and manuscript editing. W.R.M.: study conceptualization and design and manuscript editing.
Contributor Information
William R. Miller, Email: wrmiller@houstonmethodist.org.
Jared A. Silverman, Bill & Melinda Gates Medical Research Institute, Cambridge, Massachusetts, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.01009-23.
Non-synonymous polymorphisms in each isolate.
Non-synonymous mutations in resistance associated genes.
Modified carbapenem inactivation method using cefiderocol disks.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Parsels KA, Mastro KA, Steele JM, Thomas SJ, Kufel WD. 2021. Cefiderocol: a novel siderophore cephalosporin for multidrug-resistant gram-negative bacterial infections. J Antimicrob Chemother 76:1379–1391. doi: 10.1093/jac/dkab015 [DOI] [PubMed] [Google Scholar]
- 2. Luscher A, Moynié L, Auguste PS, Bumann D, Mazza L, Pletzer D, Naismith JH, Köhler T. 2018. TonB-dependent receptor repertoire of Pseudomonas aeruginosa for uptake of siderophore-drug conjugates. Antimicrob Agents Chemother 62:e00097-18. doi: 10.1128/AAC.00097-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Streling AP, Al Obaidi MM, Lainhart WD, Zangeneh T, Khan A, Dinh AQ, Hanson B, Arias CA, Miller WR. 2021. Evolution of cefiderocol non-susceptibility in Pseudomonas aeruginosa in a patient without previous exposure to the antibiotic. Clin Infect Dis 73:e4472–e4474. doi: 10.1093/cid/ciaa1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ito A, Nishikawa T, Ota M, Ito-Horiyama T, Ishibashi N, Sato T, Tsuji M, Yamano Y. 2018. Stability and low induction propensity of cefiderocol against chromosomal AmpC beta-lactamases of Pseudomonas aeruginosa and Enterobacter cloacae. J Antimicrob Chemother 73:3049–3052. doi: 10.1093/jac/dky317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bassetti M, Echols R, Matsunaga Y, Ariyasu M, Doi Y, Ferrer R, Lodise TP, Naas T, Niki Y, Paterson DL, Portsmouth S, Torre-Cisneros J, Toyoizumi K, Wunderink RG, Nagata TD. 2021. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant gram-negative bacteria. Lancet Infect Dis 21:226–240. doi: 10.1016/S1473-3099(20)30796-9 [DOI] [PubMed] [Google Scholar]
- 6. Choby JE, Ozturk T, Satola SW, Jacob JT, Weiss DS. 2021. Widespread cefiderocol heteroresistance in carbapenem-resistant gram-negative pathogens. Lancet Infect Dis 21:597–598. doi: 10.1016/S1473-3099(21)00194-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Band VI, Crispell EK, Napier BA, Herrera CM, Tharp GK, Vavikolanu K, Pohl J, Read TD, Bosinger SE, Trent MS, Burd EM, Weiss DS. 2016. Antibiotic failure mediated by a resistant subpopulation in Enterobacter cloacae. Nat Microbiol 1:16053. doi: 10.1038/nmicrobiol.2016.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Egge SL, Rizvi SA, Simar SR, Dinh A, Hanson BM, Arias CA, Cecilia Tran T, Miller WR. 2022. 146. Heteroresistance to cefiderocol among Pseudomonas aeruginosa clinical isolates harboring changes in TonB-dependent receptor pathways and AmpC. Open Forum Infect Dis 9. doi: 10.1093/ofid/ofac492.224 [DOI] [Google Scholar]
- 9. Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feldgarden M, Brover V, Gonzalez-Escalona N, Frye JG, Haendiges J, Haft DH, Hoffmann M, Pettengill JB, Prasad AB, Tillman GE, Tyson GH, Klimke W. 2021. AMRFinderPlus and the reference gene catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci Rep 11:12728. doi: 10.1038/s41598-021-91456-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. CLSI . 2022. M-100: performance standards for antimicrobial susceptibility testing. 32nd ed. Clinical and Laboratory Standards Institute. [Google Scholar]
- 12. Höfte M, Dong Q, Kourambas S, Krishnapillai V, Sherratt D, Mergeay M. 1994. The sss gene product, which affects pyoverdin production in Pseudomonas aeruginosa 7NSK2, is a site-specific recombinase. Mol Microbiol 14:1011–1020. doi: 10.1111/j.1365-2958.1994.tb01335.x [DOI] [PubMed] [Google Scholar]
- 13. Baggett NS, Bronson AS, Cabeen MT. 2021. SOS-independent pyocin production in P.aeruginosa is induced by XerC recombinase deficiency. mBio 12:e0289321. doi: 10.1128/mBio.02893-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peña JM, Prezioso SM, McFarland KA, Kambara TK, Ramsey KM, Deighan P, Dove SL. 2021. Control of a programmed cell death pathway in Pseudomonas aeruginosa by an antiterminator. Nat Commun 12:1702. doi: 10.1038/s41467-021-21941-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tian ZX, Yi XX, Cho A, O’Gara F, Wang YP. 2016. CpxR activates MexAB-OprM efflux pump expression and enhances antibiotic resistance in both laboratory and clinical nalB-type isolates of Pseudomonas aeruginosa. PLoS Pathog 12:e1005932. doi: 10.1371/journal.ppat.1005932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smoke SM, Brophy A, Reveron S, Iovleva A, Kline EG, Marano M, Miller LP, Shields RK. 2023. Evolution and transmission of cefiderocol-resistant Acinetobacter baumannii during an outbreak in the burn intensive care unit. Clin Infect Dis 76:e1261–e1265. doi: 10.1093/cid/ciac647 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Non-synonymous polymorphisms in each isolate.
Non-synonymous mutations in resistance associated genes.
Modified carbapenem inactivation method using cefiderocol disks.