ABSTRACT
This study is aimed to evaluate the safety, tolerability, and pharmacokinetics (PK), as well as to select an appropriate dosing regimen for the pivotal clinical trial of GST-HG171, an orally bioavailable, potent, and selective 3CL protease inhibitor by a randomized, double-blind, and placebo-controlled phase I trial in healthy subjects. We conducted a Ph1 study involving 78 healthy subjects to assess the safety, tolerability, and PK of single ascending doses (150–900 mg) as well as multiple ascending doses (MADs) (150 and 300 mg) of GST-HG171. Additionally, we examined the food effect and drug–drug interaction of GST-HG171 in combination with ritonavir through a MAD regimen of GST-HG171/ritonavir (BID or TID) for 5 days. Throughout the course of these studies, no serious AEs or deaths occurred, and no AEs necessitated study discontinuation. We observed that food had no significant impact on the exposure of GST-HG171. However, the presence of ritonavir substantially increased the exposure of GST-HG171, which facilitated the selection of the GST-HG171/ritonavir dose and regimen (150/100 mg BID) for subsequent phase II/III trials. The selected dose regimen was achieved through concentrations continuously at 6.2–9.9-fold above the levels required for protein-binding adjusted 50% inhibition (IC50) of viral replication in vitro. The combination of 150 mg GST-HG171/100 mg ritonavir demonstrated favorable safety and tolerability profiles. The PK data obtained from GST-HG171/ritonavir administration guided the selection of appropriate dose for a pivotal phase II/III trial currently in progress. (This study has been registered at ClinicalTrials.gov under identifier NCT05668897).
KEYWORDS: clinical trial, population pharmacokinetics, SARS-CoV-2, GST-HG171, COVID-19
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus responsible for the global coronavirus disease 2019 (COVID-19) pandemic, which emerged in December 2019 and quickly spread worldwide (1 – 3). Various antiviral strategies have been explored, including direct inhibition of viral proteins such as RNA-dependent RNA polymerase (RdRp) and the main protease (Mpro or 3C-like protease, 3CLpro), as well as interference with host enzymes like angiotensin-converting enzyme two and immunoregulatory pathways, such as the Janus kinase/signal transducer and activator of transcription pathway (1).
The Mpro or 3CL protease of SARS-CoV-2 is an attractive target for viral inhibitors due to its crucial role in processing viral polyproteins, its high conservation in SARS-CoV-2, and its limited potential for off-target activity in humans (4 – 7). Nirmatrelvir, an oral antiviral treatment authorized for treating COVID-19 in 2021, is an inhibitor of the SARS-CoV-2 Mpro (4). However, given the large number of infections globally, many patients cannot afford antiviral drugs. In addition, SARS-CoV-2 has strong potential to escape human immune system via constant variations/mutations of its viral genome. Therefore, there is still an urgent need for developing more orally administered therapies that are safe, effective, and affordable, and can be self-administered at home, particularly for individuals at high risk of severe illness. In this context, GST-HG171, designed for oral administration, has shown potent and specific inhibition of Mpro enzymatic activity and antiviral activity across a diverse spectrum of coronaviruses in preclinical assays.
In preclinical cellular assays, we determined the in vitro 50% effective concentrations (EC50) of nirmatrelvir with protein-binding adjustment were 840.25, 190.07, and 560.17 ng/mL toward wild type, omicron BA.4, and omicron BA.5 variants of SARS-CoV-2, respectively, whereas the corresponding values for GST-HG171 were 149.68, 93.22, and 131.87 ng/mL, respectively (to be published elsewhere). Thus, the antiviral activity of GST-HG171 is 2.0–5.6 times greater than that of nirmatrelvir (8). Indeed, the difference in efficacy of GST-HG171 depends on the specific sub-variants of SARS-CoV-2. In a murine SARS-CoV-2 model, the oral administration of GST-HG171 demonstrated dose-dependent reduction of pulmonary viral titers and tissue pathology, with similar or better effects compared to nirmatrelvir at the same dose (to be published elsewhere). Since GST-HG171 is primarily metabolized by cytochrome P450 3A4 (CYP3A4), it was unclear if the concentrations of GST-HG171 could achieve sufficient higher coverage of the in vitro EC50 with protein-binding adjustment in humans. To assess this, the study included groups with or without a combination with ritonavir, a pharmacokinetic (PK) enhancing agent, which could lead to the attainment of efficacious concentrations (8 – 10). Additionally, population PK models were used in this study to monitor drug concentrations in real time and to predict dosing regimens to achieve ECs, ultimately informing the design of the phase II/III clinical studies.
This study aimed to examine the safety, tolerability, and PK of GST-HG171, with or without a combination with ritonavir (a PK enhancer) in healthy adults. The study facilitated the selection of an appropriate dose of GST-HG171 with its plasma concentrations sufficiently above the in vitro EC50 with protein-binding adjustment. The dose 150 mg BID of GST-HG171 in combination with 100 mg of ritonavir may achieve an optimal therapeutic effect for treating COVID-19 patients and has been recommended for the large pivotal phase II/III trial currently in progress in China.
RESULTS
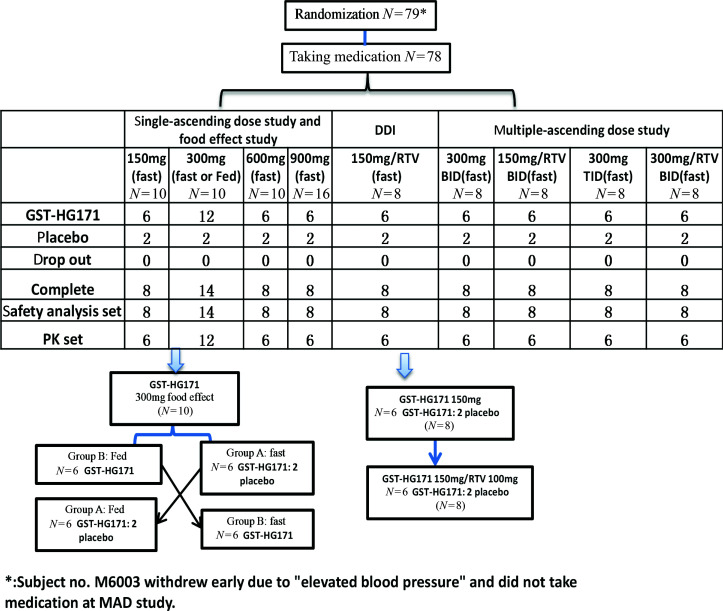
A total of 78 subjects were enrolled in this study, all of whom completed the drug administration and were included in the safety analysis set. Among them, 32 subjects were enrolled in the single ascending dose (SAD) study, 8 subjects were enrolled in the drug–drug interaction (DDI) study, 14 subjects were enrolled in the food effect study, and 32 subjects were enrolled in the multiple ascending dose (MAD) study (Fig. 1).
Fig 1.
Flow-chart depicting the parameters examined in this study.
Demographics
The mean ages of the participants were 34.4 ± 9.6, 37.8 ± 11.9, 32.3 ± 7.6, and 33.6 ± 7.3 years in the SAD, food effect, DDI, and MAD groups, respectively. The mean body mass index (BMI) values of the subjects in these groups were 23.48 ± 2.50, 23.84 ± 2.69, 24.19 ± 2.55, and 23.45 ± 2.12 kg/m2, respectively. In these groups, the sex ratios were similar, with a 1:1 male-to-female ratio. Almost all participants were Han Chinese. The demographic data were balanced among the different study groups. All participants successfully completed the study, and the demographic data of the MAD study are presented in Table 1.
TABLE 1.
Baseline demographics of the multiple ascending dose study a
| Baseline parameter | GST-HG171 (300 mg) BID (N = 6) | GST-HG171 (150 mg)/RTV (100 mg) BID (N = 6) | GST-HG171 (300 mg) TID (N = 6) | GST-HG171 (300 mg)/RTV (100 mg) (N = 6) | Placebo (N = 8) |
|---|---|---|---|---|---|
| Age, mean ± SD (years) | 36.2 ± 8.6 | 33.2 ± 8.2 | 7 ± 6.6 | 37.5 ± 8.0 | 37.0 ± 6.5 |
| Sex (N (%)) | 3 (50.0) | 3 (50.0) | 3 (50.0) | 3 (50.0) | 4 (50.0) |
| Ethnicity of Asians (N (%)) | 5 (83.3) | 6 (100) | 6 (100) | 5 (83.3) | 7 (87.5) |
| Weight, mean ± SD (kg) | 65.42 ± 11.98 | 59.03 ± 6.49 | 65.98 ± 7.48 | 63.72 ± 12.46 | 63.55 ± 7.37 |
| BMI, mean ± SD (kg/m2) | 23.58 ± 2.39 | 22.20 ± 1.72 | 24.53 ± 1.59 | 23.32 ± 2.53 | 23.59 ± 2.20 |
BID, drug administration every 12 h; BMI, body mass index; RTV, ritonavir; TID, drug administration every 8 h.
Safety and tolerability
The results showed that the drug was well tolerated after administration, with no reports of serious adverse events (AEs) or AEs that led to withdrawal after dosing. There were no severe AEs of grade III or above (CTC-AE version 5.0). Five drug-related AEs were categorized as grade II (hypertriglyceridemia at MAD study of GST-HG171 300 mg BID and DDI study of GST-HG171 150 mg, low white blood cell count and low neutrophil count at MAD study of GST-HG171 150 mg/RTV BID, and hypoglycemia at MAD study of GST-HG171 300 mg/RTV). The grade II occurred in four subjects, while the other drug-related AEs were classified as grade I. There was no clear correlation between the incidence of AEs and the dose of GST-HG171. The majority of AEs resulted in complete recovery without any lasting effects.
SAD study
In SAD study, 5 out of 8 subjects (62.5%) in the placebo group experienced AEs; while in the GST-HG171 group, 5 out of 24 subjects (20.8%) had six drug-related AEs. The incidence of AEs was slightly lower in the GST-HG171 group compared to the placebo group (Table S1).
DDI study
Five out of eight subjects (62.5%) experienced seven drug-related AEs, including hypertriglyceridemia (25%), abdominal pain (12.5%), diarrhea (12.5%), rash (12.5%), and hypoalbuminemia (12.5%) in the DDI study. In the placebo group, one subject (50.0%) experienced two AEs; while in the GST-HG171 group, four subjects (66.7%) experienced five AEs. The incidence of AEs was similar between the two groups. In the GST-HG171 group, four subjects either in the first period treated with GST-HG171 or in the second period treated with GST-HG171/ritonavir had drug-related AEs, and the incidence was similar in both treatment groups.
Food effect study
Five subjects (35.7%) experienced seven drug-related AEs in food effect study, including hypertriglyceridemia (21.4%), increased alanine aminotransferase (7.1%), increased aspartate aminotransferase (7.1%), albuminuria (7.1%), and increased creatinine (7.1%). In the GST-HG171 group, two subjects (16.7%) experienced two AEs under the fasting conditions, and two subjects had three AEs under the fed conditions. The incidences of AEs under the fasting and fed conditions were comparable.
MAD study results
Within the placebo group of the MAD study, seven drug-related AEs were observed in four subjects (4/8, 50%). In the GST-HG171 group, 25 drug-related AEs occurred in 13 subjects (13/24, 54.2%). The incidence of drug-related AEs in the GST-HG171 group was similar to that in the placebo group. Notably, the incidence of increased alanine aminotransferase was the highest (50%, 3/6) in the GST-HG171 (300 mg)/ritonavir (100 mg) BID group, surpassing the rates observed in the placebo and other dose groups. Furthermore, oral bitterness occurred in 33.3% (2/6) of the subjects in the GST-HG171 (300 mg)/ritonavir (100 mg) BID group, while no occurrences were reported in the other dose groups. This finding warrants further investigation in phase II/III clinical trials. Among the drug-related AEs, hypertriglyceridemia had an incidence of 66.7% (4/6) in the GST-HG171 (300 mg) BID group but its occurrence was reduced in the GST-HG171 (150 mg)/ritonavir (100 mg) BID and GST-HG171 (300 mg)/ritonavir (100 mg) BID groups. Therefore, hypertriglyceridemia may not be a true drug-related AE. The remaining drug-related AEs were sporadic (Table 2).
TABLE 2.
| GST-HG171 (300 mg) BID (N = 6) | GST-HG171 (150 mg)/RTV (100 mg) BID (N = 6) | GST-HG171 (300 mg) TID (N = 6) | GST-HG171 (300 mg)/RTV (100 mg) (N = 6) | Placebo (N = 8) | |
|---|---|---|---|---|---|
| Hypertriglyceridemia | 66.7% (4/6) | 0 | 33.3% (2/6) | 33.3% (2/6) | 50% (4/8) |
| Increased alanine aminotransferase | 16.7% (1/6) | 16.7% (1/6) | 16.7% (1/6) | 50% (3/6) | 12.5% (1/8) |
| Increased aspartate aminotransferase | 16.7% (1/6) | 0 | 0 | 33.3% (2/6) | 0 |
| Low white blood cell count | 0 | 16.7% (1/6) | 0 | 0 | 0 |
| Decreased neutrophil count | 0 | 16.7% (1/6) | 0 | 0 | 0 |
| Hypoglycemia | 0 | 0 | 0 | 16.7% (1/6) | 0 |
| Hypoalbuminemia | 0 | 0 | 16.7% (1/6) | 0 | 0 |
| Bitter mouth | 0 | 0 | 0 | 33.3% (2/6) | 0 |
| Dizziness | 0 | 0 | 0 | 0 | 12.5% (1/8) |
| Fatigue | 0 | 0 | 0 | 0 | 12.5% (1/8) |
| Diarrhea | 16.7% (1/6) | 0 | 0 | 16.7% (1/6) | 0 |
The data are presented as n (%).
RTV, ritonavir.
PK profiles of GST-HG171
SAD study
Following a single oral administration of 150–900 mg of GST-HG171, a rapid and steep absorption and elimination phase was observed. A power model was employed to analyze the linear relationship between the drug doses and the PK parameters (C max, AUC0–t , and AUC0–∞). The slopes (β) of the linear equation were 0.75 (0.51–0.99), 0.89 (0.72–1.05), and 0.89 (0.72–1.05) for the dose range of 150–900 mg. Higher doses exhibited a saturation trend.
DDI study
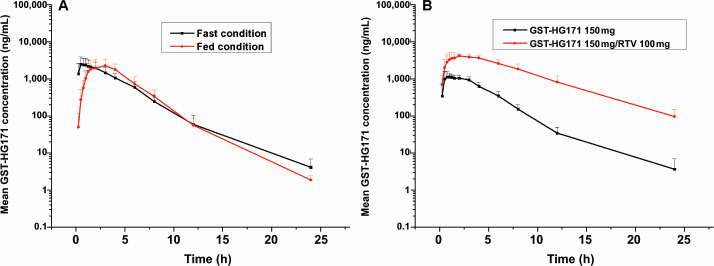
The combined treatment of GST-HG171 with ritonavir slightly extended the absorption phase, resulting in an extended T max of 1.75 h, compared to GST-HG171 treatment alone (1 h). The V and CL of GST-HG171 in the plasma decreased, while the exposure (C max, AUC) of GST-HG171 increased by ~3–6 times with the addition of ritonavir. The concentration at 12-h post-administration was 34.5 ± 14.6 ng/mL in the mono-treatment group and 816.1 ± 385.4 ng/mL in the combined treatment group. The geometric mean ratios and 90% CIs for C max, AUC0–t , and AUC0–∞ between the combined and mono-treatment groups were 330.16% (283.64%–384.32%), 619.81% (512.84%–749.10%), and 619.30% (512.77%–747%), respectively. These findings indicate significantly higher exposure in the combination group, compared to GST-HG171 mono-treatment group (Fig. 2; Table S2).
Fig 2.
Mean values of plasma GST-HG171 concentration–time profiles in different treatment groups, including a food effect study of GST-HG171 300 mg (A), and a drug–drug interaction study (B). Data are presented as the mean (±SD).
Food effect study
The absorption phase of GST-HG171 was slightly extended, resulting in an extended T max of 2 h when administered with food. The t 1/2 values in the fasting and fed conditions were 3.55 ± 3.64 h and 1.90 ± 0.31 h, respectively. The in vivo exposure (C max and AUC) of GST-HG171 was similar between the fasting and fed conditions, with geometric mean ratios and 90% CIs for AUC0–t and AUC0–∞ falling within the range of 80%–125%. Although C max was slightly outside this range, the deviation was small. Therefore, it was determined that both fasting and fed conditions are suitable for subsequent clinical trials (Fig. 2; Table S3).
In the fasted state, following a single oral administration of GST-HG171 (300 mg) in six healthy subjects, the mean cumulative excretions of GST-HG171 in the urine and feces within 96 h were ~19 and 0.7 mg, respectively. The mean cumulative excretion rates of GST-HG171 were 6.48% in urine and 0.25% in feces.
MAD study
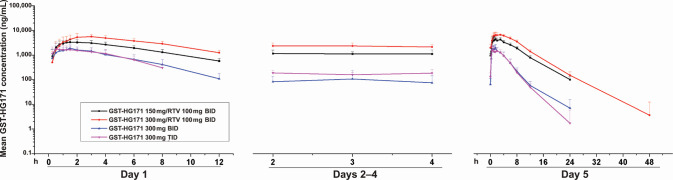
In the MAD study, the plasma T max values of GST-HG171 were 1.50 and 2.25 h in healthy subjects on day 5 after the oral administration of GST-HG171 (150 mg)/ritonavir (100 mg) and GST-HG171 (300 mg)/ritonavir (100 mg), respectively. GST-HG171 reached steady state at day 2, and there was no obvious accumulation after continuous administration. The average accumulation index (C max and AUC) ranged from 123% to 141%. After administration on day 5, the mean ratios of C max and AUC0–t for GST-HG171 at doses of 300 and 150 mg were ~1.0:1.45 and 1.0:1.72, respectively, slightly lower than the dosage increase ratio.
Compared to GST-HG171 mono-treatment, the combined treatment of GST-HG171 and ritonavir showed a slightly extended absorption phase and increased T max. In the combined treatment, the CL of GST-HG171 in the plasma decreased, while the exposure (C max and AUC) of GST-HG171 increased by ~3–6 fold. The fluctuation coefficient decreased, and the steady-state trough concentration also increased in the combined treatment group. The mean steady-state t 1/2 values were similar across each group, at around 4 h after administration (Table 1; Fig. 3).
Fig 3.
Mean values of plasma GST-HG171 concentration–time profiles of different treatment groups in the multiple ascending dose study. Data are presented as the mean (±SD).
The exposure (C max, AUC), T max, and t 1/2 values were consistent between male and female subjects in the SAD, DDI, food effect, and MAD studies, with the ratio being ~1. No sex effect was observed (data not shown).
Dose selection for phase II/III trials
For dose selection in phase II/III trials, the PK values of GST-HG171 with ritonavir (100 mg) following oral administration were extensively characterized using a two-compartment disposition model with parallel zero-order and first-order absorption as well as first-order elimination and the mixed error model. The covariance of the time for the zero-order absorption (T1) and V was included in the model but no covariates such as demographic or biochemical data were successfully selected and included in the final population PK model. The model parameters are listed in Table S4. The goodness-of-fit is shown in Fig. S1. The GST-HG171 concentrations were simulated for 1,000 subjects, and the simulated steady-state trough concentration closely matched the measured trough concentration in this study (921 ng/mL vs 854.79 ± 245.95 ng/mL, Tables 3 and 4).
TABLE 3.
Pharmacokinetic parameters of GST-HG171 of the multiple ascending dose study in each treatment group in the fasted state at day 5 (mean ± SD)
| PK parameter | GST-HG171 (300 mg) BID (N = 6) | GST-HG171 (150 mg)/RTV (100 mg) BID (N = 6) | GST-HG171 (300 mg) TID (N = 6) | GST-HG171 (300 mg)/RTV (100 mg) (N = 6) |
|---|---|---|---|---|
| C max,ss (ng/mL) | 2272.81 ± 519.89 | 5019.08 ± 1106.20 | 2037.05 ± 696.16 | 7276.06 ± 1311.29 |
| Trough concentration (ng/mL) | 65.02 ± 45.02 | 854.79 ± 245.95 | 129.15 ± 53.44 | 1892.21 ± 656.96 |
| AUC0–t,ss (h × ng/mL) | 8561.44 ± 2886.72 | 35992.89 ± 5834.64 | 8874.28 ± 3607.54 | 61752.17 ± 7744.63 |
| AUC0–∞,ss (h × ng/mL) | 8733.43 ± 2986.66 | 36564.86 ± 5979.17 | 9521.77 ± 4901.67 | 62441.62 ± 8046.32 |
| t 1/2,ss (h) | 4.13 ± 3.75 | 3.84 ± 0.29 | 6.56 ± 10.77 | 3.85 ± 0.87 |
| T max,ss (h) | 1.13 (0.500, 1.500) | 1.50 (1.000, 3.001) | 0.63 (0.250, 3.000) | 2.25 (1.500, 3.000) |
| DF (%) | 336.78 ± 68.58 | 162.69 ± 23.66 | 210.94 ± 69.24 | 125.53 ± 28.96 |
| CL,ss
(L/h) |
39642.31 ± 11059.76 | 4998.38 ± 781.51 | 42443.87 ± 6223.18 | 5863.32 ± 868.12 |
| R cmax (%) | 99.72 ± 34.45 | 140.54 ± 33.27 | 89.93 ± 35.30 | 123.06 ± 21.18 |
| R auc(%) | 85.71 ± 8.24 | 139.02 ± 23.03 | 87.55 ± 16.73 | 133.71 ± 20.79 |
Median (min–max); R cmax, accumulation index for C max; R auc, accumulation index for AUC; DF, degree of fluctuation.
TABLE 4.
Predicted C min in ng/mL and percentage of simulated subjects achieving C min ≥ EC50 of 149.68 ng/mL a
| Dose (mg) plus ritonavir (100 mg) | Time point | Median | Fifth percentile | 95th percentile | Min | Participants achieving C min ≥ EC50 (%) |
|---|---|---|---|---|---|---|
| 75 | First dose | 406 | 195 | 689 | 92 | 99 |
| Steady state | 465 | 212 | 881 | 100 | 99.5 | |
| 100 | First dose | 544 | 271 | 880 | 122 | 99.8 |
| Steady state | 622 | 293 | 1131 | 131 | 99.9 | |
| 150 | First dose | 807 | 395 | 1345 | 166 | 100 |
| Steady state | 921 | 429 | 1711 | 179 | 100 | |
| 300 | First dose | 1632 | 836 | 2649 | 415 | 100 |
| Steady state | 1860 | 905 | 3361 | 445 | 100 |
C min, minimum concentration during the dosing interval (12 h); EC50, concentration at which 50% inhibition of viral replication is observed; that is, concentration required for 50% effect.
Based on the above population PK model using preliminary data collected from this clinical study, simulations of GST-HG171 (150 mg)/ritonavir (100 mg) BID showed that 100% of future trial participants would achieve the target trough concentration above the in vitro EC50 with protein-binding adjustment after the first and subsequent dosing. The projected median trough concentrations on day 1 and at steady-state were 807 and 921 ng/mL, respectively. These concentrations were 5.39–8.66 and 6.15–9.88 times higher than the in vitro EC50 with protein-binding adjustment, indicating a high potential for robust antiviral effects. The simulated steady-state (day 5) trough concentrations for different dosing regimens are shown in Table 4.
DISCUSSION
To combat the progression of COVID-19, more effective and safer therapeutic treatments by means of reducing the viral load and transmission, shortening the clinical recovery time, and preventing the development of adverse outcomes are urgently needed (11 – 15). To this end, the US Food and Drug Administration authorized Nirmatrelvir/ritonavir, a 3CL protease inhibitor, for the treatment of patients with mild-to-moderate COVID-19 in 2021 (16, 17). The clinical validation of 3CL as a therapeutic target in treating COVID-19 has led to the discovery of more potent and selective 3CL inhibitors such as GST-HG171, currently at various stages of clinical development. In preclinical studies, GST-HG171 demonstrated potent antiviral activity, good oral bioavailability, and a favorable toxicity profile, and its efficacy is similar or better than that of nirmatrelvir, making it as a compelling drug candidate for clinical development. In addition, GST-HG171 has shown promising results against COVID-19 variants, including new emerging Omicron variants (18, 19) XBB.1.15, XBB.1, and BQ.1.1 (data not shown).
To expedite the clinical development of GST-HG171, a flexible and efficient Ph1 experimental approach was adopted (20). A coherent, seamless study plan was designed, comprising SAD, food effect, DDI, and MAD studies. This interleaving design allowed for rapid dose escalation and efficient data generation. This study was able to be completed within 60 days, which is significantly shorter than the typical timeline of about 6 months for a first-in-human study (20). The implementation of this accelerated Ph1 study design enabled the timely acquisition and analysis of data, facilitating the rapid progress of GST-HG171 into advanced clinical development.
The PK profile of GST-HG171 demonstrated a resemblance to a two-compartment model with first-order (two-stage) absorption (21). Our findings indicate the rapid oral absorption and metabolism of GST-HG171. The C max and area under the concentration–time curve (AUC) values of GST-HG171 increased proportionally, albeit slightly lower than the dose in the 900 mg group. Since the metabolism of GST-HG171 is mediated by the CYP3A4 enzyme, its concentration decreases rapidly as expected, posing challenges in maintaining an effective trough concentration to inhibit the SARS-CoV-2 virus. To address this issue, we explored the potential of combining it with ritonavir, a known CYP3A4 inhibitor, and achieved an increase of the exposure (C max and AUC) of GST-HG171 in vivo by up to 3–6 times. This enables GST-HG171 to maintain multiple-fold exposures above the in vitro EC50, adjusted for protein binding. Furthermore, the enhanced drug exposure may offer a potential barrier against the development of resistance. Co-administration of ritonavir resulted in a significant increase in GST-HG171 trough concentrations at 12 h after dosing, ~24-fold higher (816.11 vs 34.46 ng/mL). Similarly, co-administration also led to an ~8-fold increase in nirmatrelvir concentrations when ritonavir was co-administered (20). These effects can be attributed to the inhibitory impact of ritonavir on CYP3A4 (22).
In the nirmatrelvir treatment group, treatment-related AEs such as increased blood thyroid-stimulating hormone, dysgeusia, and nausea were reported although these events were mild in severity (20). However, these AEs were not found after GST-HG171 treatment. Furthermore, there were no clinically significant changes (grade II or higher) in laboratory values, vital sign measurements, or electrocardiography measurements in GST-HG171 treatment group, which align closely with the safety profile of nirmatrelvir (4, 20, 23).
This study, conducted in healthy Chinese subjects, demonstrated that GST-HG171 is safe and well tolerated. Most of the drug-related AEs were sporadic and not dose-dependent. Nevertheless, there was a dose-dependent increase in alanine aminotransferase levels in one group of the MAD study. Multiple incidences of increased alanine aminotransferase were observed in GST-HG171 (300 mg)/ritonavir (100 mg) BID group, while only isolated cases occurred in the other dose groups and the placebo group. These increases were all classified as grade I and considered mild, and are likely representing fluctuations in transaminase levels. It is important to note that this study had a small sample size and included healthy subjects only, and further research is needed to evaluate the safety of GST-HG171 tablets in COVID-19 patients.
The AUC on the fifth day of multiple administration of GST-HG71 at 300 mg in combination with ritonavir in the MAD study was 61.75 h × μg/mL (Table 3), which was lower than the no-observed-adverse-effect levels (NOAELs) observed in animal tox studies. In pre-clinical studies, the NOAELs of GST-HG171 in Sprague Dawley rats and beagle dogs were determined to be 600 and 300 mg/kg, respectively. The average AUC0–t of GST-HG171 at NOAEL ranged from 126.05 to 539.88 h × μg/mL. The data provides assurance for the safety of GST-HG171 at 150 mg in combination with ritonavir as the recommended dose for phase II/III clinical studies (24).
The effectiveness of oral anti-viral agents such as non-nucleoside polymerase inhibitors and protease inhibitors is often assessed with the multiples of the trough concentrations in human PK studies over their corresponding protein-binding adjusted EC50s determined in cellular assays in vitro (25 – 28). The EC50 values of nirmatrelvir, the first approved 3CL protease inhibitor for treating COVID-19, with plasma protein correction, for wild type, Omicron BA.4, and Omicron BA.5 were 840.25, 190.07, and 560.17 ng/mL, respectively. Therefore, an steady-state trough concentration of nirmatrelvir (300 mg)/ritonavir (100 mg) (1800 ng/mL) could provide a coverage of ~2.1, 9.5, and 3.2 times above the EC50 with plasma protein correction (20, 29). For GST-HG171, the EC50 value, with plasma protein correction, for wild type, Omicron BA.4, and Omicron BA.5 were 149.68, 93.22, and 131.87 ng/mL, respectively. The ss trough concentration (921 ng/mL) achieved with GST-HG171 (150 mg)/ritonavir (100 mg) treatment could provide a coverage of ~6.2, 9.9, and 7.0 times above the EC50 with plasma protein correction. The predictions, derived from the population PK model, suggest that the clinical efficacy with this regimen (150 mg GST-HG171/100 mg ritonavir) could be close to or even better than that of nirmatrelvir (300 mg)/ritonavir (100 mg). Based on the data, GST-HG171 (150 mg)/ritonavir (100 mg) administered BID was proposed for phase II/III trials and is predicted to continuously achieve efficacious concentrations in 100% of the participants in clinic (15, 25, 30, 31).
Conclusion
The combination of GST-HG171 (150 or 300 mg) and ritonavir (100 mg) has demonstrated good safety and tolerability in healthy subjects in the Ph1 study. Ritonavir played a crucial role in enhancing the PK of GST-HG171, allowing for the attainment of plasma concentrations and maintenance of trough concentration values that cover with multiple folds above the EC50 with plasma protein correction. Based on these findings, we have a high level of confidence in selecting the regimen of GST-HG171 (150 mg)/ritonavir (100 mg) administered BID for phase II/III clinical trials in patients with COVID-19.
MATERIALS AND METHODS
Our study was conducted in compliance of the standards of the Good Clinical Practice guidelines, and its protocol was approved by the independent Ethics Committee of The First Hospital of Jilin University (Changchun, Jilin, China). Written informed consent was obtained from all subjects before their inclusion in the study.
Subjects
A total of 78 healthy Chinese subjects were enrolled in this study, and 78 subjects completed the safety assessment. This study aimed to evaluate the PK, safety, and tolerability of single and multiple escalating doses of GST-HG171 in healthy subjects; this is the first-in-human study on GST-HG171 to assess these parameters. The evaluation was conducted in the presence or absence of food and ritonavir, a PK enhancer. The main inclusion criteria included an age range of 18–50 years old, a BMI of 18–28 kg/m2, no clinically relevant conditions based on physical examination results as well as eligible laboratory test results, electrocardiography, and medical history. The main exclusion criteria included the use of alcohol or drugs known to influence drug-metabolizing enzymes within 4 weeks before dosing and during the study, except for ritonavir in the study, as well as pregnancy or breastfeeding.
Study design
This clinical trial was a randomized, double-blind, placebo-controlled, and ascending-dose study (Clinical Trial Registration Number: NCT05668897) conducted at the Phase I Clinical Research Center, The First Hospital of Jilin University (Jilin, China), during the period from 1 October 2022 to 30 November 2022. A flow chart illustrating the study design is presented in Fig. 1.
Subject compliance and drug administration
The subjects were asked to keep fasting for at least 10 h before blood sample collection for clinical laboratory tests. They were also instructed to comply with the guidelines of the Phase I Clinical Research Center at the First Hospital of Jilin University regarding diet and activity regulations. The investigational drug was administered orally while subjects drank 240 mL of water. GST-HG171 and placebo were synthesized and provided by Fujian Guangsheng Zhonglin Biotechnology Co., Ltd. and Fujian Guangsheng Tang Pharmaceutical Co., Ltd. Ritonavir and placebo were synthesized and provided by Abbott GmbH & Co., Ltd. and Fujian Guangsheng Tang Pharmaceutical Co., Ltd. (Lot # 1150459 and # 422001Y, respectively).
SAD study
The SAD and food effect study involved 40 healthy Chinese subjects who were randomly assigned to four groups receiving a single dose of GST-HG171 (150, 300, 600, or 900 mg, respectively). Each group contained eight subjects (GST-HG171 : placebo = 6:2). All of them received the drug administration under fasted conditions. Tolerance assessments were conducted on days 2 and 5. The lowest dose group was initiated first, and the next highest dose level was administered only after the safety data were reviewed by the principal investigator and the sponsor and the drug was deemed safe and tolerable.
Food effect study
The food effect study included the 300 mg group, comprising 14 subjects, of which 12 subjects received GST-HG171 and two subjects received placebo. The study was designed as a randomized, single-dose, two-sequence, and two-period crossover study to evaluate the effect of food on the PK of GST-HG171. The first period, with a dosage of 300 mg, under the fasting condition was the same as those in the SAD study. Urine and fecal samples were collected during the second period under the fasting conditions for investigation of drug metabolism. During the fed state portion of the study, the subjects consumed a high-fat meal ~30 min prior to dosing. The washout period was 14 days, and tolerance assessments were conducted on days 7 and 21. Moreover, the calorie content of the high-fat meal contains carbohydrate 256.76 kcal (28%), fat 532.44 kcal (58%), and protein 125.88 kcal (14%).
DDI study
The DDI study involved the 600 mg dose of GST-HG171 treatment for tolerance evaluation, followed by the DDI study. Eight subjects were enrolled including two receiving a placebo. GST-HG171 was administered in the first period, and in the second period, GST-HG171 (150 mg) and ritonavir (100 mg) were co-administered under fasting conditions. The subjects in the placebo group received placebo in both periods, and there was a washout period of 5 days.
MAD study
Based on the data of safety, tolerability, and PK, four dose groups were established for the MAD study: (i) 300 mg of GST-HG171 twice daily (BID), (ii) 150 mg of GST-HG171 and 100 mg of ritonavir BID, (iii) 300 mg of GST-HG171 three times daily (TID), and (iv) 300 mg of GST-HG171 and 100 mg of ritonavir BID. The drug was administered continuously for 5 days under fasting conditions. On day 5, the drug was administered only once in the morning. Each group consisted of eight subjects receiving multiple doses (two received a placebo). Tolerance assessments were conducted on days 3, 6, and 9, with dose escalation occurring if the drug was tolerated according to the evaluation.
PK analysis
Venous blood samples (4 mL) were collected and added to tubes containing K2EDTA as an anticoagulant. After centrifugation (1,300 × g at 4°C for 10 min), plasma was collected and stored at −80°C until use for PK analysis. The detailed information on time points of blood collection is provided in the supplementary material.
Various PK parameters were analyzed, including the maximum observed plasma concentration (C max), terminal elimination half-life of the drug in plasma (t ½), time to maximum observed plasma concentration (T max), AUC from time of dosing (0 h) to t h (AUC0–t ), and AUC from time of dosing extrapolated to infinity (AUC0–∞), which were assessed at the first dose. Plasma samples were also collected to measure the following PK parameters at steady state (ss), such as C ss max, t ss ½, T ss max, AUCss 0–t , accumulation index, and degree of fluctuation at last dose. Plasma samples were also collected to measure the PK parameters at steady state. The PK parameters of GST-HG171 were calculated by the non-compartmental methods which are provided by professional software WinNonlin Version 8.3 (Certara).
Bioanalysis
The concentrations of GST-HG171 in plasma, urine, and fecal samples were measured by Shanghai Guanhe Pharmaceutical Technology Co., Ltd. (Shanghai, China) using high-pressure liquid chromatography and tandem mass spectrometry. The accuracy and precision of the measurements were within acceptable ranges. The standard curve ranges of GST-HG171 were 1–1,000 or 10–20,000 ng/mL in plasma; 2–4,000 ng/mL in urine; and 2–4,000 ng/g in the fecal sample. GST-HG171-D3 was employed as an internal standard for quantification. GST-HG171 and internal standard GST-HG171-D3 were extracted from human plasma by protein precipitation method, and then separated by reversed-phase high-performance liquid chromatography on a Thermo Hypersil Gold aQ column (100 mm × 3.0 mm, 3 µm). Mass spectrometry was carried out in the electrospray ionization positive ion mode. The ion mass charge ratio for GST-HG171 was monitored at m/z 512.100 → 122.100, and for GST-HG171-D3, it was m/z 516.100 → 122.100.
Statistical analysis
Descriptive statistical methods were used in this study. Analysis of variance was performed to compare C max and AUC in the food effect study. The results were presented as geometric least-square means with a 90% CI. Dose proportionality of GST-HG171 was assessed using a power model. The statistical analysis results were reported as the mean (SD). Statistical analysis was completed using SAS version 9.4 (SAS Institute Inc.) software.
ACKNOWLEDGMENTS
This work was financially supported by the Capital Construction Funds within the provincial budget for 2020 (in the category of Innovation Capacity Construction, project number: 2020C038-1) and was also sponsored by Fujian Akeylink Biotechnology Co., Ltd. and Fujian Cosunter Pharmaceutical Co., Ltd.
H.Z., J.M., G.Z., and Y.D. designed the experiment. J.Z., H.C., Y.T., W.Y., T.Z., C.L., S.C., and G.L. performed the clinic trials. H.Z. analyzed the data. H.Z., J.M., G.Z., and Y.D. wrote and edited the paper, and drew the figures. A statement indicating that all authors approved the final version of the manuscript.
John Mao, Yanan Tang, Wenhao Yan, Tianxiang Zhang, Chuanjing Li, Shikui Chen, Guoping Li, and George Zhang are employees of Fujian Akeylink Biotechnology Co., Ltd. and Fujian Cosunter Pharmaceutical Co., Ltd., which sponsored this study.
Contributor Information
Li Liu, Email: lli01@jlu.edu.cn.
Miguel Angel Martinez, IrsiCaixa Institut de Recerca de la Sida, Badalona, Barcelona, Spain .
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.01115-23.
Supplemental methods and results.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Arabi M, Al-Najjar Y, Mhaimeed N, Salameh MA, Paul P, AlAnni J, Abdelati AA, Laswi I, Khanjar B, Al-Ali D, Elshafeey A, Mhaimeed O, Burney Z, D’Souza A, Sinha P, Bhatti M, Pillai KV, Homssi M, Bshesh K, Yagan L, Zakaria D. 2023. Severity of the omicron SARS-CoV-2 variant compared with the previous lineages: a systematic review. J Cell Mol Med 27:1443–1464. doi: 10.1111/jcmm.17747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ledford H. 2021. How severe are omicron infections. Nature 600:577–578. doi: 10.1038/d41586-021-03794-8 [DOI] [PubMed] [Google Scholar]
- 3. Micheli V, Bracchitta F, Rizzo A, Mancon A, Mileto D, Lombardi A, Stefanelli P, Gismondo MR. 2022. First identification of the new severe acute respiratory syndrome coronavirus 2 omicron variant (B.1.1.529) in Italy. Clin Infect Dis 75:522–524. doi: 10.1093/cid/ciab1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hammond J, Bao W, Simón-Campos A. 2022. Nirmatrelvir for nonhospitalized adults with COVID-19. N Engl J Med 387:475–476. doi: 10.1056/NEJMc2206277 [DOI] [PubMed] [Google Scholar]
- 5. Hilgenfeld R. 2014. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J 281:4085–4096. doi: 10.1111/febs.12936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang L, Lin D, Sun X, Curth U, Drosten C, Sauerhering L, Becker S, Rox K, Hilgenfeld R. 2020. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 368:409–412. doi: 10.1126/science.abb3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. 2003. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science 300:1763–1767. doi: 10.1126/science.1085658 [DOI] [PubMed] [Google Scholar]
- 8. Huang C, Shuai H, Qiao J, Hou Y, Zeng R, Xia A, Xie L, Fang Z, Li Y, Yoon C, et al. 2023. A new generation Mpro inhibitor with potent activity against SARS-CoV-2 omicron variants. Sig Transduct Target Ther 8:128. doi: 10.1038/s41392-023-01392-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ibrahim MAA, Mohamed EAR, Abdelrahman AHM, Allemailem KS, Moustafa MF, Shawky AM, Mahzari A, Hakami AR, Abdeljawaad KAA, Atia MAM. 2021. Rutin and flavone analogs as prospective SARS-CoV-2 main protease inhibitors: in silico drug discovery study. J Mol Graph Model 105:107904. doi: 10.1016/j.jmgm.2021.107904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Owen DR, Allerton CMN, Anderson AS, Aschenbrenner L, Avery M, Berritt S, Boras B, Cardin RD, Carlo A, Coffman KJ, et al. 2021. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 374:1586–1593. doi: 10.1126/science.abl4784 [DOI] [PubMed] [Google Scholar]
- 11. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, Martín-Quirós A, Caraco Y, Williams-Diaz A, Brown ML, Du J, Pedley A, Assaid C, Strizki J, Grobler JA, Shamsuddin HH, Tipping R, Wan H, Paschke A, Butterton JR, Johnson MG, De Anda C, MOVe-OUT Study Group . 2022. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med 386:509–520. doi: 10.1056/NEJMoa2116044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jung J, Sung H, Kim SH. 2021. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med 385:1629–1630. doi: 10.1056/NEJMc2113497 [DOI] [PubMed] [Google Scholar]
- 13. Herman GA, O’Brien MP, Forleo-Neto E, Sarkar N, Isa F, Hou P, Chan K-C, Bar KJ, Barnabas RV, Barouch DH, et al. 2022. Efficacy and safety of a single dose of casirivimab and imdevimab for the prevention of COVID-19 over an 8-month period: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 22:1444–1454. doi: 10.1016/S1473-3099(22)00416-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kwon S, Joshi AD, Lo C-H, Drew DA, Nguyen LH, Guo C-G, Ma W, Mehta RS, Shebl FM, Warner ET, Astley CM, Merino J, Murray B, Wolf J, Ourselin S, Steves CJ, Spector TD, Hart JE, Song M, VoPham T, Chan AT. 2021. Association of social distancing and face mask use with risk of COVID-19. Nat Commun 12:3737. doi: 10.1038/s41467-021-24115-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diseases TLI. 2021. Unmet need for COVID-19 therapies in community settings. Lancet Infect Dis 21:1471. doi: 10.1016/S1473-3099(21)00633-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sendi P, Razonable RR, Nelson SB, Soriano A, Gandhi RT. 2022. First-generation oral antivirals against SARS-CoV-2. Clin Microbiol Infect 28:1230–1235. doi: 10.1016/j.cmi.2022.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lai C-C, Wang Y-H, Chen K-H, Chen C-H, Wang C-Y. 2022. The clinical efficacy and safety of anti-viral agents for non-hospitalized patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials. Viruses 14:1706. doi: 10.3390/v14081706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, Zhang B, Li X, Zhang L, Peng C, et al. 2020. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 582:289–293. doi: 10.1038/s41586-020-2223-y [DOI] [PubMed] [Google Scholar]
- 19. Pillaiyar T, Manickam M, Namasivayam V, Hayashi Y, Jung SH. 2016. An overview of severe acute respiratory syndrome-coronavirus (SARS-CoV) 3Cl protease inhibitors: peptidomimetics and small molecule chemotherapy. J Med Chem 59:6595–6628. doi: 10.1021/acs.jmedchem.5b01461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh RSP, Toussi SS, Hackman F, Chan PL, Rao R, Allen R, Van Eyck L, Pawlak S, Kadar EP, Clark F, Shi H, Anderson AS, Binks M, Menon S, Nucci G, Bergman A. 2022. Innovative randomized phase I study and dosing regimen selection to accelerate and inform pivotal COVID-19 trial of nirmatrelvir. Clin Pharmacol Ther 112:101–111. doi: 10.1002/cpt.2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang H, Wang F, Zhu X, Chen Y, Chen H, Li X, Wu M, Li C, Liu J, Zhang Y, Ding Y, Niu J. 2021. Antiviral activity and pharmacokinetics of the hepatitis B virus (HBV) capsid assembly modulator GLS4 in patients with chronic HBV infection. Clin Infect Dis 73:175–182. doi: 10.1093/cid/ciaa961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Montanha MC, Fabrega F, Howarth A, Cottura N, Kinvig H, Bunglawala F, Lloyd A, Denti P, Waitt C, Siccardi M. 2022. Predicting drug-drug interactions between rifampicin and ritonavir-boosted atazanavir using PBPK modelling. Clin Pharmacokinet 61:375–386. doi: 10.1007/s40262-022-01178-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petrakis V, Rafailidis P, Trypsianis G, Papazoglou D, Panagopoulos P. 2023. The antiviral effect of nirmatrelvir/ritonavir during COVID-19 pandemic real-world data. Viruses 15:976. doi: 10.3390/v15040976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang H, Gao L, Lou J, Wu M, Chen H, Yang L, Liu J, Zhu X, Li X, Li C, Wang M, Liu C, Guo W, Wang Y, Gao Z, Han L, Wang D, Jin W, Ding Y. 2022. First-in-human study on pharmacokinetics, safety, and tolerability of single and multiple escalating doses of hepenofovir, a novel hepatic targeting prodrug of tenofovir in healthy Chinese subjects. Front Pharmacol 13:873588. doi: 10.3389/fphar.2022.873588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reddy MB, Morcos PN, Le Pogam S, Ou Y, Frank K, Lave T, Smith P. 2012. Pharmacokinetic/pharmacodynamic predictors of clinical potency for hepatitis C virus nonnucleoside polymerase and protease inhibitors. Antimicrob Agents Chemother 56:3144–3156. doi: 10.1128/AAC.06283-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bertz RJ, Persson A, Chung E, Zhu L, Zhang J, McGrath D, Grasela D. 2013. Pharmacokinetics and pharmacodynamics of atazanavir-containing antiretroviral regimens, with or without ritonavir, in patients who are HIV-positive and treatment-naïve. Pharmacotherapy 33:284–294. doi: 10.1002/phar.1205 [DOI] [PubMed] [Google Scholar]
- 27. Canini L, Lemenuel-Diot A, Brennan BJ, Smith PF, Perelson AS. 2018. A pharmacokinetic/viral kinetic model to evaluate treatment of chronic HCV infection with a non-nucleoside polymerase inhibitor. Antivir Ther 23:353–361. doi: 10.3851/IMP3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boras B, Jones RM, Anson BJ, Arenson D, Aschenbrenner L, Bakowski MA, Beutler N, Binder J, Chen E, Eng H, et al. 2021. Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID19. Nat Commun 12:6055. doi: 10.1038/s41467-021-26239-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. von Delft A, Hall MD, Kwong AD, Purcell LA, Saikatendu KS, Schmitz U, Tallarico JA, Lee AA. 2023. Accelerating antiviral drug discovery: lessons from COVID-19. Nat Rev Drug Discov 22:585–603. doi: 10.1038/s41573-023-00692-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rayner CR, Smith PF, Andes D, Andrews K, Derendorf H, Friberg LE, Hanna D, Lepak A, Mills E, Polasek TM, Roberts JA, Schuck V, Shelton MJ, Wesche D, Rowland-Yeo K. 2021. Model-informed drug development for anti-infectives: state of the art and future. Clin Pharmacol Ther 109:867–891. doi: 10.1002/cpt.2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, Sami S, Sabo RT, Hall N, Foreman A, Schubert PL, Gallagher GR, Fink T, Madoff LC, Gabriel SB, MacInnis B, Park DJ, Siddle KJ, Harik V, Arvidson D, Brock-Fisher T, Dunn M, Kearns A, Laney AS. 2021. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings - Barnstable county, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep 70:1059–1062. doi: 10.15585/mmwr.mm7031e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental methods and results.