Abstract
• Context.—
The coronavirus disease 19 (COVID-19) pandemic is placing unparalleled burdens on regional and institutional resources in medical facilities across the globe. This disruption is causing unprecedented downstream effects to traditionally established channels of patient care delivery, including those of essential anatomic pathology services. With Washington state being the initial North American COVID-19 epicenter, the University of Washington in Seattle has been at the forefront of conceptualizing and implementing innovative solutions in order to provide uninterrupted quality patient care amidst this growing crisis.
Objective.—
To conduct a rapid validation study assessing our ability to reliably provide diagnostic neuropathology services via a whole slide imaging (WSI) platform as part of our departmental COVID-19 planning response.
Design.—
This retrospective study assessed diagnostic concordance of neuropathologic diagnoses rendered via WSI as compared to those originally established via traditional histopathology in a cohort of 30 cases encompassing a broad range of neurosurgical and neuromuscular entities. This study included the digitalization of 93 slide preparations, which were independently examined by groups of board-certified neuropathologists and neuropathology fellows.
Results.—
There were no major or minor diagnostic discrepancies identified in either the attending neuropathologist or neuropathology trainee groups for either the neurosurgical or neuromuscular case cohorts.
Conclusions.—
Our study demonstrates that accuracy of neuropathologic diagnoses and interpretation of ancillary preparations via WSI are not inferior to those generated via traditional microscopy. This study provides a framework for rapid subspecialty validation and deployment of WSI for diagnostic purposes during a pandemic event.
On December 29, 2019, official reports describing a cluster of patients suffering from a severe novel pneumonia of unknown etiology initially surfaced from Wuhan (Hubei province) in the People’s Republic of China.1–7 It is hypothesized that the illness, coronavirus disease 19 (COVID-19), is likely the result of an initial zoonotic event that may have occurred in the Huanan seafood wholesale wet market.6–9 On January 7, 2020, the causative infectious agent, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was officially described.2,5,7,10–12 COVID-19 has a reported incubation period of roughly 2 to 14 days, following which patients may present with fever, dry cough, and/or shortness of breath.10 While a significant subset of individuals can be asymptomatic or only mildly affected, others, particularly the elderly and those with preexisting conditions, may exhibit a moderate to severe illness, which may require mechanical ventilation and can be associated with an increased mortality risk.4,11–13 On January 20, 2020, western Washington became the first region in North America with a confirmed COVID-19 case.12–14 Subsequently, Washington state became the first major site of the COVID-19 outbreak in the United States.11,15–17 On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a pandemic: one that as of June 1, had reported more than 6 363 000 positive cases and at least 377 000 deaths across 215 countries and territories including more than 1 859 000 positive cases and at least 106 000 deaths in the United States.18
The nature of the work and interactions occurring in anatomic pathology laboratories, together with viral features such as asymptomatic carrier shedding and prolonged stability in aerosols and surfaces, can result in SARS-CoV-2 transmission events even in these nondirect patient care areas.19–26 Workplace transmission events may lead to reductions of laboratory personnel and/or physician staff owing to individuals’ need to self-quarantine because of exposure and/or sickness. This in addition to other unknowns following infection, including whether or not immunity is conferred or if there is a potential for relapse and/or reinfection of self or others following initial sickness resolution, may further complicate these workplace transmission events.27,28 Age is another complicating factor; a study conducted more than a decade ago reported that 75% of the pathologist workforce in the United States was 45 years or older, with 12% being 65 years or older.10,13,29 Ostensibly, some of these factors can disproportionately affect patient care pipelines in subspecialized practices, particularly in instances where there is no widespread diagnostic expertise outside of the affected subspecialty group and/or in subspecialty groups with high numbers of older individuals. These factors together with potential regional and institutional demands in the face of COVID-19 (eg, deployment of laboratory staff and physicians as part of institutional surge-planning initiatives) could pose unexpected systemwide complications in the delivery of anatomic pathology services and overall patient care.17,30,31 The University of Washington neuropathology division (UWNPD) covers 3 main medical centers within the University of Washington (UW) system across Seattle, Washington, and serves as a referral center for western Washington and a number of outside clients throughout the 5-state WWAMI region (Washington, Wyoming, Alaska, Montana, and Idaho). Faced with this unprecedented situation and in order to mitigate potential patient care interruptions, UWNPD proposed a plan to departmental leadership for the rapid validation and deployment of telepathology services via the Leica Aperio slide imaging system. This plan builds upon existing workflows for digital pathology that have been used by UWNPD for more than a decade for the purpose of providing intraoperative consultation support throughout our Seattle-based hospitals.
A whole slide image (WSI) is a digitized high-resolution replica of a histopathology glass slide created on a whole slide scanner (WSS), which can be manipulated through software to mimic traditional microscope review and issue diagnoses.32 Several published studies have reported low major and minor discrepancy rates, demonstrating that diagnoses rendered by WSI are not inferior to primary diagnoses via traditional histopathology.33–45 Per the College of American Pathologists (CAP; GEN.52920), each laboratory must validate WSS/WSI platforms for each clinical diagnostic usage by performing its own validation study.46 Owing to the COVID-19 pandemic, CAP initiated advocacy efforts geared toward a temporary waiver of current regulations limiting remote review of pathology slides in sites physically outside of the Clinical Laboratory Improvement Amendments (CLIA)–accredited laboratory as set by the Centers for Medicare & Medicaid Services (CMS) and the Department of Health and Human Services.47 On March 26, CMS, recognizing the need for expanded precautions to halt transmission in the workplace together with the wide-ranging operational challenges posed by the COVID-19 pandemic, issued a memorandum describing a policy of temporary enforcement relaxation of these regulations, thus allowing for remote “sign out” by pathologists via telepathology.48 Lastly, on April 24, the US Food and Drug Administration (FDA) announced to Leica Biosystems their intention to exercise enforcement discretion in regard to the use of Aperio WebViewer, a component of Aperio eSlide Manager, to examine slides acquired with an Aperio ScanScope AT2 and AT2 DX for remote pathology diagnosis.
Herein we describe a minimal resource utilization method for rapid validation and deployment of WSI technology for issuing neuropathologic diagnoses undertaken as part of our regional COVID-19 response during the upward sloping phase of regional and hospital resource utilization. This study can serve as a model for rapid and efficient telepathology subspecialty validation for laboratories that are preparing to face or are currently facing COVID-19 as part of an initial wave or potentially subsequent episodic waves, a combined flu and COVID-19 season, other mass casualty events, or workplace transmission events.14,31,49
METHODS
Study Roles and Training
The UW digital pathology manager (DPM; JH) oversaw the anatomic pathology technologists (APT; DZ, SC) operating the WSS as well as all the technical aspects of this validation study alongside the quality and compliance group (QCG; TH, TK), all under the leadership of the laboratory medical directors (CEA, DFC). The validation pathologist supervisor (VPS; LFG-C) is a board-certified neuropathologist with 8.5 years experience (YE) as an attending neuropathologist in the UWNPD. The VPS and DPM conceptualized, implemented, and oversaw execution of the rapid validation study alongside the QCG, following CAP checklists for telepathology and WSI (GEN.50057, GEN.50614, GEN.52842, GEN.52860, GEN.52900, and GEN.52920) and satisfying 9 of 12 (4 of 4 expert consensus opinions, 5 of 7 recommendations, 0 of 1 suggestion) CAP Pathology and Laboratory Quality Center guideline statements proposed by Pantanowitz and colleagues.46 The study pathologist group (SPG) is composed of 3 board-certified attending neuropathologists (CDK 10.9 YE, PJC 4.9 YE, and CSL 2.7 YE, for an average of 6.1 YE). The study trainee group (STG) consists of 1 first-year (RAY) and 1 second-year (KPS) neuropathology fellow. All members of the SPG and STG reviewed the neurosurgical (Nsx) set. SPG members who actively participate in the neuromuscular (NM) clinical service (PJC and CSL) as well as both members of the STG reviewed the NM set.
The DPM was also in charge of the training of the VPS, SPG, and STG end-users as well as the technical training of the APT. Training sessions were performed before slide digitalization and diagnostic examination of cases as required for each of the participant’s role in the study. Dated documentation of training for each specific role and each specific user is available on our laboratory’s document control system (MediaLab, Inc, Lawrenceville, Georgia) in accordance with CAP regulations (GEN.52900). In short, APT scanner training included hardware and software overview, slide preparation and loading into the scanner, review of prescan snapshots, tissue and focus point selection, quality control check of scanned images, and image assignment in Aperio eSlide Manager digital slide information system (Leica Biosystems, Inc, Buffalo Grove, Illinois). Before examination of cases, the VPS, SPG, and STG underwent training via 1 teleconference session covering the WSI system software. This session included topics on how to access the digitized slides from the UW Aperio eSlide Manager digital slide information system, operation of the Aperio viewing software programs (Aperio eSlide Manager and Aperio ImageScope), and WSI image quality control. Because the aim of this validation was to test for remote diagnostic usage of the WSI system, the study pathologists were allowed to use their own home devices when examining digital slides. The SPG and STG were provided with an overview of minimum computer monitor requirements and asked that they evaluate their home device to ensure they were confident in using the display when examining digital slides for diagnosis. Aperio eSlide Manager and Aperio ImageScope fulfill the safeguarding of patient confidentiality under CAP regulations (GEN.52842). Following the completion of the validation study, the DPM and VPS held a final meeting/training digital session to address any outstanding topics related to image manipulation and software troubleshooting of any other potential issues identified during the validation study.
Validation Study Design
Case Curation.—
The VPS selected potential validation cases out of his personal collection or from sources available within the UW neuropathology division didactic repository consisting of more than 500 cases (eg, glass slide study sets and/or cases that had been set aside for various didactic purposes). Before inclusion in the study, all glass slides from each case were reviewed by the VPS in order to (1) verify that the original neuropathologic diagnosis and ancillary study interpretation was correct, (2) confirm the presence of the desired diagnostic features, (3) assess the preservation, sectioning, and staining quality of all the slides, and (4) assess the quality of any included positive controls.
To best emulate the clinical environment of our intended use (providing diagnoses in an academic neuropathology practice) a total of 30 cases covering a wide spectrum of neuropathologic disease and slide preparations were selected (Table). These 30 cases were divided into 2 groups, then randomized within each group, deidentified, and given a unique study case ID number. The Nsx set was composed of 20 cases including neoplastic and nonneoplastic processes of the nervous system, eye, and orbit, and brain autopsy pathology, whereas the NM set was composed of 10 cases including metabolic/ mitochondrial and dystrophic processes as well as immune-mediated myopathies/neuropathies. All cases contained at least 1 hematoxylineosin (H&E)–stained slide with some cases containing variable numbers of ancillary studies (eg, special stains, immunohistochemical [IHC] stains [DAB chromogen], methylene blue–stained sections, and teased nerve fiber studies). The type and number of stains per case initially provided to the diagnosticians was at the discretion of the VPS, who selected these with the intent of providing diagnosticians with sufficient information to arrive and work through a differential diagnosis. Once case selection was finalized the DPM and VPS created a key to the randomized study case ID numbers, containing case identifiers, neuropathologic diagnoses, and ancillary study interpretations that were extracted from the pathology report, and was not shared with any other study members.
Table:
Details of Validation Cohorts Including Diagnoses and Number or Slide Preparations As Well As the Results of Diagnostic Concordance Evaluation
| Nsx Set: Category (No. of Cases) | Study Set Number | Abbreviated Classification/Diagnosis and Grade (if applicable) | H&E, FFPE | Special Stains | IHC | Total Slides | SPG Dx QCS (n = 3) | SPG SS/ IHC QCS (n = 3) | STG Dx QCS (n = 2) | STG SS/ IHC QCS (n = 2) | Diagnostic Image Quality, Yes/No |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| CNS/PNS neoplastic (10) | Nsx-01 | Central neurocytoma, WHO grade II | 1 | 0 | 4 (SYN, NeuN, GFAP, NF) | 5 | 0 | 0 | 0 | 0 | Yes |
| Nsx-02 | Ependymoma, WHO grade II | 1 | 0 | 3 (GFAP, EMA, NF) | 4 | 0 | 0 | 0 | 0 | Yes | |
| Nsx-03 | Glioblastoma, WHO grade IV | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | Yes | |
| Nsx-04 | Oligodendroglioma, IDH-mutant and 1 p/19q codeleted, WHO grade II | 1 | 0 | 5 (mlDHl, ATRX, P53, GFAP, NF) | 6 | 0 | 0 | 0 | 0 | Yes | |
| Nsx-05 | Myxopapillary ependymoma, WHO grade I | 1 | 1 (Al Bl) | 1 (GFAP) | 3 | 0 | 0 | 0 | 0 | Yes | |
| Nsx-07 | Medulloblastoma, WHO grade IV | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | Yes | |
| Nsx-09 | Hemangioblastoma, WHO grade I | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | Yes | |
| Nsx-10 | Schwannoma, WHO grade I | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | Yes | |
| Nsx-13 | Adamantinomatous craniopharyngioma, WHO grade I | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | Yes | |
| Nsx-15 | Secretory meningioma, WHO grade I | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | Yes | |
| CNS/PNS nonneoplastic (3) | Nsx-06 | Arteriovenous malformation | 1 | 1 (VVG) | 0 | 2 | 0 | 0 | 0 | 0 | Yes |
| Nsx-08 | Neurocysticercosis | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | Yes | |
| Nsx-11 | Cavernous malformation (cavernoma) | 1 | 1 (GT) | 0 | 2 | 0 | 0 | 0 | 0 | Yes | |
| Brain autopsy neoplastic (1) | Nsx-14 | Intravascular B-cell lymphoma | 1 | 0 | 1 (CD20) | 2 | 0 | 0 | 0 | 0 | Yes |
| Brain autopsy nonneoplastic (1) | Nsx-12 | Coccidioidal meningitis | 1 | 1 (GMS) | 0 | 2 | 0 | 0 | 0 | 0 | Yes |
| Ophthalmic neoplastic (2) | Oph-1 | Uveal melanoma | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | Yes |
| Oph-3 | Invasive squamous cell carcinoma | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | Yes | |
| Ophthalmic nonneoplastic (3) | Oph-2 | Hidrocy stoma | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | Yes |
| Oph-4 | Epidermal inclusion cyst | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | Yes | |
| Oph-5 | Lipogranuloma (chalazion) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | Yes | ||
| Total | 20 | 20 | 4 | 14 | 38 | ||||||
| Metabolic/mitochondrial myopathy (4) | NM-01 | GSD/acid maltase deficiency (glycogenosis type II) | 0/1 | 4 (mGT, PAS, ORO, AcP)/0 | 0/0 | 5 | 0 | 0 | 0 | 0 | Yes |
| NM-02 | GSD/McArdle disease (glycogenosis type V) | 0/1 | 4 (MP, COX/SDH, ORO, PAS)/0 | 0/0 | 5 | 0 | 0 | 0 | 0 | Yes | |
| NM-04 | Mitochondrial myopathy | 0/1 | 5 (mGT, MP, COX/ SDH, AMPD, PFK)/1 (MB) | 0/0 | 7 | 0 | 0 | 0 | 0 | Yes | |
| NM-06 | Lipid storage disease | 0/1 | 2 (ORO, PAS)/1 (MB) | 0/0 | 4 | 0 | 0 | 0 | 0 | Yes | |
| Dystrophic (1) | NM-07 | Necrotizing myopathy/dysferlinopathy | 0/1 | 0/0 | 4 (dysferlin, dystrophins (rod, C-term, N-term))/0 | 5 | 0 | 0 | 0 | 0 | Yes |
| Immune/inflammatory (5) | NM-03 | Inflammatory myopathy/immune myopathy with prominent perimysial pathology | 0/1 | 2 (AcP, AlkP)/0 | 3 (HLA-I, CD3, CD20)/0 | 6 | 0 | 0 | 0 | 0 | Yes |
| NM-05 | Inflammatory myopathy/sporadic inclusion body myositis | 0/1 | 0/1 (MB) | 4 (HLA-I, CD3, CD20, TDP43)/0 | 6 | 0 | 0 | 0 | 0 | Yes | |
| NM-08 | Immune mediated/macrophagic myofasciitis | 0/1 | 2 (PAS, AcP)/1 (MB) | 0/0 | 4 | 0 | 0 | 0 | 0 | Yes | |
| NM-09 | Immune-mediated neuropathy with axonopathy | 1/0 | 0/2 (MB, teased nerve fiber preparation) | 0/1 (CD 3) | 4 | 0 | 0 | 0 | 0 | Yes | |
| NM-10 | Immune-mediated/vasculitis with axonopathy and neurogenic atrophya | 3/1 | 0/1 (MB) | 1 (CD3)/3 (CD3, CD68, NF) | 9 | 0 | 0 | 0 | 0 | Yes | |
| Total | 10 | 4/9 | 19/7 | 12/4 | 55 | ||||||
Abbreviations: AcP, acid phosphatase; Al Bl, Alcian blue; AlK P, alkaline phosphatase; AMPD, myoadenylate deaminase; ATRX, a-thalassemia/mental retardation, X-linked; CNS, central nervous system; COX/SDH, cytochrome c oxidase/succinate dehydrogenase; DX, diagnosis; EMA, epithelial membrane antigen; FFPE, formalin fixed, paraffin embedded; Fzn, frozen; GFAP, glial fibrillary acidic protein; GMS, Grocott methenamine silver stain; GSD, glycogen storage disease; GT, Gomori trichrome; H&E, hematoxylin-eosin; HLA-I, human leukocyte antigen class I; IHC, immunohistochemical stains; MB, methylene blue; mGT, modified Gomori trichrome; mIDH, mutant isocitrate dehydrogenase; MP, myophosphorylase; NeuN, neuronal nuclear protein; NF, neurofilament; NM, neuromuscular; Nsx, neurosurgical; ORO, Oil Red O; PAS, periodic acid-Schiff; PFK, phosphofructokinase; PNS, peripheral nervous system; QCS, quality concern score; SPG, study pathologist group; SS, special stains; STG, study trainee group; SYN, synaptophysin; TDP43, TAR DNA-binding protein 43; VVG, Verhoeff-Van Gieson stain; WHO, World Health Organization.
Includes slides additionally provided per request of 1 SPG member (2 additional H&E stains and CD3 and CD68 IHC).
Study Materials Preparation.—
Each case included in our study was linked to its original neuropathology report in our laboratory information system (LIS; PowerPath, Tuscon, Arizona). For each case, the VPS extracted the patient’s clinical information, removing all patient identifiers and, when applicable, selected and provided images (eg, radiologic studies, autopsy brain cutting photographs, electron microscopy photographs). It is important to note that as part of our divisional workflow, in particular for the workup of neoplastic neurosurgical cases, representative presurgical magnetic resonance imaging images are routinely uploaded into the LIS. As such, providing these same radiologic, ultrastructural, or brain cutting images to the diagnosticians does not deviate from the information that was available at the time of the original neuropathologic diagnosis. Also similar to traditional clinical practice, if a case had an intraoperative consultation this information was also made available to the diagnosticians. The case-specific clinical information and images were assembled into 1 document per cohort and provided to the diagnosticians (Figure 1, A and B). The VPS also created an answer sheet for each set that included a section to rate each case’s WSI quality (Figure 2, A and B). The Nsx answer sheet included sections for overall disease classification and final diagnosis as well as, when applicable, tumor grading and interpretation of ancillary studies. Similarly, the NM sheet included sections for overall disease classification, final diagnosis, and ancillary study interpretation. In addition, since NM cases are typically more complex, an additional column designated “any additional diagnostic comments” was added, allowing the diagnosticians to voluntarily share any important clinicopathologic details or diagnostic comments.
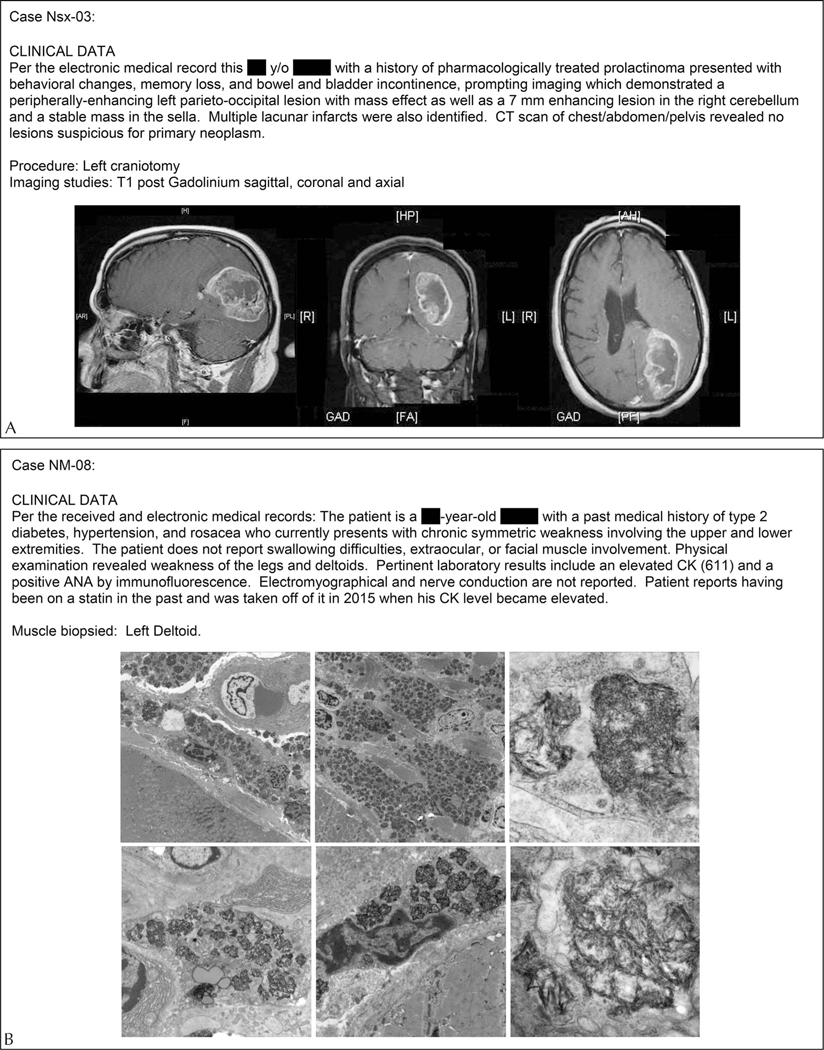
Figure 1.
Examples of the clinical information and images provided per case for the Neurosurgical (Nsx) set (A) and the Neuromuscular (NM) set (B). Abbreviations: ANA, antinuclear antibody; CT, computed tomography; CK, creatine kinase.
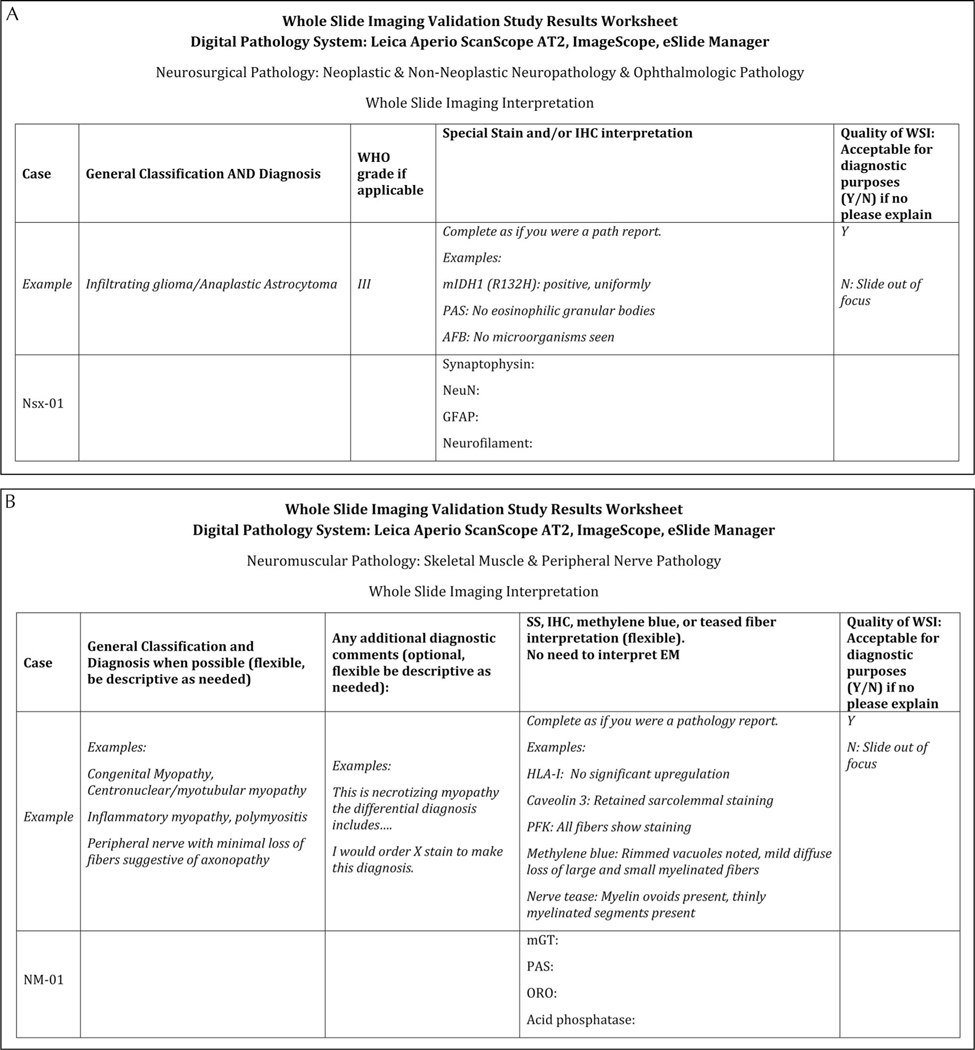
Figure 2.
Examples of the answer sheets provided for the Neurosurgical (Nsx) set (A) and the Neuromuscular (NM) set (B). Abbreviations: AFB, acid-fast bacilli/Ziehl–Neelsen stain; EM, electron microscopy; GFAP, glial fibrillary acidic protein; HLA-I, human leukocyte antigen class I; IHC, immunohistochemistry; mGT, modified Gomori trichrome; mIDH1, mutant isocitrate dehydrogenase 1; NeuN, neuronal nuclear protein; ORO, Oil Red O; PAS, periodic acid–Schiff; PFK, phosphofructokinase; SS, special stain; WHO, World Health Organization; WSI, whole slide imaging.
Slide Digitization.—
Slides from each of the previously randomized and deidentified cases were then relabeled first using opaque labels, then with a second label displaying the assigned study case ID number and slide preparation (eg, H&E, GT [Gomori trichrome], CD20). The APT cleaned the blinded glass slides so they were free of marker dotting, dust, oils, or other contaminants that may affect WSI quality. Following this, the APT members performed a whole slide digitization of the slides at ×40 magnification, using the Leica Aperio ScanScope AT2 system. During the scanning steps, an APT member reviewed the prescan snapshots of each slide to ensure all tissue on the slide was adequately scanned (including on slide positive controls for IHC and special stains when applicable). After slide scanning and image acquisition, the technologist reviewed the digitized WSIs for quality issues (eg, focus, calibration errors) to ensure image quality control. The technologist marked the time at which the slides are obtained and scanning is completed so as to measure turnaround time for scanning and ultimately interpretations.
Validation Study Execution
Each member of the SPG and STG independently reviewed the medical histories, clinical images, and digitalized slides by using the Aperio eSlide Manager and/or Aperio ImageScope and recorded their interpretations on the validation study answer sheet. In this validation study, the slide labels were deidentified; as such, each end-user must use the study case ID on the image capture of the slide label as an identifier in order to confirm positive identification of the correct case. Quality of WSI was recorded for each case as either yes or no (in terms of adequacy to render primary diagnosis). The interpretations of all SPG and STG participants were analyzed by the VPS. Similar to traditional clinical practice, SPG and STG members had the ability to request additional slide preparations that would aid them in making a diagnosis; however, dissimilar from a traditional clinical practice, diagnosticians were not allowed to seek slide review by other members of the team.
Interpretation of Results
The VPS evaluated each set of answers for diagnostic concordance between the WSI interpretations and the verified baseline diagnosis rendered via traditional histopathology. Areas of evaluation included general disease classification and final diagnosis as well as grade (when applicable). Evaluation of ancillary study interpretation was undertaken with a high level of scrutiny, emphasizing on not only the stated result but also the identification of diagnostic pertinent positive or pertinent negative features for each case. A concordance threshold of at least 95% between the original and WSI diagnoses is required for positive validation. The final diagnosis results were assigned a quality concern score (QCS); concordant results are those with QCS of 0 to 2, and discordant results are those with QCS of 3 to 5. A QCS score of 0 indicates no quality concerns, 1 indicates a variation in practice that is not associated with a clinically significant risk to the patient, and a 2 is a variation in practice associated with a clinically significant increased risk to the patient. QCS scores of 3 to 5 indicate errors that would result in additional care, were potentially life-threatening, or may cause other severe outcomes including death. Lastly, the DPM compiled the results, which are then reviewed and approved by the medical director.
RESULTS
Overview
Our rapid validation study is composed of 30 total cases (20 combined neurosurgical, autopsy neuropathology, and ophthalmic; and 10 neuromuscular cases) encompassing the wide range of pathologic processes and tissue/slide preparations that would be expected in an academic neuropathology practice. There was 100% diagnostic concordance without any major or minor diagnostic discrepancies for any of the SPG or STG members; as such discrepancy rates by diagnostician were not calculated. These results demonstrate that the WSI system was able to adequately capture the pathologic features of each slide/case and that each diagnostician was able to use these images together with the available clinical information to render correct diagnoses and ancillary study interpretations, as they would do in their traditional clinical practice. The validation study results are summarized in the Table.
Study Pathologist Group
There were no major or minor diagnostic discrepancies in either the Nsx or NM sets (100% diagnostic concordance; QCS 0) and all ancillary studies were interpreted and described correctly. For case NM-10, one diagnostician commented that while they had identified vascular changes in the muscle and nerve that were concerning for vasculitis, in a clinical setting they would request additional studies in order to arrive at that diagnosis. On the basis of their request they were provided additional WSIs generated from archival material that had been available to the original diagnostician: 2 additional formalin-fixed, paraffin-embedded (FFPE) H&E stains (muscle and nerve) and IHC for the frozen muscle (CD3) and FFPE nerve (CD68). The SPG member reviewed these new sections and arrived at the correct diagnosis in both the muscle and nerve specimens. All study participants were given the opportunity to review these additional slides and none provided diagnostic modifications. Lastly, in the NM set answer sheets, none of the additional text provided in the “any additional diagnostic comments” section showed any major or minor diagnostic discrepancies.
Study Trainee Group
There were no major or minor diagnostic discrepancies in either the Nsx or NM sets (100% diagnostic concordance; QCS 0) and all ancillary studies were interpreted and described correctly. For case NM-03, one trainee correctly classified it as an “inflammatory myopathy,” offered a short differential diagnosis including polymyositis and overlap myositis and suggested “. .correlation with autoimmune testing may be helpful.” In addition, the trainee correctly interpreted all ancillary studies (acid and alkaline phosphatases and HLA-I [human leukocyte antigen class I], CD3, and CD20 IHC). All other study participants also correctly diagnosed and characterized this case as an immune myopathy with perimysial pathology (IMPP). While IMPP is not a universally accepted term, it describes a phenotype that can be seen in some patients with antisynthetase syndrome (eg, with anti-Jo1 antibodies) who can also be at risk for developing idiopathic pulmonary fibrosis.50 As such and since this specific terminology is not uniformly accepted, a diagnosis of inflammatory myopathy and request for correlation with serologic testing (eg, myositis panel) is not considered a discrepancy nor does it pose any significant risk to the patient, as these serologic panels are routinely performed for patients with myositis. Lastly, it should be noted that STG members were given the opportunity to review the additional slides that were requested and provided for case NM-10 as part of their initial review.
Image Quality Assessment
Study participants did not defer any cases because of issues with image quality. As part of the validation study the SPG and STG members were also asked to assess WSI quality and render either a “yes” or “no” opinion as to whether the image quality was acceptable for diagnostic purposes; all members of the SPG and STG answered “yes” for all 93 slides on both the Nsx and NM sets. In general, all diagnosticians were impressed with the image quality of H&E and ancillary preparations. Additional image quality comments were requested and provided even if the diagnostician felt the WSIs were of diagnostic quality. It must be noted that even when diagnosticians provided additional comments related to image quality, these did not affect their ability to arrive at the correct pathologic classification, diagnosis, and grade, nor did it affect their ability to provide the correct interpretation for each ancillary tissue preparation.
Questions regarding minimal adjustments to image brightness and/or contrast were the most commonly provided comments. For case Nsx-04, one STG member mentioned that assessing the staining intensity for nuclear p53 immunoreactivity was perhaps “a bit harder to interpret for me than the others.” For case Nsx-06, one SPG member mentioned that VVG was dark and somewhat difficult to interpret, similarly 1 STG member mentioned that the modified Gomori trichrome stains on cases NM-01 and NM-04 were somewhat dark. In addition, 1 SPG member mentioned that for case Nsx-04, owing to the newness of using this platform for mitotic counting, a Ki-67 assay (not available for this historic case but currently customarily ordered in all infiltrating gliomas in our clinical practice) would have been useful to corroborate their impression of low mitotic activity and confirm grade assignment. As mentioned in “Study Roles and Training” in the Methods section, following the completion of the validation study, the DPM and VPS held a wrap-up digital session addressing topics related to software or troubleshooting of brightness, contrast, and color balance, using the aforementioned cases as examples, which answered the specific diagnostician’s questions.
DISCUSSION
Even with effectively deployed and adhered to community mitigation strategies, a multitude of regions and medical systems across the globe are facing unprecedented stress to their social infrastructure and health care systems during the COVID-19 pandemic.14,15,49,51–53 Services provided by anatomic pathology laboratories are a crucial component of the patient care pipeline even when not in direct contact with COVID-19 patients.54 This is especially true for other urgent non–COVID-19 patients with serious medical conditions for whom pathologic diagnoses and integration of histopathologic and molecular data are crucial for timely initiation of appropriate therapy regimens.54 The combination of the nature of the work and interactions that occur in these laboratories, together with practice subspecialization, workforce demographics, and inherent characteristics of the SARS-CoV-2 virus could potentially lead to unexpected operational challenges (eg, interruption of laboratory operations and/or diagnostic subspecialty services).10,13,27–29 This rapidly evolving situation has allowed our medical systems a variety of opportunities to meet these demands in flexible and innovative ways, while at the same time remaining compliant with regulatory guidelines, in the face of the extraordinary challenges posed by this pandemic.14,15,49,52
In preparation for any of the aforementioned potential stressors, we designed and conducted, in roughly 3 weeks, a rapid validation study to assess the possibility of providing remote diagnostic neuropathology services via telepathology for the UW Medicine system. Our intent was to conduct this study with the least amount of additional resources, as it was piloted in mid-March to early April during the upward sloping of the epidemiologic and hospital resource utilization curves during the initial COVID-19 outbreak in western Washington. This validation study included the review of 93 slide preparations (eg, H&E, special stains, immunohistochemistry) across 30 cases consisting of a mixture of neurosurgical, ophthalmic, brain autopsy, and neuromuscular pathology entities by a group of neuropathology attendings and trainees. None of the diagnosticians had major or minor diagnostic discrepancies in either cohort (Nsx and NM), as such achieving our threshold of at least 95% diagnostic concordance and successfully validating this platform within the neuropathology subspecialty service during the COVID-19 pandemic as well as the period of relaxed CMS and FDA enforcement of policies regulating remote review of pathology slides.
Our rapid validation study has some limitations. First, this project builds upon the extensive collective telepathology experience of the UWNPD members (anywhere from 2.5–11 years per member), who when on service perform remote intraoperative diagnostic consultations using a similar platform (most recently Aperio LV1). As such, this level of user experience with telepathology may not be necessarily available in all other centers attempting to rapidly implement this system with limited resource utilization. An additional point is that this validation method did not follow the recommendation to establish pathologist intraobserver concordance between traditional review of glass slides and digital examination of WSI, but rather demonstrated pathologists’ digital competency with the WSI system and interobserver concordance.46 This compromise allowed for case selection from readily available on-site slide libraries, which both limited the number of staff interactions to support the institution’s social-distancing guidelines and addressed the needs of a rapid validation timeline. A more traditional validation study would require additional case selection and slide pull steps using slide room staff, glass slide examination followed by a minimum 2-week washout period, and then digital slide examination. Another limitation is that the total number of cases evaluated in this study might be considered a constraint when compared to other larger studies; however, it should be noted that other groups have conducted validation studies that included a similar number of cases and slides (12–52 cases and 24–391 slides).37–40,55 For our group an element of this choice was due to our desire to use the least amount of resources together with the need for rapid validation, during the time of telepathology-related waivers, as a component of our COVID-19 planning response. Consequently, we turned our attention to building very inclusive case cohorts with a broad range of neuropathologic entities and slide/tissue preparations in order to ensure a wide and robust representation of pathologic processes and preparations proper to an academic neuropathology practice. While some of the study set cases, individually, may not be reflective of all the complexities found in routine clinical practice, we believe that for the reasons listed above, the case selection acts to expand the spectrum of entities and preparations being validated within 30 neuropathology cases compared to what could be achieved with a set of consecutive cases.
In addition, our validation study used ×40 magnification slide scans, and thus all digital slides used for clinical diagnostic purposes under this validation will need to be scanned at this resolution. We note this resolution may not be necessary for all subspecialty services and as such the impact of slide-scanning resolution on the daily throughput of the scanner, image storage, and bandwidth requirements should be considered when laboratories are designing their clinical workflows guided by our validation study. It should also be noted that while this study included immunohistochemical slide preparations using the DAB chromogen, preparations using different chromogens or prognostic biomarkers (HER2, ER, PR) should and will be validated separately to ensure the digital pathology system is able to capture the expected range of colors and intensities for the intended diagnostic application. Notwithstanding, we note the need for additional validations in order to study supplementary organ systems and diagnostic applications/ specimen preparations.56 To address these limitations the UW Digital Pathology section is currently working on a follow-up validation study with our colleagues in the genitourinary pathology subspecialty, which is designed to meet all recommendations on a more relaxed timeline.
Once deployed, diagnostic WSI services would need to be included in the laboratories’ quality management plan as mandated by CAP (GEN.52860). A policy for prospective review of cases in which telepathology was used to arrive at a primary diagnosis is still being formalized at a departmental and subspecialty level. Currently in UWNPD, more than 80% to 90% of neoplastic cases are reviewed at our weekly case review conference, a subset of which is then reviewed again during multidisciplinary conference preparation. Occasionally, interesting and/or challenging nonneoplastic cases may also be reviewed in this weekly forum, in our monthly ophthalmic or neuromuscular case review conferences, or in preparation of other working conferences within the UW system. For UWNPD, these safeguards would allow an adequate degree of oversight. Part of our future policy would have to include a more formalized process for prospective review of nonneoplastic cases diagnosed via WSI (eg, glass slide review of ~5% of cases for diagnostic concordance).
In conclusion, we present a method for rapid validation of diagnostic WSI services as part of our divisional and departmental COVID-19 response. This study can be completed in approximately 2 to 3 weeks and serves as a validation blueprint for other groups to efficiently deploy diagnostic telepathology services during times of diminished resources and/or staffing during the COVID-19 pandemic or other similar events.
Acknowledgments
All authors wish to thank Christine Federhart, BA, for outstanding administrative support in maintaining our neuropathology didactic case database and slides, in particular the materials related to the Neuromuscular Pathology Section teaching case collection.
Sources of support include The Nancy and Buster Alvord Endowment (CDK).
The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.International Society of Infectious Diseases. Undiagnosed pneumonia China (Hubei): request for information. Program for Monitoring Emerging Diseases (ProMED). https://promedmail.org/promed-post/?id¼6864153. Accessed April 15, 2020.
- 2.International Society of Infectious Diseases. Undiagnosed pneumonia China (HU) (07): official confirmation of novel coronavirus. Program for Monitoring Emerging Diseases (ProMED). https://promedmail.org/promed-post/?id¼20200108.6878869. Accessed April 15, 2020.
- 3.Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MUG, Khan K. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. J Travel Med. 2020;27(2):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with anew coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Morales AJ, Bonilla-Aldana DK, Balbin-Ramon GJ, et al. History is repeating itself: probable zoonotic spillover as the cause of the 2019 novel coronavirus epidemic. Infez Med. 2020;28(1):3–5. [PubMed] [Google Scholar]
- 7.Ahmad T, Khan M, Haroon, et al. COVID-19: zoonotic aspects [published online ahead of print February 27, 2020]. Travel Med Infect Dis. doi: 10.1016/j.tmaid.2020.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee PI, Hsueh PR. Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. J Microbiol Immunol Infect. 2020;53(3):365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam TT, Shum MH, Zhu HC, et al. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins [published online ahead of print March 26, 2020]. Nature. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 10.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region: case series. N Engl J Med. 2020;382(21):2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMichael TM, Clark S, Pogosjans S, et al. COVID-19 in a long-term care facility: King County, Washington, February 27-March 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21): 2005–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fausto J, Hirano L, Lam D, et al. Creating a palliative care inpatient response plan for COVID19: the UW Medicine experience. J Pain Symptom Manage. 2020;60(1):e21–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grange ES, Neil EJ, Stoffel M, et al. Responding to COVID-19: the UW Medicine Information Technology Services experience. Appl Clin Inform. 2020; 11(2):265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mossa-Basha M, Medverd J, Linnau K, et al. Policies and guidelines forCOVID-19 preparedness: experiences from the University of Washington [published online ahead of print April 8, 2020]. Radiology. doi: 10.1148/radiol.2020201326. [DOI] [PubMed] [Google Scholar]
- 16.Mossa-Basha M, Meltzer CC, Kim DC, Tuite MJ, Kolli KP, Tan BS. Radiology Department preparedness for COVID-19: Radiology Scientific Expert Panel [published online ahead of print March 16, 2020]. Radiology. doi: 10.1148/radiol.2020200988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim CS, Lynch JB, Cohen S, et al. One academic health system’s early (and ongoing) experience responding to COVID-19: recommendations from the initial epicenter of the pandemic in the United States [published online ahead of print April 9, 2020]. Acad Med. doi: 10.1097/ACM.0000000000003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Worldmeters.info COVID-19 coronavirus pandemic. worldmeters.info.2020. https://www.worldometers.info/coronavirus/. Accessed June 1, 2020.
- 19.Ling L, Wong WT, Wan WTP, Choi G, Joynt GM. Infection control in nonclinical areas during COVID-19 pandemic. Anaesthesia. 2020;75(7):962–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prem K, Liu Y, Russell TW, et al. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5(5):e261–e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020; 382(16):1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Su X, Chen W, et al. Epidemiological investigation on a cluster epidemic of COVID-19 in a collective workplace in Tianjin [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(5):649–653. [DOI] [PubMed] [Google Scholar]
- 23.Lu J, Gu J, Li K, et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pambuccian SE. The COVID-19 pandemic: implications for the cytology laboratory. J Am Soc Cytopathol. 2020;9(3):202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivett L, Sridhar S, Sparkes D, et al. Screening of healthcare workers forSARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission [published online ahead of print May 11, 2020]. doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan SS, Yan B, Saw S, et al. Practical laboratory considerations amidst theCOVID-19 outbreak: early experience from Singapore [published online ahead of print March 20, 2020]. J Clin Pathol. doi: 10.1136/jclinpath-2020-206563. [DOI] [PubMed] [Google Scholar]
- 27.Chen D, Xu W, Lei Z, et al. Recurrence of positive SARS-CoV-2 RNA inCOVID-19: a case report. Int J Infect Dis. 2020;93:297–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou L, Liu K, Liu HG. Cause analysis and treatment strategies of” recurrence” with novel coronavirus pneumonia (COVID-19) patients after discharge from hospital [in Chinese]. Zhonghua Jie He He Hu Xi Za Zhi. 2020; 43(4):281–284. [DOI] [PubMed] [Google Scholar]
- 29.Robboy SJ, Weintraub S, Horvath AE, et al. Pathologist workforce in the United States: I, development of a predictive model to examine factors influencing supply. Arch Pathol Lab Med. 2013;137(12):1723–1732. [DOI] [PubMed] [Google Scholar]
- 30.Carenzo L, Costantini E, Greco M, et al. Hospital surge capacity in a tertiary emergency referral centre during the COVID-19 outbreak in Italy. Anaesthesia. 2020;75(7):928–934. doi: 10.1111/anae.15072. [DOI] [PubMed] [Google Scholar]
- 31.Challen K, Bentley A, Bright J, Walter D. Clinical review: mass casualty triage—pandemic influenza and critical care. Crit Care. 2007;11(2):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Digital Pathology Association. Glossary of terms. https://digitalpathologyassociation.org/glossary-of-terms_1 . Accessed May 1, 2020.
- 33.Bauer TW, Behling C, Miller DV, et al. Precise identification of cell and tissue features important for histopathologic diagnosis by a whole slide imaging system. J Pathol Inform. 2020;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauer TW, Slaw RJ. Validating whole-slide imaging for consultation diagnoses in surgical pathology. Arch Pathol Lab Med. 2014;138(11):1459–1465. [DOI] [PubMed] [Google Scholar]
- 35.Borowsky AD, Glassy EF, Wallace WD, et al. Digital whole slide imaging compared with light microscopy for primary diagnosis in surgical pathology: a multicenter, double-blinded, randomized study of 2045 cases [published online ahead of print February 14, 2020]. Arch Pathol Lab Med. doi: 10.5858/arpa.2019-0569-OA. [DOI] [PubMed] [Google Scholar]
- 36.Buck TP, Dilorio R, Havrilla L, O’Neill DG. Validation of a whole slide imaging system for primary diagnosis in surgical pathology: a community hospital experience. J Pathol Inform. 2014;5(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fallon MA, Wilbur DC, Prasad M. Ovarian frozen section diagnosis: use of whole-slide imaging shows excellent correlation between virtual slide and original interpretations in a large series of cases. Arch Pathol Lab Med. 2010; 134(7):1020–1023. [DOI] [PubMed] [Google Scholar]
- 38.Furness P A randomized controlled trial of the diagnostic accuracy of internet-based telepathology compared with conventional microscopy. Histopathology. 2007;50(2):266–273. [DOI] [PubMed] [Google Scholar]
- 39.Gilbertson JR, Ho J, Anthony L, Jukic DM, Yagi Y, Parwani AV. Primary histologic diagnosis using automated whole slide imaging: a validation study. BMC Clin Pathol. 2006;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho J, Parwani AV, Jukic DM, Yagi Y, Anthony L, Gilbertson JR. Use of whole slide imaging in surgical pathology quality assurance: design and pilot validation studies. Hum Pathol. 2006;37(3):322–331. [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhyay S, Feldman MD, Abels E, et al. Whole slide imaging versus microscopy for primary diagnosis in surgical pathology: a multicenter blinded randomized noninferiority study of 1992 cases (pivotal study). Am J Surg Pathol. 2018;42(1):39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakayama I, Matsumura T, Kamataki A, et al. Development of a teledermatopathology consultation system using virtual slides. Diagn Pathol. 2012;7:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vosoughi A, Smith PT, Zeitouni JA, et al. Frozen section evaluation via dynamic real-time nonrobotic telepathology system in a university cancer center by resident/faculty cooperation team. Hum Pathol. 2018;78:144–150. [DOI] [PubMed] [Google Scholar]
- 44.Dunn BE, Choi H, Recla DL, Kerr SE, Wagenman BL. Robotic surgical telepathology between the Iron Mountain and Milwaukee Department of Veterans Affairs Medical Centers: a 12-year experience. Hum Pathol. 2009; 40(8):1092–1099. [DOI] [PubMed] [Google Scholar]
- 45.Lopez AM, Graham AR, Barker GP, et al. Virtual slide telepathology enables an innovative telehealth rapid breast care clinic. Hum Pathol. 2009;40(8):1082–1091. [DOI] [PubMed] [Google Scholar]
- 46.Pantanowitz L, Sinard JH, Henricks WH, et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137(12):1710–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.College of American Pathologists. CAP secures remote work waiver for pathologists. Advocacy Update. 2020. https://www.cap.org/advocacy/latestnews-and-practice-data/march-26-2020. Accessed April 15, 2020
- 48.Department of Health and Human Services/Centers for Medicaid and Medicare Services. Clinical Laboratory Improvement Amendments (CLIA) Laboratory Guidance During COVID-19 Public Health Emergency. https://documents.cap.org/documents/clia-cms-remote-work.pdf . Accessed April 15, 2020.
- 49.Gadzinski AJ, Ellimoottil C, Odisho AY, Watts KL, Gore JL. Implementing telemedicine in response to the 2020 COVID-19 pandemic. J Urol. 2020;204(1): 14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pestronk A Acquired immune and inflammatory myopathies: pathologic classification. Curr Opin Rheumatol. 2011;23(6):595–604. [DOI] [PubMed] [Google Scholar]
- 51.Gates B Responding to Covid-19: a once-in-a-century pandemic? N Engl J Med. 2020;382(18):1677–1679. [DOI] [PubMed] [Google Scholar]
- 52.Pagano MB, Hess JR, Tsang HC, et al. Prepare to adapt: blood supply and transfusion support during the first 2 weeks of the 2019 novel coronavirus (COVID-19) pandemic affecting Washington State. Transfusion. 2020;60(5):908–911. [DOI] [PubMed] [Google Scholar]
- 53.The Institute for Health Metrics and Evaluation (IHME). COVID-19 projections. https://covid19.healthdata.org/united-states-of-america. Accessed June 15, 2020.
- 54.Fleming KA, Naidoo M, Wilson M, et al. High-quality diagnosis: an essential pathology package. In: Jamison DT, Gelband H, Horton S, et al. , eds. Disease Control Priorities: Improving Health and Reducing Poverty. 3rd ed. Washington, DC: The International Bank for Reconstruction and Development/ The World Bank; 2017. [PubMed] [Google Scholar]
- 55.Bauer TW, Schoenfield L, Slaw RJ, Yerian L, Sun Z, Henricks WH. Validation of whole slide imaging for primary diagnosis in surgical pathology. Arch Pathol Lab Med. 2013;137(4):518–524. [DOI] [PubMed] [Google Scholar]
- 56.College of American Pathologists. CAP validating whole slide imaging (WSI) for diagnostic purposes in pathology – frequently asked questions. 2016. https://documents.cap.org/documents/validating-whole-slide-imaging-faq.pdf. Accessed April 15, 2020.