Abstract
Bacillus cereus 569 (ATCC 10876) germinates in response to inosine or to l-alanine, but the most rapid germination response is elicited by a combination of these germinants. Mutants defective in their germination response to either inosine or to l-alanine were isolated after Tn917-LTV1 mutagenesis and enrichment procedures; one class of mutant could not germinate in response to inosine as a sole germinant but still germinated in response to l-alanine, although at a reduced rate; another mutant germinated normally in response to inosine but was slowed in its germination response to l-alanine. These mutants demonstrated that at least two signal response pathways are involved in the triggering of germination. Stimulation of germination in l-alanine by limiting concentrations of inosine and stimulation of germination in inosine by low concentrations of l-alanine were still detectable in these mutants, suggesting that such stimulation is not dependent on complete functionality of both these germination loci. Two transposon insertions that affected inosine germination were found to be located 2.2 kb apart on the chromosome. This region was cloned and sequenced, revealing an operon of three open reading frames homologous to those in the gerA and related operons of Bacillus subtilis. The individual genes of this gerI operon have been named gerIA, gerIB, and gerIC. The GerIA protein is predicted to possess an unusually long, charged, N-terminal domain containing nine tandem copies of a 13-amino-acid glutamine- and serine-rich sequence.
Bacillus species have the ability, under certain nutrient stresses, to undergo a complex differentiation process resulting in the formation of a highly resistant dormant endospore (6). These spores can then persist in the environment for prolonged periods until a sensitive response mechanism detects specific environmental conditions, initiating the processes of germination and outgrowth (9, 21, 25). Germination can be initiated by a variety of agents (12), including nutrients, enzymes, or physical factors, such as abrasion or hydrostatic pressure.
The molecular genetics of spore germination has been most extensively studied in Bacillus subtilis 168 (21). B. subtilis spores can be triggered to germinate in response to either l-alanine or to a combination (29) of asparagine, glucose, fructose, and potassium ions (AGFK). Mutants of B. subtilis which are defective in germination responses to one or to both types of germinant have been isolated previously (20, 27). Analysis of these mutants suggests that the germinants interact with separate germinant-specific complexes within the spore (21). This in some way leads to activation of components of the germination apparatus common to both responses, such as germination-specific cortex lytic enzymes, leading in turn to complete germination of the spore (10, 22). The mutations within the gerA operon of B. subtilis specifically block germination initiated by l-alanine (34). The predicted amino acid sequences of the three GerA proteins encoded in the operon suggest that these proteins could be membrane associated, and they are the most likely candidates to represent the germinant receptor for alanine (21).
The amino acid l-alanine has been identified as a common but not universal germinant in a variety of Bacillus species, often requiring the presence of adjuncts such as electrolytes and sugars. Ribosides, such as inosine, represent another type of common germinant, although many species are unable to germinate rapidly in response to these without the addition of l-alanine (9).
The food-borne pathogen Bacillus cereus is a major cause of food poisoning of an emetic and diarrheal type (13, 16). The germination and growth of Bacillus cereus spores during food storage can lead to food spoilage and the potential to cause food poisoning (16). B. cereus has been shown to germinate in response to l-alanine and to ribosides (11, 18, 23). Spore germination can be triggered by l-alanine alone, but at high spore densities this response becomes inhibited by d-alanine, generated by the alanine racemase activity associated with the spores (8, 11). This auto-inhibition of l-alanine germination can be reduced by the inclusion of a racemase inhibitor (O-carbamyl-d-serine) with the germinating spores (11).
Inosine is the most effective riboside germinant for B. cereus T, while adenosine and guanosine are less potent (28). The rate of riboside-triggered germination has been reported to be enhanced dramatically by the addition of l-alanine (18). It is unclear whether ribosides can act as a sole germinant, or whether there is an absolute requirement for l-alanine (28).
An attempt has been made to analyze genetically the molecular components of the germination apparatus in B. cereus in order to dissect the germination responses of this species and to determine whether riboside-induced germination involves components related to those already described for amino acid and sugar germinants in B. subtilis.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains used in this study are listed in Table 1. B. cereus was routinely cultured in or on Oxoid nutrient broth and agar (NB and NA) containing the appropriate antibiotics (tetracycline at 50 μg ml−1 [NBTet] or erythromycin and lincomycin at 1 and 25 μg ml−1, respectively [NBEL]). CCY medium (25) was used for spore preparation.
TABLE 1.
Strain and plasmids used in this study
| Strain or plasmid | Relevant genotype and/ or phenotype | Source or reference |
|---|---|---|
| B. cereus strains | ||
| 569 UM20.1 | trp-1 Strr | 1a |
| 569 UM20.1/ pLTV1 | trp-1 Strr Tetr Cmr Eryr | This study |
| AM1310 | Tn917-LTV1::gerIB1 (ino-1) Eryrtrp-1 Strr | This study |
| AM1314 | Tn917-LTV1::gerIA5 (ino-5) Eryrtrp-1 Strr | This study |
| AM1316 | Tn917-LTV1::ger (ala-1) Eryrtrp-1 Strr | This study |
| Plasmids | ||
| pLTV1 | Cmr Eryr Tetr Ampr | 2 |
| pINO1 | Ampr | This study |
| pINO5 | Ampr | This study |
| pMOC6 | Ampr | This study |
The medium for culture of Escherichia coli strains was L broth (LB) or L agar, containing appropriate antibiotics. Plasmids used in this study are included in Table 1.
Spore preparation.
A culture of B. cereus in CCY broth (400 ml; inoculated with 10 ml of a mid-log-phase NB culture) was incubated with shaking, at 37°C for 2 days, until >90% free spores were present. Spores were harvested and washed 10 times by repeated centrifugation and resuspension in distilled water, discarding the upper layer of cellular debris in the pellet from early washing steps. The spores were stored at 8 to 10 mg (dry wt) ml of distilled water−1 at −20°C.
Spore germination assay.
Spores were heat activated in distilled water at 70°C for 30 min prior to germination. Spores were suspended in germination buffer (10 mM Tris-HCl [pH 7.4], 10 mM NaCl) at 0.05 mg (dry wt) ml−1. For l-alanine germination, 5 μg of O-carbamyl-d-serine ml−1 was added to inhibit alanine racemase activity (11). After 15 min of preincubation at 37°C, germination at 37°C was initiated by the addition of inosine (to 5 mM) or l-alanine (to 100 mM). The optical density at 580 nm (OD580) of the spore suspension was monitored continuously. The rate of germination is expressed as the maximum rate of loss of OD580 of the spore suspension, relative to the initial value.
Transposon mutagenesis.
A mid-log culture of B. cereus 569 UM20.1(pLTV1) in NBTet, generated by overnight incubation at 25°C, was diluted 25-fold into NBEL and incubated at 44°C; subculture at this temperature was repeated until the cells had grown through ca. 14 generations at this temperature. The cells were then harvested and resuspended in 25 ml of NB, and 10 ml was used to inoculate 400 ml of CCY broth for spore preparation. Because of the extended period of subculture at a selective temperature in liquid medium, it is not possible to quantitate the frequency of transposon insertion.
Identification of germination mutants.
Spore suspensions were enriched for germination mutants by a modification of methods used for B. subtilis (20). Briefly, washed spores were heat activated and diluted into 20 ml of germination buffer (10 mM Tris-HCl [pH 7.4], 10 mM NaCl) to give a spore density of 0.05 mg ml−1. For enrichment of either inosine or l-alanine germination mutants, inosine (0.3 mg ml−1) or l-alanine (4 mM) was added, respectively, and the spores were incubated for 1 h. Germinated spores were killed by addition of 3 drops of chloroform. The survivors were harvested, washed in distilled water, incubated at 37°C for 1 h in 1 ml of NB, and then inoculated into 50 ml of CCY broth, and the spores were prepared by the standard procedure for a second round of enrichment.
Potential mutants were recovered from enrichments as individual colonies on NA and were screened for germination phenotype by a modification of the tetrazolium colony transfer test (14). Spore-containing patches on CCY agar (produced by 2 days of incubation at 37°C) were transferred onto filter paper discs (Whatman no. 1), and placed colony side up on FTA agar (3% agar, 0.01% d-alanine). After incubation on this medium in a 65°C oven for 2 h, the filters were transferred colony side up onto germination agar plates and incubated at 37°C until color developed. Red patches indicated spore germination; white patches contained nongerminating spores. Germination agar was prepared from a 200-ml agar base (1.5% agar) containing 1.4% (wt/vol) K2HPO4 · 3H2O and 0.6% (wt/vol) KH2PO4 by the addition of 2 ml of the following 100 mg ml−1 solutions: l-malate, 2,3,5-triphenyltetrazolium chloride, and either l-alanine or inosine.
Phage transduction.
Phage CP51ts is a heat-sensitive derivative of generalized transducing phage CP51 (26). Methods of phage transduction were slightly modified from those of Thorne (26a). Indicator bacteria were cultured at 37°C in LB supplemented with 0.4% (wt/vol) glycerol and used as fresh mid-log-phase cultures. Phage CP51ts was stored as infected spores, and titers were determined on PA agar (26). The top agar was 0.7% PA, and incubation to plaque formation was at 30°C overnight. To prepare donor phage, five turbid plaques of CP51ts were resuspended together in PA broth, mixed with 500 μl of indicator bacteria, and allowed to adsorb at 30°C for 15 min before the addition of 3 ml of PA overlay and plating on NBY agar containing 0.4% glycerol (26), for overnight incubation at 30°C. The overlay was harvested and resuspended in 5 ml of PA broth, the agar and cell debris were pelleted from the phage lysate by centrifugation (15 min; 3,000 × g at room temperature), and the supernatant was filter sterilized. Magnesium sulfate was added to 20 mM, and the lysate was stored at 25°C.
For generalized transduction, mid-log-phase recipient cells (500 μl; grown at 37°C in LB) were incubated with 108 donor phage for 10 min at 43°C. The cells were then pelleted, resuspended in 500 μl of PA broth (43°C), and added to 2.5 ml of molten nutrient agar (Oxoid NB plus 1% [wt/vol] agar) prewarmed to 42°C containing erythromycin (0.4 μg/ml). The mixture was then poured onto a prewarmed (37°C) nutrient agar plate and incubated for 2 h at 43°C. Then, 2.5 ml of the same medium, this time containing erythromycin (1 μg ml−1) and lincomycin (25 μg ml−1), was overlaid. Transductants usually appeared after 24 h of incubation at 43°C.
Screening of B. cereus genomic library.
B. cereus 569 UM20.1 genomic DNA was prepared by the phenol extraction method of Errington (5). After partial digestion with Sau3A, fragments of 4 to 6 kb were gel separated, excised, purified with GeneClean II (Bio 101), and ligated with predigested λZAP Express BamHI-digested vector as recommended by the suppliers (Stratagene). The ligation products were packaged using Stratagene’s Gigapack II. About 20,000 plaques were screened by plaque blotting, probing, and detecting hybridization with the Digoxigenin DNA labelling and detection kit, as recommended by the manufacturer (Boehringer Mannheim).
Sequencing and analysis.
DNA sequencing was performed with the Taq DyeDeoxy Terminator cycle sequencing kit (Applied Biosystems) and an Applied Biosystems DNA sequencer. The DNA sequence was analyzed and assembled by using the Staden suite of programs (24).
Nucleotide sequence accession number.
The sequences described in this manuscript have been deposited in GenBank under accession no. AF067645.
RESULTS
Choice of strain.
B. cereus 569 was used for this work, as, in our experience, it was more stably transformable with plasmids than was B. cereus T. It is also a strain for which generalized transduction has been developed (26). It was necessary, therefore, to determine the germination characteristics of spores of this strain.
Kinetics of inosine- and l-alanine-initiated spore germination.
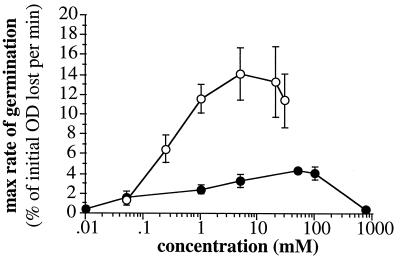
The progress of germination of spore suspensions, estimated as decrease in OD, was determined in either inosine or l-alanine in a standard buffer. The dependence of germination rate (as estimated by the rate of OD reduction, which reflects the distribution of germination times in the population) on germinant concentration was measured. Results are summarized in Fig. 1. Germination can be initiated by either l-alanine or inosine as the sole germinant, but the maximum rate of germination in inosine is at least threefold higher than that in alanine and is observed at a lower concentration of germinant (5 and 50 mM, respectively). Germination in inosine was rapid; 50% of the potential fall in OD occurred within 2.2 min, and 90% occurred within 6 min of the addition of the germinant. In l-alanine, 50% of the potential fall in OD was complete in 10 min, and 90% occurred within 32 min.
FIG. 1.
Concentration dependence of spore germination in response to l-alanine (solid circles) or inosine (open circles) in standard buffer conditions at 37°C. Values given are means (error bars represent standard deviations) of three independent experiments on the same spore preparation.
Even higher rates of germination could be achieved in the presence of both inosine and l-alanine. Preincubation in low concentrations of the second germinant, insufficient to induce rapid germination alone, stimulated germination (Table 2). When spores were preincubated and then germinated with the same germinant, no increase in the rate of germination was observed.
TABLE 2.
Stimulatory effects of subgerminal concentrations of alanine and inosine
| Germinant | Maximum rate of germination (% of initial OD lost per min) after preincubation witha:
|
||
|---|---|---|---|
| No addition | l-Alanine (10 μM) | Inosine (1 μM) | |
| None | 0 | 0 | 0 |
| l-Alanine (100 mM) | 7.6 ± 2.3 | 6.8 ± 0.8 | 38.6 ± 0.8 |
| Inosine (5 mM) | 30.6 ± 1.0 | 91.9 ± 4.7 | 32.6 ± 2.2 |
Preincubation was for 15 min before addition of the main germinant. Results are the means ± standard deviations of three independent experiments.
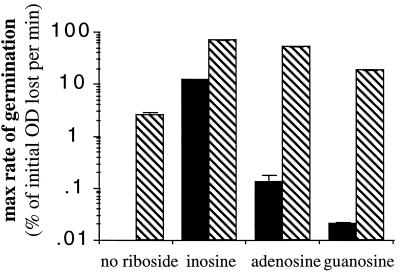
The response of spores to ribosides other than inosine was very slow (Fig. 2) unless l-alanine was also present. The presence of 1 mM l-alanine in combination with adenosine or guanosine increased the rate of germination; alanine and ribosides appear to act synergistically.
FIG. 2.
Effect of l-alanine on riboside germination response. Germination was initiated by the addition of 1 mM riboside (either inosine, guanosine, or adenosine), with (shaded bars) or without (solid bars) 1 mM l-alanine as indicated. Values given are means (error bars represent standard deviations) of three independent experiments.
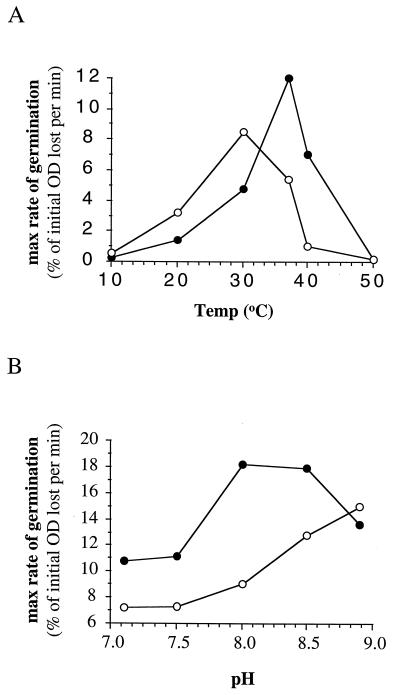
In order to define more-optimal conditions for germination in inosine or in alanine as individual germinants, the temperature of incubation (Fig. 3A) or the pH of the Tris-HCl buffer (Fig. 3B) was varied. The temperature and pH optima for inosine germination were 37°C and 8 to 8.5, respectively, while for l-alanine germination they were 30°C and >8.9, respectively. These differences suggest that either these germinants are detected by separate proteins in the spore or, possibly, by different binding sites in the same protein.
FIG. 3.
Effect of temperature (A) and pH (B) on the maximum rate of germination in inosine and l-alanine. Germination was initiated by the addition of either 5 mM inosine (solid circles) or 100 mM l-alanine (open circles).
The requirement for cations was explored (Table 3). In either alanine or inosine, the rate of OD loss was stimulated by sodium ions. As reported previously (23), ammonium ions stimulated alanine germination. Potassium ions were not stimulatory and in fact very strongly inhibited germination in inosine.
TABLE 3.
Effects of different cations on the germination response of wild-type spores
| Ion (10 mM)a | Maximum rate of germination (% of initial OD lost per min) in:
|
|
|---|---|---|
| l-Alanine (1 mM) | Inosine (0.3 mM) | |
| None | 0.7 ± 0.1 | 3.8 ± 0.4 |
| NaCl | 1.5 ± 0.2 | 14.8 ± 0.5 |
| KCl | 0.8 ± 0.2 | 0.07 ± 0.01 |
| CaCl2 | 1.5 ± 0.2 | 4.5 ± 0.3 |
| NH4Cl | 2.8 ± 0.4 | 2.4 ± 0.7 |
The salts indicated were added to the germination buffer.
Isolation of mutants defective in spore germination.
Independent pools of Tn917-LTV1 insertion mutants were enriched and then screened by a tetrazolium colony transfer assay for mutants defective in inosine or l-alanine spore germination. After two cycles of enrichment through spores, at least 10% of survivors were germination defective, although many were likely to be siblings. The phenotypes of representative mutants were characterized further by germination assays on washed spore suspensions (Table 4). The AM1310 (ino-1) and AM1314 (ino-5) Tn insertions totally abolished the ability of the spores to germinate in response to inosine as the sole germinant; germination in inosine could be initiated if the spores were preincubated with a subgerminal concentration of l-alanine prior to the addition of inosine, but the rate of response was reduced at least 60-fold compared to that of the wild type. The ino-1 and ino-5 Tn insertions also affected l-alanine-initiated germination, reducing the response by around threefold. The stimulating effect on alanine germination of a subgerminal concentration of inosine remained strong, despite essentially complete loss of the germination response to this chemical as the sole germinant.
TABLE 4.
Responses of mutant spores to germinants
| Germinant(s) | Maximum rate of germination (% of initial OD lost per min)
|
|||
|---|---|---|---|---|
| Parental strain | ino-1 | ino-5 | ala-1 | |
| 0.3 mM inosine | 8.0 | <0.01 | <0.01 | 7.8 |
| 0.3 mM inosine–0.01 mM l-alanine | 41 | 0.7 | 0.1 | 39.8 |
| 1 mM l-alanine | 2.4 | 0.6 | 0.8 | 0.8 |
| 1 mM l-alanine–0.01 mM inosine | 26.7 | 10.0 | 11.1 | 17.2 |
In contrast, the ala-1 Tn insertion resulted in an approximately threefold reduction in the maximum rate of germination in response to l-alanine compared to the wild-type strain. The maximum rate of inosine germination was unaffected by the ala-1 mutation. These data, like the physiological analysis, suggest that germination receptors exist for the separate detection of inosine and l-alanine. That represented by the ala-1 mutation is alanine specific, whereas that represented by ino-1 and ino-5 mutations can contribute to both alanine and inosine germination. This ino locus is not, however, essential for stimulation of alanine germination by a subgerminal concentration of inosine. Southern analysis and sequence studies (2a) have revealed that the ala-1 mutation lies in a separate genetic locus from that in which ino-1 and ino-5 insertions have previously been shown to occur.
ino mutations.
Any process involving separate mutagenesis and enrichment regimens is likely to give rise to some strains carrying multiple mutations. Generalized transduction with phage CP51ts was used to determine the linkage of germination defects in the mutants to the resistance marker of the transposon. This phage is extremely lytic at 30°C, but it contains a mutation which prevents phage replication at temperatures above 40°C, allowing us to perform inductions and selections in an overlay at an elevated temperature in order to select erythromycin- and/or lincomycin-resistant transductants. The Ger phenotype of transductants (100 from each cross) was assessed by the tetrazolium colony transfer test. The Ger phenotypes of several transductants from each experiment were then confirmed by preparing washed spores and measuring the rate of fall in the OD580 of the spore suspension upon addition of germinant. In the case of ino-1 and ino-5, all of the transductants had phenotypes identical to the corresponding original mutant, confirming 100% linkage of the germination defect to the transposon insertion. Of six ino mutants tested in total, in two cases the ger defect was not linked to the site of transposon insertion, confirming the importance of such tests.
Cloning and sequencing of gerI operon.
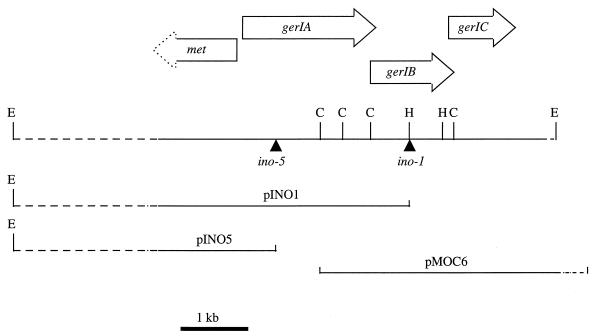
Mapping of ino-1 and ino-5 Tn insertions by Southern blot analysis revealed that the insertions were located 2.2 kb apart on a 9-kb chromosomal EcoRI fragment, therefore defining this region of the chromosome as being essential for inosine germination. EcoRI restriction of chromosomal DNA from AM1310 (ino-1) and AM1314 (ino-5) followed by ligation and transformation of E. coli allowed recovery of chromosomal DNA extending to an EcoRI site from the site of insertion as part of the plasmids recovered from the transposon sequence (2); these plasmids were named pINO1 and pINO5, respectively. The chromosomal DNA fragments present on pINO1 and pINO5 overlapped, spanning a 5.9-kb region of the wild-type EcoRI fragment (Fig. 4). A clone carrying DNA downstream of the ino-5 insertion was obtained by probing a B. cereus genomic λZapExpress library with a 1-kb ClaI/HindIII fragment purified from pINO1. The insert was recovered from this clone as a phagemid (pMOC6) which contained a 4-kb fragment of chromosomal DNA, overlapping the pINO1 insert and extending the cloned region by 2 kb downstream of the ino-1 Tn insertion.
FIG. 4.
Map and cloning strategy for characterization of the gerI operon. The arrows indicate the positions of the genes identified within the sequenced region. Restriction sites for EcoRI (E), ClaI (C), and HindIII (H) and sites of transposon insertion are indicated. The precise sites of insertion are bases 1412 and 3634 in the GenBank sequence. The extent of cloned inserts in plasmids pINO1 and pINO5, recovered from the chromosome of the transposon insertion mutants, and that in plasmid pMOC6, isolated from a B. cereus genomic library, are indicated. Dotted lines mark the unsequenced regions, which are not shown to scale.
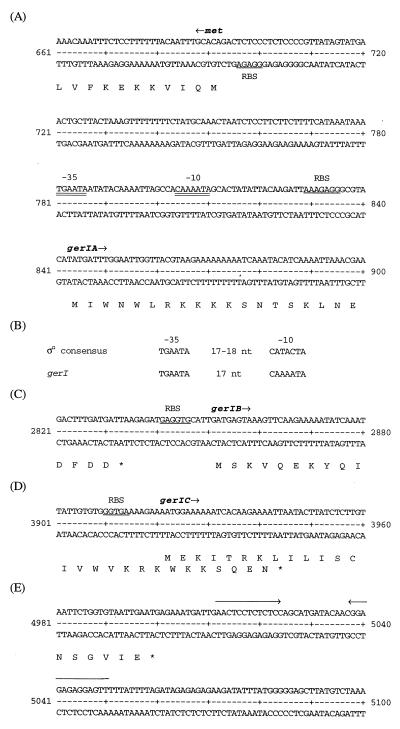
Over 5 kb of this region was sequenced fully on both strands, and contained four open reading frames (ORFs) (Fig. 4 and 5). The ino-1 and ino-5 Tn insertions were located in an operon homologous to the B. subtilis gerA family of germination operons (3, 15, 17, 33, 34). The ino-5 Tn insertion disrupts ORF2 (named gerIA), which encodes a putative 664-amino-acid polypeptide with a predicted molecular mass of 75.3 kDa. This polypeptide would be homologous to gerAA, but it contains an atypical N-terminal extension of about 160 residues. A distinctive feature of this extension is a 13-residue repeat which is repeated nine times and when translated gives a domain which is charged and relatively glutamine rich (Fig. 6). The Tn insertion ino-1 disrupted a gene (ORF3; gerIB) encoding a putative 365-amino-acid polypeptide with a predicted molecular mass of 41.9 kDa, homologous to GerAB. ORF4 (gerIC) encodes a 360-amino-acid polypeptide with a predicted molecular mass of 40.6 kDa. A third transposon insertion mutation conferring an inosine germination defect (ino-89) is located in this ORF (1). The incomplete ORF1 is upstream from gerIA and transcribed from the complementary strand; it encodes a homologue of homoserine O-acetyltransferase, involved in methionine biosynthesis (32).
FIG. 5.
Features of the gerI sequence. (A) Predicted start codon and ribosome binding sites (RBS) for the met and gerIA genes and a putative ςG-dependent promoter; (B) comparison of the ςG promoter consensus sequence to the potential gerI promoter sequence; (C) coding region and potential RBS at the start of gerIB; (D) coding region and potential RBS at the start of gerIC; (E) predicted stop codon of gerIC and a potential rho-independent transcription terminator.
FIG. 6.
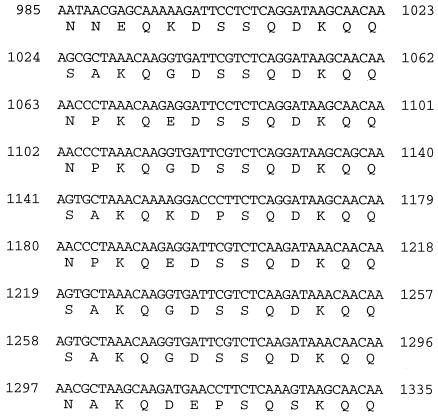
Repeated region at the start of the gerIA gene. The nine repeats of 39 bp and 13 amino acids are shown. The number at the start of each nucleotide sequence refers to the position on the GenBank sequence.
The predicted promoter, transcriptional terminator, and translation initiation signals of this region are shown in Fig. 5. A 153-bp intergenic region between the divergently transcribed orf-1 and gerIA genes contains, just upstream of the predicted gerIA ORF, a putative promoter closely matching the consensus sequence of promoters recognized by the forespore-specific ςG (7). The three gerI ORFs are closely coupled, with an overlap in the coding sequences of gerIB and gerIC. A potential rho-independent transcription terminator is located downstream of the stop codon of gerIC. These features suggest that the gerI genes are transcribed as a tricistronic operon.
DISCUSSION
B. cereus 569 spores can respond relatively independently to inosine or l-alanine, although maximal rates of germination were achieved only in a mixture of both. For germination in response to ribosides other than inosine, i.e., adenosine and guanosine, significant rates of germination were achieved only when l-alanine was added, as previously demonstrated for B. cereus T (28). The second component was required only at a very low concentration, below a level that would induce germination as a sole germinant.
Marked differences in the optimal pH and temperature profiles and in the effects of monovalent cations on l-alanine or inosine germination suggest that there are different germinant-specific signal transduction mechanisms present in the spore. This is supported by the mutational evidence of separate ala and ino loci. An inosine locus, gerI, is described in detail.
It proved possible to use as a mutagen in B. cereus the modified Tn917 derivative LTV1, which allows cloning of flanking sequences by religation of junction fragments containing transposon-derived plasmid functions. As the upper growth temperature limit of B. cereus was close to that required for inhibition of replication of the delivery plasmid, cycling was necessary and the libraries constructed were relatively small.
Despite the loss of a component apparently essential for inosine germination, incubation of the gerI mutants with subgerminal concentrations of inosine still led to enhanced rates of germination in alanine. This effect is therefore not due to synergistic function of an alanine receptor with a GerI protein. Equally, there is still a residual low germination rate in inosine when subgerminal alanine is added, suggesting that there are other inosine-sensitive components in the germination apparatus.
The existence of separate germination responses for different germinants is not unique to B. cereus, since it has also been well characterized in B. subtilis (2, 14, 20). The germinative combination AGFK in B. subtilis requires the function of the proteins encoded by both gerB and gerK operons. In B. subtilis, the gerA operon is the only one found to be important specifically for alanine germination; the situation for alanine germination in B. cereus is more complex, with the alanine response contributed by two systems, one of which involves the gerI inosine receptor. It is possible that the products of two loci, gerI and another, are both required in B. cereus for the response to a single germinant inosine, as another ino defect results from insertion in a second locus (2b). We cannot yet define whether the need for multiple GerA-like homologues in a germination response reflects a physical association of homologous “receptors” or the simultaneous stimulation of several separate complexes.
The presence of a potential promoter sequence for the sporulation-specific E · ςG form of RNA polymerase suggests that transcription of this operon may occur in the forespore, in the same fashion as the gerA and gerB operons of B. subtilis (4, 7).
The N termini of the GerAA homologues are quite variable: GerKA and the clostridial GerAA homologue contain an extension of ca. 30 residues, charged and amide rich. The N-terminal domain of GerIA is, however, particularly unusual as it is much longer and contains three perfect repeats and six almost-perfect tandem repeats of 13 residues; the DNA sequence shows the corresponding multiple tandem repeat of 39 bases, which are relatively conserved. The repeats are particularly glutamine rich and are reminiscent of Q-linkers (31). Q-linkers are sequences which link two different domains of a protein and are commonly found in two-component regulatory and signal transduction systems. Glutamine-rich regions, like proline-rich regions, may tend to form extended, conformationally restricted, polypeptide chain structures (30). The role of this N-terminal extension may therefore be to aid binding of the GerIA protein to some other component of the germinant apparatus or to some structural element in the spore.
There is now a growing family of GerA homologues in B. subtilis. GerB and GerK are involved in the AGFK germination response (3, 15), and more recently two other homologous operons, the yfkQRT operon (33) and the yndDEF operon (17), have been identified as part of the B. subtilis Genome Sequencing project. The yfk and ynd gene products could be involved in germination in response to as yet unidentified germinants. It appears, therefore, that the gerA family of genes have evolved by gene duplication and subsequent divergence, giving rise to receptors with different germinant specificity.
This study demonstrates that B. cereus, too, has at least one ger locus homologous to the gerA family of B. subtilis and that the germination response to ribosides requires proteins of this family. It is possible that related proteins are also involved in germination of the anaerobic spore formers; homologues of GerAA and GerAC, but not GerAB, have been identified as encoded in a bicistronic operon in Clostridium pasteurianum, although the role of these putative ger genes has not been proven (19). The basic mechanism of recognition of germinant by the spore, reflected in the involvement of separate homologous operons, each encoding coevolving proteins that are not functionally interchangeable, may be conserved among all bacterial endospore formers.
ACKNOWLEDGMENTS
This work was funded by the AFRC and BBSRC.
We thank E. Helen Kemp for her help and advice at early stages of this work and C. Thorne for the donation of B. cereus strains and phage and his generous help with the development of the phage protocols.
REFERENCES
- 1.Barlass, P. J., J. Butten, and A. Moir. Unpublished data.
- 1a.Battisti L, Green B D, Thorne C B. Mating system for transfer of plasmids among Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. J Bacteriol. 1985;162:543–550. doi: 10.1128/jb.162.2.543-550.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camilli A, Portnoy D A, Youngman P. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J Bacteriol. 1990;172:3738–3744. doi: 10.1128/jb.172.7.3738-3744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Clements, M. O., P. J. Barlass, and A. Moir. Unpublished data.
- 2b.Clements, M. O., P. J. Barlass, C. Houston, and A. Moir. Unpublished data.
- 3.Corfe B M, Sammons R L, Smith D A, Mauel C. The gerB region of the Bacillus subtilis 168 chromosome encodes a homologue of the gerA spore germination operon. Microbiology. 1994;140:471–478. doi: 10.1099/00221287-140-3-471. [DOI] [PubMed] [Google Scholar]
- 4.Corfe B M, Moir A, Popham D, Setlow P. Analysis of the expression and regulation of the gerB spore germination operon of Bacillus subtilis 168. Microbiology. 1994;140:3079–3083. doi: 10.1099/13500872-140-11-3079. [DOI] [PubMed] [Google Scholar]
- 5.Errington J. Efficient Bacillus subtilis cloning system using bacteriophage vector Φ-105J9. J Gen Microbiol. 1984;130:2615–2628. doi: 10.1099/00221287-130-10-2615. [DOI] [PubMed] [Google Scholar]
- 6.Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feavers I M, Foulkes J, Setlow J B, Sun D, Nicholson W, Setlow P, Moir A. The regulation of transcription of the gerA spore germination operon of Bacillus subtilis. Mol Microbiol. 1990;4:275–282. doi: 10.1111/j.1365-2958.1990.tb00594.x. [DOI] [PubMed] [Google Scholar]
- 8.Fey C, Gould G W, Hitchins A D. Identification of d-alanine as the auto-inhibitor of germination of Bacillus globigii spores. J Gen Microbiol. 1964;35:229–236. doi: 10.1099/00221287-35-2-229. [DOI] [PubMed] [Google Scholar]
- 9.Foerster H F, Foster J W. Response of Bacillus spores to combinations of germinative compounds. J Bacteriol. 1966;91:1168–1177. doi: 10.1128/jb.91.3.1168-1177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster S J, Johnstone K. Pulling the trigger: the mechanism of bacterial spore germination. Mol Microbiol. 1990;4:137–141. doi: 10.1111/j.1365-2958.1990.tb02023.x. [DOI] [PubMed] [Google Scholar]
- 11.Gould G W. Stimulation of l-alanine-induced germination of Bacillus cereus spores by d-cycloserine and O-carbamyl-d-serine. J Bacteriol. 1966;92:1261–1262. doi: 10.1128/jb.92.4.1261-1262.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gould G W, Dring G J. Biochemical mechanisms of spore germination. In: Halvorson H O, Hanson R, Campbell L L, editors. Spores V. Washington, D.C: American Society for Microbiology; 1972. pp. 401–408. [Google Scholar]
- 13.Granum P E. Bacillus cereus and its toxins. J Appl Bacteriol. 1994;76:61s–66s. [PubMed] [Google Scholar]
- 14.Irie R, Okamoto T, Fujita Y. A germination mutant of Bacillus subtilis deficient in response to glucose. J Gen Appl Microbiol. 1982;28:345–354. [Google Scholar]
- 15.Irie R, Fujita Y, Kobayashi M. Nucleotide sequence and gene organisation of the gerK spore germination locus of Bacillus subtilis 168. J Gen Appl Microbiol. 1996;42:141–153. [Google Scholar]
- 16.Kramer J M, Gilbert R J. Bacillus cereus and other Bacillus species. In: Doyle M P, editor. Foodborne bacterial pathogens. New York, N.Y: Marcel Dekker; 1989. pp. 21–70. [Google Scholar]
- 17.Kunst F, Ogasawara N, Moszer I, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence N L. The relationship between the cleavage of purine ribosides by bacterial spores and the germination of the spores. J Bacteriol. 1955;70:583–587. doi: 10.1128/jb.70.5.583-587.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer J. Sequence of a 10.5 kbp fragment of Clostridium pasteurianum genomic DNA encompassing the hydrogenase-I gene and 2 spore germination genes. Anaerobe. 1995;1:169–174. doi: 10.1006/anae.1995.1015. [DOI] [PubMed] [Google Scholar]
- 20.Moir A, Lafferty E, Smith D A. Genetic analysis of spore germination mutants of Bacillus subtilis 168: the correction of phenotype with map location. J Gen Microbiol. 1979;111:165–180. doi: 10.1099/00221287-111-1-165. [DOI] [PubMed] [Google Scholar]
- 21.Moir A, Smith D A. The genetics of bacterial spore germination. Annu Rev Microbiol. 1990;44:531–553. doi: 10.1146/annurev.mi.44.100190.002531. [DOI] [PubMed] [Google Scholar]
- 22.Moriyama R, Kudoh S, Miyata S, Nonobe S, Hattori A, Makino S. A germination-specific spore cortex-lytic enzyme from Bacillus cereus spores: cloning and sequencing of the gene and molecular characterization of the enzyme. J Bacteriol. 1996;178:5330–5332. doi: 10.1128/jb.178.17.5330-5332.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preston R A, Douthit H A. Functional relationships between l-alanine and d-alanine, inosine and NH4Cl during germination of spores of Bacillus cereus T. J Gen Microbiol. 1988;134:3001–3010. doi: 10.1099/00221287-134-11-3001. [DOI] [PubMed] [Google Scholar]
- 24.Staden R. Finding protein coding regions in genomic sequences. Methods Enzymol. 1990;183:163–180. doi: 10.1016/0076-6879(90)83012-x. [DOI] [PubMed] [Google Scholar]
- 25.Stewart G S A B, Johnstone K, Hagelberg E, Ellar D J. Commitment of bacterial spores to germinate: a measure of the trigger reaction. Biochem J. 1981;198:101–106. doi: 10.1042/bj1980101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorne C B. Transducing bacteriophage for Bacillus cereus. J Virol. 1968;2:657–662. doi: 10.1128/jvi.2.7.657-662.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Thorne, C. B. Personal communication.
- 27.Trowsdale J, Smith D A. Isolation, characterization and mapping of Bacillus subtilis 168 germination mutants. J Bacteriol. 1975;123:83–95. doi: 10.1128/jb.123.1.83-95.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren S C, Gould G W. Bacillus cereus spore germination: absolute requirement for an amino acid. Biochim Biophys Acta. 1968;170:341–350. doi: 10.1016/0304-4165(68)90014-7. [DOI] [PubMed] [Google Scholar]
- 29.Wax R, Freese E. Initiation of the germination of Bacillus subtilis spores by a combination of compounds in place of l-alanine. J Bacteriol. 1968;95:433–438. doi: 10.1128/jb.95.2.433-438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson M P. The structure and function of proline-rich regions in proteins. Biochem J. 1994;297:249–260. doi: 10.1042/bj2970249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wooton J C, Drummond M H. The Q-linker: a class of interdomain sequences found in bacterial multidomain regulatory proteins. Protein Eng. 1989;2:535–543. doi: 10.1093/protein/2.7.535. [DOI] [PubMed] [Google Scholar]
- 32.Wyman A, Paulus H. Purification and properties of homoserine transacetylases from Bacillus polymyxa. J Biol Chem. 1975;250:3897–3903. [PubMed] [Google Scholar]
- 33.Yamamoto H, Uchiyama S, Nugroho F A, Sekiguchi J. Cloning and sequencing of a 35.7 kb in the 70°–73° region of the Bacillus subtilis genome reveal genes for a new two-component system, three spore germination proteins, an iron uptake system and a general stress response protein. Gene. 1997;194:191–199. doi: 10.1016/s0378-1119(97)00130-3. [DOI] [PubMed] [Google Scholar]
- 34.Zuberi A R, Moir A, Feavers I M. The nucleotide sequence and gene organisation of the gerA spore germination operon of Bacillus subtilis 168. Gene. 1987;51:1–11. doi: 10.1016/0378-1119(87)90468-9. [DOI] [PubMed] [Google Scholar]