Abstract
Simple Summary
Gut microbiota is a key regulators of the efficacy of immune checkpoint inhibitor (ICIs). Thus, manipulating microbiota may enhance cancer treatment outcomes. Clostridium butyricum MIYAIRI 588 strain (CBM588) enhances the effects of ICI monotherapy in patients with advanced lung cancer. However, its effect on the outcomes of chemoimmunotherapy combinations in lung cancer remains unknown. We retrospectively analyzed 106 patients with stage IV or recurrent metastatic non-small cell lung cancer (NSCLC) consecutively treated with chemoimmunotherapy combination. CBM588 significantly extended the overall survival in patients with NSCLC receiving chemoimmunotherapy combinations and was associated with overall survival in patients using proton pump inhibitors and/or antibiotics. CBM588-induced manipulation of commensal microbiota has the potential to enhance the efficacy of chemoimmunotherapy combinations. The survival benefit of CBM588 in the tumor-programmed cell death ligand 1 (PD-L1) < 1% cohort was higher than that in the PD-L1 1–49% and PD-L1 ≥ 50% cohorts. Further exploration of the synergy between CBM588 and immunotherapy is warranted.
Abstract
The gut microbiota has emerged as a key regulator of immune checkpoint inhibitor (ICI) efficacy. Therapeutic approaches aimed at manipulating the microbiota through targeted reconstitution to enhance cancer treatment outcomes have garnered considerable attention. A single live microbial biotherapeutic bacterium, Clostridium butyricum MIYAIRI 588 strain (CBM588), has been shown to enhance the effects of ICI monotherapy in patients with advanced lung cancer. However, whether CBM588 affects the outcomes of chemoimmunotherapy combinations in lung cancer remains unknown. We hypothesized that CBM588 augments the effect of chemoimmunotherapy combinations and restores diminished effectiveness in patients with non-small cell lung cancer (NSCLC) receiving dysbiosis-inducing drugs. To validate this hypothesis, we retrospectively analyzed 106 patients with stage IV or recurrent metastatic NSCLC consecutively treated with chemoimmunotherapy combinations. A survival analysis was performed employing univariate and multivariate Cox proportional hazard models with inverse probability of treatment weighting (IPTW) using propensity scores. Forty-five percent of patients received Clostridium butyricum therapy. CBM588 significantly extended overall survival in patients with NSCLC receiving chemoimmunotherapy. The favorable impact of CBM588 on the efficacy of chemoimmunotherapy combinations varied based on tumor-programmed cell death ligand 1 (PD-L1) expression. The survival benefit of CBM588 in the PD-L1 <1% cohort was higher than that in the PD-L1 1–49% and PD-L1 ≥ 50% cohorts. Furthermore, CBM588 was associated with improved overall survival in patients receiving proton pump inhibitors and/or antibiotics. CBM588-induced manipulation of the commensal microbiota holds the potential to enhance the efficacy of chemoimmunotherapy combinations, warranting further exploration of the synergy between CBM588 and immunotherapy.
Keywords: antibiotics, CBM588, dysbiosis, gut microbiome, immune checkpoint inhibitor, Clostridium butyricum, lung cancer, proton pump inhibitors
1. Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of advanced lung cancer [1]. Current therapeutic options for advanced non-small cell lung cancer (NSCLC) include ICI monotherapy and the combination of ICIs with platinum-containing chemotherapy, known as chemoimmunotherapy combinations (https://www.nccn.org/guidelines, accessed on 7 December 2023) [2,3]. As the synergistic efficacy of chemoimmunotherapy combinations has been shown to be beneficial in advanced cases, these combinations have become as prevalent as ICI monotherapy. Clinical guidelines now recommend chemoimmunotherapy combinations as the standard first-line treatment for NSCLC, irrespective of the expression of tumor-programmed cell death ligand 1 (PD-L1), which is a predictive biomarker in NSCLC [2,3]. Despite the availability of these treatments, their effectiveness has been limited to a subset of patients, highlighting the necessity for improved strategies aimed at reinvigorating the antitumor immune response facilitated by ICIs [4,5,6].
The gut microbiota has emerged as a key regulator of the therapeutic efficacy of ICIs [5,6,7,8,9,10]. Medication significantly influences the structure of the gut microbiome, and its overuse is associated with increased morbidity and mortality [11,12,13]. Proton pump inhibitors (PPIs) and antibiotics are frequently administered concomitantly with cancer treatment and compromise the effectiveness of ICIs, potentially by inducing gut dysbiosis [14,15,16,17,18]. These findings have prompted interest in targeting the gut microbiota to enhance ICI efficacy. Notably, accumulating clinical evidence suggests that manipulating the gut microbiota through fecal microbiota transplantation (FMT) using stools from ICI-responsive patients enables patients with melanoma to overcome primary resistance to ICIs [19,20]. These findings lend support to therapeutic strategies that target the gut microbiota through controlled reconstitution to enhance clinical outcomes in cancer immunotherapy [5,9,21].
Dizman et al. reported that a single microbial, live biotherapeutic bacterium, Clostridium butyricum MIYAIRI 588 strain (CBM588, MIYA-BM®), modulated the gut microbiome and enhance the clinical benefits of ICIs in a randomized phase IB clinical trial comparing nivolumab/ipilimumab with or without CBM588 in patients with advanced renal cell carcinoma (NCT03829111), suggesting the potential benefits of combining CBM588 with ICIs [22]. Moreover, we previously reported that patients receiving CBM588 exhibited a reduced relative abundance of potentially harmful oral bacteria in the gut and that CBM588 could potentially enhance the efficacy of ICI monotherapy in patients with advanced NSCLC [18,23]. We revealed that the administration of CBM588, especially in patients with advanced lung cancer undergoing PPI and/or antibiotic treatments, substantially restored the efficacy of ICIs and improved patient survival in a cohort primarily (>90%) receiving ICI monotherapy [18,23]. However, the impact of CBM588 on the outcomes of chemoimmunotherapy combinations, especially in the context of patients with advanced lung cancer, remains unexplored. In addition, post hoc analyses of randomized trials demonstrated the negative impact of antibiotic exposure in patients treated with ICI monotherapy compared with those in the control arm receiving conventional chemotherapy [17,24]. However, antibiotic exposure did not adversely affect clinical outcomes in patients receiving chemoimmunotherapy combinations for NSCLC [25], confirming that the detrimental effect of antibiotic exposure is limited to ICI monotherapy. Conversely, post hoc analyses of randomized clinical trials indicated that PPIs negatively influenced the magnitude of ICI efficacy in both ICI monotherapy and chemoimmunotherapy combinations [14,26,27]. These findings raise questions about whether the impact of the live bacterial product CBM588 on the efficacy of chemoimmunotherapy combinations differs from its impact on ICI monotherapy.
While CBM588 reportedly modulates the gut microbiome and enhances the clinical benefits of ICI monotherapy and dual ICIs in patients with advanced cancers, its potential influence on the outcomes of chemoimmunotherapy combinations in lung cancer remains unexplored. We hypothesized that CBM588 amplifies the efficacy of chemoimmunotherapy combinations and restores their diminished effectiveness in patients with NSCLC receiving dysbiosis-inducing drugs through the modulation of the gut microbiota. Accordingly, we conducted a retrospective study on patients with NSCLC to assess the impact of CBM588 on chemoimmunotherapy outcomes as well as its potential to restore treatment effectiveness. This study could provide valuable insights into enhancing the efficacy of chemoimmunotherapy and improving patient outcomes.
2. Materials and Methods
2.1. Patients
Patients were eligible for inclusion in the study if they had stage IV or recurrent metastatic NSCLC classified according to Tumor–Node–Metastasis (TNM) staging classification of lung cancer, ver. 8. We conducted a retrospective analysis of 106 patients with stage IV or recurrent metastatic NSCLC who were consecutively treated with chemoimmunotherapy combinations in a routine clinical setting at Kumamoto University Hospital from 1 January 2019, to 28 July 2022. Forty-nine patients received pembrolizumab (200 mg intravenously every 3 weeks) in combination with carboplatin (area under the curve 5 mg/mL per min every 3 weeks) plus pemetrexed (500 mg/m2 intravenously every 3 weeks). Twenty-six patients received pembrolizumab (200 mg intravenously every 3 weeks) in combination with carboplatin (area under the curve 6 mg/mL per min every 3 weeks) plus nab-paclitaxel (100 mg/m2 intravenously every week). Eleven patients received atezolizumab (1200 mg intravenously every 3 weeks) in combination with bevacizumab (15 mg/kg intravenously every 3 weeks) plus paclitaxel (175–200 mg/m2 intravenously every 3 weeks) plus carboplatin (area under the curve 6 mg/mL per min every 3 weeks). Four patients received atezolizumab (1200 mg intravenously every 3 weeks) in combination with carboplatin (area under the curve 6 mg/mL per min every 3 weeks) plus nab-paclitaxel (100 mg/m2 intravenously every week). Ten patients received nivolumab (360 mg intravenously every 3 weeks) plus ipilimumab (1 mg/kg intravenously every 6 weeks) in combination with platinum doublet chemotherapy. Six patients were excluded owing to insufficient clinical information at the onset of ICI treatment or an uncertain lung cancer diagnosis. The study cohort comprised 72 men and 28 women, with a median age of 67 years (interquartile range: 38–71). All patients received ICI therapy (anti-PD-(L)1 antibody) in combination with chemotherapy. Medical records were reviewed, and treatment continued until disease progression, undesirable toxicity, or consent withdrawal. We extracted the following data from the database: date, treatment details, age, sex, histology, PD-L1 status, initial diagnosis stage, Eastern Cooperative Oncology Group (ECOG) performance status (PS), smoking status, driver mutations, liver metastases, oral or intravenous PPI use within 30 days before and after the initiation of chemoimmunotherapy (for >3 weeks during immunotherapy), oral or intravenous antibiotic use within 60 days before starting chemoimmunotherapy, and C. butyricum therapy using CBM588 (MIYA-BM®, Miyarisan Pharmaceutical Co., Ltd., Tokyo, Japan) prescribed within 3 weeks before initiating chemoimmunotherapy and/or concurrently with chemoimmunotherapy until cessation [18,23]. Time frames for PPI use, antibiotic use, and C. butyricum therapy were determined based on prior analyses [14,15,17,18,23]. In the non-CBM588 group, three patients received probiotic therapy using Bifidobacterium (BIOFERMIN TABLETS®) during the administration of chemoimmunotherapy combinations. In the CBM588 group, the patients did not receive live bacterial product other than CBM588 during the administration of chemoimmunotherapy combinations. Patient characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of patients with advanced NSCLC receiving chemoimmunotherapy combinations.
| Total n = 100 |
CBM588 n = 45 |
No CBM588 n = 55 |
p-Value (CBM588 vs. No CBM588) |
|
|---|---|---|---|---|
| Median age (IQR) | 67.0 (63.0–71.0) | 67.0 (64.5–70.5) | 66.0 (61.0–72.0) | 0.57 |
| Sex, n (%) | ||||
| Male | 72 (72) | 33 (73) | 39 (71) | 0.83 |
| Female | 28 (28) | 12 (27) | 16 (29) | |
| ECOG performance status, n (%) | ||||
| 0–1 | 90 (90) | 38 (84) | 52 (95) | 0.11 |
| 2–4 | 10 (10) | 7 (16) | 3 (5) | |
| Smoking history, n (%) | ||||
| Current/Former | 79 (79) | 39 (87) | 40 (73) | 0.14 |
| Never | 21 (21) | 6 (13) | 15 (27) | |
| Stage at initial diagnosis, n (%) | ||||
| I–III | 32 (32) | 13 (29) | 19 (35) | 0.67 |
| IV | 68 (68) | 32 (71) | 36 (65) | |
| Histology, n (%) | ||||
| Adenocarcinoma | 66 (66) | 27 (60) | 39 (71) | 0.29 |
| Squamous/others | 34 (34) | 18 (12/6, 40%) | 16 (12/4, 29%) | |
| EGFR/ALK mutation status, n (%) | ||||
| Wild-type | 73 (73) | 34 (76) | 39 (71) | 0.015 |
| Mutant | 11 (11) | 1 (2) | 10 (18) | |
| Unknown | 16 (16) | 10 (22) | 6 (11) | |
| PD-L1 status, n (%) | ||||
| TPS ≥ 50% | 35 (35) | 17 (38) | 18 (33) | 0.85 |
| TPS 1–49% | 32 (32) | 13 (29) | 19 (35) | |
| TPS < 1% | 27 (27) | 13 (29) | 14 (25) | |
| Unknown/Undeterminable | 6 (6) | 2 (4) | 4 (7) | |
| Therapy line, n (%) | ||||
| 1st line | 85 (85) | 41 (91) | 44 (80) | 0.16 |
| ≥2nd line | 15 (15) | 4 (9) | 11 (20) | |
| Immune checkpoint inhibitor, n (%) | ||||
| Pembrolizumab | 75 (75) | 33 (73) | 42 (76.4) | 0.08 |
| Atezolizumab | 15 (15) | 5 (11) | 10 (18.2) | |
| Nivolumab + Ipilimumab | 10 (10) | 7 (16) | 3 (5.4) | |
| Liver metastasis, n (%) | ||||
| Yes | 12 (12) | 7 (16) | 5 (9) | 0.37 |
| No | 88 (88) | 38 (84) | 50 (91) | |
| Antibiotic use, n (%) | ||||
| Yes | 46 (46) | 23 (51) | 23 (42) | 0.42 |
| No | 53 (54) | 22 (49) | 32 (58) | |
| PPI use, n (%) | ||||
| Yes | 55 (55) | 21 (47) | 34 (62) | 0.16 |
| No | 45 (45) | 24 (53) | 21 (38) |
Abbreviations: NSCLC, non-small cell lung cancer; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; n, number; PD-L1, programmed cell death ligand 1; TPS, tumor proportion score; PPI, proton pump inhibitor.
Histories of C. butyricum therapy, PPI use, and antibiotic use were extracted from a prescription database and manually cross-referenced with medical records. The PD-L1 tumor proportion score (TPS) was determined through PD-L1 immunohistochemistry (clone 22C3; Dako North America, Inc., Carpinteria, CA, USA).
2.2. Statistical Analysis
Patient characteristics were delineated based on CBM588 administration status and compared using Fisher’s exact test and the Wilcoxon rank-sum test for categorical and continuous data, respectively. Progression-free survival (PFS) and overall survival (OS) were assessed using the Kaplan–Meier method, with differences determined via two-tailed log-rank tests. PFS was measured from the date the chemoimmunotherapy combination was started to the date of documented progression or death from any cause. OS was calculated from the date of initiation of the chemoimmunotherapy combination to death from any cause or last follow-up, with a data cutoff date of April 7, 2023. The median (IQR) follow-up durations were 14.5 (8.0–27.3) and 5.5 (3.0–14.0) months for PFS and OS, respectively. Univariate Cox proportional hazard analyses using sex, age, ECOG PS, histology, smoking history, tumor PD-L1 status, initial stage, therapy line, driver mutation status, liver metastasis, antibiotic use, PPI use, and C. butyricum therapy using CBM588 were employed to estimate the hazard ratio (HR) and 95% confidence interval (CI). Covariate selection was based on the results of univariate Cox proportional hazard analyses, and a multivariable Cox proportional hazard analysis was conducted. Apart from these methods, survival analysis was also conducted using univariate and multivariate Cox proportional hazards regression models incorporating propensity scores to mitigate potential confounding factors affecting treatment assignment. Propensity score adjustment included sex, age, smoking history, ECOG PS, histology, tumor PD-L1 status, driver mutation status, initial stage, therapy line, liver metastasis, antibiotic use, PPI use, and C. butyricum therapy using CBM588, with each factor categorized as presented in Table 1. Propensity score adjustment helps preserve statistical power by reducing covariates into a single variable. To evaluate the adjusted effect of CBM588 or PPI, propensity scores were estimated. Propensity scores were calculated through binary logistic regression, predicting the probability of CBM588 or PPI use while accounting for background factors.
Subsequently, survival analyses were conducted using multivariate Cox proportional hazard models with inverse probability of treatment weighting (IPTW) using propensity scores, balancing relevant characteristics between the CBM588 and non-CBM588 groups or the PPI user and PPI non-user groups. To ensure statistical robustness, we implemented an alternative method employing propensity scores as covariates in multivariate Cox proportional hazards models. Statistical analyses were performed using R version 4.1.3 (The R Foundation for Statistical Computing, Vienna, Austria) [18,23], with significance set at p < 0.05. The Forester package (https://github.com/rdboyes/forester, latest accessed on 15 December 2023) was used to draw a forest plot.
3. Results
3.1. Patient Characteristics
We retrospectively analyzed 106 patients with advanced or recurrent NSCLC consecutively treated with chemoimmunotherapy combinations. Six patients were excluded owing to insufficient clinical information. Among the 100 patients with NSCLC who received chemoimmunotherapy combinations, 72% were men, the median age was 67 years, and 45 patients (45%) received C. butyricum therapy using the live bacterial product CBM588. Fifty-five patients (55%) did not receive CBM588 (Table 1). Most patients (75%) received pembrolizumab-containing chemoimmunotherapy combinations as the first-line therapy. The median OS in our cohort was 20 months. The indications and characteristics of C. butyricum therapy using CBM588 are presented in Supplementary Table S1. Of the 100 patients, 45 (45%) received CBM588, among whom 38 (84%) received CBM588 concurrently with chemoimmunotherapy combinations (median duration, 12 months) and 7 (16%) received it before and during therapy (median duration, 15 months). CBM588 was administered to alleviate constipation (62%), nonspecific abdominal symptoms (20%), and idiopathic diarrhea (7%). CBM588 was prophylactically administered with antibiotics in three patients (7%). All patients who received CBM588 for constipation were also treated with laxatives such as magnesium oxide and/or sennoside A·B calcium. Moreover, 55 patients (55%) received a PPI within the 60-day window, and 46 (46%) received antibiotic therapy within 60 days prior to ICI therapy initiation. The characteristics of the PPI and antibiotic therapies are presented in Supplementary Tables S2 and S3, respectively. Of the 55 patients with NSCLC using a PPI, 24 were using esomeprazole (44%), 14 lansoprazole (25%), 14 vonoprazan fumarate (25%), 2 rabeprazole (4%), and 1 omeprazole (2%). β-lactam- and quinolone-based antibiotic therapies were the most commonly used antibiotics. In addition, 22/55 (38%), 24/45 (53%), 23/46 (50%), and 21/54 (41%) patients received CBM588 in the PPI user, PPI non-user (Table 1), antibiotic user, and antibiotic non-user groups, respectively. Among the 25 patients who received both PPIs and antibiotic therapy within the treatment window, 11 (44%) received CBM588.
3.2. Impact of CBM588 on the Efficacy of Chemoimmunotherapy Combinations
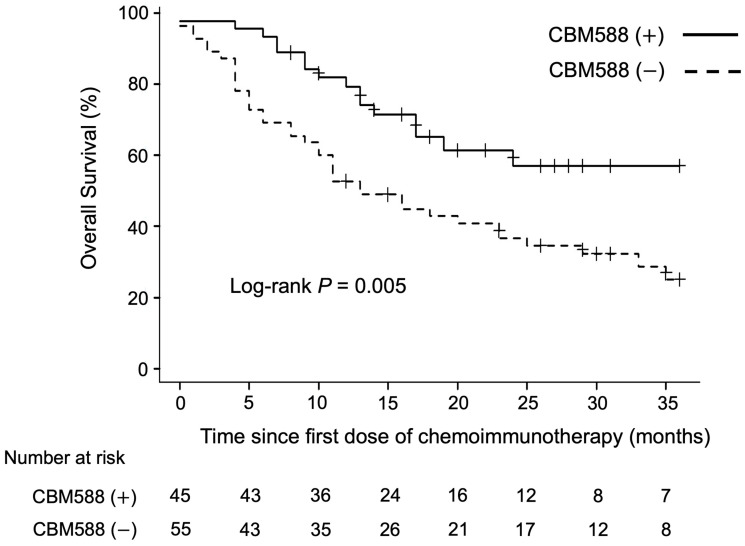
We analyzed the association between C. butyricum therapy using CBM588 and OS in patients with NSCLC treated with chemoimmunotherapy combinations. The univariate analysis revealed that CBM588 was associated with longer PFS (HR 0.62, 95% CI 0.39–0.97, p = 0.036; log-rank test p = 0.032, median PFS [mPFS] 9 vs. 5 months) and OS (HR 0.44, 95% CI 0.25–0.79, p = 0.006; log-rank test p = 0.005, median OS [mOS]) not reached vs. 13 months, Figure 1).
Figure 1.
Association between Clostridium butyricum therapy using CBM588 and overall survival (OS) in patients with advanced non-small cell lung cancer treated with chemoimmunotherapy combinations. The figure illustrates the overall survival of patients, stratified by CBM588 administration.
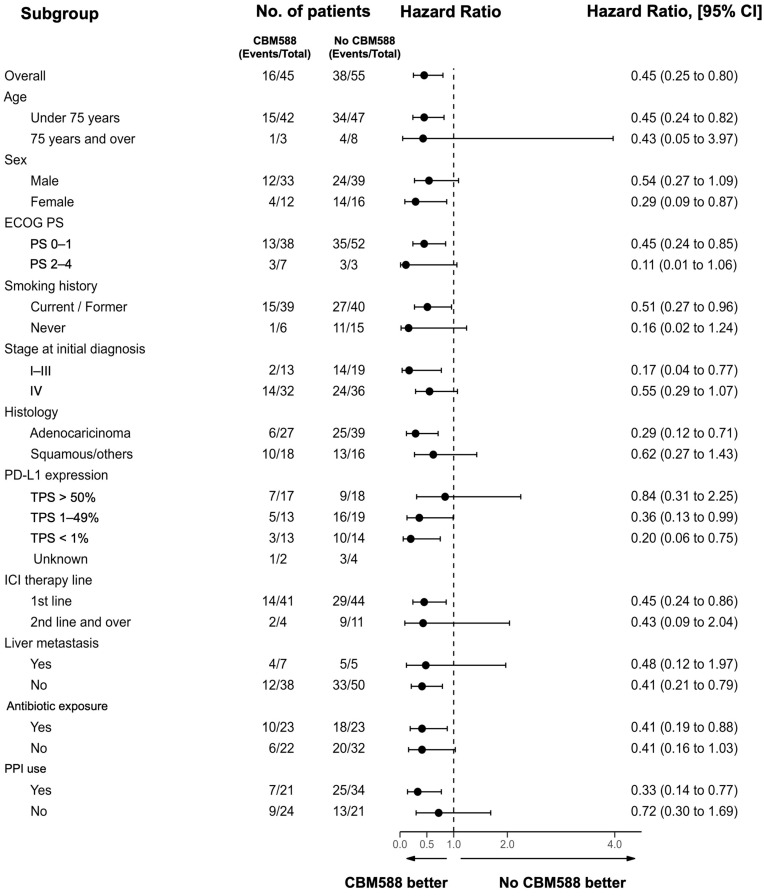
The association of PFS and OS with patient characteristics in the chemoimmunotherapy combinations is shown in Table 2. According to the multivariable Cox proportional hazards regression model, the administration of CBM588 (HR 0.55, 95% CI 0.34–0.87, p = 0.011), sex (HR 0.54, 95% CI 0.33–0.88, p = 0.013), and liver metastasis (HR 3.33, 95% CI 1.69–6.58, p = 0.001) were independently associated with PFS (Table 2). The administration of CBM588 (HR 0.41, 95% CI 0.22–0.76, p = 0.004) and histology (HR 0.32, 95% CI 0.15–0.69, p = 0.003) were independently associated with OS (Table 3).
Table 2.
Analyses of progression-free survival (PFS) in patients with NSCLC treated with chemoimmunotherapy combinations.
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| CBM588 | ||||
| No | Reference | Reference | ||
| Yes | 0.62 (0.39–0.97) | 0.036 | 0.55 (0.34–0.87) | 0.011 |
| Age (years) | 1.00 (0.97–1.03) | 0.83 | ||
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 0.59 (0.36–0.95) | 0.030 | 0.54 (0.33–0.88) | 0.013 |
| ECOG performance status | ||||
| 0–1 | Reference | |||
| 2–4 | 1.83 (0.94–3.55) | 0.08 | ||
| Smoking history | ||||
| Current/Former | 0.75 (0.44–1.26) | 0.28 | ||
| Never | Reference | |||
| Stage at initial diagnosis | ||||
| I–III | Reference | |||
| IV | 1.31 (0.80–2.16) | 0.28 | ||
| Histology | ||||
| Adenocarcinoma | 0.70 (0.44–1.12) | 0.14 | ||
| Squamous/others | Reference | |||
| EGFR/ALK mutation status | ||||
| Wild-type | Reference | |||
| Mutant | 1.96 (0.99–3.88) | 0.05 | ||
| Unknown | 0.87 (0.45–1.66) | 0.67 | ||
| PD-L1 status | ||||
| TPS < 1% | Reference | |||
| TPS 1–49% | 1.13 (0.65–1.98) | 0.66 | ||
| TPS ≥ 50% | 0.70 (0.39–1.27) | 0.25 | ||
| Unknown/Undeterminable | 1.20 (0.45–3.18) | 0.71 | ||
| Therapy line | ||||
| 1st line | Reference | |||
| ≥2nd line | 1.34 (0.74–2.44) | 0.34 | ||
| Liver metastasis | ||||
| Yes | 2.54 (1.32–4.89) | 0.005 | 3.33 (1.69–6.58) | 0.001 |
| No | Reference | Reference | ||
| Antibiotic use | ||||
| Yes | 1.25 (0.80–1.95) | 0.33 | ||
| No | Reference | |||
| PPI use | ||||
| Yes | 1.46 (0.93–2.29) | 0.10 | ||
| No | Reference | |||
Abbreviations: NSCLC, non-small cell lung cancer; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; PD-L1, programmed cell death ligand 1; TPS, tumor proportion score; PPI, proton pump inhibitor.
Table 3.
Analyses of overall survival (OS) in patients with NSCLC treated with chemoimmunotherapy combinations.
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| CBM588 | ||||
| No | Reference | Reference | ||
| Yes | 0.44 (0.25–0.79) | 0.006 | 0.41 (0.22–0.76) | 0.004 |
| Age (years) | 1.00 (0.97–1.04) | 0.94 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 0.61 (0.35–1.08) | 0.09 | ||
| ECOG performance status | ||||
| 0–1 | Reference | |||
| 2–4 | 1.64 (0.70–3.85) | 0.26 | ||
| Smoking history | ||||
| Current/Former | 0.92 (0.49–1.76) | 0.81 | ||
| Never | Reference | |||
| Stage at initial diagnosis | ||||
| I–III | Reference | |||
| IV | 1.13 (0.63–2.02) | 0.69 | ||
| Histology | ||||
| Adenocarcinoma | 0.53 (0.31–0.92) | 0.024 | 0.32 (0.15–0.69) | 0.003 |
| Squamous/others | Reference | Reference | ||
| EGFR/ALK mutation status | ||||
| Wild-type | Reference | Reference | ||
| Mutant | 2.42 (1.16–5.07) | 0.019 | 2.13 (0.93–4.86) | 0.07 |
| Unknown | 1.24 (0.61–2.51) | 0.55 | 0.52 (0.22–1.26) | 0.15 |
| PD-L1 status | ||||
| TPS < 1% | Reference | |||
| TPS 1–49% | 1.59 (0.79–3.18) | 0.19 | ||
| TPS ≥ 50% | 1.10 (0.53–2.29) | 0.80 | ||
| Unknown/Undeterminable | 1.87 (0.61–5.76) | 0.27 | ||
| Therapy line | ||||
| 1st line | Reference | |||
| ≥2nd line | 1.51 (0.78–2.93) | 0.23 | ||
| Liver metastasis | ||||
| Yes | 2.77 (1.32–5.82) | 0.007 | 2.02 (0.90–4.53) | 0.09 |
| No | Reference | |||
| Antibiotic use | ||||
| Yes | 1.40 (0.82–2.40) | 0.22 | ||
| No | Reference | |||
| PPI use | ||||
| Yes | 1.30 (0.76–2.24) | 0.34 | ||
| No | Reference | |||
Abbreviations: NSCLC, non-small cell lung cancer; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; PD-L1, programmed cell death ligand 1; TPS, tumor proportion score; PPI, proton pump inhibitor.
Moreover, the multivariate Cox proportional hazards models with IPTW using propensity scores confirmed that CBM588 significantly prolonged PFS (IPTW-adjusted HR 0.55; p = 0.012; 95% CI, 0.35–0.88) and OS (IPTW-adjusted HR 0.36; p = 0.002; 95% CI, 0.19–0.69; Supplementary Table S4). To confirm the statistical robustness, we used the propensity score as a covariate in the Cox proportional hazards regression models and confirmed that CBM588 was independently associated with longer PFS (HR, 0.55; p = 0.027; 95% CI, 0.32–0.93) and OS (HR, 0.40; p = 0.005; 95% CI, 0.21–0.75). These results are consistent with those of our previous study in which we analyzed a cohort of patients who received single-agent ICI [23]. The results of the subgroup analyses conducted based on various clinicopathological factors associated with CBM588 (Figure 2) were consistent with those of the whole-cohort analyses, with the OS being superior in the CBM588 group in most analyses. In patients who were treated with chemoimmunotherapy combinations as a first-line treatment (n = 85), the administration of CBM588 led to a trend toward improved PFS (HR 0.64, 95% CI 0.39–1.05, p = 0.08; log-rank test p = 0.07, mPFS 9 vs. 4.5 months) and significantly prolonged OS (HR 0.45, 95% CI 0.24–0.85, p = 0.014; log-rank test p = 0.012, mOS not reached vs. 13 months) (Supplementary Figure S1).
Figure 2.
Subgroup analysis of overall survival among all patients. The dashed vertical line represents the hazard ratio. Factors considered include Eastern Cooperative Oncology Group performance status (ECOG PS), tumor proportion score (TPS), and proton pump inhibitors (PPIs).
3.3. Impact of CBM588 on Tumor PD-L1 Expression-Based OS
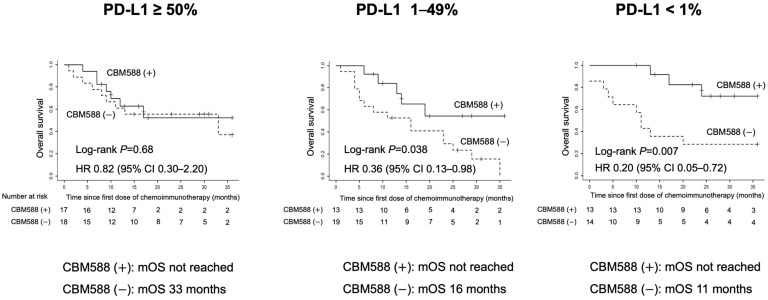
Tumor PD-L1 expression substantially affects the efficacy of chemoimmunotherapy combinations in NSCLC [2,3]; therefore, we investigated the effect of C. butyricum therapy using CBM588 on OS, based on PD-L1 expression in 94 patients for whom PD-L1 expression was determined. Kaplan–Meier curves displaying the impact of CBM588 based on tumor PD-L1 expression are presented in Figure 3.
Figure 3.
Kaplan–Meier estimates of a survival outcome in patients with advanced non-small cell lung cancer treated with chemoimmunotherapy combinations. The figure displays overall survival stratified by CBM588 administration. Kaplan–Meier curves depicting the impact of CBM588 based on tumor-programmed cell death ligand 1 (PD-L1) expression.
Similar to the whole cohort, patients who received CBM588 exhibited significantly longer OS in the tumor PD-L1 < 1% (HR 0.20, 95% CI 0.05–0.72; log-rank test, p = 0.007, mOS not reached vs. 11 months, right panel) and PD-L1 1–49% (HR 0.36, 95% CI 0.13–0.98; log-rank test, p = 0.038, mOS not reached vs. 16 months, middle panel) cohorts. The positive impact of CBM588 in the PD-L1 <1% group was higher than that in the PD-L1 1–49% group. Conversely, in the PD-L1 ≥ 50% group, the OS did not significantly differ between the patients who received or did not receive CBM588 (HR 0.82, 95% CI 0.30–2.20; log-rank test, p = 0.68, mOS not achieved vs. 33 months, left panel).
3.4. CBM588 Enhances the Efficacy of Chemoimmunotherapy Combinations in Patients with NSCLC Receiving PPIs
We evaluated the effect of PPI use on patient survival in our cohort. The PPI use was not significantly associated with poor PFS (HR 1.46, 95% CI 0.93–2.29, p = 0.10, mPFS 4 versus 8 months) and OS (HR 1.30, 95% CI 0.76–2.24, p = 0.34, mOS 19 versus 23 months; Supplementary Figure S2).
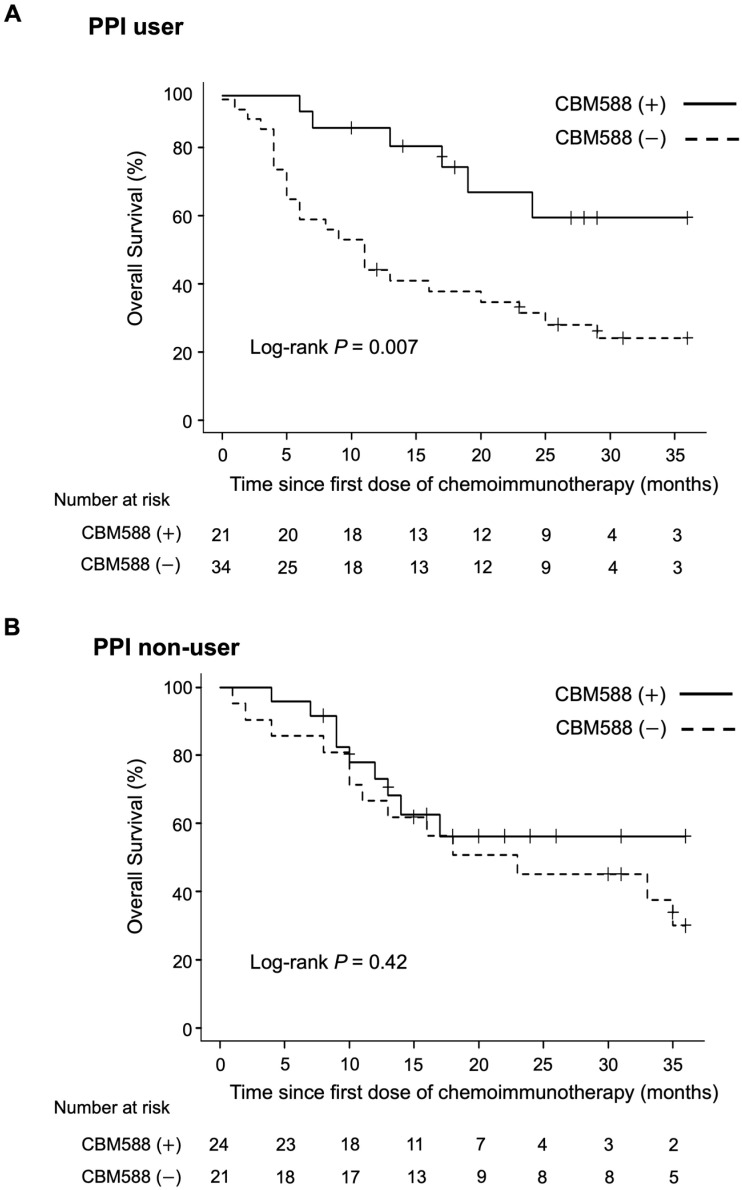
Subsequently, we assessed the impact of CBM588 on survival in patients who received or did not receive PPIs within the 60-day window. In the PPI user cohort, patients administered CBM588 had significantly improved OS when compared with those not administered CBM588 (HR, 0.33; 95% CI 0.14–0.77; p = 0.010, log-rank test p = 0.007; mOS not reached vs. 11 months; Figure 4, upper panel); however the PFS in the patients administered CBM588 was not improved (HR, 0.64; 95% CI 0.35–1.17; p = 0.15, log-rank test p = 0.15; mPFS 7 vs. 4 months).
Figure 4.
Kaplan–Meier estimates of a survival outcome in patients with non-small cell lung cancer (NSCLC) receiving proton pump inhibitors (PPIs) and those who did not receive PPIs within the 60-day window. (A) PPI user: overall survival (OS) in patients with NSCLC who received PPIs, stratified by CBM588 administration. (B) PPI non-user: OS of patients with NSCLC who did not receive PPIs, stratified by CBM588 administration.
We conducted multivariate Cox proportional hazards models with IPTW using propensity scores. The propensity score analysis revealed significantly longer OS in patients who had received CBM588 than in those who had not (IPTW-adjusted HR, 0.26; 95% CI 0.10–0.64, p = 0.004; Supplementary Table S4); however, the PFS was not significantly improved (IPTW-adjusted HR, 0.59; 95% CI 0.32–1.07, p = 0.08). To confirm statistical robustness, we reanalyzed the data using the propensity score as a covariate in the Cox proportional hazards regression models, whichh confirmed that CBM588 was independently associated with longer PFS (HR 0.47, p = 0.039; 95% CI 0.23–0.96) and OS (HR 0.29, p = 0.006; 95% CI 0.12–0.70). Consistent with our earlier findings [18], CBM588 was not significantly associated with longer OS in the PPI non-user cohort (HR 0.70, 95% CI 0.30–1.66, p = 0.42, log-rank test p = 0.42, mOS not reached vs. 23 months; Figure 4, lower panel). These results suggest that CBM588 can potentially enhance the efficacy of chemoimmunotherapy combinations, especially in patients with cancer receiving PPIs.
3.5. Impact of CBM588 on Survival Outcomes in Patients with NSCLC Receiving Chemoimmunotherapy Combinations Alongside Antibiotics
Retrospective studies have confirmed that antibiotic exposure before ICI monotherapy is detrimental to clinical outcomes [5]. However, Cortellini et al. reported that prior antibiotic exposure did not impair the clinical outcomes of chemotherapy in patients with NSCLC [25]. Concordantly, antibiotic use within 60 days before initiating the chemoimmunotherapy combination was not significantly associated with worse PFS (HR 1.25, 95% CI 0.80–1.95, p = 0.33; log-rank test p = 0.33) and OS (HR 1.40, 95% CI 0.82–2.40, p = 0.22; log-rank test p = 0.21, Supplementary Figure S3) in our study.
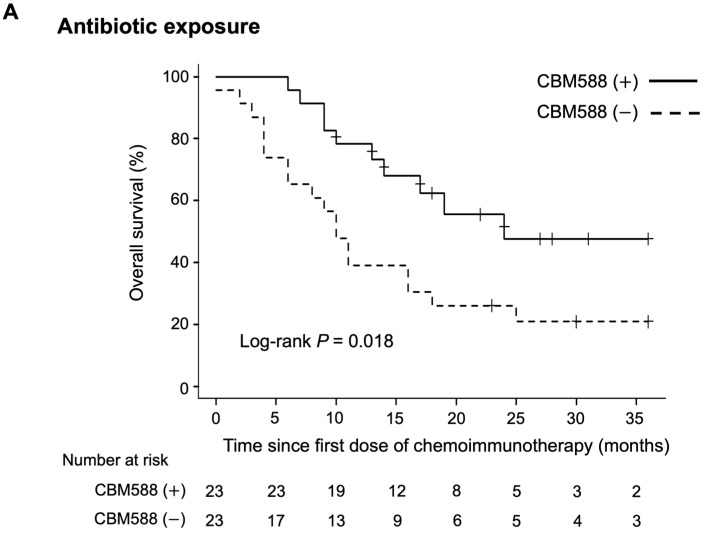
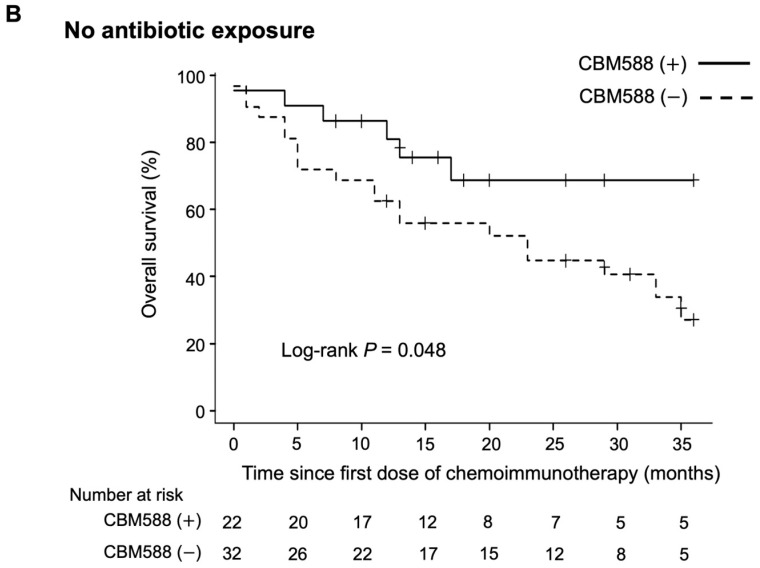
Subsequently, we evaluated the impact of CBM588 on the survival of patients who received antibiotics and those who did not. In patients who received antibiotic therapy within 60 days before the chemoimmunotherapy combination, patients administered CBM588 had longer PFS (HR 0.54, 95% CI 0.29–1.02, p = 0.06; log-rank test p = 0.018, mPFS 9 vs. 4 months) and OS (HR 0.40, 95% CI 0.18–0.87, p = 0.021; log-rank test p = 0.018, mOS 24 vs. 10 months; Figure 5, upper panel) than did those not administered CBM588. In patients who did not receive the antibiotic therapy, administration of CBM588 led to a trend toward improved PFS (HR 0.63, 95% CI 0.33–1.21, p = 0.16; log-rank test p = 0.16, mPFS 8.5 vs. 5 months) and OS (HR 0.41, 95% CI 0.16–1.02, p = 0.05; log-rank test p = 0.048, mOS not reached vs. 23 months; Figure 5, lower panel).
Figure 5.
Kaplan–Meier estimates of survival outcome in patients with non-small cell lung cancer (NSCLC) who received antibiotics and those who did not receive antibiotics within the 60-day window. (A) Antibiotic exposure: overall survival (OS) in patients with NSCLC who received antibiotics, categorized by CBM588 administration. (B) No antibiotic exposure: OS of patients with NSCLC who did not receive antibiotics, stratified by CBM588 administration.
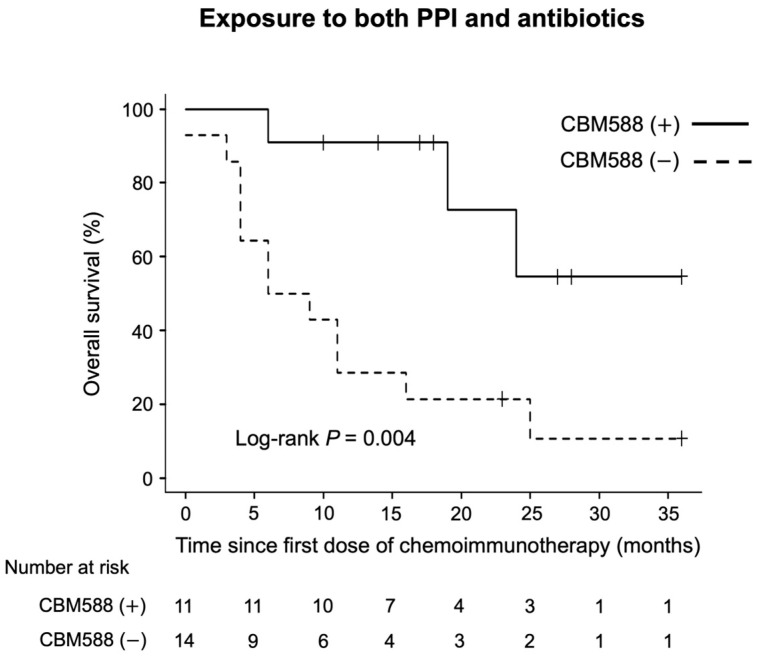
Finally, we evaluated the impact of CBM588 on the survival of the 25 patients who received both PPIs and antibiotics within the therapeutic window. We observed a significant association between CBM588 and longer PFS (HR 0.38, 95% CI 0.15–0.92, p = 0.033; log-rank test p = 0.024, mPFS 9 vs. 3 months) and OS (HR 0.19, 95% CI 0.05–0.69, p = 0.011; log-rank test p = 0.005, mOS not reached vs. 7.5 months; Figure 6) in patients exposed to both antibiotics and PPIs.
Figure 6.
Kaplan–Meier estimate of overall survival (OS) in patients with non-small cell lung cancer (NSCLC) who received both PPIs and antibiotics within the 60-day window. The figure depicts the OS of patients with NSCLC treated with chemoimmunotherapy combinations, categorized by CBM588 administration.
4. Discussion
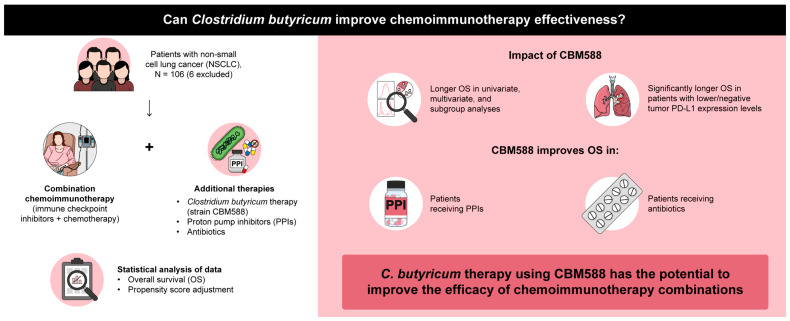
Mounting evidence suggests that the gut microbiota plays a pivotal role in influencing the response to cancer immunotherapy [5,6,8,10,28]. Recent clinical studies have demonstrated the potential of interventions, such as FMT and dietary modifications, in enhancing the effectiveness of ICIs [9,19,20]. However, the use of live biotherapeutic single bacterial strains for targeting gut microorganisms remains unestablished [5,21,29]. Additionally, treatments to restore the diminished therapeutic efficacy of ICIs in patients receiving gut dysbiosis-inducing medications, such as PPIs and antibiotics, remain unestablished [5,18,23]. Our current findings present compelling evidence for the positive impact of C. butyricum therapy using a single microbial strain, CBM588, on the efficacy of chemoimmunotherapy combinations in patients with NSCLC. CBM588 was significantly associated with prolonged OS in patients receiving chemoimmunotherapy combinations and significantly improved the OS in patients receiving both chemoimmunotherapy and dysbiosis-inducing drugs. These findings confirm that C. butyricum therapy using CBM588 may enhance the efficacy of chemoimmunotherapy combinations, thereby providing novel insights into the complex interplay among the gut microbiome, host immunity, and cancer immunotherapy (Figure 7).
Figure 7.
Overall schema of this study.
The concomitant use of dysbiosis-inducing medications can disrupt the composition of the intestinal microbiota, which plays a pivotal role in regulating both innate and adaptive immune responses [18,29,30,31]. Previous research in preclinical mouse models and clinical investigations have emphasized the importance of the gut microbiota in modulating tumor responses to chemotherapeutic agents and ICIs [32,33,34,35,36]. Retrospective analyses of clinical studies and meta-analyses involving patients with advanced lung, kidney, and bladder cancers and melanoma have indicated a reduced clinical benefit of single-agent ICIs in patients exposed to antibiotics or PPIs around the time of initiation of ICIs [5,17]. We previously reported that the administration of CBM588, particularly in patients with NSCLC receiving PPIs and/or antibiotics, improved survival in a cohort primarily (>90%) receiving ICI monotherapy [18,23]. However, whether CBM588 can enhance the efficacy of chemoimmunotherapy combinations remains unconfirmed.
PPIs reportedly alter the gut microbiome’s composition by facilitating the translocation of oral commensals into the gut of patients with cancer, thereby modifying its immunomodulatory properties and compromising ICI efficacy [14,18,30,31]. We previously reported, by analyzing fecal samples from patients with lung cancer using 16S metagenomic sequencing, that patients receiving PPIs exhibited a higher abundance of harmful oral-related pathobionts and a lower abundance of beneficial gut bacteria crucial for immunotherapy. Additionally, patients receiving CBM588 had a reduced relative abundance of harmful oral-related bacteria in the gut. Consequently, we hypothesized that CBM588 enhances the outcomes of chemoimmunotherapy combinations by modulating the gut microbiota in patients using PPIs. In the present study, PPI use was not correlated with worse OS. This finding is inconsistent with that of a previous study based on a post hoc Cox proportional hazard analysis of a phase III trial, reporting that PPI use was associated with worse OS in patients treated with chemoimmunotherapy combinations, mirroring the impact of PPIs on survival in patients treated with ICI monotherapy [14]. Moreover, in the present study, CBM588 significantly improved the efficacy of chemoimmunotherapy combinations in patients receiving PPIs but not in those not receiving PPIs, which is consistent with our previous findings in a patient cohort predominantly (>90%) treated with ICI monotherapy [18]. These findings suggest that PPIs did not exert detrimental effects on the efficacy of chemoimmunotherapy combinations in our cohort and that the positive impact of CBM588 on patient survival was enhanced under conditions of PPI-induced gut dysbiosis, ultimately leading to improved survival in patients receiving PPI.
The PD-L1 TPS serves as a pivotal predictive biomarker for NSCLC patients most likely to benefit from ICI monotherapy and chemoimmunotherapy combinations [2]. In the present study, our subgroup analyses revealed that the influence of CBM588 on the efficacy of chemoimmunotherapy combinations varied depending on tumor PD-L1 expression in patients with NSCLC. Notably, CBM588 exhibited a positive impact on patients with NSCLC in the PD-L1 < 1% and PD-L1 1–49% groups but not in those with PD-L1 ≥ 50%. This result suggests that combining CBM588 with ICIs in NSCLC may yield maximum benefits in tumors with negative or low PD-L1 expression. γδ T cells, which possess properties of innate and adaptive immune cells, contribute to the response to ICIs in patients with HLA-class-I-negative cancers [37]. CBM588 might have stimulated innate-like γδ T cells and enhanced the efficacy of ICIs in the PD-L1 < 1% and PD-L1 1–49% groups, although the potential beneficial effects of CBM588 for clinical outcomes in patients with PD-L1 ≥ 50% may have been obscured by their high response to ICIs, coupled with the limited sample size.
Previous retrospective and prospective clinical studies on NSCLC and renal cell carcinoma have reported that CBM588 significantly enhances the efficacy of ICIs, although the underlying mechanisms remain elusive [18,22,23]. C. butyricum produces substantial amounts of butyric acid [38], and preclinical investigations have revealed that microbiota-derived butyrate enhances cellular metabolism and augments the memory potential of antigen-activated CD8+ T cells [39]. Additionally, butyrate, a microbial metabolite, can directly modulate antitumor CD8+ T cell responses and enhance chemotherapy effectiveness through ID2-dependent IL-12 signaling [40]. CBM588 also expands the population of resident Bifidobacterium, a potentially beneficial bacterium that promotes antitumor immunity and enhances the efficacy of ICIs [38,41]. Moreover, CBM588 directly induces the release of tumor necrosis factor-related apoptosis-inducing ligands from polymorphonuclear neutrophils, resulting in significant antitumor effects in vitro as well as in in vivo murine bladder cancer models [42]. These direct and indirect mechanisms by which the butyrate-producing human gut symbiont C. butyricum enhances host antitumor immunity may account for the positive impact of CBM588 on the efficacy of chemoimmunotherapy combinations.
We acknowledge some limitations in our study, including its retrospective design, small sample size, and cohort heterogeneity. Moreover, we did not investigate potential factors, such as diet, dietary fiber intake, lifestyle, and genetics, which could have influenced gut microbiota composition. In addition, the number of matching variables was high; thus, there is a potential risk of overmatching in our propensity score analyses. Despite these limitations, our findings represent the first evidence that manipulating the commensal microbiota using the live bacterial product CBM588 may enhance the activity of chemoimmunotherapy combinations. This study provides a compelling rationale for further validation through prospective studies.
5. Conclusions
We present novel evidence supporting the potential of CBM588-induced manipulation of commensal microbiota to enhance chemoimmunotherapy combination activity, which warrants further exploration of the synergy between CBM588 and immunotherapies.
Acknowledgments
We are grateful to the patients for their participation in this study. This study was supported by the Kumamoto University Hospital Research Revitalization Project.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16010047/s1, Figure S1: Association between Clostridium butyricum therapy using CBM588 and overall survival (OS) and progression-free survival (PFS) in patients who were treated with chemoimmunotherapy combinations as the first-line treatment (n = 85). The figures illustrate the OS (A) and PFS (B) of patients, stratified by CBM588 administration; Figure S2: Kaplan–Meier estimate of the overall survival (OS) for proton pump inhibitor (PPI) users vs. PPI non-users in patients with non-small cell lung cancer (NSCLC) receiving chemoimmunotherapy combinations. The figure illustrates the OS of patients with NSCLC receiving chemoimmunotherapy combinations, stratified by PPI usage within the 60-day window; Figure S3: Kaplan–Meier estimate of survival outcome for patients who received antibiotics vs. those who did not receive antibiotics within the 60-day window in patients with non-small cell lung cancer (NSCLC) receiving chemoimmunotherapy combinations. The figure displays the overall survival of patients with NSCLC receiving chemoimmunotherapy combinations, stratified by antibiotic exposure within the 60-day window; Table S1: Indications and characteristics of Clostridium butyricum therapy using CBM588; Table S2: Characteristics of PPIs within the 60-day window; Table S3: Characteristics of antibiotic therapy; Table S4: Cox proportional hazards regression models with inverse probability of treatment weighting using propensity score; CBM588 vs. no CBM588 in the total cohort and protein pump inhibitor user cohort.
Author Contributions
Conceptualization, Y.T. and T.I.; Data curation, Y.T., S.S., K.I., S.I., K.S. (Koichi Saruwatari), T.J., K.A., M.A., S.H., Y.G., K.F., A.T. and T.S.; Formal analysis, Y.T. and T.I.; Funding acquisition, Y.T., S.S. and H.T.; Investigation, Y.T., S.S., K.I. and T.I.; Methodology, Y.T. and T.I.; Project administration, Y.T., S.S. and T.I.; Resources, Y.T., S.S., K.I., S.I., K.S. (Koichi Saruwatari), T.J., K.A., M.A., S.H., Y.G., K.F. and A.T. and T.S.; Software, Y.T. and T.I.; Supervision, Y.T. and T.I.; Validation, Y.T., T.I., K.S. (Kenji Sugata), C.M., Y.S., T.U. and T.S.; Visualization, Y.T. and T.I.; Writing—original draft, all authors; Writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study adhered to the principles of the Declaration of Helsinki and approval was obtained from the Kumamoto University Institutional Review Board (IRB number, 1825, Approval Date, 2 July 2021).
Informed Consent Statement
Patient consent was waived because the data were retrospectively and anonymously analyzed.
Data Availability Statement
The data generated in this study are available upon request from the corresponding authors.
Conflicts of Interest
Takuro Sakagami received research funding from Miyarisan Pharmaceutical Co., Ltd. The funder, Miyarisan Pharmaceutical Co., Ltd., was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. All authors declare no other competing interest.
Funding Statement
Yusuke Tomita received funding from JSPS KAKENHI (Grant Numbers 18K15928 and 22K08256) and the Shinnihon Foundation of Advanced Medical Treatment Research. Shinya Sakata received funding from the JSPS KAKENHI (Grant Number 20K16449). Hirotake Tsukamoto received funding from JSPS KAKENHI (Grant Numbers 21H02788).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 2.Reck M., Remon J., Hellmann M.D. First-line immunotherapy for non-small-cell lung cancer. J. Clin. Oncol. 2022;40:586–597. doi: 10.1200/JCO.21.01497. [DOI] [PubMed] [Google Scholar]
- 3.Peters S., Reck M., Smit E.F., Mok T., Hellmann M.D. How to make the best use of immunotherapy as first-line treatment of advanced/metastatic non-small-cell lung cancer. Ann. Oncol. 2019;30:884–896. doi: 10.1093/annonc/mdz109. [DOI] [PubMed] [Google Scholar]
- 4.Kraehenbuehl L., Weng C.H., Eghbali S., Wolchok J.D., Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2022;19:37–50. doi: 10.1038/s41571-021-00552-7. [DOI] [PubMed] [Google Scholar]
- 5.Derosa L., Routy B., Desilets A., Daillere R., Terrisse S., Kroemer G., Zitvogel L. Microbiota-centered interventions: The next breakthrough in immuno-oncology? Cancer Discov. 2021;11:2396–2412. doi: 10.1158/2159-8290.CD-21-0236. [DOI] [PubMed] [Google Scholar]
- 6.Helmink B.A., Khan M.A.W., Hermann A., Gopalakrishnan V., Wargo J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019;25:377–388. doi: 10.1038/s41591-019-0377-7. [DOI] [PubMed] [Google Scholar]
- 7.Zitvogel L., Kroemer G. Cross-reactivity between cancer and microbial antigens. Oncoimmunology. 2021;10:1877416. doi: 10.1080/2162402X.2021.1877416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derosa L., Routy B., Thomas A.M., Iebba V., Zalcman G., Friard S., Mazieres J., Audigier-Valette C., Moro-Sibilot D., Goldwasser F., et al. Intestinal akkermansia muciniphila predicts clinical response to pd-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 2022;28:315–324. doi: 10.1038/s41591-021-01655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer C.N., McQuade J.L., Gopalakrishnan V., McCulloch J.A., Vetizou M., Cogdill A.P., Khan M.A.W., Zhang X., White M.G., Peterson C.B., et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374:1632–1640. doi: 10.1126/science.aaz7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fluckiger A., Daillere R., Sassi M., Sixt B.S., Liu P., Loos F., Richard C., Rabu C., Alou M.T., Goubet A.G., et al. Cross-reactivity between tumor mhc class i-restricted antigens and an enterococcal bacteriophage. Science. 2020;369:936–942. doi: 10.1126/science.aax0701. [DOI] [PubMed] [Google Scholar]
- 11.Nagata N., Nishijima S., Miyoshi-Akiyama T., Kojima Y., Kimura M., Aoki R., Ohsugi M., Ueki K., Miki K., Iwata E., et al. Population-level metagenomics uncovers distinct effects of multiple medications on the human gut microbiome. Gastroenterology. 2022;163:1038–1052. doi: 10.1053/j.gastro.2022.06.070. [DOI] [PubMed] [Google Scholar]
- 12.Maier L., Pruteanu M., Kuhn M., Zeller G., Telzerow A., Anderson E.E., Brochado A.R., Fernandez K.C., Dose H., Mori H., et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savage N. The complex relationship between drugs and the microbiome. Nature. 2020;577:S10–S11. doi: 10.1038/d41586-020-00196-0. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins A.M., Kichenadasse G., McKinnon R.A., Abuhelwa A.Y., Logan J.M., Badaoui S., Karapetis C.S., Rowland A., Sorich M.J. Efficacy of first-line atezolizumab combination therapy in patients with non-small cell lung cancer receiving proton pump inhibitors: Post hoc analysis of impower150. Br. J. Cancer. 2022;126:42–47. doi: 10.1038/s41416-021-01606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins A.M., Kichenadasse G., Karapetis C.S., Rowland A., Sorich M.J. Concomitant proton pump inhibitor use and survival in urothelial carcinoma treated with atezolizumab. Clin. Cancer Res. 2020;26:5487–5493. doi: 10.1158/1078-0432.CCR-20-1876. [DOI] [PubMed] [Google Scholar]
- 16.Hakozaki T., Richard C., Elkrief A., Hosomi Y., Benlaifaoui M., Mimpen I., Terrisse S., Derosa L., Zitvogel L., Routy B., et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol. Res. 2020;8:1243–1250. doi: 10.1158/2326-6066.CIR-20-0196. [DOI] [PubMed] [Google Scholar]
- 17.Chalabi M., Cardona A., Nagarkar D.R., Scala A.D., Gandara D.R., Rittmeyer A., Albert M.L., Powles T., Kok M., Herrera F.G., et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: Pooled post hoc analyses of the oak and poplar trials. Ann. Oncol. 2020;31:525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Tomita Y., Goto Y., Sakata S., Imamura K., Minemura A., Oka K., Hayashi A., Jodai T., Akaike K., Anai M., et al. Clostridium butyricum therapy restores the decreased efficacy of immune checkpoint blockade in lung cancer patients receiving proton pump inhibitors. Oncoimmunology. 2022;11:2081010. doi: 10.1080/2162402X.2022.2081010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baruch E.N., Youngster I., Ben-Betzalel G., Ortenberg R., Lahat A., Katz L., Adler K., Dick-Necula D., Raskin S., Bloch N., et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 20.Davar D., Dzutsev A.K., McCulloch J.A., Rodrigues R.R., Chauvin J.M., Morrison R.M., Deblasio R.N., Menna C., Ding Q., Pagliano O., et al. Fecal microbiota transplant overcomes resistance to anti-pd-1 therapy in melanoma patients. Science. 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park E.M., Chelvanambi M., Bhutiani N., Kroemer G., Zitvogel L., Wargo J.A. Targeting the gut and tumor microbiota in cancer. Nat. Med. 2022;28:690–703. doi: 10.1038/s41591-022-01779-2. [DOI] [PubMed] [Google Scholar]
- 22.Dizman N., Meza L., Bergerot P., Alcantara M., Dorff T., Lyou Y., Frankel P., Cui Y., Mira V., Llamas M., et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: A randomized phase 1 trial. Nat. Med. 2022;28:704–712. doi: 10.1038/s41591-022-01694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomita Y., Ikeda T., Sakata S., Saruwatari K., Sato R., Iyama S., Jodai T., Akaike K., Ishizuka S., Saeki S., et al. Association of probiotic Clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol. Res. 2020;8:1236–1242. doi: 10.1158/2326-6066.CIR-20-0051. [DOI] [PubMed] [Google Scholar]
- 24.Derosa L., Routy B., Fidelle M., Iebba V., Alla L., Pasolli E., Segata N., Desnoyer A., Pietrantonio F., Ferrere G., et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur. Urol. 2020;78:195–206. doi: 10.1016/j.eururo.2020.04.044. [DOI] [PubMed] [Google Scholar]
- 25.Cortellini A., Ricciuti B., Facchinetti F., Alessi J.V.M., Venkatraman D., Dall F.G., Cravero P., Vaz V.R., Ottaviani D., Majem M., et al. Antibiotic-exposed patients with non-small-cell lung cancer preserve efficacy outcomes following first-line chemo-immunotherapy. Ann. Oncol. 2021;32:1391–1399. doi: 10.1016/j.annonc.2021.08.1744. [DOI] [PubMed] [Google Scholar]
- 26.Eng L., Sutradhar R., Niu Y., Liu N., Liu Y., Kaliwal Y., Powis M.L., Liu G., Peppercorn J.M., Bedard P.L., et al. Impact of antibiotic exposure before immune checkpoint inhibitor treatment on overall survival in older adults with cancer: A population-based study. J. Clin. Oncol. 2023;41:3122–3134. doi: 10.1200/JCO.22.00074. [DOI] [PubMed] [Google Scholar]
- 27.Hopkins A.M., Badaoui S., Kichenadasse G., Karapetis C.S., McKinnon R.A., Rowland A., Sorich M.J. Efficacy of atezolizumab in patients with advanced nsclc receiving concomitant antibiotic or proton pump inhibitor treatment: Pooled analysis of five randomized control trials. J. Thorac. Oncol. 2022;17:758–767. doi: 10.1016/j.jtho.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Mager L.F., Burkhard R., Pett N., Cooke N.C.A., Brown K., Ramay H., Paik S., Stagg J., Groves R.A., Gallo M., et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369:1481–1489. doi: 10.1126/science.abc3421. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes M.R., Aggarwal P., Costa R.G.F., Cole A.M., Trinchieri G. Targeting the gut microbiota for cancer therapy. Nat. Rev. Cancer. 2022;22:703–722. doi: 10.1038/s41568-022-00513-x. [DOI] [PubMed] [Google Scholar]
- 30.Imhann F., Bonder M.J., Vila A.V., Fu J., Mujagic Z., Vork L., Tigchelaar E.F., Jankipersadsing S.A., Cenit M.C., Harmsen H.J., et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson M.A., Goodrich J.K., Maxan M.E., Freedberg D.E., Abrams J.A., Poole A.C., Sutter J.L., Welter D., Ley R.E., Bell J.T., et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–756. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iida N., Dzutsev A., Stewart C.A., Smith L., Bouladoux N., Weingarten R.A., Molina D.A., Salcedo R., Back T., Cramer S., et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viaud S., Saccheri F., Mignot G., Yamazaki T., Daillere R., Hannani D., Enot D.P., Pfirschke C., Engblom C., Pittet M.J., et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A., Vicente D., Hoffman K., Wei S.C., et al. Gut microbiome modulates response to anti-pd-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matson V., Fessler J., Bao R., Chongsuwat T., Zha Y., Alegre M.L., Luke J.J., Gajewski T.F. The commensal microbiome is associated with anti-pd-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillere R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., et al. Gut microbiome influences efficacy of pd-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 37.de Vries N.L., van de Haar J., Veninga V., Chalabi M., Ijsselsteijn M.E., van der Ploeg M., van den Bulk J., Ruano D., van den Berg J.G., Haanen J.B., et al. Gammadelta t cells are effectors of immunotherapy in cancers with hla class i defects. Nature. 2023;613:743–750. doi: 10.1038/s41586-022-05593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoeva M.K., Garcia-So J., Justice N., Myers J., Tyagi S., Nemchek M., McMurdie P.J., Kolterman O., Eid J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes. 2021;13:1907272. doi: 10.1080/19490976.2021.1907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bachem A., Makhlouf C., Binger K.J., de Souza D.P., Tull D., Hochheiser K., Whitney P.G., Fernandez-Ruiz D., Dahling S., Kastenmuller W., et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated cd8(+) t cells. Immunity. 2019;51:285–297.e5. doi: 10.1016/j.immuni.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 40.He Y., Fu L., Li Y., Wang W., Gong M., Zhang J., Dong X., Huang J., Wang Q., Mackay C.R., et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic cd8(+) t cell immunity. Cell Metab. 2021;33:988–1000.e7. doi: 10.1016/j.cmet.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M., Benyamin F.W., Lei Y.M., Jabri B., Alegre M.L., et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-pd-l1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shinnoh M., Horinaka M., Yasuda T., Yoshikawa S., Morita M., Yamada T., Miki T., Sakai T. Clostridium butyricum miyairi 588 shows antitumor effects by enhancing the release of trail from neutrophils through mmp-8. Int. J. Oncol. 2013;42:903–911. doi: 10.3892/ijo.2013.1790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available upon request from the corresponding authors.