Abstract
We cloned a gene (sstT) for the Na+/serine symporter from the chromosome of Escherichia coli by using a low-copy-number vector and sequenced it. According to the deduced amino acid sequence, the transporter (SstT) consists of 414 amino acid residues. Hydropathy analysis suggested that the SstT protein possesses 9, instead of 12, hydrophobic domains.
Cells of Escherichia coli K-12 or its derivatives possess several transporters for serine. A major, constitutive serine-threonine transport system is an Na+-coupled symporter (14, 27). Members of our laboratory reported the second major transport system for serine, the serine-specific H+-coupled symporter, which is induced by leucine (15). Cells grown in the absence of leucine possess little activity of this system (15). The structural gene for this system was identified and found to be under the control of leucine-responsive regulatory protein (Lrp) (35). A third system, the leucine-isoleucine-valine transporter LIV-1, a binding protein-dependent transport system, also transports serine slowly (30). A fourth system, TdcC, is not functional in cells grown under aerobic conditions (17). An H+ conductor strongly inhibited this system (37). Recently, we found that this system is an H+/serine-threonine symporter (27).
We have isolated an E. coli mutant lacking the principal serine transport system, the Na+/serine (threonine) symporter (27). This mutant has made the cloning of a serine transporter gene(s) possible. In fact, we have cloned tdcC using the mutant as the host and pBR322 as a vector (27). However, we failed to clone the gene for the Na+/serine (threonine) symporter using the same host-vector system. Here we report cloning by the use of a low-copy-number vector, sequencing, and expression of the Na+/serine symporter gene (sstT) and some characterization of the Na+/serine (threonine) symporter.
Gene cloning.
E. coli mutant WAT9 lacks the principal serine uptake system, the Na+/serine symporter (27). Therefore, cells of WAT9 are unable to grow on serine as a major carbon source. We used this strain as a host for the cloning of the serine transport gene(s) of E. coli. In our early attempts in which we used pBR322 as a vector, we were able to clone just one gene, which encodes the TdcC system. This suggested that a high-copy-number plasmid is not suitable for cloning genes encoding serine transporters with high activity such as the Na+/serine symporter (14) and the H+/serine symporter SdaC (15, 35). Thus, we used a low-copy-number plasmid, pMW119. Chromosomal DNA was prepared from cells of E. coli W3133-2 by the method of Berns and Thomas (2). The DNA was partially digested with the restriction enzyme Sau3AI. Fragments of 4 to 10 kbp were then separated by sucrose density gradient centrifugation. The DNA fragments were ligated into pMW119 (which had been digested with BamHI and dephosphorylated with bacterial alkaline phosphatase) with a ligation kit (Takara Co.). Competent cells of E. coli WAT9 were transformed with the ligated recombinant plasmids and then spread onto agar plates containing a minimal medium consisting of 40 mM serine, 1 mM glycine, 1 mM isoleucine, 1 mM threonine, 60 μg of ampicillin per ml, and 1.5% agar and incubated at 37°C for 3 days. Plasmids were prepared from the transformants. Competent cells of E. coli WAT9 were retransformed and spread onto the same plates. The plates were incubated at 37°C for 3 days. Plasmids from the retransformants were isolated. We obtained five candidate recombinant plasmids which enabled WAT9 cells to grow on serine as a carbon source. Judging from the growth on serine and the serine transport activity (see below), it seemed that plasmid pMST3 possessed the gene for the Na+/serine symporter. Thus, we further characterized pMST3.
Properties of the transporter derived from pMST3.
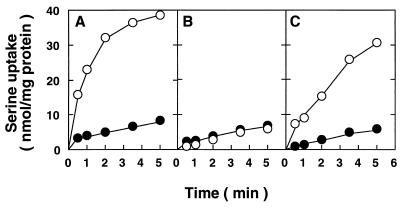
We measured serine transport activity (14) with cells of WAT9/pMST3 that were grown in a minimal medium (38) supplemented with 40 mM potassium lactate under aerobic conditions at 37°C. We observed fairly high serine transport activity with WAT9/pMST3 cells (Fig. 1C). W3133-2 cells showed slightly higher activity (Fig. 1A) than WAT9/pMST3 cells. One of the characteristics of the Na+/serine symporter is that threonine is another substrate for this transporter (14, 27). The serine uptake by W3133-2 cells was greatly reduced by excess (50-fold) threonine (Fig. 1A). In this experiment, cells were grown in minimal medium supplemented with lactate as a carbon source. Therefore, most of the serine transport activity in those cells is due to the Na+/serine symporter. The second major serine transporter in E. coli, SdaC, is not induced under such conditions. Very low serine transport activity, which was not affected by addition of excess threonine, was observed with WAT9 cells (Fig. 1B). The serine transport activity restored in WAT9/pMST3 cells was strongly inhibited by threonine (Fig. 1C), suggesting that the gene carried on plasmid pMST3 codes for a serine-threonine transport system.
FIG. 1.
Serine transport activity in parental cells (W3133-2 [A]), mutant cells (WAT9 [B]), and mutant cells harboring plasmid pMST3 (WAT9/pMST3 [C]). Cells were grown aerobically in minimal medium supplemented with 40 mM potassium lactate at 37°C. Serine transport was measured, at a final concentration of 0.1 mM, in either the absence (○) or presence (•) of a 50-fold molar excess (5 mM) of threonine at 25°C.
There are two serine-threonine transport systems in E. coli K-12: the Na+/serine symporter and the H+/serine symporter (TdcC system) (27, 37). Since we had plasmids carrying the tdcC gene (27), we checked whether the gene carried on pMST3 is tdcC. We found that the restriction patterns of the insert in pMST3 was completely different from that of tdcC (data not shown). Thus, it seemed most likely that pMST3 carried the gene for the Na+/serine symporter. If this is the case, serine transport in WAT9/pMST3 cells should be stimulated by Na+. Since WAT9 cells possess the second major serine transporter, the leucine-inducible SdaC system, cells were grown without leucine. As expected, Na+ stimulated serine uptake in WAT9/pMST3 cells (data not shown).
We confirmed that the transporter derived from the gene carried on pMST3 is the Na+/serine symporter by measuring Na+ influx into cells elicited by serine influx (14). Addition of serine to a cell suspension of WAT9/pMST3 under anaerobic conditions elicited Na+ uptake (data not shown). No Na+ uptake was observed in WAT9 cells. Thus, it became clear that the serine transport system derived from the cloned gene is really the Na+/serine symporter. We have designated the gene for the Na+/serine (threonine) transporter sstT.
Mapping of the gene.
We tried to determine the location of the sstT on the E. coli chromosome map by using a mapping membrane kit (Takara Co.) and a DNA fragment derived from the DNA insert of pMST3 as the probe. The DNA probe hybridized strongly with the 15B3 Kohara clone and faintly with the 4A1 clone (21) (data not shown). These two clones possess a region of overlap in the 68-min region of the E. coli chromosomal map (21). The restriction sites determined with pMST3 matched well with those of the overlapping region of 15B3 and 4A1 reported for the Kohara map (21). The DNA sequence of the entire chromosomal DNA of E. coli has been determined in the E. coli genome project (3). About 10 open reading frames are present in the DNA region carried on pMST3.
We constructed several deletion plasmids carrying various portions of the DNA insert of pMST3 and tested the growth of WAT9 cells harboring each one of the plasmids on serine as a carbon source as well as for serine transport activity to clarify the location of the sstT gene (data not shown). Our results clearly demonstrated that the open reading frame ygjU (3) is the serine transporter gene sstT. Sequencing of this region supported this conclusion, as is described below.
Sequences.
We determined the nucleotide sequence of the sstT region and its flanking regions by the dideoxy chain termination method (32). We found one alteration of a nucleotide in the coding region of sstT compared with the sequence of this region registered in the GenBank database (G at position 901 [A of initiation codon ATG is 1] was T in our sequence). We found two promoter-like sequences in the region upstream from sstT and a palindrome followed by T cluster which may function as a ρ-independent terminator in the region downstream from sstT (42). Thus, it seems that sstT is one component in a monocistronic operon.
According to our deduced amino acid sequence of SstT, SstT consists of 414 amino acid residues with a calculated molecular mass of 43,507 Da. There is one alteration of an amino acid residue in our sequence compared with that registered in the SwissProt database (Ala at position 301 was Thr in our sequence). SstT is very rich in hydrophobic residues. The Ser-plus-Thr content in SstT is 13.8 mol%. In two other serine transporters of E. coli, SdaC (35) and TdcC (33), the values were 14.4 and 16.4 mol%, respectively. These values are significantly higher than those in other secondary transporters. We calculated the amounts Ser plus Thr in 10 arbitrarily chosen E. coli secondary transporters for other amino acids: AroP (18), CycA (3, 31), GabP (26), GltP (40), GltS (9), LysP (36), PheP (29), ProP (6), PutP (25), and TyrP (41). We also calculated the amounts in 10 E. coli secondary transporters for sugars or other compounds: AraE (23, 24), GalP (24), GlpT (10), LacY (4), MelB (43), NhaA (20), NupC (5), PanF (19), UhpT (13), and XylE (8). The average amounts of Ser plus Thr were 11.3 mol% for the amino acid transporters and 11.4 mol% for the sugar and other transporters.
A search for sequence similarity between SstT and other proteins was conducted in several protein sequence databases. A hypothetical YgjU protein of Haemophilus influenzae (12) showed high sequence similarity: 61% identity and 88% similarity. We detected serine transport activity which was stimulated by Na+ in cells of H. influenzae (unpublished results). No other transporters showed such a high identity. Human SATT (34), an amino acid transporter, showed fairly high sequence similarity throughout the entire sequence (data not shown). Some other amino acid transporters of animal cells (ASCT1, ASCT2, and GLAST) (1, 39) showed some extent of identity and fairly high similarity. The bacterial dicarboxylic acid transporter DctA and the glutamate transporter GltP showed similar levels of identity and similarity to SstT. These transporters are members of DCT (dicarboxylate-cation symporter) family.
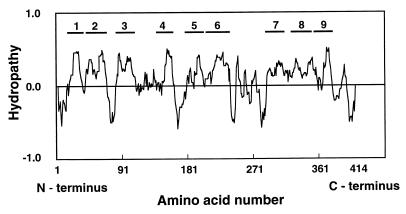
Hydropathy values were calculated by the method of Eisenberg et al. (11) and plotted along the deduced amino acid sequence of SstT (Fig. 2). There are nine hydrophobic domains with sufficient length to span the membrane. The hydropathy pattern was similar to those of human SATT (34), ASCT1 (1), and ASCT2 (39), which showed nine hydrophobic domains. It has been reported for glutamate transporters and ASCT2 that a long hydrophobic stretch is present at the C-terminal portion (39). The authors calculated the hydropathy of the ASCT2 by the method of Kyte and Doolittle (22), with a window length of 21. We calculated the hydropathy along the sequence of SstT by the same method, and the pattern revealed the presence of a similar long hydrophobic stretch at the C-terminal portion, which includes hydrophobic regions 7, 8, and 9 in Fig. 2.
FIG. 2.
Hydropathy patterns of SstT. Hydropathy values were calculated by the method of Eisenberg et al. (11) along the deduced amino acid sequence of the SstT. Portions above and below the midpoint line indicate hydrophobic and hydrophilic regions, respectively. The nine hydrophobic domains of SstT are indicated.
Defect in the sstT region of WAT9.
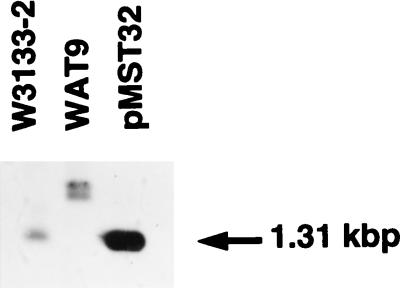
WAT9 cells have no Na+/serine (threonine) symporter activity (27). Cloning of the sstT gene has enabled us to test whether the genetic defect in WAT9 is in the sstT gene. Chromosomal DNA of parental W3133-2 and the mutant WAT9 and the plasmid pMST32 DNA which carries the cloned gene for the Na+/serine symporter were digested with restriction enzymes, and Southern blot analysis was done with a DNA fragment derived from the sstT gene as the probe. As shown in Fig. 3, a 1.31-kbp band from W3133-2 DNA and pMST32 hybridized with the probe whereas a 2.0-kbp band and a 2.2-kbp band from WAT9 DNA hybridized with the probe when digested with AccI. Thus, it seems that a 2.9-kpb DNA insertion occurred in the sstT gene of WAT9, in which one AccI site exists.
FIG. 3.
Southern blot analysis. Chromosomal DNA of E. coli W3133-2 and WAT9 and plasmid DNA of pMST321, which carries the sstT gene, were digested with restriction enzyme AccI. The digests were applied to a 1% agarose gel, separated by electrophoresis, and blotted onto a nylon membrane, Hybond-N (Amersham Corp.). After blotting, hybridized bands were detected with the enhanced chemiluminescence system of Amersham by using a AccI-AccI fragment which covers the sstT gene as the probe, as suggested by the manufacturer. The probe DNA was from pMST321.
Effect of tryptophan on expression of sstT.
Members of our laboratory reported previously that tryptophan, but not other amino acids, in the growth medium reduced serine transport activity (14). It should be noted that tryptophan itself is not a substrate for the Na+/serine symporter. We tested whether tryptophan reduces the serine transport activity in cells of WAT9/pMST3. As expected, cells grown in the presence of 5 mM tryptophan in lactate minimal medium showed about twofold-lower serine transport activity than cells grown in its absence (data not shown). These results suggest that expression of the sstT gene is repressed by tryptophan (or its metabolite). A Northern blot analysis revealed that addition of tryptophan to the growth medium reduced expression of the sstT gene both in W3133-2 cells and in WAT9/pMST321 cells (data not shown). sstT may be a member of the tryptophan regulon. In fact, we found a sequence in the region upstream from the sstT gene (TTATACTCG) in the −10 region of a putative promoter which is very similar to the sequence of the binding site of the tryptophan repressor in the regulatory region of the mtr gene (TTGTACTCG) (7, 16), a gene repressed by tryptophan. It has been reported that a CTAG or CTCG sequence is an important region for repressor binding (7, 28).
Acknowledgments
We thank Manuel F. Varela of Eastern New Mexico University for critically reading the manuscript.
This study was supported in part by a grant from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Arriza J L, Kavanaugh M P, Fairman W A, Wu Y, Murdoch G H, North R A, Amara S G. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem. 1993;268:15329–15332. [PubMed] [Google Scholar]
- 2.Berns K I, Thomas C A. Isolation of high molecular weight DNA from Haemophilus influenzae. J Mol Biol. 1965;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- 3.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 4.Buchel D E, Gronenborn B, Muller-Hill B. Sequence of the lactose permease gene. Nature. 1980;283:541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- 5.Craig J E, Zhang Y, Gallagher M P. Sequencing of the gene ant which affects the Na+/H+ antiporter activity in Escherichia coli. Mol Microbiol. 1992;11:1159–1168. [Google Scholar]
- 6.Culham D E, Lasby B, Marangoni A G, Milner J L, Steer B A, van Nues R W, Wood J M. Isolation and sequencing of Escherichia coli gene proP reveals unusual structural features of the osmoregulatory proline/betaine transporter, ProP. J Mol Biol. 1993;229:268–276. doi: 10.1006/jmbi.1993.1030. [DOI] [PubMed] [Google Scholar]
- 7.Czernik P J, Shin D S, Hurlbert B K. Functional selection and characterization of DNA binding sites for trp repressor of Escherichia coli. J Biol Chem. 1994;269:27869–27875. [PubMed] [Google Scholar]
- 8.Davis E O, Henderson P J. The cloning and DNA sequence of the gene xylE for xylose-proton symport in Escherichia coli K12. J Biol Chem. 1987;262:13928–13932. [PubMed] [Google Scholar]
- 9.Deguchi Y, Yamato I, Anraku Y. Nucleotide sequence of gltS, the Na+/glutamate symport carrier gene of Escherichia coli B. J Biol Chem. 1990;265:21704–21708. [PubMed] [Google Scholar]
- 10.Eiglmeier K, Boos W, Cole S T. Nucleotide sequence and transcriptional startpoint of the glpT gene of Escherichia coli: extensive sequence homology of the glycerol-3-phosphate transport protein with components of the hexose-6-phosphate transport system. Mol Microbiol. 1987;1:251–258. doi: 10.1111/j.1365-2958.1987.tb01931.x. [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg D, Schwarz E, Komaromy M, Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984;179:125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- 12.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C A, Gocayne J D, Scott J D, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich M J, Kadner R J. Nucleotide sequence of the uhp region of Escherichia coli. J Bacteriol. 1987;169:3556–3563. doi: 10.1128/jb.169.8.3556-3563.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hama H, Shimamoto T, Tsuda M, Tsuchiya T. Properties of a Na+-coupled serine-threonine transport system in Escherichia coli. Biochim Biophys Acta. 1987;905:231–239. doi: 10.1016/0005-2736(87)90451-2. [DOI] [PubMed] [Google Scholar]
- 15.Hama H, Shimamoto T, Tsuda M, Tsuchiya T. Characterization of a novel l-serine transport system in Escherichia coli. J Bacteriol. 1988;170:2236–2239. doi: 10.1128/jb.170.5.2236-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heatwole V M, Somerville R L. Cloning, nucleotide sequence, and characterization of mtr, the structural gene for a tryptophan-specific permease of Escherichia coli K-12. J Bacteriol. 1991;173:108–115. doi: 10.1128/jb.173.1.108-115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hobert E H, Datta P. Synthesis of biodegradative threonine dehydratase in Escherichia coli: role of amino acids, electron acceptors, and certain intermediary metabolites. J Bacteriol. 1983;155:586–592. doi: 10.1128/jb.155.2.586-592.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honore N, Cole S T. Nucleotide sequence of the aroP gene encoding the general aromatic amino acid transport protein of Escherichia coli K-12: homology with yeast transport proteins. Nucleic Acids Res. 1990;18:653. doi: 10.1093/nar/18.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackowski S, Alix J-H. Cloning, sequence, and expression of the pantothenate permease (panF) gene of Escherichia coli. J Bacteriol. 1990;172:3842–3848. doi: 10.1128/jb.172.7.3842-3848.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karpel R, Olami Y, Taglicht D, Schuldiner S, Padan E. Sequencing of the gene ant which affects the Na+/H+ antiporter activity in Escherichia coli. J Biol Chem. 1988;263:10408–10414. [PubMed] [Google Scholar]
- 21.Kohara Y, Akiyama K, Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 22.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 23.Maiden M C, Davis E O, Baldwin S A, Moore D C, Henderson P J. Mammalian and bacterial sugar transport proteins are homologous. Nature. 1987;325:641–643. doi: 10.1038/325641a0. [DOI] [PubMed] [Google Scholar]
- 24.Maiden M C, Jones-Mortimer M C, Henderson P J. The cloning, DNA sequence, and overexpression of the gene araE coding for arabinose-proton symport in Escherichia coli K12. J Biol Chem. 1988;263:8003–8010. [PubMed] [Google Scholar]
- 25.Nakao T, Yamato I, Anraku Y. Nucleotide sequence of putP, the proline carrier gene of Escherichia coli K12. Mol Gen Genet. 1987;208:70–75. doi: 10.1007/BF00330424. [DOI] [PubMed] [Google Scholar]
- 26.Nigemann E, Schulz A, Bartsch K. Molecular organization of the Escherichia coli gab cluster: nucleotide sequence of the structural genes gabD and expression of the GABA permease gene. Arch Microbiol. 1993;160:454–460. doi: 10.1007/BF00245306. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa W, Kayahara T, Tsuda M, Mizushima T, Tsuchiya T. Isolation and characterization of an Escherichia coli mutant lacking the major serine transporter, and cloning of a serine transporter gene. J Biochem. 1997;122:1241–1245. doi: 10.1093/oxfordjournals.jbchem.a021887. [DOI] [PubMed] [Google Scholar]
- 28.Phillios S E, Stogkley P G. Structure and function of Escherichia coli met repressor: similarities and contrasts with trp repressor. Phil Trans R Soc Lond B. 1996;351:527–535. doi: 10.1098/rstb.1996.0051. [DOI] [PubMed] [Google Scholar]
- 29.Pi J, Wookey P J, Pittard A J. Cloning and sequencing of the pheP gene, which encodes the phenylalanine-specific transport system of Escherichia coli. J Bacteriol. 1991;173:3622–3629. doi: 10.1128/jb.173.12.3622-3629.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbins J C, Oxender D L. Transport systems for alanine, serine, and glycine in Escherichia coli K-12. J Bacteriol. 1973;116:12–18. doi: 10.1128/jb.116.1.12-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell R R B. Mapping of a d-cycloserine resistance locus in Escherichia coli K-12. J Bacteriol. 1972;111:622–624. doi: 10.1128/jb.111.2.622-624.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweizer H P, Datta P. The complete nucleotide sequence of the tdc region of Escherichia coli. Nucleic Acids Res. 1989;17:3994. doi: 10.1093/nar/17.10.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shafqat S, Tamarappoo B K, Kilberg M S, Puranam R S, McNamara J O, Guadano-Ferraz A, Fremeau R T. Cloning and expression of a novel Na+-dependent neutral amino acid transporter structurally related to mammalian Na+/glutamate cotransporters. J Biol Chem. 1993;268:15351–15355. [PubMed] [Google Scholar]
- 35.Shao Z, Lin R T, Newman E B. Sequencing and characterization of the sdaC gene and identification of the sdaCB operon in Escherichia coli K12. Eur J Biochem. 1994;222:901–907. doi: 10.1111/j.1432-1033.1994.tb18938.x. [DOI] [PubMed] [Google Scholar]
- 36.Steffes C, Ellis J, Wu J, Rosen B P. The lysP gene encodes the lysine-specific permease. J Bacteriol. 1992;174:3242–3249. doi: 10.1128/jb.174.10.3242-3249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sumantran V N, Schweizer H P, Datta P. A novel membrane-associated threonine permease encoded by the tdcC gene of Escherichia coli. J Bacteriol. 1990;172:4288–4294. doi: 10.1128/jb.172.8.4288-4294.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka S, Lerner S A, Lin E C C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967;93:642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ustunomiya-Tate N, Endou H, Kanai Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J Biol Chem. 1996;271:14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- 40.Wallace B, Yang Y J, Hong J S, Lum D. Cloning and sequencing of a gene encoding a glutamate and aspartate carrier of Escherichia coli K-12. J Bacteriol. 1990;172:3214–3220. doi: 10.1128/jb.172.6.3214-3220.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wookey P J, Pittard A J. DNA sequence of the gene (tyrP) encoding the tyrosine-specific transport system of Escherichia coli. J Bacteriol. 1988;170:4946–4949. doi: 10.1128/jb.170.10.4946-4949.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yager T D, von Hippel P H. Transcript elongation and termination in Escherichia coli. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 2. Washington, D.C: American Society for Microbiology; 1987. pp. 1241–1275. [Google Scholar]
- 43.Yazyu H, Shiota-Niiya S, Shimamoto T, Kanazawa H, Futai M, Tsuchiya T. Nucleotide sequence of the melB gene and characteristics of deduced amino acid sequence of the melibiose carrier in Escherichia coli. J Biol Chem. 1984;259:4320–4326. [PubMed] [Google Scholar]