Abstract
Insertion of an 18-amino-acid-encoding sequence within the fpvA gene identified permissive sites at residues Y350, A402, R451, R521, and R558, consistent with these residues occurring in extramembranous loop regions of the protein. Insertions at R451, R521, and R558 did not adversely affect receptor function, although insertions at Y350 and A402 compromised ferric pyoverdine binding and uptake. The latter region likely contributes to or interacts with the ligand-binding site.
Iron is an essential nutrient whose acquisition by some bacteria is promoted by low-molecular-mass, high-affinity iron-chelating molecules termed siderophores (27). In conjunction with cell surface receptors specific for the Fe(III) complex of these siderophores (27, 28), they serve to facilitate iron acquisition under iron-limited conditions that predominate in animal and plant hosts (8, 19, 24). Indeed, siderophore production by human pathogens correlates with enhanced in vivo iron acquisition and growth and, thus, virulence (12, 14, 18).
Pseudomonas aeruginosa synthesizes two known siderophores, pyoverdine (13) and pyochelin (11), in response to iron limitation in vitro and in vivo (20). Pyoverdine displays higher affinity than does pyochelin for iron in buffered solutions (31) and is by far the superior siderophore in removing iron from transferrin (40) and in supporting growth in human serum (2). Moreover, the production of pyoverdine is correlated with enhanced in vivo growth and virulence (25, 31). The receptor for ferric pyoverdine is a ca. 80- to 90-kDa outer membrane protein (32) encoded by the fpvA gene (33). FpvA is a member of a family of receptors dependent on a cytoplasmic membrane-associated protein, TonB, which apparently couples the energized state of this membrane to the operation of these receptors (7, 35). A TonB homologue has been identified in P. aeruginosa (34).
Ferric siderophore receptors are described as gated channels, with a surface-exposed loop contributing to gate formation and acting as ligand-binding site (22, 23, 26, 36). In an effort to identify a potential gate/ligand-binding region of FpvA, we mutagenized putative external loop regions of the protein. We report here the identification of a region of FpvA necessary for ferric pyoverdine binding.
Strains and procedures.
P. aeruginosa PAO1 is a wild-type strain and parent of the FpvA-deficient mutant K691, in which fpvA was disrupted by insertion of the tetracycline-resistant derivative of the Ω interposon (ΩTc) of plasmid pHP45ΩTc (16). The interposon was recovered on a 2-kb SmaI fragment and inserted into the ScaI site of fpvA on pPVR2 (a derivative of the cloning vector pAK1900) (33). This necessitated partial digestion of pPVR2 with ScaI and isolation of a 9-kb fragment representing full-length pPVR2. Transformants (Escherichia coli DH5α [3]) carrying pPVR2 with an ΩTc insert were selected on L agar containing ampicillin and tetracycline, and insertion of ΩTc within the fpvA gene was assessed by restriction analysis. The ΩTc-mutagenized fpvA gene was subsequently recovered on a 6.5-kb PstI fragment and cloned into the unique PstI site on plasmid pSUP202ΔTc (15). Following introduction into E. coli S17-1 (38), the vector was mobilized into P. aeruginosa PAO1 via conjugation (15). PAO1 derivatives carrying a chromosomal fpvA::ΩTc mutation were selected on L agar containing tetracycline and screened for the absence of plasmid-encoded carbenicillin resistance. The lack of FpvA in putative mutants was confirmed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis of isolated outer membranes. The iron-deficient succinate minimal medium has been described previously (32). Luria broth (Luria broth base; Difco) was employed as the rich medium throughout. Ampicillin (100 μg/ml), tetracycline (for P. aeruginosa, 200 μg/ml; for E. coli, 10 μg/ml), and carbenicillin (100 μg/ml) were included in growth media where appropriate. Due to the instability of the ΩTc insert in K691, K691 and plasmid-containing derivatives of this strain were always cultured in the presence of tetracycline. Bacteria were cultured at 37°C, with shaking (200 rpm) for broth cultures.
To generate a tet-linked DNA fragment encoding the cleavage site for the factor Xa protease, oligonucleotides T1 (5′- AGATCTTTATCGAGGGGCGGTTATCGAGGGGCGGT TATCGAGGGGCGGCCCGGGTGGCGCCCTGCA-3′), T2 (5′-GGGCGCCACCCGGGCCGCCCCTCGATAAC CGCCCCTCGATAACCGCCCCTCGATAAAGATCT-3′), T3 (5′-AATTAGATCTCCCGGGTGGCGCCGAGCT-3′), and T4 (5′-CGGCGCCACCCGGGAGATCT-3′) were syn-thesized. T1 and T2 (50 pmol/μl each) were incubated at 70°C for 3 min followed by a 30-min incubation at room temperature to allow annealing of the oligonucleotides. The resultant duplex DNA carried internal restriction sites for BglII and SmaI, the coding sequence for the factor Xa cleavage site in all three reading frames, and a PstI-compatible 3′ extension at one end; the other end was blunt. Oligonucleotides T3 and T4 were similarly annealed, yielding a duplex molecule possessing internal cleavage sites for BglII and SmaI and an SstI-compatible 3′ extension at one end and an EcoRI-compatible 5′ extension at the other. The duplex molecules were ligated to a 2.1-kb EcoRI-PvuII fragment of pBR322 (37) carrying the tet gene of this vector and cloned into SstI-PstI-restricted pAK1900 to yield pXa-1. The tet-factor Xa cartridge was used, ultimately, to insert 54 bp into various sites within the fpvA gene of plasmid pPVR2, thereby disrupting the FpvA receptor by the addition of 18 amino acids (including the factor Xa cleavage site). Although the intent was to use the inserted factor Xa cleavage sites to assess the topology of FpvA, the protease failed to cleave any of the modified FpvA. Still, the insertion of 54 bp/18 amino acids did afford the opportunity to examine the influence of the disruptions on FpvA receptor activity. Initial-ly, then, the factor Xa sequence was recovered with the tet gene on a 2.1-kb SmaI fragment of pXa-1. This fragment was inserted into various sites within the fpvA gene of plasmid pPVR2 following partial digestion of pPVR2 with various enzymes whose digestion products were blunt ended (MstI, NruI, RsaI, EcoRV, and HincII). Briefly, 1 μg of plasmid was restricted with 5 to 10 units of enzyme in the presence of 25 to 50 μg of ethidium bromide per ml for 15 to 30 min at 37°C. The conditions were optimized to produce a maximal yield of unit-length plasmids which were each cut once. Transformants (E. coli DH5α) carrying pPVR2 with an insert of the tet-factor Xa cartridge were selected on L agar supplemented with tetracycline and ampicillin. The site and orientation of the inserts were determined by restriction analysis and by sequencing with a primer (5′-GTGCCTGACTGCGTTAGC-3′) which anneals upstream of the pBR322 tet gene. The tet gene was subsequently excised by digestion with BglII followed by religation of the plasmids and selection of transformants which were ampicillin resistant but tetracycline sensitive. This resulted in insertion of 54 bp within fpvA encoding either the factor Xa cleavage site in all three reading frames (orientation 1) and insertion of 18 amino acids in FpvA or translational stop signals in all three reading frames (orientation 2) and no FpvA product.
Protocols for preparation of plasmid DNA, restriction digests, ligations, transformations and isolation of restriction fragments from agarose gels have been described previously (39, 42). DNA to be sequenced was purified with the Wizard Minipreps DNA purification system (Promega). DNA sequencing and oligonucleotide synthesis were performed by Cortec DNA Service Laboratories, Inc., Queen’s University. Outer membranes were prepared as Triton X-100-insoluble cell envelopes isolated following disruption of cells with a French pressure cell (33) or a cell sonicator (Vibra Cell; Sonics & Materials, Inc.) (two bursts of 25 s at 50% power on ice) (39). Whole-cell protein extracts were prepared as described previously (30) with modifications. Briefly, 100 μl of overnight cell culture was harvested by centrifugation, resuspended in 30 μl of gel-loading buffer (2% [wt/vol] SDS–62.5 mM Tris-HCl [pH 8.0]–1% [vol/vol] glycerol), heated at 95°C for 5 min, and sonicated briefly. Following centrifugation (5 min at 15,000 rpm) to remove insoluble material, the whole-cell protein-containing supernatants were recovered. SDS-polyacrylamide gel electrophoresis was carried out as described previously (39) with 9% (wt/vol) acrylamide in the running gel. Western immunoblotting was carried out as described previously (39) with a rabbit anti-FpvA antiserum (33).
Uptake of ferric pyoverdine was assayed as described previously (32) with modifications. Cells were grown in iron-deficient succinate minimal medium supplemented with Casamino Acids (0.1% [wt/vol]; Difco) and antibiotics. Once cultures reached an A600 of 0.8 to 0.9, cells (5 ml) were harvested by centrifugation and resuspended in the same volume of nitrogen-free iron-deficient succinate minimal medium. Following incubation at 37°C with shaking for 30 min, aliquots (1 ml) were removed and added to 20 μl of a mixture of pyoverdine (3.5 mM) and 55FeCl3 (0.29 μM), which had been previously incubated for 5 min at room temperature in a 10-ml disposable culture tube. Cells were vortexed gently, and aliquots (200 μl) were removed at intervals, harvested on membrane filters, washed with 10 ml of distilled water, dried, and counted in a scintillation counter (32).
Insertion mutagenesis of fpvA.
Alignment of the predicted FpvA primary amino acid sequence with homologous receptor proteins identified several potential membrane-spanning and loop regions of FpvA (31, 33). Insertions of the 54-bp sequence encoding the factor Xa cleavage site within the fpvA gene of pPVR2 were achieved as outlined above, and insertion derivatives were expressed in the FpvA-deficient strain K691. Several hundred inserts within pPVR2 were screened, and many occurred outside the fpvA coding region. Those harboring the factor Xa coding sequences in the proper orientation within fpvA are described in Table 1. Although these derivatives were ultimately not susceptible to digestion with factor Xa, the insertion derivatives, but not FpvA itself, were cleaved by a nonspecific protease (subtilisin) in intact cells (data not shown), suggesting that the insertion sites were, nonetheless, surface accessible.
TABLE 1.
FpvA insertion derivativesa
| Plasmid | Restriction endonucleaseb | Insertion site
|
Growth in EDDHA-containing medium (A600 after 10 h)e | 55Fe-pyoverdine binding (pmol ± SD)f | |
|---|---|---|---|---|---|
| Nucleotidec | Amino acidd | ||||
| pLK141-1 | RsaI | 1134 | Y350 | 0.29 | 0.54 ± 0.21 |
| pLK127-2 | MstI (FspI) | 1288 | A402 | 0.17 | 0.42 ± 0.26 |
| pLK161-3 | MstI (FspI) | 1434 | R451 | 0.95 | 6.19 ± 1.72 |
| pLK16-1 | RsaI | 1502 | G473 | NDg | ND |
| pLK21S-1 | NruI | 1645 | R521 | 0.95 | 8.2 ± 0.59 |
| pLK39S-1 | NruI | 1757 | R558 | 0.95 | 9.97 ± 1.66 |
| pPVR2 | 0.99 | 11.80 ± 2.13 | |||
| pAK1900 | 0.24 | 0.25 ± 0.21 | |||
54 bp of DNA was inserted into a variety of sites within the fpvA gene present on plasmid pPVR2, as described in the text. Insertion sites were determined by sequencing and restriction analysis.
Indicates the enzyme which was used to cleave pPVR2 in the process of inserting 54 bp into fpvA and, thus, the restriction site at which the insert is found in the indicated plasmids.
Indicates the nucleotide position of the 54-bp insert within the fpvA coding region, where the A of the ATG start codon is bp 1.
Indicates the site of insertion of the 54-bp-encoded 18 amino acids within the mature FpvA protein, assuming that amino acids 1 to 28 of the precursor are cleaved by signal peptidase and that the mature protein begins with a Q residue. The indicated amino acid was actually lost as a result of the insertion.
P. aeruginosa K691 harboring the indicated plasmids was inoculated into iron-deficient minimal medium supplemented with EDDHA (150 μg/ml) at an A600 of 0.05 to 0.10 and incubated at 37°C. Culture density (A600) was monitored hourly for 10 h, and the final A600 value obtained is reported. The data are representative of three separate experiments carried out in duplicate.
Outer membranes (100 μg) prepared from P. aeruginosa K691 carrying the indicated plasmids and cultured under iron-limiting conditions were incubated in the presence of 55FeCl3-pyoverdine and harvested on membrane filters. Binding of 55Fe was then assessed as described previously (21). Values reported are means of three determinations ± standard deviations and have been corrected for background levels of 55Fe associating with membrane filters in the absence of added outer membranes.
ND, not determined.
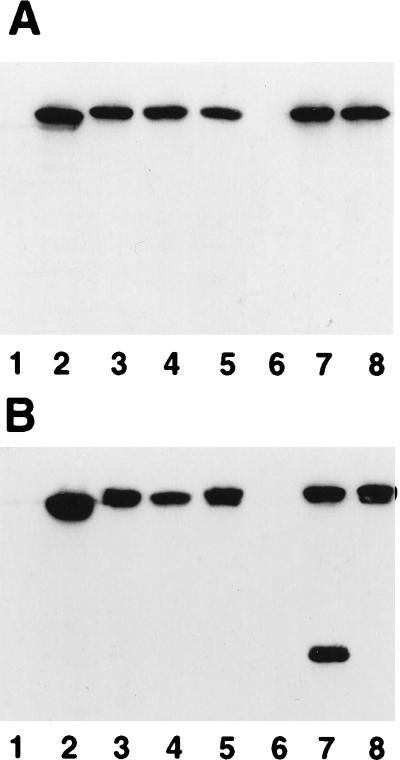
Most insertions yielded an FpvA product of slightly lower mobility than native FpvA in whole-cell extracts (Fig. 1A), consistent with the insertion of 18 additional amino acids, although an insertion at an RsaI site at bp 1502 (pLK16-1 [Table 1]) failed to yield an FpvA product (Fig. 1A, lane 6). Regions of integral membrane proteins which tolerate amino acid insertions typically correspond to extramembranous loops (1, 4, 6, 10, 17) rather than membrane-spanning regions (1, 5). Indeed, the insertion site in most derivatives in Table 1 except FpvAG473 was predicted to be a surface-exposed loop (31, 33). In each case, the proteins fractionated with the outer membrane (Fig. 1B), indicating that the insertions did not adversely affect proper export and localization of FpvA. The FpvA protein in each instance was also detectable in intact cells by using an FpvA-specific antiserum and indirect immunofluorescence (41) (data not shown). Some breakdown of the FpvA protein carrying an insertion at R451 (pLK161-3 [Table 1]) was evident in outer membrane extracts (Fig. 1B, lane 7), although this was not seen in whole-cell extracts (Fig. 1A, lane 7) and, therefore, must have occurred during the fractionation procedure.
FIG. 1.
Western immunoblot of whole-cell extracts (A) and outer membrane proteins (B) of P. aeruginosa K691 (lanes 1) and K691 harboring plasmids pPVR2 (lanes 2), pLK127-2 (lanes 3), pLK21S (lanes 4), pLK39S-1 (lanes 5), pLK16-1 (lanes 6), pLK161-3 (lanes 7), and pLK141-1 (lanes 8) probed with an FpvA-specific antiserum. Cells were cultured overnight in iron-deficient minimal medium and used to prepare whole-cell or outer membrane protein extracts which were subsequently electrophoresed on SDS-polyacrylamide gels and immunoblotted. In a typical experiment, 2.5 μl of outer membrane and 10 to 20 μl of whole-cell protein extracts were loaded onto SDS-polyacrylamide gels.
Activity of FpvA derivatives.
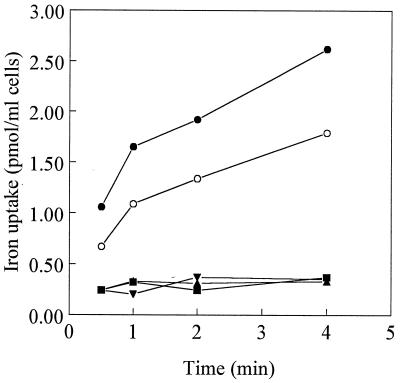
We then assessed the impact of the insertions on FpvA receptor activity. Growth of P. aeruginosa in minimal medium supplemented with the nonmetabolizable iron chelator EDDHA [ethylene diamine di(o-hydroxyphenyl acetic acid)] is dependent upon the production of pyoverdine and the presence of a functional ferric pyoverdine uptake system (32). Thus, the growth of K691 harboring the various fpvA insertion derivatives in medium containing EDDHA was measured. As expected, K691 itself grew very poorly, if at all, while the same strain harboring and expressing the wild-type fpvA gene (pPVR2) grew well (Table 1). Most of the FpvA insertion derivatives also provided for excellent growth of K691 in EDDHA-containing medium (Table 1), including those with insertions at R451 (pLK161-3), R521 (pLK32S-1), and R558 (pLK39S-1), indicating that insertions in these regions did not interfere with receptor function. In contrast, insertions at Y350 (pLK141-1) and A402 (pLK127-2) abolished the ability of these FpvA derivatives to support the growth of K691 in this medium (Table 1), consistent with these regions being important for FpvA activity. The apparent defect in activity of receptors FpvAY350 and FpvAA402 was confirmed in pyoverdine-mediated iron uptake assays (Fig. 2). As expected, K691 harboring wild-type fpvA (present on pPVR2) or an fpvA insertion derivative which did not adversely affect growth in EDDHA-containing medium (e.g., FpvAR521 [pLK21S-1]) was proficient in ferric pyoverdine uptake (Fig. 2).
FIG. 2.
Pyoverdine-mediated iron uptake by P. aeruginosa K691 harboring pAK1900 (■), pPVR2 (•), pLK127-2 (▴), pLK141-1 (▾), and pLK21S-1 (○). The data are representative of three separate experiments carried out in duplicate and are reported for cells at an A600 of 1.0.
Given the functional importance of the region(s) of FpvA in the vicinity of residues Y350 and A402, it seemed likely that these regions were somehow involved in ligand (i.e., ferric pyoverdine) binding. To test this, outer membranes were prepared from K691 harboring native FpvA and various insertion derivatives and examined for binding of ferric pyoverdine, as described previously (21). As expected, K691 harboring the native receptor (pPVR2) demonstrated binding of ferric pyoverdine, as did K691 harboring insertion derivative FpvAR521 (Table 1). FpvAR521-expressing cells did demonstrate less binding of ferric pyoverdine than did cells expressing native FpvA, probably reflecting the decreased production of FpvAR521 relative to the pPVR2-encoded native protein (Fig. 1, cf. lanes 3 and 5). In contrast, K691 harboring FpvAA402 or FpvAY350 showed minimal ferric pyoverdine binding, comparable to levels observed for K691 carrying vector only (Table 1) or PAO1 cultured under iron-rich conditions (under which conditions FpvA is not induced) (0.64 ± 0.40 pmol of Fe). These data suggest that the region(s) of FpvA neighboring A402 and Y350 is important for ferric pyoverdine binding and may contribute to a binding site. Still, it cannot be ruled out that this region(s) interacts with the ligand-binding domain(s) of FpvA and its disruption indirectly impacts the ligand-binding site.
Ferric siderophore receptors appear to be gated porins, with the gate region functioning both to control access to the channel and as a ligand-binding site. Indeed, deletion of a single aspartic acid residue (D348) within the E. coli FhuA ferrichrome receptor obviates ligand binding (23), and this residue occurs in a region of the receptor whose deletion converts FhuA into a diffusion channel (22). Similarly, deletion of a region of the ferric enterobactin receptor FepA, implicated in ligand binding, also converts the protein into a nonspecific channel, indicating that the binding domain exists as part of a gate region in this receptor as well (26, 36). The identification here of a region of FpvA which is likely extramembranous and possibly involved in ligand binding suggests that the Y350-A402 region of FpvA may comprise part of a gate region for this receptor. Despite repeated attempts to delete this region of FpvA, however, we have failed to express the deletion derivative in E. coli or P. aeruginosa. Certain deletions of other ferric siderophore receptors appear also to be unobtainable (9). Intriguingly, the insertions at Y350 and A402 occur adjacent to basic amino acids, and arginine residues of the FepA gate region have recently been implicated in ferric siderophore binding (29). We are currently using site-directed mutagenesis to assess the significance of these and other residues near Y350 and A402 in ferric pyoverdine binding.
Acknowledgments
We thank P. Klebba for helpful suggestions regarding the ferric pyoverdine binding assay.
This work was supported by an operating grant from the Medical Research Council of Canada. K.P. is a Natural Sciences and Engineering Research Council University Research Fellow.
REFERENCES
- 1.Agterberg M, Adriaanse H, Tijhaar E, Resink A, Tommassen J. Role of the cell surface-exposed regions of outer membrane protein PhoE of Escherichia coli K12 in the biogenesis of the protein. Eur J Biochem. 1989;185:365–370. doi: 10.1111/j.1432-1033.1989.tb15124.x. [DOI] [PubMed] [Google Scholar]
- 2.Ankenbauer R, Sriyosachati S, Cox C D. Effects of siderophores on the growth of Pseudomonas aeruginosa in human serum and transferrin. Infect Immun. 1985;49:132–140. doi: 10.1128/iai.49.1.132-140.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Short protocols in molecular biology. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1992. [Google Scholar]
- 4.Bosch D, Tommassen J. Effects of linker insertions on the biogenesis and functioning of the Escherichia coli outer membrane pore protein PhoE. Mol Gen Genet. 1987;208:485–489. doi: 10.1007/BF00328144. [DOI] [PubMed] [Google Scholar]
- 5.Bosch D, Voorhout W, Tommassen J. Export and localization of N-terminally truncated derivatives of Escherichia coli K-12 outer membrane protein PhoE. J Biol Chem. 1988;263:9952–9957. [PubMed] [Google Scholar]
- 6.Boulain J C, Charbit A, Hofnung M. Mutagenesis by random linker insertion into the lamB gene of Escherichia coli K12. Mol Gen Genet. 1986;205:339–348. doi: 10.1007/BF00430448. [DOI] [PubMed] [Google Scholar]
- 7.Braun V. Energy-coupled transport and signal transduction through the Gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev. 1995;16:295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 8.Brown M R W, Anwar H, Lambert P A. Evidence that mucoid Pseudomonas aeruginosa in the cystic fibrosis lung grows under iron-restricted conditions. FEMS Microbiol Lett. 1984;21:113–117. [Google Scholar]
- 9.Carmel G, Coulton J W. Internal deletions in the FhuA receptor of Escherichia coli K-12 define domains of ligand interactions. J Bacteriol. 1991;173:4394–4403. doi: 10.1128/jb.173.14.4394-4403.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charbit A, Ronco J, Michel V, Werts C, Hofnung M. Permissive sites and topology of an outer membrane protein with a reporter epitope. J Bacteriol. 1991;173:262–275. doi: 10.1128/jb.173.1.262-275.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox C D. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol. 1980;142:581–587. doi: 10.1128/jb.142.2.581-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox C D. Effect of pyochelin on the virulence of Pseudomonas aeruginosa. Infect Immun. 1982;36:17–23. doi: 10.1128/iai.36.1.17-23.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox C D, Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. 1985;48:130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosa J H. The relationship of plasmid-mediated iron transport and bacterial virulence. Annu Rev Microbiol. 1984;38:69–89. doi: 10.1146/annurev.mi.38.100184.000441. [DOI] [PubMed] [Google Scholar]
- 15.Dean C R, Poole K. Expression of the ferric enterobactin receptor (PfeA) of Pseudomonas aeruginosa: involvement of a two-component regulatory system. Mol Microbiol. 1993;8:1095–1103. doi: 10.1111/j.1365-2958.1993.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 16.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of Gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 17.Freudl R. Insertion of peptides into cell-surface-exposed area of the Escherichia coli OmpA protein does not interfere with export and membrane assembly. Gene. 1989;82:229–236. doi: 10.1016/0378-1119(89)90048-6. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths E. The iron-uptake systems of pathogenic bacteria. In: Bullen J J, Griffiths E, editors. Iron and infection. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 69–137. [Google Scholar]
- 19.Griffiths E, Stevenson P, Joyce P. Pathogenic Escherichia coli express new outer membrane proteins when growing in vivo. FEMS Microbiol Lett. 1983;16:95–99. [Google Scholar]
- 20.Haas B, Kraut J, Marks J, Cassin Zanker S, Castignetti D. Siderophore presence in sputa of cystic fibrosis patients. Infect Immun. 1991;59:3997–4000. doi: 10.1128/iai.59.11.3997-4000.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hohnadel D, Meyer J-M. Specificity of pyoverdine-mediated iron uptake among fluorescent Pseudomonas strains. J Bacteriol. 1988;170:4865–4873. doi: 10.1128/jb.170.10.4865-4873.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Killmann H, Benz R, Braun V. Conversion of the FhuA transport protein into a diffusion channel through the outer membrane of Escherichia coli. EMBO J. 1993;12:3007–3016. doi: 10.1002/j.1460-2075.1993.tb05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Killmann H, Braun V. An aspartate deletion mutation defines a binding site of the multifunctional FhuA outer membrane receptor of Escherichia coli K-12. J Bacteriol. 1992;174:3479–3486. doi: 10.1128/jb.174.11.3479-3486.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam C, Turnowsky F, Schwarzinger E, Neruda W. Bacteria recovered without subculture from infected human urines expressed iron-regulated outer membrane proteins. FEMS Microbiol Lett. 1984;24:255–259. [Google Scholar]
- 25.Meyer J-M, Neely A, Stintzi A, Georges C, Holder I A. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy C K, Kalve V I, Klebba P E. Surface topology of the Escherichia coli K-12 ferrienterobactin receptor. J Bacteriol. 1990;172:2736–2746. doi: 10.1128/jb.172.5.2736-2746.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neilands J B. Iron absorption and transport in microorganisms. Annu Rev Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- 28.Neilands J B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- 29.Newton S M, Allen J S, Cao Z, Qi Z, Jiang X, Sprencel C, Igo J D, Foster S B, Payne M A, Klebba P E. Double mutagenesis of a positive charge cluster in the ligand-binding site of the ferric enterobactin receptor, FepA. Proc Natl Acad Sci USA. 1997;94:4560–4565. doi: 10.1073/pnas.94.9.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicas T I, Hancock R E W. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980;143:872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poole K, Dean C, Heinrichs D, Neshat S, Krebes K, Young L, Kilburn L. Siderophore-mediated iron transport in Pseudomonas aeruginosa. In: Nakazawa T, Furukawa K, Haas D, Silver S, editors. Molecular biology of pseudomonads. Washington, D.C: American Society for Microbiology; 1996. pp. 371–383. [Google Scholar]
- 32.Poole K, Neshat S, Heinrichs D. Pyoverdine-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. FEMS Microbiol Lett. 1991;78:1–5. [PubMed] [Google Scholar]
- 33.Poole K, Neshat S, Krebes K, Heinrichs D E. Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J Bacteriol. 1993;175:4597–4604. doi: 10.1128/jb.175.15.4597-4604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poole K, Zhao Q, Neshat S, Heinrichs D E, Dean C R. The tonB gene of Pseudomonas aeruginosa encodes a novel TonB protein. Microbiology. 1996;142:1449–1458. doi: 10.1099/13500872-142-6-1449. [DOI] [PubMed] [Google Scholar]
- 35.Postle K. TonB and the gram-negative dilemma. Mol Microbiol. 1990;4:2019–2025. doi: 10.1111/j.1365-2958.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 36.Rutz J M, Liu J, Lyons J A, Goranson J, Armstrong S K, McIntosh M, Feix J B, Klebba P E. Formation of a gated channel by a ligand-specific transport protein in the bacterial outer membrane. Science. 1992;258:471–475. doi: 10.1126/science.1411544. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Simon R, Priefer U, Puehler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 39.Srikumar R, Kon T, Gotoh N, Poole K. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob Agents Chemother. 1998;42:65–71. doi: 10.1128/aac.42.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sriyosachati S, Cox C D. Siderophore-mediated iron acquisition from transferrin by Pseudomonas aeruginosa. Infect Immun. 1986;52:885–891. doi: 10.1128/iai.52.3.885-891.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong K K Y, Poole K, Gotoh N, Hancock R E W. Influence of OprM expression on multiple antibiotic resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2009–2012. doi: 10.1128/aac.41.9.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]