Abstract
Studies on the nitrite uptake capability of a mutant of Synechococcus sp. strain PCC 7942 lacking the ATP-binding cassette-type nitrate-nitrite-bispecific transporter revealed the occurrence of a nitrite-specific active transport system with an apparent Km (NO2−) of about 20 μM. Similar to the nitrate-nitrite-bispecific transporter, the nitrite-specific transporter was reversibly inhibited by ammonium in the medium.
Cyanobacteria can utilize nitrate, nitrite, and ammonium as sole sources of nitrogen for growth (4, 7). Nitrate is transported into the cell by an ATP-binding cassette-type transporter (NRT) encoded by the nrtABCD genes (15–17) and is reduced to nitrite by nitrate reductase. Nitrite is reduced to ammonium by nitrite reductase (NiR), and the resulting ammonium is converted into amide nitrogen of Gln. Mutants with deletions of the nrt genes require high concentrations (>30 mM) of nitrate for sustained growth on nitrate and are totally defective in uptake of low concentrations (<1 mM) of nitrate in medium, indicating that NRT is essential for nitrate assimilation in the natural environment (16, 17). Unlike nitrate, nitrite added to the medium at low concentrations is transported into the cyanobacterial cell by two distinct mechanisms: (i) by active transport of nitrite (NO2−), which is sensitive to N,N′-dicyclohexylcarbodiimide (DCCD), and (ii) by passive diffusion of nitrous acid (HNO2), whose contribution to the net uptake of nitrite decreases as the pH of the medium is raised and the nitrite concentration in medium is lowered (2). The nitrate transporter encoded by the nrt genes has been shown to be a nitrate-nitrite-bispecific transporter (11, 14) which effectively transports nitrate and nitrite at micromolar concentrations (14). An nrtABCD deletion mutant (NA3) of Synechococcus sp. strain PCC 7942 was, however, found to have significant activity of nitrite uptake at pH 9.6 and nitrite concentrations of <100 μM (14), under which conditions passive diffusion of HNO2 is null (2). The results were in conflict with the previous report that the nrtD insertional mutants are totally defective in uptake of low concentrations of nitrite (11). In this work, we characterized the uptake of nitrite by the NA3 cells, and here we present evidence for the occurrence of a nitrite-specific active transport system.
A derivative of Synechococcus sp. strain PCC 7942, which is cured of the resident small plasmid pUH24 (strain SPc, hereafter designated simply as strain PCC 7942 [8]), and a mutant with a deletion of the nrtABCD genes (NA3) derived therefrom (14) were grown photoautotrophically under continuous illumination provided by fluorescent lamps (70 μE m−2 s−1) at 30°C. The basal medium used was a nitrogen-free medium obtained by modification of BG-11 medium (19) as previously described (20). Ammonium-containing medium and nitrate-containing medium were prepared by addition of 3.75 mM (NH4)2SO4 and 60 mM KNO3, respectively, to the basal medium. All growth media were buffered with 20 mM HEPES-KOH (pH 8.2). The cultures were aerated with 2% (vol/vol) CO2 in air.
Uptake of nitrite by Synechococcus cells was measured at pH 9.6 and at pH 7.2, by monitoring the decrease in the concentration of nitrite in the medium. Cells were grown with 60 mM nitrate as the nitrogen source and harvested at the mid-logarithmic phase of growth by centrifugation at 3,000 × g for 10 min at 25°C. The collected cells were washed with the nitrogen-free medium supplemented with 20 mM HEPES-KOH (pH 8.2) by resuspension and recentrifugation and then suspended to a chlorophyll (Chl) concentration of 5 μg per ml in assay media with pH values of 9.6 and 7.2, which were prepared by supplementation of the nitrogen-free medium with 20 mM 2-(N-cyclohexylamino)ethanesulfonic acid (CHES)-KOH (pH 9.6) and 20 mM HEPES-KOH (pH 7.2), respectively, and with 5 mM NaHCO3. When the effects of ammonium on nitrite uptake were examined at pH 9.6, 20 mM HEPES-KOH was used in place of 20 mM CHES-KOH as the buffer because CHES interferes with the determination of the ammonium concentration. The reaction was started by addition of NaNO2 to the cell suspensions kept at 30°C in the light (70 μE m−2 s−1). Aliquots of 0.8 ml were withdrawn from the cell suspensions at regular intervals, and after immediate centrifugation for 60 s at 15,000 × g to sediment the cells, the concentration of nitrite in the supernatant was determined as described previously (14). For DCCD treatment, 10 μM DCCD was added to the cell suspensions and the cells were incubated in the light at 30°C for 10 min prior to the addition of nitrite. Treatment of the cells with l-methionine–dl-sulfoximine was done in a similar way, with the final concentration of the reagent and the preincubation time being 1 mM and 30 min, respectively. The in vitro activities of nitrate reductase and NiR were determined at 30°C, using toluene-permeabilized cells with dithionite-reduced methyl viologen as the electron donor, as described by Herrero et al. (5) and Herrero and Guerrero (6), respectively. The Chl concentration was determined as described by Mackinney (12). Ammonium concentrations were determined as described by Anderson and Little (1).
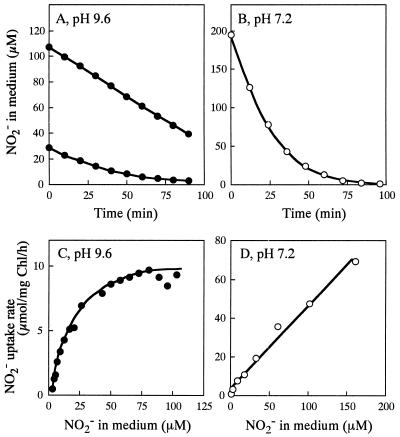
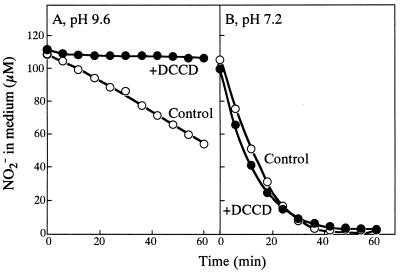
As previously shown (14), cells of NRT-less mutant NA3, grown in a medium containing 60 mM nitrate, took up nitrite at pH 9.6 and external nitrite concentrations of <100 μM, under which conditions passive diffusion of nitrous acid (HNO2) into the cells is negligible (Fig. 1A). Nitrite uptake followed a saturation-type kinetics with respect to the nitrite concentration in medium, with the substrate concentration required for half-saturation being 20 μM (Fig. 1C). The nitrite uptake rate under saturation (i.e., the rate at 100 μM external nitrite) was 11.4 ± 0.9 μmol per mg of Chl per h (mean ± standard deviation for seven determinations), which corresponded to 27% of the rate of nitrite uptake by the wild-type cells having NRT (41.6 ± 2.1 μmol per mg of Chl per h [n = 4]). At pH 7.2, by contrast, the time course of the decrease of the external nitrite concentration followed an exponential curve (Fig. 1B), and the rate of nitrite uptake was a linear function of the nitrite concentration in medium (Fig. 1D), confirming that nitrite enters the cells mainly by passive diffusion of nitrous acid at pH 7.2. As can be seen from the plot in Fig. 1D, the in vivo NiR activity of NA3 cells was determined to be no less than 70 μmol per mg of Chl per h. The saturation level of the nitrite uptake rate at pH 9.6 (Fig. 1C) was hence less than 16% of the in vivo NiR activity, indicating that transport of nitrite into the cell limits the utilization of the low concentrations (<100 μM) of nitrite at pH 9.6. The nitrite uptake by NA3 was sensitive to DCCD at pH 9.6 (Fig. 2A) but not at pH 7.2 (Fig. 2B), indicating the involvement of energy metabolism in the nitrite transport at pH 9.6. These findings showed that Synechococcus sp. strain PCC 7942 has an active nitrite transport system distinct from NRT (designated NIT).
FIG. 1.
Uptake of nitrite by nitrate (60 mM)-grown cells of NA3 at pH 9.6 and at pH 7.2. Changes in nitrite concentration in the medium after addition of nitrite to the cell suspensions containing 5 μg of Chl per ml are shown. For panel A, the cell suspension was separated into two portions and nitrite uptake was measured with initial nitrite concentrations of ca. 110 (upper curve) and 30 (lower curve) μM. (C and D) Plots of nitrite uptake rate versus nitrite concentration in medium at pH 9.6 and at pH 7.2, respectively. The nitrite uptake rate was calculated as the rate of uptake between each sampling time shown in panels A and B.
FIG. 2.
Effects of DCCD on nitrite uptake by NA3 cells at pH 9.6 and at pH 7.2. Cells grown in nitrate (60 mM)-containing medium were suspended in nitrogen-free media at the indicated pH values to a Chl concentration of 5 μg per ml and then were preincubated for 10 min in the light in the presence or absence of DCCD (10 μM). Nitrite (ca. 100 μM) was added at time zero to the cell suspensions, and changes in the nitrite concentration in the medium were monitored.
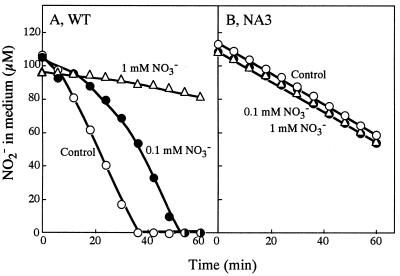
In accordance with the bispecific nature of NRT (11, 14), the uptake of nitrite by the wild-type cells was progressively inhibited by increasing amounts of nitrate as previously reported (Fig. 3A) (13). By contrast, the nitrite uptake by NA3 was not affected by nitrate added at a concentration 10-fold higher than that of nitrite (Fig. 3B). Other anions, i.e., sulfate, sulfite, chlorate, and chlorite, added at a 10-fold-higher concentration (1 mM) did not interfere with nitrite uptake by the mutant, showing that NIT is specific to nitrite (data not shown).
FIG. 3.
Effects of nitrate on the uptake of nitrite at pH 9.6 by nitrate (60 mM)-grown cells of the wild-type strain (WT) and NA3. Nitrite (100 μM) was added at time zero to the cell suspensions containing 5 μg of Chl per ml, with and without simultaneous addition of nitrate. Changes in the nitrite concentration in the medium are shown.
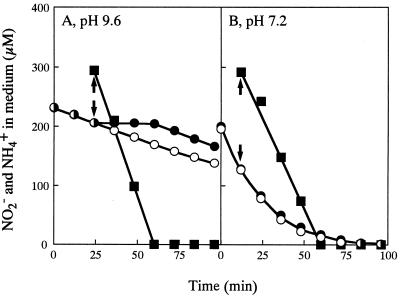
Ammonium, when added to cultures of NA3, inhibited uptake of low concentrations of nitrite at pH 9.6 but not at pH 7.2 (Fig. 4). Since the cells assimilated nitrite and ammonium simultaneously at pH 7.2 (Fig. 4B), it is clear that ammonium does not inhibit NiR. The inhibition by ammonium of nitrite uptake at pH 9.6 therefore indicates posttranslational regulation of NIT. The inhibition was reversible, and nitrite uptake resumed after consumption of ammonium in the medium (Fig. 4A). Similar to the case of regulation of NRT (9), ammonium did not inhibit NIT in l-methionine–dl-sulfoximine-treated cells (data not shown), showing that ammonium has to be converted into Gln to cause inhibition of NIT.
FIG. 4.
Effects of ammonium on the uptake of nitrite by NA3 cells at pH 9.6 and at pH 7.2. Cells grown with nitrate (60 mM) were suspended in nitrogen-free media at the indicated pH values to a Chl concentration of 5 μg per ml. Nitrite (ca. 200 μM) was added at time zero to the cell suspensions and ammonium (300 μM) was added at the times indicated by the arrows. Changes in the nitrite (circles) and ammonium (squares) concentrations in the medium are shown. Open circles, control; closed symbols, plus ammonium.
The occurrence of a nitrite-specific transporter, in addition to an NRT-like nitrate-nitrite transporter, in Klebsiella oxytoca has been suggested (10, 21). The unicellular green alga Chlamydomonas reinhardtii also has a nitrite-specific transporter in addition to a nitrate-specific transporter and a nitrate-nitrite-bispecific transporter (3, 18). It thus seems common that the organisms performing nitrate assimilation have a nitrite-specific transporter. However, no gene(s) or protein(s) involved in nitrite-specific transport has been identified, and the physiological significance of the transport is unclear. Identification of the gene(s) encoding the nitrite transport system and characterization of the relevant mutants are required for elucidation of the role of the nitrite-specific transporter(s).
Acknowledgments
This work was supported by Grants in Aid for Scientific Research in Priority Areas (09274101 and 09274103) from the Ministry of Education, Science and Culture, Japan.
REFERENCES
- 1.Anderson P M, Little R M. Kinetic properties of cyanase. Biochemistry. 1986;25:1621–1626. doi: 10.1021/bi00355a026. [DOI] [PubMed] [Google Scholar]
- 2.Flores E, Herrero A, Guerrero M G. Nitrite uptake and its regulation in the cyanobacterium Anacystis nidulans. Biochim Biophys Acta. 1987;896:103–108. [Google Scholar]
- 3.Galván A, Quesada A, Fernández E. Nitrate and nitrite are transported by different specific transport systems and by a bispecific transporter in Chlamydomonas reinhardtii. J Biol Chem. 1996;271:2088–2092. doi: 10.1074/jbc.271.4.2088. [DOI] [PubMed] [Google Scholar]
- 4.Guerrero M G, Lara C. Assimilation of inorganic nitrogen. In: Fay P, Baalen C V, editors. The cyanobacteria. Amsterdam, The Netherlands: Elsevier Science Publishers B. V.; 1987. pp. 163–186. [Google Scholar]
- 5.Herrero A, Flores E, Guerrero M G. Regulation of nitrate reductase levels in the cyanobacteria Anacystis nidulans, Anabaena sp. strain 7119, and Nostoc sp. strain 6719. J Bacteriol. 1981;145:175–180. doi: 10.1128/jb.145.1.175-180.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrero A, Guerrero M G. Regulation of nitrite reductase in the cyanobacterium Anacystis nidulans. J Gen Microbiol. 1986;132:2463–2468. [Google Scholar]
- 7.Kuhlemeier C J, Logtenberg T, Stoorvogel W, van Heugten H A A, Borrias W E, van Arkel G A. Cloning of nitrate reductase genes from the cyanobacterium Anacystis nidulans. J Bacteriol. 1984;159:36–41. doi: 10.1128/jb.159.1.36-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhlemeier C J, Thomas A A M, van der Ende A, van Leen R W, Biorrias W E, van den Hondel C A M J J, van Arkel G A. A host-vector system for gene cloning in the cyanobacterium Anacystis nidulans R2. Plasmid. 1983;10:156–163. doi: 10.1016/0147-619x(83)90068-9. [DOI] [PubMed] [Google Scholar]
- 9.Lara C, Romero J M, Guerrero M G. Regulated nitrate transport in the cyanobacterium Anacystis nidulans. J Bacteriol. 1987;169:4376–4378. doi: 10.1128/jb.169.9.4376-4378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin J T, Goldman B S, Stewart V. The nasFEDCBA operon for nitrate and nitrite assimilation in Klebsiella pneumoniae M5al. J Bacteriol. 1994;176:2551–2559. doi: 10.1128/jb.176.9.2551-2559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luque I, Flores E, Herrero A. Nitrate and nitrite transport in the cyanobacterium Synechococcus sp. PCC 7942 are mediated by the same permease. Biochim Biophys Acta. 1994;1184:296–298. [Google Scholar]
- 12.Mackinney G. Absorption of light by chlorophyll solutions. J Biol Chem. 1941;140:315–322. [Google Scholar]
- 13.Madueño F, Borrias W E, van Arkel G A, Guerrero M G. Isolation and characterization of Anacystis nidulans R2 mutants affected in nitrate assimilation: establishment of two new mutant types. Mol Gen Genet. 1988;213:223–228. [Google Scholar]
- 14.Maeda S, Omata T. Substrate-binding lipoprotein of the cyanobacterium Synechococcus sp. strain PCC 7942 involved in the transport of nitrate and nitrite. J Biol Chem. 1997;272:3036–3041. doi: 10.1074/jbc.272.5.3036. [DOI] [PubMed] [Google Scholar]
- 15.Omata T. Cloning and characterization of the nrtA gene that encodes a 45-kDa protein involved in nitrate transport in the cyanobacterium Synechococcus PCC 7942. Plant Cell Physiol. 1991;32:151–157. [Google Scholar]
- 16.Omata T, Andriesse X, Hirano A. Identification and characterization of a gene cluster involved in nitrate transport in the cyanobacterium Synechococcus sp. PCC 7942. Mol Gen Genet. 1993;236:193–202. doi: 10.1007/BF00277112. [DOI] [PubMed] [Google Scholar]
- 17.Omata T, Ohmori M, Arai N, Ogawa T. Genetically engineered mutant of the cyanobacterium Synechococcus PCC 7942 defective in nitrate transport. Proc Natl Acad Sci USA. 1989;86:6612–6616. doi: 10.1073/pnas.86.17.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quesada A, Galván A, Fernández E. Identification of nitrate transporter genes in Chlamydomonas reinhardtii. Plant J. 1994;5:407–419. doi: 10.1111/j.1365-313x.1994.00407.x. [DOI] [PubMed] [Google Scholar]
- 19.Stanier R Y, Kunisawa R, Mandel M, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriol Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki I, Sugiyama T, Omata T. Regulation by cyanate of the genes involved in carbon and nitrogen assimilation in the cyanobacterium Synechococcus sp. strain PCC 7942. J Bacteriol. 1996;178:2688–2694. doi: 10.1128/jb.178.9.2688-2694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Q, Stewart V. NasFED proteins mediate assimilatory nitrate and nitrite transport in Klebsiella oxytoca (pneumoniae) M5al. J Bacteriol. 1998;180:1311–1322. doi: 10.1128/jb.180.5.1311-1322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]