Abstract
Modifiable risk factors play an important role in the premature mortality among patients undergoing coronary artery bypass grafting (CABG). The aim of this study was to examine the factors that influence the early death of patients who had CABG. We conducted a prospective cohort study and followed 2863 patients after their CABG, and collected data on their characteristics and blood tests. We used the Cox proportional hazards regression model in Stata, version 16, to identify the predictors of early mortality. Out of 2863 patients, 162 died during the follow-up period. The survival rate was 99.2% within the first three days after the surgery, 96.2% from the fourth day to the end of the first year, 94.9% at the end of the second year, and 93.6% at the end of the third year. After adjusting for confounding factors, we found that older age (hazard ratio [HR] 1.05, 95% CI 1.02, 1.08 for one year increase in age), obesity (HR 2.16, 95% CI 1.25, 3.72), ejection fraction < 50% (HR 1.61, 95% CI 1.06, 2.44), number of rehospitalizations (HR 2.63, 95% CI 1.35, 5.12 for two or more readmissions), history of stroke (HR 2.91, 95% CI 1.63, 5.21), living in rural areas (HR 1.58, 95% CI 1.06, 2.34), opium use (HR 2.08, 95% CI 1.40, 3.09), and impaired glomerular filtration rate increased the risk of early death after CABG, while taking a beta-blocker (HR 0.59, 95% CI 0.38, 0.91) reduced the risk. We conclude that modifiable risk factors such as excess body mass, high blood glucose, opium use, and kidney dysfunction should be monitored and managed in patients who had CABG to improve their survival outcomes.
Keywords: cohort study, coronary artery bypass grafting, glomerular filtration rate, obesity, risk factors
1. Introduction
Patients with coronary artery disease often undergo coronary artery bypass grafting (CABG), a surgical procedure that restores blood flow to the heart. In the United States, about 500,000 CABG surgeries are performed every year [1]. However, CABG is not without risks, and can lead to postoperative complications [2,3]. Postoperative complications are a major cause of readmission after CABG, despite the improvements in surgical techniques [2]. According to previous research, 14% of patients who had CABG experienced complications, and 2% died within 30 days after the surgery [4]. Another study found that the increased risk of death and rehospitalization persisted for up to 7 years after CABG [5]. The mortality rate of CABG patients at 1 year after the surgery is around 5% [6]. According to research in Iran, a study on all kinds of heart surgeries revealed that isolated CABG was the most common procedure with the lowest in-hospital mortality rate of 0.5% [7]. The percentage of patients who survived one year and five years after CABG was 92% and 83%, respectively [8]. Another Iranian study reported that the survival rate of patients who underwent CABG with coronary endarterectomy was about 80% after 20 months of follow-up [9].
Previous research has shown that black patients have a higher mortality rate than white patients after CABG [10], and that socioeconomic factors such as low education, low income, and being single [11] are also linked to higher mortality. Smoking history [12] is another factor that increases the risk of death from any cause in patients with coronary artery disease who undergo CABG. On the other hand, some studies have reported lower mortality and fewer adverse cardiovascular events and hospital readmissions after CABG in overweight patients [13,14,15]. Postoperative complications such as atrial fibrillation [16] and acute kidney injury [17] are associated with higher short-term and long-term mortality after CABG, as well as longer stays in the intensive care unit and hospital [2]. However, few studies have examined the modifiable risk factors for early death after CABG in Iranian patients with coronary artery disease [18,19,20], and none have focused on long-term outcomes. Understanding the causes of early death after CABG is crucial for developing better treatment and prevention strategies to improve survival rates [21]. The purpose of this study was to identify the factors that influence early death after CABG in Iranian patients with coronary artery disease.
2. Subjects and Methods
We conducted a cohort study of CABG patients at Farshchian Heart Centre, the main specialized cardiovascular centre in western Iran. We retrospectively reviewed the medical records of eligible patients who underwent CABG from September 2015 to October 2017, and prospectively followed them up from October 2017 to March 2020. We collected the baseline characteristics from the medical records and the survival and mortality data from phone calls with patients or their families. We included patients who underwent CABG between October 2015 and March 2020 (N = 3156 patients). We excluded patients who had other cardiac surgeries such as heart valve replacement or repair, atrial and ventricular wall repair, or cardiac tumour resection (N = 293 patients). Patients who had typical chest pain or positive noninvasive tests for ischemia underwent angiography. Patients who had one-, two-, or three-vessel disease or left main coronary artery disease and were not suitable for angioplasty according to the guidelines underwent CABG. The final sample size was 2863 patients. In this study, we obtained the patients’ data from their medical records, and followed-up with them once by phone. For 638 patients, the follow-up ended until the patients’ discharge from the hospital, and we were not able to contact them further. The remaining patients were contacted at various time points after surgery, ranging from a few days to five years.
We collected data on demographic, behavioural, and clinical variables of the patients, such as age, sex, weight, height, residence area, smoking status, opium use, hypertension, diabetes, previous angioplasty or surgery, recent myocardial infarction, number of bypassed vessels, atrial fibrillation before and after surgery, beta-blocker use, stroke history, cardiopulmonary bypass (on-pump or off-pump), pulmonary diseases, and readmission. The sources of the data were the patient’s health record, medical history, clinical report, nursing notes, sonography report, angiogram, and surgical report. We also measured the blood levels of various biomarkers, including fasting blood glucose, white blood cells, neutrophil to lymphocyte ratio, red blood cells, haemoglobin, haematocrit, total cholesterol, triglycerides, LDL and HDL cholesterol, blood urea nitrogen (BUN), creatinine, glomerular filtration rate (GFR) and cardiac ejection fraction. We defined smoking as current smoking. We calculated body mass index (BMI) as weight divided by height squared (kg/m2) and categorized it as normal (BMI < 25 kg/m2), overweight (BMI 25–29.9 kg/m2), or obese (BMI ≥ 30 kg/m2). We defined normal blood glucose as fasting blood glucose level below 99 mg/dL, prediabetes as fasting blood glucose level between 100 and 125 mg/dL, and diabetes as fasting blood glucose level above 126 mg/dL. The outcome of interest was death from any cause within the five years after CABG.
We used the Sysmex kx-21 N device, which employs electrical resistance technology, to perform blood cell counts. We defined white blood cell counts of 10,000/mm3 or more as elevated. We defined low levels of red blood cells, haemoglobin, and haematocrit as less than 3.9 mL/m3, less than 11.5 g/dL, and less than 36%, respectively. A pathologist verified the neutrophil to lymphocyte ratio obtained from the device and confirmed it. We considered a ratio of 2.15 or higher as an elevated neutrophil to lymphocyte ratio [22,23]. We defined serum cholesterol levels of 200 to 239 mg/dL as borderline, and above 240 mg/dL as high. We defined triglyceride levels of 200 to 399 mg/dL as borderline, and above 400 mg/dL as high. We defined HDL levels below 30 mg/dL as low, and LDL levels above 150 mg/dL as high. We assessed kidney function with BUN, creatinine, and GFR. We defined high BUN and blood creatinine levels as above 25 mg/dL, above 1.1 in women, and above 1.4 mg/dL in men, respectively. We classified GFR into six categories: (1) normal (>90 mL/min/1.73 m2), (2) slight decrease (60 to 89), (3) mild to moderate decrease (45 to 59), (4) moderate to severe decrease (30 to 44), (5) severe reduction (15 to 29), and (6) renal failure (<15 mL/min/1.73 m2) [24]. We classified fasting blood glucose levels into three levels: normal (70 to 99 mg/dL), borderline (100 to 125 mg/dL), and diabetic (> 126 mg/dL) [25]. We measured all of the above biochemical tests using a BT 3500 device (Biotecnica Instruments S.p.A, Rome, Italy).
Statistical Analysis
We applied univariate and multivariate Cox proportional hazards regression models to analyze this time-to-event data. We first performed a univariable Cox proportional hazards regression model for each variable, and then included all the variables with p-value < 0.30 in the univariable models in the multivariable models that estimated premature mortality. The variables with p-value ≥ 0.05 were removed from the final multivariable model. We used Stata, version 16 for the analyses.
3. Results
The final analysis included 2863 patients. The mean age of the patients was 62.9 ± 9.4 years, and the majority of the patients were in the 60–69 years old age group (40.17%, N = 1150). Men accounted for 73.38% (N = 2101) of the surgical cases. Most of the patients resided in urban areas (76.39%, N = 2187). The prevalence of hypertension was 49.95% (N = 1430), and 32.06% of the patients were on beta-blocker therapy (Table 1).
Table 1.
The baseline characteristics of the study population (N = 2863).
| Characteristic | N | % | Mean | SD |
|---|---|---|---|---|
| Age group (years) | 62.94 | 9.38 | ||
| <50 | 238 | 8.31 | ||
| 50–59 | 758 | 26.48 | ||
| 60–69 | 1150 | 40.17 | ||
| 70–79 | 608 | 21.24 | ||
| ≥80 | 109 | 3.81 | ||
| Male sex | 2101 | 73.38 | ||
| Body mass index | 26.26 | 4.07 | ||
| Normal | 1159 | 40.48 | ||
| Overweight | 1199 | 41.88 | ||
| Obesity | 505 | 17.64 | ||
| Residential area | ||||
| Urban | 2187 | 76.39 | ||
| Rural | 676 | 23.61 | ||
| Current smoking | 858 | 29.97 | ||
| Hypertension | 1430 | 49.95 | ||
| Chronic obstructive pulmonary disease (COPD) | 180 | 6.29 | ||
| Atrial fibrillation | 162 | 5.66 | ||
| Diabetes (≥ 126 mg/dL) | 549 | 19.20 | ||
| Kidney failure † | 20 | 0.70 | ||
| Ejection fraction | 42.83 | 8.80 | ||
| Use of beta-blocker | 918 | 32.06 |
† Glomerular filtration rate < 15 mL/min/1.73 m2.
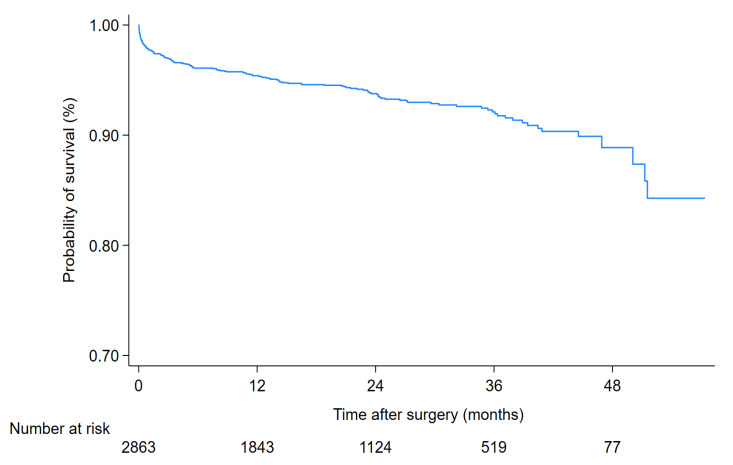
Out of 2863 patients who underwent CABG, 162 died within five years. The survival rate after CABG was 99.2% within the first three days after the surgery, 96.2% at the end of the first year, 94.9% at the end of the second year, and 93.6% at the end of the third year (Figure 1 and Table 2).
Figure 1.
Survival rate of the 2863 patients who underwent CABG.
Table 2.
Survival rate of the study population who underwent CABG by year of follow-up.
| Follow-Up Time | At Risk | Deaths | Lost | Survival (%) | 95% CI |
|---|---|---|---|---|---|
| The first 3 days after surgery | 2863 | 22 | 12 | 99.2 | 98.8–99.5 |
| The 4th day to 1 year | 2829 | 86 | 900 | 96.2 | 95.4–96.9 |
| The 2nd year | 1843 | 26 | 692 | 94.9 | 93.9–95.7 |
| The 3rd year | 1125 | 15 | 589 | 93.6 | 92.4–94.6 |
| The 4th year | 521 | 10 | 434 | 91.8 | 90.1–93.2 |
| The 5th year | 77 | 3 | 74 | 88.2 | 83.2–91.8 |
Cardiovascular causes accounted for most of the deaths (126 cases, 77.8%), while the rest (36 cases, 22.2%) were due to noncardiac causes. The noncardiac causes were stroke (n = 13), cancer (n = 10), COVID-19 (n = 5), COPD (n = 2), diabetes (n = 2), pelvic fracture (n = 2), and kidney failure (n = 2) (Table 3).
Table 3.
Outcome events among the study population.
| Outcome | N | % |
|---|---|---|
| Death | ||
| No | 2701 | 94.34 |
| Yes | 162 | 5.66 |
| Causes of death | ||
| Heart disease | 126 | 77.78 |
| Stroke | 13 | 8.02 |
| Pelvic fracture | 2 | 1.23 |
| Cancer | 10 | 6.17 |
| COVID-19 | 5 | 3.09 |
| Chronic obstructive pulmonary disease | 2 | 1.23 |
| Diabetes | 2 | 1.23 |
| Kidney disease | 2 | 1.23 |
As shown in Table 4, age, place of residence, opium use, ejection fraction, history of stroke, beta-blocker use, and the number of rehospitalizations were associated with mortality after CABG. There was no significant difference in the all-cause mortality rate between men and women.
Table 4.
Univariable hazard ratio (HR) for the associations of demographic and clinical characteristics with all-cause mortality rate following coronary artery bypass graft (CABG).
| Characteristic | Sample | Death | HR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Age group (years) | |||||
| <50 | 233 | 5 | 1 | ||
| 50–59 | 730 | 28 | 2.71 | 0.63, 11.65 | 0.179 |
| 60–69 | 1094 | 56 | 3.46 | 0.83, 14.37 | 0.088 |
| 70–79 | 555 | 53 | 7.45 | 1.80, 30.84 | 0.006 |
| ≥80 | 89 | 20 | 18.04 | 4.13, 78.92 | 0.001 |
| Sex | |||||
| Male | 1987 | 114 | 1 | ||
| Female | 714 | 48 | 0.92 | 0.60, 1.41 | 0.700 |
| Residential area | |||||
| Urban | 2075 | 112 | 1 | ||
| Rural | 626 | 50 | 1.89 | 1.24, 2.67 | 0.002 |
| Body mass index | |||||
| Normal | 1092 | 67 | 1 | ||
| Overweight | 1141 | 58 | 0.97 | 0.64, 1.48 | 0.900 |
| Obese | 468 | 37 | 1.29 | 0.78, 2.11 | 0.319 |
| Smoking status | |||||
| Never or past | 1889 | 116 | 1 | ||
| Current | 812 | 46 | 1.17 | 0.79, 1.73 | 0.429 |
| Opium use | |||||
| No | 1933 | 112 | 1 | ||
| Yes | 768 | 50 | 1.56 | 1.06, 2.28 | 0.023 |
| Ejection fraction | |||||
| ≥50% | 1531 | 47 | 1 | ||
| <50% | 1170 | 115 | 1.82 | 1.21, 2.73 | 0.004 |
| Hypertension | |||||
| No | 1362 | 71 | 1 | ||
| Yes | 1339 | 91 | 1.05 | 0.73, 1.52 | 0.784 |
| Percutaneous coronary intervention | |||||
| No | 2582 | 155 | 1 | ||
| Yes | 119 | 7 | 0.77 | 0.28, 2.09 | 0.607 |
| Myocardial infarction | |||||
| No | 2277 | 129 | 1 | ||
| Yes | 424 | 33 | 1.11 | 0.69, 1.77 | 0.666 |
| Coronary artery disease | |||||
| 1 or 2 vessels | 745 | 34 | 1 | ||
| 3 or more vessels | 1956 | 128 | 1.45 | 0.92, 2.30 | 0.109 |
| Post-CABG arrhythmia | |||||
| No | 2649 | 150 | 1 | ||
| Yes | 52 | 12 | 0.90 | 0.22, 3.60 | 0.870 |
| Pre-CABG arrhythmia | |||||
| No | 2454 | 134 | 1 | ||
| Yes | 247 | 28 | 1.68 | 0.99, 2.86 | 0.055 |
| Use of beta-blocker | |||||
| No | 1829 | 116 | 1 | ||
| Yes | 872 | 46 | 0.54 | 0.35, 0.83 | 0.005 |
| History of stroke | |||||
| No | 2627 | 146 | 1 | ||
| Yes | 74 | 16 | 4.02 | 2.29, 7.04 | 0.001 |
| Cardiopulmonary bypass | |||||
| On-Pump | 2152 | 126 | 1 | ||
| Off-Pump | 549 | 36 | 1.52 | 0.99, 2.33 | 0.056 |
| Chronic obstructive pulmonary disease (COPD) | |||||
| No | 2532 | 151 | 1 | ||
| Yes | 169 | 11 | 0.89 | 0.41, 1.91 | 0.765 |
| Readmission times | |||||
| 0 | 2352 | 115 | 1 | ||
| 1 | 268 | 32 | 2.60 | 1.65, 4.09 | 0.001 |
| ≤2 | 81 | 15 | 2.88 | 1.49, 5.58 | 0.002 |
| Atrial fibrillation | |||||
| No | 2649 | 52 | 1 | ||
| Yes | 150 | 12 | 0.98 | 0.22, 3.60 | 0.870 |
Table 5 shows the univariable hazard ratios of how laboratory characteristics relate to mortality rate after CABG.
Table 5.
Univariable hazard ratio (HR) for the associations of laboratory characteristics with all-cause mortality rate following CABG.
| Characteristic | Sample | Death | HR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Prediabetes (blood glucose 100–125 mg/dL) | |||||
| No | 2306 | 130 | 1 | ||
| Yes | 391 | 32 | 1.56 | 0.99, 2.47 | 0.057 |
| Diabetes (blood glucose ≥ 126 mg/dL) | |||||
| No | 2190 | 120 | 1 | ||
| Yes | 507 | 42 | 1.03 | 0.64, 1.66 | 0.888 |
| White blood cell (×1000/mm3) | |||||
| Normal (≤10) | 2166 | 93 | 1 | ||
| Abnormal (>10) | 535 | 69 | 1.95 | 1.32, 2.88 | 0.001 |
| Neutrophil/lymphocyte ratio | |||||
| Low (<2.15) | 932 | 28 | 1 | ||
| High (≥2.15) | 1769 | 134 | 1.71 | 1.11, 2.65 | 0.014 |
| Red blood cell (Mill/mm3) | |||||
| Low (<3.9) | 1713 | 87 | 1 | ||
| Normal (≥3.9) | 988 | 75 | 1.88 | 1.30, 2.73 | 0.001 |
| Hemoglobin (g/dL) | |||||
| Low (<11.5) | 1789 | 90 | 1 | ||
| Normal (≥11.5) | 912 | 72 | 1.77 | 1.22, 2.56 | 0.003 |
| Hematocrit (%) | |||||
| Low (<36) | 2026 | 96 | 1 | ||
| Normal (≥36) | 675 | 66 | 2.28 | 1.57, 3.31 | 0.001 |
| Cholesterol (mg/dL) | |||||
| Normal (<200) | 918 | 63 | 1 | ||
| Borderline (200–239) | 205 | 14 | 0.62 | 0.26, 1.46 | 0.275 |
| High (≥240) | 85 | 7 | 1.15 | 0.78, 3.87 | 0.177 |
| Triglyceride (mg/dL) | |||||
| Normal (<200) | 908 | 73 | 1 | ||
| Borderline (200–399) | 277 | 7 | 0.35 | 0.14, 0.90 | 0.029 |
| High (≥400) | 27 | 5 | 1.95 | 0.85, 6.61 | 0.097 |
| High-density lipoprotein (mg/dL) | |||||
| Low (<30) | 150 | 8 | 1 | ||
| Normal (≥30) | 1150 | 76 | 0.78 | 0.33, 1.84 | 0.570 |
| Low-density lipoprotein (mg/dL) | |||||
| Low (<70) | 303 | 18 | 1 | ||
| Normal (70–149) | 879 | 56 | 1.00 | 0.54, 1.85 | 0.996 |
| High (≥150) | 118 | 11 | 1.44 | 0.55, 3.77 | 0.455 |
| Blood urea nitrogen (mg/dL) | |||||
| Normal (<25) | 2460 | 103 | 1 | ||
| High (≥25) | 241 | 59 | 3.56 | 2.34, 5.41 | 0.001 |
| Creatinine (mg/dL) | |||||
| Normal (Male: <1.4, Female: <1.1) | 2386 | 97 | 1 | ||
| High (Male: ≥1.4, Female: ≥1.1 | 315 | 65 | 3.56 | 2.34, 5.41 | 0.001 |
| Kidney function | |||||
| Normal (GFR 90 mL/min/1.73 m2) | 627 | 16 | 1 | ||
| Mildly decreased (GFR 60–89 mL/min/1.73 m2) | 1213 | 45 | 1.37 | 0.72, 2.64 | 0.339 |
| Mildly to moderately decreased (GFR 45–59 mL/min/1.73 m2) | 592 | 32 | 1.82 | 0.92, 3.61 | 0.087 |
| Moderately to severely decreased (GFR 30–44 mL/min/1.73 m2) | 223 | 41 | 4.62 | 2.31, 9.23 | 0.001 |
| Severely decreased (GFR <29 mL/min/1.73 m2) | 37 | 17 | 13.85 | 6.31, 30.39 | 0.001 |
The associations of clinical and paraclinical characteristics with all-cause mortality rate after CABG are shown in Table 6, using multivariable hazard ratio (HR) analysis. Age was a significant predictor, and the mortality risk increased with age (HR 1.05, 95% CI 1.02, 1.08 for one year increase). Obese patients had a 2.16-fold higher mortality rate after CABG. The number of postsurgery hospitalizations was also an important factor, with a 2.63-fold (95% CI 1.35, 5.12) higher mortality risk for those who were hospitalized two or more times. The mortality rate after surgery increased with the decrease in ejection fraction (HR 1.61, 95% CI 1.06, 2.44). A lower glomerular filtration rate (GFR) was associated with a higher risk of death. Patients with GFR less than 29 had an 8.68-fold higher risk of death after coronary artery bypass surgery. Moreover, history of stroke, opium use, prediabetes, and living in rural areas were associated with increased risk of death after CABG. Beta-blockers had a protective effect after CABG (HR 0.59, 95% CI 0.38, 0.91).
Table 6.
Multivariable hazard ratio (HR) for the associations of clinical and paraclinical characteristics with all-cause mortality rate following CABG.
| Characteristic | HR † | 95% CI | p |
|---|---|---|---|
| Age (1-year increase) | 1.05 | 1.02, 1.08 | 0.001 |
| Body mass index (Ref: normal) | |||
| Overweight | 1.43 | 0.91, 2.24 | 0.123 |
| Obese | 2.16 | 1.25, 3.72 | 0.006 |
| Ejection Fraction (Ref: ≥50%) | |||
| <50% | 1.61 | 1.06, 2.44 | 0.026 |
| History of stroke | 2.91 | 1.63, 5.21 | 0.001 |
| Residential area (Ref: urban) | |||
| Rural | 1.58 | 1.06, 2.34 | 0.023 |
| Opium use | 2.08 | 1.40, 3.09 | 0.001 |
| Use of beta-blocker | 0.59 | 0.38, 0.91 | 0.018 |
| Readmission times (Ref: 0) | |||
| 1 | 2.28 | 1.43, 3.62 | 0.001 |
| >2 | 2.63 | 1.35, 5.12 | 0.004 |
| Prediabetes | 1.70 | 1.05, 2.75 | 0.029 |
| Diabetes | 1.07 | 0.65, 1.77 | 0.783 |
| Kidney function | |||
| Normal (GFR 90 mL/min/1.73 m2) | 1 | ||
| Mildly decreased (GFR 60–89 mL/min/1.73 m2) | 1.09 | 0.55, 2.18 | 0.803 |
| Mildly to moderately decreased (GFR 45–59 mL/min/1.73 m2) | 1.17 | 0.54, 2.55 | 0.693 |
| Moderately to severely decreased (GFR 30–44 mL/min/1.73 m2) | 2.32 | 0.99, 5.42 | 0.051 |
| Severely decreased (GFR <29 mL/min/1.73 m2) | 8.68 | 3.44, 21.91 | 0.001 |
† Adjusted for all variables in the model; GFR: glomerular filtration rate.
4. Discussion
The current study indicates that several factors, such as obesity, atrial fibrillation, reduced ejection fraction, high blood glucose, and low glomerular filtration rate increase the risk of death from any cause after CABG in patients with coronary disease.
The role of sex in short- and long-term survival after CABG is unclear. Some studies have reported longer survival for women than for men after CABG [26], while others have found the opposite [27]. Moreover, one study [21] reported higher mortality for women within 1 year after CABG, but higher mortality for men between 1 and 7 years after CABG. However, in the current study, we did not observe any sex difference in mortality rate. The mean age of patients was high for both sexes, and the present study showed that the most important predictor of mortality after CABG is the patient’s age rather than patient’s sex. This is consistent with another study in Iran that found no sex difference in postoperative mortality [20]. As the prevalence of comorbid chronic conditions rises among the elderly population, they need to have improved perioperative care procedures [28].
Our study revealed that preoperative renal disease is a significant predictor of mortality after CABG. This is consistent with a previous study that reported a higher mortality rate among patients with chronic renal failure 1 to 7 years after CABG [21]. Obesity is a well-known risk factor for cardiovascular disease. However, some studies have suggested a paradox of obesity, where overweight and obese patients with cardiovascular disease have a better outcome after CABG than normal-weight patients with cardiovascular disease [29]. A meta-analysis [30] found that overweight patients, but not obese patients, had lower short-term and long-term mortality after CABG than normal-weight patients. Another meta-analysis [31] showed that mortality after cardiac surgery was not affected by a slight increase in body mass index, but was worse for underweight and extremely obese patients. According to a study conducted on Iranian patients (N = 235), there was no difference in the mortality rates at the hospital or after 3 months of CABG between obese and nonobese patients [32]. However, that study compared patients having a BMI of 30 kg/m2 or above with patients who had a BMI below 30. The inclusion of overweight patients in the comparison group might have weakened the relationship. In contrast to these findings, we observed that obesity was associated with a 1.79-fold higher mortality rate than normal weight after CABG, while there was no difference between normal-weight and overweight patients.
Diabetes and hypertension are common comorbidities in patients with chronic renal disease and cardiovascular diseases. Diabetes [33] and arrhythmias, especially atrial fibrillation [16,34], are prevalent in patients with coronary disease who undergo CABG. In agreement with a prior study [35], we found that prediabetes was associated with a higher mortality rate than nondiabetes after CABG. This could be explained by the inadequate management of blood glucose levels in patients with borderline blood glucose.
Our study showed that beta-blockers could lower the mortality rate after CABG. However, the effect of beta-blockers on mortality and major cardiac complications in patients with coronary heart disease remains unclear. Some evidence suggests that beta-blockers do not affect major cardiovascular events in patients with coronary artery disease [36], while other studies indicate that beta-blockers could decrease the mortality rate in patients who had a recent heart attack [37] or heart failure with systolic dysfunction [38]. In addition, the use of beta-blockers in patients with recent myocardial infarction was linked to a lower risk of a heart attack [39].
We found that rural residents had a higher mortality rate after CABG than urban residents. This may be because rural residents have more risk factors for heart disease and more severe heart problems due to less access to healthcare services. Another possible factor that may account for the increased risk of death after CABG among people living in rural areas compared to those living in urban areas is the lower educational level of the former group [40]. Moreover, in people with low education and cardiometabolic comorbidities, there may be an effect on the progression of chronic diseases. It is important to improve the preventive strategies for this specific patient group [41]. Education is a key factor that influences the overall heart risk in people with high blood pressure. Therefore, it should be carefully evaluated and included in the management of high blood pressure and heart risks [42]. Smoking did not predict the mortality rate in our study. The lack of association was due to comparing current smokers with never or past smokers. Past smokers quit smoking because of their heart disease. Our study showed that lower glomerular filtration rate was associated with higher mortality risk after cardiac surgery. However, another study found that moderate but not mild renal impairment significantly affected the survival of patients with multivessel disease who underwent percutaneous coronary intervention or coronary artery bypass grafting [43]. This study found that having a stroke before undergoing CABG increased the mortality risk after the surgery. This finding is consistent with other studies that also reported a higher risk of death associated with preoperative stroke [44,45].
The current study had some limitations that should be acknowledged. We only recruited patients who underwent surgery at a single specialized centre. This centre serves more than 1.8 million people in Hamadan province. However, to increase the generalizability of our results, a multicentre study is needed to verify our findings. The 5-year survival rate that we estimated may not reflect the true situation due to a high rate of lost-to-follow up. The patients who were lost to follow-up might have a different mortality risk than those who remained. The main reason for the high rate of lost-to-follow up was the absence of electronic medical records and the incorrect contact information in the medical files. Moreover, we only contacted the patients once during the follow-up period, and we could not access the death records of the patients who were lost to follow-up. In addition, we did not assess the effects of education, income, leisure-time physical activity, depression, and psychological distress on the mortality rate because we lacked reliable data on these variables for the patients. These factors had missing data for some participants. Therefore, we could not calculate any risk scores due to the incomplete data on some risk factors.
5. Conclusions
Patients who have coronary artery disease can live longer if they monitor and control some risk factors that can worsen their condition. These include obesity, high blood glucose, opium use, and kidney problems. The results of the current study suggest that taking beta-blockers can lower the risk of death after CABG surgery.
Author Contributions
Conceptualization, F.P., J.P. and R.S.; data curation, A.M.M. and R.M.S.; formal analysis, J.P. and F.P.; investigation, F.P., A.M.M. and R.M.S.; methodology, R.S. and J.P.; project administration, J.P.; writing—original draft, F.P. and R.S.; writing—review and editing, F.P., R.S., A.M.M. and J.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The ethics committee of Hamadan University of Medical Sciences (ID IR.UMSHA.REC.1396.611) approved the study (14/08/2018).
Informed Consent Statement
The verbal consent was obtained from the patients by phone call.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Squiers J.J., Mack M.J. Coronary artery bypass grafting—Fifty years of quality initiatives since Favaloro. Ann. Cardiothorac. Surg. 2018;7:516. doi: 10.21037/acs.2018.05.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montrief T., Koyfman A., Long B. Coronary artery bypass graft surgery complications: A review for emergency clinicians. Am. J. Emerg. Med. 2018;36:2289–2297. doi: 10.1016/j.ajem.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Oshvandi K., Pakrad F., Mohamadi Saleh R., Seif Rabiei M.A., Shams A. Post-Operative Symptoms and Complications in Patients Having Undergone Coronary Artery Bypass Graft in Hamadan: A Descriptive Cross-Sectional Study. Jundishapur J. Chronic Dis. Care. 2020;9:e104180. doi: 10.5812/jjcdc.104180. [DOI] [Google Scholar]
- 4.Adelborg K., Horváth-Puhó E., Schmidt M., Munch T., Pedersen L., Nielsen P.H., Bøtker H.E., Toft Sørensen H. Thirty-Year Mortality After Coronary Artery Bypass Graft Surgery: A Danish Nationwide Population-Based Cohort Study. Circulation. Cardiovasc. Qual. Outcomes. 2017;10:e002708. doi: 10.1161/CIRCOUTCOMES.116.002708. [DOI] [PubMed] [Google Scholar]
- 5.Jawitz O.K., Gulack B.C., Brennan J.M., Thibault D.P., Wang A., O’Brien S.M., Schroder J.N., Gaca J.G., Smith P.K. Association of postoperative complications and outcomes following coronary artery bypass grafting. Am. Heart J. 2020;222:220–228. doi: 10.1016/j.ahj.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo H.D., Teoh L.K.K., Gaudino M.F., Fremes S., Kofidis T. The Asian system for cardiac operative risk evaluation for predicting mortality after isolated coronary artery bypass graft surgery (ASCORE-C) J. Card. Surg. 2020;35:2574–2582. doi: 10.1111/jocs.14836. [DOI] [PubMed] [Google Scholar]
- 7.Abbasi K., Karimi A., Abbasi S.H., Ahmadi S.H., Davoodi S., Babamahmoodi A., Movahedi N., Salehiomran A., Shirzad M., Bina P. Knowledge management in cardiac surgery: The second tehran heart center adult cardiac surgery database report. J. Tehran Univ. Heart Cent. 2012;7:111. [PMC free article] [PubMed] [Google Scholar]
- 8.Soltani M.H., Rasti M., Namayandeh S.M., Sarebanhassanabadi M. Short and long-term outcomes of patients with coronary artery bypass surgery. ARYA Atheroscler. 2021;17:1–6. doi: 10.22122/arya.v17i0.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemati M.H., Astaneh B., Khosropanah S. Outcome and graft patency in coronary artery bypass grafting with coronary endarterectomy. Korean J. Thorac. Cardiovasc. Surg. 2015;48:13. doi: 10.5090/kjtcs.2015.48.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benedetto U., Kamel M.K., Khan F.M., Angelini G.D., Caputo M., Girardi L.N., Gaudino M. Are racial differences in hospital mortality after coronary artery bypass graft surgery real? A risk-adjusted meta-analysis. J. Thorac. Cardiovasc. Surg. 2019;157:2216–2225.e4. doi: 10.1016/j.jtcvs.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen S., Giang K.W., Wallinder A., Rosengren A., Pivodic A., Jeppsson A., Karlsson M. Social factors, sex, and mortality risk after coronary artery bypass grafting: A Population-Based cohort study. J. Am. Heart Assoc. 2019;8:e011490. doi: 10.1161/JAHA.118.011490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma W.-Q., Wang Y., Sun X.-J., Han X.-Q., Zhu Y., Yang R., Liu N.-F. Impact of smoking on all-cause mortality and cardiovascular events in patients after coronary revascularization with a percutaneous coronary intervention or coronary artery bypass graft: A systematic review and meta-analysis. Coron. Artery Dis. 2019;30:367–376. doi: 10.1097/MCA.0000000000000711. [DOI] [PubMed] [Google Scholar]
- 13.Ma W.Q., Sun X.J., Wang Y., Han X.Q., Zhu Y., Liu N.F. Does body mass index truly affect mortality and cardiovascular outcomes in patients after coronary revascularization with percutaneous coronary intervention or coronary artery bypass graft? A systematic review and network meta-analysis. Obes. Rev. 2018;19:1236–1247. doi: 10.1111/obr.12713. [DOI] [PubMed] [Google Scholar]
- 14.Lv M., Gao F., Liu B., Pandey P., Feng Y., Wang Y., Zhang Y., Li Z. The Effects of Obesity on Mortality Following Coronary Artery Bypass Graft Surgery: A Retrospective Study from a Single Center in China. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021;27:e929912. doi: 10.12659/MSM.929912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang K., Wang J., Yang Y., An R. Adiposity in relation to readmission and all-cause mortality following coronary artery bypass grafting: A systematic review and meta-analysis. Obes. Rev. 2019;20:1159–1183. doi: 10.1111/obr.12855. [DOI] [PubMed] [Google Scholar]
- 16.Hung L.T., Minh Duc N.T., Nam N.H., Shah J., Tuan Anh P.T., Huan D.Q., Trang D.V., Loc L.Q., Zia S., Van Sy H. Effects of postoperative atrial fibrillation on cardiac surgery outcomes in Vietnam: A prospective multicenter study. Hosp. Pract. 2023;51:141–148. doi: 10.1080/21548331.2023.2192587. [DOI] [PubMed] [Google Scholar]
- 17.Djordjević A., Šušak S., Velicki L., Antonič M. Acute kidney injury after open-heart surgery procedures. Acta Clin. Croat. 2021;60:120–126. doi: 10.20471/acc.2021.60.01.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arabi A., Naghshtabrizi B., Baradaran H.R., Moradi Y., Asadi-Lari M., Mehrakizadeh A. Comparing clinical outcomes in patients with diabetes undergoing coronary artery bypass graft and percutaneous coronary intervention in real world practice in Iranian population. BMC Cardiovasc. Disord. 2022;22:75. doi: 10.1186/s12872-022-02521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faritous Z., Ardeshiri M., Yazdanian F., Jalali A., Totonchi Z., Azarfarin R. Hyperglycemia or high hemoglobin A1C: Which one is more associated with morbidity and mortality after coronary artery bypass graft surgery? Ann. Thorac. Cardiovasc. Surg. 2014;20:223–228. doi: 10.5761/atcs.oa.13.02282. [DOI] [PubMed] [Google Scholar]
- 20.Sadeghi R., Miri R., Kachoueian N., Sistanizad M., Hassanpour R. Differences in gender and outcomes following isolated coronary artery bypass graft (CABG) surgery. ARYA Atheroscler. 2023;19:1–11. doi: 10.48305/arya.2022.26640.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butt J.H., Sørensen R., Bäck C., Olsen P.S., Thorsteinsson K., Torp-Pedersen C., Gislason G.H., Køber L., Fosbøl E.L. Short-and long-term cause of death in patients undergoing isolated coronary artery bypass grafting: A nationwide cohort study. J. Thorac. Cardiovasc. Surg. 2018;156:54–60.e4. doi: 10.1016/j.jtcvs.2018.01.106. [DOI] [PubMed] [Google Scholar]
- 22.Kim S., Eliot M., Koestler D.C., Wu W.-C., Kelsey K.T. Association of Neutrophil-to-Lymphocyte Ratio with Mortality and Cardiovascular Disease in the Jackson Heart Study and Modification by the Duffy Antigen Variant. JAMA Cardiol. 2018;3:455–462. doi: 10.1001/jamacardio.2018.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emami F., Pakrad F., Poorolajal J., Naghshtabrizi B., Gholalikhani H.R., Alizamir A. Relation of Neutrophil to Lymphocyte Ratio with Myocardial Damage in Patients Undergoing Elective Percutaneous Coronary Intervention. Avicenna J. Clin. Med. 2019;25:185–192. doi: 10.21859/ajcm.25.4.185. [DOI] [Google Scholar]
- 24.National Institute for Health and Care Excellence Chronic Kidney Disease in Adults: Assessment and Management. [(accessed on 5 February 2023)]. Available online: https://www.nice.org.uk/guidance/cg182. [PubMed]
- 25.World Health Organization Mean Fasting Blood Glucose. [(accessed on 19 March 2023)]; Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/2380.
- 26.Nuru A., Weltzien J., Sandvik L., Tønnessen T., Bjørnstad J. Short-and long-term survival after isolated coronary artery bypass grafting, the impact of gender and age. Scand. Cardiovasc. J. 2019;53:342–347. doi: 10.1080/14017431.2019.1646430. [DOI] [PubMed] [Google Scholar]
- 27.Matyal R., Qureshi N.Q., Mufarrih S.H., Sharkey A., Bose R., Chu L.M., Liu D.C., Senthilnathan V., Mahmood F., Khabbaz K.R. Update: Gender differences in CABG outcomes—Have we bridged the gap? PLoS ONE. 2021;16:e0255170. doi: 10.1371/journal.pone.0255170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olufajo O.A., Wilson A., Zeineddin A., Williams M., Aziz S. Coronary Artery Bypass Grafting Among Older Adults: Patterns, Outcomes, and Trends. J. Surg. Res. 2021;258:345–351. doi: 10.1016/j.jss.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Lavie C.J., McAuley P.A., Church T.S., Milani R.V., Blair S.N. Obesity and cardiovascular diseases: Implications regarding fitness, fatness, and severity in the obesity paradox. J. Am. Coll. Cardiol. 2014;63:1345–1354. doi: 10.1016/j.jacc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Takagi H., Umemoto T., Group A. Overweight, but not obesity, paradox on mortality following coronary artery bypass grafting. J. Cardiol. 2016;68:215–221. doi: 10.1016/j.jjcc.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Liu X., Xie L., Zhu W., Zhou Y. Association of body mass index and all-cause mortality in patients after cardiac surgery: A dose-response meta-analysis. Nutrition. 2020;72:110696. doi: 10.1016/j.nut.2019.110696. [DOI] [PubMed] [Google Scholar]
- 32.Ardeshiri M., Faritous Z., Haghighi Z.O., Hosseini S., Baghaei R. Effect of obesity on mortality and morbidity after coronary artery bypass grafting surgery in Iranian patients. Anesthesiol. Pain Med. 2014;4:e18884. doi: 10.5812/aapm.18884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robich M.P., Sellke F.W. Diabetes and Cardiovascular Disease. Springer; Berlin/Heidelberg, Germany: 2023. Cardiac Surgery and Diabetes Mellitus; pp. 725–746. [Google Scholar]
- 34.Benedetto U., Gaudino M.F., Dimagli A., Gerry S., Gray A., Lees B., Flather M., Taggart D.P. Postoperative atrial fibrillation and long-term risk of stroke after isolated coronary artery bypass graft surgery. Circulation. 2020;142:1320–1329. doi: 10.1161/CIRCULATIONAHA.120.046940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raza S., Blackstone E.H., Houghtaling P.L., Rajeswaran J., Riaz H., Bakaeen F.G., Lincoff A.M., Sabik J.F. Influence of diabetes on long-term coronary artery bypass graft patency. J. Am. Coll. Cardiol. 2017;70:515–524. doi: 10.1016/j.jacc.2017.05.061. [DOI] [PubMed] [Google Scholar]
- 36.Arero A.G., Vasheghani-Farahani A., Soltani D. Meta-analysis of the usefulness of beta-blockers to reduce the risk of major adverse cardiovascular events in patients with stable coronary artery disease without prior myocardial infarction or left ventricular dysfunction. Am. J. Cardiol. 2021;158:23–29. doi: 10.1016/j.amjcard.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Yndigegn T., Lindahl B., Alfredsson J., Benatar J., Brandin L., Erlinge D., Haaga U., Held C., Johansson P., Karlström P. Design and rationale of randomized evaluation of decreased usage of beta-blockers after acute myocardial infarction (REDUCE-AMI) Eur. Heart J.-Cardiovasc. Pharmacother. 2023;9:192–197. doi: 10.1093/ehjcvp/pvac070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu X., Wang D.W. The progress and controversial of the use of beta blockers in patients with heart failure with a preserved ejection fraction. IJC Heart Vasc. 2020;26:100451. doi: 10.1016/j.ijcha.2019.100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersson C., Shilane D., Go A.S., Chang T.I., Kazi D., Solomon M.D., Boothroyd D.B., Hlatky M.A. Beta-blocker therapy and cardiac events among patients with newly diagnosed coronary heart disease. J. Am. Coll. Cardiol. 2014;64:247–252. doi: 10.1016/j.jacc.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 40.Meng L., Xu J., Li J., Hu J., Xu H., Wu D., Hu X., Zeng X., Zhang Q., Li J. Self-reported prevalence and potential factors influencing cardio-cerebral vascular disease among the Chinese elderly: A national cross-sectional study. Front. Cardiovasc. Med. 2022;9:979015. doi: 10.3389/fcvm.2022.979015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Chiara T., Scaglione A., Corrao S., Argano C., Pinto A., Scaglione R. Association between Low Education and Higher Global Cardiovascular Risk. J. Clin. Hypertens. 2015;17:332–337. doi: 10.1111/jch.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Chiara T., Scaglione A., Corrao S., Argano C., Pinto A., Scaglione R. Education and hypertension: Impact on global cardiovascular risk. Acta Cardiol. 2017;72:507–513. doi: 10.1080/00015385.2017.1297626. [DOI] [PubMed] [Google Scholar]
- 43.Kim T.O., Kang D.-Y., Ahn J.-M., Kim S.O., Lee P.H., Lee J., Kim J.H., Kim H.J., Kim J.B., Choo S.J. Prognostic impact of mildly impaired renal function in patients undergoing multivessel coronary revascularization. J. Am. Coll. Cardiol. 2022;79:1270–1284. doi: 10.1016/j.jacc.2022.01.035. [DOI] [PubMed] [Google Scholar]
- 44.Roy P., Brahme I., Reddy R.P., Wechsler L., Gleason T., Thirumala P.D. Meta-Analysis of Perioperative Stroke and Mortality in CABG Patients with Carotid Stenosis. Neurologist. 2020;25:113–116. doi: 10.1097/NRL.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 45.Jonsson K., Barbu M., Nielsen S.J., Hafsteinsdottir B., Gudbjartsson T., Jensen E.M., Silverborn M., Jeppsson A. Perioperative stroke and survival in coronary artery bypass grafting patients: A SWEDEHEART study. Eur. J. Cardio-Thorac. Surg. 2022;62:ezac025. doi: 10.1093/ejcts/ezac025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.