Abstract
The mainstream of the post-genome target-assisted breeding in crop plant species includes biofortification such as high-throughput phenotyping along with genome-based selection. Therefore, in this work, we used the Web-service Plant_SNP_TATA_Z-tester, which we have previously developed, to run a uniform in silico analysis of the transcriptional alterations of 54,013 protein-coding transcripts from 32,833 Arabidopsis thaliana L. genes caused by 871,707 SNPs located in the proximal promoter region. The analysis identified 54,993 SNPs as significantly decreasing or increasing gene expression through changes in TATA-binding protein affinity to the promoters. The existence of these SNPs in highly conserved proximal promoters may be explained as intraspecific diversity kept by the stabilizing natural selection. To support this, we hand-annotated papers on some of the Arabidopsis genes possessing these SNPs or on their orthologs in other plant species and demonstrated the effects of changes in these gene expressions on plant vital traits. We integrated in silico estimates of the TBP-promoter affinity in the AtSNP_TATAdb knowledge base and showed their significant correlations with independent in vivo experimental data. These correlations appeared to be robust to variations in statistical criteria, genomic environment of TATA box regions, plants species and growing conditions.
Keywords: TATA-binding protein, TATA box, noncoding polymorphism, gene expression, estimates in silico, genome-wide analysis, verification in vivo, target-assisted breeding
1. Introduction
Biofortification combines high-throughput phenotyping along with genome-based acceleration of selection [1]. This becomes a mainstream of the targeted breeding in agricultural plants [1]. Among whole-genome technologies, CRISPR/Cas-mediated editing of cis-regulatory elements is the most widespread for crop improvement [2,3,4]. For genome-editing of TATA-binding protein (TBP)-binding sites (TBP-sites) [5], the most evolutionally conservative [6] and obligatory [7,8] transcription initiation site in eukaryotic promoters [9,10], a Faecalibaculum rodentium Cas9 protein was recently discovered [5]. Moreover, all things being equal, the expression level of a gene increases with an increase in the TBP binding affinity for its 90 bp proximal promoter [11,12]. Additionally, the consensus for plant TBP-sites—i.e., tcacTATATATAg within (A+T)-rich local contextual environment—has been commonly accepted for more than a quarter of a century [13], in contrast to the TATA(a/t)A(a/t)g consensus of this site in the (G+C)-rich environment in animals [14]. The 500 bp regions just upstream of the transcription start sites (TSSs) are the commonly accepted promoters of the Arabidopsis thaliana genes because of their enrichment in cis-regulatory elements and depletion in single nucleotide polymorphisms (SNPs) [15]. In natural ecotypes of Arabidopsis, SNPs in the promoter regions between positions −40 and +40 relative to TSSs of the protein-coding genes affect expression of these genes [16]. In addition, SNPs in Arabidopsis promoters can change transcription start sites, binding of transcription factors, as well as the epigenetic status of promoters [17,18,19,20,21]. For example, the majority of natural accessions (>88%) have G at position −230 relative to the TSS of a floral repressor gene FLOWERING LOCUS C (FLC) and autumnal (high) expression in response to winter cold. On the other hand, A at this position promotes the usage of the alternative upstream (−100) transcription start site (uTSS) instead of the major one (mTSS) and by this reduces FLC expression and accelerates flowering [22]. The SNPs are unevenly distributed near the TSS; for example, in rice, the decline in SNP density was recorded at −250 bp, and this decline comes to a minimum at the TSS [23].
The 90 bp proximal promoter region covers all experimentally proven natural TBP-sites in eukaryotes, and we have previously created a Web service Plant_SNP_TATA_Z-tester (http://wwwmgs.bionet.nsc.ru/cgi-bin/mgs/tatascan_plant/start.pl, accessed 20 June 2023) for calculating in silico the effects of SNPs within the 90 bp proximal promoter region at the gene expression level [24]. It implements a three-step model of TBP-promoter molecular binding [25] such as (i) TBP slides along the promoter DNA-helix [26] ↔ (ii) TBP stops at a potential TBP-binding site (TBP-site) [6,27] ↔ (iii) the TBP–promoter complex is stabilized due to DNA bending at a 90° angle [28], as observed experimentally in vitro [29]. Additionally, the results of the Plant_SNP_TATA_Z-tester were successfully verified using twelve independent experiments measuring the effects of single, paired and multiple nucleotide substitutions, deletions and insertions, as well as artificial promoter constructs on the expression of tobacco and Arabidopsis genes in vivo, ex vivo and in vitro [24]. Finally, as a practical Plant_SNP_TATA_Z-tester implementation, we have discovered the fact of spontaneous selection in ancient times by farmers of the crop plants with reduced expression of genes encoding food allergens, namely β-amylase, albumin and globulin [30].
In the present work, using the Plant_SNP_TATA_Z-tester [24] we conducted the genome-wide in silico analysis of 871,707 Arabidopsis SNPs within 90 bp proximal promoters versus 48,321 protein-coding transcripts from 32,833 genes and calculated their effects in gene expression. We predicted 54,993 SNPs to decrease or increase TBP affinity to promoters and, consequently, gene expression. We illustrated the influence of some of the genes possessing these SNPs on plant development and responses to environmental changes with independent published experimental data in vivo and annotated the impact of some of them to plant vital traits.
2. Results and Discussion
2.1. The Genome-Wide Prediction of SNPs in Proximal Promoters
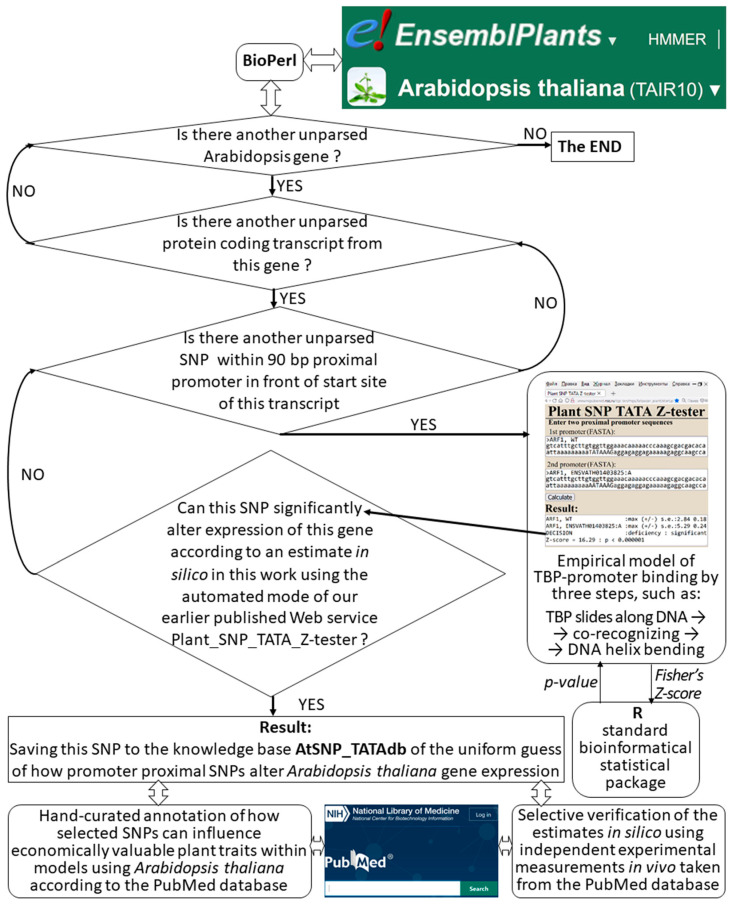
In this work, we identified in silico in the A. thaliana genome the proximal SNPs in promoters of protein-coding genes for the first time, which can significantly alter the TBP binding affinity, as schematically shown in Figure 1 and quantitatively characterized in Table 1. First of all, among Arabidopsis genes annotated in the Ensembl Plants database [31] (accessed on 16 October 2023), we selected 27,628 protein-coding genes (80%) as well as 48,321 protein-coding transcripts among 54,013 transcripts according to the current state of this database (Table 1: 89.5% of 100%). Then, using the automatic mode of Plant_SNP_TATA_Z-tester [24] (hereinafter, see “Supplementary Materials” [25,26,27,28,29,32,33,34,35,36,37,38], Section S1), we examined separately, one-by-one each of 871,707 single nucleotide substitutions (SNPs) localized within the 90 bp proximal region adjacent to the TSS of each transcript.
Figure 1.
Flowchart of AtSNP_TATAdb knowledge base development by processing genome-wide information from the Ensembl Plant database [31] by first using Plant_SNP_TATA_Z-tester [24] and, after that, selectively annotating some of the SNPs by a search in the PubMed database [39] using information about where, when and under what conditions changes in expression of these genes or their homolog genes were observed in agricultural studies. Finally, we selectively verified estimates in silico using independent experimental data in vivo taken from the PubMed database [39].
Table 1.
A summary of the search for candidate molecular markers of plant advantages related to Arabidopsis thaliana proximal promoter SNPs using the Ensembl Plant database [31] along with Plant_SNP_TATA_Z-tester [24], as schematically shown in Figure 1, the result of which is freely available in the AtSNP_TATAdb database created within this work (https://www.sysbio.ru/AtSNP_TATAdb/), as illustrated in Figure 2.
| # | Indicator | Result |
|---|---|---|
| Genome-wide analysis in silico: | ||
| 1 | Total number of the Arabidopsis thaliana genes documented in the Ensembl Plants database [31] and, thus, taken into account | 32,833 (100%) |
| 2 | Total number of the Arabidopsis thaliana protein-coding genes, which were selected for further analysis | 27,628 (80.04%) |
| 3 | Total number of the transcripts from the Arabidopsis thaliana genes taken into account | 54,013 (100%) |
| 4 | Total number of the protein-coding transcripts selected from the above amount of the Arabidopsis thaliana transcripts, which were selected for further analysis | 48,321 (89.46%) |
| 5 | Total number of the nucleotide substitutions (SNPs) localized in the proximal region of 90 bp in length just before TSS for the analyzed transcripts | 871,707 (100%) |
| 6 | Total number of candidate SNP markers having significant changes in the TBP binding affinity identified in this work | 54,993 (6.31%) |
| 7 | Total number of candidate SNP markers that down-regulated gene expression | 27,568 (3.16%) |
| 8 | Total number of candidate SNP markers that up-regulated gene expression | 27,425 (3.15%) |
| 9 | Total number of A. thaliana genes whose expression may be significantly altered by the promoter proximal SNPs changing the TBP binding affinity | 18,636 (56.78%) |
| 10 | The arithmetic mean estimate and its standard error rate of the equilibrium dissociation constant (KD) expressed in nanomoles per liter (nM) of the complexes between the TBP and only the ancestral alleles of the promoters of the Arabidopsis thaliana genes (Mean ± SEM) | 3.96 ± 0.01 nM |
| 11 | The arithmetic mean estimate and its standard error rate of the same KD-values in the case of only the minor alleles of the Arabidopsis thaliana promoters | 4.07 ± 0.01 nM |
| 12 | Statistical significance of the difference between ancestral and minor alleles of the Arabidopsis thaliana promoters examined by the arithmetic mean of the KD-values in question according to the following: | |
|
8.07 (10−7) | |
|
7.78 (10−2) | |
| 13 | The standard deviation of the same KD-values in the case of only the ancestral alleles of the Arabidopsis thaliana promoters | 1.95 nM |
| 14 | The standard deviation of the same KD-values in the case of only the minor alleles of the Arabidopsis thaliana promoters | 1.90 nM |
| 15 | Significance of the difference between ancestral and minor alleles of the A. thaliana promoters studied by the standard deviation of the KD-values by means of Fisher’s F-test and F-score (PADJ) | 1.06 (10−2) |
| 16 | Statistical significance of the difference between ancestral and minor alleles of the Arabidopsis thaliana promoters examined as the difference between the distributions of the KD-values according to the following: | |
|
298.17 (10−9) | |
|
0.04 (10−9) | |
| 17 | Total number of the Arabidopsis thaliana protein-coding genes whose SNP-related expression changes were hand annotated (Supplementary Materials: Table S1) | 109 |
| 18 | Total number of the plant benefits annotated within this work | 173 |
| 19 | Total number of the plant species whose gene expression changes were taken into account within the framework of our hand-curated annotation | 17 |
| 20 | Total number of the original articles on experimental observations of benefits from gene expression alterations cited in this work | 83 |
| 21 | Total number of the proximal promoter SNPs, which can statistically significantly alter expression of the Arabidopsis thaliana genes and their paralogs annotated within this work | 2426 |
Notes. PADJ, significance level according to Bonferroni correction for multiple comparisons.
As a result, 54,993 candidate SNP markers (6.3%) were identified as having significant changes in the TBP affinity for the promoters, namely 27,425 (3.2%) and 27568 (3.2%) SNPs, minor alleles of which significantly downregulate and upregulate the 18,636 Arabidopsis genes (56.78%), respectively.
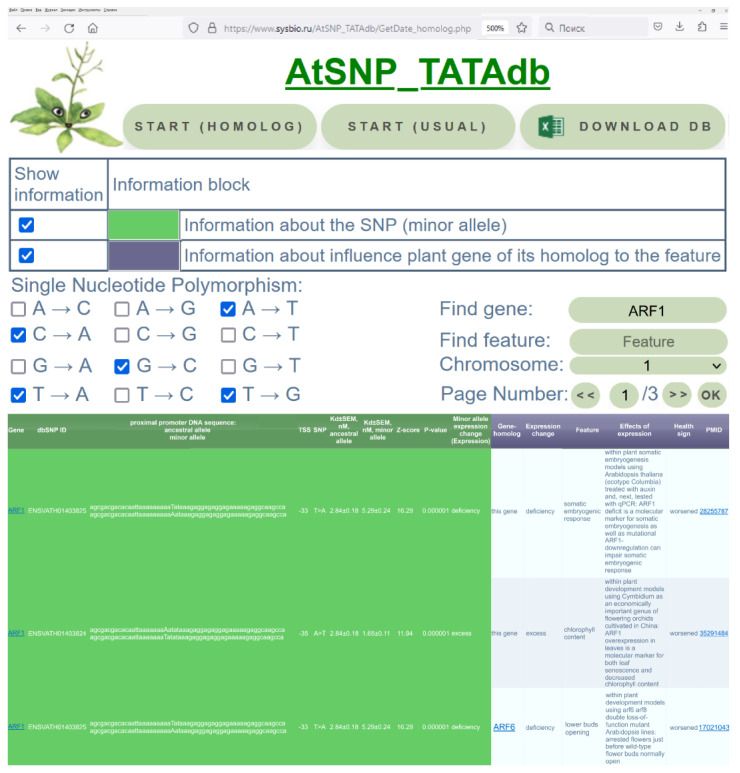
Finally, we saved these results in the database AtSNP_TATAdb, which was developed in this work as described in the “materials and methods” section and is illustrated in Figure 2. Clicking on the middle “Start (usual)” button in the interface depicted in Figure 2, one can find all these genome-wide results freely available (https://www.sysbio.ru/AtSNP_TATAdb/).
Figure 2.
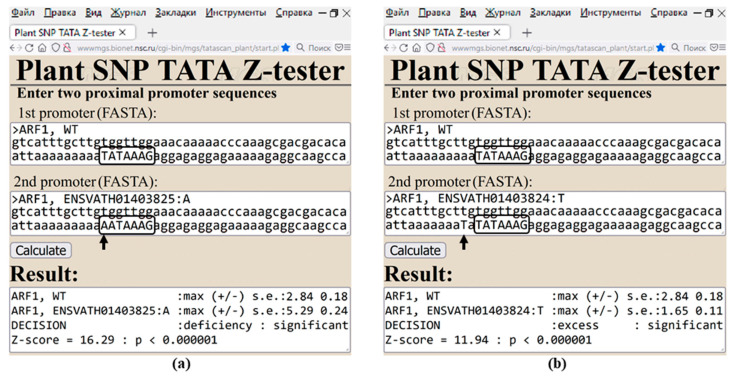
A sample entry in the AtSNP_TATAdb documents on two SNPs ENSVATH01403825:A and ENSVATH01403824:T of the ARF1 (Auxin Response Factor 1) gene, which can significantly downregulate and upregulate its expression, as calculated in this work together with their annotation (see Supplementary Materials, Table S1: first row) using the database PubMed [39] (PMIDs: 28255787, 35291484, 17021043).
2.2. Genome-Wide Statistical Analysis of How SNPs Can Affect TBP Binding to the Proximal Promoters of Arabidopsis Protein-Coding Genes
In rows 10 to 16 (Table 1), the readers can see the results of genome-wide statistical analysis of how all currently known SNPs located in the proximal promoters of protein-coding genes can affect TBP binding. First of all, within in silico calculations, the arithmetic mean and its standard error rate for the equilibrium dissociation constant (KD) of the complexes between the TBP and only ancestral alleles of the promoters (i.e., Mean ± SEM) equals 3.96 ± 0.01 nM. Additionally, in the case of only the minor alleles, this estimate was 4.07 ± 0.01 nM. Taking into account the Bonferroni correction for multiple comparisons, this difference between ancestral and minor SNP alleles is significant according to both the Student’s t-test (t = 8.07, PADJ < 10−7) and the Fisher’s Z-test (Z = 7.78, PADJ < 10−2).
Moreover, the standard deviation of KD values from their arithmetic mean estimated for minor variants unexpectedly turned out to be significantly less than that for ancestral variants of the same promoters by means of the Fisher’s F-test (respectively: 1.90 nM < 1.95 nM and F = 1.06, PADJ < 10−2). Finally, the significance of the differences between the distributions of KD-values for ancestral and minor alleles was also confirmed by both the Pearson chi-square (χ2 = 298.17, PADJ < 10−9) and Kolmogorov–Smirnov tests (D = 0.04, PADJ < 10−9).
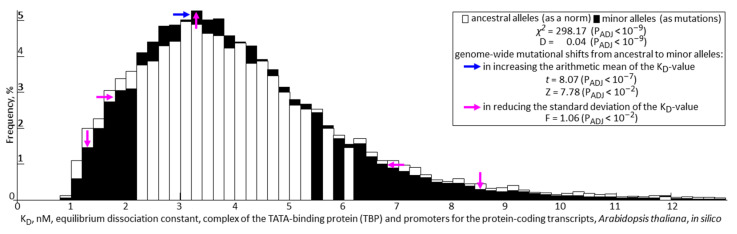
Figure 3 illustrates this result by distributions of the KD-values calculated in silico using separately either ancestral or minor alleles of the SNPs. The blue-colored right arrow (→) in this figure indicates a mutational shift from ancestral to minor alleles in the direction of increasing KD-values that corresponds to a decrease in the TBP binding affinity for minor versus ancestral alleles. The purple-colored arrows (→) symbolize mutational shifts in the direction of the reducing standard deviation of KD-values from ancestral to minor alleles that might be consistent with both types of alleles being under pressure of natural selection leading to stabilization.
Figure 3.
A comparison between the distributions of the KD-values calculated in silico using ancestral and minor alleles of the Arabidopsis SNPs. Legend: χ2 and D, the scores of Pearson’s chi-square test and Kolmogorov–Smirnov’s test for assessing the difference between two distributions, respectively; t and Z, the scores of Student’s t-test and Fisher’s Z-test for comparing the difference between two estimates of arithmetic means, respectively; F, Fisher’s F-test for comparing the difference between two estimates of the standard deviation; PADJ, statistical significance level with the Bonferroni correction for multiple comparisons calculated using STATISTICA version 10.0 (StatsoftTM, Tulsa, OK, USA).
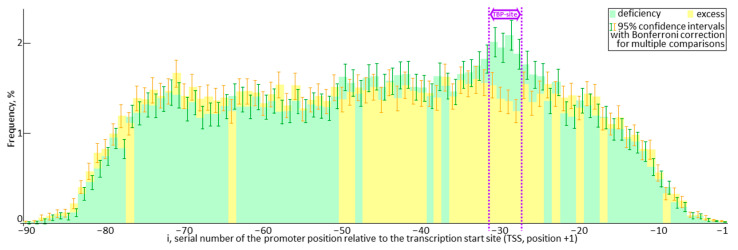
Because natural selection is usually associated with the vital traits of living organisms, it seems necessary to discuss these mutational shifts in the KD-values in more detail. Figure 4 specifies the predominant decrease in ratings in silico for the gene expression in the case of minor alleles of their proximal promoters in comparison with that for the ancestral ones. In this figure, light gray and dark gray bars at each position between −90 and −1 relative to the TSS (X-axis) display the frequency rates of SNP occurrence (Y-axis) for statistically significant decreases and increases in the expression of these genes.
Figure 4.
The frequencies of occurrence along promoters of candidate SNP markers that increase versus decrease the expression of protein-coding genes in Arabidopsis. Legend: error bars: the 95% confidence interval boundaries with the Bonferroni correction for multiple comparisons calculated using STATISTICA version 10.0 (StatsoftTM, Tulsa, OK, USA).
First of all, taking into account the 95% confidence intervals of these frequencies by means of the Bonferroni correction for multiple comparisons (error bars, “I”), there is only a region in the promoters where SNP-caused damage of TBP sites significantly prevails over those improvements of these sites (Figure 4: double-headed arrow “TBP-site” in-between two purple-colored broken lines). This may correspond to preferential mutational damage of TBP binding to minor alleles compared to the ancestral alleles as a somewhat current norm. In Figure 3, this might correspond to two purple-colored down (“↓”) and right (“→”) arrows above the X-axis region in-between 0 nM and 3 nM.
Additionally, in promoters, this TBP-site region is immediately adjacent to the area of a position-by-position trend towards SNP-related down-regulation of expression that is next followed by the area with a trend towards SNP upregulation of these genes. These latter regions of mutational improvement in TBP binding to minor alleles with respect to their ancestral norm could correspond in Figure 3 to another pair of purple-colored down (“↓”) and right (“→”) arrows, which characterize the region of the X axis where KD-values exceed 5 nM.
Thus, the only purple upward arrow (“↑”) in this figure summarizes these two opposing SNP-dependent trends in weakening the strong TBP binding at TBP sites and strengthening the weak binding of TBP to the surrounding area of these sites that forms the mutational decrease in the standard deviation of KD-values identifying the stabilizing natural selection in favor of vital plant traits.
Finally, the reader can find in this figure the leftmost and rightmost regions, approximately 20 bp long, within the boundaries of the proximal promoters, where the frequencies of SNPs that can significantly change TBP-promoter binding decrease with decreasing distance to the boundaries of the proximal promoters [12].
2.3. Manually Curated Annotation of the Promoter Proximal SNPs
To understand the unexpectedly discovered mosaic structure of the proximal promoters, we manually verified our calculations for the ARF1 gene, as shown in Figure 5. This figure represents two SNPs, ENSVATH01403825:A and ENSVATH01403824:T, which likely correspond to downregulation and upregulation of this gene, as calculated in silico.
Figure 5.
The output of Web service Plant_SNP_TATA_Z-tester [24] regarding assessment of SNPs (a) ENSVATH01403825:A and (b) ENSVATH01403824:T located in the proximal promoter of the Arabidopsis gene ARF1. One can see the results along with their annotations in Figure 2 as an illustration of how to use the knowledge base AtSNP_TATAdb.
On one hand, the first SNP (i.e., ENSVATH01403825:A) is the substitution “T => A” at position −33 relative to the transcription start site of ARF1-204 annotated in the Ensembl Plant database [31], such as “taaaaaaaaaTATAAAGagga => taaaaaaaaaAATAAAGagga” (Figure 5a, Table S1 [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123]). As readers can see, this substitution disrupts the canonical TATA box “TATAAAAG” (in CAPITAL font) and thus increases the KD-value from 2.84 ± 0.18 to 5.29 ± 0.24, reducing the affinity of TBP for this promoter, which can ultimately lead to ARF1 deficiency, the estimation of which is significant according to the Fisher’s Z-test (Z = 16.29, p < 0.000001).
On another hand, the second SNP (i.e., ENSVATH01403824:T) is the substitution “A => T” at position −35 relative to the same transcription start site of the ARF1 gene, such as “aattaaaaaaaAaTATAAAGag => aattaaaaaaaTaTATAAAGag” [31] (Figure 5b and Table S1). According to our calculations, this mutation located within the (A+T)- rich area in front of the same canonical TATA box decreases the KD-value from 2.84 ± 0.18 to 51.65 ± 0.11 that can increase both the affinity of TBP for this promoter and the ARF1 expression (Figure 5b and Table S1: Z = 11.94, p < 0.000001).
This example allows us to heuristically assume that SNPs within TBP-binding sites may more often damage these sites and, therefore, reduce the expression of the corresponding genes, in contrast to SNPs in the local (A+T)-rich environment of TBP sites, which may more often improve both TBP-promoter affinity and gene expression. With this in mind, multiple repetition of this effect along with varying the positions of TBP sites from promoter to promoter can give an interference pattern of alternating areas within proximal promoters, which predominantly trend towards SNP-related upregulation and downregulation of gene expression (Figure 4). This can reduce the standard deviation of the KD-values of TBP complexes with minor alleles (Table 1: row #15) and cause the discussed stabilizing natural selection in favor of vital plant traits (Figure 3).
With this in mind, using the PubMed database [39], we learned that both increases and decreases in ARF1 gene expression relative to the ancestral optimum, negatively affect plant development as impaired somatic embryogenesis in A. thaliana [40] and reduced chlorophyll content in Cymbidium goeringii [41] (Figure 2 and Table S1). The existence of SNPs in very conserved proximal promoters, with their ability to equally likely increase and decrease the expression of plant genes keeping them around the evolutionarily fixed optimal expression level, may indicate stabilizing natural selection in favor of intraspecific biodiversity as the gold standard for the survival of both populations and species [124].
We also described the possible effect of increased or decreased expression for another 109 genes from having SNPs in proximal promoters by the hand-curated annotation (Table S1) of 83 original articles and found 173 putative advantages (Table 1) such as fast growing [43], lateral root formation [48], inorganic phosphate deficiency stress response [52], antiviral response [59], photo-oxidative stress response [61], food allergenicity [83], susceptibility to parasites [89], flowering time [92], trichome number [97], plant–environment response [105], seed mineral concentrations [108], drought stress response [111], photosynthetic efficiency [113], cold tolerance [116], coenzyme Q10 concentration [118] and resistance to low-nutrient conditions [123]. These vital plant traits can vary depending on the proximal promoter SNPs, as observed experimentally in 17 crop species (Table 1) such as Cymbidium goeringii [41], Triticum turgidum [51], Camellia oleifera [52], Solanum lycopersicum [57], Vitis vinifera L. [58], Oryza sativa L. [59], Carica papaya [60], Betula pendula [69] and Lilium longiflorum [72] (Table S1).
All these data are freely available as our knowledge base AtSNP_TATAdb (https://www.sysbio.ru/AtSNP_TATAdb/), as accessed on 15 December 2023.
2.4. Validation of In Silico Estimates of KD-Values Documented in the AtSNP_TATAdb Knowledge Base Using Independent In Vivo Experimental Data
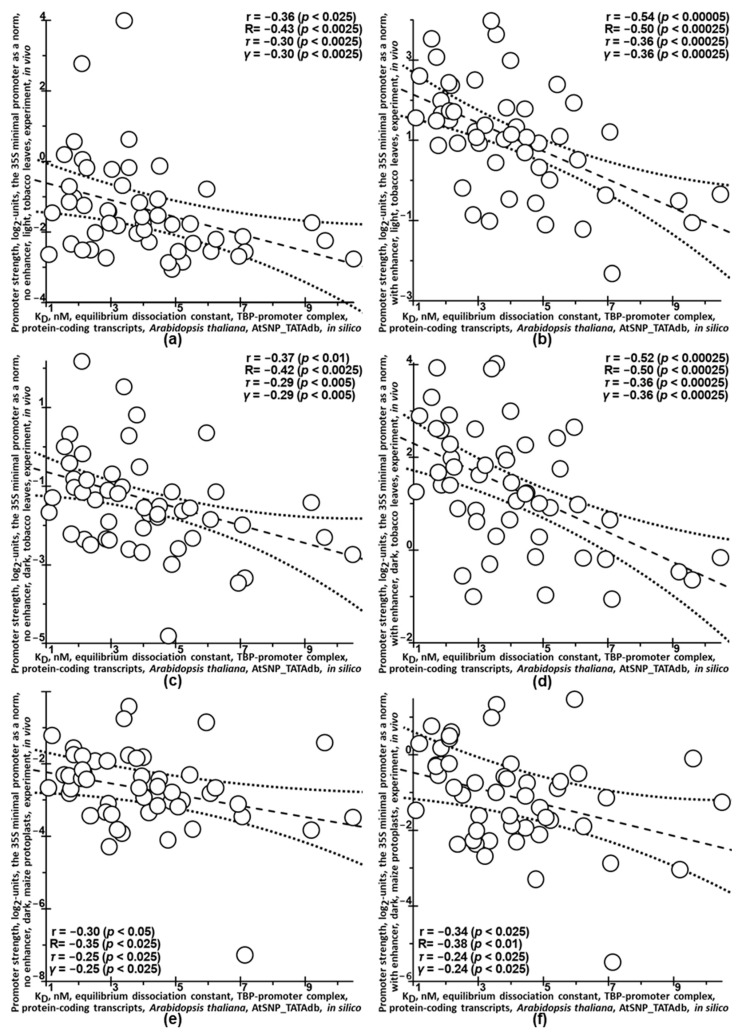
Because the most debatable are doubtless the in silico estimates of the KD-values of TBP-promoter complexes stored in the AtSNP_TATAdb knowledge base, we tested them using independent experimental data in vivo [125], as shown in Figure 6 and Table S2.
Figure 6.
Statistically significant correlations between the KD-values of the equilibrium dissociation constant expressed in nanomoles per liter (nM) of the complexes between the TBP and only the ancestral alleles of the promoters of the A. thaliana genes as evaluated in silico and stored in the AtSNP_TATAdb (X-axis) and the log2-value of the promoter strength (Y-axis), which were measured in vivo under the experimental conditions described in the caption to this axis on parts (a–f) of this figure according to article [125]. Solid and dash-and-dot lines denote linear regression and boundaries of its 95% confidence interval, calculated by means of software package STATISTICA version 10.0 (StatsoftTM, Tulsa, OK, USA). Statistics: r, R, τ, γ and p are coefficients of Pearson’s linear correlation, Spearman’s rank correlation, Kendall’s rank correlation, Goodman–Kruskal generalized correlation, and their p-values (statistical significance), respectively.
In total, for 50 ancestral alleles of 90 bp promoters before the starts of protein-coding transcripts from the A. thaliana genome, we found the log2-values of promoter strength measured in 6 variants of in vivo conditions [125], as shown in Figure 6 (the captions to the Y axes) and in Table S2 (the column headings). Here the readers can see significant correlations between the KD-values (AtSNP_TATAdb: X-axis) and the log2-values of the promoter strength (Y-axis), which are robust to variations in statistical criteria, genomic environment of promoters, species and growing conditions of tested plants [125]. Taken together, these correlations indicate that the TBP-promoter complex is an autonomous module in the gene expression regulatory machinery [11] and universally explains about 10% of the variation in eukaryotic gene expression in addition to approximately 90% of this variation, which depends on species, tissue and other factors influencing gene activity [24].
2.5. Genome-Wide Pattern of Arabidopsis Proximal Promoter SNPs in Comparison with Other Species
This work is the first to conduct a uniform in silico analysis of 871,707 proximal SNPs in promoters of 27,628 A. thaliana genes documented in the Ensembl Plant database [31]. As a result, we have predicted that 54,993 SNPs can significantly alter the TBP binding affinity of these promoters along with the gene expression levels and suggested them as candidate SNP markers for 173 plant beneficial features.
First of all, we found that all these SNPs taken together can significantly reduce the affinity of TBP for the gene promoters (Figure 3), which is consistent with both the theoretical genome-wide predictions [126] and the genome-wide data of the 1000 Genomes Project Consortium for binding sites of transcription factors, including TBP, with the human gene promoters [127] as well as with generally accepted ideas about the influence of mutations on gene functions.
Additionally, we observed the only short region 4 bp long around position −30 relative to the transcription start sites of Arabidopsis thaliana genes, where the frequencies of SNPs damaging TBP binding to the promoters are statistically significantly higher than those of SNPs that improve TBP-promoter affinity (Figure 4). This whole-genome result corresponds to both the Bucher criterion [6] for TATA boxes in the promoters of all eukaryotic genes and the predominant localization of TATA boxes in the promoters of plant [13] genes.
At last, for the first time we discovered approximately equal shares of proximal SNPs, which downregulate (3.2%) and upregulate (3.2%) the promoter activity of the protein-coding genes in Arabidopsis thaliana (Table 1) along with the mosaic structure of the proximal promoter, enriched predominantly in one or another type of these SNPs (Figure 4). Together with the (A+T)-enrichment of plant promoters, which has been generally recognized for over a quarter of a century [14], this result may fit a balance between the best efficiency of gene functioning, on the one hand, and, on another hand, a necessity of intraspecific biodiversity as a guarantor of survival kept by the stabilizing effect of natural selection.
Summarizing this discussion, we would like to emphasize that, first of all, the influence of SNP on plant gene expression goes far beyond the TBP-promoter binding studied in this work. It may include changes in both the epigenetic status of promoters and the efficiency of their binding to other transcription factors, as well as the density of packaging these promoters into chromatin. So, the annotation we have provided is only a part of an adequate genome-wide systematization of the promoter SNP roles in gene expression.
3. Materials and Methods
3.1. In Silico Analysis of DNA Sequences
DNA sequences for ancestral and minor alleles of each Arabidopsis thaliana gene promoter were retrieved from the Ensembl Plant database [31] using the BioPerl standard library [128]. Each pair of sequences, representing the ancestral and minor alleles, was analyzed in the automatic operating mode of our Web service Plant_SNP_TATA_Z-tester [24], as described in Section S1. The lists of homologous genes of Arabidopsis thaliana were taken from the paralogs section of the TAIR database [38].
3.2. The Knowledge Base AtSNP_TATAdb Created in this Work
The output data of Plant_SNP_TATA_Z-tester [24] altogether with their hand-curated annotations (Table S1) were written as a textual flat file in an Excel-compatible format. Finally, in the MariaDB 10.2.12 Web environment (MariaDB Corp AB, Espoo, Finland), this flat file was added to our knowledge base AtSNP_TATAdb (https://www.sysbio.ru/AtSNP_TATAdb/), as accessed on 15 December 2023.
3.3. How to Use the Knowledge Base AtSNP_TATAdb
We tried to develop a “user-friendly Web-based interface” [129] that could itself be intuitively clear as depicted in Figure 2. First, clicking on the far-left button “Start (Homolog)”, users can work with the knowledge base in the “On-line” mode. Furthermore, we added two buttons: the middle “Start (usual)” button, which by clicking on, anyone can obtain the complete list of 54,993 candidate SNP markers that significantly alter the TBP affinity for the promoters of A. thaliana protein-coding genes. Finally, users can download the complete Excel-formatted flat file of the AtSNP_TATAdb knowledge base by clicking on the far-right button “Download DB”.
3.4. Statistical Analysis
Statistical analysis in Plant_SNP_TATA_Z-tester [24] was carried out by the standard statistical package R [36], as shown in Figure 2 and Figure 5. To calculate the statistical estimates presented in Table 1 and Table S2 along with Figure 3, Figure 4 and Figure 6 we used the widely accepted toolbox STATISTICA version 10.0 (StatsoftTM, Tulsa, OK, USA).
4. Conclusions
In this work we identified in silico the 54,993 SNPs able to significantly alter the TBP binding affinity to promoters of A. thaliana protein-coding genes for the first time and made these genome-wide finding freely available through our knowledge base AtSNP_TATAdb, https://www.sysbio.ru/AtSNP_TATAdb/.
Additionally, we presented some evidence that these candidate SNP markers can influence plant development and responses to environmental changes. Thus, they may be a part of intraspecific diversity, which is a guarantor of species survival kept by the stabilizing natural selection. Because natural selection is commonly associated with vital traits, we selectively hand-annotated some of the genes carrying these in silico estimated SNPs and found the effects of changes in the gene expression on plant vital traits. We deposited these findings in the knowledge base AtSNP_TATAdb.
Finally, we presented the significant correlations of the in silico estimates of the KD-values of TBP-promoter complexes stored in the AtSNP_TATAdb with independent experimental data in vivo [125] that appeared to be robust to variations in statistical criteria, genomic environment of TATA boxes, plant species and growing conditions.
Here we show that SNPs in proximal promoters can change gene expression and suppose that AtSNP_TATAdb can be applied to search for molecular markers of crop advantages in plant breeding.
Acknowledgments
The authors are thankful to the Multi-Access Center “Bioinformatics” for the use of computational resources as supported by Russian government projects FWNR-2022-0020 and FWNR-2022-0015 together with the Russian Federal Science and Technology Program for the Development of Genetic Technologies.
Abbreviations
| ARF1 | auxin response factor 1 |
| ARF6 | auxin response factor 6 |
| KD | equilibrium dissociation constant |
| nM | natural logarithm units |
| SNP | single-nucleotide polymorphism |
| TAIR | The Arabidopsis Information Resource |
| TBP | TATA-binding protein |
| TSS | transcription start site |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25010607/s1.
Author Contributions
Conceptualization and supervision, N.A.K., L.S. and E.Z.; methodology, N.P.; software, S.F., A.B., B.K., D.R. and P.P.; validation, E.S.; resources, N.T. and O.P.; data curation, K.Z., I.C. and E.K.; writing—original draft preparation, M.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All original in silico data presented in this work are freely available in the knowledge base AtSNP_TATAdb (https://www.sysbio.ru/AtSNP_TATAdb/) created and presented in this work, as accessed on 15 December 2023.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This study was supported by the Russian Science Foundation, grant No. 20-14-00140.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Virk P.S., Andersson M.S., Arcos J., Govindaraj M., Pfeiffer W.H. Transition from targeted breeding to mainstreaming of biofortification traits in crop improvement programs. Front. Plant Sci. 2021;12:703990. doi: 10.3389/fpls.2021.703990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X., Cal A.J., Borevitz J.O. Genetic architecture of regulatory variation in Arabidopsis thaliana. Genome Res. 2011;21:725–733. doi: 10.1101/gr.115337.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., Sapkota M., van der Knaap E. Perspectives of CRISPR/Cas-mediated cis-engineering in horticulture: Unlocking the neglected potential for crop improvement. Hortic. Res. 2020;7:36. doi: 10.1038/s41438-020-0258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saeed S., Usman B., Shim S.H., Khan S.U., Nizamuddin S., Saeed S., Shoaib Y., Jeon J.S., Jung K.H. CRISPR/Cas-mediated editing of cis-regulatory elements for crop improvement. Plant Sci. 2022;324:111435. doi: 10.1016/j.plantsci.2022.111435. [DOI] [PubMed] [Google Scholar]
- 5.Cui Z., Tian R., Huang Z., Jin Z., Li L., Liu J., Huang Z., Xie H., Liu D., Mo H., et al. FrCas9 is a CRISPR/Cas9 system with high editing efficiency and fidelity. Nat. Commun. 2022;13:1425. doi: 10.1038/s41467-022-29089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucher P. Weight matrix descriptions of four eukaryotic RNA polymerase II promoter elements derived from 502 unrelated promoter sequences. J. Mol. Biol. 1990;212:563–578. doi: 10.1016/0022-2836(90)90223-9. [DOI] [PubMed] [Google Scholar]
- 7.Muller F., Lakatos L., Dantonel J., Strähle U., Tora L. TBP is not universally required for zygotic RNA polymerase II transcription in zebrafish. Curr. Biol. 2001;11:282–287. doi: 10.1016/S0960-9822(01)00076-8. [DOI] [PubMed] [Google Scholar]
- 8.Martianov I., Viville S., Davidson I. RNA polymerase II transcription in murine cells lacking the TATA binding protein. Science. 2002;298:1036–1039. doi: 10.1126/science.1076327. [DOI] [PubMed] [Google Scholar]
- 9.Yang M.Q., Laflamme K., Gotea V., Joiner C.H., Seidel N.E., Wong C., Petrykowska H.M., Lichtenberg J., Lee S., Welch L., et al. Genome-wide detection of a TFIID localization element from an initial human disease mutation. Nucleic Acids Res. 2011;39:2175–2187. doi: 10.1093/nar/gkq1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhee H.S., Pugh B.F. Genome-wide structure and organization of eukaryotic pre-initiation complexes. Nature. 2012;483:295–301. doi: 10.1038/nature10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mogno I., Vallania F., Mitra R.D., Cohen B.A. TATA is a modular component of synthetic promoters. Genome Res. 2010;20:1391–1397. doi: 10.1101/gr.106732.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponomarenko M., Mironova V., Gunbin K., Savinkova L. Hogness Box. In: Maloy S., Hughes K., editors. Brenner’s Encyclopedia of Genetics. 2nd ed. Volume 3. Academic Press; San Diego, CA, USA: 2013. pp. 491–494. [Google Scholar]
- 13.Joshi C.P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987;15:6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohno S., Yomo T. Various regulatory sequences are deprived of their uniqueness by the universal rule of TA/CG deficiency and TG/CT excess. Proc. Natl. Acad. Sci. USA. 1990;87:1218–1222. doi: 10.1073/pnas.87.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korkuc P., Schippers J.H., Walther D. Characterization and identification of cis-regulatory elements in Arabidopsis based on single-nucleotide polymorphism information. Plant Physiol. 2014;164:181–200. doi: 10.1104/pp.113.229716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava R., Rai K.M., Srivastava M., Kumar V., Pandey B., Singh S.P., Bag S.K., Singh B.D., Tuli R., Sawant S.V. Distinct role of core promoter architecture in regulation of light-mediated responses in plant genes. Mol. Plant. 2014;7:626–641. doi: 10.1093/mp/sst146. [DOI] [PubMed] [Google Scholar]
- 17.Hetzel J., Duttke S.H., Benner C., Chory J. Nascent RNA sequencing reveals distinct features in plant transcription. Proc. Natl. Acad. Sci. USA. 2016;113:12316–12321. doi: 10.1073/pnas.1603217113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lou S.L., Guo X., Liu L., Song Y., Zhang L., Jiang Y.Z., Zhang L.S., Sun P.C., Liu B., Tong S.F., et al. Allelic shift in cis-elements of the transcription factor RAP2.12 underlies adaptation associated with humidity in Arabidopsis thaliana. Sci. Adv. 2022;8:eabn8281. doi: 10.1126/sciadv.abn8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le N.T., Harukawa Y., Miura S., Boer D., Kawabe A., Saze H. Epigenetic regulation of spurious transcription initiation in Arabidopsis. Nat. Commun. 2020;11:3224. doi: 10.1038/s41467-020-16951-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao J., Jin R., Yu X., Shen M., Wagner J.D., Pai A., Song C., Zhuang M., Klasfeld S., He C., et al. Cis and trans determinants of epigenetic silencing by Polycomb repressive complex 2 in Arabidopsis. Nat. Genet. 2017;49:1546–1552. doi: 10.1038/ng.3937. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto Y.Y., Yoshitsugu T., Sakurai T., Seki M., Shinozaki K., Obokata J. Heterogeneity of Arabidopsis core promoters revealed by high-density TSS analysis. Plant J. 2009;60:350–362. doi: 10.1111/j.1365-313X.2009.03958.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhu P., Schon M., Questa J., Nodine M., Dean C. Causal role of a promoter polymorphism in natural variation of the Arabidopsis floral repressor gene FLC. Curr. Biol. 2023;33:4381–4391.e3. doi: 10.1016/j.cub.2023.08.079. [DOI] [PubMed] [Google Scholar]
- 23.Triska M., Solovyev V., Baranova A., Kel A., Tatarinova T.V. Nucleotide patterns aiding in prediction of eukaryotic promoters. PLoS ONE. 2017;12:e0187243. doi: 10.1371/journal.pone.0187243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasskazov D., Chadaeva I., Sharypova E., Zolotareva K., Khandaev B., Ponomarenko P., Podkolodnyy N., Tverdokhleb N., Vishnevsky O., Bogomolov A., et al. Plant_SNP_TATA_Z-Tester: A Web service that unequivocally estimates the impact of proximal promoter mutations on plant gene expression. Int. J. Mol. Sci. 2022;23:8684. doi: 10.3390/ijms23158684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponomarenko P., Savinkova L., Drachkova I., Lysova M., Arshinova T., Ponomarenko M., Kolchanov N. A step-by-step model of TBP/TATA box binding allows predicting human hereditary diseases by single nucleotide polymorphism. Dokl. Biochem. Biophys. 2008;419:88–92. doi: 10.1134/S1607672908020117. [DOI] [PubMed] [Google Scholar]
- 26.Coleman R.A., Pugh B.F. Evidence for functional binding and stable sliding of the TATA binding protein on nonspecific DNA. J. Biol. Chem. 1995;270:13850–13859. doi: 10.1074/jbc.270.23.13850. [DOI] [PubMed] [Google Scholar]
- 27.Berg O.G., von Hippel P.H. Selection of DNA binding sites by regulatory proteins. Statistical-mechanical theory and application to operators and promoters. J. Mol. Biol. 1987;193:723–750. doi: 10.1016/0022-2836(87)90354-8. [DOI] [PubMed] [Google Scholar]
- 28.Flatters D., Lavery R. Sequence-dependent dynamics of TATA-Box binding sites. Biophys. J. 1998;75:372–381. doi: 10.1016/S0006-3495(98)77521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delgadillo R., Whittington J., Parkhurst L., Parkhurst L. The TBP core domain in solution variably bends TATA sequences via a three-step binding mechanism. Biochemistry. 2009;48:1801–1809. doi: 10.1021/bi8018724. [DOI] [PubMed] [Google Scholar]
- 30.Vishnevsky O.V., Chadaeva I.V., Sharypova E.B., Khandaev B.M., Zolotareva K.A., Kazachek A.V., Ponomarenko P.M., Podkolodny N.L., Rasskazov D.A., Bogomolov A.G., et al. Promoters of genes encoding β-amylase, albumin and globulin in food plants have weaker affinity for TATA-binding protein as compared to non-food plants: In silico analysis. Vavilovskii Zhurnal Genet. Selektsii. 2022;26:798–805. doi: 10.18699/VJGB-22-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Contreras-Moreira B., Naamati G., Rosello M., Allen J.E., Hunt S.E., Muffato M., Gall A., Flicek P. Scripting analyses of genomes in Ensembl Plants. Methods Mol. Biol. 2022;2443:27–55. doi: 10.1007/978-1-0716-2067-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukumoto F., Hirose S., Imaseki H., Yamazaki K. DNA sequence requirement of a TATA element-binding protein from Arabidopsis for transcription in vitro. Plant Mol. Biol. 1993;23:95–1003. doi: 10.1007/BF00021814. [DOI] [PubMed] [Google Scholar]
- 33.Hahn S., Buratowski S., Sharp P., Guarente L. Yeast TATA-binding protein TFIID binds to TATA elements with both consensus and nonconsensus DNA sequences. Proc. Natl. Acad. Sci. USA. 1989;86:5718–5722. doi: 10.1073/pnas.86.15.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karas H., Knuppel R., Schulz W., Sklenar H., Wingender E. Combining structural analysis of DNA with search routines for the detection of transcription regulatory elements. Comput. Appli. Biosci. 1996;12:441–446. doi: 10.1093/bioinformatics/12.5.441. [DOI] [PubMed] [Google Scholar]
- 35.Ponomarenko M.P., Ponomarenko J.V., Frolov A.S., Podkolodny N.L., Savinkova L.K., Kolchanov N.A., Overton G.C. Identification of sequence-dependent features correlating to activity of DNA sites interacting with proteins. Bioinformatics. 1999;15:687–703. doi: 10.1093/bioinformatics/15.7.687. [DOI] [PubMed] [Google Scholar]
- 36.Waardenberg A., Basset S., Bouveret R., Harvey R. CompGO: An R package for comparing and visualizing Gene Ontology enrichment differences between DNA binding experiments. BMC Bioinform. 2015;16:275. doi: 10.1186/s12859-015-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sayers E.W., Bolton E.E., Brister J.R., Canese K., Chan J., Comeau D.C., Farrell C.M., Feldgarden M., Fine A.M., Funk K., et al. Database resources of the National Center for Biotechnology Information in 2023. Nucleic Acids Res. 2023;51:D29–D38. doi: 10.1093/nar/gkac1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berardini T.Z., Reiser L., Li D., Mezheritsky Y., Muller R., Strait E., Huala E. The Arabidopsis information resource: Making and mining the “gold standard” annotated reference plant genome. Genesis. 2015;53:474–485. doi: 10.1002/dvg.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Z. PubMed and beyond: A survey of web tools for searching biomedical literature. Database. 2011;2011:baq036. doi: 10.1093/database/baq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wojcikowska B., Gaj M.D. Expression profiling of AUXIN RESPONSE FACTOR genes during somatic embryogenesis induction in Arabidopsis. Plant Cell Rep. 2017;36:843–858. doi: 10.1007/s00299-017-2114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Z., Li F., Li M., He Y., Chen Y., Hu F. Functional analysis of ARF1 from Cymbidium goeringii in IAA response during leaf development. PeerJ. 2022;10:e13077. doi: 10.7717/peerj.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu M.F., Tian Q., Reed J.W. Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development. 2006;133:4211–4218. doi: 10.1242/dev.02602. [DOI] [PubMed] [Google Scholar]
- 43.Xia Y., Du K., Ling A., Wu W., Li J., Kang X. Overexpression of PagSTOMAGEN, a positive regulator of stomatal density, promotes vegetative growth in poplar. Int. J. Mol. Sci. 2022;23:10165. doi: 10.3390/ijms231710165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nada R.M., Abo-Hegazy S.E., Budran E.G., Abogadallah G.M. The interaction of genes controlling root traits is required for the developmental acquisition of deep and thick root traits and improving root architecture in response to low water or nitrogen content in rice (Oryza sativa L.) cultivars. Plant Physiol. Biochem. 2019;141:122–132. doi: 10.1016/j.plaphy.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Yang J., Tian L., Sun M.X., Huang X.Y., Zhu J., Guan Y.F., Jia Q.S., Yang Z.N. AUXIN RESPONSE FACTOR17 is essential for pollen wall pattern formation in Arabidopsis. Plant Physiol. 2013;162:720–731. doi: 10.1104/pp.113.214940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi Z.H., Zhang C., Xu X.F., Zhu J., Zhou Q., Ma L.J., Niu J., Yang Z.N. Overexpression of AtTTP affects ARF17 expression and leads to male sterility in Arabidopsis. PLoS ONE. 2015;10:e0117317. doi: 10.1371/journal.pone.0117317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia Y., Kong X., Hu K., Cao M., Liu J., Ma C., Guo S., Yuan X., Zhao S., Robert H.S., et al. PIFs coordinate shade avoidance by inhibiting auxin repressor ARF18 and metabolic regulator QQS. New Phytol. 2020;228:609–621. doi: 10.1111/nph.16732. [DOI] [PubMed] [Google Scholar]
- 48.Okushima Y., Fukaki H., Onoda M., Theologis A., Tasaka M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell. 2007;19:118–130. doi: 10.1105/tpc.106.047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J., Dai X., Zhao Y. A role for auxin response factor 19 in auxin and ethylene signaling in Arabidopsis. Plant Physiol. 2006;140:899–908. doi: 10.1104/pp.105.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao D., Jiao J., Du B., Liu K., Wang C., Ding Y. Volatile organic compounds from Lysinibacillus macroides regulating the seedling growth of Arabidopsis thaliana. Physiol. Mol. Biol. Plants. 2022;28:1997–2009. doi: 10.1007/s12298-022-01268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuluaga D.L., Liuzzi V., Curci P.L., Sonnante G. MicroRNAs in durum wheat seedlings under chronic and short-term nitrogen stress. Funct. Integr. Genom. 2018;18:645–657. doi: 10.1007/s10142-018-0619-7. [DOI] [PubMed] [Google Scholar]
- 52.Chen J., Han X., Ye S., Liu L., Yang B., Cao Y., Zhuo R., Yao X. Integration of small RNA, degradome, and transcriptome sequencing data illustrates the mechanism of low phosphorus adaptation in Camellia oleifera. Front. Plant Sci. 2022;13:932926. doi: 10.3389/fpls.2022.932926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan S., Stone J.M. Arabidopsis thaliana GH3.9 in auxin and jasmonate cross talk. Plant Signal. Behav. 2007;2:483–485. doi: 10.4161/psb.2.6.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taniguchi M., Nakamura M., Tasaka M., Morita M.T. Identification of gravitropic response indicator genes in Arabidopsis inflorescence stems. Plant Signal. Behav. 2014;9:e29570. doi: 10.4161/psb.29570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lakehal A., Chaabouni S., Cavel E., Le Hir R., Ranjan A., Raneshan Z., Novak O., Pacurar D.I., Perrone I., Jobert F., et al. A molecular framework for the control of adventitious rooting by TIR1/AFB2-Aux/IAA-dependent auxin signaling in Arabidopsis. Mol. Plant. 2019;12:1499–1514. doi: 10.1016/j.molp.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Arase F., Nishitani H., Egusa M., Nishimoto N., Sakurai S., Sakamoto N., Kaminaka H. IAA8 involved in lateral root formation interacts with the TIR1 auxin receptor and ARF transcription factors in Arabidopsis. PLoS ONE. 2012;7:e43414. doi: 10.1371/journal.pone.0043414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abe-Hara C., Yamada K., Wada N., Ueta R., Hashimoto R., Osakabe K., Osakabe Y. Effects of the sliaa9 mutation on shoot elongation growth of tomato cultivars. Front. Plant Sci. 2021;12:627832. doi: 10.3389/fpls.2021.627832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujita K., Horiuchi H., Takato H., Kohno M., Suzuki S. Auxin-responsive grape Aux/IAA9 regulates transgenic Arabidopsis plant growth. Mol. Biol. Rep. 2012;39:7823–7829. doi: 10.1007/s11033-012-1625-9. [DOI] [PubMed] [Google Scholar]
- 59.Qin Q., Li G., Jin L., Huang Y., Wang Y., Wei C., Xu Z., Yang Z., Wang H., Li Y. Auxin response factors (ARFs) differentially regulate rice antiviral immune response against rice dwarf virus. PLoS Pathog. 2020;16:e1009118. doi: 10.1371/journal.ppat.1009118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Estrella-Maldonado H., Chan-Leon A., Fuentes G., Giron-Ramirez A., Desjardins Y., Santamaria J.M. The interaction between exogenous IBA with sucrose, light and ventilation alters the expression of ARFs and Aux/IAA genes in Carica papaya plantlets. Plant Mol. Biol. 2022;110:107–130. doi: 10.1007/s11103-022-01289-2. [DOI] [PubMed] [Google Scholar]
- 61.Mielecki J., Gawronski P., Karpinski S. Aux/IAA11 is required for UV-AB tolerance and auxin sensing in Arabidopsis thaliana. Int. J. Mol. Sci. 2022;23:13386. doi: 10.3390/ijms232113386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Falkenberg B., Witt I., Zanor M.I., Steinhauser D., Mueller-Roeber B., Hesse H., Hoefgen R. Transcription factors relevant to auxin signalling coordinate broad-spectrum metabolic shifts including sulphur metabolism. J. Exp. Bot. 2008;59:2831–2846. doi: 10.1093/jxb/ern144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Padmanabhan M.S., Shiferaw H., Culver J.N. The Tobacco mosaic virus replicase protein disrupts the localization and function of interacting Aux/IAA proteins. Mol. Plant Microbe Interact. 2006;19:864–873. doi: 10.1094/MPMI-19-0864. [DOI] [PubMed] [Google Scholar]
- 64.Kohno M., Takato H., Horiuchi H., Fujita K., Suzuki S. Auxin-nonresponsive grape Aux/IAA19 is a positive regulator of plant growth. Mol. Biol. Rep. 2012;39:911–917. doi: 10.1007/s11033-011-0816-0. [DOI] [PubMed] [Google Scholar]
- 65.Akkasaeng C., Tantisuwichwong N., Chairam I., Prakrongrak N., Jogloy S., Pathanothai A. Isolation and identification of peanut leaf proteins regulated by water stress. Pak. J. Biol. Sci. 2007;10:1611–1617. doi: 10.3923/pjbs.2007.1611.1617. [DOI] [PubMed] [Google Scholar]
- 66.Siena L.A., Azzaro C.A., Podio M., Stein J., Leblanc O., Pessino S.C., Ortiz J.P.A. The auxin-response repressor IAA30 is downregulated in reproductive tissues of apomictic Paspalum notatum. Plants. 2022;11:1472. doi: 10.3390/plants11111472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park J., Kim Y.S., Kim S.G., Jung J.H., Woo J.C., Park C.M. Integration of auxin and salt signals by the NAC transcription factor NTM2 during seed germination in Arabidopsis. Plant Physiol. 2011;156:537–549. doi: 10.1104/pp.111.177071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cao M., Chen R., Li P., Yu Y., Zheng R., Ge D., Zheng W., Wang X., Gu Y., Gelova Z., et al. TMK1-mediated auxin signalling regulates differential growth of the apical hook. Nature. 2019;568:240–243. doi: 10.1038/s41586-019-1069-7. [DOI] [PubMed] [Google Scholar]
- 69.Chen K., Qu C., Zhang X.Y., Wang W., Gu C.R., Liu G.F., Yu Q.B., Yang C.P., Jiang J. Molecular mechanism of leaf adaxial upward curling caused by BpPIN3 suppression in Betula pendula. Front. Plant Sci. 2022;13:1060228. doi: 10.3389/fpls.2022.1060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spartz A.K., Lee S.H., Wenger J.P., Gonzalez N., Itoh H., Inze D., Peer W.A., Murphy A.S., Overvoorde P.J., Gray W.M. The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J. 2012;70:978–990. doi: 10.1111/j.1365-313X.2012.04946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z., Yang L., Liu Z., Lu M., Wang M., Sun Q., Lan Y., Shi T., Wu D., Hua J. Natural variations of growth thermo-responsiveness determined by SAUR26/27/28 proteins in Arabidopsis thaliana. New Phytol. 2019;224:291–305. doi: 10.1111/nph.15956. [DOI] [PubMed] [Google Scholar]
- 72.Howlader J., Robin A.H.K., Natarajan S., Biswas M.K., Sumi K.R., Song C.Y., Park J.I., Nou I.S. Transcriptome analysis by RNA-Seq reveals genes related to plant height in two sets of parent-hybrid combinations in Easter lily (Lilium longiflorum) Sci. Rep. 2020;10:9082. doi: 10.1038/s41598-020-65909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chae K., Isaacs C.G., Reeves P.H., Maloney G.S., Muday G.K., Nagpal P., Reed J.W. Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. Plant J. 2012;71:684–697. doi: 10.1111/j.1365-313X.2012.05024.x. [DOI] [PubMed] [Google Scholar]
- 74.He S.L., Hsieh H.L., Jauh G.Y. SMALL AUXIN UP RNA62/75 are required for the translation of transcripts essential for pollen tube growth. Plant Physiol. 2018;178:626–640. doi: 10.1104/pp.18.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao P., Zhang J., Chen S., Zhang Z., Wan G., Mao J., Wang Z., Tan S., Xiang C. ERF1 inhibits lateral root emergence by promoting local auxin accumulation and repressing ARF7 expression. Cell Rep. 2023;42:112565. doi: 10.1016/j.celrep.2023.112565. [DOI] [PubMed] [Google Scholar]
- 76.Hu L.Q., Chang J.H., Yu S.X., Jiang Y.T., Li R.H., Zheng J.X., Zhang Y.J., Xue H.W., Lin W.H. PIN3 positively regulates the late initiation of ovule primordia in Arabidopsis thaliana. PLoS Genet. 2022;18:e1010077. doi: 10.1371/journal.pgen.1010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mira M.M., Huang S., Kapoor K., Hammond C., Hill R.D., Stasolla C. Expression of Arabidopsis class 1 phytoglobin (AtPgb1) delays death and degradation of the root apical meristem during severe PEG-induced water deficit. J. Exp. Bot. 2017;68:5653–5668. doi: 10.1093/jxb/erx371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waldie T., Leyser O. Cytokinin targets auxin transport to promote shoot branching. Plant Physiol. 2018;177:803–818. doi: 10.1104/pp.17.01691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Di Mambro R., Svolacchia N., Dello Ioio R., Pierdonati E., Salvi E., Pedrazzini E., Vitale A., Perilli S., Sozzani R., Benfey P.N., et al. The Lateral Root Cap Acts as an auxin sink that controls meristem size. Curr. Biol. 2019;29:1199–1205.e4. doi: 10.1016/j.cub.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 80.Roychoudhry S., Sageman-Furnas K., Wolverton C., Grones P., Tan S., Molnar G., De Angelis M., Goodman H.L., Capstaff N., Lloyd J.P.B., et al. Antigravitropic PIN polarization maintains non-vertical growth in lateral roots. Nat. Plants. 2023;9:1500–1513. doi: 10.1038/s41477-023-01478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schroeder M.M., Gomez M.Y., McLain N., Gachomo E.W. Bradyrhizobium japonicum IRAT FA3 alters Arabidopsis thaliana root architecture via regulation of auxin efflux transporters PIN2, PIN3, PIN7, and ABCB19. Mol. Plant Microbe Interact. 2022;35:215–229. doi: 10.1094/MPMI-05-21-0118-R. [DOI] [PubMed] [Google Scholar]
- 82.Dal Bosco C., Dovzhenko A., Liu X., Woerner N., Rensch T., Eismann M., Eimer S., Hegermann J., Paponov I.A., Ruperti B., et al. The endoplasmic reticulum localized PIN8 is a pollen-specific auxin carrier involved in intracellular auxin homeostasis. Plant J. 2012;71:860–870. doi: 10.1111/j.1365-313X.2012.05037.x. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y., Weng J., Zhu C., Ai R., Zhou J., Wang C., Chen Q., Fu L. Allergenicity assessment and allergen profile analysis of different Chinese wheat cultivars. World Allergy Organ. J. 2021;14:100559. doi: 10.1016/j.waojou.2021.100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shaikhali J., de Dios Barajas-Lopez J., Otvos K., Kremnev D., Garcia A.S., Srivastava V., Wingsle G., Bako L., Strand A. The CRYPTOCHROME1-dependent response to excess light is mediated through the transcriptional activators ZINC FINGER PROTEIN EXPRESSED IN INFLORESCENCE MERISTEM LIKE1 and ZML2 in Arabidopsis. Plant Cell. 2012;24:3009–3025. doi: 10.1105/tpc.112.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duan Z., Zhang Y., Tu J., Shen J., Yi B., Fu T., Dai C., Ma C. The Brassica napus GATA transcription factor BnA5.ZML1 is a stigma compatibility factor. J. Integr. Plant Biol. 2020;62:1112–1131. doi: 10.1111/jipb.12916. [DOI] [PubMed] [Google Scholar]
- 86.Velez-Bermudez I.C., Salazar-Henao J.E., Fornale S., Lopez-Vidriero I., Franco-Zorrilla J.M., Grotewold E., Gray J., Solano R., Schmidt W., Pages M., et al. A MYB/ZML complex regulates wound-induced lignin genes in maize. Plant Cell. 2015;27:3245–3259. doi: 10.1105/tpc.15.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim Y.S., Kim S.G., Lee M., Lee I., Park H.Y., Seo P.J., Jung J.H., Kwon E.J., Suh S.W., Paek K.H., et al. HD-ZIP III activity is modulated by competitive inhibitors via a feedback loop in Arabidopsis shoot apical meristem development. Plant Cell. 2008;20:920–933. doi: 10.1105/tpc.107.057448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Somers D.E., Kim W.Y., Geng R. The F-box protein ZEITLUPE confers dosage-dependent control on the circadian clock, photomorphogenesis, and flowering time. Plant Cell. 2004;16:769–782. doi: 10.1105/tpc.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verma A., Lee C., Morriss S., Odu F., Kenning C., Rizzo N., Spollen W.G., Lin M., McRae A.G., Givan S.A., et al. The novel cyst nematode effector protein 30D08 targets host nuclear functions to alter gene expression in feeding sites. New Phytol. 2018;219:697–713. doi: 10.1111/nph.15179. [DOI] [PubMed] [Google Scholar]
- 90.Higgins J.D., Sanchez-Moran E., Armstrong S.J., Jones G.H., Franklin F.C. The Arabidopsis synaptonemal complex protein ZYP1 is required for chromosome synapsis and normal fidelity of crossing over. Genes Dev. 2005;19:2488–2500. doi: 10.1101/gad.354705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vallarino J.G., Merchante C., Sanchez-Sevilla J.F., de Luis Balaguer M.A., Pott D.M., Ariza M.T., Casanal A., Pose D., Vioque A., Amaya I., et al. Characterizing the involvement of FaMADS9 in the regulation of strawberry fruit receptacle development. Plant Biotechnol. J. 2020;18:929–943. doi: 10.1111/pbi.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xie W., Huang J., Liu Y., Rao J., Luo D., He M. Exploring potential new floral organ morphogenesis genes of Arabidopsis thaliana using systems biology approach. Front. Plant Sci. 2015;6:829. doi: 10.3389/fpls.2015.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dinkins R., Pflipsen C., Thompson A., Collins G.B. Ectopic expression of an Arabidopsis single zinc finger gene in tobacco results in dwarf plants. Plant Cell Physiol. 2002;43:743–750. doi: 10.1093/pcp/pcf086. [DOI] [PubMed] [Google Scholar]
- 94.Tavva V.S., Palli S.R., Dinkins R.D., Collins G.B. Applications of EcR gene switch technology in functional genomics. Arch. Insect. Biochem. Physiol. 2007;65:164–179. doi: 10.1002/arch.20193. [DOI] [PubMed] [Google Scholar]
- 95.Cai S., Lashbrook C.C. Stamen abscission zone transcriptome profiling reveals new candidates for abscission control: Enhanced retention of floral organs in transgenic plants overexpressing Arabidopsis ZINC FINGER PROTEIN2. Plant Physiol. 2008;146:1305–1321. doi: 10.1104/pp.107.110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joseph M.P., Papdi C., Kozma-Bognar L., Nagy I., Lopez-Carbonell M., Rigo G., Koncz C., Szabados L. The Arabidopsis ZINC FINGER PROTEIN3 interferes with abscisic acid and light signaling in seed germination and plant development. Plant Physiol. 2014;165:1203–1220. doi: 10.1104/pp.113.234294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou Z., An L., Sun L., Zhu S., Xi W., Broun P., Yu H., Gan Y. Zinc finger protein5 is required for the control of trichome initiation by acting upstream of zinc finger protein8 in Arabidopsis. Plant Physiol. 2011;157:673–682. doi: 10.1104/pp.111.180281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gan Y., Liu C., Yu H., Broun P. Integration of cytokinin and gibberellin signalling by Arabidopsis transcription factors GIS, ZFP8 and GIS2 in the regulation of epidermal cell fate. Development. 2007;134:2073–2081. doi: 10.1242/dev.005017. [DOI] [PubMed] [Google Scholar]
- 99.Haydon M.J., Cobbett C.S. A novel major facilitator superfamily protein at the tonoplast influences zinc tolerance and accumulation in Arabidopsis. Plant Physiol. 2007;143:1705–1719. doi: 10.1104/pp.106.092015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee S., Ricachenevsky F.K., Punshon T. Functional overlap of two major facilitator superfamily transporter, ZIF1, and ZIFL1 in zinc and iron homeostasis. Biochem. Biophys. Res. Commun. 2021;560:7–13. doi: 10.1016/j.bbrc.2021.04.120. [DOI] [PubMed] [Google Scholar]
- 101.Remy E., Cabrito T.R., Batista R.A., Teixeira M.C., Sa-Correia I., Duque P. The major facilitator superfamily transporter ZIFL2 modulates cesium and potassium homeostasis in Arabidopsis. Plant Cell Physiol. 2015;56:148–162. doi: 10.1093/pcp/pcu157. [DOI] [PubMed] [Google Scholar]
- 102.Wu C.Q., Hu H.H., Zeng Y., Liang D.C., Xie K.B., Zhang J.W., Chu Z.H., Xiong L.Z. Identification of novel stress-responsive transcription factor genes in rice by cDNA array analysis. J. Integr. Plant Biol. 2006;48:1216–1224. doi: 10.1111/j.1744-7909.2006.00305.x. [DOI] [Google Scholar]
- 103.Kawachi M., Kobae Y., Mori H., Tomioka R., Lee Y., Maeshima M. A mutant strain Arabidopsis thaliana that lacks vacuolar membrane zinc transporter MTP1 revealed the latent tolerance to excessive zinc. Plant Cell Physiol. 2009;50:1156–1170. doi: 10.1093/pcp/pcp067. [DOI] [PubMed] [Google Scholar]
- 104.Maathuis F.J. The role of monovalent cation transporters in plant responses to salinity. J. Exp. Bot. 2006;57:1137–1147. doi: 10.1093/jxb/erj001. [DOI] [PubMed] [Google Scholar]
- 105.Zhao Y.N., Li C., Li H., Liu X.S., Yang Z.M. OsZIP11 is a trans-Golgi-residing transporter required for rice iron accumulation and development. Gene. 2022;836:146678. doi: 10.1016/j.gene.2022.146678. [DOI] [PubMed] [Google Scholar]
- 106.Henriques R., Jasik J., Klein M., Martinoia E., Feller U., Schell J., Pais M.S., Koncz C. Knock-out of Arabidopsis metal transporter gene IRT1 results in iron deficiency accompanied by cell differentiation defects. Plant Mol. Biol. 2002;50:587–597. doi: 10.1023/A:1019942200164. [DOI] [PubMed] [Google Scholar]
- 107.Niihama M., Takemoto N., Hashiguchi Y., Tasaka M., Morita M.T. ZIP genes encode proteins involved in membrane trafficking of the TGN-PVC/vacuoles. Plant Cell Physiol. 2009;50:2057–2068. doi: 10.1093/pcp/pcp137. [DOI] [PubMed] [Google Scholar]
- 108.Wu J., Zhao F.J., Ghandilyan A., Logoteta B., Olortegui Guzman M., Schat H., Wang X., Aarts M.G.M. Identification and functional analysis of two ZIP metal transporters of the hyperaccumulator Thlaspi caerulescens. Plant Soil. 2009;325:79–95. doi: 10.1007/s11104-009-0151-6. [DOI] [Google Scholar]
- 109.Lee S., Lee J., Ricachenevsky F.K., Punshon T., Tappero R., Salt D.E., Guerinot M.L. Redundant roles of four ZIP family members in zinc homeostasis and seed development in Arabidopsis thaliana. Plant J. 2021;108:1162–1173. doi: 10.1111/tpj.15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kilambi H.V., Kumar R., Sharma R., Sreelakshmi Y. Chromoplast-specific carotenoid-associated protein appears to be important for enhanced accumulation of carotenoids in hp1 tomato fruits. Plant Physiol. 2013;161:2085–2101. doi: 10.1104/pp.112.212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luo J., Tang S., Peng X., Yan X., Zeng X., Li J., Li X., Wu G. Elucidation of cross-talk and specificity of early response mechanisms to salt and PEG-simulated drought stresses in Brassica napus using comparative proteomic analysis. PLoS ONE. 2015;10:e0138974. doi: 10.1371/journal.pone.0138974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trentmann S.M., Kende H. Analysis of Arabidopsis cDNA that shows homology to the tomato E8 cDNA. Plant Mol. Biol. 1995;29:161–166. doi: 10.1007/BF00019127. [DOI] [PubMed] [Google Scholar]
- 113.Awad J., Stotz H.U., Fekete A., Krischke M., Engert C., Havaux M., Berger S., Mueller M.J. 2-cysteine peroxiredoxins and thylakoid ascorbate peroxidase create a water-water cycle that is essential to protect the photosynthetic apparatus under high light stress conditions. Plant Physiol. 2015;167:1592–1603. doi: 10.1104/pp.114.255356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kriechbaumer V., Maneta-Peyret L., Fouillen L., Botchway S.W., Upson J., Hughes L., Richardson J., Kittelmann M., Moreau P., Hawes C. The odd one out: Arabidopsis reticulon 20 does not bend ER membranes but has a role in lipid regulation. Sci. Rep. 2018;8:2310. doi: 10.1038/s41598-018-20840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang S., Xue M., He C., Shen D., Jiang C., Zhao H., Niu D. AtMC1 associates with Lsm4 to regulate plant immunity through modulating pre-mRNA splicing. Mol. Plant Microbe Interact. 2021;34:1423–1432. doi: 10.1094/MPMI-07-21-0197-R. [DOI] [PubMed] [Google Scholar]
- 116.Raza A., Su W., Hussain M.A., Mehmood S.S., Zhang X., Cheng Y., Zou X., Lv Y. Integrated analysis of metabolome and transcriptome reveals insights for cold tolerance in rapeseed (Brassica napus L.) Front. Plant Sci. 2021;12:721681. doi: 10.3389/fpls.2021.721681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eudes A., Teixeira Benites V., Wang G., Baidoo E.E., Lee T.S., Keasling J.D., Loque D. Precursor-directed combinatorial biosynthesis of cinnamoyl, dihydrocinnamoyl, and benzoyl anthranilates in Saccharomyces cerevisiae. PLoS ONE. 2015;10:e0138972. doi: 10.1371/journal.pone.0138972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Soubeyrand E., Kelly M., Keene S.A., Bernert A.C., Latimer S., Johnson T.S., Elowsky C., Colquhoun T.A., Block A.K., Basset G.J. Arabidopsis 4-COUMAROYL-COA LIGASE 8 contributes to the biosynthesis of the benzenoid ring of coenzyme Q in peroxisomes. Biochem. J. 2019;476:3521–3532. doi: 10.1042/BCJ20190688. [DOI] [PubMed] [Google Scholar]
- 119.Goyer A., Collakova E., Diaz de la Garza R., Quinlivan E.P., Williamson J., Gregory J.F., III, Shachar-Hill Y., Hanson A.D. 5-Formyltetrahydrofolate is an inhibitory but well tolerated metabolite in Arabidopsis leaves. J. Biol. Chem. 2005;280:26137–26142. doi: 10.1074/jbc.M503106200. [DOI] [PubMed] [Google Scholar]
- 120.Ercetin M.E., Ananieva E.A., Safaee N.M., Torabinejad J., Robinson J.Y., Gillaspy G.E. A phosphatidylinositol phosphate-specific myo-inositol polyphosphate 5-phosphatase required for seedling growth. Plant Mol. Biol. 2008;67:375–388. doi: 10.1007/s11103-008-9327-3. [DOI] [PubMed] [Google Scholar]
- 121.Ercetin M.E., Gillaspy G.E. Molecular characterization of an Arabidopsis gene encoding a phospholipid-specific inositol polyphosphate 5-phosphatase. Plant Physiol. 2004;135:938–946. doi: 10.1104/pp.104.040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Y., Lin W.H., Chen X., Xue H.W. The role of Arabidopsis 5PTase13 in root gravitropism through modulation of vesicle trafficking. Cell Res. 2009;19:1191–1204. doi: 10.1038/cr.2009.105. [DOI] [PubMed] [Google Scholar]
- 123.Ananieva E.A., Gillaspy G.E., Ely A., Burnette R.N., Erickson F.L. Interaction of the WD40 domain of a myoinositol polyphosphate 5-phosphatase with SnRK1 links inositol, sugar, and stress signaling. Plant Physiol. 2008;148:1868–1882. doi: 10.1104/pp.108.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chadaeva I., Ponomarenko P., Kozhemyakina R., Suslov V., Bogomolov A., Klimova N., Shikhevich S., Savinkova L., Oshchepkov D., Kolchanov N.A., et al. Domestication explains two-thirds of differential-gene-expression variance between domestic and wild animals; the remaining one-third reflects intraspecific and interspecific variation. Animals. 2021;11:2667. doi: 10.3390/ani11092667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jores T., Tonnies J., Wrightsman T., Buckler E.S., Cuperus J.T., Fields S., Queitsch C. Synthetic promoter designs enabled by a comprehensive analysis of plant core promoters. Nat. Plants. 2021;7:842–855. doi: 10.1038/s41477-021-00932-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kasowski M., Grubert F., Heffelfinger C., Hariharan M., Asabere A., Waszak S., Habegger L., Rozowsky J., Shi M., Urban A., et al. Variation in transcription factor binding among humans. Science. 2010;328:232–235. doi: 10.1126/science.1183621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.1000 Genomes Project Consortium. Abecasis G., Auton A., Brooks L., DePristo M., Durbin R., Handsaker R., Kang H., Marth G., McVean G., et al. An integrated map of genetic variation from 1.092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stajich J.E., Block D., Boulez K., Brenner S.E., Chervitz S.A., Dagdigian C., Fuellen G., Gilbert J.G., Korf I., Lapp H., et al. The Bioperl toolkit: Perl modules for the life sciences. Genom. Res. 2002;12:1611–1618. doi: 10.1101/gr.361602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Blackmore C.C., Richardson M.L., Linnau K.F., Schwed A.M., Lomoschitz F.M., Escobedo E.M., Hunter J.C., Jurkovich G.J., Cummings P. Web-based image review and data acquisition for multiinstitutional research. AJR Am. J. Roentgenol. 2003;180:1243–1246. doi: 10.2214/ajr.180.5.1801243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All original in silico data presented in this work are freely available in the knowledge base AtSNP_TATAdb (https://www.sysbio.ru/AtSNP_TATAdb/) created and presented in this work, as accessed on 15 December 2023.