Abstract
Purpose of review
The vestibular system provides three-dimensional idiothetic cues for updating of one's position in space during head and body movement. Ascending vestibular signals reach entorhinal and hippocampal networks via head-direction pathways, where they converge with multisensory information to tune the place and grid cell code.
Recent findings
Animal models have provided insight to neurobiological consequences of vestibular lesions for cerebral networks controlling spatial cognition. Multimodal cerebral imaging combined with behavioural testing of spatial orientation and navigation performance as well as strategy in the last years helped to decipher vestibular-cognitive interactions also in humans.
Summary
This review will update the current knowledge on the anatomical and cellular basis of vestibular contributions to spatial orientation and navigation from a translational perspective (animal and human studies), delineate the behavioural and functional consequences of different vestibular pathologies on these cognitive domains, and will lastly speculate on a potential role of vestibular dysfunction for cognitive aging and impeding cognitive impairment in analogy to the well known effects of hearing loss.
Keywords: cognition, hippocampus, navigation, spatial orientation, vestibular
INTRODUCTION
Preclinical and clinical studies in the past decades have clearly shown a contribution of vestibular signals to cognitive processes foremost in the domains of spatial orientation, navigation and cognition [1,2▪]. Based on theoretical considerations, it is reasonable that target-oriented navigation relies on both allothetic, that is, mostly visual cues from the environment, and idiothetic, that is, vestibular and somatosensory cues for a continuous updating of one's own position in space during active and passive movement [3–5]. Spatial orientation and navigation is guided by a widespread network of brain regions (prefrontal cortex, basal ganglia, thalamus, cerebellum, posterior parietal cortex, retrosplenial cortex, posterior parahippocampus, lingual gyrus, hippocampus, and entorhinal cortex) [6]. Creating a mental representation of a novel environment seems to depend critically on the hippocampus, entorhinal cortex, and retrosplenial cortex with its highly specialized cell ensembles (place cells, grid cells, and head direction cells) [6]. Given the extensive ascending projections of the vestibular system to neocortical and hippocampal networks [7▪▪,8▪], there is an overlap of vestibular/multisensory networks with hubs and circuits involved in spatial cognition [1]. In this review, we will give an update on the behavioural consequences of vestibular lesions for spatial orientation and navigation in conjunction with considerations on the underlying cellular and circuitry mechanisms including both animal and human studies.
Box 1.
no caption available
VESTIBULAR INPUT TO HIPPOCAMPAL AND EXTRAHIPPOCAMPAL NAVIGATION NETWORKS
Vestibular input from the semicircular canals and otolith organs gets transmitted to the hippocampus via the vestibular nucleus, vestibulocerebellum and ascending multisynaptic pathways involving the dorsal brainstem tegmentum, anterior thalamus, subiculum and entorhinal cortex [9]. By this route, information on heading direction reaches hippocampal networks, which is relevant for the updating of one's own position in space during movement in interaction with other sensory sources [10,11▪]. Furthermore, vestibular input to hippocampal place cells and grid cells has been well documented [12]. Modulation of vestibular afferent signals during head and body movement has instantaneous consequences on hippocampal and entorhinal theta rhythm [13–15]. Selective electrical stimulation experiments of the semicircular canals and otolith organs in rats have demonstrated a rather widespread and variable bilateral induction of hippocampal local field potentials arising from single sensor stimulation [16▪]. In addition, a vestibular contribution to extrahippocampal head-direction networks, for example, in the retrosplenial cortex has been described [17,18▪,19▪▪,20▪▪].
BEHAVIOURAL CONSEQUENCES OF VARIOUS VESTIBULAR DISEASES ON SPATIAL COGNITION
Vestibular animal models are optimally suited to experimentally control for the extent (complete vs. incomplete), endurance (transient vs. permanent), and laterality (unilateral vs. bilateral) of a vestibular deficit, as well as the timely dynamics and magnitude of spatial orientation and navigation deficits following peripheral vestibular lesions [21]. Consequently, vestibular contribution to spatial orientation has been studied predominantly in animal models over the last decades.
Several studies have shown that bilateral vestibular damage in rodents leads to severe and persistent navigation deficits in real space by disrupting the head direction cell code in the dorsal brainstem tegmentum, anterior thalamus, subiculum and entorhinal cortex, the place cell code in the hippocampus, and the grid cell code in the entorhinal cortex. Recently, it could be shown that the implantation of a vestibular prosthesis can inversely improve spatial orientation in animals with vestibular deficits [22]. Galvanic vestibular stimulation in mice had some effect on visuospatial cognition, if the bilateral peripheral vestibular deficits were incomplete [23▪]. However, it is unclear how persistent such an effect would be, as repetitive subthreshold noisy galvanic vestibular stimulation (nGVS) in rats with incomplete bilateral vestibular loss had no enduring effect on the movement pattern in an open field and no modulatory effects on hippocampal energy metabolism in vivo[24▪].
Recently, long-lasting egocentric and allocentric spatial memory deficits and impaired ipsilesional hippocampal plasticity have also been found following complete unilateral vestibular loss in rats [25▪▪]. Nguyen et al. [26] furthermore documented an impact of the lesion side, with left-sided unilateral labyrinthectomy having a more severe effect on short-term and long-term spatial cognition. Galvanic vestibular stimulation was able to improve spatial cognition after unilateral labyrinthectomy in mice [27].
Following the seminal publication of Brandt et al. [28] in 2005, which showed selective deficits of spatial cognition in a virtual Morris Water Task in patients with a complete bilateral vestibular loss, several studies have been published over the last years reporting differential spatial orientation and navigation deficits in patients with various vestibular pathologies in real-space as well as desktop and immersive virtual reality setups [29,30,31▪,32▪]. As a general note, these results may be interpreted in consideration of different vestibular inputs and reference frames inherent to the respective setups, that is desktop virtual reality or paper-pencil applications may rather probe two-dimensional static vestibular spatial memory [29,33], while real-space or immersive virtual reality navigation allows for multisensory and specifically 3D vestibular inputs induced by translational and rotational head and body movements [3,34].
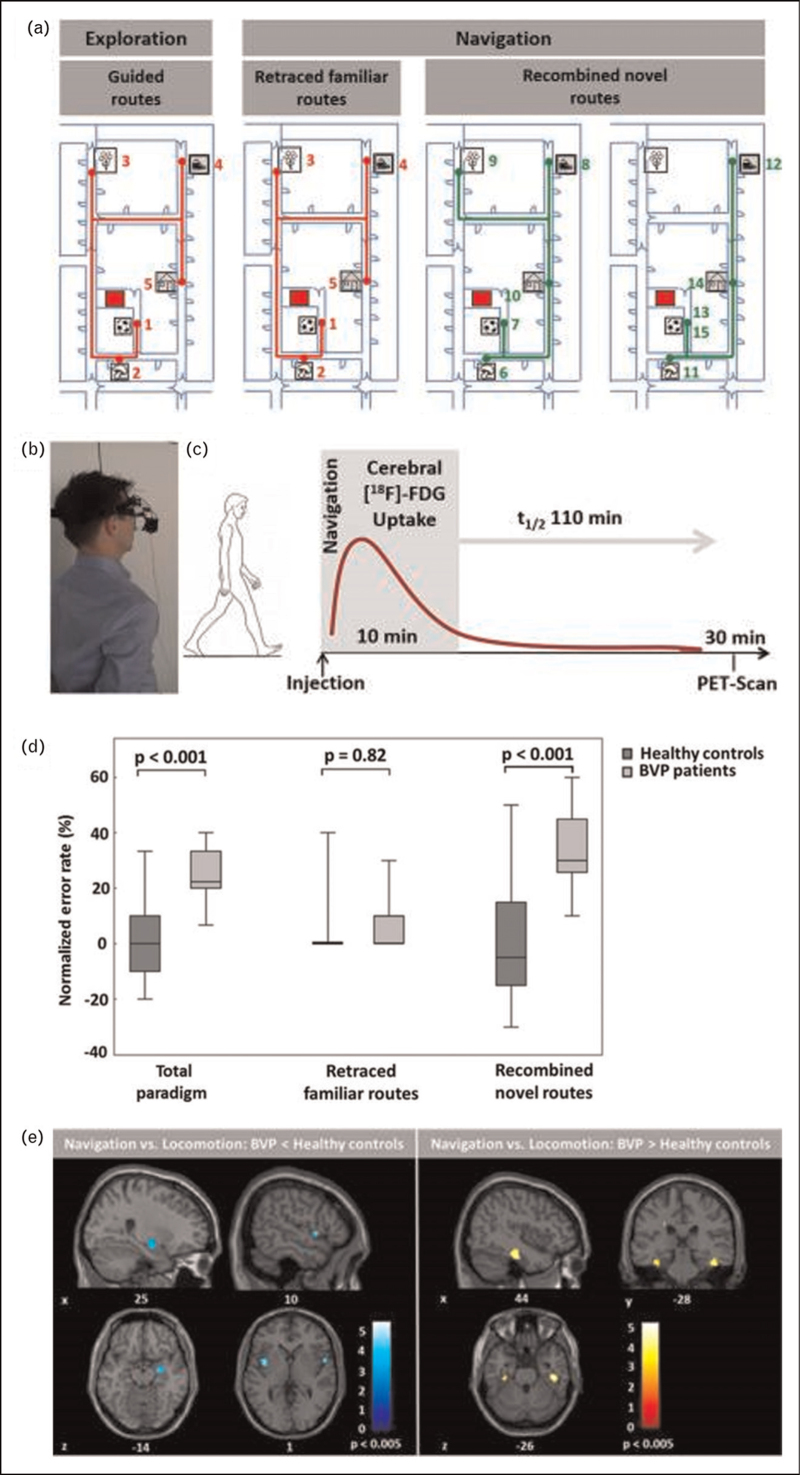
In a recent study, real-space navigation performance and strategy, as well as visual exploration and landmark fixation was tested in patients with bilateral vestibulopathy [4]. Patients performed worse than matched healthy controls, when recombining novel routes (allocentric navigation), whereas retracing of familiar routes (egocentric navigation) was normal (Fig. 1 a--d). Importantly, these deficits correlated with the severity of the bilateral vestibular loss. Patients exhibited higher gait fluctuations, spent less time at crossroads, and used path optimization strategies such as possible shortcuts less often. Overall, this pattern reflected a selective deficit in allocentric navigation strategies, which rely on a mental representation of a cognitive map, while visual landmark-based and stimulus-response strategies were used more abundantly, likely in an attempt of functional compensation. Similar results were reported in a recent study, which applied a virtual reversed T-maze paradigm to document a preference for egocentric or allocentric navigation strategies in patients with bilateral or unilateral vestibular loss [35▪▪]. It was observed that patients with bilateral vestibulopathy had a generally reduced ability to select a specific navigation strategy and specifically had some reduced odds to use an allocentric strategy. In contrast, patients with chronic unilateral vestibular loss tended to use an egocentric navigation strategy less frequently. Only right-sided vestibular loss affected the ability to adopt a specific spatial orientation strategy [35▪▪]. In another recent cohort study, patients with a chronic unilateral or bilateral vestibulopathy performed significantly worse in a triangle completion test for path integration and rotational memory test, but showed no differences in terms of the visuospatial subset of the Berlin intelligence structure test (BIS-4) and d2-R for attention and concentration [36]. Lately, Oh et al.[37▪▪] tested performance in visuospatial perception and memory tasks by paper-pencil tests (i.e., the Visual and Object Space Perception battery, Block design test, Corsi block-tapping test) in 72 patients with acute unilateral peripheral vestibulopathy at 2 days and 4 weeks after symptom onset. In the acute phase, patients performed significantly worse on visuospatial memory, but not perception tasks. At this time, patients with right-sided vestibular lesions had decreased visuospatial memory and perception compared to left-sided lesions. Visuospatial abilities crossly recovered within 4 weeks of compensation.
FIGURE 1.
Real-space navigation testing in patients with bilateral vestibulopathy. (a) Navigation paradigm in real space. The area, in which five target items had been placed, was shown to the individuals first on an investigator-guided walk (exploration, left side). Afterwards, individuals had to find the items in a defined pseudo-randomised order over the next 10 min beginning from the starting point (red square). The first five routes in the navigation paradigm were identical to the previous exploration routes and therefore had to be simply retraced (middle). Then, the order of target items was changed in a way that required recombining novel routes (right side); (b) Individuals wore a gaze-monitoring head camera to allow posthoc analysis of their visual exploration; c) [18F]-FDG was injected at the start of the 10-min navigation phase to depict the cerebral glucose utilization during navigation; (d) The normalized error rate during the navigation is depicted for the total paradigm, as well as separately for retraced familiar and recombined novel routes. (e) During navigation, BVP patients had a decrease of rCGM in the right hippocampal formation and bilateral insular cortex, and an increase of rCGM in the bilateral parahippocampus and lingual gyrus (i.e., parahippocampal place are, PPA) compared to healthy controls. BVP, bilateral vestibulopathy. Figure adapted from [4].
PATHOPHYSIOLOGY OF SPATIAL NAVIGATION DISORDERS IN VESTIBULOPATHIES
Recent animal studies have given further insight in the consequences of vestibular lesions on hippocampal structure and function. Serial in vivo whole-brain imaging of neuronal activity after a unilateral chemical labyrinthectomy in rats depicted a decrease in the ipsilesional posterior and anterior hippocampus as well as entorhinal cortex glucose metabolism and a relative increase in the contralesional anterior hippocampus and entorhinal cortex from day 1 to 15 after unilateral chemical labyrinthectomy [38,39▪]. Recently, long-lasting impaired plasticity mechanisms in the ipsilesional hippocampal formation were documented following unilateral vestibular neurectomy in the rat [25▪▪]. The overly ipsilateral effects on hippocampal neurobiology could speak for a functional lateralization of vestibulo-hippocampal projections, similar to the well known ipsilateral dominance of vestibular projections to the parieto-insular cortex [40]. In a bilateral chemical labyrinthectomy rat model, hippocampal glucose metabolism decreased on both sides from days 1–60 post lesion [24▪]. In vivo synaptic density imaging depicted no major synaptic loss in the hippocampus during this time period [41▪]. This finding is in accordance to older in vitro studies, which indicated no major synaptic loss in the hippocampus following bilateral vestibular deafferentation, but only a minor change in dendritic structure [42].
In humans with peripheral vestibular disorders, multimodal imaging studies in the last years have added to the understanding of functional and structural hippocampal alterations. During real-space navigation, the right hippocampus and entorhinal cortex were less active in patients with bilateral vestibular loss, while the bilateral parahippocampal place area were more active than in healthy controls (Fig. 1e). Patients with a complete bilateral vestibulopathy due to neurectomy additionally showed reduced activations in the pontine brainstem, anterior thalamus, posterior insular, and retrosplenial cortex compared to patients with incomplete bilateral vestibular loss [4]. This navigation-induced brain activation pattern in BVP is compatible with deficits in creating a mental representation of a novel environment. Residual vestibular function allows recruitment of brain areas involved in head direction signalling to support navigation. Parahippocampal place area activation may reflect a compensatory visually guided navigation strategy in patients with bilateral vestibular loss. Volumetric brain imaging studies have reported differential results in terms of structural consequences of vestibular loss for hippocampal volume (for a recent review see [2▪]). While single studies showed a global atrophy of the hippocampus [28] following bilateral vestibular loss, others reported a decrease of volume only in hippocampal subregions [43▪,44▪], while some studies found no volumetric changes at all [35▪▪,45]. It is currently hypothesized that hippocampal volumetric changes depend on several cofactors such as disease duration, extent of the vestibular lesion, or concomitant hearing loss. It seems that hippocampal volume loss is not a prerequisite nor a core substrate of spatial disorientation but rather a morphological correlate of an enduring loss of hippocampal signal input [2▪].
CLINICAL RELEVANCE OF SPATIAL NAVIGATION DEFICITS IN VESTIBULOPATHIES
Patients with incomplete unilateral or bilateral vestibular loss rarely complain about functionally relevant spatial orientation deficits in everyday life. It seems that compensatory mechanisms via landmark-based strategies, residual function of the head direction system, or stimulus-response strategies can functionally cope for most of the allocentric spatial orientation deficit in these disorders. However, vestibular deafferentation may reduce the functional reserve of hippocampal, insular, and parietal networks during aging and may thus be a susceptibility factor for an earlier cognitive decline during aging. In line, patients with bilateral lateral semicircular canal or bilateral utricular dysfunction have worse rotational spatial orientation [46]. Patients with vestibular disorders may also show mild deficits of nonspatial cognitive domains ([47▪], for a recent meta-analysis see [48▪▪]). Vestibular function deficits mostly from otolith pathways are more prevalent in patients with Alzheimer's dementia especially if spatial orientation deficits are the predominant clinical phenotype [49]. While several theoretical concepts have been proposed to explain the possible impact of vestibular loss for cognitive decline, the concept is not substantiated by longitudinal cohort studies and less well established than for hearing loss.
CONCLUSION
Current studies in animal models and patients have substantiated the view that vestibular signals critically contribute to spatial cognition and orientation. Potential mechanisms may be the following: a deficit in creating a mental representation or cognitive map of a novel environment due to a functional disturbance of head-direction, place and grid cell systems, an impaired heuristic in topographical processing with difficulty to select the most appropriate navigation strategy for a given situation or task, or a noisier and less accurate path integration due to imprecise convergence of idiothetic and allothetic cues with a partial “multisensonsory conflict”. The extent of the spatial orientation deficits in vestibular disorders depends on multiple factors such as laterality and magnitude of vestibular loss. Overall, right-sided vestibular lesions in humans tend to have more severe effects on spatial cognition. Hippocampal effects of unilateral vestibular loss seem to have a preponderance to the lesion side. Consequences of vestibular loss for nonspatial cognitive domains have been reported, but their relevance as a risk factor for cognitive impairment remains elusive. Future studies need to address the long-term effects by a longitudinal and comprehensive assessment of visuospatial function in patients with vestibular disorders to answer these questions. A further topic will be whether novel methods for restoration or augmentation of vestibular function (i.e. vestibular implant, galvanic vestibular simulation) in patients with bilateral vestibular failure can effectively improve spatial navigation performance and also nonspatial cognitive function.
Acknowledgements
The authors thank Katie Göttlinger for copyediting the manuscript.
Financial support and sponsorship
German Federal Ministry of Education and Research (BMBF, number EO1401).
Conflicts of interest
The authors declare no conflict of interest related to the manuscript.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Brandt T, Dieterich M. The dizzy patient: don’t forget disorders of the central vestibular system. Nat Rev Neurol 2017; 13:352–362. [DOI] [PubMed] [Google Scholar]
- 2▪.Smith PF. Interpreting the meaning of changes in hippocampal volume associated with vestibular loss. Front Integr Neurosci 2023; 17:1254972. [DOI] [PMC free article] [PubMed] [Google Scholar]; This current review addresses the relevance of structural hippocampal changes in peripheral vestibular lesions.
- 3.Taube JS, Valerio S, Yoder RM. Is navigation in virtual reality with FMRI really navigation? J Cogn Neurosci 2013; 25:1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schöberl F, Pradhan C, Grosch M, et al. Bilateral vestibulopathy causes selective deficits in recombining novel routes in real space. Sci Rep 2021; 11:2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teaford M, Keller K, Merfeld DM. The contribution of interoceptive signals to spatial orientation: a mini-review. Neurosci Biobehav Rev 2022; 143:104943. [DOI] [PubMed] [Google Scholar]
- 6.Epstein RA, Patai EZ, Julian JB, Spiers HJ. The cognitive map in humans: spatial navigation and beyond. Nat Neurosci 2017; 20:1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪▪.Neumann N, Fullana MA, Radua J, et al. Common neural correlates of vestibular stimulation and fear learning: an fMRI meta-analysis. J Neurol 2023; 270:1843–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides an actual meta-analysis of functional imaging studies applying a vestibular stimulation.
- 8▪.Conrad J, Baier B, Eberle L, et al. Network architecture of verticality processing in the human thalamus. Ann Neurol 2023; 94:133–145. [DOI] [PubMed] [Google Scholar]; This multimodal magnetic resonance imaging study depicts the structural and functional connectivity network architecture of the vestibular representations in the thalamus and cortex.
- 9.Hitier M, Besnard S, Smith PF. Vestibular pathways involved in cognition. Front Integr Neurosci 2014; 8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angelaki D, Laurens J. The head direction cell network: attractor dynamics, integration within the navigation system, and three-dimensional properties. Curr Opin Neurobiol 2020; 60:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11▪.Grieves RM, Shinder ME, Rosow LK, et al. The neural correlates of spatial disorientation in head direction cells. eNeuro 2022; 9: ENEURO.0174-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]; This rat study provides evidence for a multisensory integration from a variety of inputs to the head direction system in order to prevent disorientation.
- 12.Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input. Hippocampus 2002; 12:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neo P, Carter D, Zheng Y, et al. Septal elicitation of hippocampal theta rhythm did not repair cognitive and emotional deficits resulting from vestibular lesions. Hippocampus 2012; 22:1176–1187. [DOI] [PubMed] [Google Scholar]
- 14.Jacob P, Poucet B, Liberge M, et al. Vestibular control of entorhinal cortex activity in spatial navigation. Front Integr Neurosci 2014; 8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aitken P, Zheng Y, Smith PF. The modulation of hippocampal theta rhythm by the vestibular system. J Neurophysiol 2018; 119:548–562. [DOI] [PubMed] [Google Scholar]
- 16▪.Hitier M, Zhang Y, Sato G, et al. Stratification of hippocampal electrophysiological activation evoked by selective electrical stimulation of different angular and linear acceleration sensors in the rat peripheral vestibular system. Hear Res 2021; 403:108173. [DOI] [PubMed] [Google Scholar]; This rat study shows a complex pattern of bilateral hippocampal local field potentials during stimulation of single vestibular sensors in the inner ear.
- 17.Angelaki DE, Ng J, Abrego AM, et al. A gravity-based three-dimensional compass in the mouse brain. Nat Commun 2020; 11:1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪.Hennestad E, Witoelar A, Chambers AR, Vervaeke K. Mapping vestibular and visual contributions to angular head velocity tuning in the cortex. Cell Rep 2021; 37:110134. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study in mice reports neurons that signal the angular velocity of head movements (AHV cells) in the motor, somatosensory, visual, and posterior parietal cortex.
- 19▪▪.Keshavarzi S, Bracey EF, Faville RA, et al. Multisensory coding of angular head velocity in the retrosplenial cortex. Neuron 2022; 110:532–543. [DOI] [PMC free article] [PubMed] [Google Scholar]; This mouse study provides evidence that cortical AHV cell coding required vestibular input but also uses visual signals to optimize heading estimation during navigation.
- 20▪▪.Sit KK, Goard MJ. Coregistration of heading to visual cues in retrosplenial cortex. Nat Commun 2023; 14:1992. [DOI] [PMC free article] [PubMed] [Google Scholar]; The results from this this study in mice suggest a circuit in the retrosplenial cortex, which anchors heading representations to environmental visual landmarks.
- 21.Straka H, Zwergal A, Cullen KE. Vestibular animal models: contributions to understanding physiology and disease. J Neurol 2016; 263: (Suppl 1): S10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karmali F, Haburcakova C, Gong W, et al. An implanted vestibular prothesis improves spatial orientation in animals with severe vestibular damage. J Neurosci 2021; 41:3879–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪.Nguyen TT, Nam GS, Han GC, et al. The effect of galvanic vestibular stimulation on visuospatial cognition in an incomplete bilateral deafferentation mouse model. Front Neurol 2022; 13:857736. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study demonstrates that early administration of sinusoidal galvanic vestibular stimulation in rats accelerates the recovery of locomotor and spatial memory deficiencies.
- 24▪.Wuehr M, Eilles E, Lindner M, et al. Repetitive low-intensity vestibular noise stimulation partly reverses behavioral and brain activity changes following bilateral vestibular loss in rats. Biomolecules 2023; 13:1580. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study in rats discloses the potential neurophysiological and neuroanatomical substrates that mediate previously observed therapeutic effects of nGVS on postural deficits in bilateral vestibular loss.
- 25▪▪.El Mahmoudi N, Laurent C, Péricat D, et al. Long-lasting spatial memory deficits and impaired hippocampal plasticity following unilateral vestibular loss. Prog Neurobiol 2023; 223:102403. [DOI] [PubMed] [Google Scholar]; This study highlights the crucial role of symmetrical vestibular information in spatial memory and links deficits in spatial cognitive domains following unilateral vestibular neurectomy in rats to long-lasting impairment of plasticity in the ipsilesional hippocampus.
- 26.Nguyen TT, Nam GS, Kang JJ, et al. The differential effects of acute right- vs. left-sided vestibular deafferentation on spatial cognition in unilateral labyrinthectomized mice. Front Neurol 2021; 12:789487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen TT, Nam GS, Kang JJ, et al. Galvanic vestibular stimulation improves spatial cognition after unilateral labyrinthectomy in mice. Front Neurol 2021; 12:716795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandt T, Schautzer F, Hamilton DA. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain 2005; 128:2732–2741. [DOI] [PubMed] [Google Scholar]
- 29.Schöberl T, Zwergal A, Brandt T. Testing navigation in real space: contributions to understanding the physiology and pathology of human navigation control. Front Neural Circuits 2020; 14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerb J, Brandt T, Dieterich M. Different strategies in pointing tasks and their impact on clinical bedside tests of spatial orientation. J Neurol 2022; 269:5738–5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31▪.Gerb J, Brandt T, Dieterich M. Different approaches to test orientation of self in space: comparison of a 2D pen-and-paper test and a 3D real-world pointing task. J Neurol 2023; 270:642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports a comparison of different spatial orientation tests based on questionnaires, paper-pencil applications, and a 3D real-world pointing task in 121 participants.
- 32▪.Huang Y, Zhang X, Tang J, et al. Vestibular cognition assessment system: tablet-based computerized visuospatial abilities test battery. Front Psychol 2023; 14:1095777. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study applies a newly developed mobile tablet-based vestibular cognitive assessment system to evaluate the visuospatial cognitive ability of patients with vestibular dysfunction.
- 33.Brandt T, Dieterich M. Vestibular contribution to three-dimensional dynamic (allocentric) and two-dimensional static (egocentric) spatial memory. J Neurol 2016; 263:1015–1016. [DOI] [PubMed] [Google Scholar]
- 34.Cullen KE, Taube JS. Our sense of direction: progress, controversies and challenges. Nat Neurosci 2017; 20:1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35▪▪.Gammeri R, Léonard J, Toupet M, et al. Navigation strategies in patients with vestibular loss tested in a virtual reality T-maze. J Neurol 2022; 269:4333–4348. [DOI] [PubMed] [Google Scholar]; This study reports differential alterations in the selection of navigation strategies in patients with unilateral and bilateral vestibular disorders using a virtual reality setup.
- 36.Dordevic M, Sulzer S, Barche D, et al. Chronic, mild vestibulopathy leads to deficits in spatial tasks that rely on vestibular input while leaving other cognitive functions and brain volumes intact. Life 2021; 11:1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37▪▪.Oh SY, Nguyen TT, Kang JJ, et al. Visuospatial cognition in acute unilateral peripheral vestibulopathy. Front Neurol 2023; 14:1230495. [DOI] [PMC free article] [PubMed] [Google Scholar]; This prospective cohort study describes acute and delayed effects of right and left-sided acute vestibular loss in patients.
- 38.Grosch M, Lindner M, Bartenstein P, et al. Dynamic whole-brain metabolic connectivity during vestibular compensation in the rat. Neuroimage 2021; 226:117588. [DOI] [PubMed] [Google Scholar]
- 39▪.Zwergal A, Lindner M, Grosch M, Dieterich M. In vivo neuroplasticity in vestibular animal models. Mol Cell Neurosci 2022; 120:103721. [DOI] [PubMed] [Google Scholar]; This review summarizes recent in vivo evidence for whole-brain adaptive neuroplasticity in vestibular disorders.
- 40.Dieterich M, Bense S, Lutz S, et al. Dominance for vestibular cortical function in the nondominant hemisphere. Cereb Cortex 2003; 13:994–1007. [DOI] [PubMed] [Google Scholar]
- 41▪.Antons M, Lindner M, Grosch M, et al. Longitudinal [18]UCB-H/[18F]FDG imaging depicts complex patterns of structural and functional neuroplasticity following bilateral vestibular loss in the rat. Sci Rep 2022; 12:6049. [DOI] [PMC free article] [PubMed] [Google Scholar]; This rat study reports complex multilevel dynamic alterations of neuronal activity and synaptic density in rats following bilateral chemical labyrinthectomy.
- 42.Balabhadrapatruni S, Zheng Y, Napper R, et al. Basal dendritic length is reduced in the rat hippocampus following bilateral vestibular deafferentation. Neurobiol Learn Mem 2016; 131:56–60. [DOI] [PubMed] [Google Scholar]
- 43▪.Schöne C, Rebsamen M, Wyssen G, et al. Hippocampal volume in patients with bilateral and unilateral vestibular dysfunction. Neuroimage Clin 2022; 36:103212. [DOI] [PMC free article] [PubMed] [Google Scholar]; This imaging study indicates volumetric changes in hippocampal subregions in patients with peripheral vestibular disorders.
- 44▪.Lee E, Weon Y, Kim J, et al. Functional and anatomical alterations in bilateral vestibulopathy: a multimodal neuroimaging study and clinical correlation. Front Neurol 2023; 14:1157931. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study displays hippocampal volume reductions and alterations in functional and anatomical connectivity in patients with bilateral vestibulopathy, which correlated with the degree of health-related disability.
- 45.Cutfield N, Scott G, Waldman A, et al. Visual and proprioceptive interaction in patients with bilateral vestibular loss. Neuroimage Clin 2014; 4:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anson E, Ehrenburg MR, Simonsick EM, Agrawal Y. Association between vestibular function and rotational spatial orientation perception in older adults. J Vestib Res 2021; 31:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47▪.Obermann M, Gebauer A, Arweiler-Harbeck D, et al. Cognitive deficits in patients with peripheral vestibular dysfunction. Eur J Neurol 2023; doi: 10.1111/ene.15907. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes deficits in multiple spatial and nonspatial cognitive domains in patients with unilateral and bilateral vestibular deficits.
- 48▪▪.Chari DA, Madhani A, Sharon JD, Lewis RF. Evidence for cognitive impairment in patients with vestibular disorders. J Neurol 2022; 269:5831–5842. [DOI] [PubMed] [Google Scholar]; This current review of literature presents evidence for the presence of deficits in different cognitive domains due to vestibular loss.
- 49.Smith PF. Recent developments in the understanding of the interactions between the vestibular system, memory, the hippocampus, and the striatum. Front Neurol 2022; 13:986302. [DOI] [PMC free article] [PubMed] [Google Scholar]