Abstract
The Lactococcus lactis adhE gene, which encodes a multifunctional alcohol dehydrogenase, has been cloned and characterized. A DNA fragment encoding the putative alcohol dehydrogenase domain of the AdhE protein was cloned by screening an L. lactis genomic library in a fermentative mutant of Escherichia coli and selecting for the ability to grow anaerobically. Further analysis of the clone obtained allowed the cloning of the entire adhE gene sequence. Analysis of adhE expression in L. lactis during anaerobiosis showed induction at the transcriptional level, especially in medium containing glucose. Constructed mutant strains produced reduced amounts of ethanol under anaerobic conditions. With the L. lactis gene as a probe, adhE homologs were found in other industrially relevant lactic acid bacteria.
During fermentation in Lactococcus lactis, pyruvate metabolism leads to the predominant formation of lactate and a number of minor metabolites. Anaerobic growth in milk requires the presence of pyruvate formate-lyase (PFL) and lactate dehydrogenase (LDH) for growth, acidification, and the regeneration of reduced cofactors. Pyruvate is converted into acetyl coenzyme A (CoA) and formate via PFL. In Escherichia coli, acetyl-CoA is further metabolized to acetaldehyde and ethanol by a multifunctional dehydrogenase encoded by the adhE gene (15). AdhE is also responsible for deactivation of PFL, to ensure protection against irreversible inactivation of PFL in the presence of oxygen (14). To date, only one adhE homolog has been reported for Clostridium acetobutylicum (20) based on functional analysis, and putative homologs have been identified for Salmonella typhimurium and Actinobacillus pneumoniae (5, 25). Eukaryotic adhE genes have also been characterized for amitochondriate protozoan parasites (21).
E. coli AdhE is a multifunctional protein of 890 amino acids (aa) that catalyzes the conversion of acetyl-CoA into ethanol and has acetaldehyde dehydrogenase and alcohol dehydrogenase (ADH) activities. Additionally, AdhE shows PFL-deactivase activity, which is involved in the inactivation of PFL, a key enzyme in anaerobic metabolism (14). L. lactis is an aerotolerant anaerobic gram-positive bacterium in which the presence of a PFL-deactivase may represent a selective advantage. We have recently reported the cloning and characterization of the L. lactis pfl gene (4). Gene inactivation of pfl resulted in enhanced production of diacetyl without formate under anaerobic conditions.
It has recently been postulated that Streptococcus thermophilus does not have an ADH and that production of acetaldehyde, characteristic of this organism, may be obtained via conversion of threonine to glycine with equimolar production of acetaldehyde (18). However, if the presence of an adhE homolog in lactic acid bacteria could be demonstrated, metabolic and genetic engineering could be applied to increase levels of acetaldehyde, a relevant component in yogurt production (18), during fermentation and to modulate PFL activity.
We report here the cloning of the ADH domain of the L. lactis adhE gene by genetic complementation of a fermentative mutant of E. coli and characterization of the complete adhE gene. We also report the presence of adhE homologs in other lactic acid bacteria, including S. thermophilus.
MATERIALS AND METHODS
Bacteria and plasmids.
E. coli MC1000 (7) and DH5α (Stratagene) were used for cloning. E. coli NZN111 (pfl ldh), also referred to as FMJ39 (19), was used for genetic complementation experiments. L. lactis subsp. cremoris MG1363 (11) and L. lactis subsp. lactis biovar diacetylactis DB1341 (kindly provided by Chr. Hansen A/S, Hørsholm, Denmark) were used in this study. S. thermophilus ATCC 19258, Leuconostoc mesenteroides subsp. mesenteroides ATCC 10878, and Lactobacillus acidophilus ATCC 4796 were used for the identification of adhE homologs. PCR fragments were cloned into pBluescript KS+ (Stratagene) or pSMA500 (17).
Media and growth conditions.
E. coli strains were grown in Luria-Bertani medium at 37°C. Erythromycin (250 μg ml−1), kanamycin (50 μg ml−1), or ampicillin (50 μg ml−1) was added as required. L. lactis strains were grown in 1.5× M17 (24) supplemented with 0.5% (wt/vol) glucose (GM17), galactose (GalM17), or lactose (LacM17). The media were supplemented with 10 mM citric acid for growth of L. lactis subsp. lactis biovar diacetylactis DB1341 and transformants derived from this strain. We also used a defined medium, SA (13a), which was supplemented with 1% (wt/vol) glucose or lactose and is referred to as GSA or LSA, respectively. Acetate was omitted, and 100 mM lipoic acid was added to SA for fermentation to allow the synthesis of acetyl-CoA via pyruvate dehydrogenase during aerobic growth (23). Erythromycin (1 μg ml−1) was added as required. Fermentation was carried out with six benchtop fermentors (Applikon), each containing 1 liter of medium and set to operate at 30°C. Stirring was applied with a supply of air (aerobic conditions) or nitrogen (anaerobiosis). Oxygen levels were monitored with an Ingold oxygen electrode (Mettler Toledo) and were kept above 20% saturation with air. The fermentors were inoculated with 1 to 10 ml of a fresh overnight culture grown in GM17 or GSA. Growth was monitored by measuring the optical density at 600 nm (OD600), and samples were taken at an OD600 of 1.0 ± 0.1 for RNA studies and metabolite analysis. Additionally, cultures were grown statically in tightly closed 50-ml tubes containing 50 ml of medium without shaking and were considered anaerobic. Aerobic cultures were grown in 50 ml of medium in 250-ml flasks with shaking (250 rpm). Cultures grown in flasks or tubes were used for metabolite analysis and in mRNA studies to compare the levels of expression observed in fermentors. S. thermophilus, Leuconostoc mesenteroides, and Lactobacillus acidophilus were grown in MRS as specified by the procedures of the American Type Culture Collection.
Protein extraction and NADH oxidation measurement in E. coli.
Protein extraction was carried out by adding 100 μl of 100 mM MOPS (morpholinepropanesulfonic acid) buffer (pH 6.5)–2% Triton X-100 to the cell pellets from 1.5-ml overnight cultures, which had been washed in fresh ice-cold medium and frozen at −80°C for 15 min. The pellets were dissolved and transferred to Eppendorf tubes. Lysozyme (5 mg) was added, and samples were incubated on ice for 30 min. Subsequently, glass beads (diameter, 100 μm; Sigma) were added and samples were vortexed for 30 s and kept on ice for 30 s. This step was repeated 10 to 15 times, and samples were centrifuged in an Eppendorf centrifuge at maximum speed for 2 min. Supernatants were transferred to a new Eppendorf tube and kept at −80°C until assayed. To measure NADH oxidation, the following components were mixed in a quartz cuvette: 700 μl of 100 mM MOPS (pH 6.5), 100 μl of 120 mM Na-pyruvate, 50 μl of 2.56 mM NADH, and 50 μl H2O. The decrease in OD340, as a result of the oxidation of NADH to NAD, was monitored after the addition of 100 μl of sample. As a control, pyruvate was omitted from the reaction. No significant decrease in OD was observed in the control.
Measurement of metabolites.
The amounts of acetaldehyde and ethanol produced after overnight growth were analyzed by headspace gas chromatography with a flame ionization detector (Perkin-Elmer model HS-40, Chrompack CP9000).
DNA manipulations and transformation.
Chromosomal DNA was isolated from L. lactis, S. thermophilus, Leuconostoc mesenteroides, and Lactobacillus acidophilus as previously described (3). Plasmid DNA was isolated from E. coli with Jet Prep kits (Genomed). DNA restriction, dephosphorylation, and cloning were performed according to standard procedures (22). DNA fragments were purified from agarose gels with Jet Sorb kits (Genomed). Probe labeling was carried out with a Ready-to-go kit (Pharmacia). Unincorporated nucleotides were removed with Nick columns (Pharmacia). Heterologous hybridization experiments were carried out overnight at 65°C. Filters were washed twice in 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at room temperature for 30 min and subsequently once in 3× SSC–0.1% sodium dodecyl sulfate at 65°C for 30 min.
DNA sequencing was carried out with T3 and T7 primers and subsequently with primers derived from the obtained sequences. Sequencing primers were Cy-5 labeled, and reactions were run on an ALF Express sequencer (Pharmacia). E. coli was transformed by electroporation as described previously (22). L. lactis strains were transformed essentially as described previously (13).
Construction and screening of an L. lactis genomic library.
A genomic library was constructed with partially Sau3AI-digested DNA of L. lactis subsp. lactis biovar diacetylactis strain DB1341 and cloning into BamHI-digested plasmid pSMA500 (17). The genomic library consisted of approximately 10,000 independent recombinant clones with an average insert size of 4 kb. A mixed culture, containing all clones obtained, was grown in Luria-Bertani medium supplemented with erythromycin, and plasmid DNA was isolated for genetic complementation with E. coli NZN111 (pfl::Tn5 ldh Kmr). This strain is unable to grow in the absence of O2, due to mutation in the pfl and ldh genes (19). Additionally, a λZAP Express genomic library of strain DB1341 (4) was used to characterize the full-length adhE gene. Plasmid DNA isolation from positive clones followed in vivo excision (Stratagene), and sequence analysis (ALF Express; Pharmacia) was carried out.
PCR amplifications.
With the adhE sequence of strain DB1341 obtained from pC1, an adhE DNA fragment was amplified from L. lactis MG1363 with primers adhe-mg1 (5′-CGCAAGCCCTCTCGGTGTACTT-3′ [positions 2211 to 2232 in Fig. 2]) and adhe-mg2 (5′-ACGTTCAAGGTGAGCTTTACG-3′ [positions 4452 to 4431 in Fig. 2]). The fragment was sequenced with the relevant primers derived from the sequence of the DB1341 adhE fragment. Subsequently, inverse PCR was carried out on HindIII-digested and religated chromosomal DNA from strain DB1341 with primers adhe-350 (5′-CTGTTGATGTTGGATTAGTC-3′ [positions 2269 to 2250 in Fig. 2]) and adhe-700 or adhe-1300x (respectively, 5′-AAGGTCTTCTTCGGCACGTTCAAT-3′ [positions 2613 to 2590 in Fig. 2] or 5′-GAGATTGTACGTAGCTTACTTGC-3′ [positions 2986 to 3008 in Fig. 2]) to clone the upstream region of the adhE gene (Fig. 1). Sequence analysis of the obtained PCR products, with primer adhe-1300x, allowed the identification of the upstream region of the adhE gene. A second inverse PCR was also carried out with PstI-digested DNA from strain DB1341 to clone the upstream region including orfB (Fig. 1), and primers derived from the sequence obtained were used to sequence the corresponding chromosomal region of strain MG1363. Primers adhe-mg1 and adhe-1697 (5′-TGACGAGCGATTGCAATTGCTT-3′ [positions 3491 to 3512 in Fig. 2]) were used to amplify a 1.5-kb fragment from this strain, including the 5′ region of the adhE gene, and primers adhe-1300x and adhe-mg2 (5′-ACGTTCAAGGTGAGCTTTACG-3′ [positions 4452 to 4431 in Fig. 2]) were used to amplify an overlapping 1.5-kb fragment that included the 3′ region of this gene. PCR amplifications were carried out with 50-μl samples and a GeneAmp DNA amplification reagent kit from Perkin-Elmer Cetus, with the addition of 0.5% Tween 20.
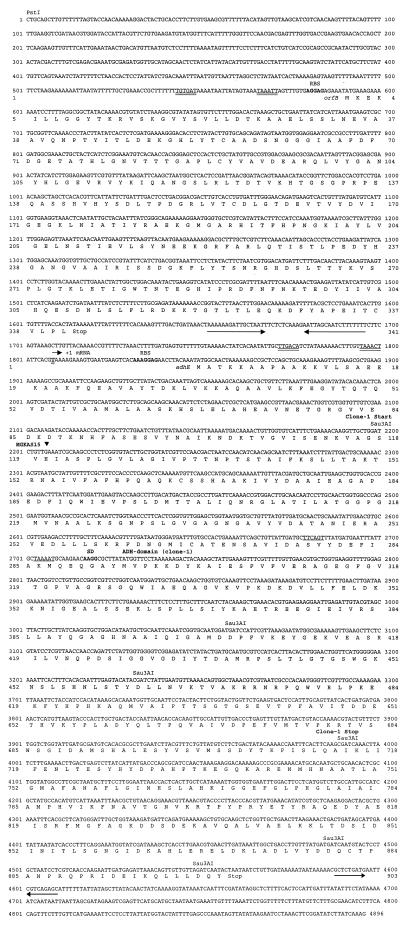
FIG. 2.
Sequence of the L. lactis DB1341 adhE gene. The sequence begins upstream of orfB and includes the adhE gene and ca. 350 bp of sequence downstream of adhE. +1 mRNA refers to the adhE transcription start site. Ribosome binding sites (RBS) for orfB and adhE are shown in boldface type. Promoter regions, −35 and −10, are indicated with double underlines. Pairs of arrows depict putative transcriptional terminator sequences. The deduced protein sequences are shown in single-letter code. The region included in pC1 is shown (Clone-1 Start to Clone-1 Stop) above the sequence. Consensus E. coli expression signals (Shine-Dalgarno sequence [SD; boldface type] and −35 and −10 promoter regions [underlined]) just upstream of the ADH domain in pC1 [ADH-domain (clone-1)] are shown. An inverted triangle above the sequence depicts the position of gene inactivation in the adhE strain MGKAS15.
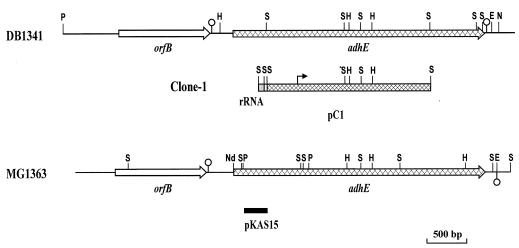
FIG. 1.
Genetic maps of the adhE chromosomal regions of L. lactis MG1363 and DB1341. Only relevant restriction sites are shown. E, EcoRI; H, HindIII; N, NsiI; Nd, NdeI; P, PstI; S, Sau3AI. The DNA fragments included in pC1 are shown, indicating the three different Sau3AI DNA fragments that were ligated together under library construction and that included an rRNA gene sequence (rRNA box in clone 1). The position of the consensus E. coli expression signals (Shine-Dalgarno sequence and promoter region) in pC1 is shown as a bent arrow (see also Fig. 2). The fragment used for gene inactivation of the MG1363 adhE gene is depicted as a filled box (plasmid construction pKAS15 [see Materials and Methods]). Putative transcriptional terminators for orfB and adhE are shown as open circles.
DNA sequence analysis.
Analysis of the DNA sequence data and homology searches were performed with the Genetics Computer Group (University of Wisconsin) program package, version 8.
Northern blot and primer extension analysis.
RNAs isolated from exponentially growing cultures (OD600 = 1) were separated on 0.8 to 1.2% agarose-formaldehyde gels. Blotting, hybridization, and washes were carried out as described previously (3). Strain-specific adhE probes were used for hybridization (see below; Fig. 1). Induction (mRNA) levels were estimated with an Instant Imager 2024 (Packard) by measuring the radioactivity in each full lane directly from the nylon membrane. Primer extension analysis was carried out with 10 μg of total RNA; the 32P-end-labeled primers mgadhe-PE1 (5′-AAGAACAGCAGCTTGTGCTTT-3′ [positions 1974 to 1954 in Fig. 2]), mgadhe-PE2 (5′-AACTTTCTTTGCAGCTGGAGCGG-3′ [positions 1887 to 1865 in Fig. 2]), and mgadhe-PE3 (5′-AAAGAAGTCAAGCGATATTGTGATGAAATTT-3′ [located 106 bp further upstream from mgadhe-PE2 in the orfB-adhE intergenic region, which is highly divergent between DB1341 and MG1363]); and the avian myeloblastosis virus reverse transcriptase primer extension system (Promega). Products of the primer extension reactions were separated on sequencing gels. Sequencing reactions were performed simultaneously with a Thermosequenase radiolabeled terminator cycle sequencing kit (Amersham) on an 864-bp PCR product that included the upstream region of the adhE gene. The gene was amplified with primers adhe-268 (5′-GGCTGGACAAATACTGAAGG-3′ [positions 1428 to 1447 in Fig. 2]) and adhe-350, their corresponding primers, and α-33P-labeled dideoxynucleotides (Amersham).
Construction of an L. lactis adhE mutant in MG1363.
A PCR fragment containing an internal upstream region of the adhE gene was amplified with primers mgadhe-null2-XhoI (5′-CATGCCTCGAGCCGCAAAATTCCAAGAAG-3 [XhoI site underlined, positions 1906 to 1924 in Fig. 2]) and mgadhe-BamHI (5′-CATGCGGATCCAACAGAACCAGCAACTTTGTT-3′ [BamHI site underlined, positions 2206 to 2185 in Fig. 2]) and with genomic DNA from strain MG1363 as the template. The expected 300-bp DNA fragment was digested with XhoI and BamHI, cloned into similarly digested pSMA500, and transformed into E. coli MC1000. The plasmid construction pKAS15 (Fig. 1) was introduced into L. lactis MG1363, which resulted in strain MGKAS15 (Fig. 2). PCR was used to verify the integration of pKAS15 into the adhE gene in MGKAS15 by Campbell-like recombination (16).
Nucleotide sequence accession numbers.
The sequences of the adhE regions of strains MG1363 (4,741 bp) and DB1341 (4,896 bp) have been deposited in EMBL under accession no. AJ001007 and AJ001008, respectively.
RESULTS
Genetic complementation in E. coli NZN111 with an L. lactis genomic library.
E. coli NZN111 harbors mutations in the pfl and ldh genes (19). This strain cannot grow under strict anaerobic conditions, under which PFL and LDH activities participate in the fermentative regeneration of NAD. NZN111 can therefore be used for the cloning of genes involved in fermentation from other organisms, if the gene is expressed in E. coli and the product is functional. Thus, enzymatic activities that convert NADH into NAD should complement the defect in NZN111. We decided to study the presence of such activities in L. lactis using genetic complementation. E. coli NZN111 was transformed with 200 ng of plasmid DNA from a genomic library made from L. lactis subsp. lactis biovar diacetylactis strain DB1341 (see Materials and Methods). Transformation mixtures were incubated at 37°C in anaerobic jars. As a control, pSMA500-transformed strain NZN111 was used. After 2 days, transformation plates were incubated aerobically a further two days, to allow weak complementing clones to grow. One clone, clone 1, was identified in the library-transformed plates, but no transformants were obtained in the pSMA500 control. In a preliminary screening, protein extracts of clone 1 were used in a modified LDH assay (9), where the pyruvate-dependent conversion of NADH to NAD was monitored to demonstrate that complementation of the fermentative defects in strain NZN111 occurred. A relatively high rate of conversion (approximately twofold compared to that of the NZN111::pSMA500 control) was observed for clone 1.
The plasmid present in clone 1, pC1, was isolated and used to retransform E. coli NZN111. Duplicate plates were incubated aerobically for 4 days or anaerobically for 2 days and then for 2 days aerobically at 37°C. Similar numbers of transformants, ca. 7 × 105 per μg of DNA, were obtained by both procedures, compared with 1 × 106 transformants per μg of DNA obtained with pSMA500 under aerobic incubation. No transformants were obtained with pSMA500 under anaerobic selection as indicated above for clone 1. These results clearly indicated that pC1 complements the mutations in strain NZN111.
Sequence analysis of pC1 and identification of a DNA fragment homologous to the E. coli adhE gene.
Plasmid pC1 was further characterized by restriction enzyme analysis and included a 2.1-kb insert. Sequence analysis determined that it included a 1.8-kb Sau3AI fragment. The 1.8-kb fragment included an open reading frame (ORF) homologous to the E. coli adhE gene (12) (Fig. 1 and 2). Two additional Sau3AI fragments, 0.1 and 0.2 kb in size, that were ligated together during library construction were also present in pC1 (Fig. 1). The 0.2-kb fragment corresponded to a fragment of the L. lactis 23S rRNA gene (data not shown). As shown in Fig. 1 and 2, pC1 included the ADH domain of an L. lactis AdhE homolog, and it contained by chance the necessary signals for expression in E. coli (Shine-Dalgarno sequence and −35 and −10 regions) (Fig. 2). The putative gene product encoded in pC1, a 427-aa protein, is highly homologous to a number of other iron-dependent ADHs. Comparison at the protein level showed 41.4% identity (78% similarity) with E. coli AdhE, in addition to significant homology to the C. acetobutylicum AdhE homolog, Aad, and other ADHs of both eukaryotic and prokaryotic origin.
Characterization of the full-length L. lactis adhE sequence.
Sequence comparison of pC1 with the two previously cloned bacterial adhE genes indicated that approximately the first 300 bp and the last 600 bp of the putative L. lactis adhE homolog were not present in this DNA fragment (Fig. 1 and 2). Therefore, a λZAP genomic library of strain DB1341 was screened with a 0.8-kb Sau3AI fragment from pC1 (positions 2198 to 3062 in Fig. 2) as a probe. Two positive clones (named λadhE1 and λadhE3) were selected for characterization. Following in vivo excision of the pBK plasmid version of the clones (Stratagene), restriction mapping and sequencing were carried out. Clone λadhE1 included a 1.8-kb Sau3AI insert that was identical to the adhE fragment of pC1 (positions 2198 to 3993 in Fig. 2). Clone λadhE3 contained a 4-kb insert that included the region starting from the Sau3AI site at position 3231 in Fig. 2. Sequence analysis of this clone confirmed that it included the 3′ end of the L. lactis adhE gene, which ends with a double stop codon (TAATAA; positions 4557 to 4562 in Fig. 2). Downstream from this position, a putative transcription terminator was found with an estimated ΔG of −12.8 kcal/mol (10) (positions 4587 to 4609 in Fig. 2). To obtain the upstream sequence of the adhE gene of strain DB1341, inverse PCR was used (see Materials and Methods). PCR and sequencing with primers derived from this sequence were used to obtain the entire sequence of the adhE gene of strain MG1363. A limited sequence variation at the DNA level was observed (202 base changes in the adhE coding region, corresponding to 92.5% sequence homology), resulting in only 18 amino acid substitutions (or 98% identity).
The L. lactis adhE gene of strain DB1341 encodes a 903-aa protein, as was deduced from the DNA sequence (Fig. 2), with an estimated molecular mass of 98.2 kDa. A putative ribosome binding site (AAAGGAG; positions 1831 to 1837 in Fig. 2) is found 11 bp upstream of the start codon (10). Homology comparisons showed 44% identity of the L. lactis AdhE to the E. coli protein and 42.4% identity to the C. acetobutylicum Aad protein throughout an approximately 750-aa-long fragment (Fig. 3). A significantly lower level of homology was observed in the C-terminal regions of these three proteins.
FIG. 3.
Multiple alignment of AdhE proteins. The lactococcal AdhE sequence from strain DB1341 was aligned with the sequences of all AdhE entries in the databases by using the Clustal alignment of the MEGALIGN program (DNAstar, Lasergene). Shaded boxes show positions of residues identical to the consensus. Abbreviations: dbadhe, strain DB1341 AdhE; ecadhe, E. coli AdhE; caaad, C. acetobutylicum Aad; ehadhe, E. histolytica AdhE; gladhe, G. lamblia AdhE.
An ORF, named orfB, that may encode a putative 341-aa protein was identified upstream of adhE. A consensus ribosome binding site (AGGAG; positions 580 to 584 in Fig. 2) and −35 and −10 promoter regions (positions 542 to 547 and 566 to 571, respectively, in Fig. 2) were found upstream from the orfB start codon. A putative transcriptional terminator was found downstream of orfB (ΔG of −12.9 kcal/mol; positions 1652 to 1696 in Fig. 2), suggesting that orfB and adhE are independently transcribed in L. lactis (Fig. 2 and 3). The E. coli adhE gene is transcribed independently (8), whereas the C. acetobutylicum homolog, aad, is transcribed as part of an operon together with three acetone formation genes (20). An ORF, orf1, that encodes a putative protein similar in size (319 aa) to L. lactis OrfB was also found upstream of the aad gene, and it is placed in the same orientation as the aad gene in the C. acetobutylicum chromosome (20). However, no homology was found between orfB and orf1. Only a putative 36.3-kDa E. coli protein without a known function, YbhE (6), showed 34.4% identity to OrfB. Thus, the function of orfB remains to be elucidated.
Analysis of adhE expression in L. lactis MG1363.
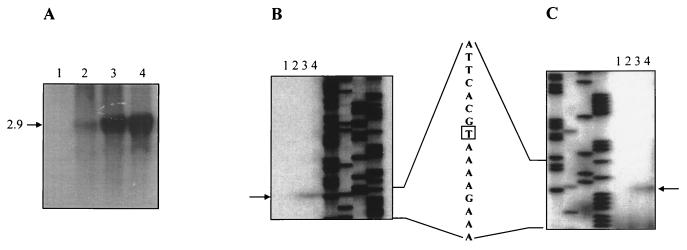
In E. coli, adhE expression is anaerobically induced (8). The expression of the L. lactis adhE gene was studied in exponentially growing cultures of strain MG1363 in M17 medium, containing either glucose or galactose, under anaerobic or aerobic conditions in a fermentor. Northern blot analysis showed a very low level of expression during aerobiosis and a sevenfold induction during anaerobiosis in glucose. In medium containing galactose, a very high level of expression was observed during aerobiosis (20-fold higher than that observed under aerobiosis in glucose) and a slightly higher (1.2-fold) level of expression was observed during anaerobiosis (Fig. 4). A single transcript (2.9 kb) was detected in all samples tested (Fig. 4A). The size of the transcript corresponded to the size of the adhE gene, confirming that transcription is independent of orfB. Primer extension studies were carried out with three different primers, mgadhe-PE1, mgadhe-PE2, and mgadhe-PE3. Single primer extension products of 167 and 80 bp were detected with mgadhe-PE1 and mgadhe-PE2, respectively (Fig. 4B and C). No extension products were observed with primer mgadhe-PE3 (data not shown). Thus, the transcription start site (tss) of the L. lactis adhE gene is located 41 bp upstream of the start codon (Fig. 2 and 4). A much larger nontranslated region (292 bp) has been reported for the E. coli adhE mRNA, which is involved in modulation of translation efficiency (2).
FIG. 4.
Northern blot and primer extension analysis of the L. lactis adhE gene. (A) Northern blot analysis of total RNA from L. lactis MG1363 grown in fermentors. Lane 1, aerobic growth in GM17; lane 2, anaerobic growth in GM17; lane 3, aerobic growth in GalM17; lane 4, anaerobic growth in GalM17. The size of the adhE transcript is shown on the left in kilobases. (B and C) Primer extension carried out with primers mgadhe-PE1 (B) and mgadhe-PE2 (C) on total RNA of strain MG1363. Lanes 1 to 4, MG1363 grown as described for panel A. A sequence ladder was run, in each case, with the corresponding primer. The adhE transcription start site is indicated with a box in the sequence line. Extension products are indicated with arrows.
Construction and analysis of adhE mutant strains in L. lactis MG1363.
As described recently, the introduction of pSMA500 derivatives to achieve gene inactivation in L. lactis DB1341 resulted in apparent genetic instability of the endogenous plasmids (4). Therefore, genetic inactivation of the adhE gene was carried out with L. lactis MG1363 (16). In order to ensure a null mutant, an adhE strain, MGKAS15, that is expected to synthesize a truncated AdhE protein at residue Val119 was constructed. The position of genetic disruption of the adhE gene in MGKAS15 is shown in Fig. 2 and was verified by PCR analysis (data not shown). The truncated AdhE produced in MGKAS15 should lack both acetaldehyde dehydrogenase and ADH activities, judging from the location of conserved dehydrogenase motifs in the primary sequence (Fig. 3). Acetaldehyde and ethanol levels from overnight cultures grown in GM17 or GalM17 with and without aeration were determined (Table 1). Relatively small amounts of acetaldehyde and no ethanol were produced during aerobic growth in either MG1363 or the adhE strains, regardless of the carbon source. Interestingly, a large amount of ethanol was observed in MG1363 growing anaerobically in GalM17. Under these conditions, expression of the L. lactis pfl and adhE genes is maximal, which may account for the high levels observed (4) (Fig. 4). A larger amount of acetaldehyde was produced by the adhE strain under anaerobic conditions in GM17 than by MG1363. The levels observed, however, are similar to the amounts of acetaldehyde produced by both strains during aerobiosis (Table 1). Thus, it is likely that these amounts represent the acetaldehyde generated elsewhere in the metabolism of L. lactis, especially when it grows in GM17. Under anaerobic conditions, the activity of wild-type AdhE may convert the acetaldehyde produced into ethanol, accounting for the lack of acetaldehyde observed in MG1363. MGKAS15 did not produce ethanol during anaerobic growth in GM17 (Table 1), demonstrating that the cloned gene encodes an ADH. The differences in the amounts of ethanol produced were not due to growth differences, since both strains reached identical OD600 values (Table 1). Growth in GalM17 was generally poorer, especially for MGKAS15. The high level of lactate produced under these conditions by MGKAS15 might account for the observed differences (not shown). It remains unclear whether the low level of ethanol produced by the adhE strains in GalM17 resulted from residual ADH activity of the truncated AdhE protein or was generated by an alternative ADH (Table 1).
TABLE 1.
Characterization of the L. lactis MG1363 adhE strain MGKAS15
| Strain | Growth medium | Result under:
|
|||||
|---|---|---|---|---|---|---|---|
| Aerobic conditions
|
Anaerobic conditions
|
||||||
| Acetaldehyde (mM) | Ethanol (mM) | Final OD600 | Acetaldehyde (mM) | Ethanol (mM) | Final OD600 | ||
| MG1363 | GM17 | 0.05 | ≤0.001 | 4.1 | ≤0.001 | 1.4 | 3.7 |
| GalM17 | 0.04 | ≤0.001 | 3.6 | 0.01 | 12.97 | 3.6 | |
| MGKAS15 | GM17 | 0.06 | ≤0.001 | 3.8 | 0.05 | 0.05 | 3.7 |
| GalM17 | 0.05 | ≤0.001 | 3.2 | ≤0.001 | 0.18 | 3.2 | |
Identification of adhE homologs in other lactic acid bacteria with an L. lactis adhE probe.
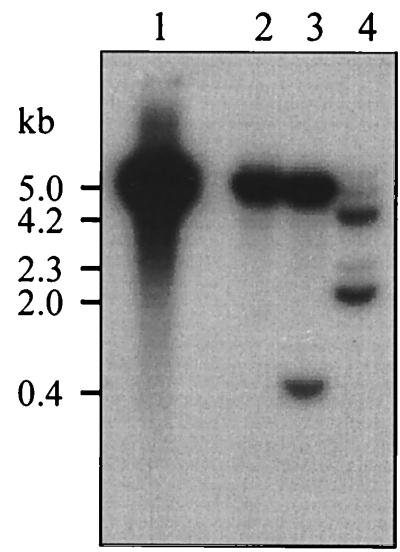
Two Sau3AI fragments including most of the L. lactis subsp. lactis biovar diacetylactis DB1341 adhE coding sequence contained in pC1 (Fig. 1) were used as probes in Southern hybridization experiments with EcoRI-digested total DNA from S. thermophilus, Leuconostoc mesenteroides subsp. mesenteroides, and Lactobacillus acidophilus. As shown in Fig. 5, a single EcoRI DNA fragment, approximately 5 kb in size, including the L. lactis MG1363 adhE sequence was detected, as was expected from the genetic map of the adhE locus in this strain (Fig. 1). Strongly hybridizing bands were detected in S. thermophilus (5 kb) and Leuconostoc mesenteroides (5 and 0.4 kb). More weakly hybridizing bands (4.2 and 2 kb) and two minor bands (5 and 2.3 kb) were identified in Lactobacillus acidophilus. These results suggest that an adhE homolog is present in these three industrially relevant lactic acid bacteria, and experiments are in progress to clone their respective genes.
FIG. 5.
Detection of an adhE homolog in other lactic acid bacteria. Southern blot analysis of genomic DNA digested with EcoRI by using the L. lactis adhE probe (Fig. 1). Lane 1, L. lactis MG1363; lane 2, S. thermophilus; lane 3, Leuconostoc mesenteroides; lane 4, Lactobacillus acidophilus. Bands sizes are shown.
DISCUSSION
We have cloned the L. lactis adhE gene, which encodes a multifunctional ADH. A plasmid library clone, pC1, containing the putative ADH domain of the L. lactis ADHE was able to complement the fermentative mutant E. coli NZN111, restoring its ability to grow anaerobically. The primary structure of the known AdhE homologs might contain separate functional domains as previously suggested (20). The putative lactococcal gene product encoded in the obtained pC1 clone showed a high degree of homology to a large number of iron-dependent ADHs, including all cloned AdhE homologs (Fig. 3).
Limited sequence variation was observed between the adhE gene of DB1341 (a diacetylactis strain) and MG1363 (an L. lactis subsp. cremoris strain), representing a 2% protein sequence divergence. The deduced L. lactis AdhE showed a high degree of identity to the previously characterized AdhE homologs, with over 40% sequence identity. Interestingly, the degree of homology of the C-terminal regions of the different AdhE proteins was significantly lower (Fig. 3). It is tempting to suggest that this region may be involved in recognition and deactivation of PFL. The N-terminal region of the lactococcal AdhE is homologous to known aldehyde dehydrogenases, despite the sequence variation observed for this class of enzymes. L. lactis AdhE shows the conserved 15-aa peptide (positions 123 to 137 in Fig. 2), including two strictly conserved Pro residues that are found in other aldehyde dehydrogenases (20). Additionally, two unique features found in other known ADHs are present in L. lactis AdhE: (i) a conserved glycine-rich region with a GXGXXG pattern located in the NADH-binding domain (20) (positions 446 to 451 in Fig. 3) and (ii) a highly conserved GXGXXVXXA motif (GTGSEVTPFA; positions 636 to 645 in Fig. 3), present in other ADHs, including all known AdhE homologs with the exception of Giardia lamblia AdhE (21), where a serine is found at the last position (Fig. 3).
Starter cultures with increased acetaldehyde production could be obtained by alteration of the AdhE primary sequence. We constructed strains with a genetic disruption in the adhE gene at different positions further downstream than in MGKAS15. These strains showed very similar phenotypes, i.e., reduced ethanol amounts and no acetaldehyde production (not shown). Interestingly, E. coli AdhE forms a multimeric structure that can be visualized by electron microscopy (14). This multimeric conformation has been suggested as a requisite for activity. Our results with the constructed disruption mutants support this hypothesis, since a truncated AdhE protein is not expected to form multimers.
A large promoter region with an unusually long nontranslated region that requires cleavage by RNase III for translation has been reported for the E. coli adhE gene (2). These features are not evident in the promoter region of L. lactis adhE, as confirmed by mapping of a unique transcription start site for the adhE mRNA (Fig. 4). A consensus FNR box, the recognition site for the global transcriptional activator FNR, is present just upstream of the −35 promoter region of E. coli adhE, although no alteration in gene expression was observed in an fnr strain (8). We have recently suggested the presence of FNR-like boxes in the promoter region of the L. lactis pfl gene on the basis of its sequence and location being similar to those of the E. coli pfl gene. However, sequence variation was observed between the different putative FNR-like boxes in the L. lactis pfl promoter region (4). A sequence with homology to the proposed lactococcal consensus FNR box is present upstream of the adhE gene in DB1341 [TTGTC(N4)ATCAA (where N is any base); positions 1495 to 1508 in Fig. 2]. Further work is required to elucidate the role of these sequences in the expression of the L. lactis pfl and adhE genes. A gene, orfB, that is transcribed in the same orientation was found upstream of adhE. The presence of a transcriptional terminator downstream of the orfB coding region (Fig. 2) together with Northern blot analysis of adhE expression (Fig. 4) indicated that adhE is transcribed independently of orfB. We have studied adhE transcription during exponential growth with glucose or galactose in rich medium. Anaerobic induction in medium containing glucose and high levels of expression during both aerobic and anaerobic growth with galactose was observed. Interestingly, no ethanol formation was detected in aerobiosis, in spite of the high level of transcription of the adhE gene (Table 1; Fig. 4). NADH and other cofactors needed for AdhE activity might be present at low concentrations, precluding enzymatic activity. Oxygen and carbon source dependence have also been observed for the expression of the L. lactis pfl gene, although strong anaerobic induction was detected also during growth in galactose (4).
In E. coli PFL activation occurs via free-radical formation through an activase that contains an Fe-S cluster (26). Thus, PFL activity and AdhE-mediated NAD regeneration require iron. Milk, the growth medium for L. lactis during industrial fermentation, is a poor source of iron. Therefore, the activities of L. lactis AdhE and activase (and consequently PFL) in milk remain to be demonstrated. Indirect evidence for activity has been obtained by comparison of wild-type and pfl mutated starter strains (4), since the mutant produced a higher level of diacetyl and acetoin than the wild type and no formate during anaerobic growth in milk (not shown), indicating a certain degree of PFL activity in the wild type.
The absence of ethanol production in GM17 of the adhE strain MGKAS15 confirmed that the L. lactis adhE gene encodes an ADH. For the bacterium Zymomonas mobilis, two nonrelated ADHs, ADH-I and ADH-II, respectively encoded by the adhA and adhB genes, have been characterized. ADH-II has been identified as a major stress protein in Z. mobilis and is synthesized in response to heat shock and exposure to ethanol (1). Experiments are in progress to characterize the enzyme(s) responsible for the limited ethanol production observed during anaerobic growth of the mutants in GalM17 and to study whether L. lactis contains a protein with a role similar to that of Z. mobilis ADH-II.
The presence of putative adhE homologs was detected in other industrially relevant lactic acid bacteria by Southern blot analysis (Fig. 5). Our results strongly indicate the presence of adhE homologs in all three species investigated. Further work to clone and characterize the adhE genes of these bacteria is being carried out.
Site-directed mutagenesis of the conserved ADH consensus regions present in L. lactis AdhE will be carried out to attempt the engineering of AdhE for increased acetaldehyde formation. Current work concerns the study of the putative PFL-deactivase function of L. lactis AdhE. Physiological analysis of the constructed adhE strains to study the effect of a truncated AdhE on the activity of PFL in L. lactis grown under anaerobic and aerobic conditions will be carried out.
ACKNOWLEDGMENTS
This work is the result of a collaborative project of the Biotechnological Institute, Hørsholm, Denmark, and Chr. Hansen A/S, which was sponsored and partially financed by a Danish MPU grant from the Ministry of Agriculture.
Pernille Smith, Annemette Jørgensen, and Anne Cathrine Steenbjerg are thanked for excellent technical assistance. We thank Anne Maria Hansen (Biotechnological Institute, Kolding, Denmark) for metabolite measurements. We also thank Mogens Kilstrup (Technical University of Denmark) for quantification of results from Northern blots. Eric Johansen (Chr. Hansen A/S) is thanked for providing the L. lactis subsp. lactis biovar diacetylactis DB1341 strain.
REFERENCES
- 1.An H, Scopes R K, Rodriguez M, Keshav K F, Ingram L O. Gel electrophoretic analysis of Zymomonas mobilis glycolytic and fermentative enzymes: identification of alcohol dehydrogenase II as a stress protein. J Bacteriol. 1991;173:5975–5982. doi: 10.1128/jb.173.19.5975-5982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aristarkhov A, Mikulskis A, Belasco J G, Lin E C C. Translation of the adhE transcript to produce ethanol dehydrogenase requires RNase III cleavage in Escherichia coli. J Bacteriol. 1996;178:4327–4332. doi: 10.1128/jb.178.14.4327-4332.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnau J, Sørensen K I, Appel K F, Vogensen F K, Hammer K. Analysis of heat shock gene expression in Lactococcus lactis MG1363. Microbiology. 1996;142:1685–1691. doi: 10.1099/13500872-142-7-1685. [DOI] [PubMed] [Google Scholar]
- 4.Arnau J, Jørgensen F, Madsen S M, Vrang A, Israelsen H. Cloning, expression, and characterization of the Lactococcus lactis pfl gene, encoding pyruvate formate-lyase. J Bacteriol. 1997;179:5884–5891. doi: 10.1128/jb.179.18.5884-5891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bäumler A J, Kusters J G, Stojiljkovic I, Heffron F. Salmonella typhimurium loci involved in survival within macrophages. Infect Immun. 1994;62:1623–1630. doi: 10.1128/iai.62.5.1623-1630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 7.Casadaban M J, Cohen S N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138:179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y-M, Lin E C C. Regulation of the adhE gene, which encodes ethanol dehydrogenase in Escherichia coli. J Bacteriol. 1991;173:8009–8013. doi: 10.1128/jb.173.24.8009-8013.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crow V L, Pritchard G G. Fructose 1,6-diphosphate-activated l-lactate dehydrogenase from Streptococcus lactis: kinetic properties and factors affecting activation. J Bacteriol. 1977;131:82–91. doi: 10.1128/jb.131.1.82-91.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vos W M, Simons G. Gene cloning and expression systems in lactococci. In: Gasson M, de Vos W, editors. Genetics and biotechnology of lactic acid bacteria. London, United Kingdom: Chapman and Hall; 1994. pp. 52–105. [Google Scholar]
- 11.Gasson M J. Plasmid complements of Streptococcus lactis NCDO712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodlove P E, Cunningham P R, Parker J, Clark D P. Cloning and sequence analysis of the fermentative alcohol-dehydrogenase-encoding gene of Escherichia coli. Gene. 1989;85:209–214. doi: 10.1016/0378-1119(89)90483-6. [DOI] [PubMed] [Google Scholar]
- 13.Holo H, Nes I F. High-frequency transformation by electroporation of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Jensen P R, Hammer K. Minimal requirements for exponential growth of Lactococcus lactis. Appl Environ Microbiol. 1993;59:4363–4366. doi: 10.1128/aem.59.12.4363-4366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessler D, Leibrecht I, Knappe J. Pyruvate-formate-lyase-deactivase and acetyl-coA reductase activities of Escherichia coli reside on a polymeric protein particle encoded by adhE. FEBS Lett. 1991;281:59–63. doi: 10.1016/0014-5793(91)80358-a. [DOI] [PubMed] [Google Scholar]
- 15.Kessler D, Herth W, Knappe J. Ultrastructure and pyruvate formate-lyase radical quenching of the multienzymic AdhE protein of Escherichia coli. J Biol Chem. 1992;267:18073–18079. [PubMed] [Google Scholar]
- 16.Leenthous K, Kok J, Venema G. Replacement recombination in Lactococcus lactis. J Bacteriol. 1991;173:4794–4798. doi: 10.1128/jb.173.15.4794-4798.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madsen S M, Albrechtsen B, Hansen E, Israelsen H. Cloning and transcriptional analysis of two threonine biosynthetic genes from Lactococcus lactis MG1614. J Bacteriol. 1996;178:3689–3694. doi: 10.1128/jb.178.13.3689-3694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall V M E, Tamime A Y. Physiology and biochemistry of fermented milks. In: Law B A, editor. Microbiology and biochemistry of cheese and fermented milk. 2nd ed. London, United Kingdom: Chapman and Hall; 1997. pp. 153–192. [Google Scholar]
- 19.Mat-Jan F, Alam K Y, Clark D P. Mutants of Escherichia coli deficient in the fermentative lactate dehydrogenase. J Bacteriol. 1988;171:342–348. doi: 10.1128/jb.171.1.342-348.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair R V, Bennett G N, Papoutsakis E T. Molecular characterization of an aldehyde/alcohol dehydrogenase gene from Clostridium acetobutylicum ATCC 824. J Bacteriol. 1994;176:871–885. doi: 10.1128/jb.176.3.871-885.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenthal B, Mai Z, Caplivski D, Ghosh S, de la Vega H, Graf T, Samuelson J. Evidence for the bacterial origin of genes encoding fermentation enzymes of the amitochondriate protozoan parasite Entamoeba histolytica. J Bacteriol. 1997;179:3736–3745. doi: 10.1128/jb.179.11.3736-3745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 23.Snoep J L, de Mattos M J T, Starrenburg M J C, Hugenholtz J. Isolation, characterization, and physiological role of the pyruvate dehydrogenase complex and α-acetolactate synthase of Lactococcus lactis subsp. lactis biovar diacetylactis. J Bacteriol. 1992;174:4838–4841. doi: 10.1128/jb.174.14.4838-4841.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terzaghi B A, Sandine W E. Improved medium for lactic acid streptococci and their bacteriophages. Appl Environ Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vezina G, Sirois M, Clairoux N, Boissinot M. Cloning and characterization of the groE locus from Actinobacillus pleuropneumoniae. FEMS Microbiol Lett. 1997;147:11–16. doi: 10.1111/j.1574-6968.1997.tb10213.x. [DOI] [PubMed] [Google Scholar]
- 26.Wagner A, Frey M, Neugebauer F, Schafer W, Knappe J. The free radical in pyruvate formate-lyase is located on glycine-734. Proc Natl Acad Sci USA. 1992;89:996–1000. doi: 10.1073/pnas.89.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]