Abstract
We report the identification of the promoter region of the Escherichia coli O7-specific lipopolysaccharide (LPS) gene cluster (wbEcO7). Typical −10 and −35 sequences were found to be located in the intervening region between galF and rlmB, the first gene of the wbEcO7 cluster. Data from RNase protection experiments revealed the existence of an untranslated leader mRNA segment of 173 bp, including the JUMPStart and two ops sequences. We characterized the structure of this leader mRNA by using the program Mfold and a combination of nested and internal deletions transcriptionally fused to a promoterless lac operon. Our results indicated that the leader mRNA may fold into a series of complex stem-loop structures, one of which includes the JUMPStart element. We have also found that one of the ops sequences resides on the predicted stem and the other resides on the loop region, and we confirmed that these sequences are essential for the RfaH-mediated regulation of the O polysaccharide cluster. A very similar stem-loop structure could be predicted in the promoter region of the LPS core operon encoding the waaQGPSBIJYZK genes. We observed another predicted stem-loop, located immediately downstream from the wbEcO7 transcription initiation site, which appeared to be involved in premature termination of transcription. This putative stem-loop is common to many other O polysaccharide gene clusters but is not present in core oligosaccharide genes. wbEcO7-lac transcriptional fusions in single copy numbers were also used to determine the effects of various environmental cues in the transcriptional regulation of O polysaccharide synthesis. No effects were detected with temperature, osmolarity, Mg2+ concentration, and drugs inducing changes in DNA supercoiling. We therefore conclude that the wbEcO7 promoter activity may be constitutive and that regulation takes place at the level of elongation of the mRNA in a RfaH-mediated manner.
Lipopolysaccharide (LPS) is a complex cell surface glycolipid that consists of three regions: lipid A, core oligosaccharide, and the O-specific polysaccharide chain or O antigen (50). LPS is the most abundant lipid in the outer leaflet of the outer membrane in the majority of gram-negative bacteria. While the lipid A is embedded in the membrane, the O antigen extends towards the surface and, in pathogenic organisms, contributes to the evasion of the lytic action of serum complement (20). The biosynthesis of LPS takes place in two separate pathways, one resulting in the formation of the lipid A core and another one resulting in the formation of the O antigen (49, 50). The O antigen intermediates are synthesized attached to undecaprenyl phosphate, and the complete O subunit is translocated across the inner membrane, polymerized, and transferred onto the lipid A core to complete the LPS molecule (49).
The synthesis of LPS involves a large number of genes, many of which are parts of various clusters located on different regions of the bacterial chromosome and, in some organisms, also in plasmids (40). Therefore, it is conceivable that LPS gene expression has to be coordinated to ensure that all necessary components are available at any given time. However, the regulation of LPS synthesis is not well understood. It has been shown recently that certain chemical modifications of lipid A are regulated by the Mg2+ concentration through the phoP-phoQ regulon (16). Regulation of LPS biosynthesis by amino acid starvation has also been reported (18), but there is no information on how this regulation is accomplished.
Gene expression of the core biosynthetic gene cluster is regulated by the RfaH protein (11, 34) and also by the heat shock response (21). RfaH is a homolog of the NusG factor that regulates gene expression of the hemolysin operon (6, 23, 24, 31), polysaccharide capsule genes (43), and genes for the transfer of the F plasmid (8). RfaH regulation is exerted at the level of elongation of mRNA (5) and depends on cis-acting sequences known as ops (for operon polarity suppressor) located upstream of the coding regions of the RfaH-regulated operons (4, 23, 31). One such operon is waaQGPSBIJYZK, which includes 10 genes of the core LPS gene cluster (10, 34). Similar, presumably untranslated, leader mRNA segments containing ops exist in the O polysaccharide gene clusters of Escherichia coli, Shigella flexneri, Salmonella enterica, and Yersinia enterocolitica (17), but no direct evidence exists on their role in regulating gene expression.
Regulation of the O-specific polysaccharide gene expression has not been systematically investigated despite the availability of completely sequenced O polysaccharide gene clusters. In at least one case, O polysaccharide gene expression is regulated by temperature via changes in DNA supercoiling (39), and in another case it is regulated by osmolarity (1). Posttranscriptional regulation occurs via the cld (rol) determinant, whose product controls the length distribution of O polysaccharide chains by a mechanism that is not completely understood (7, 12, 47).
We are using the E. coli O7 polysaccharide as a model system to understand the synthesis, assembly, and regulation of O polysaccharide gene expression (2, 27, 28, 30, 45). We have previously reported the DNA sequence and gene organization of the upstream portion of the E. coli O7 antigen biosynthesis gene cluster, wbEcO7 (previously called rfb; see reference 37 for a description of the new nomenclature adopted for bacterial polysaccharide genes), containing a promoter region and four biosynthetic genes, rlmBADC, which are involved in the biosynthesis of the nucleotide sugar precursor dTDP-rhamnose (27). In this work we report the characterization of this promoter and also of an upstream leader segment with several predicted stem-loop structures. These regions appear to be important as a site for the regulation of the elongation of the wbEcO7 mRNA transcript in a RfaH-dependent manner. We also show that the wbEcO7 promoter activity is expressed constitutively.
MATERIALS AND METHODS
Bacterial strains, plasmids, and general methods.
The E. coli strains and plasmids used in this study are described in Table 1. CLM13 and CLM12 are rfaH+ and rfaH-deficient derivatives constructed by P1 transduction of strain ED3869 by using a lysate prepared from strain PN130 (fadA::Tn10). The presence of the rfaH mutation was confirmed by examining the lipid A core banding pattern (38) as well as by sensitivity to bacteriophage C21 and concomitant resistance to bacteriophage U3. Bacteria were cultured in Luria broth (LB) supplemented with ampicillin (100 μg/ml), kanamycin (50 μg/ml), and tetracycline (20 μg/ml) as appropriate. For some experiments bacteria were cultured on MacConkey agar plates. LPS was extracted as previously described (30) and analyzed by Tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (9). Tricine SDS-PAGE gels (16%) were purchased from Novex, San Diego, Calif. DNA sequencing of plasmid constructs was carried out with an automated sequencer at MOBIX, McMaster’s University, Hamilton, Ontario.
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Relevant propertiesa | Source or reference |
|---|---|---|
| Strains | ||
| DH5α | F− φ80lacZΔM15 endA recA hsdR(rK−mK−) supE thi gyrA relA? Δ(lacZYA-argF)U169 | Laboratory stocks |
| CLM4 | lacZ trp Δ(sbcB-rfb) upp rel rpsL recA | 28 |
| CLM12 | ED3869 rfaH11 fadA::Tn10 | This work |
| CLM13 | ED3869 fadA::Tn10 | This work |
| ED3869 | Δ(codB-lac)3 tsx-80 galK λdef trp-52 lys-30 rpsL160(Strr) rfaH11 malA30 | 8 |
| JT4000 | SG20250 Δlon-510 | 41 |
| MV103 | VW187 Δlac | 46 |
| PN130 | LE392 fadA30 (fadA::Tn10) | P. Black |
| SG20250 | Δlac rpsL relA1 araD139 flbB lon+ | 41 |
| Plasmids | ||
| pEX1 | Expression vector; Apr | 33 |
| pFZY1 | Promoterless cloning vector; AproriF | 22 |
| pGEM3 | Cloning and sequencing vector; Apr | Promega |
| pKZ17 | 6.8-kb BamHI fragment containing rfaH gene cloned into pBR322; Apr | 38 |
| pTL61T | Promoterless cloning vector; Apr | 25 |
| pCM10 | 8.1-kb HindIII fragment containing wbEcO7 promoter region cloned into pACYC184; Cmr | 26 |
| pCM111 | 5.6-kb EcoRI fragment containing wbEcO7 promoter region cloned into pGEM3; Apr | This work |
| pCM117 | 1.2-kb EcoRI-HindIII fragment from pCM10 cloned into pTL61T; Apr Lac+ | 27 |
| pCM131 | 1.2-kb EcoRI-HindIII fragment from pCM117 cloned into pFZY1; Apr Lac+ | This work |
| pCM141 | 1.2-kb EcoRI-HindIII fragment containing wbEcO7 promoter region with a 71-bp internal deletion cloned into pTL61T; Apr Lac+ | This work |
| pCM143 | 412-bp amplicon (primers 25-26) from pCM111 cloned into pGEM3; Apr | This work |
| pCM144 | 1.2-kb EcoRI-HindIII fragment from pCM141 cloned into pFZY1; Apr Lac+ | This work |
| pCM145 | 1.2-kb EcoRI-HindIII fragment containing wbEcO7 promoter region with an 8-bp internal deletion cloned into pTL61T; Apr Lac+ | This work |
| pCM147 | 1.2-kb EcoRI-HindIII fragment containing wbEcO7 promoter region with a 26-bp internal deletion cloned into pTL61T; Apr Lac+ | This work |
| pCM148 | 1.2-kb EcoRI-HindIII fragment from pCM145 cloned into pFZY1; Apr Lac+ | This work |
| pCM152 | 1.2-kb EcoRI-HindIII fragment from pCM147 cloned into pFZY1; Apr Lac+ | This work |
| pCM153 | 1.2-kb EcoRI-HindIII fragment containing wbEcO7 promoter region with a 5-bp internal deletion cloned into pTL61T; Apr Lac+ | This work |
| pCM154 | 1.2-kb EcoRI-HindIII fragment from pCM153 cloned into pFZY1; Apr Lac+ | This work |
| pCM160 | 490-bp amplicon (primers 50-51) from pKZ17 containing rfaH gene cloned into pEX1; Apr | This work |
| pCM162 | Kmr cassette inserted into Apr gene of pCM160; Kmr Aps | This work |
| pCM163 | 606-bp amplicon (primers 28-42) from pCM10 cloned into SmaI site from pTL61T; Apr Lac+ | This work |
| pCM164 | 588-bp amplicon (primers 28-44) from pCM10 cloned into SmaI site from pTL61T; Apr Lac+ | This work |
| pCM166 | 572-bp amplicon (primers 28-29) from pCM10 cloned into SmaI site from pTL61T; Apr Lac+ | This work |
| pCM169 | 653-bp amplicon (primers 28-60) from pCM10 cloned into SmaI site from pTL61T; Apr Lac+ | This work |
| pCM173 | 632-bp EcoRI-HindIII fragment from pCM166 cloned into pFZY1; Apr Lac+ | This work |
| pCM174 | 648-bp EcoRI-HindIII fragment from pCM164 cloned into pFZY1; Apr Lac+ | This work |
| pCM175 | 666-bp EcoRI-HindIII fragment from pCM163 cloned into pFZY1; Apr Lac+ | This work |
| pCM176 | 713-bp EcoRI-HindIII fragment from pCM169 cloned into pFZY1; Apr Lac+ | This work |
| pCM180 | 737-bp EcoRI-HindIII fragment from pCM177 cloned into pFZY1; Apr Lac+ | This work |
| pCM182 | 410-bp amplicon (primers 74-76) from strain CLM4 containing waaQ promoter region cloned into pTL61T; Apr Lac+ | This work |
| pCM183 | 310-bp amplicon (primers 74-75) from strain CLM4 containing waaQ promoter region cloned into pTL61T; Apr Lac+ | This work |
| pCM186 | 423-bp EcoRI-HindIII fragment from pCM182 cloned into pFZY1; Apr Lac+ | This work |
| pCM187 | 323-bp EcoRI-HindIII fragment from pCM183 cloned into pFZY1; Apr Lac+ | This work |
| pCM188 | 561-bp amplicon (primers 28-89) from pCM10 cloned into SmaI site from pTL61T; Apr Lac+ | This work |
| pCM190 | 574-bp EcoRI-HindIII fragment from pCM188 cloned into pFZY1; Apr Lac+ | This work |
| pCM192 | 1.2-kb EcoRI-HindIII fragment containing wbEcO7 promoter region with a 24-bp internal deletion cloned into pTL61T; Apr Lac+ | This work |
| pCM193 | 1.2-kb EcoRI-HindIII fragment containing wbEcO7 promoter region with a 99-bp internal deletion cloned into pTL61T; Apr Lac+ | This work |
| pCM194 | 1.2-kb EcoRI-HindIII fragment from pCM192 cloned into pFZY1; Apr Lac+ | This work |
| pCM195 | 1.2-kb EcoRI-HindIII fragment from pCM193 cloned into pFZY1; Apr Lac+ | This work |
| pCM196 | 1.2-kb EcoRI-HindIII fragment containing wbEcO7 promoter region with a 130-bp internal deletion cloned into pFZY1; Apr Lac+ | This work |
| pCM197 | 0.8-kb fragment containing sequences downstream from −35 region of wbEcO7 promoter to HindIII site on rlmB cloned into pTL61T | This work |
Internal and nested deletions of the wbEcO7 and waaQ untranslated leader sequences.
Internal deletions of the wbEcO7 leader sequence were constructed by using a PCR strategy described elsewhere (3). Briefly, two independent PCRs were conducted with primers flanking the deletion endpoints. After phosphorylation with T4 polynucleotide kinase, both amplicons were ligated and the ligation mix was further amplified with the primers annealing to the distal ends of the deletion junction. The product of the second amplification was cleaved with EcoRI and HindIII, purified from a 0.7% agarose gel with the QIAquick gel extraction kit (Qiagen, Chatsworth, Calif.), and ligated to the same sites in pTL61T, followed by transformation into E. coli DH5α. Recombinant plasmids were characterized by restriction endonuclease analysis and PCR amplification, and the appropriate constructs were verified by DNA sequencing. The primers used are shown in Table 2. pCM10 and pCM111 (Table 1) were used as DNA templates for these experiments. To maintain the same levels of expression as found in the chromosome, each construct was cleaved with EcoRI and HindIII, and the fragments containing the various deletions were cloned into the single-copy-number vector pFZY1. Constructs in pFZY1 were verified by PCR with internal primers and a primer corresponding to the upstream sequence of the lacZ gene.
TABLE 2.
Primers used in construction of plasmids
| Primer | Sequence | Source |
|---|---|---|
| 25 | 5′-CTGGAAGTGTCGGTCAGTA | wbEcO7 |
| 26 | 5′-TGACGAACAACAGCAGAACC | wbEcO7 |
| 28 | 5′-AGTCAGCCGCATTGTTG | wbEcO7 |
| 29 | 5′-TTCCACGATAGTGACGAG | wbEcO7 |
| 30 | 5′-CTGGAGCAGTCTATTTCAC | wbEcO7 |
| 31 | 5′-TTCCATGCGCGAACCAA | wbEcO7 |
| 41 | 5′-CGTGCATTAATGCATC | wbEcO7 |
| 42 | 5′-CCTGGCTTAACAG | wbEcO7 |
| 43 | 5′-CTGTTAAGCCAGG | wbEcO7 |
| 44 | 5′-AGAGCACTGCGTACAT | wbEcO7 |
| 50 | 5′-ATGCAATCCTGGTAT | rfaH |
| 51 | 5′-GTAGAGTTTGCGGAA | rfaH |
| 52 | 5′-GGTAAGACAATTAGCGT | wbEcO7 |
| 57 | 5′-TGGCAAACAGAGATTGTG | galK |
| 58 | 5′-TCATGGTCATAGCTGTT | lacZ |
| 60 | 5′-GACTGCTCCAGATTTGAT | wbEcO7 |
| 74 | 5′-GCTGACTTATGG | waaQ |
| 75 | 5′-ACGCACTTACTTTGACG | waaQ |
| 76 | 5′-TTCCACTAGCGACTCTT | waaQ |
| 89 | 5′-CGTGCCAAATCCGAAA | wbEcO7 |
| 90 | 5′-AATTCAAACGCTAATTGTC | wbEcO7 |
| 95 | 5′-GGAAATGTACGCAGTGCT | wbEcO7 |
| 110 | 5′-GGCTATATGGAATAAAAAAGTGAAGATAC | wbEcO7 |
| 117 | 5′-CTACAGCTGTTTGGTAAG | wbEcO7 |
Nested deletions of the untranslated leader sequences in the wbEcO7 and waaQ promoter regions were also constructed by a PCR strategy. For deletions along the wbEcO7 promoter region, PCR amplifications were carried out with primer sets 28-29, 28-44, 28-42, and 28-60 (Table 2). The products were phosphorylated and cleaved with EcoRI followed by ligation in pTL61T digested with EcoRI and SmaI. For deletions of the waaQ leader sequence, PCR amplifications were carried out with primer sets 74-75 and 74-76 (Table 2). After phosphorylation the products were cloned into pTL61T digested with SmaI. As in the construction of the internal deletions, all the fragments containing the nested deletions were verified by DNA sequencing and cloned in pFZY1. All PCRs were carried out with a Hybaid Omnigene temperature cycler (Interscience, Markham, Ontario, Canada) with PwoI DNA polymerase (Boehringer Mannheim, Dorval, Quebec, Canada), since this enzyme does not have a terminal transferase activity and possesses a high polymerization fidelity.
β-Galactosidase assays.
The units of β-galactosidase were determined as described by Putnam and Koch (36). Each sample was analyzed in triplicate during at least three independent experiments. To compare results from different experiments, the units of activity were corrected for the background level determined in cells containing the negative control pFZY1, which were included in every experiment. The values were expressed as a percentage of the activity of the construct containing the wild-type promoter region (pCM131).
RNase protection assay.
The RNA probe was prepared in vitro with plasmid pCM143 linearized with HindIII as a template. The in vitro RNA synthesis reaction mixture consisted of 0.7 μg of DNA template, 10 mM dithiothreitol, 800 μM ribonucleoside 5′-triphosphate, 40 U of RNasin (Promega), 100 μg of bovine serum albumin, 20 μCi of [α32P]CTP, and 40 U of T7 RNA polymerase. The mixture was incubated for 30 min at 37°C followed by the addition of 2 U of RNase-free DNase. After incubation for another 30 min, the probe was purified with a Bio-Spin chromatography column (Bio-Rad, Mississauga, Ontario, Canada). The radiolabeled RNA probe was used for an RNase protection assay performed with the ribonuclease protection kit (United States Biochemical Corp., Cleveland, Ohio). Lysates containing the target RNA were prepared from exponential cultures of strain CLM4(pCM117) and also from strain CLM4(pGEM3) as a negative control. Probe (106 cpm) was added to 45 μl of cell lysate, and the hybridization was carried out overnight at 37°C. Samples were processed and analyzed on a sequencing gel as indicated by the supplier.
Analysis of mRNA secondary structure.
The secondary structures of the leader untranslated mRNA sequences were predicted with the program Mfold version 2.3 (19) and the on-line Mfold server at http://www.ibc.wustl.edu/~zuker/rna/form1.cgi. The default parameters (37°C, 1 M NaCl, no divalent ions, and 5% suboptimality) and the energy parameters given by Walter et al. (48) were used for the predictions.
Transcriptional regulation of the wbEcO7 promoter.
In all of the following experiments, aliquots were taken from cultures at various times and examined for β-galactosidase activity. The following conditions were investigated.
(i) Temperature.
Overnight cultures of DH5α cells carrying either pCM131 or pFZY1 were inoculated into fresh LB medium and incubated at 22, 37, or 42°C until the cells reached the stationary phase. In some experiments, cells were first incubated at 37°C for 2 h, at which time the cultures were rapidly shifted to either 22 or 42°C.
(ii) Osmolarity.
Osmolarity experiments were carried out with MV103(pCM131) and MV103(pFZY1) cells grown in M9 medium (35). An overnight culture was diluted into fresh medium containing 50, 100, 200, 400, or 800 mM NaCl and incubated for 2 h, at which time the levels of β-galactosidase activity were measured.
(iii) Regulation by Mg2+ concentration.
MV103(pCM131) and MV103(pFZY1) bacterial cells grown in N medium (42) with 40 μM MgCl2 were washed three times with N salts, inoculated into fresh N medium containing no magnesium or 0.05, 0.25, 1.25, or 6.25 mM MgCl2, and incubated for 2 h.
(iv) DNA supercoiling.
SG20250(pCM131) and SG20250(pFZY1) cells were used for DNA supercoiling experiments. The cells were inoculated into LB with 0, 12.5, 25, 50, 75, and 100 μg of novobiocin/ml (13) and incubated for 2 h at 37°C before their β-galactosidase levels were determined.
(v) Effect of the lon mutation.
In the absence of the Lon protease, there is a higher availability of a positive regulator, RcsA, which increases the production of colanic acid capsule (15). To test whether the wbEcO7 promoter is regulated by this mechanism, we examined β-galactosidase production over the growth curve in SG20250(pCM131), SG20250(pFZY1), JT4000(pCM131), and JT4000 (pFZY1) cells.
RESULTS
Characterization of the wbEcO7 promoter region.
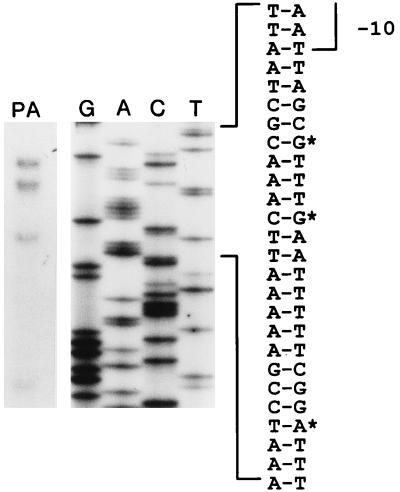
The intergenic region between galF and the first gene of the O7-specific LPS gene cluster, rlmB, in E. coli O7:K1 consists of a 367-bp segment (Fig. 1) and contains a promoter (27). To localize the promoter element precisely, we determined the site of transcription initiation by an RNase protection assay. This involved the in vitro synthesis of a radiolabeled antisense RNA probe that was derived from pCM143 (Fig. 2). The probe was used for hybridization with total RNA isolated from strains CLM4(pCM117) and CLM4(pGEM3) as a negative control. Strain CLM4, containing a deletion of the E. coli K-12 wb cluster, was utilized to rule out detection of protected RNA fragments from transcripts initiated at the chromosomal wbEcK12 promoter region, which is almost identical to that of wbEcO7 (51). Three protected fragments of 225, 220, and 210 bp were identified (Fig. 3), which corresponded to transcripts initiated at two G residues (positions +1 and +5) and an A residue (position +16), respectively (Fig. 1 and 3). A fourth, 180-bp fragment was also observed (Fig. 3), corresponding to a transcript initiated at an A residue at position +45. This pattern of RNase protection was reproducible, since the same four protected fragments were detected in two independent experiments.
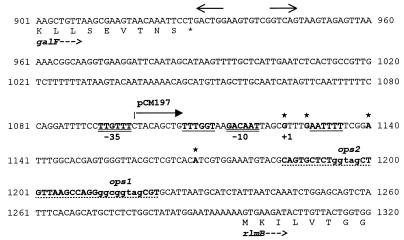
FIG. 1.
DNA sequence of the wbEcO7 promoter region. The sequence has been published previously (GenBank accession no. U23775 [26]). The end of galF and the beginning of rlmB are indicated. The diverging arrows indicate the locations of inverted repeats at the end of galF that presumably are involved in transcription termination. The −35 and −10 sequences of the wbEcO7 promoter are in boldface and double underlined. The underlined sequences overlapping the −35 and −10 regions correspond to the −35 and −10 sequences of a putative second promoter. The stars above boldface G and A letters denote alternative sites of initiation of transcription according to the RNase protection data shown in Fig. 3, although all but the G at +1 probably correspond to sites of RNA processing (see the text). The endpoint of the DNA insert in pCM197 that lacks the −35 sequences is indicated by the labeled arrow. The nucleotides of the JUMPStart sequence are in boldface and underlined with dots. The ops1 and ops2 subsequences are indicated in lowercase. The asterisks indicate the 5′ ends of the RNase-protected fragments.
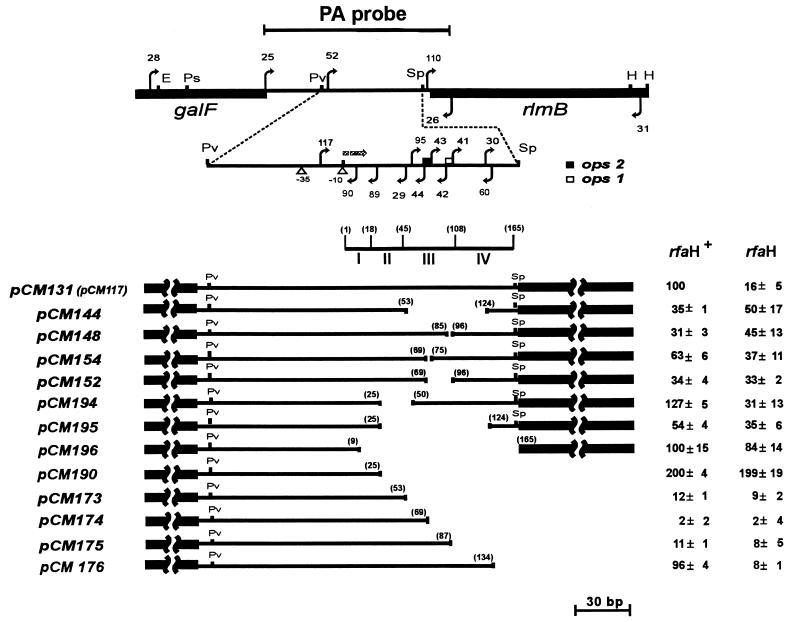
FIG. 2.
Internal and nested deletions of the wbEcO7 promoter region. A partial restriction map of the 1.2-kb EcoRI-HindIII fragment containing part of galF and part of rlmB is shown. The arrows indicate the locations and directions (upper arrows, sense; lower arrows, antisense) of the primers used for construction of the deletions. The primers are numbered, and their DNA sequences are indicated in Table 2. “PA probe” indicates the boundaries of the insert in pCM143 that was used for the RNase protection experiments. The −35 and −10 regions of P1, the initiation of transcription (shaded arrow), and the locations of the ops1 and ops2 sequences are indicated. E, EcoRI; Ps, PstI; Pv, PvuII; Sp, SphI; H, HindIII. The region indicated with roman numerals corresponds to the predicted stem-loop structures I, II, III, and IV (Fig. 5) in the transcribed but untranslated leader sequence, and the numbers in parentheses indicate the boundaries of each structure, starting with 1 to indicate the first transcribed nucleotide. The boundaries of the deletions in the various constructs are also indicated. The β-galactosidase activities mediated by each deletion construct were determined in rfaH+ and rfaH backgrounds and are expressed as percentages of the values obtained for the wild-type construct pCM131 in the rfaH+ strain. The means and standard deviations were calculated based on data from four determinations.
FIG. 3.
RNase protection assay. The left side of the figure shows an autoradiograph of a 4% polyacrylamide DNA-sequencing gel. Lane PA contains the RNase-protected fragments. The bases corresponding to each protected fragment are indicated with asterisks.
Upstream of the G at the +1 position, −10 and −35 regions, also predicted by the computer algorithm of O’Neill (32), were found, suggesting that transcription is initiated at this nucleotide. A putative second promoter was identified upstream of the A at +16 (Fig. 1), which could explain initiation of transcription at this site. The putative −35 sequence of the second promoter was located just upstream of the −10 sequence shown in Fig. 1. The existence of this second promoter was investigated by the construction of plasmid pCM197 (Table 1), which harbors a fusion to a promoterless lac operon and lacks the −35 sequence indicated in Fig. 1 but contains the intact putative second promoter region. The level of β-galactosidase activity mediated by this plasmid was identical to that of the vector control (data not shown), ruling out the existence of a second promoter overlapping the one indicated in Fig. 1. Therefore, we conclude that the three protected fragments of 220, 210, and 180 bp shown in Fig. 3 may have been created by enzymatic processing near the 5′ end of the mRNA. This interpretation is plausible, considering that the 5′ segment of this mRNA remains untranslated and has a good probability of folding into several stem-loops (see below). The sizes of these three smaller protected fragments were consistent with those expected for possible endonucleolytic cleavage sites in AT-rich regions between predicted stem-loops.
To investigate the level of expression of the promoter in its normal gene dosage, the 1.2-kb EcoRI-HindIII fragment from pCM117 was subcloned into the single-copy-number vector pFZY1, resulting in pCM131 (Table 1 and Fig. 2). DH5α cells containing this plasmid expressed around 250 U of β-galactosidase, indicating that the wbEcO7 promoter has a relatively low activity in vivo at its normal dosage.
The wbEcO7 promoter is regulated by the product of the rfaH gene.
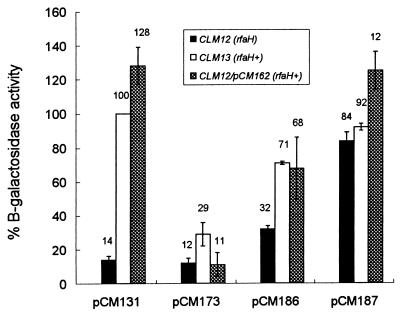
The localization of the transcription initiation sites in the wbEcO7 promoter also served to confirm that there is an untranslated mRNA segment of 173 bp (Fig. 1). The untranslated segment includes a 39-bp sequence that has been found to be conserved in many other polysaccharide clusters and has been designated JUMPStart (17). Recently, an 8-bp subsequence within JUMPStart has been shown to be involved in increasing the transcription of distal genes of the E. coli hemolysin gene cluster (4, 31), and it has been designated ops. This sequence is present at one end of the JUMPStart sequence in wbEcO7 (ops1 [Fig. 1]), whereas a partial ops containing only 5 bp is found near the other end (ops2 [Fig. 1]). ops sequences are widely recognized in the untranslated regions of RfaH-regulated gene clusters (4, 23, 43). Work by several laboratories suggested that RfaH is involved in controlling the elongation of bacterial transcripts, probably acting as a transcriptional antiterminator (4, 5, 11, 34). Also, it has been shown that the RfaH-mediated effect requires an intact ops (4, 5, 23). To investigate whether rfaH plays any role in the regulation of the wbEcO7 promoter, we constructed the isogenic strains CLM12 and CLM13, the former containing a mutated rfaH gene (rfaH11 allele). We used these strains to compare the levels of expression of β-galactosidase directed by plasmid pCM131. Figure 4 shows that in the presence of the rfaH mutation the expression of the wbEcO7 promoter (contained in pCM131) is reduced about sevenfold with respect to wild-type levels, reaching values similar to those found in strain CLM12(pFZY1). The expression of β-galactosidase was restored in cells containing pCM162, which carries a functional E. coli K-12 rfaH gene cloned in the vector pEX1. In this case, the expression was higher than that mediated by pCM131 alone, probably due to the higher gene dosage of RfaH, as has been shown previously with the hemolysin system (4). From these experiments we concluded that the expression of wbEcO7 is regulated by the RfaH protein.
FIG. 4.
Regulation of the wbEcO7 and waaQ promoters by RfaH. β-Galactosidase assays were conducted on the indicated E. coli strains carrying various plasmids. The results are expressed as percentages of the activity of the wild-type promoter fusion in CLM13(pCM131) and represent the means of three determinations.
Interestingly, a deletion that eliminated the JUMPStart region, including both ops sequences (pCM173 [Fig. 2]) displayed a low level of transcriptional activity irrespective of the presence of RfaH (Fig. 4). This result was unexpected, since the wbEcO7 promoter region as well as the site of transcription initiation remained intact in this plasmid. As a control, we constructed analogous transcriptional fusions with the waaQ promoter region of the lipopolysaccharide core gene cluster (10), which is also regulated by RfaH (34) and contains a JUMPStart sequence with ops elements (17). The data in Fig. 4 show that a deletion of most of the waaQ untranslated region, leaving only the first 15 bp (pCM187, Δ+15 [Fig. 5]), resulted in a level of β-galactosidase expression higher than that determined by pCM186 (Δ+110), containing an intact JUMPStart (Fig. 5). The deletion endpoint in pCM187 is roughly similar to that of pCM173 in the wbEcO7 promoter region (Fig. 2 and 5). Also, β-galactosidase expression by the fusion containing the entire waaQ promoter region and JUMPStart sequences (pCM186) was regulated in a RfaH-dependent fashion (Fig. 4), although the expression dropped only 1.5-fold in the rfaH mutant strain. In contrast, the expression of β-galactosidase by pCM187 was RfaH independent (Fig. 4). These results suggest that the JUMPStart sequence is important for the RfaH-mediated effect. Also, since both the wbEcO7 and waaQ promoters are cloned in the same cloning vector and determine similar levels of β-galactosidase activity, these results cannot be explained by differences in promoter strength. Therefore, other differences between these two apparently similar RfaH-regulated promoter regions may exist.
FIG. 5.
Predicted RNA secondary structure of the untranslated leader sequences of the wbEcO7 and waaQ promoter regions calculated with the program Mfold. The asterisk in each structure indicates the first transcribed nucleotide. Double lines above the UGGA sequence in wbEcO7 indicate a probable Shine-Dalgarno sequence. The predicted stem-loops are indicated with roman numerals. The endpoints of nested deletions as well as the number of nucleotides remaining after each deletion are also indicated. The plasmids containing the deletions are indicated in parentheses and are shown in Fig. 2. The boxed sequences in both regions denote the JUMPStart sequences. The individually boxed nucleotides indicate the ops1 and ops2 subsequences.
Prediction of the secondary structure of the wbEcO7 untranslated leader mRNA.
Although a relationship has been established between the JUMPStart sequence and regulation of transcription by RfaH (4, 23), the untranslated mRNA regions in RfaH-regulated genes usually contain other sequences in addition to JUMPStart. These sequences could potentially involve other cis-regulatory elements specific for each system that could explain the differences we observed between the expression of wbEcO7 and waaQ transcriptional fusions. We examined the structural properties of the wbEcO7 173-bp upstream leader sequence with the program Mfold in an attempt to develop a model for the analysis of this sequence. Mfold predicted four stem-loop structures, with an overall minimal free energy of −46.6 ± 2.3 kcal (Fig. 5). Of these, stem-loop I is probably the least stable structure, since it contains only one G-C pair. In contrast, structures II, III, and IV are likely to be more stable and probably occur in vivo, since they contain many paired G-C bases. These structures consistently appeared under different conditions predicted to be suboptimal. The JUMPStart sequence spanned both the stem and the loops of the predicted structure III (Fig. 5). The ops1 subsequence was located partly in the stem and contained three paired G-C bases, while the five bases of ops2 formed part of the predicted loop (Fig. 5).
For comparison we also modelled the secondary structure of the untranslated mRNA of the waaQ promoter region. In this case, the JUMPStart sequence begins at position +18 (Fig. 5) instead of position +61, as in the case of the wbEcO7 promoter region (Fig. 1 and 5). The secondary structure analysis of the untranslated mRNA of waaQ predicts a structure similar to stem-loop III of the wbEcO7 promoter (Fig. 5, stem-loop I), while there is no structure similar to stem-loop II.
Deletion analysis of the upstream untranslated leader sequences in wbEcO7 and waaQ.
To investigate the possible role of the predicted stem-loops in transcription by the wbEcO7 promoter, we constructed a series of nested deletion derivatives spanning the region from bases +25 to +134 that were transcriptionally fused to a promoterless lac operon (Fig. 2 and 5). The deletions were constructed as described in Materials and Methods and were subcloned into the single-copy-number vector pFZY1. The levels of expression of β-galactosidase mediated by the resulting recombinant plasmids in rfaH and rfaH+ backgrounds, as well as the effects of the deletions on the predicted stem-loops shown in Fig. 5, are indicated in Fig. 2. Only pCM176 expressed a β-galactosidase level comparable with that of the wild-type promoter in pCM131, and it was the only nested deletion whose expression was regulated by RfaH (Fig. 2). This deletion contained 134 of the 173 bases of the untranslated segment and completely spanned the predicted stem-loops I, II, and III (Fig. 2 and 5). Therefore, we concluded that stem-loop IV does not play a significant role in transcriptional regulation of the expression of the wbEcO7 promoter under these experimental conditions. However, stem-loop IV could be important in modulating the translation of rmlB, since the Shine-Dalgarno sequence is folded within the stem (Fig. 5).
The other deletions, resulting in the elimination of ops1 (Δ+87; pCM175), ops2 (Δ+69; pCM174), and both ops1 and ops2 as well as the complete stem-loop III (Δ+53; pCM173) (Fig. 2 and 5), caused dramatic reductions in the expression of β-galactosidase as well as loss of RfaH-mediated regulation (Fig. 2). These observations could be explained by an effect of the JUMPStart element on transcription initiation or by premature transcription termination at predicted stem-loops I and II (Fig. 5). pCM190 (Fig. 2), containing a deletion-eliminating structure II (Δ+25 [Fig. 5]) determined a level of β-galactosidase expression twofold higher than the expression mediated by the wild-type promoter regions in pCM131 and deletion plasmid pCM176. Since pCM190 contains the complete predicted stem-loop I, we concluded that this structure does not play a role in transcription termination and, since it has only one G-C pair, may not even exist in vivo. In contrast, the increased level of β-galactosidase expression when predicted stem-loop II is disrupted suggests this region alone is important for determining a reduction in β-galactosidase expression, which is consistent with premature termination of the initiated transcript. In conclusion, the experiments with the nested deletions show that predicted stem-loop II may serve as a site for premature termination of transcription and that a complete stem-loop III, including the complete JUMPStart sequence, is required for wild-type levels of transcriptional activity. This interpretation is also consistent with the fact that a stem-loop II-like structure is absent in the waaQ leader sequence (Fig. 5) and may explain the higher expression of β-galactosidase detected with pCM186 and pCM187 even in the absence of RfaH (Fig. 4).
Relative contributions of stem-loop structures and ops sequences to wbEcO7 promoter expression.
Although the nested deletion experiments permitted us to detect a functional role for the predicted stem-loop II in the wbEcO7 promoter, they did not address the relative contribution of each predicted stem-loop to the promoter activity. For this purpose, we constructed a series of internal deletions (Fig. 2). The cloning strategy was similar to that described above except that the deletions contained the remainder of the untranslated leader sequence and 512 bp of the coding region of the rlmB gene. In this manner, the results could be better compared with those for the plasmid pCM131. The predicted stem-loop structures affected by each deletion are also indicated in Fig. 2. The internal deletion of the predicted stem-loop II (pCM194) resulted in an expression level 30% higher than that mediated by pCM131 containing the wild-type wbEcO7 promoter. In contrast, the internal deletion of stem-loop III (pCM144) caused a 65% reduction in β-galactosidase activity (Fig. 2).
Other internal deletions eliminating specific sequences within the predicted stem-loop III were constructed. Figure 2 shows that deletion of ops1 alone (pCM148) caused a 70% reduction in the expression of β-galactosidase with respect to wild-type levels while the deletion of ops2 (pCM154) caused a 40% reduction. As expected, deletion of both ops1 and ops2 and the intervening sequences (pCM152) also resulted in a marked decrease in β-galactosidase production, and they all lost RfaH-mediated regulation (Fig. 2). From these experiments, we concluded that the ops1 subsequence, and to a lesser degree ops2, is a critical cis-regulatory element involved in the transcriptional activity of the wbEcO7 promoter region. Furthermore, the effects of these deletions on the expression of β-galactosidase were similar to the effect of the rfaH mutation on the transcriptional activity of the wild-type wbEcO7 promoter (Fig. 4), suggesting that at least an intact ops1 subsequence is required for the RfaH-mediated function.
Interestingly, a deletion removing both predicted structures II and III (pCM195) gave a level of transcriptional activity higher than that of pCM144, again suggesting that structure II may play a role in premature termination. Also, sequences downstream from structure III may be important for the stability of the message, since removing the majority of the untranslated segment (pCM196) resulted in a level of expression similar to that of the wild-type promoter region in pCM131, albeit the expression was not regulated by RfaH (Fig. 2).
Expression of the wbEcO7 promoter region under different conditions.
The cloning and characterization of the promoter region as a transcriptional fusion in a single-copy-number vector allowed us to examine the possible roles of different conditions of growth in the transcriptional activity of this promoter. We tested the effects of growth temperature, Mg2+ concentration, and osmolarity because these conditions have been shown to be important for regulation of LPS in other systems (1, 16, 39). We also investigated whether the wbEcO7 promoter expression changes under conditions affecting the DNA topology, especially DNA supercoiling (39), as well as in the presence of the lon mutation that is known to increase the expression of colanic acid capsule (15). No differences were observed in the expression of β-galactosidase under any of the conditions tested (data not shown). Therefore, we conclude that wbEcO7 promoter activity is expressed constitutively.
DISCUSSION
In the present work we have characterized the promoter region of the wbEcO7 gene cluster and confirmed the existence of a relatively large untranslated leader sequence. Secondary structure prediction analysis combined with transcriptional fusions served to define several regions, possibly folding into complex stem-loop structures, some of which may be functional in vivo. The leader sequence contains a predicted stem-loop that spans the JUMPStart sequence and carries two ops elements. We demonstrated that the ops elements, especially ops1, are sufficient for an RfaH-mediated regulation of wbEcO7 promoter activity. This is in agreement with previous studies by other laboratories of other RfaH-regulated gene clusters (6, 23, 24, 31, 43). However, unlike previous results of others (6, 31), we demonstrate that the partial ops sequence (ops2) also plays a role in the RfaH-mediated function. Although it is clear that RfaH and ops sequences cooperate to control elongation of the initiated transcript, the actual mechanism of this interaction remains to be elucidated. Recently, Bailey et al. (5) discussed several models to explain how RfaH and the ops element interact. These authors suggest that the ops element may recruit RfaH and possibly other factors to the transcription complex, resulting in a modification of the RNA polymerase into a termination-resistant form. It has been shown by others (11) that rfaH mutants can be suppressed by a mutation in the Rho termination factor. Therefore, it is possible that in addition to RfaH, Rho and another factor(s) may be required for the continuation of the elongation of the transcript. It is not clear from the published literature whether RfaH interacts directly with DNA. We were unable to determine direct binding of purified RfaH in vitro to a synthetic RNA oligonucleotide containing the JUMPStart segment (29), suggesting that either more factors are needed or the binding takes place in the context of the entire transcription complex and perhaps involves single-stranded DNA.
We also showed in this work that RfaH acts on the leader segment of the waaQ operon of the core oligosaccharide synthesis cluster. More importantly, deletion of this region is associated with an expression of promoter activity higher than wild-type levels, as determined by production of β-galactosidase. This is in agreement with recent observations by Leeds and Welch (23), suggesting that the JUMPStart region is a site for the RNA polymerase to pause. In contrast to the observations with the waaQ leader segment, deletions of JUMPStart and ops1 in the corresponding leader sequence of wbEcO7 were associated with dramatic decreases in transcriptional activity. These findings could not be explained by differences in the strengths of the two promoters. Experiments using nested deletion-fusion suggested the existence in the wbEcO7 leader region of a short segment with a predicted stem-loop structure located upstream of the JUMPStart sequence (predicted stem-loop II [Fig. 5]). There was no similar predicted stem-loop in the corresponding region in waaQ. Deletion of this segment in the wbEcO7 leader sequence resulted in an increased level of transcription. Internal deletion-fusion experiments revealed that untranslated sequences downstream from the JUMPStart region (corresponding to predicted stem-loop IV [Fig. 5]) are also important to maintain a level of transcriptional activity similar to that of the wild-type wbEcO7 leader. Interestingly, the effect of the regions flanking the ops sequences on the expression of the transcriptional fusions is apparent only when the ops sequences are deleted or in the rfaH mutant.
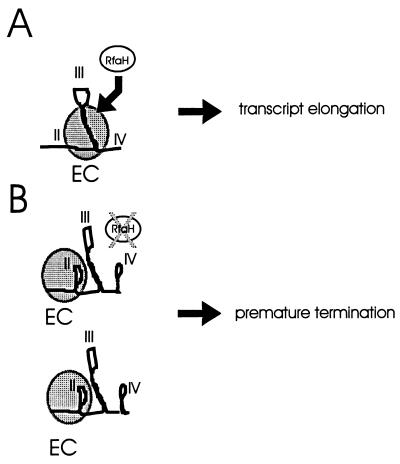
We propose a working model (Fig. 6) where the predicted stem-loop II and IV regions may serve as sites for transcription termination, but only in the absence of RfaH or in the presence of a mutation(s) compromising the ops sequences contained in stem-loop III. In the presence of RfaH, the ops sequences presumably would serve to bring RfaH itself or another transcription factor(s) together with the RNA polymerase elongation complex, and the binding of all these factors may prevent the formation of stable stem-loops II and IV. Since the putative stem-loop IV includes the predicted Shine-Dalgarno sequence of rlmB, it is possible that in the presence of the elongation complex this sequence is exposed to the ribosome and the stem-loop does not form. Further studies using site-directed mutagenesis are required to confirm the existence of the proposed structures and their roles as sites for premature transcription termination.
FIG. 6.
Working model explaining the possible roles of structures II and IV. (A) Diagram showing that binding of the elongation complex (EC) to the ops sequences present in stem-loop III in the presence of RfaH avoids the formation of structures II and IV, allowing for the progression of the transcript. (B) In the absence of RfaH or in the presence of mutations in the ops sequences, structures II and IV promote premature termination of the initiated transcript.
In support of our hypothesis, structures similar to stem-loop II, also located upstream of the JUMPStart sequence and ops elements, can be modelled in the case of the Y. enterocolitica wb gene cluster (29). To our knowledge, this is the only other O polysaccharide gene cluster in which the boundaries of the leader sequence have been determined experimentally (52). Also, the structures are present in the untranslated regions of O polysaccharide gene clusters of S. flexneri, E. coli K-12, and S. enterica, which are very similar to the wbEcO7 untranslated region (29). We propose that these structures play a general role in maintaining a tight regulation of the transcription of O polysaccharide genes in the absence of RfaH. The lack of equivalent structures not only in the E. coli K-12 waaQ but also in all the other E. coli core types and the core region of S. enterica (28) would explain why the expression of core lipid A is not completely blocked in rfaH mutants. Our hypothesis is consistent with the general view that core lipid A is more important for the bacterial cell than the O-specific polysaccharide chain. In fact, expression of O polysaccharide in the absence of core would be energetically unfavorable and also may lead to a rapid consumption of the bactoprenyl phosphate that is essential for the synthesis of peptidoglycan (14).
Interestingly, the leader sequences in capsule polysaccharide clusters, such as colanic acid capsule and region II of K5 capsular polysaccharide genes (43, 44), are also longer and more complex than the waaQ leader region. This may be related to the fact that these genes may be subjected to additional regulatory controls, such as RcsA and RcsB in the case of the colanic acid capsule and other yet-undiscovered factors in the case of the K5 capsule.
Attempts to identify regulatory factors other than RfaH acting on the wbEcO7 promoter were unsuccessful. The wbEcO7 promoter was not upregulated in a lon mutant, suggesting that it is not under the control of the RcsA and RcsB regulators. Likewise, the wbEcO7 promoter was not regulated by DNA supercoiling, temperature shifts, osmolarity, and the Mg2+ concentration, suggesting that this promoter is constitutive in terms of transcription initiation and is regulated only at the level of mRNA elongation by RfaH, at least under our experimental conditions. These findings do not preclude the existence of regulation at the level of translation of gene products or enzyme feedback interactions. Further experiments are required to examine these possibilities as well as other regions within the wbEcO7 cluster that may play a role in the regulation of gene expression.
ACKNOWLEDGMENTS
We are grateful to the colleagues mentioned in the text or referenced in Table 1 for kindly supplying strains and plasmids. Special thanks are due to the members of the lab for useful discussions and to C. Whitfield and D. E. Heinrichs for supplying us with the sequences of the E. coli core clusters for core chemotypes 1, 2, 3, and 4 prior to publication.
This investigation was supported by grant MT10206 from the Medical Research Council of Canada.
REFERENCES
- 1.Aguilar A, Merino S, Rubires X, Tomas J M. Influence of osmolarity on lipopolysaccharides and virulence of Aeromonas hydrophila serotype O:34 strains grown at 37°C. Infect Immun. 1997;65:1245–1250. doi: 10.1128/iai.65.4.1245-1250.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander D C, Valvano M A. Role of the rfe gene in the biosynthesis of the Escherichia coli O7-specific lipopolysaccharide and other O-specific polysaccharides containing N-acetylglucosamine. J Bacteriol. 1994;176:7079–7084. doi: 10.1128/jb.176.22.7079-7084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali S A, Steinkasserer A. PCR-ligation-PCR mutagenesis: a protocol for creating gene fusions and mutations. BioTechniques. 1995;18:746–750. [PubMed] [Google Scholar]
- 4.Bailey M J A, Hughes C, Koronakis V. Increased distal gene transcription by the elongation factor RfaH, a specialized homologue of NusG. Mol Microbiol. 1996;22:729–737. doi: 10.1046/j.1365-2958.1996.d01-1726.x. [DOI] [PubMed] [Google Scholar]
- 5.Bailey M J A, Hughes C, Koronakis V. RfaH and the ops element, components of a novel system controlling bacterial transcription elongation. Mol Microbiol. 1997;26:845–851. doi: 10.1046/j.1365-2958.1997.6432014.x. [DOI] [PubMed] [Google Scholar]
- 6.Bailey M J A, Koronakis V, Schmoll T, Hughes C. Escherichia coli HlyT protein, a transcriptional activator of haemolysin synthesis and secretion, is encoded by the rfaH (sfrB) locus required for expression of sex factor and lipopolysaccharide genes. Mol Microbiol. 1992;6:1003–1012. doi: 10.1111/j.1365-2958.1992.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 7.Batchelor R A, Haraguchi G E, Hull R A, Hull S I. Regulation by a novel protein of the bimodal distribution of lipopolysaccharide in the outer membrane of Escherichia coli. J Bacteriol. 1991;173:5699–5704. doi: 10.1128/jb.173.18.5699-5704.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beutin L, Achtman M. Two Escherichia coli chromosomal cistrons, sfrA and sfrB, which are needed for expression of F factor tra functions. J Bacteriol. 1979;139:730–737. doi: 10.1128/jb.139.3.730-737.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooke J S, Valvano M A. Biosynthesis of inner core lipopolysaccharide in enteric bacteria: identification and characterization of a conserved phosphoheptose isomerase. J Biol Chem. 1996;271:3608–3614. doi: 10.1074/jbc.271.7.3608. [DOI] [PubMed] [Google Scholar]
- 10.Clementz T. The gene coding for 3-deoxy-manno-octulosonic acid transferase and the rfaQ gene are transcribed from divergently arranged promoters in Escherichia coli. J Bacteriol. 1992;174:7750–7756. doi: 10.1128/jb.174.23.7750-7756.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farewell A, Brazas R, Davie E, Mason J, Rothfield L I. Suppression of the abnormal phenotype of Salmonella typhimurium rfaH mutants by mutations in the gene for transcription termination factor Rho. J Bacteriol. 1991;173:5188–5193. doi: 10.1128/jb.173.16.5188-5193.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franco A V, Liu D, Reeves P R. A Wzz (Cld) protein determines the chain length of K lipopolysaccharide in Escherichia coli O8 and O9 strains. J Bacteriol. 1996;178:1903–1907. doi: 10.1128/jb.178.7.1903-1907.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Free A, Dorman C J. Escherichia coli tyrT gene transcription is sensitive to DNA supercoiling in its native chromosomal context: effect of DNA topoisomerase IV overexpression on tyrT promoter function. Mol Microbiol. 1994;14:151–161. doi: 10.1111/j.1365-2958.1994.tb01275.x. [DOI] [PubMed] [Google Scholar]
- 14.Gaspar, J. A., J. A. Thomas, and M. A. Valvano. The TolA protein has a role in the translocation of O antigen subunits across the cytoplasmic membrane. Submitted for publication.
- 15.Gottesman S, Stout V. Regulation of capsule polysaccharide synthesis in Escherichia coli K-12. Mol Microbiol. 1991;5:1599–1606. doi: 10.1111/j.1365-2958.1991.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 16.Guo L, Lim K B, Gunn J S, Bainbridge B, Darveau R P, Hackett M, Miller S I. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276:250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 17.Hobbs M, Reeves P R. The JUMPstart sequence: a 39 bp element common to several polysaccharide gene clusters. Mol Microbiol. 1994;12:855–856. doi: 10.1111/j.1365-2958.1994.tb01071.x. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 18.Ishiguro E E, Vanderwel D, Kusser W. Control of lipopolysaccharide biosynthesis and release by Escherichia coli and Salmonella typhimurium. J Bacteriol. 1986;168:328–333. doi: 10.1128/jb.168.1.328-333.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaeger J A, Turner D H, Zuker M. Predicting optimal and suboptimal secondary structure for RNA. Methods Enzymol. 1990;183:281–306. doi: 10.1016/0076-6879(90)83019-6. [DOI] [PubMed] [Google Scholar]
- 20.Joiner K A. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- 21.Karow M, Raina S, Georgopoulos C, Fayet O. Complex phenotypes of null mutations in the htr genes, whose products are essential for Escherichia coli growth at elevated temperatures. Res Microbiol. 1991;142:289–294. doi: 10.1016/0923-2508(91)90043-a. [DOI] [PubMed] [Google Scholar]
- 22.Koop A H, Hartley M E, Bourgeois S. A low-copy-number vector utilizing β-galactosidase for the analysis of gene control elements. Gene. 1987;52:245–256. doi: 10.1016/0378-1119(87)90051-5. [DOI] [PubMed] [Google Scholar]
- 23.Leeds J A, Welch R A. Enhancing transcription through the Escherichia coli hemolysin operon, hlyCABD: RfaH and upstream JUMPStart DNA sequences function together via a postinitiation mechanism. J Bacteriol. 1997;179:3519–3527. doi: 10.1128/jb.179.11.3519-3527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leeds J A, Welch R A. RfaH enhances elongation of Escherichia coli hlyCABD mRNA. J Bacteriol. 1996;178:1850–1857. doi: 10.1128/jb.178.7.1850-1857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linn T, St. Pierre R. Improved vector system for constructing fusions that ensures independent translation of lacZ. J Bacteriol. 1990;172:1077–1084. doi: 10.1128/jb.172.2.1077-1084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marolda C L, Valvano M A. The GalF protein of Escherichia coli is not an UDP-glucose pyrophosphorylase but interacts with the GalU protein to possibly regulate cellular levels of UDP-glucose. Mol Microbiol. 1996;22:827–840. doi: 10.1046/j.1365-2958.1996.01531.x. [DOI] [PubMed] [Google Scholar]
- 27.Marolda C L, Valvano M A. Genetic analysis of the dTDP-rhamnose biosynthesis region of the Escherichia coli VW187 (O7:K1) rfb gene cluster: identification of functional homologs of rfbB and rfbA in the rff cluster and correct location of the rffE gene. J Bacteriol. 1995;177:5539–5546. doi: 10.1128/jb.177.19.5539-5546.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marolda C L, Valvano M A. Identification, expression, and DNA sequence of the GDP-mannose biosynthesis genes encoded by the O7 rfb gene cluster of strain VW187 (Escherichia coli O7:K1) J Bacteriol. 1993;175:148–158. doi: 10.1128/jb.175.1.148-158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marolda, C. L., and M. A. Valvano. Unpublished observations.
- 30.Marolda C L, Welsh J, Dafoe L, Valvano M A. Genetic analysis of the O7-polysaccharide biosynthesis region from the Escherichia coli O7:K1 strain VW187. J Bacteriol. 1990;172:3590–3599. doi: 10.1128/jb.172.7.3590-3599.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nieto J M, Bailey M J A, Hughes C, Koronakis V. Suppression of transcription polarity in the Escherichia coli haemolysin operon by a short upstream element shared by polysaccharide and DNA transfer determinants. Mol Microbiol. 1996;19:705–713. doi: 10.1046/j.1365-2958.1996.446951.x. [DOI] [PubMed] [Google Scholar]
- 32.O’Neill M C. Consensus methods for finding and ranking DNA binding sites—application to Escherichia coli promoters. J Mol Biol. 1989;207:301–310. doi: 10.1016/0022-2836(89)90256-8. [DOI] [PubMed] [Google Scholar]
- 33.Passador L, Linn T. Autogenous regulation of the RNA polymerase β subunit of Escherichia coli occurs at the translational level in vivo. J Bacteriol. 1989;171:6234–6242. doi: 10.1128/jb.171.11.6234-6242.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pradel E, Schnaitman C A. Effect of rfaH (sfrB) and temperature on expression of rfa genes of Escherichia coli K-12. J Bacteriol. 1991;173:6428–6431. doi: 10.1128/jb.173.20.6428-6431.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Provence D L, Curtiss R., III . Gene transfer in gram-negative bacteria. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C: American Society for Microbiology; 1994. pp. 317–347. [Google Scholar]
- 36.Putnam S L, Koch A L. Complication in the simplest cellular enzyme assay: lysis of Escherichia coli for the assay of β-galactosidase. Anal Biochem. 1975;63:350–360. doi: 10.1016/0003-2697(75)90357-7. [DOI] [PubMed] [Google Scholar]
- 37.Reeves P R, Hobbs M, Valvano M A, Skurnik M, Whitfield C, Coplin D, Kido N, Klena J, Maskell D, Raetz C R H, Rick P D. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 1996;4:495–503. doi: 10.1016/s0966-842x(97)82912-5. [DOI] [PubMed] [Google Scholar]
- 38.Rehemtulla A, Kadam S K, Sanderson K E. Cloning and analysis of the sfrB (sex factor repression) gene of Escherichia coli K-12. J Bacteriol. 1986;166:651–657. doi: 10.1128/jb.166.2.651-657.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohde J R, Fox J M, Minnich S A. Thermoregulation in Yersinia enterocolitica is coincident with changes in DNA supercoiling. Mol Microbiol. 1994;12:187–199. doi: 10.1111/j.1365-2958.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 40.Schnaitman C A, Klena J D. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol Rev. 1993;57:655–682. doi: 10.1128/mr.57.3.655-682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon L D, Gottesman M, Tomczak K, Gottesman S. Hyperdegradation of proteins in Escherichia coli rho mutants. Proc Natl Acad Sci USA. 1979;76:1623–1627. doi: 10.1073/pnas.76.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snavely M D, Gravina S A, Cheung T-B T, Miller C G, Maguire M E. Magnesium transport in Salmonella typhimurium: regulation of mgtA and mgtB expression. J Biol Chem. 1991;266:824–829. [PubMed] [Google Scholar]
- 43.Stevens M P, Clarke B R, Roberts I S. Regulation of the Escherichia coli K5 capsule gene cluster by transcription antitermination. Mol Microbiol. 1997;24:1001–1012. doi: 10.1046/j.1365-2958.1997.4241780.x. [DOI] [PubMed] [Google Scholar]
- 44.Stout V. Identification of the promoter region for the colanic acid polysaccharide biosynthetic genes in Escherichia coli K-12. J Bacteriol. 1996;178:4273–4280. doi: 10.1128/jb.178.14.4273-4280.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valvano M A, Crosa J H. Molecular cloning and expression in Escherichia coli K-12 of chromosomal genes determining the O7 lipopolysaccharide antigen of a human invasive strain of E. coli O7:K1. Infect Immun. 1989;57:937–943. doi: 10.1128/iai.57.3.937-943.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valvano M A, Crosa J H. Molecular cloning, expression, and regulation in Escherichia coli K-12 of a chromosome-mediated aerobactin iron transport system from a human invasive isolate of E. coli K1. J Bacteriol. 1988;170:5529–5538. doi: 10.1128/jb.170.12.5529-5538.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van den Bosch L, Manning P A, Morona R. Regulation of O-antigen chain length is required for Shigella flexneri virulence. Mol Microbiol. 1997;23:765–775. doi: 10.1046/j.1365-2958.1997.2541625.x. [DOI] [PubMed] [Google Scholar]
- 48.Walter A E, Turner D H, Kim J, Lyttle M H, Muller P, Mathews D H, Zuker M. Coaxial stacking of helixes enhances binding of oligoribonucleotides and improves predictions on RNA folding. Proc Natl Acad Sci USA. 1994;91:9218–9222. doi: 10.1073/pnas.91.20.9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitfield C. Biosynthesis of lipopolysaccharide O antigens. Trends Microbiol. 1995;3:178–185. doi: 10.1016/s0966-842x(00)88917-9. [DOI] [PubMed] [Google Scholar]
- 50.Whitfield C, Valvano M A. Biosynthesis and expression of cell-surface polysaccharides in gram-negative bacteria. Adv Microb Physiol. 1993;35:135–246. doi: 10.1016/s0065-2911(08)60099-5. [DOI] [PubMed] [Google Scholar]
- 51.Yao Z, Valvano M A. Genetic analysis of the O-specific lipopolysaccharide biosynthesis region (rfb) of Escherichia coli K-12 W3110: identification of genes that confer group 6 specificity to Shigella flexneri serotypes Y and 4a. J Bacteriol. 1994;176:4133–4143. doi: 10.1128/jb.176.13.4133-4143.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, al-Hendy A, Toivanen P, Skurnik M. Genetic organization and sequence of the rfb gene cluster of Yersinia enterocolitica serotype O:3: similarities to the dTDP-l-rhamnose biosynthesis pathway of Salmonella and to the bacterial polysaccharide transport systems. Mol Microbiol. 1993;9:309–321. doi: 10.1111/j.1365-2958.1993.tb01692.x. [DOI] [PubMed] [Google Scholar]