Abstract
Pathogenic members of the family Neisseriaceae produce specific receptors facilitating iron acquisition from transferrin (Tf) and lactoferrin (Lf) of their mammalian host. Tf receptors are composed of two outer membrane proteins, Tf-binding proteins A and B (TbpA and TbpB; formerly designated Tbp1 and Tbp2, respectively). Although only a single Lf-binding protein, LbpA (formerly designated Lbp1), had previously been recognized, we recently identified additional bacterial Lf-binding proteins in the human pathogens Neisseria meningitidis and Moraxella catarrhalis and the bovine pathogen Moraxella bovis by a modified affinity isolation technique (R. A. Bonnah, R.-H. Yu, and A. B. Schryvers, Microb. Pathog. 19:285–297, 1995). In this report, we characterize an open reading frame (ORF) located immediately upstream of the N. meningitidis B16B6 lbpA gene. Amino acid sequence comparisons of various TbpBs with the product of the translated DNA sequence from the upstream ORF suggests that the region encodes the Lf-binding protein B homolog (LbpB). The LbpB from strain B16B6 has two large stretches of negatively charged amino acids that are not present in the various transferrin receptor homologs (TbpBs). Expression of the recombinant LbpB protein as a fusion with maltose binding protein demonstrated functional Lf-binding activity. Studies with N. meningitidis isogenic mutants in which the lbpA gene and the ORF immediately upstream of lbpA (putative lbpB gene) were insertionally inactivated demonstrated that LbpA, but not LbpB, is essential for iron acquisition from Lf in vitro.
Elemental iron is essential for the sustained growth of nearly all living organisms. When infecting their mammalian host, invading microbes are confronted with an environment with iron levels far too low to permit their proliferation. The iron stores of the host are primarily intracellular, and extracellular iron in serum and cerebrospinal fluid is bound by the glycoprotein transferrin (Tf), whereas the glycoprotein lactoferrin (Lf) serves to sequester iron on mucosal surfaces. To attain iron, many microbes produce and secrete siderophores, small molecules which are able to chelate both soluble and insoluble environmental iron. The resulting iron-siderophore complex is subsequently bound at the bacterial cell surface by a specific receptor and then transported to the periplasmic region of the cells, where it can be shuttled to the cytoplasm for storage and utilization (15, 32).
Some bacteria do not produce siderophores but acquire iron by alternative mechanisms that are seemingly advantageous in their ecological niche. Pathogenic organisms such as the strict human pathogens Neisseria meningitidis (45, 47), Neisseria gonorrhoeae (28), Moraxella (Branhamella) catarrhalis (10–12, 45), and Moraxella lacunata (11, 34) and the bovine pathogen Moraxella bovis (10) have been shown to possess host Tf- and Lf-specific receptors to circumvent the iron sequestration of the host (see reference 21 for a review of this mechanism of iron acquisition). Thus, capture of iron from these host iron-binding glycoproteins permits proliferation of these organisms in an otherwise iron-restricted environment.
There are two outer membrane components of the receptor-mediated mechanism of iron acquisition: Tf-binding proteins A and B (TbpA and TbpB). Although only a TbpA functional homolog was described for the Lf receptor (LbpA) (37, 39), recent genetic (36, 38) and biochemical (10, 12) evidence implies that bacterial Lf receptors have a second constituent (LbpB) with attributes comparable to those of TbpB.
The objective of this study was to examine the role of N. meningitidis LbpA in iron acquisition from Lf and characterize an open reading frame (ORF) located immediately upstream of the lbpA structural gene which has significant sequence homology to the B component of the bacterial Tf receptor (putative lbpB gene). For this purpose, we have prepared a series of isogenic mutants which lack either or both Lf receptor proteins. Given that the Tf receptor proteins characterized to date appear to have an operonic organization (21), we also address the possibility that LbpA and LbpB are cotranscribed and perform preliminary biochemical characterization of the proteins encoded by both ORFs.
MATERIALS AND METHODS
Growth conditions, bacterial strains, and plasmids.
Neisseria and Moraxella sp. cells were propagated in brain heart infusion (BHI) media, whereas Escherichia coli strains were cultured in L broth. Iron starvation of bacterial cells (46) and preparation of crude membranes were achieved as specified previously (45). Growth studies were performed by in vitro plate assays described previously (10) in which BHI agar plates containing 100 μM ethylenediamine-di(o-hydroxyphenylacetic acid) (EDDA) were examined after 24 and 48 h of incubation for cell growth surrounding the disks containing 200 μg of iron-loaded Lf or Tf. N. meningitidis B16B6 and its derivatives N16T1E (tbpA::erm), N16T2K (tbpB::kan), and N16T12EK (tbpB::kan tbpA::erm) have been described previously (24). Plasmid pAM23, which contains a functional copy of the lbpA gene from N. meningitidis BNCV (37), was provided by J. Tommassen, University of Utrecht (Utrecht, The Netherlands). The lbp genes were insertionally inactivated by using either a chloramphenicol acetyltransferase gene with a transcriptional terminator (catΩ) (26) or a gentamicin resistance cassette (gent) (48) provided by H. Schweizer, Colorado State University (Boulder). The pMal-c2 vector (which lacks the normal signal sequence of the malE gene) and the amylose affinity resin were purchased from New England BioLabs (Mississauga, Ontario, Canada). All enzymes and growth media were purchased from Gibco BRL (Burlington, Ontario, Canada), whereas chemical reagents were purchased from Sigma (St. Louis, Mo.), unless otherwise indicated.
Protein analysis.
The high-stringency solid-phase binding assay using horseradish peroxidase (HRP)-labeled human Tf (Jackson Immunoresearch Laboratories, West Grove, Pa.), human Lf, or bovine Tf has been described previously (10), except that Tf-binding assays were routinely performed with 50 mM Tris buffer (pH 8.0), containing 1 M NaCl, whereas the Lf-binding assays were performed in the same buffer, at pH 9.0. Alternatively, where indicated, binding assays were performed in low-pH, low-stringency buffer containing 50 mM Tris-HCl (pH 6.0) plus 0.1 M NaCl to enhance LbpB-Lf interactions (10). Covalent linkage of Tf or Lf to cyanogen bromide (CNBr)-activated CH Sepharose 4B (Pierce Chemical, Rockford, Ill.) or HRP was performed as described previously (33). Prevention of nonspecific binding to either Nitro ME-nitrocellulose (Micron Separations, Westboro, Mass.), used for solid-phase binding assays, or to Immobilon P (Millipore, Bedford, Mass.), used for Western blot analysis, was achieved by using blotting-grade nonfat dry milk blocker, whereas color development was performed with HRP development reagent, which was purchased from Bio-Rad (Richmond, Calif.).
For sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), samples were boiled in Laemmli sample buffer unless otherwise indicated, separated by using a 10% acrylamide gel and the Tris-HCl–glycine buffer system (27), and then transferred to Immobilon P (Western blot) and probed with specific antisera, as described previously for Lbps (10). Protein concentrations were determined by the Bio-Rad DC protein assay.
DNA manipulations.
PCR was employed to amplify specific regions of plasmid or chromosomal DNA from E. coli or N. meningitidis strains. For PCR screening, supernatants from boiled bacterial colonies suspended in small aliquots of sterile water were used as a template. Microscale purification of bacterial chromosomal DNA was performed as described previously (6), whereas the Promega (Madison, Wis.) Wizard Plus miniprep system was used to isolate plasmid DNA. Electrophoretically separated DNA fragments were purified by using the QIAquick gel extraction kit (Qiagen, Hilden, Germany). For Southern blot analysis (4), the PCR digoxigenin labeling mix was used to label DNA probes (Boehringer GmbH, Mannheim, Germany).
Determination of the N. meningitidis B16B6 lbpB gene sequence.
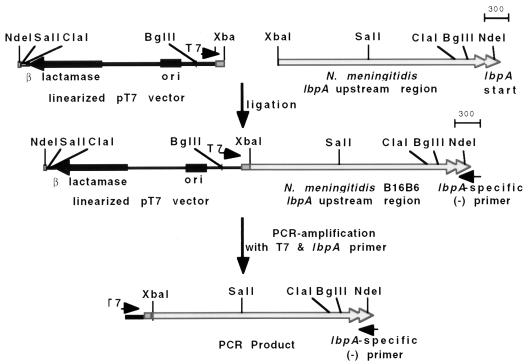
To delineate the DNA sequence of regions upstream of lbpA, ligation-based PCR was performed (see Fig. 1). Briefly, after Southern hybridization was used to map the region upstream of N. meningitidis B16B6 lbpA, chromosomal DNA was linearized with XbaI (Fig. 1) (2.6 kb upstream of the lbpA start site). Separated DNA fragments of the preferred size were excised from the agarose gel, purified, and ligated to XbaI-linearized pT7 vector. The ligation mixture was used in a standard PCR with the T7 primer and an lbpA-specific primer. Both strands of the PCR product were sequenced directly (University of Calgary Core DNA Services Laboratory) by using a Taq Dye Deoxy Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, Calif.) with a series of sequencing primers (Table 1) and analyzed with the ABI Sequence Editor program. DNAsis (Hitachi Software) and Gene Construction Kit software (Textco, Inc.) were used for analysis of DNA sequence data and determination of ORFs, protein molecular weights, or isoelectric points. Images were scanned with a Hewlett-Packard ScanJet IIp, with accompanying DeskScan software, imported into Adobe Photoshop for manipulation, and then subsequently labeled by using MacDraw Pro.
FIG. 1.
Southern hybridization map of the N. meningitidis B16B6 lbpA upstream region and diagrammatic representation of ligation-based PCR protocol. A genetic map of the regions immediately upstream of the N. meningitidis B16B6 lbpA gene (lbpA start is indicated) was determined by Southern hybridization analysis using an lbpB-specific DNA probe (36). Ligation-based PCR was used to amplify the region upstream of lbpA. Depicted is a single-step ligation-based PCR protocol in which the chromosomal DNA from N. meningitidis B16B6 was digested with XbaI (XbaI site situated approximately 2.6 kb upstream of lbpA start) and ligated to XbaI-linearized pT7 vector. The ligated fragments were used as templates in a PCR using the T7 primer and an lbpA-specific primer. Scale is in base pairs.
TABLE 1.
Oligonucleotide primer sequences used for PCR amplification
| Primer
|
Oligonucleotide sequence | aa sequenceb | Reference | |
|---|---|---|---|---|
| Namea | No. | |||
| PLBP-A7 | 212 | AAGCTTAAAACTTCATTTCGAGCGCGAG | N/A | 39 |
| PLBP-A10 | 215 | CGTCATATCGCGGATATCGTAACGCTG | QRYDIRDMT | 39 |
| PLBP-A16 | 539 | TGCCGCCGTTTCCGGATTTGCCGCA | AANPETAA | 39 |
| PLBP-A22 | 287 | GGCTTTCTCGAGATCAGCAGCTTCCGCAAC | GFLEISSFRN | 39 |
| PSEL-A3 | 218 | CAGCTTACCCTGACTGCCTTGGCTGTTGCCGCCGCCTTCCCGTCCTATGCGGC | QLTLTALAVAAAFPSYA | 39; this study |
| PSEL-A4 | 219 | CATATGAATAAGAAACACGGTTTTCAGCTTACCCTGACTGCCTTG | MNKKHGFQLTLTAL | 39; this study |
| PLBP-B14 | 536 | CATATGTGTAAACCGAATTATGGCGGC | MCKPNYGG | This study |
| PLBP-B8 | 513 | TTAGCGAAAAGCCATGCCAAT | LAKSHAN | This study |
| PLBP-B9R | 526 | ACCTAAGCGGTTGGAAAACTG | QFSNRLG | This study |
| PLBP-B10R | 527 | CGCTTGTTTTAAGTTTCGGG | LbpB upstream | This study |
| PLBP-B12 | 528 | CTCGTCGCCTTGTCCTGATT | LbpB upstream | This study |
| PLBP-B13R | 535 | GTTGACCGGCGTGATTTCTAT | IEITPVN | This study |
| PLBP-B7R | 537 | ATGGCTTTTCGCTAAATC | DLAKSH | This study |
| PLBP-B1 | 318 | CCTGAAAGGTATCCGCACGGCGGAAGCCG | LKGIRTAEA | 36 |
| PLBP-B6R | 538 | GTCCTGCGCCTTCGGTTTGGCG | AKPKAQD | This study |
| PLBP-B-XmnI | 591 | GAAGGGGTTCAGGCGGCAATTTCGGCGTGCAGCCTG | GGNFG | This study |
| T7 | 027 | TAATACGACTCACTATAGGG | 50, 51 | |
| PGM-1 | 262 | GTTAGGTGGCGGTACTTGGGTC | 48 | |
| PGM-2 | 263 | CGAATTGACATAAGCCTGTTCGGTT | 48 | |
| PCAT-1 | 394 | TCGATCCCTTTAGGGTTCCGATT | 22 | |
| PCAT-2 | 395 | AGTGAATTTTCGCTGCCGGGT | 22 | |
The designation A (as in A-16) or B (as in B-8) refers to either the lbpA or lbpB gene, respectively.
aa, amino acid. N/A, not applicable.
Construction of N. meningitidis isogenic mutants.
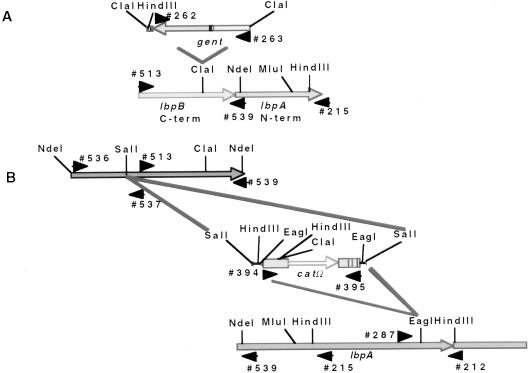
To insertionally inactivate the lbpB gene, a gent cassette with flanking ClaI sites was subcloned into the ClaI site of the partial lbpB gene (see Fig. 3A). In addition, a catΩ resistance determinant was ligated into the SalI site of the cloned (pCR2.1) full-length lbpB gene (see Fig. 3B), as well as the EagI site of the strain BNCV lbpA gene on plasmid pAM23, to insertionally inactivate the lbpA gene.
FIG. 3.
(A) Construction of an N. meningitidis N16T12EK lbpB::gent isogenic mutant. The lbpB gene was amplified by PCR using primers 513 and 215 and cloned into pCR2.1. A gentamicin (gent) resistance marker with flanking ClaI sites was ligated into the unique ClaI site. An E. coli colony was isolated with the gent cassette in the orientation opposite to that of the lbpB reading frame (determined by PCR analysis). The DNA was linearized by using unique restriction enzymes sites in the pCR2.1 polylinker sequence, and the DNA was electroporated into N. meningitidis N16T12EK (TbpB− TbpA−). (B) Construction of N. meningitidis N16T12EK lbpB::catΩ and lbpA::catΩ isogenic mutants. The entire N. meningitidis B16B6 lbpB gene was amplified by PCR using primers 536 and 539 and cloned into pCR2.1. A chloramphenicol acetyltransferase omega (catΩ) resistance marker with flanking SalI sites was ligated into the unique SalI site of the B16B6 lbpB gene, and the catΩ with flanking EagI sites was ligated into the unique EagI site of the strain BNCV lbpA gene (37). Orientation of the inserted catΩ gene was determined by PCR analysis. The DNA was linearized by using NdeI sites that flanked the DNA inserts and used for natural transformation into N. meningitidis N16T12EK. The NdeI site at the 5′ end of the B16B6 lbpB gene was incorporated by using site-specific mutagenesis with primer 536 (Table 1). The gent and catΩ cassette insertions are as shown. The catΩ cassette was inserted in the orientation opposite to that of the lbpA gene.
For biosafety concerns (introduction of the cat gene), N. meningitidis N16T12EK (24), a strain B16B6 derivative which lacks both TbpA and TbpB and is unlikely to proliferate in humans (14), was chosen as a host strain for gene replacement. DNA was linearized and then introduced into strain N16T12EK by either natural transformation (lbpB::catΩ or pAM23::catΩ plasmids) (49) or electroporation (lbpB::gent) (43) with a Bio-Rad Gene Pulser and standard parameters. Transformants were selected on BHI agar with appropriate antibiotic selection.
LbpB and LbpA expression and isolation.
To prepare Mbp::LbpB fusion protein, we used N. meningitidis B16B6 DNA as a template with primers 591 and 539 (Table 1) to amplify the lbpB gene lacking the region encoding the signal peptide, as well as the signal peptidase II recognition site and N-terminal Cys. Primer 591 incorporated an XmnI site immediately upstream of the region coding for mature LbpB protein to allow in-frame ligation to the malE gene of pMal-c2. Expression of Mbp::LbpB fusion protein was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to E. coli DH5α cells containing the pMal-c2lbpB plasmid in accordance with the supplier’s instructions.
For expression of the full-length LbpB, the strain B16B6 lbpB gene was amplified by PCR using oligonucleotide primers 536 and 539 (Table 1). Primer 536 was specific for the predicted native 5′-end lbpB gene, with an NdeI site incorporated immediately upstream of the ATG start codon to facilitate direct cloning into the pT7 expression system (50, 51). Primer 539 was specific for the noncoding strand of the N. meningitidis BNCV lbpA gene (37, 39) at a region which encodes the mature N terminus of LbpA.
Prior to ligation into pT7, the N. meningitidis BNCV lbpA (iroA) gene from plasmid pAM23 (37, 39) was modified by using overlapping oligonucleotide primers 218 and 219 (Table 1). In successive PCRs, the native NdeI site was eliminated and an NdeI site was incorporated immediately upstream of the lbpA ATG start codon (confirmed by sequence analysis) to facilitate ligation to pT7. Expression of the pT7 derivatives (pT7lbpB and pT7lbpA) was achieved by addition of CE6 bacteriophage as described previously (50).
Mbp::LbpB isolation was achieved by lysing cells with a French press, removing cellular debris by centrifugation, and then using the supernatant for affinity purification with amylose resin as described previously (4). LbpA from N. meningitidis B16B6 was affinity purified by using standard high-stringency affinity isolation conditions described previously, with human Lf-Sepharose as the affinity matrix (10).
Antiserum production.
Rabbit polyclonal antiserum specific for either Mbp::LbpB or LbpA was generated by standard techniques in female New Zealand White rabbits. Briefly, 50 μg of purified protein emulsified in complete Freund’s adjuvant was given by intramuscular injection. Subsequent subcutaneous booster injections containing the same dose of protein antigen emulsified in incomplete Freund’s adjuvant were performed at 17 and 30 days after the primary injection, and sera were collected 1 week later.
RESULTS
Analysis of DNA nucleotide sequence upstream of lbpA.
Limited DNA sequence information suggested that a putative ORF with corresponding amino acid sequence homology to TbpB may reside upstream of the N. meningitidis lbpA gene (36). Thus, we sought to characterize and determine the role of the putative upstream ORF in iron acquisition from human Lf. Due to difficulties encountered when directly cloning the tbpB gene from several bacterial species, we were inclined to utilize an approach that would avoid these problems. One solution was to utilize ligation-based PCR (Fig. 1) to amplify genetic elements adjacent to known DNA sequences. By PCR amplification of regions upstream of the lbpA gene, we could sequence the PCR product directly and avoid anticipated difficulties in cloning the DNA elements.
We utilized a ligation-based PCR strategy to amplify 2.6 kb upstream of the strain B16B6 lbpA gene, and the PCR product was sequenced (see Materials and Methods). Analysis of the DNA sequence upstream of the N. meningitidis B16B6 lbpA gene (GenBank accession no. AF031432) revealed a large putative ORF, encoding either 739 (ORF1a) or 726 (ORF1b) amino acids, depending on which ATG start codon was considered authentic. We suspect that the second start codon (ORF1b) is authentic since it has an appropriately positioned ribosomal binding site and promoter region, which is in contrast to the first putative lbpA start codon. In addition, the ORF1b product possesses a hydrophobic signal peptide or preprotein peptide of 18 amino acids with significant homology to that of lipoproteins posttranslationally modified by the addition of fatty acyl chains to their N-terminal Cys residues before cleavage by signal peptidase II (53).
Compared to E. coli promoter DNA sequences (23), ORF1b has 5 of 6 and 6 of 6 identical bases at the −35 and −10 regions, respectively, and exactly 17 bases between the two sites, reflecting a conserved interaction with RNA polymerases (23). Also, a putative Fur box (ferric uptake regulation) with 16 of 19 bases identical to consensus sequences (5) overlaps with the proposed −10 region. In addition, two nearly identical stretches of DNA sequence found either upstream or downstream from a number of meningococcal and gonococcal genes were identified. No known function has been attributed to this sequence; however, its presence upstream, or in some cases downstream, of Neisseria structural genes suggests a potential role in genetic recombinational events.
Amino acid sequence comparison of LbpB and TbpB.
The predicted amino acid sequence of N. meningitidis B16B6 LbpB was aligned with those of known TbpB proteins (Fig. 2). Sequences of TbpB from N. meningitidis B16B6 (GenBank accession no. Z15129) and M982 (Z15130) (29), N. gonorrhoeae FA19 (U05205) (2), Haemophilus influenzae DL63 (U10882) (20), Actinobacillus pleuropneumoniae S-tp1 (Z46775) (18) and H49 (U16017) (19), and Pasteurella haemolytica H196 (U73302) (35) were used in this analysis. Several regions of amino acid sequence identity between all TbpBs and LbpB were found (Fig. 2). The 708-amino-acid mature LbpB protein from strain B16B6 differed from all TbpB homologs in two distinct areas which contained one large stretch and one smaller stretch of negatively charged amino acids (Fig. 2). Amino acids 434 to 515 (QDEED…RDID) contained only 6 positively charged residues (1 Arg, 4 Asn, and 1 Gln) but 39 negatively charged residues (13 Asp and 26 Glu). A second negatively charged region encompassing amino acids 675 to 700 (Fig. 2) (EGTEN…DADVE) contained only 2 positively charged residues (1 Arg and 1 Lys) and 13 negatively charged residues (8 Asp and 4 Glu). It is interesting to note that the negatively charged regions were both localized to the C-terminal portion of LbpB. In addition, a poorly conserved region speculated to be a phosphate-binding loop (P-loop motif) for TbpB (30) is shown (Fig. 2).
FIG. 2.
Comparison of the putative N. meningitidis LbpB protein sequence with sequences of TbpB proteins from related organisms. The putative amino acid sequence of N. meningitidis B16B6 LbpB was compared to the known amino acid sequences of other known TbpBs, namely, N. meningitidis B16B6 and M982 (29), N. gonorrhoeae FA19 (2), A. pleuropneumoniae H49 (19) and S-tp1 (18), P. haemolytica H196 (35), and H. influenzae DL63 (20). The basic alignment was performed by using the program DNAsis. ∗, homology with at least three other TbpBs; Δ, absolute homology with all selected TbpBs. Shaded boxes indicate negatively charged regions of LbpB not present in TbpBs; unshaded box indicates proposed site of P-loop motif.
Construction of N. meningitidis mutants and confirmation of gene replacement.
As a first step in characterizing the roles of LbpA and LbpB in iron acquisition from Lf, we constructed isogenic mutants lacking either or both receptor proteins. We interrupted the reading frames of the cloned lbpA and lbpB genes with antibiotic resistance cassettes (insertional inactivation) (Fig. 3) conferring resistance to either gentamicin (gent) or chloramphenicol (catΩ). Appropriate antibiotic selection was used to initially identify clones in which the mutated DNA had undergone homologous recombination with the native gene. For biosafety considerations, we used a previously described N. meningitidis B16B6 mutant (strain N16T12EK) (24) which was deficient for both of the Tf receptor proteins, TbpB and TbpA (tbpA::mTn3erm and tbpB::kan), as a recipient host strain to introduce the mutated lbpA and lbpB genes.
PCR amplifications with primers specific for the gent gene, the cat gene, and the lbpB and lbpA genes, adjacent to the sites of insertion, were used to verify the identity of the isogenic mutants. Primers flanking the ClaI site (primers 513 and 539) (Table 1; Fig. 3A) in lbpB amplified a 1.35-kb PCR product from the parental strain N16T12EK (Fig. 4, lane 1) as well as the lbpB::catΩ (lane 3) and lbpA::catΩ (lane 4) mutant derivatives. In the lbpB::gent mutant, a 2.6-kb PCR product was obtained, consistent with insertion of the 1.35-kb gentamicin resistance determinant into the ClaI site. As further proof of gene replacement of the native lbpB gene with lbpB::gent, we used a primer specific for the region encoding the C terminus of the gent cassette (primer 262) and a primer specific for the noncoding strand of the signal peptide region of the lbpA gene (primer 539) and obtained a 1.7-kb PCR product with the lbpB::gent mutant (lane 13), demonstrating that the gent cassette was in the opposite orientation of the lbpB gene. We were unable to amplify a PCR product with the B16B6 wild-type strain, strain N16T12EK, or with the N16T12EK lbpB::catΩ and lbpA::catΩ mutants by using this primer pair (data not shown).
FIG. 4.
PCR analysis. Gentamicin-resistant or chloramphenicol-resistant N. meningitidis B16B6 colonies were selected for PCR analysis, and PCR products were separated by electrophoresis. Products in lanes 1 to 4 were obtained with primers 513 and 539 (Table 1), which flank the ClaI site of the lbpB gene. Products in lanes 5 to 8 were obtained with primers 536 and 537, which flank the unique SalI site of the lbpB gene. Lanes 9 to 12 were obtained with primers 287 and 212, which flank the EagI site of the meningococcal lbpA gene (39). The PCR product in lane 13 was obtained with primer 262, which is specific for the 5′ end of the gent cassette, and primer 539, which is specific for the lbpA gene (39). Lane 14 contains the PCR product obtained with primer 536, which is specific for the 5′ end of the lbpB gene, and primer 395, which is specific for the 3′ end of the catΩ gene (22). The PCR product in lane 15 was obtained with primers 287 and 394, which are specific for regions upstream of the 3′ end of the catΩ gene (22). Lanes 1, 5, and 9, N16T12EK; 2, 6, 10, and 13, N16T12EK lbpB::gent; 3, 7, 8, and 14, N16T12EK lbpB::catΩ; 4, 8, 12, and 15, N16T12EK lbpA::catΩ. A negative image of the ethidium bromide-stained agarose gel is shown. STDS, molecular weight standards.
Primers flanking the SalI site of the lbpB gene (Fig. 3B) were used to verify the identity of the lbpB::catΩ mutants. By using primers 536 and 537 (Table 1), a 0.95-kb PCR product was obtained with the parental strain N16T12EK (Fig. 4, lane 5) and the lbpB::gent (lane 6) and lbpA::catΩ (lane 8) mutants that were unaffected at this region of the lbpB gene. In contrast, a 2.4-kb product was obtained with the lbpB::catΩ mutant (lane 7), consistent with insertion of the SalI catΩ cassette (1.5 kb). In addition, by using primer 536 and a primer specific for the C terminus of the cat cassette (primer 395), we amplified a 2.1-kb PCR product from the lbpB::catΩ mutant (lane 14), which accounts for the insertion of the 1.5-kb catΩ gene in the same orientation as the lbpB gene. PCR products were not obtained with either the lbpB::gent or lbpA::catΩ N16T12EK derivatives (data not shown) by using this primer pair.
By using primers 287 and 212, which flank the EagI insertion site of the catΩ cassette in the lbpA gene (Fig. 3B; Table 1), a 0.67-kb PCR product was obtained with strain N16T12EK (Fig. 4, lane 9) and with the lbpB::gent (lane 10) and lbpB::catΩ (lane 11) N16T12EK derivatives which were unaffected at this region of the lbpA gene. In contrast, a 2.0-kb PCR product was obtained with the N16T12EK lbpA::catΩ mutant (lane 12), which accounts for insertion of the 1.3-kb catΩ cassette into the EagI site of the lbpA gene. In addition, by using primer 287 and a primer specific for regions upstream of the 5′ end of the catΩ cassette (primer 394), we were able to amplify a 1,572-bp PCR product from the N16T12EK lbpA::catΩ mutant (lane 15), demonstrating that the catΩ cassette had been inserted in the orientation opposite to that of the reading frame of the lbpA gene. No PCR products were obtained with strain N16T12EK or the lbpB::gent or lbpB::catΩ derivatives when this primer pair was used (data not shown).
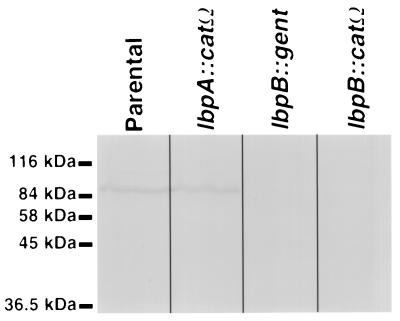
Western blot analysis of mutants.
To demonstrate that the insertionally inactivated lbpB mutants did not produce truncated LbpB, we prepared Western blots of the separated total cellular proteins from the individual mutants which were probed with Mbp::LbpB-specific polyclonal antisera. A single 85-kDa protein was detected in both the parental strain (Fig. 5) and the lbpA::catΩ mutant. In contrast, LbpB was not detected in either the lbpB::gent mutant or the lbpB::catΩ mutant (Fig. 5).
FIG. 5.
Western blot analysis of N. meningitidis mutants. N. meningitidis cells that were grown under iron-limiting conditions to similar optical densities were boiled in Laemmli sample buffer prior to SDS-PAGE and Western blotting. The blots were blocked and incubated with rabbit polyclonal antisera specific for Mbp::LbpB. Binding of the rabbit polyclonal antibodies was detected by using anti-rabbit antibody-HRP conjugate and developed.
Characterization of mutants.
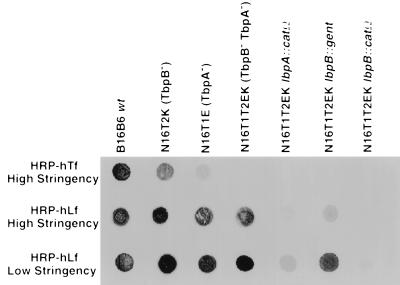
To evaluate the ligand-binding properties of the isogenic mutant strains, we used a solid-phase binding assay (10). Lysates of cells grown in iron-deficient media were spotted onto nitrocellulose, incubated with HRP-labeled human Lf or human Tf under either high- or low-stringency buffer conditions (see Materials and Methods), and then developed. The low-stringency conditions were used because they are necessary for detection of Lf interactions with LbpB (10). As mentioned previously, the parental meningococcal strain used for introduction of the insertionally inactivated lbpB and lbpA genes (strain N16T12EK) was deficient in the production of both TbpB (tbpB::kan) and TbpA (tbpA::mTn3erm) and can neither bind (Fig. 6) nor utilize Tf (Table 2) as a sole iron source (24). Additional control meningococcal strains deficient in the production of either TbpA (strain N16T1E; tbpA::mTn3erm) or TbpB (strain N16T2K; tbpB::kan) (24) were also included in our analysis to compare and contrast the Tf- and Lf-binding properties of the mutants lacking the individual receptor proteins (Fig. 6).
FIG. 6.
Analysis of binding of various mutants to Lf- and Tf-HRP conjugates. Equal amounts of lysed cell suspension obtained from bacteria grown under iron-restricted conditions were spotted directly onto nitrocellulose membranes (in triplicate). After blocking, the blots were incubated in either high-stringency Tf-binding buffer (50 mM Tris, 1 M NaCl [pH 8.0]), high-stringency Lf-binding buffer (50 mM Tris, 1 M NaCl [pH 9.0]), or low-stringency Lf-binding buffer (50 mM Tris, 0.1 M NaCl [pH 6.0]), containing 1:1,000 dilutions of peroxidase-conjugated human Lf (hLf-HRP) or human Tf (hTf-HRP). After repeatedly being washed in the binding buffer, the blots were subsequently developed. The N. meningitidis TbpA− and TbpB− mutants used as controls have been characterized previously (24).
TABLE 2.
Ability of various defined mutants to utilize human Lf and Tf as a sole iron source and production of Lf or Tf receptor proteins
| N. meningitidis B16B6 derivative | Growtha
on:
|
Productionb
of:
|
Reference | ||||
|---|---|---|---|---|---|---|---|
| hLf | hTf | LbpB | LbpA | TbpA | TbpB | ||
| Wild type | +++ | +++ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | 24, 46 |
| TbpB− TbpA− | +++ | − | ∗∗∗ | ∗∗∗ | ND | ND | 24 |
| TbpB− TbpA−lbpB::gent | ++ | − | ND | ∗∗ | ND | ND | This study |
| TbpB− TbpA−lbpA::catΩ | − | − | ∗∗∗ | ND | ND | ND | This study |
| TbpB− TbpA−lbpB::catΩ | − | − | ND | ND | ND | ND | This study |
+++, zone of growth surrounding disk greater than 3 mm; ++, zone of growth surrounding disk less than 3 mm; −, no observable growth.
∗∗∗, wild-type levels of protein present; ∗∗, reduced but detectable levels of protein present; ND, no protein detected.
In accordance with previous results with an N. meningitidis B16B6 lbpA::gent mutant, (11) strain N16T12EK lbpA::catΩ displayed no evident binding to human Lf-HRP when the high-stringency solid-phase binding assay was used (Fig. 6). This implies that the Lf-binding activity displayed under these assay conditions can be attributed solely to LbpA, as suggested previously (10). In contrast to the lbpA::catΩ mutant, the tbpA::erm mutant (strain N16T1E) displayed some Tf-binding activity, under high-stringency conditions (Fig. 6). We subsequently examined the Lf-binding properties of the N16T12EK lbpA::catΩ mutant under the low-stringency conditions and were able to detect weak Lf binding that is attributable to the additional Lf-binding protein (LbpB). The absence of this Lf-binding activity with the N16T12EK lbpB::catΩ mutant confirms that this binding activity is not due to nonspecific interactions by the cells under the low-stringency assay conditions.
The binding activities of the two LbpB− mutants (lbpB::gent and lbpB::catΩ) are clearly different under high- and low-stringency conditions (Fig. 6). Since the binding activity under high-stringency conditions is solely attributable to LbpA, the results illustrate that insertion of the gent cassette into the lbpB gene reduces, but does not eliminate, Lf-binding activity by LbpA. Similar results were obtained when the N. meningitidis tbpB gene was insertionally inactivated with a kan cassette (strain N16T2K; TbpB+), where binding to human Tf-HRP was also dramatically impaired relative to that of the wild type (Fig. 6). In contrast, the lbpB::catΩ insertion mutant, which contains a transcriptional terminator, completely eliminates Lf-binding activity under either high- or low-stringency assay conditions.
Growth analysis of N. meningitidis mutants.
The defined mutants were examined for their ability to utilize various iron sources by using a previously described plate growth assay (10); in this assay, iron-starved organisms were spread onto BHI media containing an iron chelator and sterile disks containing the individual iron sources were placed on the media to allow localized diffusion. Strain B16B6 (wild type) and the bovine pathogen M. bovis served as controls for utilization of iron from human and bovine forms of Lf and Tf. Under these assay conditions, growth of M. bovis was observed only when bovine Lf or Tf, and not human Lf or Tf, was provided as an iron source (data not shown). In contrast to strain B16B6, strain N16T12EK (TbpB− TbpA−) and derivatives of this strain (lbpB::gent, lbpB::catΩ, and lbpA::catΩ) were unable to grow when human Tf was supplied as the sole iron source (Table 2).
When we tested the meningococcal lbpA::catΩ derivative of strain N16T12EK, we did not observe any growth of this TbpB− TbpA− LbpA− meningococcal mutant surrounding the human Lf-impregnated disk (Table 2). This data supports the conclusion that the TonB-dependent membrane-spanning constituent of the bacterial Lf receptor (LbpA) is essential for utilization of iron bound to Lf, as demonstrated previously (11, 37, 40). Similarly, the N16T12EK lbpB::catΩ mutant displayed no detectable growth when human Lf was supplied as the sole iron source, consistent with the lack of LbpA produced in this strain. A small intense zone of growth surrounding the human Lf-impregnated disk (approximately 1.0 to 1.5 mm in diameter) was observed with the N16T12EK lbpB::gent mutant (TbpA− TbpB− LbpB−), suggesting that this mutant retains the capacity to utilize Lf, provided that the iron source is present in a sufficiently high concentration. In contrast, the zones of growth surrounding the human Lf-impregnated disks for strain B16B6 and strain N16T12EK (TbpA− TbpB−) appeared as disperse areas of growth (approximately 4 to 5 mm in diameter), presumably indicative of the ability to use Lf at lower concentrations.
Recombinant LbpA and LbpB analysis.
In view of prior difficulties in cloning the tbpB structural gene and upstream regions from N. meningitidis, we designed PCR primers (Table 1) that would allow amplification of the entire coding sequence of the lbpB and lbpA genes, lacking the putative ribosomal binding site and promoter. As well, we included NdeI sites at the start codons to facilitate subcloning into pT7-7 (50) for controlled expression of recombinant LbpB. Ligation of the lbpA gene into pT7 necessitated the removal of an NdeI site located 70 bases downstream of the lbpA gene ATG start codon (37) (see Materials and Methods). PCR products were ligated into pCR2.1, and then the gene products of modified lbpB and lbpA genes were subcloned in the pT7 vector (NdeI/HindIII), transformed into a nonexpression host (E. coli DH5α), and expressed by the addition of CE6 bacteriophage as described previously (50). For production of recombinant LbpB that could be readily purified, we prepared oligonucleotide primers (primers 591 and 539) (Table 1) to allow PCR amplification of the region encoding the mature LbpB lacking the N-terminal cysteine. The resulting PCR product was subcloned into the pMal-c2 vector for production of Mbp::LbpB fusion protein. It has been previously demonstrated that stable Mbp::TbpB fusions produced in E. coli retained salient characteristics of native TbpB (41).
To evaluate the expression of the recombinant proteins, samples of induced whole cells were boiled in Laemmli sample buffer, subjected to SDS-PAGE, electroblotted, and detected with appropriate antisera. Expression of LbpA (Fig. 7, lane 2) was detected in the CE6-infected E. coli pT7lbpA cells with LbpA-specific polyclonal antisera, whereas no protein was detected in uninfected cells (lane 1). Similarly, expression of LbpB was detected only in CE6-infected E. coli pT7lbpB cells by using Mbp::LbpB-specific polyclonal antisera (compare lanes 3 and 4). The same antisera detected production of Mbp::LbpB in E. coli pMal-c2lbpB cells with both IPTG-induced (lane 6) and uninduced (lane 5) cells; however, the uninduced cells produced considerably less fusion protein. It is interesting to note that the quantity of recombinant fusion protein present in IPTG-induced E. coli pMal-c2 cells was considerably greater than the LbpB produced with the phage-infected E. coli pT7lbpA and pT7lbpB cells as detected by Coomassie blue staining of the gels (data not shown) or serological analysis of the electroblotted proteins (Fig. 7; compare lanes 6 and 7 and lanes 2 and 4). It is important to note that considerable amounts of degradation products were seen in all of the E. coli cells producing recombinant Lbps (Fig. 7, lanes 2, 4, 6, and 7).
FIG. 7.
Western blot analysis of recombinant LbpB and LbpA.
E. coli cells with the meningococcal lbpA
(lanes 1 and 2) or lbpB (lanes 3 and 4) gene under
control of the T7 promoter were either infected (+phage) with CE6
phage or uninfected (−phage). E. coli cells with the
pMal-c2lbpB plasmid (lanes 5, 6, 7, and 10) were either
induced (+IPTG) or left uninduced (−IPTG). The IPTG-induced cells with
pMal-c2lbpB were subjected to affinity isolation using
immobilized amylose. Specifically bound proteins were eluted by the
addition of 10 mM maltose and lyophilized. The collected cells (lanes 1
to 7 and 10) or isolated Mbp::LbpB fusion protein (lanes 8
and 9) was then incubated with Laemmli sample buffer and either boiled
(lanes 1 to 7) or left unboiled (lanes 8 to 10) and subjected to
SDS-PAGE. The gel was either stained for protein (lane 8 only) or
Western blotted. For serological detection of proteins, the blot was
first incubated with a 1:5,000-diluted rabbit polyclonal antiserum
raised against (i) B16B6 LbpA (lanes 1 and 2), (ii) Mbp::LbpB
(lanes 3 to 6), or (iii) MBP (lane 7). The blots were washed, incubated
in the presence of goat anti-rabbit-HRP immunoglobulin G, and
developed. Alternatively, the blots were incubated in the presence of
50 mM Tris–0.10 M NaCl (pH 6.0) containing human Lf-HRP (lanes 9 and
10) and then washed and developed. Symbols indicate LbpA (•),
LbpB ( ), and
proteins with molecular masses of 123 (▸), 95
(░⃞), and 85
(▪) kDa.
), and
proteins with molecular masses of 123 (▸), 95
(░⃞), and 85
(▪) kDa.
The Mbp::LbpB was affinity purified by using amylose resin, and the maltose-eluted fraction was incubated without boiling in Laemmli sample buffer and separated by SDS-PAGE. The gel was either stained for protein (Fig. 7, lane 8) or electrophoretically transferred to Immobilon-P (Western blot; see below). The Coomassie-stained gel of the isolated samples (Fig. 7, lane 8) revealed that the majority of protein isolated had an approximate molecular mass of 123 kDa; however, significant quantities of two additional proteins with approximate molecular masses of 95 and 85 kDa were also isolated.
Western blots of the affinity-isolated Mbp::LbpB were incubated with polyclonal antiserum specific for Mbp (New England BioLabs), and the antiserum detected all three of the isolated proteins (data not shown), suggesting that the two smaller-molecular-mass proteins (95 and 85 kDa) were proteolytic breakdown products of Mbp::LbpB. Also, we prepared Western blots of total cellular proteins from IPTG-induced E. coli pMal-c2lbpB cells and incubated the blots with the Mbp-specific polyclonal antiserum (Fig. 7, lane 7). The Mbp-specific antiserum avidly detected the 123-kDa Mbp::LbpB, but we also observed reactivity with a large number of proteolytic degradation products (Fig. 7, lane 7).
Lf-binding activity following electroblotting has been demonstrated for N. meningitidis (38) and M. catarrhalis (12) LbpB. We wanted to assess whether recombinant LbpB also retained Lf-binding activity by using this approach. Western blots of either the affinity-purified, unboiled Mbp::LbpB (Fig. 7, lane 9) or unboiled, IPTG-induced E. coli pMal-c2lbpB whole cells (Fig. 7, lane 10) were incubated with human Lf-HRP in low-stringency buffer. Human Lf-HRP was found to bind avidly to all three of the amylose resin affinity-isolated electroblotted proteins (Fig. 7, lane 9). In addition, we observed avid Lf-HRP binding activity to a single protein with an approximate molecular mass of 123 kDa from the IPTG-induced E. coli pMal-c2lbpB whole cells (Fig. 7, lane 10). In contrast, after SDS-PAGE and Western blotting, we were unable to detect Lf binding to recombinant LbpB produced with the T7 system (with or without boiling) (data not shown). To assess the stability of the Lf-binding domain of LbpB, we briefly boiled (1 to 2 min) the purified Mbp::LbpB in Laemmli sample buffer prior to SDS-PAGE or Western blot analysis. No Lf-binding activity was detected on the blots of the boiled Mbp::LbpB samples (data not shown). None of the LbpA preparations displayed any Lf-binding activity after SDS-PAGE and Western blotting (data not shown).
To evaluate the ligand-binding properties of the recombinant Lbps, we performed solid-phase binding studies using either high- or low-stringency conditions. These results were compared with the results obtained with the solid-phase binding analysis with the meningococcal isogenic mutants (Fig. 6). Our initial analysis consisted of utilizing French press lysates of E. coli strains with recombinant LbpA, LbpB, and Mbp::LbpB. As expected, E. coli cells producing LbpA (pT7lbpA) demonstrated avid Lf-binding activity under either high- or low stringency-buffer conditions (Fig. 8). E. coli cells producing LbpB (pT7lbpB) or Mbp::LbpB (pMal-c2lbpB) displayed little Lf-binding activity under the high-stringency buffer conditions (Fig. 8); however, under the low-stringency buffer conditions, weak Lf-binding activity was detected by the E. coli cells producing Mbp::LbpB (Fig. 8, pMal-c2lbpB). Since these modified buffer conditions could potentially foster interactions between Lf and E. coli cell surface constituents (17), we wanted to ensure that the Lf binding was specific. Therefore, we assessed the Lf-binding capacity of amylose resin affinity-purified Mbp::LbpB by using the low-stringency solid-phase assay system. Avid binding by the recombinant fusion protein was detected (Fig. 8), and under these conditions, there was no detectable binding by a control Mbp protein (data not shown).
FIG. 8.
Analysis of binding of recombinant Lbps to Lf-HRP conjugates with high- or low-stringency buffer. E. coli DH5α cells producing recombinant LbpA, LbpB, or Mbp::LbpB were grown to midlogarithmic phase in L broth and induced for production of recombinant protein either by the addition of CE6 bacteriophage (pT7lbpA and pT7lbpB) or by the addition of IPTG (pMal-c2lbpB). The collected cells were lysed, and insoluble cellular debris was removed by centrifugation. Lysed cell suspension or amylose resin affinity-purified Mbp::LbpB was spotted onto the nitrocellulose membranes. The membrane was blocked and then incubated in either high-stringency binding buffer (50 mM Tris, 1 M NaCl [pH 9.0]) or low-stringency binding buffer (50 mM Tris, 0.1 M NaCl [pH 6.0]) containing 1:1,000 dilutions of peroxidase-conjugated human Lf (HRP-hLf). The blots were washed in the respective binding buffer and developed.
DISCUSSION
Analysis of previously described meningococcal (11, 37, 40) and gonococcal (9) LbpA− mutants suggested that the bacterial Lf receptor had a single constituent. More recently, biochemical and preliminary genetic evidence (10, 12, 36) suggested that the Lf receptor also has a B-component homolog (LbpB). In addition, both this report and a recent publication by Pettersson et al. (38) have identified a complete ORF immediately upstream of the lbpA gene from N. meningitidis which encodes LbpB. In addition to having nearly identical lbpB genes, strains BNCV (38) and B16B6 (this report) are of the same serotype, serogroup, and TbpB group (42), suggesting that the two strains are from a similar lineage.
Amino acid sequence comparisons of LbpB from N. meningitidis B16B6 with TbpB proteins from other related bacterial species (Fig. 2) strongly suggest that the two proteins may serve as functional homologs. Similarly, meningococcal outer membrane proteins involved in iron acquisition from hemoglobin-haptoglobin (HpuA and HpuB) are also likely composed of two antigenically distinct proteins (30). In these bipartite receptor systems, one component (TbpA, LbpA, and HpuB) has regions of homology to other TonB-dependent receptors and likely has several regions which span the outer membrane, allowing the protein to serve as a gated pore. In contrast, the second component (TbpB, HpuA, and presumably LbpB) is an accessory lipoprotein, the role of which is not clearly defined. The N terminus of LbpB displays significant homologies to the N termini of lipoproteins that are known to be posttranslationally modified by the addition of fatty acyl chains to their N-terminal Cys residues before cleavage by signal peptidase II (53), and TbpB lipidation has been demonstrated previously (2, 18, 20, 29). Lipid modification has been postulated to allow the protein to retain an association with the outer membrane of the bacteria (29).
For N. meningitidis (29), N. gonorrhoeae (2), H. influenzae (20), P. haemolytica (35), and A. pleuropneumoniae (19), the tbpB gene immediately precedes the tbpA gene in an operonic organization where the products of these genes are believed to be cotranscribed during conditions of limiting iron (21). There are several lines of evidence that suggest that the N. meningitidis lbpB and lbpA genes appear to have similar organizations. First, the catΩ gene with an fd terminator sequence inserted into the lbpB gene (approximately 1.4 kb upstream of lbpA [Fig. 3]) abolishes LbpA production as detected by Lf binding (Fig. 6). Second, growth using Lf as a sole iron source was either abolished (lbpB::catΩ) or impaired (lbpB::gent) in these meningococcal TbpA− TbpB− mutants (Table 2; see below). Finally, analysis of the lbpB and lbpA ORFs reveals that the TGA stop codon of the lbpB gene also overlaps the predicted ATG start codon of the lbpA gene of the strain, suggesting that production of LbpB and LbpA is translationally coupled. In contrast, tbpB and tbpA genes are predicted to have an operonic organization but have a variable intergenic region separating the two genes which range from 14 bp (H. influenzae DL63 [20] and A. pleuropneumoniae H49 [19]) to 87 bp (N. meningitidis B16B6 [29]). Ultimately, mRNA transcript analysis of the lbpB and lbpA genes will confirm this hypothesis.
Although a Fur box consensus sequence in the purported lbpA promoter region was previously reported (39), subsequent studies have shown this to be incorrect (36). In support of the hypothesis that both lbpB and lbpA genes are transcribed in an iron-regulated operon, a Fur box with 16 of 19 bases identical to those of the consensus sequences (5) and overlapping the proposed −10 region was located upstream of the lbpB gene from strains BNCV (38) and B16B6 (this report). The fur gene product (Fur) described for Neisseria spp. (8, 25) is likely to bind at this site, although this remains to be experimentally validated. This observation also correlates with the increased Lf-binding activity (47) and enhanced LbpB and LbpA expression (38) observed with N. meningitidis cells grown under iron-limiting conditions.
In an attempt to characterize the proteins encoded by the N. meningitidis lbpA and lbpB ORFs, we placed the structural genes under control of inducible promoters for heterologous expression analysis in E. coli DH5α. We were able to demonstrate that both the lbpA and lbpB ORFs could be translated by using a heterologous promoter and expression system in vitro (Fig. 7, pT7lbpA, pT7lbpB, and pMal-c2lbpB). By using a solid-phase binding assay, E. coli cells expressing recombinant LbpA (Fig. 7, lane 2) were shown to readily bind Lf under both high- and low-stringency buffer conditions (Fig. 8). Although we were able to demonstrate expression of recombinant LbpB from the T7 promoter (Fig. 7, lane 4), we were unable to demonstrate that these cells had acquired the capacity to bind Lf with the solid-phase binding assay using either high- or low-stringency buffer conditions (Fig. 8). We suspected that the inability of recombinant LbpB produced in the T7 system to bind Lf was due to improper folding and/or protein instability. Therefore, we constructed an Mbp::LbpB fusion to overcome these limitations, as described previously for meningococcal TbpB (41). We detected weak Lf binding by E. coli cells expressing Mbp::LbpB (Fig. 8, pMal-c2lbpB), whereas the E. coli cells expressing Mbp alone (Fig. 8, pMal-c2) were not able to bind Lf under either high- or low-stringency buffer conditions. We also tested the amylose resin affinity-purified Mbp::LbpB for Lf-binding activity and observed avid Lf-binding activity under the low-stringency buffer conditions (Fig. 8); however, no Lf-binding activity was detected by purified Mbp (data not shown).
To assess the stability of the Lf-binding domain of LbpA and LbpB, we incubated the E. coli cells expressing LbpA, LbpB, or Mbp::LbpB with Laemmli sample buffer (with or without boiling) and then subjected the samples to SDS-PAGE and Western blot analysis. Only Mbp::LbpB was shown to bind Lf (Fig. 7, lane 10), suggesting that recombinant Mbp::LbpB has a stable Lf-binding domain that is retained even following SDS-PAGE and Western blotting. These results are consistent with bacterial Tf receptors, in which the B constituent is resistant to denaturation, and all Tf-binding activity of TbpA is destroyed by even mild denaturation, suggesting a conformationally dependent ligand-binding epitope(s). However, it is important to note that the Lf-binding activity of LbpB was abolished when the samples were boiled (data not shown), implying that the Lf-binding epitope of LbpB may not be as stable as the Tf-binding epitope of TbpB. Similarly, it has been recently demonstrated that insertional inactivation of the lbpB ORF in the native organism was correlated with the loss of a 95-kDa protein which displayed Lf-binding activity after SDS-PAGE and Western blotting, providing the samples had not been boiled (38).
The majority of isolated Mbp::LbpB had an approximate molecular mass of 123 kDa (Fig. 7, lane 8); however, two additional proteins with approximate molecular masses of 95 and 85 kDa (lane 8) were also isolated. The ability of all three proteins to bind to amylose, and be detected by Mbp-specific antisera, suggests that the two smaller-molecular-mass proteins (95 and 85 kDa) were proteolytic breakdown products of 123-kDa Mbp::LbpB in which regions of the C terminus were removed. All three proteins bind Lf after Western blotting (Fig. 7, lane 9), suggesting that a stable Lf-binding domain is retained in the N-terminal region of LbpB. Similarly, the N-terminal region of TbpB contains a stable Tf-binding domain (52).
To evaluate the role of LbpA and LbpB for in vitro iron acquisition from Lf, we prepared a series of isogenic mutants lacking either or both of the receptor proteins. For our analysis, iron-starved organisms were spread on solid BHI growth media containing the iron chelator EDDA and then sterile disks containing the individual iron sources were placed on the media. As shown previously by ourselves (10) and others (37, 40), meningococcal mutants lacking a functional copy of the lbpA gene (lbpA::catΩ) were incapable of utilizing human Lf as a sole iron source, suggesting that LbpA plays an essential role (Table 2). In contrast, we observed zones of growth surrounding the Lf-impregnated disks by the N. meningitidis mutant lacking LbpB (lbpB::gent) (Table 2). Thus, unlike meningococcal TbpB isogenic mutants, LbpB isogenic mutants retain the ability to utilize human Lf as a sole iron source. Our results suggest that LbpB plays a facilitative role for in vitro iron acquisition, since the zone of growth surrounding the Lf-impregnated disk was considerably smaller for the lbpB::gent mutants, as compared to that for the N16T12EK parental strain. Similar results have been reported for N. gonorrhoeae (2) and H. influenzae (20) TbpB− mutants.
Lewis et al. suggest that the lipoprotein constituents of the Tf receptor possess a phosphate-binding loop (P-loop motif) and may contain adenine or guanine nucleotide binding sites (30). Presumptively, ATP or GTP hydrolysis at the outer membrane may occur at some point to allow iron removal from the host iron-carrier protein (30). Although this was not explicitly stated, the authors were presumably referring to the region of the M982 TbpB with the amino acid sequence GDTNGKT (amino acids 469 to 475 of the mature protein). One argument against this theory is the apparent lack of conservation of the P-loop motif among TbpBs and LbpBs known to date (Fig. 2). In addition, the M982 TbpB sequence does not contain the appropriate number of amino acid residues associated with the P-loop motif consensus sequence [GXXXXGK(TS)]. Further, it has been stated that the P-loop motif consensus sequence with a large number of false positives is found in many proteins (44).
Evidence of a B homolog for the Lf receptor (LbpB) with biochemical attributes similar to that of TbpB has been documented for M. catarrhalis (10–12). However, M. catarrhalis appears to produce several Lf-binding proteins, including LbpA, LbpB, and CopB (OMPB2), which directly or indirectly play a role in iron acquisition from Lf (1, 12). Like the M. catarrhalis OMPB1 (presumably TbpB) (10, 12, 13), the M. catarrhalis LbpB has a stable ligand-binding epitope and displays Lf binding even after SDS-PAGE and Western blotting (12), a property unique to TbpB-like molecules (12, 18, 31, 46, 52). In addition, following M. catarrhalis infection, a strong immune response to LbpB occurs (12), suggesting that LbpB is expressed and accessible to the immune system in vivo. Thus, LbpB may represent a novel antigen for immunization purposes.
The availability of cloned lbpB and lbpA genes, as well as specific isogenic mutants lacking these proteins, will substantially aid in further characterization of these unique gene products. Their individual roles in iron acquisition need further evaluation, but based on prior models (21), it appears that the Lf receptor system has numerous parallels to the Tf receptor complex. The functional expression of these proteins in a heterologous system will aid in production of significant quantities of these proteins. Large amounts may be useful for vaccination purposes, although it is evident that the problem of proteolytic degradation of the recombinant protein may need to be addressed.
Lf is found in high concentrations on mucosal surfaces, and apo-Lf is found in the granules of polymorphonuclear cells in vivo. Lf is postulated to have several biological roles, including sequestration of iron on mucosal surfaces (bacteriostatic effects). There is also evidence that cationic peptides generated from proteolytic digestion of the N-terminal region of Lf are involved in direct killing of bacteria (bactericidal effects) at sites of infection (7, 16, 17). An interesting feature of the putative amino acid sequence of the N. meningitidis B16B6 LbpB protein is the two regions of negatively charged amino acids. A speculative role for these regions would be to bind to the Lf-derived cationic peptides (7), thus preventing the bactericidal effects exhibited when these peptides bind to Lipid A (3) and disrupt the integrity of the outer membrane (16). Cornelissen et al. have recently reported that without Tf receptors, N. gonorrhoeae FA1090 was unable to colonize human male volunteers in an experimental urethritis model (14). It is important to note that these mutants also lacked the ability to utilize human Lf (undefined mutation), and it is unknown if these mutants produce LbpB. This may be an important consideration, since LbpB may play a role in preventing the bactericidal effects of Lf and peptides derived from Lf.
Lf receptors are not ubiquitous among organisms having Tf receptors. Although members of the family Neisseriaceae are able to utilize both Tf and Lf as a sole iron source, (10) members of the related family Pasteurellaceae produce host-specific Tf receptors but none have been shown to produce Lf receptors. Due to the similar genetic organization of tbpBA and lbpBA genes, it is likely the lbpBA genes disseminated from duplication of the tbpBA genes after divergence of the Pasteurellaceae and Neisseriaceae. In addition, disease isolates producing only Lf receptors have not been reported to date. Thus, Lbps may be less evolved and, consequently, less antigenically diverse than Tbps and could represent a more conserved vaccine target. However, should a disseminated organism lose the ability to produce Lf receptors, it is unknown whether this would affect further growth since Tf is likely to be a prominent iron source. Thus, studies aimed at determining both the antigenic heterogeneity and ubiquity of LbpBs among disease isolates are of considerable interest and should be pursued.
ACKNOWLEDGMENTS
This work was supported by the Medical Research Council of Canada grant MT10350.
We thank Rainer Haas for his helpful E-mail discussions and for providing plasmid pTnMax4 and Annika Pettersson and Jan Tommassen for provision of plasmid pAM23 and for their useful comments. We also acknowledge Henry Wong for his technical assistance in preparing the cloned catΩ cassette with flanking SalI sites.
REFERENCES
- 1.Aebi C, Stone B, Beucher M, Cope L D, Maciver I, Thomas S E, McCracken G H, Jr, Sparling P F, Hansen E J. Expression of the CopB outer membrane protein by Moraxella catarrhalisis regulated by iron and affects iron acquisition from transferrin and lactoferrin. Infect Immun. 1996;64:2024–2030. doi: 10.1128/iai.64.6.2024-2030.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson J A, Sparling P F, Cornelissen C N. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J Bacteriol. 1994;176:3162–3170. doi: 10.1128/jb.176.11.3162-3170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelmelk B J, An Y-Q, Geerts M, Thijs B G, De Boer H A, Maclaren D M, De Graaff J, Nuijens J H. Lactoferrin is a lipid A-binding protein. Infect Immun. 1994;62:2628–2632. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1989. [Google Scholar]
- 5.Bagg A, Neilands J B. Molecular mechanisms of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987;51:509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barcak G J, Chandler M S, Redfield R J, Tomb J-F. Genetic systems in Haemophilus influenzae. Methods Enzymol. 1991;204:321–342. doi: 10.1016/0076-6879(91)04016-h. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. Identification of the bactericial domain of lactoferrin. Biochim Biophys Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- 8.Berish S A, Subbarao S, Chen C-Y, Trees D L, Morse S A. Identification and cloning of a fur homolog from Neisseria gonorrhoeae. Infect Immun. 1993;61:4599–4606. doi: 10.1128/iai.61.11.4599-4606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biswas G D, Sparling P F. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect Immun. 1995;63:2958–2967. doi: 10.1128/iai.63.8.2958-2967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnah R A, Yu R-H, Schryvers A B. Biochemical analysis of lactoferrin receptors in the Neisseriaceae: identification of a second bacterial lactoferrin receptor protein. Microb Pathog. 1995;19:285–297. doi: 10.1016/s0882-4010(96)80002-7. [DOI] [PubMed] [Google Scholar]
- 11.Bonnah R A, Yu R-H, Schryvers A B. Bacterial lactoferrin receptors in the Neisseriaceae. In: Lonnerdol B, Hutchens W, editors. Lactoferrin: interactions and biological functions. Totowa, N.J: Humana Press; 1997. pp. 277–301. [Google Scholar]
- 12.Bonnah R A, Yu R-H, Wong H, Schryvers A B. Biochemical and immunological properties of lactoferrin binding proteins from Moraxella (Branhamella) catarrhalis. Microb Pathog. 1998;24:89–100. doi: 10.1006/mpat.1997.0173. [DOI] [PubMed] [Google Scholar]
- 13.Campagnari A A, Ducey T F, Rebmann C A. Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect Immun. 1996;64:3920–3924. doi: 10.1128/iai.64.9.3920-3924.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornelissen C N, Kelley M, Hobbs M M, Anderson J E, Cannon J G, Cohen M S, Sparling P F. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 15.Crosa J H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Lett. 1989;53:517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellison R T, III, Giehl T J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest. 1991;88:1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellison R T, III, LaForce F M, Giehl T J, Boose D S, Dunn B E. Lactoferrin and transferrin damage of the gram-negative outer membrane is modulated by Ca2+ and Mg2+ J Gen Microbiol. 1990;136:1437–1446. doi: 10.1099/00221287-136-7-1437. [DOI] [PubMed] [Google Scholar]
- 18.Gerlach G F, Anderson C, Potter A A, Klashinsky S, Willson P J. Cloning and expression of a transferrin-binding protein from Actinobacillus pleuropneumoniae. Infect Immun. 1992;60:892–898. doi: 10.1128/iai.60.3.892-898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez G C, Yu R-H, Rosteck P, Schryvers A B. Sequence, genetic analysis, and expression of Actinobacillus pleuropneumoniaetransferrin receptor genes. Microbiology. 1995;141:2405–2416. doi: 10.1099/13500872-141-10-2405. [DOI] [PubMed] [Google Scholar]
- 20.Gray-Owen S D, Loosmore S, Schryvers A B. Identification and characterization of genes encoding the human transferrin-binding proteins from Haemophilus influenzae. Infect Immun. 1995;63:1201–1210. doi: 10.1128/iai.63.4.1201-1210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray-Owen S D, Schryvers A B. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–191. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 22.Haas R, Kahrs A F, Facius D, Allmeier H, Schmitt R, Meyer T F. TnMax—a versatile mini-transposon for the analysis of cloned genes and shuttle mutagenesis. Gene. 1993;130:23–31. doi: 10.1016/0378-1119(93)90342-z. [DOI] [PubMed] [Google Scholar]
- 23.Hawley D K, McClure W R. Compilation and analysis of Escherichia colipromoter DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irwin S W, Averill N, Cheng C Y, Schryvers A B. Preparation and analysis of isogenic mutants in the transferrin receptor protein genes, tbp1 and tbp2, from Neisseria meningitidis. Mol Microbiol. 1993;8:1125–1133. doi: 10.1111/j.1365-2958.1993.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 25.Karkhoff-Schweizer R R, Schryvers A B, Schweizer H P. Cloning and sequence analysis of the fur gene encoding an iron-regulatory protein of Neisseria meningitidis. Gene. 1994;141:139–140. doi: 10.1016/0378-1119(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 26.Khun H H, Kirby S D, Lee B C. A Neisseria meningitidis fbpABCmutant is incapable of using nonheme iron for growth. Infect Immun. 1998;66:2330–2336. doi: 10.1128/iai.66.5.2330-2336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Lee B C, Schryvers A B. Specificity of the lactoferrin and transferrin receptors in Neisseria gonorrhoeae. Mol Microbiol. 1988;2:827–829. doi: 10.1111/j.1365-2958.1988.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 29.Legrain M, Jacobs E, Irwin S W, Schryvers A B, Quentin-Millet M J. Molecular cloning and characterization of Neisseria meningitidisgenes encoding the transferrin binding proteins Tbp1 and Tbp2. Gene. 1993;130:73–80. doi: 10.1016/0378-1119(93)90348-7. [DOI] [PubMed] [Google Scholar]
- 30.Lewis L A, Gray E, Wang Y P, Roe B A, Dyer D W. Molecular characterization of hpuAB, the haemoglobin-haptoglobin-utilization operon of Neisseria meningitidis. Mol Microbiol. 1997;23:737–749. doi: 10.1046/j.1365-2958.1997.2501619.x. [DOI] [PubMed] [Google Scholar]
- 31.Loosmore S M, Yang Y P, Coleman D C, Shortreed J M, England D M, Harkness R E, Chong P S C, Klein M H. Cloning and expression of the Haemophilus influenzaetransferrin receptor genes. Mol Microbiol. 1996;19:575–586. doi: 10.1046/j.1365-2958.1996.406943.x. [DOI] [PubMed] [Google Scholar]
- 32.Nikaido H. Uptake of iron-siderophore complexes across the bacterial outer membrane. Trends Microbiol. 1993;1:5–8. doi: 10.1016/0966-842x(93)90016-k. [DOI] [PubMed] [Google Scholar]
- 33.Ogunnariwo J A, Schryvers A B. Correlation between the ability of Haemophilus paragallinarumto acquire ovotransferrin-bound iron and the expression of ovotransferrin-specific receptors. Avian Dis. 1992;36:655–663. [PubMed] [Google Scholar]
- 34.Ogunnariwo J A, Schryvers A B. Rapid identification and cloning of bacterial transferrin and lactoferrin receptor protein genes. J Bacteriol. 1996;178:7326–7328. doi: 10.1128/jb.178.24.7326-7328.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogunnariwo J A, Woo T K W, Lo R Y C, Gonzalez G C, Schryvers A B. Characterization of the Pasteurella haemolyticatransferrin receptor genes and the recombinant receptor proteins. Microb Pathog. 1997;23:273–284. doi: 10.1006/mpat.1997.0156. [DOI] [PubMed] [Google Scholar]
- 36.Pettersson A, Klarenbeek V, van Deurzen J, Poolman J T, Tommassen J. Molecular characterization of the structural gene for the lactoferrin receptor of the meningococcal strain H44/76. Microb Pathog. 1994;17:395–408. doi: 10.1006/mpat.1994.1085. [DOI] [PubMed] [Google Scholar]
- 37.Pettersson A, Maas A, Tommassen J. Identification of the iroA gene product of Neisseria meningitidisas a lactoferrin receptor. J Bacteriol. 1994;176:1764–1766. doi: 10.1128/jb.176.6.1764-1766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pettersson A, Prinz T, Umar A, vanderBlezen J, Tommassen J. Molecular characterization of LbpB, the second lactoferrin-binding protein of Neisseria meningitidis. Mol Microbiol. 1998;27:599–610. doi: 10.1046/j.1365-2958.1998.00707.x. [DOI] [PubMed] [Google Scholar]
- 39.Pettersson A, Van der Ley P, Poolman J T, Tommassen J. Molecular characterization of the 98-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect Immun. 1993;61:4724–4733. doi: 10.1128/iai.61.11.4724-4733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinn M L, Weyer S J, Lewis L A, Dyer D W, Wagner P M. Insertional inactivation of the gene for the meningococcal lactoferrin binding protein. Microb Pathog. 1994;17:227–237. doi: 10.1006/mpat.1994.1068. [DOI] [PubMed] [Google Scholar]
- 41.Renauld-Mongénie G, Poncet D, Von Olleschik-Elbheim L, Cournez T, Mignon M, Schmidt M A, Quentin-Millet M J. Identification of human transferrin-binding sites within meningococcal transferrin-binding protein B. J Bacteriol. 1997;179:6400–6407. doi: 10.1128/jb.179.20.6400-6407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rokbi B, Mazarin V, Maitre-Wilmotte G, Quentin-Millet M J. Identification of two major families of transferrin receptors among Neisseria meningitidisstrains based on antigenic and genomic features. FEMS Microbiol Lett. 1993;110:51–58. doi: 10.1111/j.1574-6968.1993.tb06294.x. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 44.Saraste M, Sibbald P R, Wittinghofer A. The P-loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990;15:430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- 45.Schryvers A B, Lee B C. Comparative analysis of the transferrin and lactoferrin binding proteins in the family Neisseriaceae. Can J Microbiol. 1989;35:409–415. doi: 10.1139/m89-063. [DOI] [PubMed] [Google Scholar]
- 46.Schryvers A B, Morris L J. Identification and characterization of the transferrin receptor from Neisseria meningitidis. Mol Microbiol. 1988;2:281–288. doi: 10.1111/j.1365-2958.1988.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 47.Schryvers A B, Morris L J. Identification and characterization of the human lactoferrin-binding protein from Neisseria meningitidis. Infect Immun. 1988;56:1144–1149. doi: 10.1128/iai.56.5.1144-1149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schweizer H P. Small broad-host-range gentamycin resistance gene cassettes for site-specific insertion and deletion mutagenesis. BioTechniques. 1993;15:831–833. [PubMed] [Google Scholar]
- 49.Seifert H S, Ajioka R S, Paruchuri D, Heffron F, So M. Shuttle mutagenesis of Neisseria gonorrhoeae: pilin null mutations lower DNA transformation competence. J Bacteriol. 1990;172:40–46. doi: 10.1128/jb.172.1.40-46.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 51.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 52.Vonder Haar R A, Legrain M, Kolbe H V J, Jacobs E. Characterization of a highly structured domain in Tbp2 from Neisseria meningitidisinvolved in binding to human transferrin. J Bacteriol. 1994;176:6207–6213. doi: 10.1128/jb.176.20.6207-6213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu H C, Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]