Abstract
The bacterial Na+(Li+)/H+ antiporter NhaA has been expressed in the yeast Saccharomyces cerevisiae. NhaA was present in both the plasma membrane and internal membranes, and it conferred lithium but not sodium tolerance. In cells containing the yeast Ena1-4 (Na+, Li+) extrusion ATPase, the extra lithium tolerance conferred by NhaA was dependent on a functional vacuolar H+ ATPase and correlated with an increase of lithium in an intracellular pool which exhibited slow efflux of cations. In yeast mutants without (Na+, Li+) ATPase, lithium tolerance conferred by NhaA was not dependent on a functional vacuolar H+ ATPase and correlated with a decrease of intracellular lithium. NhaA was able to confer sodium tolerance and to decrease intracellular sodium accumulation in a double mutant devoid of both plasma membrane (Na+, Li+) ATPase and vacuolar H+ ATPase. These results indicate that the bacterial antiporter NhaA expressed in yeast is functional at both the plasma membrane and the vacuolar membrane. The phenotypes conferred by its expression depend on the functionality of plasma membrane (Na+, Li+) ATPase and vacuolar H+ ATPase.
The homeostatic mechanisms which maintain intracellular ion concentrations within the range compatible with biochemical processes are essential for living cells (14, 36). These mechanisms, composed of membrane transporters and regulatory components, are relatively well understood in bacteria (1) and animal cells (14, 36) but in the case of fungal and plant cells they have only started to be elucidated (31). Sodium plays an important role in animal cells, which are adapted to live with an extracellular salt concentration of approximately 150 mM (14). The Na+/K+ ATPase found in all animals couples the sodium transport out of the cell to potassium influx utilizing ATP as a driving force (19, 34, 36). In addition to controlling several physiological functions (such as osmoregulation, water and salt balance, membrane potential, and K+ homeostasis), this Na+/K+ exchange pump establishes a Na+ gradient across the plasma membrane. The dissipation of the Na+ gradient through secondary transport systems (Na+ symporters and Na+ antiporters) can be used for the uptake of nutrients (sugars and amino acids), the expulsion of metabolic end products, the regulation of internal pH via the Na+/H+ antiporters, or the modulation of Ca2+ homeostasis via Na+/Ca2+ antiporters (34, 36).
In contrast to animals, sodium is not essential for most fungi and plants (21), organisms in which a Na+/K+ ATPase is not present. In these organisms, evolution took another course and a plasma membrane H+ ATPase generates the proton gradient which drives secondary active H+ cotransport (29). With the exception of halophytic species (the native flora of saline soils), which grow and develop optimally at high salt concentrations (21), most plants and fungi cannot tolerate high NaCl concentrations in soils and water (8, 31). The toxic effects of salinity on cells may be mediated by osmotic inhibition of water absorption, specific and nonspecific effects of high Na+ and Cl− concentrations, and nutritional imbalance (8, 31, 43).
One of the major deleterious effects of high salinity is caused by Na+ accumulation in the cytoplasm, where many metabolic activities are sensitive to Na+ inhibition (31). Expression of heterologous sodium efflux transporters could be a useful approach to improve salt tolerance in sensitive organisms such as nonhalophytic fungi and plants. However, the complexities of higher organisms, with several intracellular compartments and, in the case of plants, with interconnected organs, put a note of caution on the anticipated results. As a first step for exploring the capability of this approach to alter ion homeostasis, we have expressed the bacterial cation antiporter NhaA in the yeast Saccharomyces cerevisiae. This combination of recipient cell and sodium transporter offers special advantages for the interpretation of results. NhaA is a well-characterized sodium and lithium extrusion system composed of a single polypeptide which operates as an electrogenic antiporter (2H+ exchanged for each Li+ or Na+) (25). Yeast is a useful model system because (i) it shares basic bioenergetic mechanisms with plant cells (31), (ii) it has been extensively studied as a host of heterologous membrane proteins (32), and (iii) its transport mechanisms at the plasma membrane and vacuolar membrane are relatively well characterized at the molecular level (16, 30).
Many strains of the yeast S. cerevisiae contain a major sodium and lithium extrusion P ATPase encoded by the ENA1-4/PMR2 gene cluster (12, 41). Lithium is a sodium analog with higher toxicity and can be used as a growth inhibitor, at lower concentrations than sodium, to reduce osmotic effects (11, 31). We demonstrate in the present work the capability of the bacterial NhaA secondary transporter to replace the yeast ENA1-4 pump in terms of lithium but not sodium tolerance. In addition, by using mutants deficient in vacuolar H+ ATPase, we provide evidence for the role of this proton pump in the altered ion homeostasis conferred by NhaA.
MATERIALS AND METHODS
Yeast strains and culture conditions.
Standard methods for yeast culture and manipulation were used (9). The Saccharomyces cerevisiae strains used in the present work are described in Table 1. The standard growth medium contained 2% glucose, 0.7% yeast nitrogen base without amino acids (Difco), 50 mM succinic acid adjusted to pH 5.5 with Tris, 50 μg of adenine/ml, 100 μg of tryptophan/ml, 100 μg of histidine/ml, and 100 μg of leucine/ml. Solid medium contained 2% bacteriological-grade agar. For media at pH 7.2, the succinate-Tris buffer was replaced by 50 mM MOPS [3-(N-morpholino)propanesulfonic acid] adjusted to pH 7.2 with Tris. Media were supplemented with NaCl and LiCl as indicated.
TABLE 1.
Yeast strains used
| Strain | Genotype | Source or referencea |
|---|---|---|
| DBY746 | MATα his3-Δ1 leu2-3,112 trp1-289 ura3-52 | YGSC |
| W303-1A | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 tpr1-1 ura3-1 | 40 |
| RH16.6 | DBY746 ena1-4/pmr2::LEU2 | 12 |
| K633 | W303-1A ena1-4/pmr2::HIS3 | 4 |
| RS-1140 | W303-1A ena1-4/pmr2::HIS3 tfp1::LEU2 | |
| RS-1142 | W303-1A ena1-4/pmr2::HIS3 LEU2 | |
| RS-1144 | W303-1A tfp1::LEU2 | |
| RS-1146 | W303-1A LEU2 | |
| RS-997 | DBY746(pRS-699) | |
| RS-1045 | DBY746(pRS-824) | |
| RS-1043 | RH16.6(pRS-699) | |
| RS-1041 | RH16.6(pRS-824) | |
| RS-1175 | RS-1146(pRS-699) | |
| RS-1176 | RS-1146(pRS-824) | |
| RS-1177 | RS-1144(pRS-699) | |
| RS-1178 | RS-1144(pRS-824) | |
| RS-1170 | RS-1142(pRS-699) | |
| RS-1171 | RS-1142(pRS-824) | |
| RS-1179 | RS-1140(pRS-699) | |
| RS-1180 | RS-1140(pRS-824) |
Unless otherwise indicated, the source for strains was this study. YGSC, Yeast Genetic Stock Center, Berkeley, Calif.
Construction of the yeast vacuolar mutants.
Plasmid pPK8, containing a 5-kb XbaI fragment with the tfp1::LEU2 null allele of the catalytic subunit of vacuolar H+ ATPase (13, 33), was a gift of T. H. Stevens, University of Oregon, Eugene. It was digested with XbaI and utilized to transform (15) yeast strain W303-1A and its ena1-4/pmr2::HIS3 derivative K633 (Table 1). To check the vacuolar ATPase phenotype, Leu+ transformants were replicated to plates buffered at either pH 5.5 or 7.2. Disruption of vacuolar ATPase causes conditional lethality at neutral pH (24, 42). Disruption of the TFP1 gene was confirmed by amplification of a 0.53-kb fragment of the gene using as sense primer 5′-GGACTATCAAGTGGCAATTTACTC and as antisense primer 5′-CCCATGACCTTATTACCAACCTC. Transformation was also made with the 2.3-kb XhoI-SalI LEU2 fragment of plasmid YEp13 (3) to generate control strains without leucine auxotrophy.
Construction of expression plasmid pRS-824.
The open reading frame of the bacterial Na+/H+ antiporter gene nhaA was amplified from plasmid pGM36 (17) using as sense primer 5′-GCGCTCGAGATGAAACATCTGCATC and as antisense primer 5′-GCGCTCGAGTCAAACTGATGGACG, containing XhoI sites (underlined) before the start and stop codons, respectively. The GTG start codon of the nhaA gene (37) was changed to ATG to ensure that the yeast cells would recognize methionine as the first encoded amino acid. In order to minimize amplification errors, the reaction included a relatively large amount of plasmid pGM36 (2 μg/ml) and only 15 temperature cycles. The amplified fragment of 1.2 kb (about 20 μg/ml) was extracted with phenol-chloroform, precipitated with ethanol, digested with XhoI, and purified free of residual plasmid by preparative electrophoresis and extraction with GeneClean (Bio 101, La Jolla, Calif.). The purified 1.2-kb XhoI fragment was ligated to the yeast expression plasmid pRS-699 linearized with XhoI. pRS-699 is a yeast multicopy plasmid of 7.05 kb containing the URA3 marker and the strong constitutive promoter of the PMA1 gene before the XhoI cloning site (32). Three recombinant plasmids with the correct orientation (BamHI fragments of 6.5 and 1.75 kb) were tested for lithium tolerance after transformation into yeast strain RH16.6 (see below) and produced similar lithium tolerance. One of them (pRS-824) was selected to transform all the different yeast strains (see Table 1).
Salt tolerance tests.
Cultures were pregrown in solid medium without salt because expression of NhaA was found to decrease viability in stationary phase more in liquid cultures than in solid cultures. After 2 days of growth, one small loop of cells was diluted in water and the absorbance at 660 nm (measured with Spectronic 20D; Milton Roy, Rochester, N.Y.) was adjusted to 1 (2 × 107 cells/ml). After dilutions of 1/4, 1/16, and 1/50 were made, about 1 μl was spotted with an 8 by 6 stainless steel replica plater (Sigma, St. Louis, Mo.) on plates containing the indicated concentrations of salt. The growth of the intermediate dilution (about 103 cells) is shown in the figures.
Sucrose gradient fractionation and Western blot analysis.
Yeast membranes were isolated, fractionated by isopycnic centrifugation in linear sucrose gradients (16 to 49% sucrose [wt/wt]), and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% acrylamide gels) and Western immunoblotting with antibodies against the plasma membrane marker Pma1p as previously described (38). Antibodies against the C terminus of NhaA (6) were utilized to localize the bacterial antiporter in the different fractions. Purification of the NhaA protein from overproducing bacterial cultures was performed as previously described (37). Protein concentration was determined by the method of Bradford (2) with the protein assay reagent from Bio-Rad (Hercules, Calif.).
Measurement of intracellular ion concentrations and efflux of lithium.
Cultures were grown to exponential phase (absorbance at 660 nm, approximately 0.5) with the indicated concentrations of salt, and aliquots of 10 ml were centrifuged (2 min at 2,000 × g) and then washed three times by resuspension and centrifugation with ice-cold 10 mM MgCl2 containing sorbitol at the same osmotic concentration as the salt present in the growth medium. Washed cells were resuspended in 1 ml of 10 mM MgCl2 without sorbitol. After the cell concentration was determined by the absorbance at 660 nm of a 10-fold dilution, intracellular ions were extracted by addition of concentrated HCl to a 0.1 M final concentration and incubation for 10 min at 95°C. After removal of cell debris by centrifugation, potassium, sodium, and lithium concentrations in the supernatant were determined with an atomic absorption spectrometer (Varian) in flame emission mode. Intracellular water (1.4 μl/ml per absorbance unit) was estimated as previously described (5).
For the determination of lithium efflux, cultures were grown to exponential phase (absorbance at 660 nm of about 0.3) with either 2 or 50 mM LiCl, washed twice with 10 mM MgCl2 by vacuum filtration on nitrocellulose circles (Millipore HATF08250), and resuspended in the same volume of fresh medium without LiCl. After incubation for the times indicated in the figures, samples were processed for the determination of intracellular lithium as described above.
RESULTS
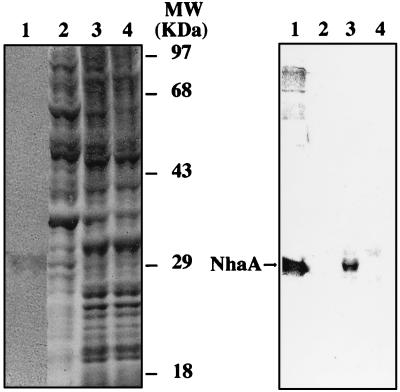
The yeast expression plasmid pRS-699, a multicopy episome with the strong PMA1 promoter (32), was utilized to express the Escherichia coli nhaA gene in the yeast S. cerevisiae. As indicated in Fig. 1, the bacterial NhaA antiporter could be expressed in yeast membranes with an electrophoretic mobility similar to that of the NhaA protein purified from bacteria (about 30 kDa). No NhaA protein was detected in the soluble fraction of the yeast homogenate. We estimate the NhaA protein to represent about 3% of total yeast membrane protein (0.5% of total yeast protein), a level comparable to that obtained with the plant plasma membrane H+ ATPase (38).
FIG. 1.
Immunological evidence for expression of NhaA in yeast membranes. Cellular homogenization, centrifugation to resolve soluble and membrane fractions, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western blot analysis were performed as described in Materials and Methods. Lanes 1, purified NhaA protein (2 μg); lanes 2, soluble fraction from strain RS-1045 (expressing NhaA) (30 μg of protein); lanes 3, membrane fraction from strain RS-1045 (expressing NhaA) (30 μg of protein); lanes 4, membrane fraction from control strain RS-997 (not expressing NhaA) (30 μg of protein). (Left) Coomassie blue-stained gel. (Right) Western immunoblot with antibody against the NhaA protein.
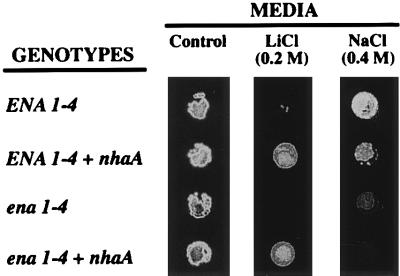
Yeast cells expressing NhaA exhibited no significant growth difference with respect to control cells when tested in normal medium (Fig. 2). However, in medium with a highly toxic LiCl concentration, the expression of NhaA in both ENA1-4 and ena1-4 cells dramatically improved yeast growth (Fig. 2). This suggested that NhaA expressed in yeast was functional as a Li+/H+ antiporter and was apparently more active for lithium extrusion than the endogenous yeast ENA1-4 ATPase. One puzzling result was that the expression of NhaA did not increase sodium tolerance. On the contrary, yeast cells expressing NhaA were slightly more sensitive to NaCl than controls (Fig. 2).
FIG. 2.
Lithium tolerance (but not sodium tolerance) is conferred by expression of NhaA in yeast. Strains RS-997 (ENA1-4), RS-1045 (ENA1-4 nhaA), RS-1043 (ena1-4), and RS-1041 (ena1-4 nhaA) were tested for salt tolerance as described in Materials and Methods. Plates contained either no salt (control) or 0.2 LiCl or 0.4 M NaCl as indicated. Similar results were obtained in three independent experiments.
In order to investigate the mechanisms of salt tolerance conferred by NhaA, we determined the intracellular levels of Na+ and Li+ in cells growing in the presence of these cations (Table 2). The concentration of salt was adjusted to allow for equivalent growth rates with all strains. In medium with NaCl, expression of NhaA produced no significant changes in the intracellular sodium level of ENA1-4 cells (grown with 0.5 M NaCl) and a small decrease (13%) in ena1-4 cells (grown with 0.3 M NaCl). This could explain the absence of a sodium tolerance phenotype upon expression of NhaA. On the other hand, in medium with LiCl, two different responses were observed: in cells with ENA1-4 ATPase, grown with 50 mM LiCl, the intracellular Li+ was increased about threefold upon expression of NhaA, while in ena1-4 cells grown with 2 mM LiCl, the intracellular Li+ was decreased about threefold by NhaA expression. Since in both cases lithium tolerance was improved by NhaA expression (Fig. 2), a simple correlation between tolerance and total intracellular concentrations of toxic cations was unlikely.
TABLE 2.
Potassium, sodium, and lithium contents of yeast cells grown in liquid medium supplemented with different concentrations of Na+ or Li+
| Ion concn in medium | Genotypea | Ion concn in cells (mM)b
|
||
|---|---|---|---|---|
| K+ | Na+ | Li+ | ||
| 0.5 M Na+ | ENA1-4 | 328 ± 25 | 90 ± 9 | |
| ENA1-4 nhaA | 318 ± 22 | 113 ± 13 | ||
| 50 mM Li+ | ENA1-4 | 329 ± 8 | 7.7 ± 0.5 | |
| ENA1-4 nhaA | 268 ± 8 | 24 ± 2 | ||
| 0.3 M Na+ | ena1-4 | 189 + 21 | 402 + 39 | |
| ena1-4 nhaA | 222 ± 19 | 350 ± 15 | ||
| 2 mM Li+ | ena1-4 | 406 ± 42 | 13.0 ± 1.8 | |
| ena1-4 nhaA | 311 ± 22 | 3.9 ± 0.6 | ||
Strains used were RS-997 (ENA1-4), RS-1045 (ENA1-4 nhaA), RS-1043 (ena1-4), and RS-1041 (ena1-4 nhaA).
Results are means ± standard errors of results from at least three independent experiments.
Transmission electron microscopy and selective permeabilization of the yeast plasma membrane have been used to demonstrate that a large fraction of cellular cations are compartmentalized into the vacuole (16, 27). The operation of NhaA as transporter of toxic cations into the vacuole was a plausible explanation for the discrepancy between salt tolerance and total concentration of intracellular cations. Growth inhibition by toxic cations depends on their cytoplasmic concentrations (31) and their accumulation into the vacuole may distort measurements of total intracellular cations.
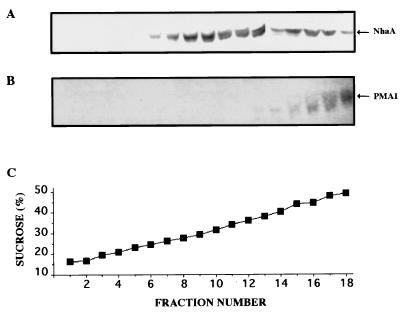
The subcellular distribution of NhaA was investigated by equilibrium sucrose gradient centrifugation (Fig. 3). In these gradients the plasma membrane (identified by the anti-Pma1p immunological marker) equilibrates at the highest density and internal membranes, including the vacuole, equilibrate at intermediate densities (38). A dual localization of NhaA was apparent, with approximately 30% of the protein at the plasma membrane and 70% in internal membranes. These results were compatible with the operation of NhaA at the vacuolar compartment. Unfortunately, the available antibodies against NhaA do not recognize the protein in formaldehyde-fixed cells utilized for immunofluorescence localization (38a).
FIG. 3.
Dual subcellular localization of NhaA expressed in yeast. Membranes from strain RS-1045 (expressing NhaA) were fractionated by equilibrium sucrose gradient centrifugation as described in Materials and Methods. The top of the gradient is fraction 1. Aliquots (15 μl) of the fractions were analyzed by Western blotting with antibodies against either NhaA (A) or the plasma membrane marker Pma1 (B). The sucrose concentration of every fraction is also shown (C).
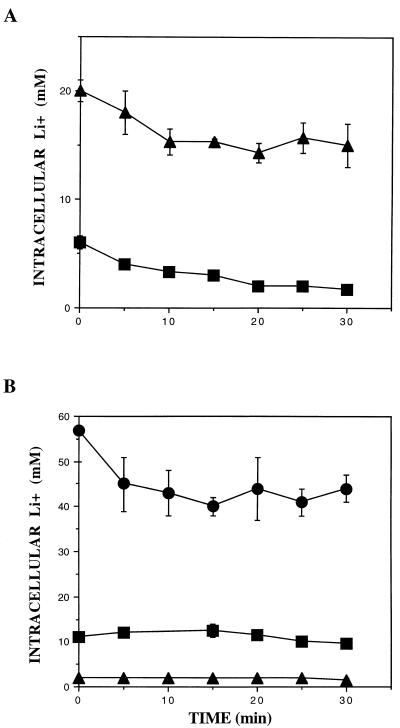
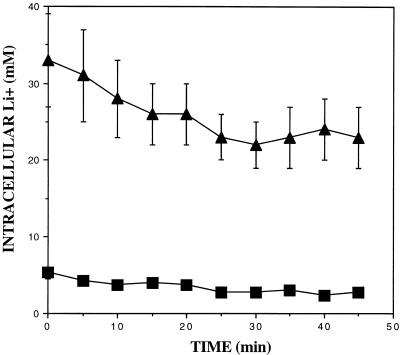
In order to further investigate the activity of NhaA in intracellular compartments, we performed efflux studies using control and NhaA-expressing cells. Efflux kinetics may differentiate between a fast-efflux cytoplasmic pool and a slow-efflux vacuolar compartment (39). As indicated in Fig. 4A, most of the intracellular lithium in control ENA1-4 yeast cells grown with 50 mm LiCl exits from the cells in 20 min. Expression of NhaA resulted in a greater level of intracellular lithium, with most of it constituting a slow-efflux pool. These results are compatible with an NhaA-mediated accumulation of lithium within the yeast vacuole. In ena1-4 cells grown with 2 mM LiCl no lithium efflux was observed and expression of NhaA greatly reduced lithium accumulation (Fig. 4B). After growth with 50 mM LiCl, ena1-4 cells expressing NhaA exhibited two kinetic pools of intracellular lithium; a small fraction effluxed within 20 min but most of the intracellular lithium constituted a slow-efflux pool. Control ena1-4 cells without NhaA cannot grow with 50 mM LiCl.
FIG. 4.
Effect of the expression of NhaA on lithium accumulation and efflux kinetics. (A) Cells from strain RS-1045 (ENA1-4 NhaA) (triangles) and RS-997 (ENA1-4, control) (squares) were grown on medium with 50 mM LiCl and washed, and lithium efflux was measured as described in Materials and Methods. (B) Cells from strain RS-1041 (ena1-4 NhaA) (triangles) and RS-1043 (ena1-4, control) (squares) were grown on medium with 2 mM LiCl and washed, and lithium efflux was measured as described in Materials and Methods. Circles correspond to strain RS-1041 grown on medium with 50 mM LiCl. Values are averages from three experiments and the error bars indicate standard deviations.
The role of the yeast vacuole in cation accumulation mediated by NhaA was investigated by testing the effect of mutations in the vacuolar H+ ATPase. The vacuolar proton pump should be necessary to energize cation transport mediated by a cation/H+ antiporter, (16, 30). As indicated in Fig. 5, a null mutation in the catalytic subunit of the vacuolar H+ ATPase dramatically decreased intracellular accumulation of lithium in NhaA-expressing cells. Therefore, the NhaA antiporter seems to transport lithium into the vacuolar compartment.
FIG. 5.
Effect of a functional vacuolar H+ ATPase on lithium accumulation and efflux kinetics. Cells from strains RS-1178 (ENA1-4 tfp1 nhaA) (squares) and RS-1176 (ENA1-4 TFP1 nhaA) (triangles) were grown on medium with 50 mM LiCl and washed, and lithium efflux was measured as described in Materials and Methods. Values are averages from three experiments and the error bars indicate standard deviations.
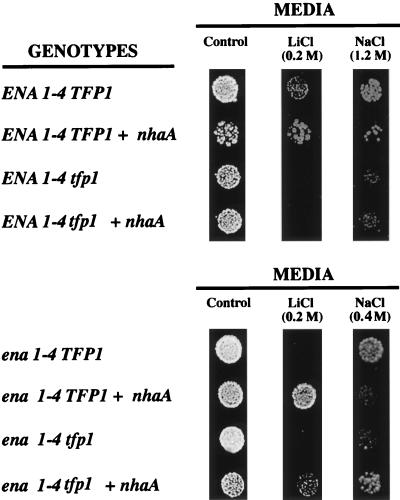
The salt tolerance phenotypes conferred by expression of NhaA were reinvestigated in the mutant without functional vacuolar H+ ATPase. As indicated in Fig. 6, the lithium tolerance conferred by NhaA in ENA1-4 yeast cells was dependent on a functional vacuolar H+ ATPase. Therefore, this tolerance is explained by lithium accumulation at the energized vacuole mediated by the Li+/H+ exchange activity of NhaA. Expression of NhaA produced no sodium tolerance in ENA1-4 yeast cells, independently of the presence of a functional vacuolar H+ ATPase. In the ena1-4 tfp1 double mutant, devoid of both plasma membrane (Na+, Li+) ATPase and vacuolar H+ ATPase, the expression of NhaA produced a clear tolerance to both lithium and sodium (Fig. 6). As described above, in the ena1-4 mutant with functional vacuolar H+ ATPase, NhaA conferred lithium tolerance but sodium sensitivity.
FIG. 6.
Lithium and sodium tolerance in yeast without functional vacuolar H+ ATPase. Strains RS-1175 (ENA1-4 TFP1), RS-1176 (ENA1-4 TFP1 nhaA), RS-1177 (ENA1-4 tfp1), and RS-1178 (ENA1-4 tfp1 nhaA) (top) and strains RS-1170 (ena1-4 TFP1), RS-1171 (ena1-4 TFP1 nhaA), RS-1179 (ena1-4 tfp1), and RS-1180 (ena1-4 tfp1 nhaA) (bottom) were tested for salt tolerance as described in Materials and Methods. Plates contained either no salt (control) or 0.2 M LiCl, 0.4 M NaCl, or 1.2 M NaCl as indicated. Similar results were obtained in three independent experiments.
DISCUSSION
It is remarkable that despite large differences in the mechanisms of membrane biogenesis between bacteria and eukaryotic cells (28), we could achieve the functional expression of an integral membrane protein from E. coli, the (Na+, Li+/H+) antiporter NhaA, in the yeast S. cerevisiae. The E. coli glycerol channel GlpF can partially substitute for the yeast glycerol channel Fps1 (20) but we are not aware of previous reports on the functional expression in yeast of bacterial ion transporters.
The bacterial NhaA antiporter seems to be active at both the plasma membrane and the vacuolar membrane of the recipient yeast cells. Vacuolar membranes have been proposed as the default destination for membrane proteins in yeast (35). In yeast containing a plasma membrane (Na+, Li+) ATPase, the lithium tolerance conferred by expression of NhaA depends on a functional vacuolar H+ ATPase and is correlated with increased lithium accumulation into a compartment of slow efflux. The most plausible explanation for these results is that lithium transport at the plasma membrane mediated by the ENA1-4 ATPase is very active and cannot be improved by expression of NhaA. On the other hand, lithium accumulation at the yeast vacuole (probably mediated by the NHX1 antiporter [23]) is limiting for lithium tolerance, and such accumulation can be improved by a heterologous antiporter such as NhaA.
The lithium tolerance conferred by NhaA in the ena1-4 mutant correlates with reduced levels of intracellular lithium and is still observed in the double mutant ena1-4 tfp1, which is devoid of both plasma membrane (Na+, Li+) ATPase and vacuolar H+ ATPase. These results suggest that NhaA is also active at the yeast plasma membrane and that it can substitute for the (Na+, Li+) ATPase. It has recently been reported that sod2, the plasma membrane Na+/H+ antiporter of the yeast Schizosaccharomyces pombe, can also substitute for the S. cerevisiae (Na+, Li+) ATPase (10). It remains a puzzle why S. cerevisiae has an (Na+, Li+) ATPase as a major cation efflux system while bacteria and other yeasts such as S. pombe employ an antiporter mechanism.
Despite the clear phenotype of lithium tolerance conferred by NhaA, this bacterial antiporter could not increase the sodium tolerance of yeast. This was difficult to explain because sodium transport is limiting for sodium tolerance in S. cerevisiae (31) and the purified NhaA protein can transport both sodium and lithium (25). Interestingly, NhaA conferred sodium tolerance to S. cerevisiae mutants devoid of both plasma membrane (Na+, Li+) ATPase and vacuolar H+ ATPase. Also, in these double mutants we could detect sodium efflux mediated by NhaA (data not shown). Apparently, the activity of NhaA in energized vacuolar membranes is deleterious for the cell in the presence of sodium but not lithium. We have no clues about the mechanisms involved but this phenomenon points to important differences between lithium and sodium homeostasis in the vacuolar compartment. As this compartment includes not only the large vacuole but also the Golgi apparatus and intermediary vesicles, the toxic effects of increased sodium accumulation may occur at specific subcompartments and not necessarily at the large central vacuole. The potassium dependency of some membrane assembly and secretory functions (26) may provide targets for sodium toxicity in the vacuolar compartment.
The sod2 antiporter from Schizosaccharomyces pombe can be functionally expressed in S. cerevisiae and it gives an additive tolerance phenotype to both sodium and lithium (10). One possible explanation for the different results in terms of sodium tolerance obtained with NhaA and sod2 is that the latter antiporter could be confined to the plasma membrane and be absent from the vacuolar compartment, where sodium accumulation may be deleterious. The fact that sod2 is electroneutral (10) while NhaA is electrogenic (25) could also contribute to the observed phenotypic differences.
The manipulation of sodium transport in transgenic fungi and plants could be a useful approach to improve salt tolerance in these organisms (31). However, the present results with the yeast model system and the bacterial NhaA antiporter suggest that a better understanding of intracellular ion homeostasis is needed before this manipulation can produce useful results. In particular, the effect of sodium accumulation in the vacuolar compartment should be further investigated. Transgenic plants with a reduced level of vacuolar H+ ATPase have been generated by antisense methodologies (7), and they could be useful in characterizing the role of the plant vacuole in the phenotypes conferred by heterologous sodium transporters. In addition, strategies for the targeting of heterologous sodium transporters to either the plasma membrane or the vacuolar membrane should be developed. Fusions with vacuolar pyrophosphatase have been employed for the targeting of soluble proteins to the plant vacuolar membrane (18), and this approach could also be applied in the case of heterologous membrane proteins. The observation that the heterologous expression of ion transporters perturbs the yeast secretory pathway (22, 38) adds further complications to the genetic engineering of ion homeostasis in eukaryotic cells.
ACKNOWLEDGMENTS
This work was supported by a grant from the “Conselleria de Agricultura y Medio Ambiente de la Generalitat Valenciana,” Autonomous Government of Valencia, Spain.
We thank T. H. Stevens (University of Oregon, Eugene) for the tfp1::LEU2 disruption casette, Alonso Rodriguez-Navarro (Universidad Politecnica de Madrid, Madrid, Spain) for the RH16.6 strain, G. R. Fink (Whitehead Institute, Cambridge, Mass.) for the K633 strain, and Avelino Corma (Instituto de Tecnologia Quimica, Valencia, Spain) for making available his atomic absorption spectrometer.
REFERENCES
- 1.Bakker E P, editor. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press; 1993. [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quatitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 3.Broach J R, Strathern J N, Hicks J B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979;8:121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- 4.Ferrando A, Kron S J, Rios G, Fink G R, Serrano R. Regulation of cation transport in Saccharomyces cerevisiae by the salt tolerance gene HAL3. Mol Cell Biol. 1995;15:5470–5481. doi: 10.1128/mcb.15.10.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaxiola R, Larrinoa I F, Villalba J M, Serrano R. A novel and conserved salt-induced protein is an important determinant of salt tolerance in yeast. EMBO J. 1992;11:3157–3164. doi: 10.1002/j.1460-2075.1992.tb05392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerchman Y, Olami Y, Rimon A, Taglicht D, Schuldiner S, Padan E. Histidine-226 is part of the pH sensor of NhaA, a Na+/H+ antiporter in Escherichia coli. Proc Natl Acad Sci USA. 1993;90:1212–1216. doi: 10.1073/pnas.90.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gogarten J P, Fichmann J, Braun Y, Morgan L, Styles P, Taiz S L, DeLapp K, Taiz L. The use of antisense mRNA to inhibit the tonoplast H+ ATPase in carrot. Plant Cell. 1992;4:851–864. doi: 10.1105/tpc.4.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenway H, Munns R. Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Physiol. 1980;31:149–190. [Google Scholar]
- 9.Guthrie C, Fink G R. Guide to yeast genetics and molecular biology. New York, N.Y: Academic Press; 1991. [Google Scholar]
- 10.Hahnenberger K M, Jia Z, Young P G. Functional expression of the Schizosaccharomyces pombe Na+/H+ antiporter gene, sod2, in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5031–5036. doi: 10.1073/pnas.93.10.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haro R, Bañuelos M A, Quintero F J, Rubio F, Rodriguez-Navarro A. Genetic basis for sodium exclusion and sodium tolerance in yeast. A model for plants. Physiol Plant. 1993;89:868–874. [Google Scholar]
- 12.Haro R, Garciadeblas B, Rodriguez-Navarro A. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 1991;291:189–191. doi: 10.1016/0014-5793(91)81280-l. [DOI] [PubMed] [Google Scholar]
- 13.Hirata R, Ohsumi Y, Nakano A, Kawasaki H, Suzuki K, Anraku Y. Molecular structure of a gene, VMA1, encoding the catalytic subunit of H(+) translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem. 1990;265:6726–6733. [PubMed] [Google Scholar]
- 14.Hoffman J F, editor. The cellular functions of membrane transport. Englewood Cliffs, N.J: Prentice-Hall; 1964. [Google Scholar]
- 15.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones E W, Webb G C, Hiller M A. Biogenesis and function of the yeast vacuole. In: Pringle J R, Broach J R, Jones E W, editors. The molecular and cellular biology of the yeast Saccharomyces. Cell cycle and cell biology. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 363–470. [Google Scholar]
- 17.Karpel R, Olami Y, Taglicht D, Schuldiner S, Padan E. Sequencing of the gene ant which affects the Na+/H+ antiporter activity in Escherichia coli. J Biol Chem. 1988;263:10408–10414. [PubMed] [Google Scholar]
- 18.Knight H, Trewavas A J, Knight M R. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lingrel J B, Kuntzweiler T. Na+,K+-ATPase. J Biol Chem. 1994;269:19659–19662. [PubMed] [Google Scholar]
- 20.Luyten K, Albertyn J, Skibbe W F, Prior B A, Ramos J, Thevelein J M, Hohmann S. Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J. 1995;14:1360–1371. doi: 10.1002/j.1460-2075.1995.tb07122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marschner H. Mineral nutrition of higher plants. Berlin, Germany: Springer; 1995. [Google Scholar]
- 22.Morsomme P, d’Exaerde A de K, De Meester S, Thines D, Goffeau A, Boutry M. Single point mutations in various domains of a plant plasma membrane H(+)-ATPase expressed in Saccharomyces cerevisiae increase H(+) pumping and permit yeast growth at low pH. EMBO J. 1996;15:5513–5526. [PMC free article] [PubMed] [Google Scholar]
- 23.Nass R, Cunningham K W, Rao R. Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane ATPase. Insights into mechanisms of sodium tolerance. J Biol Chem. 1997;272:26145–26152. doi: 10.1074/jbc.272.42.26145. [DOI] [PubMed] [Google Scholar]
- 24.Nelson H, Nelson N. Disruption of genes encoding subunits of yeast vacuolar H+-ATPases causes conditional lethality. Proc Natl Acad Sci USA. 1990;87:3503–3507. doi: 10.1073/pnas.87.9.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padan E, Schuldiner S. Bacterial Na+/H+ antiporters: molecular biology, biochemistry and physiology. In: Konings W N, Kaback H R, Lolkema J S, editors. Handbook of biological physics. Vol. 2. Amsterdam, The Netherlands: Elsevier Science; 1996. pp. 501–531. [Google Scholar]
- 26.Perier F, Coulter K L, Liang H, Radeke C M, Gaber R F, Vandenberg C A. Identification of a novel mammalian member of the NSF/CDC48p/Pas1p/TBP-1 family through heterologous expression in yeast. FEBS Lett. 1994;351:286–290. doi: 10.1016/0014-5793(94)00879-5. [DOI] [PubMed] [Google Scholar]
- 27.Perkins J, Gadd G M. Accumulation and intracellular compartmentation of lithium ions in Saccharomyces cerevisiae. FEMS Microbiol Lett. 1993;107:255–260. doi: 10.1111/j.1574-6968.1993.tb06039.x. [DOI] [PubMed] [Google Scholar]
- 28.Rapoport T A, Jungnicker B, Kutay U. Protein transport across the eukaryotic endoplasmic reticulum and bacterial inner membranes. Annu Rev Biochem. 1996;65:271–303. doi: 10.1146/annurev.bi.65.070196.001415. [DOI] [PubMed] [Google Scholar]
- 29.Serrano R. Structure and function of plasma membrane ATPase. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:61–94. [Google Scholar]
- 30.Serrano R. Transport across yeast vacuolar and plasma membranes. In: Pringle J R, Broach J R, Jones E W, editors. The molecular and cellular biology of the yeast Saccharomyces. Cell cycle and cell biology. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1991. pp. 523–585. [Google Scholar]
- 31.Serrano R. Salt tolerance in plants and microorganisms: toxicity targets and defense responses. Int Rev Cytol. 1996;165:1–51. doi: 10.1016/s0074-7696(08)62219-6. [DOI] [PubMed] [Google Scholar]
- 32.Serrano R, Villalba J M. Expression and localization of plant membrane proteins in Saccharomyces. Methods Cell Biol. 1995;50:481–496. doi: 10.1016/s0091-679x(08)61052-3. [DOI] [PubMed] [Google Scholar]
- 33.Shih C-K, Wagner R, Feinstein S, Kanik-Ennulat C, Neff N. A dominant trifluoperazine resistance gene from Saccharomyces cerevisiae has homology with F0F1 ATP synthase and confers calcium-sensitive growth. Mol Cell Biol. 1988;8:3094–3103. doi: 10.1128/mcb.8.8.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skou J C. The Na, K pump. Methods Enzymol. 1988;156:1–25. doi: 10.1016/0076-6879(88)56004-4. [DOI] [PubMed] [Google Scholar]
- 35.Stack J H, Horazdovsky B, Emr S D. Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu Rev Cell Dev Biol. 1995;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- 36.Stein W D. Channels, carriers and pumps. New York, N.Y: Academic Press; 1990. [Google Scholar]
- 37.Taglicht D, Padan E, Schuldiner S. Overproduction and purification of a functional Na+/H+ antiporter coded by nhaA (ant) from Escherichia coli. J Biol Chem. 1991;266:11289–11294. [PubMed] [Google Scholar]
- 38.Villalba J M, Palmgren M G, Berberian G E, Ferguson C, Serrano R. Functional expression of plant plasma membrane H+-ATPase in yeast endoplasmic reticulum. J Biol Chem. 1992;267:12341–12349. [PubMed] [Google Scholar]
- 38a.Villalba, J. M., and R. Serrano. Unpublished data.
- 39.Walker N A, Pitman M G. Measurement of fluxes across membranes. In: Lüttge U, Pitman M G, editors. Encyclopedia of plant physiology, new series. 2, part A. Berlin, Germany: Springer Verlag; 1976. pp. 93–117. [Google Scholar]
- 40.Wallis J W, Chrebet G, Brodsky G, Rolfe M, Rothstein R. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell. 1989;58:409–419. doi: 10.1016/0092-8674(89)90855-6. [DOI] [PubMed] [Google Scholar]
- 41.Wieland J, Nitsche A M, Strayle J, Steiner H, Rudolph H K. The PMR2 gene cluster encodes functionally distinct isoforms of a putative Na+ pump in the yeast plasma membrane. EMBO J. 1995;14:3870–3882. doi: 10.1002/j.1460-2075.1995.tb00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashiro C T, Kane P M, Wolczyk D F, Preston R A, Stevens T H. Role of vacuolar acidification in protein sorting and zymogen activation: a genetic analysis of the yeast vacuolar protein-translocating ATPase. Mol Cell Biol. 1990;10:3737–3749. doi: 10.1128/mcb.10.7.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu J-K, Hasegawa P M, Bressan R A. Molecular aspects of osmotic stress in plants. Crit Rev Plant Sci. 1997;16:253–277. [Google Scholar]