Abstract
Pulmonary hypertension (PH) is a serious condition in which there is an abnormally high pressure in the pulmonary arteries that can occur as a complication of connective tissue diseases. Although the relationship between PH and systemic lupus erythematosus or systemic sclerosis has been well-characterized, PH rarely occurs in patients with anti-synthetase syndrome (ASS), and little is known about the pathophysiology and clinical outcome of patients with ASS-PH. We herein report a patient with anti-Jo-1-positive ASS complicated by PH and discuss the treatment strategy through a review of previously reported cases.
Keywords: pulmonary hypertension, anti-synthetase syndrome, anti-Jo-1 antibody, idiopathic inflammatory myopathy, connective tissue diseases
Introduction
Pulmonary hypertension (PH) is a relentless, progressive disease associated with an abnormally high blood pressure in the pulmonary arteries. PH is classified into five groups based on the etiology (1). Group 1 involves pulmonary arterial hypertension (PAH), which is comprised of diverse diseases, such as familial, drug- and toxin-triggered, and autoimmune diseases, and is characterized by aggressive vascular remodeling and a poor prognosis. The remaining four PH groups are secondary to other conditions; Group 3, for example, is associated with chronic lung disease, as represented by interstitial lung disease (ILD).
Recently, PH has been established as a severe complication of connective tissue diseases (CTDs); PH groups 1 and 3 are particularly common (2). Specifically, group 1 PAH is a common disorder, especially in patients with systemic sclerosis (SSc) and systemic lupus erythematosus (SLE), while group 3 PH occurs as a complication in patients with severe CTD-ILD.
Anti-synthetase syndrome (ASS) is a subset of idiopathic inflammatory myopathies (IIMs) that is characterized by specific clinical features, such as ILD, Raynaud's phenomenon, skin involvement, and arthritis, as well as the presence of anti-aminoacyl-tRNA-synthetase (anti-ARS) autoantibodies (3). PH rarely occurs in patients with ASS, and little is known about the pathophysiology and clinical outcomes of patients with ASS-PH.
We herein report a patient with anti-Jo-1-positive ASS complicated by PH and discuss the efficacy of immunosuppressive therapy and vasodilators for ASS-PH based on a literature review.
Case Report
A 53-year-old woman was admitted to our cardiovascular intensive care unit with severe dyspnea and pitting edema of the extremities of 3 months duration. She admitted to having had Raynaud's phenomenon and finger stiffness for three years before admission and a persistent low-grade fever and proximal muscle myalgia one year before hospitalization. She subsequently became aware of bilateral lower limb edema, exertional dyspnea, and muscle weakness.
At the time of admission, a physical examination revealed distended jugular veins, lip cyanosis, and pitting edema in the lower extremities. Chest auscultation revealed a regular heart rhythm with an increased intensity of the second heart sound in the pulmonary area and a systolic murmur in the tricuspid area. Digital ulcers and tenderness in the biceps brachii muscle bilaterally were also noted. Capillaroscopy demonstrated nail fold bleeding and avascular areas under low magnification and giant loop-like irregular capillaries under high magnification. Neither arthritis nor skin rashes were observed.
Laboratory testing was significant for the following: decreased hemoglobin, 8.7 g/dL (normal range, 13.5-17.0 g/dL); elevated uric acid, 9.5 mg/dL (normal range, 2.6-5.5 mg/dL); slightly elevated aldolase, 8.2 U/L (normal range, 2.7-7.5 U/L); elevated C-reactive protein (CRP), 2.98 mg/dL (normal range, <0.3 mg/dL); hypergammaglobulinemia, 2,687 mg/dL (normal range, 861-1,747 mg/dL); elevated brain natriuretic peptide (BNP), 309.8 pg/mL (normal range, 0-18.4 pg/mL); elevated Krebs von den Lungen-6 (KL-6), 3,861 U/mL (normal range, 0-464 U/mL); rheumatoid factor, 134 IU/mL (normal range, 0-15 IU/mL); antinuclear antibody with an ×320 speckled and cytoplasmic pattern; anti-ARS antibody, 169 IU/mL (normal range, 0-24.9 IU/mL); anti-Jo-1 antibody, 208 IU/mL (normal range, 0-17.9 IU/mL); and anti-SSA antibody, >240 IU/mL (normal range, 0-10 IU/mL). Anti-SSB antibody and other autoantibodies associated with IIMs (anti-MDA-5, anti-TIF1-γ, anti-SRP, and anti-Mi-2 antibodies), anti-cyclic citrullinated peptide, anti-neutrophil cytoplasmic, and anti-cardiolipin antibody titers, and lupus anti-coagulant and angiotensin-converting enzyme levels were negative. Creatine kinase, cardiac troponin I, D-dimer, and thyroid-stimulating hormone levels were within normal limits.
There was no evidence of current infections with severe acute respiratory syndrome-coronavirus-2 or human immunodeficiency virus. Blood testing was not suggestive of any underlying protein S, protein C, or antithrombin III deficiency.
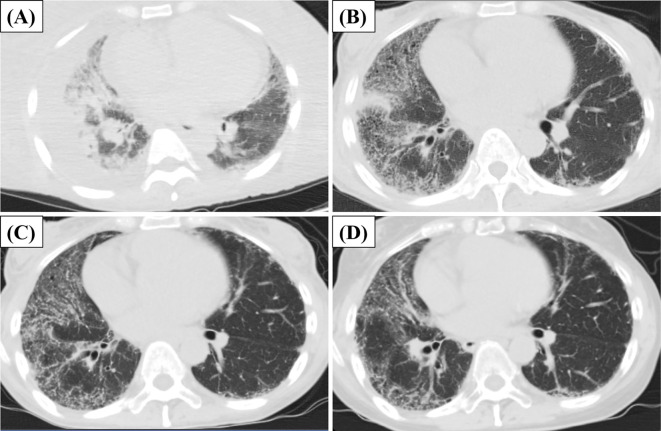
An electrocardiogram showed right-axis deviation and a high P wave amplitude in the inferior leads. An echocardiogram demonstrated enlarged ventricular chambers and dilation of the inferior vena cava. Chest computed tomography (CT) showed bilateral pleural effusion and ground-glass opacities (GGOs) (Figure A). GGOs persisted after the administration of diuretics (Figure B), suggesting concurrent ILD. Right heart catheterization (RHC) revealed an elevated mean pulmonary artery pressure (mPAP=31 mmHg), normal pulmonary arterial wedge pressure (PAWP=6 mmHg), and high pulmonary vascular resistance [PVR=390 dyn s-1 cm-5 (Fick method)], which together supported the diagnosis of precapillary PH (4). Pulmonary perfusion scintigraphy was normal with no ventilation-perfusion mismatch. Pulmonary function tests (PFTs) showed a decrease in the forced vital capacity (%FVC=47%), forced expiratory volume in 1 second (%FEV1=50.8%), and diffusing capacity for carbon monoxide (%DLCO=31.7%), as well as an increase in the %FVC/%DLCO ratio (%FVC/%DLCO=1.48). Simple magnetic resonance imaging (MRI) indicated that the short tau inversion recovery (STIR) had a high signal area in the upper limbs and the trunk, including weakened biceps brachii muscles.
Figure.
Chest computed tomography findings during follow-up. (A) Image at the time of admission. (B) Image on the treatment (immunosuppressive therapy), after the improvement of pulmonary edema by diuretics. (C) Image 1 month after treatment. (D) Image 6 months after treatment.
A muscle biopsy of the left biceps brachii showed inflammatory infiltrates, mainly in the perimysium expressing HLA-DR. Those findings were consistent with ASS (5). Based on these findings, PH associated with anti-Jo-1-positive ASS was diagnosed (3). Although potential overlap with Sjögren's syndrome was considered due to the positive findings for anti-SSA antibodies and hypergammaglobulinemia, she did not meet the classification criteria, as Sicca syndrome was lacking and a Shirmer test was negative.
The patient was started on high-dose glucocorticoids (prednisolone, 1 mg/kg/day) with intravenous cyclophosphamide (IVCY) pulse therapy (500 mg/m2 monthly) promptly after the diagnosis (height, 157 cm; weight, 52.3 kg; body mass index, 21.2 kg/m2). The dyspnea, systemic edema, and digital ulcers gradually improved a few weeks after starting treatment. Repeat chest CT performed one month after the start of immunosuppressive therapy revealed gradual improvement in the GGOs (Figure C). However, RHC performed at the same time revealed no apparent improvement in the pulmonary arterial pressure (mPAP=28 mmHg). PAH was suspected as the cause of the persistent precapillary PH.
Macitentan, a dual endothelin receptor antagonist (ERA), was added at a dose of 10 mg/day for vasodilatory effects. IVCY was administered for a total of six courses, and then tacrolimus was started as maintenance therapy. Six months after the start of immunosuppressive therapy, chest CT revealed remarkable improvement in interstitial pneumonia (Figure D). PFTs showed remarkable improvement in the %FVC, but the %DLCO remained high (%FVC/%DLCO=1.44). The subjective symptoms of dyspnea continued to improve. The right ventricular load, as measured by RHC, also gradually declined but was still high [mPAP=24 mmHg; PAWP=13 mmHg; PVR=193 dyn s-1 cm-5 (Fick method)]. Based on these results, sildenafil, a phosphodiesterase 5 inhibitor (PDE-5i), was started at a dose of 60 mg/day.
The patient is continuing treatment with both immunosuppressive therapy and vasodilators without worsening of PH symptoms, such as dyspnea or edema, or ASS disease activity flare. The temporal changes in RHC and PFTs are summarized in Table 1.
Table 1.
Hemodynamic Parameters in RHC and Results of PFTs and Laboratory Tests.
| On admission | On the treatment | One month after the treatment | Seven months after the treatment | ||
|---|---|---|---|---|---|
| RHC | mPAP (mmHg) | 31 | 28 | 28 | 24 |
| PAWP (mmHg) | 6 | 13 | 9 | 13 | |
| CO (L/min) | 5.89 | 3.93 | 2.99 | 4.55 | |
| PVR (dyn/s/cm5) | 390 | 385 | 508 | 193 | |
| PFTs | %VC | 44.7 | 55 | 72.4 | |
| %FVC | 47 | 57.5 | 73.9 | ||
| %FEV1 | 88.6 | 93.2 | 87.1 | ||
| %DLCO | 31.7 | 48 | 51.3 | ||
| %FVC/%DLCO | 1.48 | 1.20 | 1.44 | ||
| Laboratory | KL-6 (U/mL) | 3,861 | 3,111 | 3,058 | 1,443 |
| tests | BNP (pg/mL) | 310 | 181 | 90 | 21 |
| NYHA classification | IV | III | II | I | |
| 6MWT (m/6min) | 451 | ||||
RHC: right heart catheterization, PFTs: pulmonary function tests, NYHA: New York heart association, 6MWT: 6-minute walk test, mPAP: mean pulmonary artery pressure, PAWP: pulmonary arterial wedge pressure, CO: cardiac output (Fick method), PVR: pulmonary vascular resistance, VC: vital capacity, FVC: forced vital capacity, FEV1: forced expiratory volume in 1 second, DLCO: diffusing capacity for carbon monoxide, KL-6: Krebs von den Lungen-6 , BNP: brain natriuretic peptide
Discussion
PH is reported to be complicated by IIM in approximately 3% of cases (5); ASS, a type of IIM, has a higher frequency (10-20%) (6). PH is a poor prognostic factor for ASS, but the optimal treatment has yet to be established due to an insufficient number of cases and its unclear etiology. Group 1 PAH requires the use of vasodilators, while group 3 PH prioritizes the treatment of lung diseases due to concerns about V/Q mismatch. Different doses of immunosuppressive drugs are recommended in group 1 PAH patients when used with vasodilators, depending on the type of CTD; however, the optimum treatment for ASS has not been established (7).
A limited number of cases of precapillary PH concomitant with ASS have been reported in detail (Table 2). We reviewed reports where precapillary PH was confirmed by RHC or autopsy and where we could also confirm the details of ASS, such as the treatment applied and organ involvement, including ILD. The %FVC and %FVC/%DLCO values were noted in the available cases to estimate the ILD severity and PH involvement. Outcomes of PH and ILD are listed separately in the “outcome after treatment” column along with the measurement used for the evaluation of the treatment response in each case. Although all 10 cases had a history of ILD, in cases 8, 9, and 10, PH occurred without ILD progression or disease activity of ASS. In these cases, the use of vasodilators was effective for managing precapillary PH, suggesting that remodeling of the pulmonary artery itself may have greatly contributed to the pathogenesis.
Table 2.
Published Cases of Anti-synthetase Syndrome-associated Pulmonary Hypertension.
| Age/sex | specific autoantibody | SS-A | PH onset from ASS diagnosis (mon) | Initial mPAP (mmHg) | Initial PAWP (mmHg) | Initial PVR (dyne/s/cm5) | Perfusion scintigraphy | Intercurrent ILD | Other active organ involvement by ASS | Glucocorticoids and immunosuppressants | Vasodilators | Outcome after treatment | Comment | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 69F | Jo-1 | (N/A) | 0 | 50 | 14 | (N/A) | Normal | New onset (PaO2=51 mmHg) | - | mPSL+ AZA | Sildenafil | PH: improved at TTE ILD: outcome N/A | PAH confirmed by vasoreactivity test negative | (10) |
| Case 2 | 56F | Jo-1 | - | 0 | 52 | 16 | 250 | Not performed, but no emboli on CT | Progressive from ASS diagnosis (PFTs: N/A) | - | PSL+ CY | Sildenafil+ treprostinil | PH: progressed clinically (fatal course) | (N/A) | (11) |
| Case 3 | 56F | Jo-1 | + | 60 | 34 | 6 | 552 | (N/A) | stable (%FVC= 59%) | - | PSL+ MMF | Sildenafil+ bosentan | PH: improved at RHC (mPAP: 21 mmHg after 2 years) | %FVC/%DLCO=2.6 | (12) |
| Case 4 | 64F | Jo-1 | - | 48 | 35 | 10 | 464 | (N/A) | Stable (%FVC= 47%) | - | PSL+ MTX | Bosentan | PH: improved at RHC (mPAP: 21 mmHg after 1 year) | %FVC/%DLCO=2.6 | (12) |
| Case 5 | 51F | PL-7 | + | 204 | 37 | 9 | 328 | (N/A) | Stable (%FVC= 66%) | Pericardium | PSL+ HCQ+ COL | Sitaxentan, ambrisentan | PH: stable for 15 months, relapsed after 4 years (fatal course) | %FVC/%DLCO=1.2 | (12) |
| Case 6 | 51F | Jo-1 | - | 32 | 29 | 15 | 388 | (N/A) | Stable (%FVC= 61%) | Muscle | RTX | - | PH: stable at RHC (under follow-up) | (N/A) | (13) |
| Case 7 | 77F | EJ | - | 0 | 33 | 13 | 4.6 Wood Units | (N/A) | New onset (%FVC= 81.4%) | Muscle, skin | PSL+ TAC | - | PH: improved clinically ILD: improved at CT | %FVC/%DLCO=1.3 | (14) |
| Case 8 | 56M | Jo-1 | + | 84 | 25 | 7 | 210 | (N/A) | Stable for 7 years (%FVC= 58%) | - | - | Sildenafil | PH: improved clinically | %FVC/%DLCO=1.9 | (15) |
| Case 9 | 78F | Jo-1 | + | 72 | 25 | 8 | 240 | (N/A) | Stable for 6 years (%FVC= 87%) | - | - | Sildenafil | PH: improved at RHC (mPAP: normalized, details N/A) | %FVC/%DLCO=1.7 | (15) |
| Case 10 | 50F | PL-12 | (N/A) | 108 | 36 | (N/A) | 500 | (N/A) | Stable for 9 years (%FVC= 71%) | - | - | Sildenafil+ bosentan | PH: improved clinically | (N/A) | (13) |
| Present Case | 53F | Jo-1 | + | 0 | 31 | 6 | 390 | Normal | New onset (%FVC= 47%) | Muscle, skin | PSL+ IVCY | Sildenafil+ macitentan | PH: improved with mild PH remaining at RHC ILD: improved at CT | %FVC/%DLCO=1.4 |
F, female, M: male, mPAP: mean pulmonary artery pressure, PAWP: pulmonary arterial wedge pressure, PVR: pulmonary vascular resistance, ILD: interstitial ling disease, RHC: right heart catheterization, VC: vital capacity, DLCO: diffusing capacity for carbon monoxide, PSL: prednisolone, mPSL: methylprednisolone, AZA: azathioprine, (IV)CY: (intravenous) cyclophosphamide, MMF: mycophenolate mofetil, MTX: methotrexate, HCQ: hydroxychloroquine, COL: colchicine, RTX: rituximab, PFTs: pulmonary function tests, (N/A): not available
*: pulmonary hypertension onset from the time of anti-synthetase syndrome diagnosis
In our case, precapillary PH persisted independent of ILD, so PAH was considered to be involved in precapillary PH to some degree. It is challenging to determine to what extent the components of group 1 PAH are involved in the PH that develops in cases with ILD. In contrast, the use of vasodilators should be considered when impairment of the pulmonary function is moderate (%FEV1>60%, and %FVC >70%) (8). Although the therapeutic goal for CTD-PH has been inadequately established, several studies have suggested that strict control of PH may improve the prognosis. A recent retrospective study confirmed the poor prognostic relevance of mPAP ≥20 mmHg in patients with CTD-ILD (9). The advantage of combination therapy with ERA and PDE-5i over each monotherapy for PAH treatment was proven in a meta-analysis (16). Therefore, macitentan and sildenafil were administered in our case.
The effectiveness of immunosuppressive therapy for ASS-PH is also controversial. In cases 6 and 7, precapillary PH and myositis were successfully controlled with immunosuppressive therapy without vasodilators. In contrast, there are cases, such as case 2, in which multidisciplinary therapy with immunosuppressants and vasodilators is ineffective. Autopsy of case 2 confirmed extensive remodeling of the pulmonary arteries without inflammation, so it may be necessary to carefully evaluate the overall disease activity of ASS and determine whether or not to implement immunosuppressive therapy for ASS-precapillary PH.
To achieve a better prognosis in ASS-PH patients, a prompt diagnosis and early intervention are desirable, as well as individualized drug selection.
The absence of arthritis and presence of Raynaud's phenomenon is relevant to the occurrence of precapillary PH in ASS (7). IIM-PAH is reported to have higher frequency of digital ulcers or anti-SSA antibody positivity than IIM without PAH (17). These findings are compatible with our case and may be useful as indicators of ASS-PH and the potential efficacy of vasodilators.
Conclusion
The risk of developing PH should always be taken into consideration with respect to the disease course of ASS. Despite the absence of substantial evidence or guidelines, desirable multidisciplinary intervention against ASS-PH may include the active use of both immunosuppressants and vasodilators. The further accumulation of ASS-PH cases is needed to identify the most appropriate therapeutic strategy.
notes
The patient gave her informed consent in a signed format prior to the report.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J 53: 1801904, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fayed H, Coghlan JG. Pulmonary hypertension associated with connective tissue disease. Semin Respir Crit Care Med 40: 173-183, 2019. [DOI] [PubMed] [Google Scholar]
- 3.Solomon J, Swigris JJ, Brown KK. Myositis-related interstitial lung disease and antisynthetase syndrome. J Bras Pneumol 37: 100-109, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galiè N, Humbert M, Vahiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 46: 903-975, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Aouizerate J, Antonio MD, Bassez G, et al. Myofiber HLA-DR expression is a distinctive biomarker for antisynthetase-associated myopathy. Acta Neuropathol Commun 2: 154, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albrecht K, Huscher D, Callhoff J, et al. Trends in idiopathic inflammatory myopathies: cross-sectional data from the German National Database. Rheumatol Int 40: 1639-1647, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hervier B, Meyer A, Dievial C, et al. Pulmonary hypertension in antisynthetase syndrome: prevalence, aetiology and survival. Eur Respir J 42: 1271-1282, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 53: 1801914, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi K, Taniguchi H, Ando M, et al. Mean pulmonary arterial pressure as a prognostic indicator in connective tissue disease associated with interstitial lung disease: a retrospective cohort study. BMC Pulm Med 16: 55, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erçen Diken Ö, Çiledağ A, Küçükşahin O, Özdemir Kumbasar Ö. Pulmonary arterial hypertension in antisynthetase syndrome without myositis. Tuberk Toraks 61: 170-173, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee S, Farver C. Severe pulmonary hypertension in anti-Jo-1 syndrome. Arthritis Care Res 62: 425-429, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Lecomte R, Perrin F, Journeau L, et al. [Antisynthetase syndrome with pulmonary hypertension: 4 original observations]. Rev Med Interne 36: 794-799, 2015. (in French). [DOI] [PubMed] [Google Scholar]
- 13.García-Fernández A, Quezada-Loaiza CA, Puente-Bujidos C. Antisynthetase syndrome and pulmonary hypertension: report of two cases and review of the literature. Mod Rheumatol Case Rep 5: 152-155, 2021. [DOI] [PubMed] [Google Scholar]
- 14.Okiyama N, Iwasaki R, Fukuzono M, et al. Successful treatment of pulmonary hypertension with immunosuppressive therapy in a case of anti-synthetase syndrome. J Dermatol 48: e545-e546, 2021. [DOI] [PubMed] [Google Scholar]
- 15.Cavagna L, Prisco E, Montecucco C, Caporali R. Pulmonary arterial hypertension in antisynthetase syndrome: comment on the article by Chatterjee and Farver. Arthritis Care Res 63: 633-634, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Kirtania L, Maiti R, Srinivasan A, et al. Effect of combination therapy of endothelin receptor antagonist and phophodiesterase-5 inhibitor on clinical outcome and pulmonary haemodynamics in patients with pulmonary arterial hypertension: a meta-analysis. Clin Drug Investig 39: 1031-1044, 2019. [DOI] [PubMed] [Google Scholar]
- 17.Sanges S, Yelnik CM, Sitbon O, et al. Pulmonary arterial hypertension in idiopathic inflammatory myopathies: data from the French pulmonary hypertension registry and review of the literature. Medicine (Baltimore) 95: e4911, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]