Abstract
We present the case of a 42-year-old woman with rheumatoid arthritis and Sjögren's syndrome treated with adalimumab who developed immune-mediated necrotizing myopathy (IMNM) and trigeminal neuropathy after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccination. Trigeminal neuralgia and elevated serum creatine kinase levels emerged 12 days post-vaccination, followed by myalgia in the femoral muscles. IMNM was histologically diagnosed. The pathogenesis may involve molecular mimicry between the SARS-CoV-2 spike glycoprotein and autologous tissues triggered by vaccination. This case emphasizes the association between SARS-CoV-2 vaccination, tumor necrosis factor inhibitor, IMNM, and trigeminal neuropathy, as well as the importance of monitoring immune-mediated adverse events following SARS-CoV-2 vaccination in patients with autoimmune disease.
Keywords: SARS-CoV-2 mRNA vaccine, immune-mediated necrotizing myopathy, trigeminal neuropathy, rheumatoid arthritis, Sjögren's syndrome
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccine is highly effective in preventing the onset and reducing the severity of the novel coronavirus infection, and vaccination is recommended to prevent the spread of the infection (1,2). However, cases of immune-mediated inflammatory diseases caused by SARS-CoV-2 mRNA vaccine have been reported (3-7).
We herein report a patient with rheumatoid arthritis (RA) complicated by Sjögren's syndrome who developed immune-mediated necrotizing myopathy (IMNM) and trigeminal neuropathy following SARS-CoV-2 mRNA vaccination.
Case Report
A 42-year-old woman had been diagnosed with RA 21 years prior to vaccination for the novel coronavirus. She had been receiving treatment from her previous rheumatologist, which included a subcutaneous injection of 40 mg adalimumab, a human anti-tumor necrosis factor (TNF) monoclonal antibody, every 2 weeks, along with oral administration of 6 mg methotrexate per week for a duration of 11 years. The patient had no significant medical, family, or social history. The patient was a medical assistant. There were no particular changes in her work or living environment or symptoms of infection, such as a fever, malaise, cough and sputum. The patient had also been taking mosapride citrate, rabeprazole, and trimethoprim-sulfamethoxazole for several years. No additional medications, including statins, had been administered in the past.
The patient had received her third BNT162b2 mRNA vaccination 68 days before admission. The previous vaccinations had also been with the BNT162b2 mRNA vaccine, with the first vaccination received seven months before the third vaccination and the second received three weeks after the first. There had been no fever, but pain and swelling had appeared at the injection site; the first vaccination had provoked the most intense reaction, while the third dose had been the mildest. Twelve days after receiving the third vaccination, the patient experienced numbness in her tongue, followed by numbness, paresthesia, and pain on the left side of her face 28 days after vaccination. Her serum creatine kinase (CK) level had been 120 U/L 49 days prior to vaccination; however, at a routine outpatient visit 14 days after vaccination, her serum CK level had increased to 1,137 U/L. Adalimumab and methotrexate were discontinued due to suspected drug-induced myopathy. Pain in the proximal muscles of the upper and lower extremities bilaterally developed 48 days after vaccination. A markedly high serum CK level of 4,092 U/L was observed 67 days after vaccination, leading to a referral to our hospital. Pain in the muscles of her bilateral proximal extremities, indicative of possible inflammatory muscle disease, was observed at our outpatient clinic. Subsequently, the patient was admitted to our department for a further assessment. Following admission, our neurologist diagnosed her facial problems as a left trigeminal neuropathy.
Upon admission, the patient's height was measured at 155.0 cm, and her body weight was 47 kg, indicating a body mass index of 19.6 kg/m2. She was conscious and alert, with a body temperature of 36.3°C, blood pressure of 119/74 mmHg, pulse rate of 86 bpm, and oxygen saturation level of 97%, as measured using a pulse oximeter (ambient). No signs of anemia were observed in the palpebral conjunctiva, and there was no icteric appearance in the ocular conjunctiva. The cervical lymph nodes were not swollen, and the thyroid gland was not palpable. Chest auscultation revealed fine crackles bilaterally in the lower lung fields. The heart sounds were clear, no heart murmurs were detected, her abdomen was soft and flat with no tenderness, and there was no edema in her bilateral legs.
Grasping pain was noted in the upper and lower proximal muscles bilaterally. Manual muscle testing revealed normal strength (grade 5) in both the upper and lower extremities, and Gower's sign was negative. No skin rash was observed. Finger deformities, including a swan neck and buttonhole deformities, were noted; however, no arthritis was observed. The patient reported numbness, paresthesia, and pain on the left side of her face, which was consistent with the second and third branches of the left trigeminal nerve.
The patients' laboratory findings upon admission are presented in Table. Notably, her serum CK, aldolase, myoglobin, lactate dehydrogenase, transaminase, and rheumatoid factor levels were elevated. Antinuclear antibody tests revealed positive results for speckled and NuMA-1 patterns (160×), and EUROLINE Myositis Profile 3 (BML, Tokyo, Japan) was positive for anti-signal recognition particle (SRP) antibodies. Other autoantibody tests, including an enzyme-linked immunosorbent assay and chemiluminescent enzyme immunoassay, revealed positive findings for anti-cyclic citrullinated peptide antibody at 45.8 U/mL and anti-mitochondrial M2 antibody at 10.7 index, while anti-dsDNA, anti-SS-A, anti-SS-B, anti-Scl-70, anti-RNP, anti-ARS, anti-MDA5, anti-TIF1-γ, anti-Mi-2, and anti-HMGCR antibodies were negative. Human leukocyte antigen (HLA) typing revealed the presence of A11, A26, B54, B62, DR4, and DR14.
Table.
Laboratory Findings upon Admission.
| Variable | Reference range | On admission | EUROLINE Myositis Profile 3 | ||
|---|---|---|---|---|---|
| Total protein (g/dL) | 6.5-8.0 | 7.4 | Mi-2 | 1+ | |
| Albumin (g/dL) | 3.9-4.9 | 3.9 | Ku | - | |
| Aspartate aminotransferase (U/L) | 10-37 | 209 | PM-Scl 100 | - | |
| Alanine aminotransferase (U/L) | 5-40 | 131 | PM-Scl 75 | - | |
| Lactate dehydrogenase (U/L) | 107-220 | 804 | SRP | 3+ | |
| Blood urea nitrogen (mg/dL) | 8.0-20.0 | 8.0 | Jo-1 | - | |
| Creatinine (mg/dL) | 0.34-0.79 | 0.63 | PL-7 | - | |
| Creatine kinase (U/L) | 24-195 | 4,852 | PL-12 | - | |
| Aldolase (U/L) | 2.7-7.5 | 79.1 | OJ | - | |
| Myoglobin (ng/mL) | 0-106.0 | 844.9 | EJ | - | |
| Sodium (mEq/L) | 138-147 | 138 | Ro-52 | 3+ | |
| Potassium (mEq/L) | 3.3-4.8 | 3.9 | |||
| C-reactive protein (mg/dL) | <0.25 | <0.1 | |||
| Sialylated carbohydrate antigen KL-6 (U/mL) | <500 | 287 | |||
| Surfactant protein-D (ng/mL) | <110 | 123 | |||
| Immunoglobulin G (mg/dL) | 870-1,700 | 1,497 | |||
| Ferritin (ng/mL) | 3-120 | 9 | |||
| Rheumatoid factor (IU/mL) | 0-15.0 | 36.0 | |||
| White blood cell count (/μL) | 3,250-8,570 | 6,410 | |||
| Neutrophils (%) | 40.3-71.7 | 68.8 | |||
| Lymphocytes (%) | 20.9-50.5 | 23.4 | |||
| Hemoglobin (g/dL) | 11.1-15.5 | 9.7 | |||
| Hematocrit (%) | 35.1-44.4 | 32.4 | |||
| Mean corpuscular volume | 83.6-98.2 | 72.8 | |||
| Mean corpuscular hemoglobin | 27.5-33.2 | 21.8 | |||
| Mean corpuscular hemoglobin concentration | 31.7-35.3 | 29.9 | |||
| Platelet count (/μL) | 148,000-336,000 | 385,000 | |||
| Anti-nuclear antibody titer | <1:40 | 1:160 (speckled and NuMA-1 type) |
|||
| Anti-Scl-70 antibody (U/mL) | <10.0 | <1.0 | |||
| Anti-U1-RNP antibody (INDEX) | <10.0 | <7.0 | |||
| Anti-dsDNA antibody (U/mL) | <10.0 | <1.0 | |||
| Anti-SS-A antibody (U/mL) | <10.0 | <1.0 | |||
| Anti-SS-B antibody (U/mL) | <10.0 | <1.0 | |||
| Anti-ARS antibody (index) | <24.9 | <5.0 | |||
| Anti-MDA5 antibody (index) | <31.0 | <4.0 | |||
| Anti-TIF1-γ antibody (index) | <31.0 | <5.0 | |||
| Anti-Mi-2 antibody (index) | <52.0 | <5.0 | |||
| Anti-mitochondrial M2 antibody (index) | <7.0 | 10.7 | |||
| Anti-cyclic citrullinated peptide antibody (U/mL) | <4.5 | 45.8 | |||
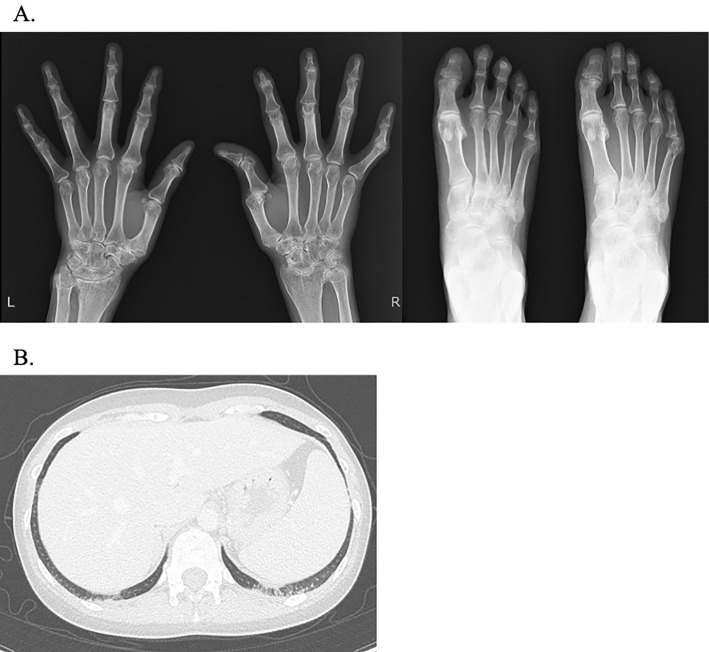
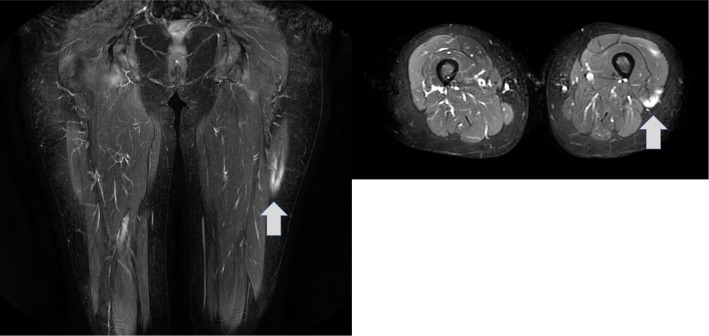
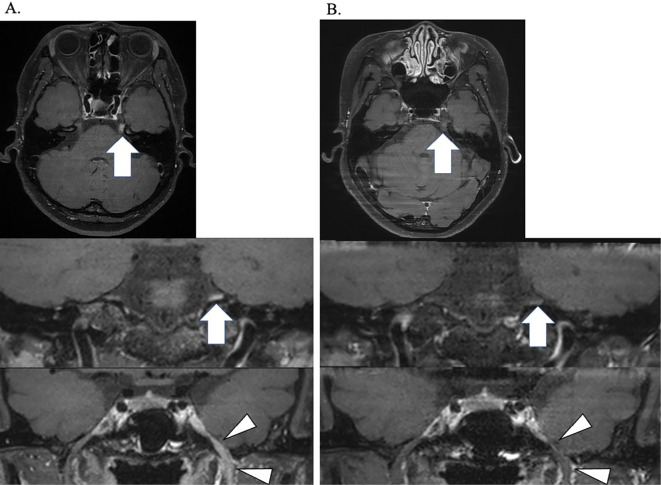
A hand and finger radiograph showed subluxation of the right thumb metacarpal and basal phalanx, along with narrowing of the joint space, particularly in the right carpal bone (Fig. 1A). Contrast-enhanced computed tomography (CT) of the chest and abdomen revealed slight ground-glass opacities in the bilateral dorsal inferior lung fields (Fig. 1B). Plain magnetic resonance imaging (MRI) showed a high-intensity lesion in the left quadriceps muscle on a short tau inversion recovery imaging (Fig. 2). In addition, gadolinium contrast-enhanced MRI of the brain showed an abnormal contrast effect that appeared non-neoplastic in the left trigeminal nerve (Fig. 3A). Furthermore, a cerebrospinal fluid examination revealed no cell counts, a protein level of 20 mg/dL, no evidence of protein-cell dissociation, no oligoclonal bands, no increase in immunoglobulin G index (0.43), and no varicella zoster virus DNA. Based on a positive Schirmer test of 0 mm in 5 minutes, a positive fluorescent dye test, a positive Saxon test (1.1 g in 2 minutes, and findings of moderate hyposalivation on salivary gland scintigraphy, Sjögren's syndrome was diagnosed. Echocardiography showed no abnormal findings, and abdominal echography revealed a 5-mm cystic lesion in the S5 region of the liver.
Figure 1.
A: Hand, finger and toe X-ray findings. B: Chest computed tomography findings.
Figure 2.
Femoral muscle plain magnetic resonance imaging findings. The left rectus femoris muscle displayed a strong signal on short tau inversion recovery imaging (arrow).
Figure 3.
Gadolinium-based contrast-enhanced brain magnetic resonance imaging findings. A: An abnormal signal was evident in the left cisternal trigeminal region (arrow) and the left third branches of the trigeminal region (arrowheads) on T1-weighted imaging before treatment. B: Contrast effects in the trigeminal region decreased after 80 days of treatment.
On day 18 of hospitalization, a muscle biopsy was performed at the site of inflammation in the left quadriceps. Based on positive anti-SRP antibody results, the patient was considered to have IMNM, and prednisolone (PSL) was initiated at 50 mg/day (1 mg/kg/day).
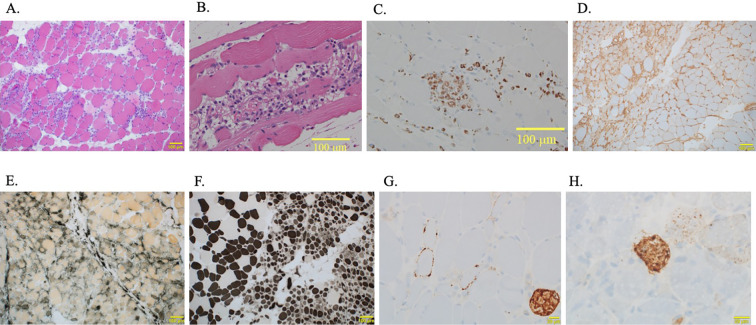
In the muscle pathology image (Fig. 4), Hematoxylin-Eosin staining partially showed myofiber necrosis and inflammatory cell infiltration, including minimal lymphocytic infiltration, in addition to myofiber size irregularities. Furthermore, immunostaining showed the infiltration of CD68-positive cells. Positive staining was observed for major histocompatibility complex (MHC) class I, alkaline phosphatase, myosin ATPase (pH 10.6), membrane attack complex meaning complement deposition, and p62 which is the autophagy marker. Based on these findings, the patient was definitively diagnosed with IMNM.
Figure 4.
Pathological imaging findings of the left femoral muscle. A: Hematoxylin and Eosin (H&E) staining (100×). Unevenly large and small muscle fibers and lymphocytic infiltration were observed. B: H&E staining (200×). Myofiber necrosis and inflammatory cell infiltration were also observed. C: Assessing the CD68 immunostaining revealed CD68-positive cells (200×). D: The sample shows positive staining for major histocompatibility complex class I (100×). E: The sample shows positive staining for alkaline phosphatase (100×). F: The sample shows positive staining for myosin ATPase (pH 10.6) (100×). G: The sample shows positive staining for membrane attack complex (200×). H: The sample shows positive staining for p62 (200×).
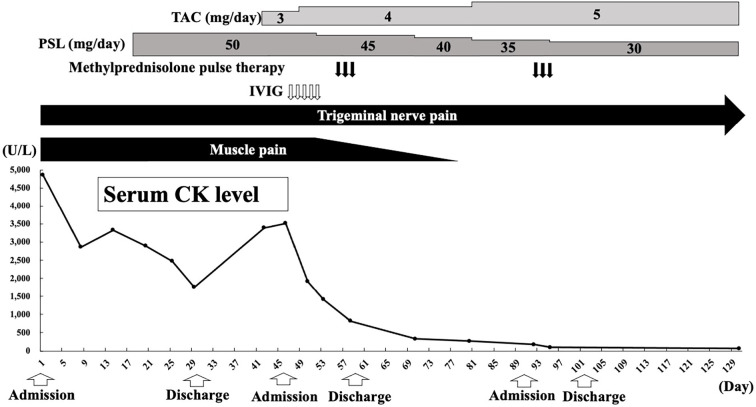
The clinical course of the patient is shown in Fig. 5. Over time, her muscle symptoms became milder, and her serum CK level decreased. Consequently, the PSL dosage was gradually reduced, and the patient was discharged on day 30 of hospitalization. At the outpatient clinic, 12 days after discharge, she was started on 3 mg/day of tacrolimus in combination with PSL owing to the presence of residual muscle symptoms, elevated serum CK levels of 3,393 U/L, and pre-existing interstitial pneumonia. Seventeen days after discharge, intravenous immunoglobulin therapy was administered to the patient to treat steroid-resistant myositis. Concurrently, methylprednisolone pulse therapy was administered to address residual left trigeminal neuropathy starting 25 days after discharge. Subsequently, the tacrolimus dosage was maintained within a blood concentration range of 5-10 ng/mL, whereas the PSL dosage was gradually tapered.
Figure 5.
Clinical course after admission. CK: creatine kinase, IVIG: intravenous immunoglobulin, PSL: prednisolone, TAC: tacrolimus
Despite the successful resolution of muscle symptoms, normalization of serum CK levels after 78 days of treatment, and decreased contrast effect in the trigeminal region on gadolinium contrast-enhanced brain MRI after 80 days of treatment (Fig. 3B), the patient continued to experience persistent left trigeminal neuropathy. Despite carbamazepine and mirogabalin, the neuropathy remained unresolved.
Discussion
We herein report a case of IMNM and trigeminal neuropathy after SARS-CoV-2 mRNA vaccination in a patient with RA complicated by Sjögren's. IMNM is a type of inflammatory myopathy characterized by necrosis of the muscle fibers and is commonly positive for anti-SRP or anti-HMGCR antibodies (8) as well as antimitochondrial antibodies (9,10). In this case, the diagnosis of IMNM was confirmed based on histopathological findings, including weakness of the proximal muscles, grasping pain, high myogenic enzyme levels, positive anti-SRP antibodies, and positive anti-mitochondrial M2 antibodies. Furthermore, concerning trigeminal neuropathy, the patient exhibited not only pain consistent with the left trigeminal nerve area but also abnormal signals in that region, as observed by contrast-enhanced MRI of the head. Although it is challenging to establish a definitive causal relationship between SARS-CoV-2 mRNA vaccination and the development of IMNM and trigeminal neuropathy, findings such as a normal serum CK level 49 days before vaccination and a markedly elevated serum CK level 2 weeks after vaccination, along with findings suggestive of left trigeminal neuropathy, suggest that vaccination may have contributed to the development of these symptoms.
To our knowledge, only seven cases of IMNM after SARS-CoV-2 mRNA vaccination have been reported (11-17). The principal mechanism suggested for the development of autoimmune diseases after SARS-CoV-2 infection is molecular mimicry between the SARS-CoV-2 spike glycoprotein and autologous tissues (18-20). Suh et al. reported that 9 of 35 autopsy cases of COVID-19 (coronavirus disease 2019) presented findings of IMNM, and in all 9 cases, there was increased expression of MHC class I in iliopsoas muscle tissues (21). Aschman et al. also reported that 23 of 43 autopsy cases of COVID-19 showed increased MHC class I expression in the quadriceps and deltoid muscles (22). Although the precise mechanism by which SARS-CoV-2 mRNA vaccine causes autoimmune diseases such as IMNM is unknown, it is speculated that autoimmune diseases may develop after SARS-CoV-2 mRNA vaccination because of molecular mimicry with autologous tissues, similar to the mechanism observed in autoimmune diseases resulting from SARS-CoV-2 infection (23,24).
Several studies have suggested that the human leukocyte antigen (HLA) genotype may affect COVID-19 severity and immune responses to vaccines (25). A recent study showed that the SARS-CoV-2 mRNA vaccine encodes a spike protein with multiple epitopes that bind to HLA alleles and haplotypes significantly associated with autoimmune diseases with molecular mimicry to SARS-CoV-2 mRNA vaccine. However, the link between the HLA genotype and vaccine-induced autoimmune disease development has not been established and needs further validation (26). RA was shown to be associated with HLA-DR4, which was also noted in the present case, but the arthritis did not worsen after vaccination. Previous studies have shown that Sjögren's syndrome was associated with HLA-DR3 (HLA-DRB1*03, HLA-DRB1*03:01) (27), and trigeminal neuropathy was associated with HLA-B15 (HLA-B15*02) (28), but these were absent in the present case. Conversely, IMNM was shown to be associated with not only HLA-DR8 (HLA-DRB1*08:03) (29) but also HLA-DR14 (HLA-DRB1*14:03) (30), which was present in our patient. Membrane attack complex deposition in IMNM was considered to be a direct effect of anti-SRP antibody (31). Although this deposition can also occur in hereditary skeletal muscle diseases, it is a typical finding in IMNM regardless of vaccination status (32) and was reported in a Japanese case of IMNM (33). Our patient had HLA-DR14, which is associated with IMNM. In addition, the abnormal expression of MHC-class II by molecular mimicry may have led to a breakdown of autoimmune tolerance and autoantibody production, anti-SRP antibody production, and complement deposition in the muscle.
In the present case, a TNF inhibitor was prescribed and continued as treatment for RA. To our knowledge, only one case of IMNM during treatment with a TNF inhibitor has been reported (34). TNF inhibitors have been reported to induce autoimmune diseases, such as lupus-like syndrome, through the production of anti-dsDNA antibodies (35) and vasculitis (36). Although the precise mechanism underlying these events is unknown, it has been postulated that TNF inhibitors can modulate the balance of Th1/Th2 cytokine production (37) and potentially increase autoantibody production by inhibiting apoptosis (38). Considering that IMNM is an immune-mediated inflammatory disease, it is plausible that a similar mechanism may contribute to its occurrence.
In addition to IMNM, the patient also developed refractory trigeminal neuropathy. To our knowledge, there have been no reports of simultaneous IMNM and trigeminal neuropathy complications. However, some cases of trigeminal neuropathy have been reported after SARS-CoV-2 mRNA vaccination (39-41). Considering the reports of central nervous system demyelinating disease following SARS-CoV-2 mRNA vaccination (42,43) and TNF inhibitor-induced demyelinating disease (44,45), we considered the possibility of demyelinating trigeminal neuropathy. However, this case was considered atypical for demyelinating disease, as a cerebrospinal fluid examination showed no cell-protein dissociation, and treatments such as glucocorticoids and intravenous immunoglobulin therapy did not prove effective in addressing trigeminal neuropathy.
The present patient was diagnosed with Sjögren's syndrome during hospitalization. As trigeminal neuropathy has been reported to be associated with Sjögren's syndrome (46-48), it was possibly involved in this case. The mechanism underlying neuropathy following SARS-CoV-2 mRNA vaccination remains unclear. However, similar to the development of other autoimmune diseases, molecular mimicry and immune-mediated inflammatory reactions may also play a role (49). Although we did not find any specific reports of Sjögren's syndrome with trigeminal neuropathy after SARS-CoV-2 mRNA vaccination, we did find a case of peripheral neuropathy due to immune activation after SARS-CoV-2 infection in a patient with Sjögren's syndrome (50). Considering this finding, we cannot rule out the possibility that the vaccine-induced immune activation in the present case contributed to the manifestation of trigeminal neuropathy associated with Sjögren's syndrome.
It has been previously suggested that the mechanism underlying IMNM and trigeminal neuropathy after SARS-CoV-2 mRNA vaccination involves the induction of autoimmunity through molecular mimicry with the SARS-CoV-2 spike glycoprotein. In this context, whether or not the present patient had pre-existing Sjögren's syndrome or anti-SRP and anti-mitochondrial M2 antibodies prior to vaccination is unclear. In addition, the TNF inhibitors used for RA may have played a role as inducing factors in the development of this autoimmune disease.
In May 2023, the World Health Organization declared the end of the state of emergency previously instated for the new coronavirus infections. However, it is important to maintain vigilance and continue to promote vaccination. While the occurrence of multiple autoimmune diseases following vaccination, as observed in this case, is rare, caution should be exercised when administering vaccines to individuals with a history of autoimmune disease. Furthermore, considering that mRNA vaccines are currently the mainstream SARS-CoV-2 vaccines, it is crucial to gather more cases and investigate the mechanisms underlying autoimmune disease development following mRNA vaccination.
notes
Written informed consent for the publication of this case report and associated research was obtained from the patient with approval from the Ethics Committee of Saitama Medical University (Approval No. 2021-142).
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We express our gratitude to Dr. Keisuke Ishizawa from the Division of Diagnostic Pathology, Saitama Medical University Hospital, and Dr. Ichizo Nishino from the Department of Neuromuscular Research, National Institute of Neuroscience, National Center of Neurology and Psychiatry (NCNP) for their valuable contributions in providing pathological diagnoses.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383: 2603-2615, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384: 403-416, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 326: 1390-1399, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velikova T, Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int 41: 509-518, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 80: 1330-1338, 2021. [DOI] [PubMed] [Google Scholar]
- 6.Furer V, Eviatar T, Zisman D, et al. Predictors of immunogenic response to the BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases treated with rituximab. Vaccines (Basel) 10: 901, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda M, Funakubo Asanuma YF, Yokota K, et al. New-onset adult-onset Still's disease following COVID-19 vaccination: three case reports and a literature review. Intern Med 62: 299-305, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allenbach Y, Benveniste O, Stenzel W, Boyer O. Immune-mediated necrotizing myopathy: clinical features and pathogenesis. Nat Rev Rheumatol 16: 689-701, 2020. [DOI] [PubMed] [Google Scholar]
- 9.Albayda J, Khan A, Casciola-Rosen L, Corse AM, Paik JJ, Christopher-Stine L. Inflammatory myopathy associated with anti-mitochondrial antibodies: a distinct phenotype with cardiac involvement. Semin Arthritis Rheum 47: 552-556, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Yang H, Lei J, et al. Muscle pathological features and extra-muscle involvement in idiopathic inflammatory myopathies with anti-mitochondrial antibody. Semin Arthritis Rheum 51: 741-748, 2021. [DOI] [PubMed] [Google Scholar]
- 11.Li JC, Siglin J, Marshall MS, Stemmer-Rachamimov A, Bloom SM, Blumenthal KG. Successful treatment of delayed localized necrotizing inflammatory myositis after severe acute respiratory syndrome coronavirus 2 mRNA-1273 vaccine: a case report. Open Forum Infect Dis 9: ofac499, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CH, Gupta R, Setyono D, Cuevas-Ocampo AK, Khoshnoodi MA. Refractory seronegative immune-mediated necrotizing myopathy after receiving mRNA-1273 SARS-CoV-2 vaccine: a case report. J Clin Neuromuscul Dis 24: 168-169, 2023. [DOI] [PubMed] [Google Scholar]
- 13.Dodig D, Fritzler MJ, Naraghi A, Tarnopolsky MA, Lu JQ. Immune-mediated necrotizing myopathy after BNT162b2 vaccination in a patient with antibodies against receptor-binding domain of SARS-CoV-2 and signal recognition particle. Muscle Nerve 65: E11-E13, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durucan I, Guner S, Kilickiran Avci BK, Unverengil G, Melikoglu M, Ugurlu S. Post-COVID-19 vaccination inflammatory syndrome: a case report. Mod Rheumatol Case Rep 7: 280-282, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaise M, Rocher F, Spittler H, et al. Severe necrotizing myopathy after COVID-19 vaccine with BNT162b2 and regimen with ipilimumab plus nivolumab in a patient with advanced melanoma. J Eur Acad Dermatol Venereol 36: e100-e102, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanemoto M, Oda R, Toyama Y, et al. Anti-signal recognition particle antibody-positive immune-mediated myopathy after mRNA-1273 SARS-CoV-2 vaccination. Intern Med 61: 3605-3609, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mufti Z, Dietz N, Pearson L, et al. Immune-mediated necrotizing myopathy with concurrent statin use after routine COVID-19 inoculation: a case report. Cureus 15: e37876, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanduc D, Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol Res 68: 310-313, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons-Weiler J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J Transl Autoimmun 3: 100051, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol 11: 617089, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suh J, Mukerji SS, Collens SI, et al. Skeletal muscle and peripheral nerve histopathology in COVID-19. Neurology 97: e849-e858, 2021. [DOI] [PubMed] [Google Scholar]
- 22.Aschman T, Schneider J, Greuel S, et al. Association between SARS-CoV-2 infection and immune-mediated myopathy in patients who have died. JAMA Neurol 78: 948-960, 2021. [DOI] [PubMed] [Google Scholar]
- 23.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin Immunol 224: 108665, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saud A, Naveen R, Aggarwal R, Gupta L. COVID-19 and myositis: what we know so far. Curr Rheumatol Rep 23: 63, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin F, Lin X, Fu B, Xiong Y, Zaky MY, Wu H. Functional studies of HLA and its role in SARS-CoV-2: stimulating T cell response and vaccine development. Life Sci 315: 121374, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talotta R. Molecular mimicry and HLA polymorphisms may drive autoimmunity in recipients of the BNT-162b2 mRNA vaccine: a computational analysis. Microorganisms 11: 1686, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz-Tapias P, Rojas-Villarraga A, Maier-Moore S, Anaya JM. HLA and Sjögren's syndrome susceptibility. A meta-analysis of worldwide studies. Autoimmun Rev 11: 281-287, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Saepoo J, Pangsomboon K, Tianviwat S. Awareness of HLA-B* 15:02 screening in trigeminal neuralgia and the gene screening policy among dentists in Southern Thailand. Spec Care Dentist 43: 286-293, 2023. [DOI] [PubMed] [Google Scholar]
- 29.Ohnuki Y, Suzuki S, Uruha A, et al. Association of immune-mediated necrotizing myopathy with HLA polymorphisms. HLA 101: 449-457, 2023. [DOI] [PubMed] [Google Scholar]
- 30.Kang EH, Go DJ, Mimori T, et al. Novel susceptibility alleles in HLA region for myositis and myositis specific autoantibodies in Korean patients. Semin Arthritis Rheum 49: 283-287, 2019. [DOI] [PubMed] [Google Scholar]
- 31.Bergua C, Chiavelli H, Allenbach Y, et al. In vivo pathogenicity of IgG from patients with anti-SRP or anti-HMGCR autoantibodies in immune-mediated necrotising myopathy. Ann Rheum Dis 78: 131-139, 2019. [DOI] [PubMed] [Google Scholar]
- 32.Allenbach Y, Benveniste O, Stenzel W, Boyer O. Immune-mediated necrotizing myopathy: clinical features and pathogenesis. Nat Rev Rheumatol 16: 689-701, 2020. [DOI] [PubMed] [Google Scholar]
- 33.Shimada T, Higashida-Konishi M, Akiyama M, et al. Immune-mediated necrotizing myopathy which showed deposition of C5b-9 in the necrotic muscle fibers and was successfully treated with intensive combined therapy with high-dose glucocorticoids, tacrolimus, and intravenous immunoglobulins. Immunol Med 45: 175-179, 2022. [DOI] [PubMed] [Google Scholar]
- 34.Chavarría-Miranda A, Hernández Lain A, Toldos González O, Pedraza Hueso MI. Immune-mediated necrotising myopathy after treatment with adalimumab in a patient with HLA-B27 ankylosing spondylitis. Neurologia (Engl Ed) 36: 631-632, 2021. [DOI] [PubMed] [Google Scholar]
- 35.Eriksson C, Engstrand S, Sundqvist KG, Rantapää-Dahlqvist S. Autoantibody formation in patients with rheumatoid arthritis treated with anti-TNF alpha. Ann Rheum Dis 64: 403-407, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramos-Casals M, Brito-Zerón P, Muñoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 86: 242-251, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Singh VK, Mehrotra S, Agarwal SS. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol Res 20: 147-161, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Lorenz HM, Herrmann M, Winkler T, Gaipl U, Kalden JR. Role of apoptosis in autoimmunity. Apoptosis 5: 443-449, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Kaya A, Kaya SY. A case of trigeminal neuralgia developing after a COVID-19 vaccination. J Neurovirol 28: 181-182, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onoda K, Sashida R, Fujiwara R, et al. Trigeminal neuropathy after tozinameran vaccination against COVID-19 in postmicrovascular decompression for trigeminal neuralgia: illustrative case. J Neurosurg Case Lessons 3: CASE22101, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S, Hor JY, Koh KL, Chia YK. Central nervous system demyelination following COVID-19 mRNA-based vaccination: two case reports and literature review. J Cent Nerv Syst Dis 14: 11795735221102747, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khayat-Khoei M, Bhattacharyya S, Katz J, et al. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J Neurol 269: 1093-1106, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Havla J, Schultz Y, Zimmermann H, Hohlfeld R, Danek A, Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol 269: 55-58, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohan N, Edwards ET, Cupps TR, et al. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum 44: 2862-2869, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Kumar N, Abboud H. Iatrogenic CNS demyelination in the era of modern biologics. Mult Scler 25: 1079-1085, 2019. [DOI] [PubMed] [Google Scholar]
- 46.Gono T, Kawaguchi Y, Katsumata Y, et al. Clinical manifestations of neurological involvement in primary Sjögren's syndrome. Clin Rheumatol 30: 485-490, 2011. [DOI] [PubMed] [Google Scholar]
- 47.Horai Y, Nishino A, Nakashima Y, et al. Case of Sjögren's syndrome presenting as trigeminal nerve palsy. Nihon Rinsho Meneki Gakkai Kaishi 35: 199-202, 2012. [DOI] [PubMed] [Google Scholar]
- 48.Farran MZ, Kesserwani H. A case of Sjögren's syndrome associated with trigeminal neuropathy and enhancement of the mandibular nerve at the foramen ovale: a case report and a review of the differential diagnosis and mechanisms of the disease. Cureus 13: e19463, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Narasimhalu K, Lee WC, Salkade PR, De Silva DA. Trigeminal and cervical radiculitis after tozinameran vaccination against COVID-19. BMJ Case Rep 14: e242344, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panagiotides NG, Zimprich F, Machold K, et al. A case of autoimmune small fiber neuropathy as possible post COVID sequelae. Int J Environ Res Public Health 20: 4918, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]