Abstract
The purpose of this study was to compare the reconstructive outcomes of soft‐tissue defects around foot and ankle with vaccum sealing drainage (VSD) or induction membrane (IM) of cement formation and attempt to provide an optimal strategy for elderly patients. A retrospective review of all continuous patients with foot and ankle reconstruction using different flaps from October of 2016 and October of 2020 was performed. Based on the different way, the patients were divided into two groups: VSD group (n = 26) and IM group (n = 27). Outcomes were assessed according to the size of the defect, frequency of debridement procedures, hospitalization time, duration of healing, the healing rate, major amputation rate, functional outcomes and complications. Immunohistochemistry (IHC) detection of vascular endothelial growth factor (VEGF) was also be completed. We found that there was no difference in demographic characteristics, size of the defect, debridement times and functional outcomes between the two groups (p > 0.05); however, a significant difference in the wound healing time, hospitalization time and complications were noted between them(p < 0.05). The fresh granulation tissue of both groups showed abundant positive expression of VEGF. Thus, the VSD and IM are both available for foot and ankle reconstruction in elderly patients. However, the IM group offers short hospitalization time, duration of healing and lower frequency of postoperative complications. Thus, we advocate the IM for reconstruction of defects of the foot and ankle region in the elderly patients.
Keywords: foot and ankle, induction membrane, soft‐tissue defects, VSD
1. INTRODUCTION
Foot and ankle soft‐tissue defects frequently occur due to road traffic accidents, gunshots, bomb blast injuries, and infections that may expose the bone, tendon, and neurovascular structures. 1 These cases commonly constitute a real challenge to reconstructive surgeons because of restricted local soft‐tissue availability. 2 There are many techniques such as vaccum sealing drainage (VSD), 3 local flaps, 4 cross‐leg flaps, 5 and free flaps 4 to reconstruct such defects on the distal region of the leg and foot. Free tissue transfer allows the greatest surface area coverage and the most freedom in terms of flap placement, but it requires longer, more technically demanding surgery. 6 , 7 VSD has been widely used in acute and chronic complex wounds, and has achieved acceptable results, but there are the disadvantages of long hospital stay and high cost. 8 , 9 Recently, the induction membrane (IM) of cement formation in the treatment of complex wounds has been reported. 10 It is a two‐stage strategy for wound management, comprising initial coverage of a fresh wound with PMMA, followed by grafting with an autologous split‐thickness skin graft under the IM. The purpose of this study is to compare VSD and IM in the treatment of foot and ankle soft‐tissue defects.
2. PATIENTS AND METHODS
2.1. Study design
This study was conducted in a tertiary hospital, approved by the Human Research Ethics Committee of our affiliated university hospital, and adhered to the principles of the Declaration of Helsinki for medical research involving humans. The pictures and information of the patients involved in this article have been approved by patients in writing to publish these cases. The retrospective review was undertaken of all patients who presented with foot and ankle soft‐tissue defect treated with VSD or IM between October of 2016 and October of 2020.
The inclusion criteria were patients who had a unilateral foot or ankle injury with soft‐tissue defect and who had undergone VSD or IM with a follow‐up of no less than 1 year. All patients' general condition may be too poor to tolerate prolonged free flap surgery, and a donor site may not be available for an effective flap. A total of eight patients (three patients in VSD group and five patients IM in the group) were lost to follow‐up because of various reasons. As a result, 26 patients were in the VSD group; 27 patients were in the IM group. All procedures were performed in a single institution by the same team.
2.2. Surgical strategy
2.2.1. VSD group
General, spinal, or regional anaesthesia was given to the patients depending on the anesthesiologist, and a tourniquet was required. Surgical debridement is performed in all patients, in addition to the removal of devitalised tissues. For wounds without periosteal coverage, the bony or tendon surface is lightly abraded. The edges of debridement were reached until the soft tissue and bone appeared macroscopically normal. The negative pressure wound therapy system (VSD Medical Science and Technology Co. Ltd., Wuhan, China) was applied to cover the wound. With medicalgrade polyurethane foam, this system sealed the wound with adhesive drapes and created a closed microenvironment by the connector tube connected to a wall‐mounted suction device. Patients underwent serial debridement at weekly intervals until there were no clinical signs and symptoms of infection. At that time, fresh granulation tissue will coat the exposed tendon or bone. A piece of fresh tissue is removed for laboratory testing of immunohistochemistry (IHC) detection of vascular endothelial growth factor (VEGF). Then, the wound was closed with an autologous split‐thickness piece of skin. A typical case is shown in Figure 1.
FIGURE 1.
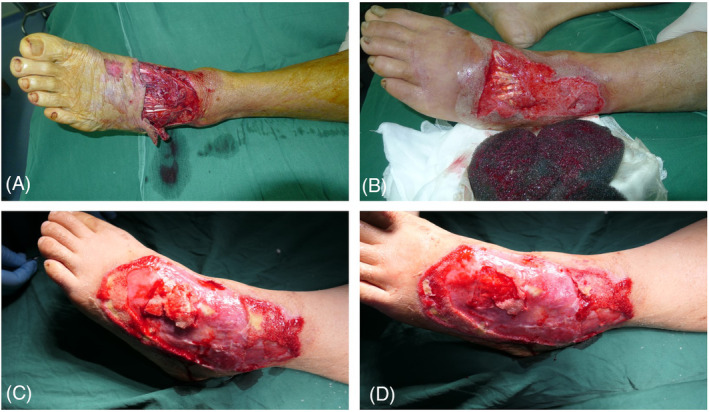
Case 1. A 65‐year‐old male with a machine‐related crush injury. (A) Soft‐tissue defect in the dorsum of foot exposing the tendon; (B) After a thorough debridement, the wound covered with VSD. After removal of VSD, fresh granulation tissue was seen. (C, D) After three times of VSD covering, a large amount of granulation tissue can be seen covering the wound, and survival can be achieved after skin grafting alone.
2.2.2. IM group
After aggressive debridement, the freshly treated wound is covered with PMMA bone cement (Zimmer, USA) loaded with antibiotics (vancomycin), the dose is 40:4 g (PMMA: vancomycin). The cement is anchored tightly before it solidifies by stapling along the wound edges. After 4–6 weeks, the PMMA cement is removed and the wound base lightly abraded to determine the adequacy of revascularisation; light blood seepage is usually observed in a mature wound bed. If the wound remains infected or necrotic, more debridement will be performed. Fresh granulation tissue will satisfactorily receive a split‐thickness skin graft to cover the wound above the IM. A 1 × 1 cm piece of IM is removed for laboratory testing of immunohistochemistry (IHC) detection of VEGF. The surgical area is closed using pressure. A well‐padded plaster or fibreglass splint or cast can provide adequate immobilization following lower extremity wound grafting. A typical case is shown in Figure 2.
FIGURE 2.
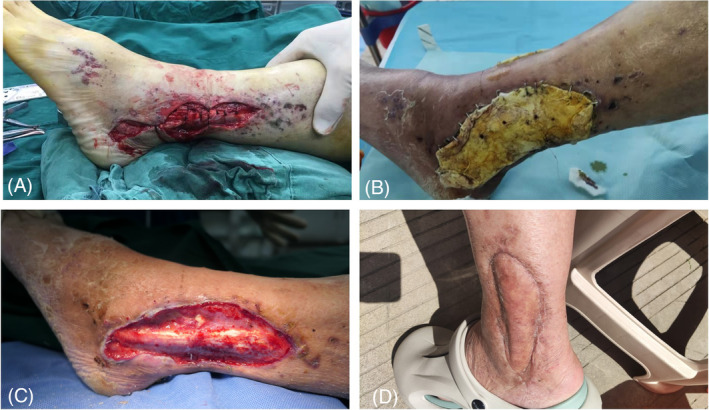
Case 2. A 72‐year‐old male with a crush injury from a heavy object. (A) Skin contusion of the left ankle with exposed tendon at the lateral malleolus; (B) After thorough debridement, the wound with exposed bone was covered with polymethyl methacrylate cement. (C) Removal of the bone cement clearly revealing the induced membrane. (D) Proper ankle healing and patient satisfaction with the outcome.
The antibiotic therapy was first managed empirically and then modified according to the results of an antibiogram from the intraoperative culture. The patient can then be discharged after wound covering with PMMA. All patients were regularly tracked by our multidisciplinary team more than 12 months.
2.3. Evaluation of outcomes
In both groups, we recorded patients' characteristics, that is, age, gender, aetiology, active use of tobacco, alcohol abuse, size of the defect, frequency of debridement procedures, hospitalization time, duration of healing, the healing rate, major amputation rate. Healing was determined to be complete epithelialization of the surgical wound. Nonhealing was defined as no significant reduction in the wound size or no significant decrease or worsening of secretions, with no need or refusal for major amputation. Major amputation (above the ankle) resulting from a life‐threatening limb during the treatment period was considered to be failure of limb salvage. The duration of healing mean the number of weeks from the initial surgical intervention to the date of complete healing. We also assessed functional outcomes in the final follow‐up by Kofoed ankle scores 11 ranging from 0 to 100 with sub‐scores for pain (0–50), function (0–30), and mobility (0–20) (Kofoed score 85–100 as excellent, 75–85 as good, 70–74 as moderate, and <70 as poor). Patients were also monitored for the following complications: skin graft necrosis, infections, blisters, chronic ulcers, deep venous thrombosis (DVT), pneumonia. DVT was determined by postoperative B‐ultrasonography of lower extremity vessels.
2.4. Statistical analysis
The data are presented as the mean ± standard error of the mean. Data were analysed using Student's t unpaired test and the chi‐squared test. p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 18.0 software.
3. RESULTS
3.1. Demographic and perioperative data
The 53 patients included in this study were followed for at least 12 months (range: 12–20 months). Average age for patients in VSD group was 63.73 ± 7.53 years and in IM group was 61.37 ± 7.35 years. There were 20 males and 6 females in VSD group and 19 males and 8 females in IM group. There were 15 smokers, 17 diabetics and 17 alcohol abusers in the VSD group. While, there are 17 smokers, 20 diabetics and 18 alcohol abusers in the IM group. About the vascular disease of lower extremity, there were 16 patients in VSD group and 17 patients in IM group. The most common cause of injury was car accident in both groups (19/26 vs. 20/27). There were no significant differences between the two groups in demographic characteristics such as age, sex, smoking, diabetes, alcohol abuse, vascular disease of lower extremity, injury mechanism (p > 0.05, Table 1).
TABLE 1.
Comparison of the characteristics of the two groups.
| Variable | VSD group (n = 26) | IM group (n = 27) | T or X 2 | p value |
|---|---|---|---|---|
| Age (mean ± SD, years) | 63.73 ± 7.53 | 61.37 ± 7.35 | T = 1.15 | p = 0.254 |
| Sex | X 2 = 0.29 | p = 0.59 | ||
| Male | 20 | 19 | ||
| Female | 6 | 8 | ||
| Smoking | X 2 = 0.15 | p = 0.70 | ||
| Yes | 15 | 17 | ||
| No | 11 | 10 | ||
| Diabetes | X 2 = 0.58 | p = 0.49 | ||
| Yes | 17 | 20 | ||
| No | 9 | 7 | ||
| Alcohol abuse | X 2 = 0.01 | p = 0.92 | ||
| Yes | 17 | 18 | ||
| No | 9 | 9 | ||
| Vascular disease of lower extremity | X 2 = 0.01 | p = 0.92 | ||
| Yes | 16 | 17 | ||
| No | 10 | 10 | ||
| Cause of injury | X 2 = 0.43 | p = 0.81 | ||
| Car accident | 19 | 20 | ||
| Crash injury | 5 | 6 | ||
| Other case | 2 | 1 |
The perioperative data are shown in Table 2. All patients underwent thorough debridement and two different interventions covered the wound. Size of the defect of VSD group was 43.54 ± 4.62 cm2 and it was 41.52 ± 5.62 cm2 in IM group. In VSD group, the frequency of debridement was 2.31 ± 0.79. In IM group, the debridement times was 2.37 ± 0.84. Finally, 22 patients in the VSD group achieved wound healing at 5.95 ± 1.36 months and four patients experienced amputation. In IM group, all patients achieved wound healing at 4.74 ± 1.72 months. The wound healing time of the two groups was statistically different (p = 0.01). There was no significant difference between the two groups in other comparative data (p > 0.05). The hospitalization time of VSD was more than that in the IM group (23.92 ± 4.80d vs. 13.37 ± 4.62d, p < 0.05).
TABLE 2.
Comparison of the perioperative data of the two groups.
| Variable | VSD group (n = 26) | IM group (n = 27) | T or X 2 | p value |
|---|---|---|---|---|
| Size of defect (cm2) | 43.54 ± 4.62 | 41.52 ± 5.62 | T = 1.43 | p = 0.16 |
| Frequency of debridement | 2.31 ± 0.79 | 2.37 ± 0.84 | T = 0.28 | p = 0.78 |
| Hospitalization time (mean ± SD, d) | 23.92 ± 4.80 | 13.37 ± 4.62 | T = 8.16 | p = 0.001* |
| Duration of healing (mean ± SD, months) | 5.95 ± 1.36 | 4.74 ± 1.72 | T = 2.69 | p = 0.01* |
| Healing rate | T = 0.39 | p = 0.53 | ||
| Wound healing | 22 | 27 | ||
| Amputation | 4 | 0 |
p < 0.05.
Vascularisation in the two groups was assessed by IHC to determine the percentages of VEGF positive cells, containing brown or yellow particles. As shown in Figure 3, the positive cells were mainly scattered and formed tube‐like vascular structures in the fresh tissue. The qualitative results indicated similar numbers of VEGF positive cells in the two group.
FIGURE 3.
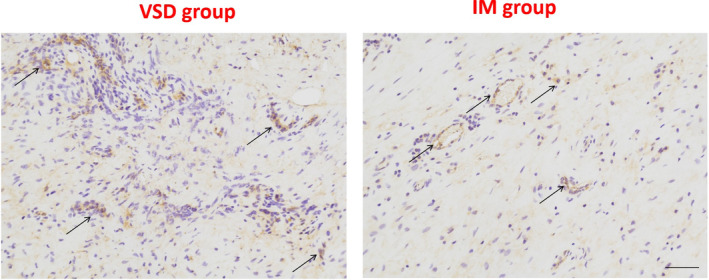
Immunohistochemical analyses of VEGF protein levels in the two group. Black arrows indicate positive cells. Scale bar: 500 μm.
3.2. Functional outcome scores
The function of the foot and ankle assessment was evaluated in 22 patients (VSD group) and 27 patients (IM group) because of four patients undergoing major amputation in VSD group. It showed that there was no significant difference between the two groups (Table 3. p > 0.05). In the VSD group, 50% (11/22) took their functional outcomes as excellent, 27.3% (6/22) as good, 13.6% (3/22) as moderate, and 9.1% (2/22) as poor. In the IM group, 55.6% (15/27) took their functional outcomes as excellent, 25.9% (7/27) as good, 11.1% (3/27) as moderate, and 7.4% (2/27) as poor.
TABLE 3.
Comparison of the functional data of the two groups.
| Variable | VSD group (n = 22) | IM group (n = 27) | X 2 | p value |
|---|---|---|---|---|
| Functional evaluation | X 2 = 0.88 | p = 0.83 | ||
| Excellent | 11 | 15 | ||
| Good | 6 | 7 | ||
| Moderate | 3 | 3 | ||
| Poor | 2 | 2 |
3.3. Complications
Four patients in the VSD group suffered major amputation. Of these, one was aggravated by an infection; one was for financial reasons; one had severe arterial occlusion of the lower extremity; one was afraid of multiple surgeries. While, there was no one amputation in the IM group. Although the amputation rate was higher in the VSD group than in the IM group, the difference was not statistically significant (p = 0.051 > 0.05). There were six DVT cases and five pneumonia cases in the VSD group. One DVT case and one pneumonia case were observed in the IM group. There was no significant difference between the two groups in the pneumonia term and the DVT term became markedly different (p < 0.05). while, the total cases in the VSD group (15/26) is more than it in the IM group (2/27, p = 0.01). There were no other wound‐healing complications. (Table 4).
TABLE 4.
Complication of the two groups.
| Variable | VSD group (n = 26) | IM group (n = 27) | X 2 | p value |
|---|---|---|---|---|
| Major amputation | 4 | 0 | p = 0.051 | |
| DVT | 6 | 1 | X 2 = 4.34 | p = 0.037* |
| Pneumonia | 5 | 1 | X 2 = 3.18 | p = 0.075 |
| Total | 15 | 2 | X 2 = 15.37 | p = 0.001* |
p < 0.05.
4. DISCUSSION
At present, reconstructing complex soft‐tissue defects of the foot and ankle in elderly patients poses a major challenge for surgeons because of the limited local soft tissue availability and weight‐bear requirement. Current literature 2 , 12 indicates that no one particular technique was superior or ideal. Especially for the elderly, 13 as they often suffer from basic diseases and have poor surgical tolerance. Although there are many treatment methods for skin defects of the foot, each method has its own advantages and disadvantages. It is inevitable that skin necrosis, defects, and deep‐tissue exposure will inevitably occur after the operation. 14
More recently, VSD has been used in the management of traumatic soft‐tissue wounds. 15 Use of a bead pouch consistently resulted in a lower infection rate than wound management without a bead pouch. In addition, the VSD allows the evacuation of interstitial fluids that accumulate in post‐traumatic wounds. 16 These fluids contain inhibitory factors that suppress the formation of fibroblasts, vascular endothelial cells, and keratinocytes, which are crucial to wound healing. It may allow the use of less extensive surgical techniques to obtain adequate soft tissue coverage in persons who require further soft‐tissue procedures. Although a VSD sponge may be placed over exposed hardware and tendon, we recommend limiting the time that hardware is exposed to the sponge (<72 h). 17 Complications associated with use of a VSD device are infrequent in properly selected wounds. 18 Bleeding from the wound may occur, therefore, in the presence of a VSD covering wound, we need to perform a thorough haemostasis, while the tourniquet is relaxed. 19 Kaushik et al. 20 recommend its regular use in all patients presenting with post‐traumatic, soft‐tissue injuries when primary coverage is not possible. However, it requires prolonged bed rest, which can lead to complications including pneumonia, bedsores, and deep venous thrombosis of the lower extremities. 21 In this study, the incidence of DVT was significantly lower in the IM group than in the VSD group.
The angiogenic potential of the IM surrounding the PMMA cement 22 was exploited in combination with skin grafting to repair exposed open wounds in patients with injuries resulting in exposed bone or tendon wounds. The advantage of our approach is that because the wound surface is covered by bone cement, the patient can be discharged from the hospital before IM formation, thus reducing the length and cost of hospitalization. 10 The IM is an initially avascular bed that supports the survival of the skin graft. IM vascularisation has also been demonstrated in other human and animal studies. 23 , 24 , 25 Compared with VSD, IM can significantly shorten the length of hospital stay and wound healing time, and there is no significant difference in the functional recovery of foot and ankle. Most surprisingly, there were no amputations in the IM group. This suggests that PMMA can produce blood‐transported granulation tissue from tissues without blood transport, and the success rate is very high. Immunohistochemical results showed that IM had a large amount of VEGF and had strong angiogenesis ability.
For elderly patients with skin defects of the foot, the most critical factors are the safety of the operation and less hospitalization time. 26 , 27 Therefore, we recommend the IM for the treatment of foot wounds for elderly, based on the following advantages: (1) Simple and convenient operation, no need to carry out the complicated wound closure process like VSD, which is conducive to reducing the operation time; (2) After PMMA covered the wound, patients could be discharged from the hospital, which reduces the length of hospital stay; (3) After PMMA coverage, patients could carry out foot movement, even walking down the ground, with a low incidence of complications; (4) PMMA has a stronger ability to induce angiogenesis and is more conducive to wound healing. During the operation, the following details should be paid attention to: (1) Thorough debridement is the premise of covering, and for exposed wounds, bone surface desortication is required. 28 If debridement is not thorough, multiple debridement is required; (2) PMMA will generate a lot of thermal damage 29 when covering the wound surface, which needs to be cooled with wet gauze, so as to reduce the damage to normal soft tissue; (3) When PMMA is fixed, it is recommended to use skin stapler for fixing to reduce the later shedding; (4) PMMA is generally removed after 4 weeks, small wounds can be directly re‐epithelialized with IM, and large wounds can be covered by skin grafting.
A limit of our study is the small sample size; although a statistical comparison of complication rates and healing time was performed, the results need to be confirmed on a larger series to achieve a higher statistical power. In addition, our research just is with the experience of only one medical centre. Prospective multiple‐centre investigations in future are advisable to compare the ways. Randomized controlled trials comparing the ways are desirable to better assess advantages, disadvantages, and indications for the use of VSD and IM in lower limb reconstruction.
5. CONCLUSION
In conclusion, the VSD and IM are both available for foot and ankle reconstruction in elderly patients. However, the IM group offers short hospitalization time, duration of healing and lower frequency of postoperative complications. Thus, we advocate the IM for reconstruction of defects of the foot and ankle region in the elderly patients.
CONFLICT OF INTEREST STATEMENT
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Zhou F, Zhang Y, Gao J, Xiang G, Li Z, Cai L. Reconstruction of foot and ankle defects using the vaccum sealing drainage versus the induced‐membrane the elderly: A retrospective comparative study. Int Wound J. 2024;21(1):e14362. doi: 10.1111/iwj.14362
Feiya Zhou, Yingying Zhang, and Jianyuan Gao contributed equally to this work and should be considered co‐first authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ring A, Kirchhoff P, Goertz O, et al. Reconstruction of soft‐tissue defects at the foot and ankle after oncological resection. Front Surg. 2016;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noaman HH, Soroor YO. Foot salvage using microsurgical free muscle flaps in severely crushed foot with soft tissue defects. Injury. 2019;50(Suppl 5):S17‐S20. [DOI] [PubMed] [Google Scholar]
- 3. Lv Z, Wang Q, Jia R, Ding W, Shen Y. Pelnac(R) artificial dermis assisted by VSD for treatment of complex wound with bone/tendon exposed at the foot and ankle, A prospective study. J Investig Surg. 2020;33(7):636‐641. [DOI] [PubMed] [Google Scholar]
- 4. Yuan K, Zhang F, Lineaweaver WC, Chen X, Li Z, Yan H. The coverage of soft‐tissue defects around the foot and ankle using free or local flaps: a comparative cohort study. Ann Plast Surg. 2021;86(6):668‐673. [DOI] [PubMed] [Google Scholar]
- 5. van Boerum MS, Wright T, McFarland M, Fiander M, Pannucci CJ. Cross‐leg flaps for lower extremity salvage: a scoping review. J Reconstr Microsurg. 2019;35(7):505‐515. [DOI] [PubMed] [Google Scholar]
- 6. Al Deek NF. Comparative effectiveness analysis of complex lower extremity reconstruction: outcomes and costs for biologically based, local tissue rearrangement, and free flap reconstruction. Plast Reconstr Surg. 2021;147(1):171e‐172e. [DOI] [PubMed] [Google Scholar]
- 7. Suominen S, Asko‐Seljavaara S. Free flap failures. Microsurgery. 1995;16(6):396‐399. [DOI] [PubMed] [Google Scholar]
- 8. Yuan XG, Zhang X, Fu YX, et al. Sequential therapy with ‘vacuum sealing drainage‐artificial dermis implantation‐thin partial thickness skin grafting’ for deep and infected wound surfaces in children. Orthop Traumatol Surg Res. 2016;102(3):369‐373. [DOI] [PubMed] [Google Scholar]
- 9. Cai L, Mei Y, Chen C, Wang J, Wang X, Zheng W. Comparison of vacuum sealing drainage and conventional drainage for postoperative drainage in closed calcaneal fracture: a randomized controlled trial. Injury. 2022;53(2):777‐783. [DOI] [PubMed] [Google Scholar]
- 10. Cai L, Hong Z, Zhang Y, et al. Management of wounds with exposed bone structures using an induced‐membrane followed by polymethyl methacrylate and second‐stage skin grafting in the elderly with a 3‐year follow‐up. Int Wound J. 2022;20:1020‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koivu H, Kohonen I, Mattila K, Loyttyniemi E, Tiusanen H. Long‐term results of Scandinavian total ankle replacement. Foot Ankle Int. 2017;38(7):723‐731. [DOI] [PubMed] [Google Scholar]
- 12. Li P, Zhang H, Zhu J, et al. Foot and ankle reconstruction using the lateral supramalleolar flap versus the anterolateral thigh flap in the elderly: a comparative study. Int Wound J. 2022;19(6):1518‐1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reddy M, Skin and wound care: important considerations in the older adult. Adv Skin Wound Care. 2008;21(9):424‐436. [DOI] [PubMed] [Google Scholar]
- 14. Sung IH, Jang DW, Kim SW, Kim YH, Kim SW. Reconstruction of diabetic lower leg and foot soft tissue defects using thoracodorsal artery perforator chimeric flaps. Microsurgery. 2018;38(6):674‐681. [DOI] [PubMed] [Google Scholar]
- 15. Liu X, Liang J, Zao J, et al. Vacuum sealing drainage treatment combined with antibiotic‐impregnated bone cement for treatment of soft tissue defects and infection. Med Sci Monit. 2016;22:1959‐1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X, Chen Y, Xiao X, Wang Z, Yang X, Zhao Z. Application of VSD technique in adults with chronic osteomyelitis of the extremities combined with soft tissue defects. Int Wound J. 2023;20(3):768‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friedrich JB, Katolik LI, Hanel DP. Reconstruction of soft‐tissue injury associated with lower extremity fracture. J Am Acad Orthop Surg. 2011;19(2):81‐90. [DOI] [PubMed] [Google Scholar]
- 18. Normandin S, Safran T, Winocour S, et al. Negative pressure wound therapy: mechanism of action and clinical applications. Semin Plast Surg. 2021;35(3):164‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Acosta S, Bjorck M, Wanhainen A. Negative‐pressure wound therapy for prevention and treatment of surgical‐site infections after vascular surgery. Br J Surg. 2017;104(2):e75‐e84. [DOI] [PubMed] [Google Scholar]
- 20. Kaushik D, Joshi N, Kumar R, Gaba S, Sapra R, Kumar K. Negative pressure wound therapy versus gauze dressings for the treatment of contaminated traumatic wounds. J Wound Care. 2017;26(10):600‐606. [DOI] [PubMed] [Google Scholar]
- 21. Liu Z, Tao X, Chen Y, Fan Z, Li Y. Bed rest versus early ambulation with standard anticoagulation in the management of deep vein thrombosis: a meta‐analysis. PLoS One. 2015;10(4):e0121388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang W, Zuo R, Long H, et al. Advances in the Masquelet technique: myeloid‐derived suppressor cells promote angiogenesis in PMMA‐induced membranes. Acta Biomater. 2020;108:223‐236. [DOI] [PubMed] [Google Scholar]
- 23. Niikura T, Jimbo N, Komatsu M, et al. Histological analysis of induced membranes in patients whose bone defects were treated with the Masquelet technique to identify factors affecting the vascularity of induced membranes. J Orthop Surg Res. 2021;16(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie J, Liu D, Wang H, et al. Effects of topical mechanical stability on the formation of Masquelet membrane in a rabbit radial defect model. Sci Rep. 2020;10(1):18939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu C, You JX, Chen YX, et al. Effect of induced membrane formation followed by polymethylmethacrylate implantation on diabetic foot ulcer healing when revascularization is not feasible. J Diabetes Res. 2019;2019:2429136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sommer R, Hampel‐Kalthoff C, Kalthoff B, et al. Differences between patient‐ and proxy‐reported HRQoL using the wound‐QoL. Wound Repair Regen. 2018;26(3):293‐296. [DOI] [PubMed] [Google Scholar]
- 27. McMahon DJ, Schwab CW, Kauder D. Comorbidity and the elderly trauma patient. World J Surg. 1996;20(8):1113‐1119. discussion 9–20, 1120. [DOI] [PubMed] [Google Scholar]
- 28. Falabella AF. Debridement and wound bed preparation. Dermatol Ther. 2006;19(6):317‐325. [DOI] [PubMed] [Google Scholar]
- 29. Gundapaneni D, Goswami T. Thermal isotherms in PMMA and cell necrosis during total hip arthroplasty. J Appl Biomater Funct Mater. 2014;12(3):193‐202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


