Abstract
Purpose
This systematic review and meta-analysis compared the outcomes of the watch-and-wait (WW) approach versus radical surgery (RS) in rectal cancers with clinical complete response (cCR) after neoadjuvant chemoradiotherapy.
Methods
This study followed the PRISMA guidelines. Major databases were searched to identify relevant articles. WW and RS were compared through meta-analyses of pooled proportions. Primary outcomes included overall survival (OS), disease-free survival (DFS), local recurrence, and distant metastasis rates. Pooled salvage surgery rates and outcomes were also collected. The Newcastle-Ottawa scale was employed to assess the risk of bias.
Results
Eleven studies including 1,112 rectal cancer patients showing cCR after neoadjuvant chemoradiation were included. Of these patients, 378 were treated nonoperatively with WW, 663 underwent RS, and 71 underwent local excision. The 2-year OS (risk ratio [RR], 0.95; P=0.94), 5-year OS (RR, 2.59; P=0.25), and distant metastasis rates (RR, 1.05; P=0.80) showed no significant differences between WW and RS. Local recurrence was more frequent in the WW group (RR, 6.93; P<0.001), and 78.4% of patients later underwent salvage surgery (R0 resection rate, 97.5%). The 2-year DFS (RR, 1.58; P=0.05) and 5-year DFS (RR, 2.07; P=0.02) were higher among RS cases. However, after adjustment for R0 salvage surgery, DFS showed no significant between-group difference (RR, 0.82; P=0.41).
Conclusion
Local recurrence rates are higher for WW than RS, but complete salvage surgery is often possible with similar long-term outcomes. WW is a viable strategy for rectal cancer with cCR after neoadjuvant chemoradiation, but further research is required to improve patient selection.
Keywords: Rectal cancer, Clinical complete regression, Neoadjuvant chemoradiation, Nonoperative management, Watch and wait
INTRODUCTION
Treatment of locally advanced rectal cancer, defined as T3–T4 or node-positive disease, is evolving. The current mainstay of curative treatment is neoadjuvant chemoradiation followed by radical surgery (RS) based on the principles of total mesorectal excision (TME) [1]. However, resectional colorectal surgery has significant morbidity, including 2% risk of perioperative mortality, 11% risk of anastomotic leak, 5% risk of reoperation for complications, and some risk of sexual and urinary dysfunction [2–5]. The risks of long-term genitourinary and bowel impairments are especially pronounced with the addition of pelvic irradiation for locally advanced rectal cancers [6, 7]. Sphincter preservation may be possible in only about 50% of patients with low rectal cancer [8], with the rest experiencing impaired quality of life associated with permanent colostomy [9].
Although organ preservation has gained appeal given the drawbacks of surgery, it faces resistance based on unfavorable oncological outcomes and is likely to benefit only patients with certain T1 tumors with favorable histology [10]. A combination of local excision (LE) and chemoradiotherapy has been associated with unacceptable rates of local recurrence in both neoadjuvant and adjuvant settings [11, 12]. Furthermore, breaching the TME plane in a local resection may compromise eventual salvage surgery [13]. An alternative form of nonoperative management termed watch and wait (WW), first reported by Habr-Gama et al. [14] in 2004, had exceptional outcomes (100% 5-year survival and 92% disease-free survival [DFS]) in a carefully selected group of patients. The strict selection protocol required patients to have clinical complete response (cCR) after neoadjuvant chemoradiation and to undergo close follow-up with repeated clinical examinations, endoscopy, and imaging. The current selection criteria for cCR are stringent and include 5 requirements: no residual tumor and a white scar on endoscopy, negative biopsy of the white scar, no palpable tumor on a digital rectal exam, no suspicious lymph nodes on magnetic resonance imaging (MRI), and substantial downsizing with no residual tumor on MRI [15].
Given the rising interest in total neoadjuvant therapy (TNT) regimes, as well as a recent meta-analysis showing significantly higher rates of pathologic complete response (pCR) relative to long-course chemoradiotherapy [16], increased interest in the consideration of organ preservation is inevitable.
The results of Habr-Gama et al. [14] have not been replicated in all studies, nor have any randomized trials been conducted. Previously published meta-analyses on this topic [7–18] include only small numbers of studies available at the time, along with significant heterogeneity arising from varying comparator groups, such as patients with pCR or those with a matched surgical cohort without cCR. We posit the latter to be an unfair comparison, as patients without cCR who undergo radical resection are likely to have poor tumor biology relative to those with cCR who undergo watchful waiting.
The aim of this systematic review and meta-analysis was to assess the available evidence comparing the postoperative and oncological outcomes of WW versus RS among patients determined to have cCR after neoadjuvant chemoradiation.
METHODS
Search process
This study was performed in accordance with the Cochrane Handbook of Systematic Reviews of Interventions [19], as well as the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement guidelines [20]. Using an exhaustive combination of the search terms “rectal cancer,” “watch and wait,” and “non-operative,” the following electronic databases were searched: MEDLINE (via PubMed), the Cochrane Library, Embase, and the ClinicalTrials.gov website. The goal was to identify all published studies and abstracts comparing outcomes of WW and RS for rectal cancers with cCR after neoadjuvant therapy. The reference lists of relevant articles were searched to identify additional studies.
Inclusion and exclusion criteria
Since no randomized trials published on this topic were found, retrospective and prospective studies were included in the analysis. Case reports and series were excluded.
The inclusion criteria for this study were comparison of outcomes between WW and RS for rectal cancer after neoadjuvant therapy, an intervention arm of WW for rectal cancers with cCR after neoadjuvant therapy, and a comparator arm of RS for rectal cancers with cCR after neoadjuvant therapy. The diagnostic criteria for cCR were the guidelines mentioned above.
The exclusion criteria were irrelevant outcomes reported and an irrelevant comparator group (including pCR or all/matched resection cases without cCR). Given the lack of an available translator, non-English-language studies were excluded. Abstracts with no extractable data were also excluded.
Selection of studies and data extraction
As shown in Fig. 1, study selection was performed in 2 stages by 2 independent reviewers. Studies were first screened for preliminary inclusion by their titles and abstracts, and the full texts of the resulting studies were then reviewed in their entirety to confirm inclusion in the final analysis. Resolution of any conflicts was first attempted by consensus; if none could be reached, the senior author served as the final arbiter regarding study eligibility.
Fig. 1.
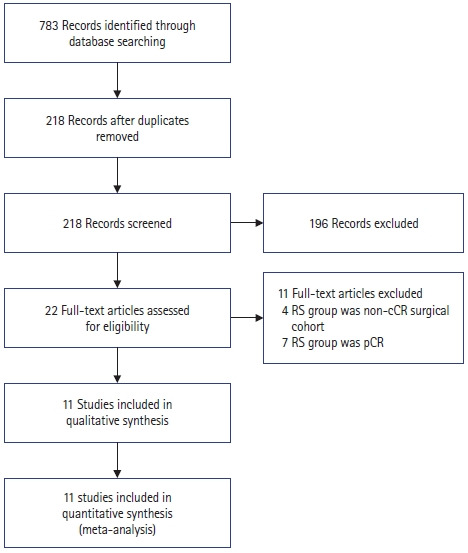
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart depicting search process and reasons for exclusion. RS, radical surgery; cCR, clinical complete response; pCR, pathologic complete response.
The primary outcomes of interest were the rates of local recurrence, distant metastasis, overall survival (OS), and DFS. In addition, the following were abstracted from each study: first author, year, country of origin, study design, mean age of patients in each arm, neoadjuvant treatment regimen, evaluation timeframe postneoadjuvant treatment, mean follow-up duration, and rate of salvage surgery after local recurrence.
Statistical analysis
RevMan ver. 5.4 (The Nordic Cochrane Centre) was used to perform all statistical analyses. Risk ratios (RRs) were reported for dichotomous variables. I2 values were computed to estimate statistical heterogeneity, with a random-effects model applied when the value exceeded 50%. Results were reported with 95% confidence intervals (CIs), and a P-value of less than 0.05 was regarded as indicating statistical significance.
Assessment of bias
The Newcastle-Ottawa scale (NOS) for nonrandomized controlled trials was employed to assess patient selection, comparability of study groups, and outcome assessment. Funnel plots were created to ascertain publication bias.
RESULTS
Systematic search
Initially, 783 papers were retrieved by a systematic search across multiple databases, and 218 remained after duplicates were removed. After title and abstract review, 22 remained, the full texts of which were reviewed. Eleven studies were excluded based on reasons stated in Fig. 1. The remaining 11 studies included 8 retrospective and 3 prospective studies [21–31].
Study characteristics
A total of 1,112 patients with cCR of rectal cancer after neoadjuvant therapy were included. Of these patients, 378 were treated nonoperatively with WW, 663 underwent RS, and 71 underwent LE. The mean age ranged from 50 to 74 years in the WW and 55 to 64 years in the RS group. The pCR rate among those who underwent resection was reported in 10 studies (pooled rate, 57.3%; range, 16.7%–96.1%).
Neoadjuvant regimes, posttreatment reassessment timeframes, and reassessment modalities are presented in Table 1 [21–31]. Tumor characteristics from each individual study is presented in Table 2 [22–29, 30, 31]. Among the patients treated with WW, a pooled local recurrence rate of 15.5% was observed. Salvage surgery rates, R0 salvage resection rates, and the reasons for not performing salvage surgery are presented in Table 3 [22–31].
Table 1.
Characteristics of included studies
| Study | Country | Study design | No. of patients |
Mean age (yr) |
Follow-up (mo) |
Neoadjuvant regimen | Reevaluation (wk) | cCR diagnostic criteria |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WW | RS | LE | WW | RS | LE | WW | RS | LE | Endoscopy | CT | MRI | |||||
| Ayloor Seshadri et al. [21] (2013) | India | Retrospective | 23 | 10 | - | 50.0 | 55.0 | - | 72.0 | 72.0 | - | Capecitabine (50.4 Gy) | 4–6 | Yes | Yes | No |
| Beard et al. [22] (2020) | USA | Retrospective | 53 | 42 | - | 64.0 | 56.5 | - | 35.0 | 35.0 | - | Capecitabine (50.4 Gy) or 5-FU (50.4 Gy)a | 8–12 | Yes | Yes | Yes |
| Dalton et al. [23] (2012) | UK | Retrospective | 6 | 6 | - | 68.0 | 69.0 | - | 25.3 | 39.3 | - | Capecitabine (45.0–50.4 Gy) | 6–8 | Yes | Yes | Yes |
| Han et al. [24] (2021) | China | Prospective | 58 | 26 | - | 57.6 | 58.4 | - | 34.9 | 34.5 | - | Capecitabine (30.0–50.6 Gy) | Not stated | Yes | Yes | Yes |
| Lai et al. [25] (2016) | Taiwan | Retrospective | 18 | 26 | - | 67.5 | 63.7 | - | 49.0 | 42.0 | - | 5-FU (45.0–50.4 Gy) | 8–12 | Yes | Yes | Yes |
| Lee et al. [26] (2015) | Korea | Prospective | 8 | 28 | 16 | 70.0 | 64.0 | 70.0 | 41.0 | 41.0 | 41.0 | 5-FU (50.4 Gy) | 6–10 | Yes | Yes | Yes |
| Lee et al. [27] (2021) | Korea | Retrospective | 14 | 31 | 30 | 64.0 | 60.0 | 64.0 | 64.7 | 64.7 | 64.7 | Capecitabine (50.4 Gy) or 5-FU (50.4 Gy) | 4–12 | Yes | Yes | Yes |
| Li et al. [28] (2015) | China | Prospective | 30 | 92 | - | 62.0 | 56.0 | - | 58.0 | 58.0 | - | Capecitabine (50.0 Gy) | 8–10 | Yes | Yes | Yes |
| Wang et al. [29] (2020) | China | Retrospective | 59 | 179 | - | 58.0 | 57.0 | - | 41.8 | 41.8 | - | 5-FU+capecitabine (50.4 Gy)a | 6–12 | Yes | Yes | Yes |
| Wang et al. [30] (2021) | China | Retrospective | 94 | 94 | - | 57.5 | 56.0 | - | 38.2 | 38.2 | - | CAPOX + 5FU (50.0 Gy) or capecitabine + 5-FU (50.0 Gy)a | Not stated | Yes | Yes | Yes |
| Yeom et al. [31] (2019) | Korea | Retrospective | 15 | 129 | 25 | 74.0 | 63.8 | 73.0 | 20.0 | 48.0 | 30.0 | Capecitabine (50.4 Gy), CAPOX (50.4 Gy), or 5-FU (50.4 Gy)a | 8 | Yes | Yes | Yes |
cCR, clinical complete response; WW, watch and wait; RS, radical surgery; LE, local excision; CT, computed tomography; MRI, magnetic resonance imaging; FU, fluorouracil; CAPOX, capecitabine and oxaliplatin.
Including total neoadjuvant therapy regime.
Table 2.
Tumor characteristics by study
| Study quality | Group |
Distance from AV |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WW |
RS |
LE |
WW | RS | LE | ||||||||||||||||
| T1 | T2 | T3 | T4 | N0 | N+ | T1 | T2 | T3 | T4 | N0 | N+ | T1 | T2 | T3 | T4 | N0 | N+ | ||||
| Beard et al. [22] (2020) | 3 | 10 | 39 | 1 | 29 | 24 | 6 | 7 | 29 | 0 | 9 | 33 | - | - | - | - | - | - | 3.00 | 8.00 | - |
| Dalton et al. [23] (2012) | 0 | 1 | 4 | 1 | 1 | 5 | - | - | - | - | - | - | 5.06 | 5.53 | - | ||||||
| Han et al. [24] (2021) | 0 | 6 | 47 | 5 | 42 | 16 | 0 | 0 | 23 | 3 | 24 | 2 | - | - | - | - | - | - | - | - | - |
| Lai et al. [25] (2016) | 0 | 0 | 15 | 3 | 11 | 7 | 0 | 0 | 25 | 1 | 8 | 18 | - | - | - | - | - | - | 3.35 | 4.81 | |
| Lee et al. [26] (2015) | 0 | 5 | 2 | 1 | 5 | 3 | 0 | 6 | 21 | 1 | 13 | 15 | 0 | 7 | 9 | 0 | 14 | 2 | 4.00 | 4.00 | 2.00 |
| Lee et al. [27] (2021) | 0 | 20 | 24 | 0 | 29 | 15 | 0 | 8 | 23 | 0 | 9 | 22 | - | - | - | - | - | - | - | - | - |
| Li et al. [28] (2015) | 3 | 5 | 15 | 7 | 14 | 16 | 10 | 14 | 48 | 20 | 39 | 53 | - | - | - | - | - | - | 3.50 | 3.70 | - |
| Wang et al. [30] (2021) | 0 | 9 | 68 | 17 | 24 | 70 | 0 | 8 | 71 | 15 | 24 | 70 | - | - | - | - | - | - | 4.00 | 4.00 | - |
| Yeom et al. [31] (2019) | 0 | 3 | 12 | 5 | 10 | 0 | 8 | 121 | 75 | 54 | 0 | 2 | 23 | 0 | 19 | 6 | 4.29 | 4.67 | 3.50 | ||
AV, anal verge; WW, watch and wait; RS, radical surgery; LE, local excision.
Table 3.
Rates of pCR and salvage surgery
| Study | pCR in RS | Salvage surgery in WW | R0 salvage rate | Reason for no surgery |
|---|---|---|---|---|
| Beard et al. [22] (2020) | 21 (50.0) | 4 (66.7) | 4 (100) | 2 Refused surgery |
| Dalton et al. [23] (2012) | 1 (16.7) | 0 (0) | 0 (0) | - |
| Han et al. [24] (2021) | 20 (76.9) | 9 (100) | 9 (100) | - |
| Lai et al. [25] (2016) | 25 (96.1) | 2 (100) | 2 (100) | - |
| Lee et al. [26] (2015) | 12 (42.9) | 1 (50.0) | 1 (100) | 1 Distant metastasis |
| Lee et al. [27] (2021) | 16 (51.6) | 2 (66.7) | 2 (100) | 1 Refused surgery |
| Li et al. [28] (2015) | 81 (88.0) | 2 (100) | 2 (100) | - |
| Wang et al. [29] (2020) | 113 (63.1) | 6 (85.7) | 5 (83.3) | 1 Distant metastasis |
| Wang et al. [30] (2021) | 48 (51.0) | 12 (85.7) | 12 (100) | 2 Distant metastasis |
| Yeom et al. [31] (2019) | 43 (33.3) | 2 (33.3) | 2 (100) | 4 Refused surgery |
Values are presented as number (%).
pCR, pathologic complete response; RS, radical surgery; WW, watch-and-wait.
Study quality
Based on the NOS assessment, all studies were deemed methodologically robust in terms of selection bias, comparability, and outcome assessment, with overall NOS scores of 6 and above out of 9 (Table 4) [21–31].
Table 4.
NOS scores for study selection
| Study | NOS score |
|||
|---|---|---|---|---|
| Selection | Comparability | Outcome | Total | |
| Ayloor Seshadri et al. [21] (2013) | 3 | 2 | 1 | 6 |
| Beard et al. [22] (2020) | 3 | 2 | 3 | 8 |
| Dalton et al. [23] (2012) | 3 | 2 | 2 | 7 |
| Han et al. [24] (2021) | 3 | 2 | 3 | 8 |
| Lai et al. [25] (2016) | 3 | 2 | 1 | 6 |
| Lee et al. [26] (2015) | 3 | 2 | 1 | 6 |
| Lee et al. [27] (2021) | 3 | 2 | 2 | 7 |
| Li et al. [28] (2015) | 3 | 1 | 2 | 6 |
| Wang et al. [29] (2020) | 2 | 2 | 2 | 6 |
| Wang et al. [30] (2021) | 3 | 3 | 2 | 8 |
| Yeom et al. [31] (2019) | 2 | 3 | 2 | 7 |
NOS, Newcastle-Ottawa scale.
Comparison of WW and RS groups
All 11 studies reported local recurrence and distant metastasis rates. Using a fixed-effects model, a significantly higher risk of local recurrence was observed in the WW group than in the RS group (RR, 6.93; 95% CI, 3.79–12.67; P<0.001; I2=0%) (Fig. 2) [21–31]. However, the risk of distant metastasis was comparable between groups (RR, 1.05; 95% CI, 0.70–1.58; P=0.80; I2=25%) (Fig. 3) [21–31].
Fig. 2.
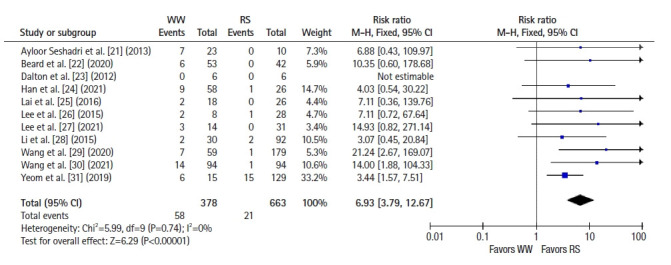
Forest plot depicting local recurrences. WW, watch and wait; RS, radical surgery; M-H, Mantel-Haenszel test; CI, confidence interval.
Fig. 3.
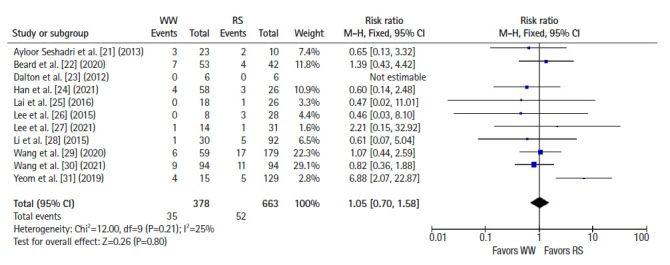
Forest plot depicting distant metastases. WW, watch and wait; RS, radical surgery; M-H, Mantel-Haenszel test; CI, confidence interval.
Six studies [22–25, 28, 30] reported 2-year OS, and a pooled analysis using a fixed-effects model showed no significant difference between groups (RR 1.09, 95% CI 0.46–2.59, P=0.85; I2=42%) (Fig. 4). Seven studies [22–26, 28, 30] reported 2-year DFS, and a pooled analysis using a fixed-effects model showed better DFS in the RS group than in the WW group (RR, 1.58; 95% CI, 1.01–2.48; P =0.05; I2=0%) (Fig. 5). Five studies [25, 27–29, 31] reported 5-year OS, and a pooled analysis using a random-effects model showed no significant difference between groups (RR, 2.59; 95% CI, 0.51–13.26; P=0.25; I2=62%) (Fig. 6). Four studies [25, 27, 28, 31] reported 5-year DFS, and a pooled analysis using a fixed-effects model demonstrated better DFS in the RS group than in the WW group (RR, 2.07; 95% CI, 1.13–3.78; P=0.02; I2=0%) (Fig. 7).
Fig. 4.
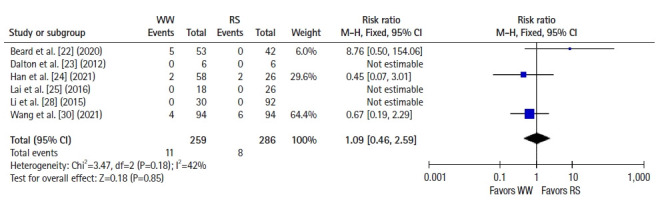
Forest plot depicting 2-year overall survival. WW, watch and wait; RS, radical surgery; M-H, Mantel-Haenszel test; CI, confidence interval.
Fig. 5.
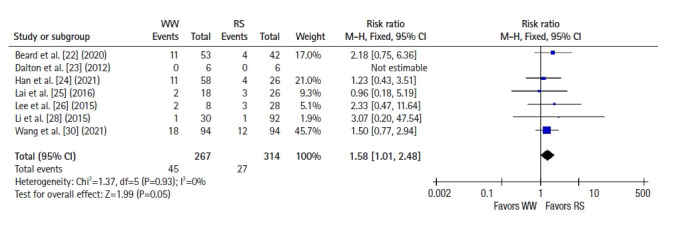
Forest plot depicting 2-year disease-free survival. WW, watch and wait; RS, radical surgery; M-H, Mantel-Haenszel test; CI, confidence interval.
Fig. 6.
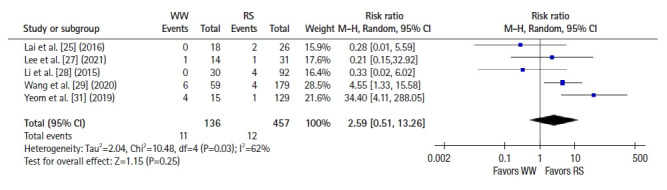
Forest plot depicting 5-year overall survival. WW, watch and wait; RS, radical surgery; M-H, Mantel-Haenszel test; CI, confidence interval.
Fig. 7.
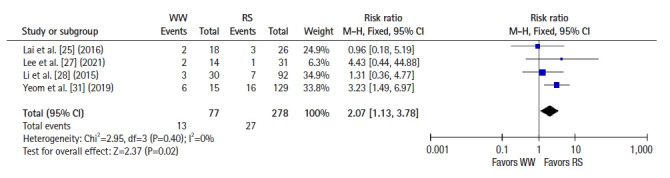
Forest plot depicting 5-year disease-free survival. WW, watch and wait; RS, radical surgery; M-H, Mantel-Haenszel test; CI, confidence interval.
Ten studies [22–31] reported salvage surgery rates (pooled rate, 78.4%) in the WW group after local recurrence, and the R0 salvage surgery rate was 97.5%. Reasons for not attempting salvage surgery were also reported and are presented in Table 3 [21–31]. Adjusted DFS rates after successful R0 salvage surgery were compared between the WW and RS groups, and no significant difference was observed (RR, 0.82; 95% CI, 0.52–1.30; P =0.41; I2=18%) (Fig. 8) [22–31].
Fig. 8.
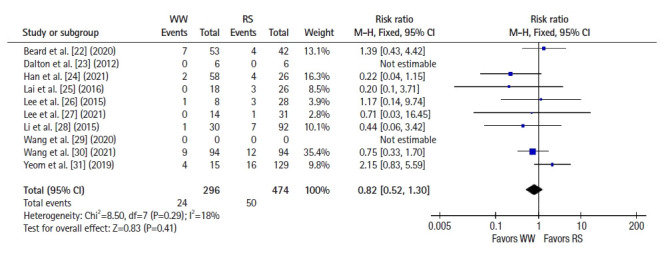
Forest plot depicting adjusted disease-free survival after accounting for R0 salvage resection in watch-and-wait (WW) group. RS, radical surgery; M-H, Mantel-Haenszel test; CI, confidence interval.
Comparison of WW and LE groups
Three studies [26, 27, 31] reported LE as a treatment arm. No significant differences were observed in the rates of local recurrence (RR, 1.38; P=0.34), distant metastasis (RR, 0.96; P=0.93), and DFS (RR, 0.91; P=0.86) (Supplementary Figs. 1–3) [26, 27, 31].
DISCUSSION
The results of this study concur with previously published metaanalyses on this topic [17, 18, 32] in terms of local recurrence and distant metastasis. The similarity in 2- and 5-year OS findings between groups is also congruent with previous research. Notably, previous studies published on this topic had limitations in study selection. In a meta-analysis, Dossa et al. [17] reported no significant differences in OS, DFS, and distant metastasis rates when comparing WW with cCR and pCR separately. However, only 2 studies were available at that time for the cCR comparison arm, and this small number limited interpretation. Zhao et al. [18] attempted to compare WW with RS in rectal cancer patients after neoadjuvant therapy, but they included studies that used cCR, pCR, and matched resection (that is, non-cCR patients) in the analysis, which confounded interpretation. Similarly, Yu et al. [32] reported a higher local recurrence rate with no significant difference in OS or DFS in WW relative to RS among rectal cancer patients after neoadjuvant therapy, but they also included studies that incorporated pCR and matched resection (non-cCR patients) in the analysis. Details are provided in the Supplementary Table 1 [14, 18, 22, 24, 26, 30, 32–36].
The present study is the first to our knowledge to specifically compare surgical and oncological outcomes between WW and RS in patients with cCR after neoadjuvant therapy. The use of non-cCR cases as controls does not make for an ideal comparator group because WW is not a treatment option for those patients, and the cancers involved are likely to differ from those with cCR to neoadjuvant therapy. The use of pCR cases as a comparator group also introduces selection bias, as patients with pCR are likely to experience better outcomes than those without pCR. Based on the pooled analysis in this paper, only 57.3% of cCR patients who underwent resection exhibited pCR. Hence, a comparator group including only cCR cases, which was used in the present study, allows for the best comparison of outcomes. This approach should also aid in clinical decision-making, as cCR will need to be diagnosed before WW can be considered as a treatment option.
A higher rate of local recurrence among WW cases is not unexpected and has been reported in multiple previous studies; this is the main reason for close follow-up for early detection of treatable local regrowths. In this study, we found a pooled local recurrence rate of 15.3% in the WW group, which is consistent with the local regrowth rate of 21.6% reported in a systematic review of WW outcomes [37]. In that study, 88% of patients with regrowth underwent salvage surgery, with R0 resection achieved in 93% of those. In comparison, the salvage surgery rate in the present meta-analysis was 78.4%, with R0 resection achieved in 97.5% of those. The slightly lower rate of salvage surgery in the present analysis is attributable mainly to patient refusal of surgery (7 patients), while 4 cases were not suitable for salvage due to distant metastasis.
In this meta-analysis, we report a significant difference in 2- and 5-year DFS that has not been previously detected. This can be attributed to a higher rate of local recurrence in the WW group, as this effect was negated after adjusting for R0 salvage surgery, showing no significant difference in DFS thereafter. As most local recurrences are amenable to R0 salvage surgery (97.5%), this discrepancy carries no change in long-term outcomes.
The major oncological anxiety regarding the WW approach following cCR is the possibility of development of untreatable metastasis as a result of waiting [36]. This study shows that watchful waiting did not lead to a higher risk of distant metastasis. Notably, the usage of chemotherapy after cCR was not consistently reported in this group of studies and merits further investigation, especially with the increasing popularity of TNT approaches to rectal cancer.
If a WW approach is to be undertaken for a patient with cCR, a robust close surveillance program is essential because the rate of local recurrence is relatively high, and most cases are amenable to curative salvage surgery. Early detection, work-up, and management are crucial to ensure that these recurrences are treated before further progression. Perhaps local regrowths should not be termed “recurrences,” but instead considered local failures of disease control that are treatable with salvage surgery. Patients initially treated with WW who had local recurrence and subsequent salvage surgery displayed no difference in OS, DFS, or distant metastasis rates compared to their RS counterparts in this meta-analysis. Meanwhile, 238 of the 378 WW patients avoided surgery with no local recurrence or distant metastasis detected on follow-up.
Our analysis was limited by heterogeneity among the included studies, specifically with regard to different neoadjuvant treatment regimes, different reassessment timeframes after neoadjuvant therapy, and the lack of fixed follow-up protocols. Most studies were retrospective in design, carrying inherent selection bias regarding treatment with WW or RS (that is, was this the patient’s choice for function preservation and avoidance of surgery, or the surgeon’s choice due to high surgical risk?). While we attempted to address statistical heterogeneity by employing the random-effects model when indicated, some residual clinical heterogeneity inevitably remained.
Nevertheless, given the multimodality of treatment available, the potential association of cCR with nonoperative management is gaining traction for this select group of patients with rectal cancer. Particularly given the increasing interest in TNT and the higher pCR rates compared to long-course chemoradiotherapy (29.9% vs. 14.9%) reported in a meta-analysis [16], nonoperative management of rectal cancers is expected to be of great clinical interest. This study demonstrated that WW may be feasible based on comparable OS and adjusted DFS rates after salvage surgery relative to RS, and it hence may not compromise oncological outcomes. While it is then tempting to jump on the WW bandwagon, caution must not be thrown to the wind; cCR still comprises a minority of all treated rectal cancers, and neoadjuvant chemoradiation is associated with side effects that may be long-lasting [38]. Future studies should focus on the selection of patients who will benefit from neoadjuvant chemoradiotherapy, TNT, and methods to sustain the response, as well as identifying the subgroup of patients who will benefit from surgery despite cCR.
This meta-analysis discusses the advantages and disadvantages of WW compared to RS in patients with cCR after neoadjuvant chemoradiation. While TME surgery is still the standard of care, this study indicates that WW can be safe, with similar rates of OS and distant metastasis. Local recurrences associated with WW were largely salvageable, with R0 resection achievable in most cases, and the adjusted DFS after salvage was similar to that among the RS group. With TNT increasing in popularity, more cCR cases can be expected, and the WW strategy may play a more prominent role in the future.
Footnotes
Conflict of interest
Emile Kwong-Wei Tan is an Editorial Board member of Annals of Coloproctology, but was not involved in the reviewing or decision process of this manuscript. No other potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: all authors; Data curation: WL, IJYW; Formal analysis: WL, IJYW; Investigation: WL, IJYW; Methodology: all authors; Validation: EKWT; Writing–original draft: WL; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Supplementary materials
Summary of limitations of previous studies
Forest plot depicting local recurrence of wait-and-watch (WW) group versus local excision (LE) group.
Forest plot depicting distant metastasis of wait-and-watch (WW) group versus local excision (LE) group.
Forest plot depicting disease-free survival of wait-and-watch (WW) group versus local excision (LE) group.
Supplementary materials are available from https://doi.org/10.3393/ac.2022.01221.0174.
REFERENCES
- 1.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–82. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 2.Alves A, Panis Y, Mathieu P, Kwiatkowski F, Slim K, Mantion G, et al. Mortality and morbidity after surgery of mid and low rectal cancer: results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509–14. doi: 10.1016/s0399-8320(05)82121-9. [DOI] [PubMed] [Google Scholar]
- 3.Paun BC, Cassie S, MacLean AR, Dixon E, Buie WD. Postoperative complications following surgery for rectal cancer. Ann Surg. 2010;251:807–18. doi: 10.1097/SLA.0b013e3181dae4ed. [DOI] [PubMed] [Google Scholar]
- 4.Hendren SK, O’Connor BI, Liu M, Asano T, Cohen Z, Swallow CJ, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg. 2005;242:212–23. doi: 10.1097/01.sla.0000171299.43954.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lange MM, Maas CP, Marijnen CA, Wiggers T, Rutten HJ, Kranenbarg EK, et al. Urinary dysfunction after rectal cancer treatment is mainly caused by surgery. Br J Surg. 2008;95:1020–8. doi: 10.1002/bjs.6126. [DOI] [PubMed] [Google Scholar]
- 6.Lange MM, van de Velde CJ. Urinary and sexual dysfunction after rectal cancer treatment. Nat Rev Urol. 2011;8:51–7. doi: 10.1038/nrurol.2010.206. [DOI] [PubMed] [Google Scholar]
- 7.Battersby NJ, Juul T, Christensen P, Janjua AZ, Branagan G, Emmertsen KJ, et al. Predicting the risk of bowel-related quality-oflife impairment after restorative resection for rectal cancer: a multicenter cross-sectional study. Dis Colon Rectum. 2016;59:270–80. doi: 10.1097/DCR.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 8.Battersby NJ, How P, Moran B, Stelzner S, West NP, Branagan G, et al. Prospective validation of a low rectal cancer magnetic resonance imaging staging system and development of a local recurrence risk stratification model: the MERCURY II Study. Ann Surg. 2016;263:751–60. doi: 10.1097/SLA.0000000000001193. [DOI] [PubMed] [Google Scholar]
- 9.Vonk-Klaassen SM, de Vocht HM, den Ouden ME, Eddes EH, Schuurmans MJ. Ostomy-related problems and their impact on quality of life of colorectal cancer ostomates: a systematic review. Qual Life Res. 2016;25:125–33. doi: 10.1007/s11136-015-1050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bach SP, Hill J, Monson JR, Simson JN, Lane L, Merrie A, et al. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg. 2009;96:280–90. doi: 10.1002/bjs.6456. [DOI] [PubMed] [Google Scholar]
- 11.Hallam S, Messenger DE, Thomas MG. A systematic review of local excision after neoadjuvant therapy for rectal cancer: are ypT0 tumors the limit? Dis Colon Rectum. 2016;59:984–97. doi: 10.1097/DCR.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 12.Borstlap WA, Coeymans TJ, Tanis PJ, Marijnen CA, Cunningham C, Bemelman WA, et al. Meta-analysis of oncological outcomes after local excision of pT1-2 rectal cancer requiring adjuvant (chemo)radiotherapy or completion surgery. Br J Surg. 2016;103:1105–16. doi: 10.1002/bjs.10163. [DOI] [PubMed] [Google Scholar]
- 13.Perez RO, Habr-Gama A, São Julião GP, Proscurshim I, Fernandez LM, de Azevedo RU, et al. Transanal endoscopic microsurgery (TEM) following neoadjuvant chemoradiation for rectal cancer: outcomes of salvage resection for local recurrence. Ann Surg Oncol. 2016;23:1143–8. doi: 10.1245/s10434-015-4977-2. [DOI] [PubMed] [Google Scholar]
- 14.Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Jr, Silva e Sousa AH, Jr, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–8. doi: 10.1097/01.sla.0000141194.27992.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gérard JP, Chamorey E, Gourgou-Bourgade S, Benezery K, de Laroche G, Mahé MA, et al. Clinical complete response (cCR) after neoadjuvant chemoradiotherapy and conservative treatment in rectal cancer: findings from the ACCORD 12/PRODIGE 2 randomized trial. Radiother Oncol. 2015;115:246–52. doi: 10.1016/j.radonc.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Kasi A, Abbasi S, Handa S, Al-Rajabi R, Saeed A, Baranda J, et al. Total neoadjuvant therapy vs standard therapy in locally advanced rectal cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2030097. doi: 10.1001/jamanetworkopen.2020.30097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:501–13. doi: 10.1016/S2468-1253(17)30074-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhao GH, Deng L, Ye DM, Wang WH, Yan Y, Yu T. Efficacy and safety of wait and see strategy versus radical surgery and local excision for rectal cancer with cCR response after neoadjuvant chemoradiotherapy: a meta-analysis. World J Surg Oncol. 2020;18:232. doi: 10.1186/s12957-020-02003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions [Internet]. Version 6.3. Cochrane; 2022 [cited 2022 Dec 21]. Available from: https://www.training.cochrane.org/handbook.
- 20.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayloor Seshadri R, Kondaveeti SS, Jayanand SB, John A, Rajendranath R, Arumugam V, et al. Complete clinical response to neoadjuvant chemoradiation in rectal cancers: can surgery be avoided? Hepatogastroenterology. 2013;60:410–4. doi: 10.5754/hge12354. [DOI] [PubMed] [Google Scholar]
- 22.Beard BW, Rettig RL, Ryoo JJ, Parker RA, McLemore EC, Attaluri V. Watch-and-wait compared to operation for patients with complete response to neoadjuvant therapy for rectal cancer. J Am Coll Surg. 2020;231:681–92. doi: 10.1016/j.jamcollsurg.2020.08.775. [DOI] [PubMed] [Google Scholar]
- 23.Dalton RS, Velineni R, Osborne ME, Thomas R, Harries S, Gee AS, et al. A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis. 2012;14:567–71. doi: 10.1111/j.1463-1318.2011.02752.x. [DOI] [PubMed] [Google Scholar]
- 24.Han Z, Li M, Chen J, Ji D, Zhan T, Peng Y, et al. Surgery may not benefit patients with locally advanced rectal cancer who achieved clinical complete response following neoadjuvant chemoradiotherapy. Asian J Surg. 2022;45:97–104. doi: 10.1016/j.asjsur.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Lai CL, Lai MJ, Wu CC, Jao SW, Hsiao CW. Rectal cancer with complete clinical response after neoadjuvant chemoradiotherapy, surgery, or “watch and wait”. Int J Colorectal Dis. 2016;31:413–9. doi: 10.1007/s00384-015-2460-y. [DOI] [PubMed] [Google Scholar]
- 26.Lee SY, Kim CH, Kim YJ, Kim HR. Oncologic outcomes according to the treatment strategy in radiologic complete responders after neoadjuvant chemoradiation for rectal cancer. Oncology. 2015;89:311–8. doi: 10.1159/000439279. [DOI] [PubMed] [Google Scholar]
- 27.Lee JK, Cho JR, Song KS, Oh JH, Jeong SY, Kim MJ, et al. Oncologic comparison between nonradical management and total mesorectal excision in good responders after chemoradiotherapy in patients with mid-to-low rectal cancer. Ann Surg Treat Res. 2021;101:93–101. doi: 10.4174/astr.2021.101.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Liu H, Yin J, Liu S, Hu J, Du F, et al. Wait-and-see or radical surgery for rectal cancer patients with a clinical complete response after neoadjuvant chemoradiotherapy: a cohort study. Oncotarget. 2015;6:42354–61. doi: 10.18632/oncotarget.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Zhao YM, Sun TT, Xu YL, Li SJ, Zhang XY, et al. Total neoadjuvant therapy followed by watch and wait approach or organ preservation for MRI stratified low-risk rectal cancer: early result from a prospective, single arm trial. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:258–65. doi: 10.3760/cma.j.cn.441530-20200222-00070. [DOI] [PubMed] [Google Scholar]
- 30.Wang QX, Zhang R, Xiao WW, Zhang S, Wei MB, Li YH, et al. The watch-and-wait strategy versus surgical resection for rectal cancer patients with a clinical complete response after neoadjuvant chemoradiotherapy. Radiat Oncol. 2021;16:16. doi: 10.1186/s13014-021-01746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeom SS, Lee SY, Kim CH, Kim YJ, Nam TK, Kim HR. Non-operative treatment outcome for rectal cancer patient with clinical complete response after neoadjuvant chemoradiotherapy. Asian J Surg. 2019;42:823–31. doi: 10.1016/j.asjsur.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Yu G, Lu W, Jiao Z, Qiao J, Ma S, Liu X. A meta-analysis of the watch-and-wait strategy versus total mesorectal excision for rectal cancer exhibiting complete clinical response after neoadjuvant chemoradiotherapy. World J Surg Oncol. 2021;19:305. doi: 10.1186/s12957-021-02415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633–40. doi: 10.1200/JCO.2011.37.7176. [DOI] [PubMed] [Google Scholar]
- 34.Smith JD, Ruby JA, Goodman KA, Saltz LB, Guillem JG, Weiser MR, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256:965–72. doi: 10.1097/SLA.0b013e3182759f1c. [DOI] [PubMed] [Google Scholar]
- 35.Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17:174–83. doi: 10.1016/S1470-2045(15)00467-2. [DOI] [PubMed] [Google Scholar]
- 36.Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol. 2015;33:1797–808. doi: 10.1200/JCO.2014.60.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dattani M, Heald RJ, Goussous G, Broadhurst J, São Julião GP, Habr-Gama A, et al. Oncological and survival outcomes in watch and wait patients with a clinical complete response after neoadjuvant chemoradiotherapy for rectal cancer: a systematic review and pooled analysis. Ann Surg. 2018;268:955–67. doi: 10.1097/SLA.0000000000002761. [DOI] [PubMed] [Google Scholar]
- 38.Verseveld M, de Graaf EJ, Verhoef C, van Meerten E, Punt CJ, de Hingh IH, et al. Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study) Br J Surg. 2015;102:853–60. doi: 10.1002/bjs.9809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of limitations of previous studies
Forest plot depicting local recurrence of wait-and-watch (WW) group versus local excision (LE) group.
Forest plot depicting distant metastasis of wait-and-watch (WW) group versus local excision (LE) group.
Forest plot depicting disease-free survival of wait-and-watch (WW) group versus local excision (LE) group.


