Abstract
Background
Exposure to air pollutants and other environmental factors increases the risk of adverse pregnancy outcomes. There is growing evidence that adverse outcomes related to air pollution disproportionately affect racial and ethnic minorities. The objective of this paper is to explore the importance of race as a risk factor for air pollution-related poor pregnancy outcomes.
Methods
Studies investigating the effects of exposure to air pollution on pregnancy outcomes by race were reviewed. A manual search was conducted to identify missing studies. Studies that did not compare pregnancy outcomes among two or more racial groups were excluded. Pregnancy outcomes included preterm births, small for gestational age, low birth weight, and stillbirths.
Results
A total of 124 articles explored race and air pollution as risk factors for poor pregnancy outcome. Thirteen percent of these (n=16) specifically compared pregnancy outcomes among two or more racial groups. Findings across all reviewed articles showed more adverse pregnancy outcomes (preterm birth, small for gestational age, low birth weight, and stillbirths) related to exposure to air pollution among Blacks and Hispanics than among non-Hispanic Whites.
Conclusion
Evidence support our general understanding of the impact of air pollution on birth outcomes and, specifically, of disparities in exposure to air pollution and birth outcomes for infants born to Black and Hispanic mothers. The factors driving these disparities are multifactorial, mostly social, and economic factors. Reducing or eliminating these disparities require interventions at individual, community, state, and national level.
Keywords: Air pollution, Race, Ethnicity, Racial disparities, Pregnancy outcomes, Public health policy
Introduction
According to the World Health Organization (WHO), 91% of the world’s population lives in places where air pollution exceeds recommended limits [1]. Globally, about 4.2 million deaths are attributed to exposure to ambient air pollution and an additional 3.8 million deaths to exposure to household air pollution, with a disproportionate burden among women and children [2, 3].
There is evidence that exposure to air pollution adversely affects human health [4–8]. Additionally, in the last two decades, evidence has emerged that exposure to a variety of pollutants in the air (carbon monoxide, nitrogen oxides, particulate matter, sulfur dioxide, etc.) and other environmental factors increase the risk of adverse pregnancy outcomes [9–12]. Further evidence is accumulating that adverse pregnancy outcomes such as low birth weight (LBW), preterm birth (PTB) and small for gestational age (SGA), maternal and infant mortality are more common in racial minority groups [13–15]. These pregnancy outcomes have been shown to have serious health consequences during the neonatal period and infancy [16–18], childhood [19–21], and adulthood [22–24]. Public health studies in the USA often incorporate race and ethnicity as factors to adjust for. Alternatively, results are often reported stratified by race/ethnicity. The adverse impact of air pollution in racial and ethnic minorities is attributed to factors including age, geographical location, socioeconomic status, and education [25–27]. Although this accumulating research offers solid evidence that exposure to air pollution increases the risk of adverse pregnancy outcomes in general, there is still a need to assess its specific impact on pregnancy outcomes in minority populations, considering growing racial disparities.
Here, we reviewed the epidemiologic studies investigating the effects of exposure to air pollution on pregnancy outcomes in the USA with results stratified by race and/or ethnicity to provide a comprehensive view of exiting research, including types of measurement and study designs, to inform future research directions and to deepen insight into this important topic.
Methods
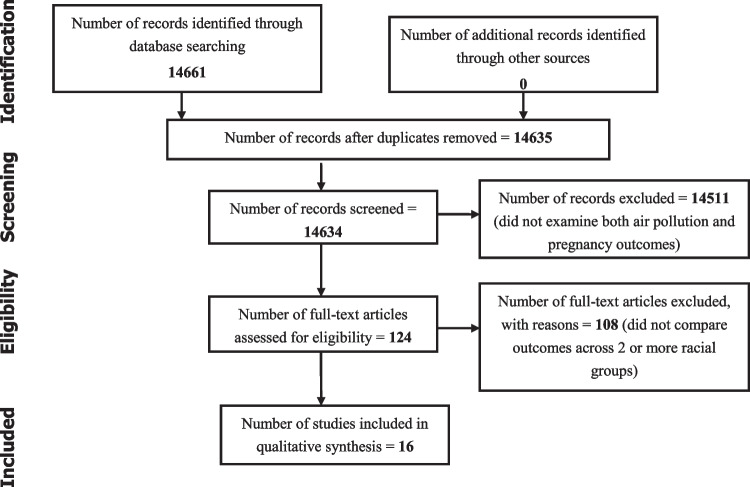
The study process followed the recommendations of the PRISMA checklist for reporting systemic reviews and meta-analysis, where registration of the protocol is not mandated [28]. We searched the PubMed database for eligible studies that explored the effects of pregnancy outcomes on women across all races by combining Medical Subject Headings (MeSH). Key search terms used for the search included a combination of “air pollution” and one or more of the following: “pregnancy outcomes,” “birth outcomes,” “complications of pregnancy,” “race,” “ethnicity,” “black,” “white,” “African American,” and “continental population groups.” We included studies, published in English language, that explored air pollution and racial disparities in pregnancy outcomes and excluded articles that did not have racial comparisons and studies that did not specifically explore the effects of air pollution. We also excluded non-original reports (reviews, letters to the editor, commentaries), articles with unavailable full texts, and duplicated records. Searches with the above terms yielded 14,661 articles. The primary author filtered for studies in the English language, followed by filtering for studies that examined effects of air pollution on pregnancy outcomes and retained 124 eligible articles. Titles and abstracts of all retained studies were then reviewed for eligibility criteria by two reviewers. Any conflicts were resolved through a discussion between the authors. Full articles for eligible studies were accessed via journal sites. Two authors developed the data extraction sheet using Microsoft Excel. Data extraction was performed by the primary reviewer and was cross-checked by two reviewers to ensure accuracy. All discrepancies were resolved via a discussion between the authors. Quality assessment was done by two reviewers who evaluated risk for bias in the included studies using the National Institute of Health (NIH) risk tool [29]. Due to wide variations in methods of exposure capture, a meta-analysis was not performed. Of the 124 studies reviewed, 16 compared pregnancy outcomes among two or more racial groups. For each, we report the study design, sample size, type of pollutant, and method used to capture exposure. We also report the type of adverse pregnancy outcome, specifically, preterm births (PTB), small for gestational age (SGA), low birth weight (LBW), and stillbirths. We compared findings across non-Hispanic white, Hispanic, and Black race/ethnicity (Fig. 1).
Fig. 1.
PRISMA flow diagram
Results
All 16 studies that met review criteria were conducted between 1990 and 2014. Of these, 15 were retrospective cohort studies using birth registries and one was a case control study (Table 1). There was a considerable representation of states across regions of the USA. Sample sizes ranged from 1761 to 1,548,904 births. Different methods were used to estimate levels of air pollution exposure, including Bayesian measurement, Community Multi-scale Air Quality (CMAQ) model, data from United States Environmental Protection Agency (EPA), installation of ambient air monitors, proximity to major roads, and mixed methods. While most studies looked at a single pregnancy outcome, others considered multiple outcomes. Specific details of racial disparities in pregnancy outcomes across race are described below.
Table 1.
Air pollution and racial disparities in pregnancy outcomes in the USA
| Reference | Study design | Study years | Country | Study population/sample size | Total black | Total white | Total Hispanic | Method used to capture exposure | Outcome measure | Adverse pregnancy outcome Total |
Adverse outcome Blacks |
Adverse outcome Whites |
Adverse outcome Hispanics |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benmarhnia T et al | Retrospective cohort | 2005–2010 | California USA | 1,066,783 | 175,297 | 891,486 | Not specified | Measurement of PM2.5 and NO2 by EPA | PTB, SGA | Prevalence PTB=10.9%, SGA=2.2% | PTB=15%; SGA=18.3% | PTB=11.3%; SGA=9.9% | Not specified |
| Gray SC et al | Retrospective cohort | 2001–2006 | North Carolina USA | 457,642 | 22.8% | 62.4% | 14.8% | Bayesian measurement of PM2.5 and O3 | LBW, SGA, PTB | LBW=7.3%; SGA=10.2%; PTB=9.8% | OR LBW=2.13(2.05,2.22); PTB=1.46(1.42,1.50) | Reference | LBW=0.99 (0.93, 1.05); PTB=0.78 (0.75, 0.81) |
| Wallace ME et al | Retrospective cohort study | 2000–2008 | Multi national | 223,375 | 50,255 | 110,541 | 38,811 | CMAQ model | PROM | PROM=7% | PROM=7.6% | PROM=6.9% | PROM=6.4% |
| Hao H, et al | Retrospective cohort | 2002–2006 | Georgia USA | 511,658 | 161,583 | 329,152 | Not Specified |
CMAQ model CO, SO2, O3, NO2, PM2.5 |
PTB | PTB=9.3% | PTB=20,283 | PTB=25,514 | Not specified |
| Woodruff TJ et al | Retrospective cohort | 1998–1999 | California USA | 4,098,740 | 15.0% | 61.2% | 18.8% | U.S. EPA ambient air quality measurement PM10, O3, CO, NO2, SO2 | SGA, PTB | SGA=9.0%, PTB=9.9% |
AOR for SGA=2.0 OR for PTB-1.9 |
Reference | Not specified |
| Kingsley SL et al | Retrospective cohort | 2002–2012 | Rhode Island USA | 61,640 | 4,706 | 36,510 | Not specified | Land-use regression and satellite remote sensing. PM2.5, BC | PTB | PTB=478 | PTB=2,853 | Not specified | |
| DeFranco E et al | Retrospective cohort | 2006–2010 | Ohio USA | 351,036 | 46.6% | 44.8% | 5.8% | U.S. EPA local measurement of PM2.5 | Stillbirths | Stillbirths=0.5% | Stillbirth=8.6 per 1000 | Stillbirth=3.7 per 1000 | Stillbirth=5.7 per 1000 |
| Nobles CJ et al | Retrospective cohort | 2002–2010 | Maryland USA | 109,126 | 216 | 43,055 | 5,328 | CMAQ model, SO2, NO2, PM10 | FGR | FGR=1.5% (825) | FGR=8 | FGR=708 | FGR=88 |
| Le HQ et al | Retrospective cohort | 1990–2001 | Michigan USA | 164,905 | 93,078 | 68,164 | Not specified | Ambient air monitors, SO2, O3CO, NO2, , PM10 | PTB, SGA |
nSGA=13,754 nPTB=24,954 |
SGA=69.1%, PTB=71.1% | SGA=28.6%, PTB=27.1% | |
| Miranda ML et al | Retrospective cohort | 2004–2008 | North Carolina USA | 468,517 | 23.3% | 60.3% | 16.4% | Proximity to major road | LBW, PTB, SGA | LBW=6.8%, PTB=10.5%, 10.2% | LBW=11.6%, PTB=14.6%, SGA=16.1% | LBW=5.5%, PTB=9.1%, SGA=8.1% |
LBW=5.0% PTB=9.9% SGA=9.3% |
| Laurent O et al | Retrospective cohort | 1997–2006 | California USA | 70,000 | 6,261 | 30,548 | 23,678 |
Mixed method NOx, CO, O3, PM |
LBW | LBW=3.19% | LBW=1.23% | LBW=1.59% | |
| Bell ML et al | Retrospective cohort | 1999–2002 | California USA | 358,504 | 10.7% | 83.4% | Not specified | US EPA measurements on NO2, SO2, PM, CO | LBW | LBW=4.01% | LBW=−97.8 (−102.9 to −92.7) | Reference | Not specified |
| Rich DQ et al | Retrospective cohort | 1999–2003 | New Jersey USA | 350,107 | 38,978 | 84,747 | 54,473 |
US EPA measurements PM2.5, NO2, SO2, CO |
SGA | SGA=8%, VSGA=2% | SGA=24% | SGA=46% | SGA=30% |
| Geer LA et al | Retrospective cohort | 1998–2004 | Texas USA | 1,548,904 | 10.6% | 34.7% | 50.7% | US EPA measurements PM, SO2 NO2, CO | LBW | LBW=2.8% | LBW=−168.1 (−170 to −165) | Reference | LBW=−64.1 (−66.0 to −62.3) |
| Ghosh JK et al | Case control | 2003 | CaliforniaUSA | 1,761 | 7% | 10.9% | 73.6% | Air quality monitoring data | LBW | LBW=3.85 (1.54,9.79) | Reference | LBW=2.75 (1.32,5.72) | |
| Kahr MK et al | Retrospective chohort | 2011–2014 | Texas USA | 9004 | 10.7% | 30.5% | 69.5% | People commuting to work | PTB | PTB=10% | PTB=11% | PTB=32% | PTB=68% |
Air Pollution and Preterm Birth
Preterm birth was assessed in eight out of sixteen of the studies reviewed. Benmarhnia et al. [30] used decomposition analysis to understand the racial disparities in PTB in California. Two pollutants were included fine-grained particulate matter (PM2.5) and nitrogen dioxide (NO2). A higher prevalence of PTB was observed for non-Hispanic Black (NHB) mothers when compared with non-Hispanic White mothers. The predicted difference in probability of PTB between Black and White infants was 0.056 (95% CI: 0.054, 0.058). All included predictors explained 37.8% of the Black–White disparity. Overall, individual variables (17.5% for PTB) such as age and level of education, and neighborhood-level variables (16.1% for PTB) such as socioeconomic environment explained a greater proportion of the Black–White difference in birth outcomes than air pollution (5.7% for PTB).
In a cohort of 457,642 births in North Carolina, using Bayesian measurement of PM2.5 and ozone (O3), Gray et al. [27] reported that NHB and Hispanic mothers were more likely to have infants born with lower birth weight when compared with NHW mothers (−188.2 g, 95% CI: −184.3 to −192.1 and −47.3 g, 95% CI: −42.4 to −52.3, respectively).
Using the CMAQ model and measurements from stationary monitors, Hao et al. [31] investigated the association between 11 ambient air pollutants and the risk of PTB in the state of Georgia. They observed that all traffic-related pollutants (carbon monoxide (CO), NO2 PM2.5, elemental carbon) were associated with PTB (e.g., odds ratios for interquartile range increases in CO during the first, second, and third trimesters and total pregnancy were 1.005 (95% CI: 1.001, 1.009), 1.007 (95% CI: 1.002, 1.011), 1.010 (95% CI: 1.006, 1.014), and 1.011 (95% CI: 1.006, 1.017), respectively). Associations were higher for African American mothers when compared to white mothers.
Woodruff et al. [32] using data from the EPA Ambient Air Monitoring observed that Hispanic, African-American, and Asian/Pacific Islander mothers experienced higher mean levels of air pollution and were more than twice as likely to live in the most air polluted counties across the country compared with White mothers. In addition, there was an increase in the odds of preterm delivery (AOR = 1.05; 95% CI, 0.99–1.12) in a county with high air pollution.
Le et al. [33] reported a stronger association between air pollutants and PTB for Blacks than Whites. PTB was associated with SO2 (OR 1.07, 1.01–1.14) exposure in the last month of pregnancy, while O3 exposures exceeding 92 parts per billion (OR 1.08, 1.02–1.14) were associated with PTB in the first months of pregnancy.
Air Pollution and Small for Gestational Age
Of all 16 studies reviewed, six assessed SGA. Benmarhnia et al. [30] explored racial disparities in SGA in California using data provided by the U.S. Environmental Protection Agency (EPA) and the California Air Resources Board (CARB) to assign chronic air pollution exposures to each birth record, linked by maternal zip code of residence. The predicted difference in probability of SGA between Black and White infants was 0.084 (95% CI: 0.081, 0.087). Together, individual demographics, neighborhood socioeconomic environment (such as unemployment and poverty rates), and neighborhood air pollution explained 37.8% of the Black–White disparity. There was a higher prevalence of SGA among non-Hispanic Blacks (18.3%) as compared to non-Hispanic Whites (9.9%).
In a cohort of 457,642 births in North Carolina, using Bayesian measurement of PM2.5 and ozone, Gray et al. [27] reported that infants born to NHB mothers and Hispanic mothers were at an increased odds of SGA (AOR = 2.18, 95% CI: 2.12 to 2.24 and AOR = 1.21, CI: 1.17 to 1.26, respectively), compared to NHW mothers. After controlling for race and individual and area-level socio-economic status, this difference persisted, suggesting that air pollution is an additional contributor to the observed outcomes.
In a retrospective cohort study of 4,098,750 births in California, Woodruff et al. [32] observed that Hispanic, African-American, and Asian/Pacific Islander mothers experienced higher mean levels of air pollution, determined from strategically placed EPA monitors. They were also more than twice as likely to live in the most polluted counties compared with White mothers, after controlling for maternal risk factors, region, and educational status (Hispanic mothers: AOR = 4.66; 95% CI: 1.92–11.32; African-American mothers: AOR = 2.58; 95% CI: 1.00–6.62; Asian/Pacific Islander mothers: AOR = 2.82; 95% CI, 1.07–7.39]. However, there was no significant increase in the odds of SGA (AOR = 0.96; 95% CI, 0.86–1.07) in counties with higher air pollution.
In a cohort of 164,905 births in Michigan, Le et al. [33] showed that there was an association between term SGA with exposure to CO and NO2 during the first trimester of pregnancy. They also reported an association between term SGA and exposure to O3 and PM10 during the later stages of pregnancy. There was evidence of stronger associations between CO and term-SGA, NO2 and term-SGA, and SO2 and term-SGA for infants of Black mothers as compared to White mothers.
Rich et al. [34] found significantly increased risk of SGA associated with first and third trimester exposures to PM2.5 and increased risk of very small for gestational age (VSGA) associated with first, second, and third trimester exposures to high NO2 concentrations. According to this study, mothers of SGA and VSGA infants were more likely to be less than 25 years old and less likely to have completed high school, compared to mothers of appropriate-size births. They were also more likely to be single, African American, and to have smoked during pregnancy.
Air Pollution and Low Birth Weight
Low birth weight was assessed in five of the 16 studies reviewed. Gray et al. [27] used multivariate analysis of factors including PM2.5 and O3 to assess their association with weight differences in grams and 95% CI for all births. PM2.5 exposures were associated with LBW among infants born to NHB and Hispanic mothers more than those born to NHW mothers (−187.5 g, 95% CI: −183.6 to −191.4 and −46.8 g, 95% CI: −41.8 to −51.7, respectively).
Miranda et al. [35] characterized maternal exposure to traffic-related air pollution during pregnancy by using residential proximity to major road ways as a proxy. Women residing within 250 m of a major roadway were at 3–5% increased odds of having a LBW baby than women residing more than 250 meters away (p<0.05). The mean birth weight was 3376 g for NHW, 2114 g for NHB, and 3330 g for Hispanics.
In a retrospective cohort study in California [36], African Americans, despite being a significantly lower percentage of the study population, had 3.19% of the LBW infants, compared to 1.23% for whites and 1.59% for Hispanics. Bell et al. [37] also reported that the association between air pollutants (especially PM2.5) and LBW for infants of Black mothers was stronger than for White mothers.
In a large cohort study of 1,548,904 births in Texas, Geer et al. [38] reported that interquartile increases in ambient air pollutant concentrations of SO2 and O3 were associated with a 4.99 g (95% CI, 1.87–8.11) and 2.72 g (95% CI, 1.11–4.33) decreases in birth weight, respectively. Lower birth weight was associated with exposure to O3 in the first and second trimester, whereas results for other pollutants did not differ significantly by trimester.
In a case-control study, Ghosh et al. [39] observed that women who were exposed to secondhand smoke at home had increased odds of term LBW (AOR = 1.36; 95% CI: 0.85, 2.18) compared to unexposed women. Blacks and Hispanic had higher odds of having LBW babies compared to White mothers.
Air Pollution and Stillbirths
Only one cohort study among reviewed studies assessed stillbirths in relation to air pollution. According to DeFranco et al. [11], high average PM2.5 exposure through pregnancy was not associated with a significant increase in stillbirth risk (AOR 1.21; 95% CI: 0.96, 1.53). There was also no higher risk of stillbirth associated with exposure in either the first or second trimester. However, exposure to high levels of PM2.5 in the third trimester of pregnancy was associated with 42% increased risk of stillbirth, (AOR = 1.42; 1.06, 1.91). Stillbirth rates were higher among mothers older than 40 years (11.6 per 1000), NHB mothers (8.6 per 1000), and mothers with lower education level and tobacco use.
Discussion
The objective of this review was to explore the importance of race/ethnicity as a risk factor for air pollution-related poor pregnancy outcomes.
In recent years, issues of racial disparities and air pollution exposure have received increasing attention in the USA and globally. Trends in air pollution and racial disparities suggest worse exposure in people of color (POC) when compared to non-Hispanic whites (NHW). One of the most comprehensive and informative analysis is that of Liu and colleagues [40] who quantified exposure disparities among racial/ethnic groups (NHW, NHB, Hispanic (any race), non-Hispanic Asian) and by income for multiple spatial units (contiguous United States, states, urban vs. rural areas) and years (1990, 2000, 2010) for carbon monoxide, nitrogen dioxide, ozone, particulate matter with aerodynamic diameter ≤2.5 μm and ≤10 μm (PM2.5 and PM10), and sulfur dioxide. They concluded that for all years and pollutants, the racial/ethnic group with the highest national average exposure was a racial/ethnic minority group (NHB), with a national mean air pollution exposure higher for all three racial/ethnic minorities than for NHW. The degree of these disparities varied by pollutant and state. These findings are consistent with other studies that have explored this topic [13, 32, 41–44].
Similarly, trends in outcomes of pregnancy suggest worse outcomes in POC when compared to NHW. Approximately 700 women die in the USA each year as a result of pregnancy or its complications [45]. Pregnancy-related mortality rates are three times higher in black women than white women and two times higher in American Indian/Alaska natives [46]. POC are more likely to have other factors that contribute to maternal and infant mortality including: teenage pregnancy, preterm birth, low birth weight, late or no prenatal care [46–48]. The factors driving these disparities are multifactorial, mostly social and economic factors — income, transportation, education, access to food, differences in access to healthcare including pre-natal care, and differences in health insurance coverage as well as structural and systemic racism and discrimination [14, 45, 46, 49–51].
This review suggests that air pollution is associated with adverse pregnancy outcomes in the USA and higher rates of adverse outcomes among people of color, than among Whites. These findings are consistent with studies conducted in other countries [13, 14, 52]. There are a multitude of factors other than exposure to air pollution that have also been shown to be associated with poor pregnancy outcomes, including socioeconomic status, level of education, and maternal smoking. These factors also tend to be independently associated with increased exposure to air pollutants and poor pregnancy outcomes [53–55]. However, when stratified by race, the exposures and outcomes tend to be worse in minority groups suggesting racial disparities in both exposures to air pollutants and outcomes of pregnancy. Almost all the studies we reviewed were large retrospective cohort studies; therefore, other factors (socio-economic) at least partially explain the disparities observed in the studies reviewed. We point, however, to evidence from molecular epidemiologic studies that suggest biological mechanisms to explain the effects of air pollution on pregnancy outcomes [56–58]. There is little data to suggest that Black mothers have any greater genetic predisposition to adverse effects of air pollution than Whites [26, 30, 58]. Finally, while studies suggest that PTB is associated with SO2, and O3 exposure, the effects of such pollutants may in part be due to their roles in exacerbating other disease conditions that are known to cause PTB, e.g., hypertension, diabetes, asthma, and preeclampsia [59, 60]. This is also another area for further research. In fact, Casey and colleagues in a recent study found that power plant retirements were associated with a decrease in the proportion of preterm birth within 5 km (−0.019, 95% CI: −0.031, −0.008) and 5–10 km (−0.015, 95% CI: −0.024, −0.007), controlling for secular trends with mothers living 10–20 km away [61].
Even though none of the studies reviewed explored household air pollution, there is evidence that it also contributes to poor pregnancy outcomes, especially in developing countries where cooking is done by burning of biomass in poorly ventilated homes [12, 62–64]. Several measures can be taken to improve air quality and reduce the negative impacts of air pollution on overall health and on gestational health in particular. At individual and household levels, these measures include avoidance of cooking or heating with biomass fuel indoors, ensuring adequate household ventilation, quitting smoking, use of chimneys, and the use of air filters [65]. At state and national levels, policy measures could include carbon tax and subsidies for alternative energy sources [66]. Lu and colleagues [67] proposed a comprehensive 12-point plan to close the black-white gap in birth outcomes that includes increased pre-conception care to POC, expanding access to healthcare to underserved areas, strengthening father involvement in minority families, closing the education gap, supporting working mothers and families, fighting systemic racism, among others.
A potential shortcoming in our review is the limited number of available published studies. We cannot rule out that many studies on this topic with null results may have remained unpublished. We also could not assess how differences in the methods by which air pollution was captured in different studies may have contributed to the reported results. Potential exposure misclassification is certainly an important possibility to consider when evaluating research in this area of investigation if we are to build a corpus of studies reliable enough to guide changes in practice and policy.
We have reviewed existing evidence that suggests a need for further investigation of the impact of air pollution on pregnancy outcomes and how these vary by race and ethnicity. Despite the limitations noted, we believe that this review of 16, US-based studies increases our understanding of the impact of air pollution on maternal-child health and of disparities in maternal-child health across races and ethnicities. With the goal of enhancing the literature and filling gaps in knowledge on this topic, on the basis of our review, we suggest future studies that explore the biologic factors underlying air pollution’s adverse effects; incorporate measurement of household air pollution and personalized exposure measurement; establish the causal mechanisms of air pollution’s impact on specific outcomes, including prematurity, birthweight, IUGR, and fetal deaths; determine the period during pregnancy in which women are most likely to be affected by air pollutants and to establish the specific effects of different pollutants and pollutant mixtures; and explore differential effects of specific air pollutants on Blacks versus Whites.
Acknowledgements
This review paper is made possible through the support of the University of Chicago Center for Global Health and the Graduate Program in Health Administration and Policy (GPHAP). Special thanks to Laura Botwinick for mentorship and guidance during this project and to Susan Duncan for editorial assistance.
Data Availability
Not applicable
Declarations
Ethics Approval
N/A — This was a review of published articles requiring no ethical approval.
Consent to Participate
All authors have consented to participate in this publication.
Consent for Publication
All authors have given their consent for this article to be published.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global ambient air pollution [Internet]. [cited 2019 Apr 19]. Available from: https://www.who.int/health-topics/air-pollution#tab=tab_1.
- 2.WHO | Air pollution [Internet]. WHO. [cited 2019 Apr 19]. Available from: http://www.who.int/airpollution/en/.
- 3.Oluwole O, Arinola GO, Ana GR, Wiskel T, Huo D, Olopade OI, et al. Relationship between household air pollution from biomass smoke exposure, and pulmonary dysfunction, oxidant-antioxidant imbalance and systemic inflammation in rural women and children in Nigeria. Glob J Health Sci. 2013;5(4):28–38. doi: 10.5539/gjhs.v5n4p28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shima M. Health effects of air pollution: a historical review and present status. Nihon Eiseigaku Zasshi Jpn J Hyg. 2017;72(3):159–165. doi: 10.1265/jjh.72.159. [DOI] [PubMed] [Google Scholar]
- 5.Mannucci PM, Franchini M. Health effects of ambient air pollution in developing countries. Int J Environ Res Public Health. 2017;14(9):1048. doi: 10.3390/ijerph14091048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coker E, Kizito S. A narrative review on the human health effects of ambient air pollution in Sub-Saharan Africa: an urgent need for health effects studies. Int J Environ Res Public Health. 2018;15(3):427. doi: 10.3390/ijerph15030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowler D, Dise N, Sheppard L. Committee on air pollution effects research: 40 years of UK air pollution. Environ Pollut Barking Essex 1987. 2016;208(Pt B):876–878. doi: 10.1016/j.envpol.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Khilnani GC, Tiwari P. Air pollution in India and related adverse respiratory health effects: past, present, and future directions. Curr Opin Pulm Med. 2018;24(2):108–116. doi: 10.1097/MCP.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 9.Glinianaia SV, Rankin J, Bell R, Pless-Mulloli T, Howel D. Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiol Camb Mass. 2004;15(1):36–45. doi: 10.1097/01.ede.0000101023.41844.ac. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Laurent O, Li L, Hu J, Kleeman M. Adverse reproductive health outcomes and exposure to gaseous and particulate-matter air pollution in pregnant women. Res Rep Health Eff Inst. 2016;188:1–58. [PMC free article] [PubMed] [Google Scholar]
- 11.DeFranco E, Hall E, Hossain M, Chen A, Haynes EN, Jones D, et al. Air pollution and stillbirth risk: exposure to airborne particulate matter during pregnancy is associated with fetal death. PLoS ONE. 2015;10(3):e0120594. doi: 10.1371/journal.pone.0120594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arinola GO, Dutta A, Oluwole O, Olopade CO. Household air pollution, levels of micronutrients and heavy metals in cord and maternal blood, and pregnancy outcomes. Int J Environ Res Public Health. 2018;15(12):2891. doi: 10.3390/ijerph15122891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grobman WA, Parker CB, Willinger M, Wing DA, Silver RM, Wapner RJ, et al. Racial disparities in adverse pregnancy outcomes and psychosocial stress. Obstet Gynecol. 2018;131(2):328–335. doi: 10.1097/AOG.0000000000002441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Cardarelli K, Shim R, Ye J, Booker KL, Rust G. Racial disparities in economic and clinical outcomes of pregnancy among Medicaid recipients. Matern Child Health J. 2013;17(8):1518–1525. doi: 10.1007/s10995-012-1162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones MR, Diez-Roux AV, O’Neill MS, Guallar E, Sharrett AR, Post W, et al. Ambient air pollution and racial/ethnic differences in carotid intima-media thickness in the Multi-Ethnic Study of Atherosclerosis (MESA) J Epidemiol Community Health. 2015;69(12):1191–1198. doi: 10.1136/jech-2015-205588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paneth NS. The problem of low birth weight. Future Child Cent Future Child David Lucile Packard Found. 1995;5(1):19–34. doi: 10.2307/1602505. [DOI] [PubMed] [Google Scholar]
- 17.Kavurt S, Celik K. Incidence and risk factors of postnatal growth restriction in preterm infants. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2018;31(8):1105–1107. doi: 10.1080/14767058.2017.1306512. [DOI] [PubMed] [Google Scholar]
- 18.Catov JM, Scifres CM, Caritis SN, Bertolet M, Larkin J, Parks WT. Neonatal outcomes following preterm birth classified according to placental features. Am J Obstet Gynecol. 2017;216(4):411.e1–411.e14. doi: 10.1016/j.ajog.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Jefferis BJMH, Power C, Hertzman C. Birth weight, childhood socioeconomic environment, and cognitive development in the 1958 British birth cohort study. Br Med J. 2002;325(7359):305–308. doi: 10.1136/bmj.325.7359.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korten I, Ramsey K, Latzin P. Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev. 2017;21:38–46. doi: 10.1016/j.prrv.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Moreira RS, Magalhães LC, Alves CRL. Effect of preterm birth on motor development, behavior, and school performance of school-age children: a systematic review. J Pediatr (Rio J). 2014;90(2):119–134. doi: 10.1016/j.jped.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Kaijser M, Bonamy AKE, Akre O, Cnattingius S, Granath F, Norman M, et al. Perinatal risk factors for ischemic heart disease: Disentangling the roles of birth weight and preterm birth. Circulation. 2008;117(3):405–410. doi: 10.1161/CIRCULATIONAHA.107.710715. [DOI] [PubMed] [Google Scholar]
- 23.Crump C, Sundquist K, Sundquist J. Adult outcomes of preterm birth. Prev Med. 2016;91:400–401. doi: 10.1016/j.ypmed.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Raju TNK, Buist AS, Blaisdell CJ, Moxey-Mims M, Saigal S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatr Oslo Nor 1992. 2017;106(9):1409–1437. doi: 10.1111/apa.13880. [DOI] [PubMed] [Google Scholar]
- 25.Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am J Prev Med. 2010;39(3):263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 26.McKinnon B, Yang S, Kramer MS, Bushnik T, Sheppard AJ, Kaufman JS. Comparison of black–white disparities in preterm birth between Canada and the United States. CMAJ Can Med Assoc J. 2016;188(1):E19–E26. doi: 10.1503/cmaj.150464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray SC, Edwards SE, Schultz BD, Miranda ML. Assessing the impact of race, social factors and air pollution on birth outcomes: a population-based study. Environ Health. 2014;29(13):4. doi: 10.1186/1476-069X-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement | Annals of Internal Medicine [Internet]. [cited 2023 Feb 9]. Available from: https://www.acpjournals.org/doi/full/10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed]
- 29.Study Quality Assessment Tools | NHLBI, NIH [Internet]. [cited 2023 Feb 9]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 30.Benmarhnia T, Huang J, Basu R, Wu J, Bruckner TA. Decomposition analysis of Black–White disparities in birth outcomes: the relative contribution of air pollution and social factors in California. Environ Health Perspect. 2017;125(10):107003. doi: 10.1289/EHP490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao H, Chang HH, Holmes HA, Mulholland JA, Klein M, Darrow LA, et al. Air pollution and preterm birth in the U.S. State of Georgia (2002–2006): associations with concentrations of 11 ambient air pollutants estimated by combining Community Multiscale Air Quality Model (CMAQ) simulations with stationary monitor measurements. Environ Health Perspect. 2016;124(6):875–880. doi: 10.1289/ehp.1409651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodruff TJ, Parker JD, Kyle AD, Schoendorf KC. Disparities in exposure to air pollution during pregnancy. Environ Health Perspect. 2003;111(7):942–946. doi: 10.1289/ehp.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le HQ, Batterman SA, Wirth JJ, Wahl RL, Hoggatt KJ, Sadeghnejad A, et al. Air pollutant exposure and preterm and term small-for-gestational-age births in Detroit, Michigan: Long-term trends and associations. Environ Int. 2012;44:7–17. doi: 10.1016/j.envint.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rich DQ, Demissie K, Lu SE, Kamat L, Wartenberg D, Rhoads GG. Ambient air pollutant concentrations during pregnancy and the risk of fetal growth restriction. J Epidemiol Community Health. 2009;63(6):488–496. doi: 10.1136/jech.2008.082792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miranda ML, Edwards SE, Chang HH, Auten RL. Proximity to roadways and pregnancy outcomes. J Expo Sci Environ Epidemiol. 2013;23(1):32–38. doi: 10.1038/jes.2012.78. [DOI] [PubMed] [Google Scholar]
- 36.Laurent O, Wu J, Li L, Chung J, Bartell S. Investigating the association between birth weight and complementary air pollution metrics: a cohort study. Environ Health. 2013;17(12):18. doi: 10.1186/1476-069X-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115(7):1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geer LA, Weedon J, Bell ML. Ambient air pollution and term birth weight in Texas from 1998 to 2004. J Air Waste Manag Assoc 1995. 2012;62(11):1285–1295. doi: 10.1080/10962247.2012.707632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh JKC, Wilhelm M, Su J, Goldberg D, Cockburn M, Jerrett M, et al. Assessing the influence of traffic-related air pollution on risk of term low birth weight on the basis of land-use-based regression models and measures of air toxics. Am J Epidemiol. 2012;175(12):1262–1274. doi: 10.1093/aje/kwr469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Clark LP, Bechle MJ, Hajat A, Kim SY, Robinson AL, et al. Disparities in air pollution exposure in the United States by race/ethnicity and income, 1990–2010. Environ Health Perspect. 129(12):127005. [DOI] [PMC free article] [PubMed]
- 41.Clark LP, Millet DB, Marshall JD. Changes in transportation-related air pollution exposures by race-ethnicity and socioeconomic status: outdoor nitrogen dioxide in the United States in 2000 and 2010. Environ Health Perspect. 2017;125(9):097012. doi: 10.1289/EHP959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kravitz-Wirtz N, Crowder K, Hajat A, Sass V. THE LONG-TERM DYNAMICS OF RACIAL/ETHNIC INEQUALITY IN NEIGHBORHOOD AIR POLLUTION EXPOSURE, 1990-2009. Bois Rev Soc Sci Res Race. 2016;13(2):237–259. doi: 10.1017/S1742058X16000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Disparities in Distribution of Particulate Matter Emission Sources by Race and Poverty Status | AJPH | Vol. 108 Issue 4 [Internet]. [cited 2022 Sep 5]. Available from: https://ajph.aphapublications.org/doi/full/10.2105/AJPH.2017.304297?casa_token=ZBMYrbfQDCkAAAAA%3ATKFsmeG_WjACSfpo1ewdKg2IwWAYThnp0t2TzUNZ4yyxuYxAX92ULCJqxVeEG3gZhuu4WOLeAlo. [DOI] [PMC free article] [PubMed]
- 44.Collins MB, Munoz I, JaJa J. Linking ‘toxic outliers’ to environmental justice communities. Environ Res Lett. 2016;11(1):015004. doi: 10.1088/1748-9326/11/1/015004. [DOI] [Google Scholar]
- 45.Petersen EE. Racial/ethnic disparities in pregnancy-related deaths — United States, 2007–2016. MMWR Morb Mortal Wkly Rep [Internet]. 2019;68 [cited 2022 Sep 18]. Available from: https://www.cdc.gov/mmwr/volumes/68/wr/mm6835a3.htm. [DOI] [PMC free article] [PubMed]
- 46.Pham O, Nov 10 URP, 2020. Racial disparities in maternal and infant health: an overview - issue brief [Internet]. KFF. 2020 [cited 2022 Sep 18]. Available from: https://www.kff.org/report-section/racial-disparities-in-maternal-and-infant-health-an-overview-issue-brief/.
- 47.About Teen Pregnancy | CDC [Internet]. 2021 [cited 2022 Sep 18]. Available from: https://www.cdc.gov/teenpregnancy/about/index.htm.
- 48.Gadson A, Akpovi E, Mehta PK. Exploring the social determinants of racial/ethnic disparities in prenatal care utilization and maternal outcome. Semin Perinatol. 2017;41(5):308–317. doi: 10.1053/j.semperi.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Hung P, Henning-Smith CE, Casey MM, Kozhimannil KB. Access to obstetric services in rural counties still declining, with 9 percent losing services, 2004–14. Health Aff (Millwood). 2017;36(9):1663–1671. doi: 10.1377/hlthaff.2017.0338. [DOI] [PubMed] [Google Scholar]
- 50.Badreldin N, Grobman WA, Yee LM. Racial disparities in postpartum pain management. Obstet Gynecol. 2019;134(6):1147–1153. doi: 10.1097/AOG.0000000000003561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woo B, Kravitz-Wirtz N, Sass V, Crowder K, Teixeira S, Takeuchi DT. Residential segregation and racial/ethnic disparities in ambient air pollution. Race Soc Probl. 2019;11(1):60–67. doi: 10.1007/s12552-018-9254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morello-Frosch R, Shenassa ED. The environmental “riskscape” and social inequality: implications for explaining maternal and child health disparities. Environ Health Perspect. 2006;114(8):1150–1153. doi: 10.1289/ehp.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Šrám RJ, Binková B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113(4):375–382. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Victora CG, Barros F, Huttly SR, Teixeira AMB, Vaughan P. Early childhood mortality in a brazilian cohort: the roles of birthweight and socioeconomic status. Int J Epidemiol. 1992;21(5):911–915. doi: 10.1093/ije/21.5.911. [DOI] [PubMed] [Google Scholar]
- 55.How racism may cause black mothers to suffer the death of their infants [Internet]. NPR.org. [cited 2019 May 18]. Available from: https://www.npr.org/sections/health-shots/2017/12/20/570777510/how-racism-may-cause-black-mothers-to-suffer-the-death-of-their-infants
- 56.Song J, Chen Y, Wei L, Ma Y, Tian N, Huang SY, et al. Early-life exposure to air pollutants and adverse pregnancy outcomes: protocol for a prospective cohort study in Beijing. BMJ Open [Internet]. 2017;7(9):e015895. doi: 10.1136/bmjopen-2017-015895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dejmek J, Solanský I, Benes I, Lenícek J, Srám RJ. The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. Environ Health Perspect. 2000;108(12):1159–1164. doi: 10.1289/ehp.001081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perera F, Hemminki K, Jedrychowski W, Whyatt R, Campbell U, Hsu Y, et al. In utero DNA damage from environmental pollution is associated with somatic gene mutation in newborns. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2002;11(10 Pt 1):1134–1137. [PubMed] [Google Scholar]
- 59.Padula AM, Huang H, Baer RJ, August LM, Jankowska MM, Jellife-Pawlowski LL, Sirota M, Woodruff TJ. Environmental pollution and social factors as contributors to preterm birth in Fresno County. Environ Health. 2018;17(1):70. 10.1186/s12940-018-0414-x. [DOI] [PMC free article] [PubMed]
- 60.Lavigne E, Yasseen AS, Stieb DM, Hystad P, van Donkelaar A, Martin RV, et al. Ambient air pollution and adverse birth outcomes: differences by maternal comorbidities. Environ Res. 2016;148:457–466. doi: 10.1016/j.envres.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 61.Retirements of coal and oil power plants in California: association with reduced preterm birth among populations nearby | American Journal of Epidemiology | Oxford Academic [Internet]. [cited 2023 Feb 9]. Available from: https://academic.oup.com/aje/article/187/8/1586/4996680?login=false. [DOI] [PMC free article] [PubMed]
- 62.Alexander D, Northcross A, Wilson N, Dutta A, Pandya R, Ibigbami T, et al. Randomized controlled ethanol cookstove intervention and blood pressure in pregnant Nigerian women. Am J Respir Crit Care Med. 2017;195(12):1629–1639. doi: 10.1164/rccm.201606-1177OC. [DOI] [PubMed] [Google Scholar]
- 63.Ghosh JKC, Wilhelm M, Ritz B. Effects of residential indoor air quality and household ventilation on preterm birth and term low birth weight in Los Angeles County. California. Am J Public Health. 2013;103(4):686–694. doi: 10.2105/AJPH.2012.300987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan MN, Nurs B, CZ, Mofizul Islam M, Islam MR, Rahman MM. Household air pollution from cooking and risk of adverse health and birth outcomes in Bangladesh: a nationwide population-based study. Environ Health Glob Access Sci. Source. 2017;16(1):57. doi: 10.1186/s12940-017-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.New Guidance for Residential Air Cleaners - ProQuest [Internet]. [cited 2020 Jun 30]. Available from: https://search.proquest.com/openview/411efdabd7ff51ce0fbe480b039313e0/1?pq-origsite=gscholar&cbl=41118.
- 66.US EPA O. Economic Incentives [Internet]. US EPA. 2014 [cited 2020 Jun 30]. Available from: https://www.epa.gov/environmental-economics/economic-incentives.
- 67.Lu MC, Kotelchuck M, Hogan V, Jones L, Wright K, Halfon N. Closing the Black-White gap in birth outcomes: a life-course approach. Ethn Dis. 2010;20(1 0 2):S2-62–S2-76. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable