Abstract
Tau proteins accumulation and their spreading pattern were affected by gender in cognitive impairment patients, especially in the progression of Alzheimer’s disease (AD). However, it was unclear whether the gender effects for tau deposition influenced by amyloid deposition. The aim of this study was to investigate gender differences for tau depositions in Aβ positive (A+) subjects. In this study, tau and amyloid positron emission tomography images, structural magnetic resonance imaging images, and demographic information were collected from 179 subjects in Alzheimer’s Disease Neuroimaging Initiative (ADNI) database and 63 subjects from Huashan Hospital. Subjects were classified as T+ or T− according to the presence or absence of tau (T) biomarkers. We used two-sample t test and one-way analysis of variance test to analyze the effect of gender with adjusting for age, years of education, and Minimum Mental State Examination. In the ADNI cohort, we found differences in Tau deposition in fusiform gyrus, inferior temporal gyrus, middle temporal gyrus and parahippocampal gyrus between the female T+ (FT+) and male T+ (MT+) groups (p < 0.05). Tau deposition did not differ significantly between female T− (FT−) and male T− (MT−) subjects (p > 0.05). In the Huashan Hospital cohort, there was no difference in Tau deposition between FT+ and MT+ (p > 0.05). The results show that tau depositions significantly increased in females in above brain regions. Our findings suggest that tau deposition is influenced by gender in the A+ subjects. This result has important clinical implications for the development of gender-guided early interventions for patients with both Tau and Amyloid depositions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s43657-022-00076-9.
Keywords: Alzheimer’s disease, Gender, Amyloid positron emission computed tomography, Tau positron emission computed tomography
Introduction
Alzheimer’s disease (AD) is one of the major neurodegeneration diseases in the elderly, and it has become an important medical challenge due to its incurability and irreversibility (Jia et al. 2020; Long and Holtzman 2019). According to the 2018 AT N research framework proposed by the National Institute on Aging and Alzheimer’s Association, the diagnosis of AD is defined by the presence of amyloid β (Aβ), phosphorylated tau, and neurodegeneration. Among them, Aβ and tau protein deposition in the brain are two most important AD biomarkers (Ossenkoppele et al. 2018). Especially, while Aβ-positive (A+) subjects are considered to enter the AD continuum, tau pathology, as a major driver of local neurodegeneration (La Joie et al. 2020), has high clinical relevance in AD late stages (Jack et al. 2013).
Gender plays an important role in brain development and aging (Kaczkurkin et al. 2019), as well as the occurrence and development of neurodegeneration (Ferretti et al. 2018). In AD, gender is believed to be a crucial factor for heterogeneity. While the impact of gender on AD epidemiology is the subject of intense current investigation, the concept of sex-specific clinicopathological AD phenotypes is largely unexplored (Ferretti et al. 2018). In many AD studies, gender is usually considered as a covariable similar to age, without further exploration. Fortunately, gender differences in AD pathological biomarkers have attracted people’s attention in recent years (Demetrius et al. 2021; Ferretti et al. 2018), which is of great significance for the development of AD precision medicine. Additionally, the effect of gender on brain tau deposition has also been explored in a few in vitro studies but limited in vivo studies with controversial findings (Barnes et al. 2005; Hu et al. 2021; Jack et al. 2017a; Mattsson et al. 2017; Oveisgharan et al. 2018; Salehi et al. 1998). Hence, to expand the current knowledge of gender effect on brain tau accumulation in the AD continuum, we conducted a two-cohort study including Caucasian and Asian subjects with two different tau positron emission computed tomography (PET) tracers in A+ populations (Jack et al. 2018). By addressing such phenotypic variation, the current study may have significant implications for the development of precise and effective therapeutics in AD.
Materials and Methods
Subjects
The subjects of this study were drawn from two cohorts, the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and Huashan cohorts. The study was also approved by the Ethics Committee of Huashan Hospital, Fudan University, Shanghai, China. All subjects from Huashan Hospital provided written informed consent.
Table 1 shows the characteristics of all 242 participants. The ADNI cohort consisted of 179 subjects and the Huashan cohort consisted of 63 subjects. Notably, all 63 subjects from Huashan cohort are with A+ and Tau-positive (T+). Each subject provided detailed clinical information including: gender, age, years of education, Mini-Mental State Examination (MMSE), Clinical Dementia Rating-Sum of Boxes (CDR-SB), the Alzheimer’s Disease Assessment Scale-Cognitive 13 (ADAS-Cog 13; ADNI only) and Montreal Cognitive Assessment (MoCA; ADNI only), and imaging data, including 18F-florbetapir (18F-AV-45) PET,18F-flortaucipir (18F-AV-1451, ADNI only) PET, 18F-florzolotau PET [also known as 18F-APN-1607, or 18F-PM-PBB3 (Lu et al. 2020; Mattsson et al. 2017); Huashan only] and T1-weighted structural MRI.
Table 1.
The demographic and clinical information of all subjects
| ADNI | Huashan Hospital | |||||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | |||
| n | 94 | 85 | 35 | 28 | ||
| T−/T+ | 63/31 | 64/21 | 0/35 | 0/28 | ||
| APOE ε4 carriers | 50 (53.2%) | 27 (31.8%) | 22 (62.9%) | 19 (67.9%) | ||
| Diagnosis | ||||||
| NC | 48 (51.1%) | 35 (41.2%) | 0 | 0 | ||
| CI | 46 (48.9%) | 50 (58.8%) | 35 (100%) | 28 (100%) | ||
| Age, years ± SD (range) | 74.1 ± 8.0 (57–89) | 74.3 ± 6.8 (63–89) | 77.4 ± 7.1 (66–92)a | 80.0 ± 8.5 (63–94)b | 66.0 ± 9.4 (51–80) | 66.1 ± 9.9 (44–83) |
| Education, years ± SD | 15.9 ± 2.3 | 15.6 ± 2.0 | 16.9 ± 2.4a | 18.1 ± 2.4b | 10.3 ± 3.2 | 10.9 ± 4.1 |
| MMSE, score ± SD | 28.1 ± 2.4 | 24.6 ± 5.2 | 27.9 ± 2.7 | 26.4 ± 3.3 | 21.0 ± 5.4 | 21.1 ± 5.7 |
| CDR-SB, score ± SD | 0.9 ± 1.8 | 2.5 ± 2.8 | 1.1 ± 1.7 | 2.4 ± 2.9 | 6.5 ± 3.1 | 6.4 ± 2.7 |
| ADAS13, score ± SD | 15.1 ± 8.0 | 24.2 ± 12.1 | 17.6 ± 8.2 | 21.7 ± 6.6 | – | – |
| MoCA, score ± SD | 24.9 ± 3.9 | 20.1 ± 6.5 | 24.4 ± 3.0 | 22.4 ± 3.9b | – | – |
ADAS13 Alzheimer’s Disease Assessment Scale-Cognitive 13, APOE Apolipoprotein E, CDR-SB Clinical Dementia Rating Scale-Sum of Boxes, CI cognitive impairment, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment, NC normal controls, SD Standard Deviation
ap < 0.05 compared to FT−
bp < 0.05 compared to FT+
In the ADNI cohort, the inclusion criteria of normal controls (NC), mild cognitive impairment (MCI) and AD refer to ADNI_GeneralProceduresManual.pdf (https://adni.loni.usc.edu/wp-content/uploads/2010/09/ADNI_GeneralProceduresManual.pdf). The NC group was the subjects with clinical diagnosis of NC in the ADNI cohort. The cognitive impairment (CI) group included MCI and AD subjects. In Huashan cohort, NC was defined as the subjects with normal performance in neuropsychological tests with visual confirmation of amyloid negative on 18F-AV-45 or 11C-PiB PET. The CI group comprised subjects with MCI and AD. The inclusion criteria for MCI were based on the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) criteria (Samuel 1995). Only those who were not diagnosed with dementia were considered for a diagnosis of MCI, which was defined according to Petersen’s criteria (Petersen 2004). The inclusion of AD dementia was based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria (McKhann et al. 1984).
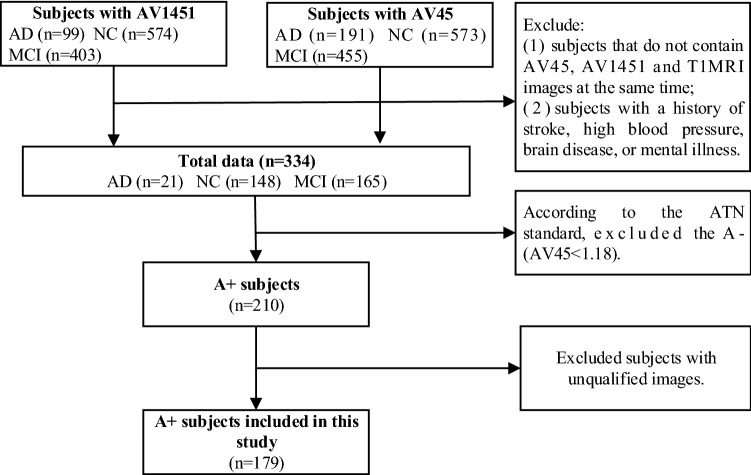
The inclusion criteria of the subjects in the ADNI cohort were as follows: (1) no history of stroke, hypertension, brain disease or mental illness; (2) all subjects were A+ [18F-AV-45 PET standardized uptake value ratio (SUVR) of cerebral relative to cerebellum is higher than 1.18] (Chen et al. 2015). For Huashan cohort, all subjects were with visual confirmation of A+ on 18F-AV-45 or 11C-PiB PET (Lundeen et al. 2018). The other inclusion criteria were same as ADNI cohort. Figure 1 shows the data inclusion and exclusion process for all subjects in the ADNI cohort.
Fig. 1.
Flowchart of the inclusion and exclusion of subjects in this study (ADNI dataset)
Image Acquisition and Preprocessing
The detailed information of image acquisition for ADNI data was available on the LONI website (https://ida.loni.usc.edu/login.jsp). The details of the image acquisition for Huashan data were described previously (Li et al. 2021; Shi et al. 2020).
The preprocessing steps were as follows. First, the original digital imaging and communications in medicine (DICOM) data were converted to the neuroimaging informatics technology initiative (NIFTI) file format using DCM2NII (http://people.cas.sc.edu/rorden/mricron/index.html). Second, the gray matter (GM), white matter, and cerebrospinal fluid images were segmented from T1-weighted images by using the CAT12 toolbox (http://dbm.neuro.uni-jena.de/cat/). Third, the PET images were co-registered with their corresponding T1-weighted images and corrected for partial volume effects (PVE) based on the Muller-Gartner algorithm (Gonzalez-Escamilla et al. 2017) using the PETPVE12 toolbox (http://www.fil.ion.ucl.ac.uk/spm/ext/#PETPVE12). Fourth, the GM images were normalized to the Montreal Neurological Institute (MNI) standard space, and the PVE-corrected PET images were normalized to the MNI space using the forward transformation parameters determined by the GM image spatial normalization. Finally, these PET images were smoothed with an 8-mm full width at half-maximum (FWHM) Gaussian kernel. We also performed preprocessing without PVE correction (PVC) in parallel. All procedures were implemented using the Statistical Parametric Mapping (SPM8) software (www.fil.ion.ac.uk/spm).
More details on the image acquisition and Muller–Gartner correction method are described in the Supplement material.
Extraction of Meta-ROI and SUVR Calculation
According to previous studies (Chotipanich et al. 2020), we selected 10 meta-regions of interest (meta-ROI) based on the Anatomical Automatic Labeling (AAL) template, including fusiform gyrus, inferior temporal gyrus, lingual gyrus, middle temporal gyrus, occipital gyrus, parahippocampal, superior parietal gyrus, posterior cingulate gyrus, and precuneus. The tau SUVR of the ROIs was calculated using the cerebellar gray matter as a reference brain region in standard MNI space (Jack et al. 2017b).
Definition of A+/A−/T+/T−
In the ADNI cohort, the definitions of A+/A−/T+/T− were from previous studies; For 18F-AV45 PET, the whole cerebellum was used as the reference region, and the whole cerebral cortex was used as the ROI to calculate the SUVR; The SUVR of 1.18 was considered as a cut-off point for A+/A− (Chen et al. 2015).
For 18F-AV1451 PET, based on previous study, a bilateral weighted SUVR value from a composite temporal meta-ROI (entorhinal, amygdala, parahippocampal, fusiform, inferior temporal, and middle temporal regions) was calculated by a reference region (cerebellar GM); The SUVR of 1.33 was considered as a cut-off point for T+/T− (Jack et al. 2017b). We also performed a visual assessment to validate the results. In Huashan cohort, due to the lack of cut-off criterion of T+/T− for 18F-florzolotau PET images, and the ununified amyloid tracers for A+/A− (18F-AV-45 or 11C-PiB), grouping was done with visual assessment by three experienced neuroradiologists (CTZ: 25 years of experience; HWZ: 15 years of experience; JYL: 5 years of experience), who were blind to the clinical diagnosis. 18F-Florzolotau PET images were displayed with the Mango viewer software (version 4.1; Research Imaging Institute, The University of Texas Health Science Center, San Antonio, TX, USA; http://ric.uthscsa.edu/mango). The maximum intensity was manually adjusted so that the predominant color in the inferior cerebellar cortex would be the midpoint of the spectrum color scale. Images were evaluated in the transverse, sagittal, and coronal views, and the overall pattern of each scan was scored as positive or negative for global cortical binding. In particular, the reader was instructed to evaluate binding in hippocampus, temporal lobe, parietal lobe, occipital lobe, frontal lobe, and cingulate gyrus, and only scans with negativity in all assessed regions would be defined as T−. The final decision of T+/T− was based on the consensus of at least two independent assessors.
Statistical Analysis
Quantitative values of SUVR to be analyzed for all subjects were calculated using MATLAB 2017a, and subjects’ clinical information and quantitative results were statistically analyzed by SPSS 25.0 (SPSS Inc., IBM Corporation, Chicago, USA), and Prism software (available from Prism-GraphPad) was used to plot and visualize the statistical data of this study. Differences of clinical variables in different groups were analyzed using two-sample t test and one-way Analysis of Variance (ANOVA) test. For tau deposition, in the ADNI cohort, the differences of Tau SUVR in different ROIs were analyzed at the gender level without distinguishing T+ and T−, as well as at gender-specific population with homologous tau pathology [female tau positive (FT+) versus male tau positive (MT+), female tau negative (FT−) versus male tau negative (MT−)]. A two-sample t test was used to analyze the effect of gender on the SUVR for the 10 ROIs after adjusting for tau SUVR of the 10 ROIs according to age, years of education, and MMSE. In the Huashan cohort, since only patients with T+ were included, the comparison between genders was just performed between FT+ and MT+ using the same method as the ADNI cohort analysis. The above experiments were repeated using data without PVC and the results are summarized in the Supplementary material. p < 0.05 was considered statistically significant.
Results
Demographic Characteristics
Table 1 lists demographic information and related indicators of AD cognitive ability assessment. In the ADNI cohort, compared to FT+, MT+ was slightly older (p = 0.005) and more educated (p < 0.001). In addition, the MoCA scale score of group MT+ was slightly higher than that of FT+ (p = 0.043). There was no statistical difference in global disease severity as per MMSE (p = 0.57), CDR-SB (p = 0.60), and ADAS13 (p = 0.48) between T+ groups of different genders. In the T− cohort, men were older (p = 0.012) and more educated (p = 0.016) than female, too. Similarly, no significant difference between MT− and FT− in MMSE (p = 0.57), CDR-SB (p = 0.27), ADAS13 (p = 0.057), and MoCA (p = 0.097) was found. In addition, we also calculated the cortical thickness of 10 ROIs, including entorhinal, fusiform, inferior temporal gyrus, lateral occipital, lingual gyrus, middle temporal gyrus, parahippocampal, superior parietal gyrus, posterior cingulate gyrus and precuneus. There was no significant difference for all regions for Huashan cohort (p > 0.05). Right parahippocampal gyrus was slightly thicker in females than that in males for ADNI cohort (p = 0.02). There were no significant differences in other ROIs for ADNI cohort (p > 0.05). For details, see Table S2.
In the Huashan cohort, no difference between MT+ and FT+ was found (age: p = 0.98, years of education: p = 0.27, MMSE: p = 0.86, CDR-SB: p = 0.98). In addition, only cognitively impairment patients with “A+T+” were included in Huashan cohort because in the hospital-based cohort the subjects with normal cognition and with cognitive dysfunction but showing “A+T−” were very limited. Further validation within the “A+T−” subjects will be carried out when we have enough data.
Effects of Gender on Regional Tau SUVR in ADNI Cohort
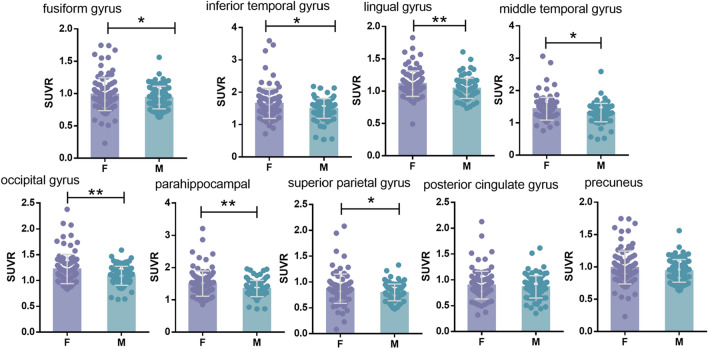
Female had higher tau SUVR values in the fusiform gyrus (p = 0.027), inferior temporal gyrus (p = 0.046), lingual gyrus (p = 0.005), middle temporal gyrus (p = 0.046), occipital lobe (p = 0.006), parahippocampal (p = 0.005), and superior parietal lobe (p = 0.027) regions (Fig. 2). There was no statistical difference in posterior cingulate gyrus (p = 0.32) and precuneus (p = 0.14) between males and females.
Fig. 2.
In the ADNI cohort, grouped by sex, there were regions of interest for differences in AV-1451 SUVR. AV-1451 SUVR for female (purple) and male (blue) are depicted. *p < 0.05, **p < 0.01, ***p < 0.001
Analysis of the data without PVC found that the lingual gyrus (p = 0.097), occipital lobe (p = 0.005), parahippocampal gyrus (p = 0.027), and superior parietal lobe (p < 0.001) preserve the effect of gender on tau SUVR (Supplementary Fig. S1).
Effects of Gender on Regional Tau SUVR in T+/T− Group in the Two Cohorts
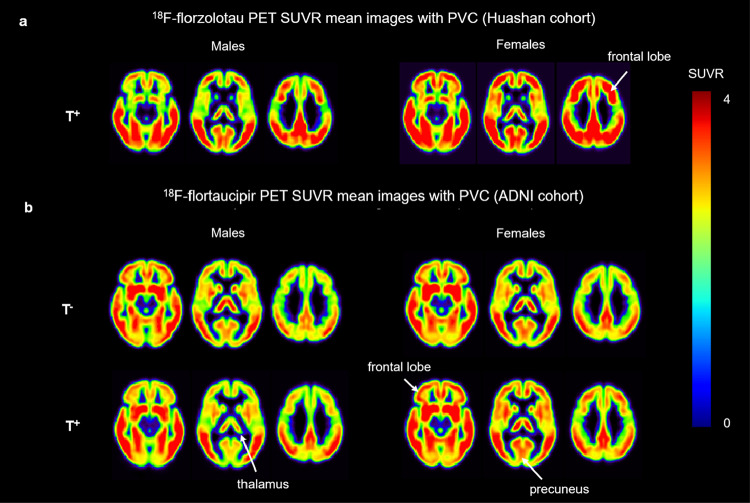
The 18F-florzolotau PET mean images with PVC (Huashan cohort) (Fig. 3a) demonstrated increased contrast among FT+ compared to MT+. The same result is also consistent in the ADNI cohort (Fig. 3b).
Fig. 3.
Mean images with PVC. a 18F-florzolotau PET SUVR mean images with PVC in Huashan cohort. b 18F-flortaucipir PET SUVR mean images with PVC in ADNI cohort. The color scale represents the SUVR
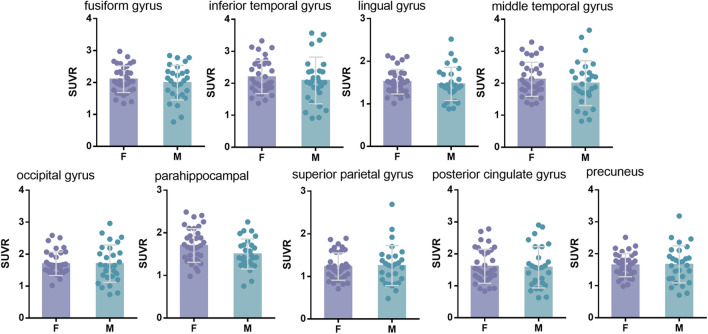
In the Huashan cohort, since only T+ patients were enrolled, the comparison between genders were only done with homologous tau pathology. In line to the above findings in the ADNI cohort, higher Tau SUVR values in the parahippocampal gyrus (p = 0.38) were found in females than that in males (Fig. 4). Table 2 shows the detailed Tau SUVR of each ROI in different groups.
Fig. 4.
In the Huashan cohort, grouped by sex, there were regions of interest for differences in florzolotau SUVR. Florzolotau SUVR for female (purple) and male (blue) are depicted. *p < 0.05, **p < 0.01, ***p < 0.001
Table 2.
The detailed tau SUVR of each ROI in different groups
| ADNI | Huashan Hospital | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FT− | FT+ | MT− | MT+ | p value | padjust | FT+ | MT+ | p value | padjust | |
| Fusiform gyrus | 1.5 ± 0.2 | 2.0 ± 0.4 | 1.5 ± 0.2 | 1.7 ± 0.3b | 0.002 | 0.009 | 2.1 ± 0.4 | 2.0 ± 0.5 | 0.38 | 0.97 |
| Inferior temporal gyrus | 1.5 ± 0.2 | 2.0 ± 0.6 | 1.5 ± 0.3 | 1.6 ± 0.3b | 0.001 | 0.009 | 2.2 ± 0.5 | 2.1 ± 0.7 | 0.49 | 0.97 |
| Lingual gyrus | 1.0 ± 0.1 | 1.2 ± 0.4 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.00 | 1.00 | 1.5 ± 0.3 | 1.5 ± 0.4 | 0.55 | 0.97 |
| Middle temporal gyrus | 1.3 ± 0.2 | 1.7 ± 0.5 | 1.3 ± 0.3 | 1.4 ± 0.2a | 0.016 | 0.041 | 2.1 ± 0.5 | 2.0 ± 0.7 | 0.45 | 0.97 |
| Occipital gyrus | 1.1 ± 0.1 | 1.4 ± 0.6 | 1.1 ± 0.2 | 1.2 ± 0.2 | 0.12 | 0.18 | 1.7 ± 0.4 | 1.7 ± 0.6 | 0.98 | 0.98 |
| Parahippocampal | 1.4 ± 0.2 | 1.8 ± 0.5 | 1.3 ± 0.2 | 1.5 ± 0.3a | 0.018 | 0.041 | 1.7 ± 0.4 | 1.5 ± 0.3a | 0.042 | 0.38 |
| Superior parietal gyrus | 0.8 ± 0.2 | 1.0 ± 0.3 | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.06 | 0.11 | 1.2 ± 0.3 | 1.3 ± 0.5 | 0.83 | 0.97 |
| Posterior cingulate gyrus | 0.8 ± 0.2 | 1.0 ± 0.4 | 0.8 ± 0.2 | 1.0 ± 0.2 | 1.00 | 1.00 | 1.6 ± 0.5 | 1.6 ± 0.6 | 0.84 | 0.97 |
| Precuneus | 0.9 ± 0.2 | 1.2 ± 0.3 | 0.9 ± 0.2 | 1.0 ± 0.2 | 0.24 | 0.31 | 1.6 ± 0.4 | 1.7 ± 0.5 | 0.86 | 0.97 |
padjust p value after Benjamini and Hochberg correction
ap < 0.05 compared to FT+
bp < 0.01 compared to FT+
In the data without PVC, tau deposits were found to be higher in females than that in males only in the parahippocampal gyrus (p = 0.018). The results are shown in Supplementary Fig. S2.
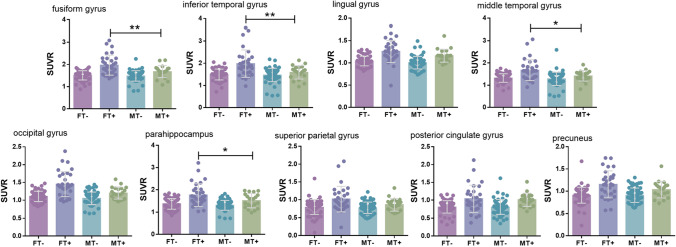
In the T+ ADNI cohort, tau SUVR values were higher in the fusiform gyrus (p = 0.009), inferior temporal gyrus (p = 0.009), middle temporal gyrus (p = 0.041), and parahippocampal (p = 0.041) in the females than that in males, while no significant difference was found in lingual gyrus, occipital lobe, superior parietal lobe, posterior cingulate gyrus and precuneus (p > 0.05). In the T− subset, all ROIs had similar Tau SUVR values between genders (Fig. 5).
Fig. 5.
In the ADNI cohort, grouped by FT−, FT+, MT−, and MT+, there were regions of interest for differences in tau SUVR. AV-1451 SUVR for female (red and purple) and male (blue and green) are depicted. *p < 0.05, **p < 0.01, ***p < 0.001
Analysis of data without PVC found that compared with MT+, FT+ had higher tau deposition in the fusiform gyrus (p = 0.078), inferior temporal gyrus (p = 0.023), middle temporal gyrus (p = 0.023), occipital gyrus (p = 0.023) and superior parietal gyrus (p = 0.02). Gender had no effect on tau deposition in lingual gyrus, parahippocampal, posterior cingulate gyrus and precuneus (Supplementary Fig. S3).
The comparison results without PVC are summarized in the supplementary material (Supplementary Fig. S1–S4 and Table S1).
Discussion
In this study, we explored the gender effect on tau accumulation in A+ subjects using two different tau PET tracers in two different ethnicities, and we consistently found that in T+ individuals, females suffered from heavier tau burden than males after controlling other confounding factors including age, education degree, and global cognitive dysfunction.
In the study, we found more tau deposition in women than men among those who entered the AD disease spectrum and were tau-positive. Previous studies have shown that women have a greater tau burden than men, which is consistent with our findings (Yan et al. 2021). In addition, based on ROI analysis, we also found inconsistent regions of interest in the ADNI cohort and the Huashan cohort in which sex caused differences in tau deposition. In the ADNI cohort, we found higher tau SUVR values in the cingulate gyrus, inferior temporal gyrus, middle temporal gyrus, and parahippocampal gyrus FT+ groups than that in the MT+ group. The ROI difference of tau deposition in the two cohorts may be caused by ethnic differences. Ethnic differences in AD biomarkers have been reported (Meeker et al. 2021; Shadlen et al. 1999; Tang et al. 1998; Weuve et al. 2018; Xiong et al. 2022). Most of the subjects in the ADNI cohort were from Europe and the United States, while the subjects included in the Huashan cohort were all from Asia. The differences in brain structure and cognitive reserve between subjects in both cohorts also may be a reason for the different tau deposition in various brain regions of ethnic groups.
Gender has been recognized as a potential risk factor for AD and has drawn a lot of attention in the scientific community over the past few decades. Although the greater prevalence of AD in females is considered an indirect consequence of the greater longevity, other social and biological factors such as education degrees, sex hormones, and genes are also thought to contribute to the AD-related sex differences (Medeiros and Silva 2019; Zhu et al. 2021). Among these factors, deviations in brain structure and biomarkers are one of the important biological explanations for such sexual dimorphism in AD. For example, in brain development, the average brain volume of female was found to be smaller than male, and hence more sensitive to pathological agents for AD (Mielke et al. 2014). More rapid neurodegeneration (i.e., annual atrophy rate) was also found in female patients of AD than males (Ardekani et al. 2016; Hua et al. 2010). For the two key pathological mechanisms—Aβ and phosphorylated tau accumulation, several studies have been conducted to explore the differences. In AD, no clear sex differences in Aβ burden have been found (Barnes et al. 2005; Holland et al. 2013; Jansen et al. 2015; Mattsson et al. 2017), except one post-mortem study indicated a higher degree of cerebral amyloid angiopathy in men than in women (Shinohara et al. 2016). For tau pathology, most post-mortem pathological studies in patients with AD did not suggest any effect of sex on global burden of tau, while individual reports indicated possible effects in specific brain regions where women had higher numbers of neurofibrillary tangles (Barnes et al. 2005; Salehi et al. 1998). And in cognitively intact elderly individuals, significantly higher Braak stages for tangles in women than men were only seen after 80 years (Hu et al. 2021). Cerebrospinal fluid (CSF) research in vivo reported similar findings, one of which reported no difference between genders in patients with AD (Mattsson et al. 2017), and another reported higher tau concentrations in women than in men with MCI (undefined) (Holland et al. 2013). One Tau PET study in cognitively unimpaired individuals aged 50 years and older found no sex differences in prevalence of tau positive (Jack et al. 2017a),while the possible sex effects on tau accumulation in vivo in specific regions in subjects under biological AD category (A+T+) required further investigation. Furthermore, a recent post-mortem study in community-based elders found that women had higher levels on a global measure of AD pathology, especially tau tangle density than men (Oveisgharan et al. 2018), which encouraged the further exploration for the possible sex effect on brain tau accumulation in vivo in those under Alzheimer’s continuum.
Herein, we conducted a two-cohort study with two different tau PET tracers, one of which was 18F-AV1451, the same first-generation ligand as reported in the previous study (Jack et al. 2017a), and another was 18F-florzolotau, a second-generation ligand with high affinity for both 3R and 4R tau deposits (Chini et al. 2020) in A+ subjects. Whereas no gender differences were found in A+T− participants with 18F-AV1451 PET scans, and the significantly higher tau burden was observed in FT+ than that in MT+ with both tau tracers when eliminating the confounding effects of age, education degree, and global cognitive dysfunction. The specific regions with elevated tau located in early involved regions in Braak stage system (Braak et al. 2006), including fusiform gyrus, inferior temporal gyrus, middle temporal gyrus, parahippocampal gyrus in the ADNI cohort, as well as parahippocampal gyrus in the Huashan cohort, which were also previously found to harbor significant higher 18F-AV1451 bindings in female APOE ε4 carriers than male APOE ε4 carriers (Yan et al. 2020). The similar tau burden in A+T− between genders might result from the relatively young ages, since in vitro study in cognitive unimpaired elders only saw the differences in those older than 80 years (Hu et al. 2021).
It is worth noting that the FT+ suffered from higher tau deposition than MT+, but had similar age, education degree, and disease severity (global cognition). It is widely accepted that in vivo Tau SUVR is a sensitive index for disease severity monitoring and progression prediction (Ossenkoppele et al. 2016). One possible explanation for such inconsistent tau level and disease severity in females might be the different vulnerability of the downstream dysfunction of tau—neurodegeneration, given that female brain was found to have 1) a persistently younger metabolic brain age compared with the male brain on 18F-FDG PET images, which relative to the chronological age (Goyal et al. 2019), and 2) greater cortical thickness throughout the lifespan on MRI images (Sowell et al. 2007). Meanwhile, although sex is found to modify APOE ε4 dose effect on brain tau deposition in cognitively impaired individuals (Yan et al. 2021), this study did not combine gender and APOE information for analysis because of some subjects in the ADNI cohort lacked APOE ε4. The conclusion in this study is that FT+ has a higher Tau burden than MT+, and whether it is related to APOE ε4 needs further investigation. Another possible reason was sex hormones since a previous hormone-based study found that testosterone has a protective effect on tau protein, which was lower in women (Sundermann et al. 2020).
Many studies have shown that PVC can effectively increase the correspondence of the measured signal with the true regional tracer uptake (Wolters et al. 2018; Zhao et al. 2019), thus has better regulating effect on SUVRs. In our study, PVC was performed for all data. Compared with the results without partial volume adjustment, most results were consistent but little more brain regions were found to differ in the analysis of gender groups, namely the fusiform gyrus, middle temporal gyrus, and inferior temporal gyrus.
There are some limitations to this study. First, the small sample size limits the statistical ability of data. We tried to overcome this problem by including other center subjects, but the requirement to receive both Aβ PET and Tau PET scans in the A+ group greatly limited the number of potential participants. In addition, the Huashan cohort is an ongoing hospital-based investigation; therefore, the number of subjects with normal cognition and cognitively impaired patients with “A+T−” is very limited. We expect to supplement this data and carry out more comprehensive research in the future. Second, our study was cross-sectional, and long-term longitudinal follow-up data could further support our hypothesis. Third, this study included the data from the two centers, but did not consider the impact of different imaging devices on the data. Fourthly, we only carried out the experiment of calculating Tau SUVR by using cerebellar gray matter as the reference brain region, and selecting different reference brain regions may affect the results. Fifthly, in the Huashan cohort, tau PET uses a new imaging agent that currently lacks T+/T− threshold studies. Visual assessment results were affected by subjective factors, and we expect to divide T+/T− with an objective quantitative threshold to verify our results in the future. Finally, lack of APOE ε4 in some participants restricted further exploration of possible gene effect on such brain tau deposition between genders. Considering the shortcomings of this study, future multi-center cooperation is required to include more participants to verify the robustness of the results.
Conclusion
Despite these limitations, our current data represent a promising step in understanding the sex effect on brain tau accumulation. As a conclusion, gender differences effected tau deposition in A+ subject, which might help the future development of a “precision medicine” approach in AD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82020108013, 81971641, 82071200, 82021002), the research project of Shanghai Health Commission (2020YJZX0111), the Shanghai Aging and Maternal and Child Health Research Special Project (Grant 2020YJZX0111) and the Clinical Research Plan of Shanghai Hospital Development Center (Grants SHDC2020CR1038B and SHDC2020CR4007). Also, special thanks to HWZ, who helped us with the visual assessment of the images of the Huashan cohort.
Authors’ Contributions
All authors significantly contributed to planning, writing, and editing of the manuscript.
Data Availability
Data from the ADNI cohort are available on the website (https://adni.loni.usc.edu/). Data from the Huashan cohort were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Huashan Hospital.
Code Availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was approved by the Ethics Committee of Huashan Hospital, Fudan University, Shanghai, China.
Consent to participate
All subjects from Huashan Hospital provided written informed consent.
Consent for publication
Not applicable.
Footnotes
Ying Zhang and Jiaying Lu have contributed equally to this work.
Contributor Information
Chuantao Zuo, Email: zuochuantao@fudan.edu.cn.
Jiehui Jiang, Email: jiangjiehui@shu.edu.cn.
References
- Ardekani BA, Convit A, Bachman AH. Analysis of the MIRIAD data shows sex differences in hippocampal atrophy progression. J Alzheimers Dis JAD. 2016;50(3):847–857. doi: 10.3233/jad-150780. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62(6):685–691. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Roontiva A, Thiyyagura P, Lee W, Liu X, Ayutyanont N, Protas H, Luo JL, Bauer R, Reschke C, Bandy D, Koeppe RA, Fleisher AS, Caselli RJ, Landau S, Jagust WJ, Weiner MW, Reiman EM. Improved power for characterizing longitudinal amyloid-β PET changes and evaluating amyloid-modifying treatments with a cerebral white matter reference region. J Nucl Med off Publ Soc Nucl Med. 2015;56(4):560–566. doi: 10.2967/jnumed.114.149732. [DOI] [PubMed] [Google Scholar]
- Chini M, Pöpplau JA, Lindemann C, Carol-Perdiguer L, Hnida M, Oberländer V, Xu X, Ahlbeck J, Bitzenhofer SH, Mulert C, Hanganu-Opatz IL. Resolving and rescuing developmental miswiring in a mouse model of cognitive impairment. Neuron. 2020;105(1):60–74.e67. doi: 10.1016/j.neuron.2019.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotipanich C, Nivorn M, Kunawudhi A, Promteangtrong C, Boonkawin N, Jantarato A. Evaluation of imaging windows for tau PET imaging using (18)F-PI2620 in cognitively normal individuals, mild cognitive impairment, and Alzheimer's disease patients. Mol Imaging. 2020;19:1536012120947582. doi: 10.1177/1536012120947582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetrius LA, Eckert A, Grimm A. Sex differences in Alzheimer's disease: metabolic reprogramming and therapeutic intervention. Trends Endocrinol Metab. 2021;32(12):963–979. doi: 10.1016/j.tem.2021.09.004. [DOI] [PubMed] [Google Scholar]
- Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, Santuccione Chadha A, Baracchi F, Girouard H, Misoch S, Giacobini E, Depypere H, Hampel H, For the Women’s Brain P, the Alzheimer Precision Medicine I Sex differences in Alzheimer disease—the gateway to precision medicine. Nat Rev Neurol. 2018;14(8):457–469. doi: 10.1038/s41582-018-0032-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Escamilla G, Lange C, Teipel S, Buchert R, Grothe MJ. PETPVE12: an SPM toolbox for Partial Volume Effects correction in brain PET—application to amyloid imaging with AV45-PET. Neuroimage. 2017;147:669–677. doi: 10.1016/j.neuroimage.2016.12.077. [DOI] [PubMed] [Google Scholar]
- Goyal MS, Blazey TM, Su Y, Couture LE, Durbin TJ, Bateman RJ, Benzinger TLS, Morris JC, Raichle ME, Vlassenko AG. Persistent metabolic youth in the aging female brain. Proc Natl Acad Sci USA. 2019;116(8):3251–3255. doi: 10.1073/pnas.1815917116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D, Desikan RS, Dale AM, McEvoy LK. Higher rates of decline for women and apolipoprotein E epsilon4 carriers. AJNR Am J Neuroradiol. 2013;34(12):2287–2293. doi: 10.3174/ajnr.A3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YT, Boonstra J, McGurran H, Stormmesand J, Sluiter A, Balesar R, Verwer R, Swaab D, Bao AM. Sex differences in the neuropathological hallmarks of Alzheimer's disease: focus on cognitively intact elderly individuals. Neuropathol Appl Neurobiol. 2021;47(7):958–966. doi: 10.1111/nan.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Hibar DP, Lee S, Toga AW, Jack CR, Jr, Weiner MW, Thompson PM. Sex and age differences in atrophic rates: an ADNI study with n = 1368 MRI scans. Neurobiol Aging. 2010;31(8):1463–1480. doi: 10.1016/j.neurobiolaging.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Wiste HJ, Weigand SD, Therneau TM, Knopman DS, Lowe V, Vemuri P, Mielke MM, Roberts RO, Machulda MM, Senjem ML, Gunter JL, Rocca WA, Petersen RC. Age-specific and sex-specific prevalence of cerebral β-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross-sectional study. Lancet Neurol. 2017;16(6):435–444. doi: 10.1016/s1474-4422(17)30077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, Gunter JL, Senjem ML, Jones DT, Kantarci K, Machulda MM, Mielke MM, Roberts RO, Vemuri P, Reyes DA, Petersen RC. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement. 2017;13(3):205–216. doi: 10.1016/j.jalz.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, Visser PJ, Aalten P, Aarsland D, Alcolea D, Alexander M, Almdahl IS, Arnold SE, Baldeiras I, Barthel H, van Berckel BN, Bibeau K, Blennow K, Brooks DJ, van Buchem MA, Camus V, Cavedo E, Chen K, Chetelat G, Cohen AD, Drzezga A, Engelborghs S, Fagan AM, Fladby T, Fleisher AS, van der Flier WM, Ford L, Förster S, Fortea J, Foskett N, Frederiksen KS, Freund-Levi Y, Frisoni GB, Froelich L, Gabryelewicz T, Gill KD, Gkatzima O, Gómez-Tortosa E, Gordon MF, Grimmer T, Hampel H, Hausner L, Hellwig S, Herukka SK, Hildebrandt H, Ishihara L, Ivanoiu A, Jagust WJ, Johannsen P, Kandimalla R, Kapaki E, Klimkowicz-Mrowiec A, Klunk WE, Köhler S, Koglin N, Kornhuber J, Kramberger MG, Van Laere K, Landau SM, Lee DY, de Leon M, Lisetti V, Lleó A, Madsen K, Maier W, Marcusson J, Mattsson N, de Mendonça A, Meulenbroek O, Meyer PT, Mintun MA, Mok V, Molinuevo JL, Møllergård HM, Morris JC, Mroczko B, Van der Mussele S, Na DL, Newberg A, Nordberg A, Nordlund A, Novak GP, Paraskevas GP, Parnetti L, Perera G, Peters O, Popp J, Prabhakar S, Rabinovici GD, Ramakers IH, Rami L, Resende de Oliveira C, Rinne JO, Rodrigue KM, Rodríguez-Rodríguez E, Roe CM, Rot U, Rowe CC, Rüther E, Sabri O, Sanchez-Juan P, Santana I, Sarazin M, Schröder J, Schütte C, Seo SW, Soetewey F, Soininen H, Spiru L, Struyfs H, Teunissen CE, Tsolaki M, Vandenberghe R, Verbeek MM, Villemagne VL, Vos SJ, van Waalwijk van Doorn LJ, Waldemar G, Wallin A, Wallin Å K, Wiltfang J, Wolk DA, Zboch M, Zetterberg H, Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Quan M, Fu Y, Zhao T, Li Y, Wei C, Tang Y, Qin Q, Wang F, Qiao Y, Shi S, Wang YJ, Du Y, Zhang J, Zhang J, Luo B, Qu Q, Zhou C, Gauthier S, Jia J. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. 2020;19(1):81–92. doi: 10.1016/s1474-4422(19)30290-x. [DOI] [PubMed] [Google Scholar]
- Kaczkurkin AN, Raznahan A, Satterthwaite TD. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology. 2019;44(1):71–85. doi: 10.1038/s41386-018-0111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Joie R, Visani AV, Baker SL, Brown JA, Bourakova V, Cha J, Chaudhary K, Edwards L, Iaccarino L, Janabi M, Lesman-Segev OH, Miller ZA, Perry DC, O'Neil JP, Pham J, Rojas JC, Rosen HJ, Seeley WW, Tsai RM, Miller BL, Jagust WJ, Rabinovici GD. Prospective longitudinal atrophy in Alzheimer's disease correlates with the intensity and topography of baseline tau-PET. Sci Transl Med. 2020 doi: 10.1126/scitranslmed.aau5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liu FT, Li M, Lu JY, Sun YM, Liang X, Bao W, Chen QS, Li XY, Zhou XY, Guan Y, Wu JJ, Yen TC, Jang MK, Luo JF, Wang J, Zuo C. Clinical utility of (18) F-APN-1607 tau PET imaging in patients with progressive supranuclear palsy. Mov Disord off J Mov Disord Soc. 2021;36(10):2314–2323. doi: 10.1002/mds.28672. [DOI] [PubMed] [Google Scholar]
- Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179(2):312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Bao W, Li M, Li L, Zhang Z, Alberts I, Brendel M, Cumming P, Lu H, Xiao Z, Zuo C, Guan Y, Zhao Q, Rominger A. Associations of [(18)F]-APN-1607 tau PET binding in the brain of Alzheimer's disease patients with cognition and glucose metabolism. Front Neurosci. 2020;14:604. doi: 10.3389/fnins.2020.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeen TF, Seibyl JP, Covington MF, Eshghi N, Kuo PH. Signs and artifacts in amyloid PET. Radiographics. 2018;38(7):2123–2133. doi: 10.1148/rg.2018180160. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Lönneborg A, Boccardi M, Blennow K, Hansson O. Clinical validity of cerebrospinal fluid Aβ42, tau, and phospho-tau as biomarkers for Alzheimer's disease in the context of a structured 5-phase development framework. Neurobiol Aging. 2017;52:196–213. doi: 10.1016/j.neurobiolaging.2016.02.034. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Medeiros AM, Silva RH. Sex Differences in Alzheimer's disease: where do we stand? J Alzheimers Dis JAD. 2019;67(1):35–60. doi: 10.3233/jad-180213. [DOI] [PubMed] [Google Scholar]
- Meeker KL, Wisch JK, Hudson D, Coble D, Xiong C, Babulal GM, Gordon BA, Schindler SE, Cruchaga C, Flores S, Dincer A, Benzinger TL, Morris JC, Ances BM. Socioeconomic status mediates racial differences seen using the AT(N) framework. Ann Neurol. 2021;89(2):254–265. doi: 10.1002/ana.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/clep.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, O'Neil JP, Janabi M, Lazaris A, Cantwell A, Vogel J, Santos M, Miller ZA, Bettcher BM, Vossel KA, Kramer JH, Gorno-Tempini ML, Miller BL, Jagust WJ, Rabinovici GD. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain J Neurol. 2016;139(Pt 5):1551–1567. doi: 10.1093/brain/aww027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Rabinovici GD, Smith R, Cho H, Schöll M, Strandberg O, Palmqvist S, Mattsson N, Janelidze S, Santillo A, Ohlsson T, Jögi J, Tsai R, La Joie R, Kramer J, Boxer AL, Gorno-Tempini ML, Miller BL, Choi JY, Ryu YH, Lyoo CH, Hansson O. Discriminative accuracy of [18F]flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2018;320(11):1151–1162. doi: 10.1001/jama.2018.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA. Sex differences in Alzheimer's disease and common neuropathologies of aging. Acta Neuropathol. 2018;136(6):887–900. doi: 10.1007/s00401-018-1920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Salehi A, Gonzalez Martinez V, Swaab DF. A sex difference and no effect of ApoE type on the amount of cytoskeletal alterations in the nucleus basalis of Meynert in Alzheimer's disease. Neurobiol Aging. 1998;19(6):505–510. doi: 10.1016/s0197-4580(98)00106-7. [DOI] [PubMed] [Google Scholar]
- Samuel BG (1995) Diagnostic and statistical manual of mental disorders, 4th edn. (DSM-IV). 152(8):1228–1228. 10.1176/ajp.152.8.1228
- Shadlen MF, Larson EB, Gibbons L, McCormick WC, Teri L. Alzheimer's disease symptom severity in blacks and whites. J Am Geriatr Soc. 1999;47(4):482–486. doi: 10.1111/j.1532-5415.1999.tb07244.x. [DOI] [PubMed] [Google Scholar]
- Shi Z, Fu LP, Zhang N, Zhao X, Liu S, Zuo C, Cai L, Wang Y, Gao S, Ai L, Guan YH, Xu B, Ji Y. Amyloid PET in dementia syndromes: a Chinese Multicenter Study. J Nucl Med off Publ Soc Nucl Med. 2020;61(12):1814–1819. doi: 10.2967/jnumed.119.240325. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Murray ME, Frank RD, Shinohara M, DeTure M, Yamazaki Y, Tachibana M, Atagi Y, Davis MD, Liu CC, Zhao N, Painter MM, Petersen RC, Fryer JD, Crook JE, Dickson DW, Bu G, Kanekiyo T. Impact of sex and APOE4 on cerebral amyloid angiopathy in Alzheimer's disease. Acta Neuropathol. 2016;132(2):225–234. doi: 10.1007/s00401-016-1580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex (new York, NY: 1991) 2007;17(7):1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann EE, Panizzon MS, Chen X, Andrews M, Galasko D, Banks SJ. Sex differences in Alzheimer's-related Tau biomarkers and a mediating effect of testosterone. Biol Sex Differ. 2020;11(1):33. doi: 10.1186/s13293-020-00310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, Andrews H, Feng L, Tycko B, Mayeux R. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279(10):751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- Weuve J, Barnes LL, Mendes de Leon CF, Rajan KB, Beck T, Aggarwal NT, Hebert LE, Bennett DA, Wilson RS, Evans DA. Cognitive aging in Black and White Americans: cognition, cognitive decline, and incidence of Alzheimer disease. Dementia. 2018;29(1):151–159. doi: 10.1097/ede.0000000000000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters EE, Golla SSV, Timmers T, Ossenkoppele R, van der Weijden CWJ, Scheltens P, Schwarte L, Schuit RC, Windhorst AD, Barkhof F, Yaqub M, Lammertsma AA, Boellaard R, van Berckel BNM. A novel partial volume correction method for accurate quantification of [18F] flortaucipir in the hippocampus. EJNMMI Res. 2018;8(1):79. doi: 10.1186/s13550-018-0432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong C, Luo J, Schindler SE, Fagan AM, Benzinger T, Hassenstab J, Balls-Berry JE, Agboola F, Grant E, Moulder KL, Morris JC. Racial differences in longitudinal Alzheimer's disease biomarkers among cognitively normal adults. Alzheimers Dement. 2022 doi: 10.1002/alz.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Zheng C, Paranjpe MD, Li J, Benzinger TLS, Lu J, Zhou Y. Association of sex and APOE ε4 with brain tau deposition and atrophy in older adults with Alzheimer's disease. Theranostics. 2020;10(23):10563–10572. doi: 10.7150/thno.48522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Zheng C, Paranjpe MD, Li Y, Li W, Wang X, Benzinger TLS, Lu J, Zhou Y. Sex modifies APOE ε4 dose effect on brain tau deposition in cognitively impaired individuals. Brain J Neurol. 2021;144(10):3201–3211. doi: 10.1093/brain/awab160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Liu M, Ha L, Zhou Y. Quantitative (18)F-AV1451 brain tau PET imaging in cognitively normal older adults, mild cognitive impairment, and Alzheimer's disease patients. Front Neurol. 2019;10:486. doi: 10.3389/fneur.2019.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Montagne A, Zhao Z. Alzheimer's pathogenic mechanisms and underlying sex difference. Cell Mol Life Sci CMLS. 2021;78(11):4907–4920. doi: 10.1007/s00018-021-03830-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the ADNI cohort are available on the website (https://adni.loni.usc.edu/). Data from the Huashan cohort were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Huashan Hospital.
Not applicable.