Abstract
Many of the genes coding for extracellular toxins, enzymes, and cell surface proteins in Staphylococcus aureus are regulated by a 510-nucleotide (nt) RNA molecule, RNAIII. Transcription of genes encoding secreted toxins and enzymes, including hla (alpha-toxin), saeB (enterotoxin B), tst (toxic shock syndrome toxin 1), and ssp (serine protease), is stimulated, while transcription of genes encoding cell surface proteins, like spa (protein A) and fnb (fibronectin binding proteins), is repressed. Besides being a regulator, RNAIII is also an mRNA coding for staphylococcal delta-lysin. We have identified RNAIII homologs in three different coagulase-negative staphylococci (CoNS), i.e., Staphylococcus epidermidis, Staphylococcus simulans, and Staphylococcus warneri. RNAIII from these CoNS turned out to be very similar to that of S. aureus and contained open reading frames encoding delta-lysin homologs. Though a number of big insertions and/or deletions have occurred, mainly in the 5′ half of the molecules, the sequences show a high degree of identity, especially in the first 50 and last 150 nt. The CoNS RNAIII had the ability to completely repress transcription of protein A in an RNAIII-deficient S. aureus mutant and the ability to stimulate transcription of the alpha-toxin and serine protease genes. However, the stimulatory effect was impaired compared to that of S. aureus RNAIII, suggesting that these regulatory functions are independent. By creating S. epidermidis-S. aureus RNAIII hybrids, we could also show that both the 5′ and 3′ halves of the RNAIII molecule are involved in the transcriptional regulation of alpha-toxin and serine protease mRNAs in S. aureus.
In Staphylococcus aureus, transcription of many virulence genes, encoding extracellular toxins, enzymes, and cell surface proteins, is regulated by a 510-nucleotide (nt)-long RNA, called RNAIII (15, 27). Production of toxins and enzymes is generally positively controlled, while that of cell surface proteins is negatively controlled (14, 18). Synthesis of RNAIII is induced when the concentration of an autocrine octapeptide in the environment has reached a certain level (16, 17). Generally, this happens during the late exponential phase of growth in laboratory cultures, which means that cell surface proteins are produced during the early exponential phase, while secreted toxins and enzymes are produced mainly during the postexponential phase of growth (4, 21, 31). Four genes, agrB, agrD, agrC, and agrA, arranged in an operon (agr) are involved in the synthesis of the inducing octapeptide and the signal transduction that leads to activation of the RNAIII gene, which is closely linked to the agr operon and transcribed in the opposite direction (16).
A precursor of the inducing peptide is encoded by agrD and requires agrB to be properly processed and secreted (16, 17). The agrC and agrA genes code for the components of a classical two-component signal transduction system, where AgrC is the sensor and AgrA is the response regulator, which is required for transcription of the RNAIII molecule and the agr operon itself (26, 27).
Besides being a regulator, RNAIII is also an mRNA coding for staphylococcal delta-lysin (14). Delta-lysin is a 26-amino-acid polypeptide which can form pores in membranes and lyse erythrocytes (8, 12, 19). Delta-lysin is not required for the regulation of target genes by RNAIII (2, 15, 27), though translation of the delta-lysin gene may influence the regulatory function of RNA (3).
An agr locus, organized in the same way as that of S. aureus, has been demonstrated in coagulase-negative Staphylococcus lugdunensis (32). However, RNAIII from S. lugdunensis does not code for delta-lysin, and its role in gene regulation is not known (32).
The production of a deltalike hemolytic activity has been demonstrated in many coagulase-negative staphylococci (CoNS) (6, 7, 9). Amino acid sequencing of the hemolysin from Staphylococcus epidermidis revealed only two amino acids that were different from those in S. aureus delta-lysin (23). This has recently been confirmed by nucleotide sequencing of the S. epidermidis RNAIII gene (28). Based on this high degree of similarity, we asked the question whether delta-lysins from S. epidermidis and other CoNS are encoded by RNAIII-like mRNAs which also have a regulatory function. In this study, RNAIII homologs were identified in S. epidermidis, S. warneri, and S. simulans and were shown to regulate virulence gene expression in S. aureus. In all molecules, the first 50 and last 150 nt were highly conserved, suggesting that these regions are important for the regulatory function.
MATERIALS AND METHODS
Bacterial strains.
S. aureus WA400 is a mutant of 8325-4 in which the RNAIII gene has been deleted and replaced by the cat86 gene (15). Strain WA400 has an intact agr operon, but due to the lack of functional RNAIII, it has an exoprotein pattern similar to that of an agrA mutant. RN4220 (20) is a restriction-deficient mutant of strain 8325-4. Escherichia coli DH5α (11) was used as the host for plasmid constructions.
Culture conditions.
S. aureus strains were precultured overnight in tryptic soy broth (TSB; Difco), and 20 ml of preculture was collected by centrifugation and used to inoculate 100 ml of brain heart infusion (BHI; Difco) in 1-liter baffled flasks. Incubation was at 37°C on a rotary shaker. Bacterial growth was monitored by measuring the optical density at 600 nm, and cultures were harvested by centrifugation at the indicated time points. The appropriate antibiotics were added to the precultures: chloramphenicol (5 μg ml−1) and/or tetracycline (5 μg ml−1).
Protease activity was assayed by growing the bacteria on casein agar plates as described previously (1).
PCR and sequencing.
Chromosomal DNAs were prepared (22) from S. epidermidis, S. cohnii, S. haemolyticus, S. saprophyticus, S. simulans, S. warneri, and S. xylosus and used as the template in PCR with S. aureus RNAIII primers, nt 1095 to 1116 (primer 11) and nt 1554 to 1578 (primer 12) according to the nucleotide sequence numbering of Kornblum et al. (18).
A fragment containing the 5′ end and the promoter region of RNAIII in S. epidermidis and S. warneri was obtained by PCR with an S. aureus primer located in agrC from nt 3206 to 3224 (18), together with primer 11. The 3′ end and the terminator region of RNAIII in S. warneri were obtained by PCR with an S. aureus primer located in open reading frame 7 from nt 579 to 603 (18) together with primer 12.
To obtain flanking regions, inverted PCR was performed on chromosomal DNAs from S. epidermidis and S. simulans. DNA from S. epidermidis was cleaved with EcoRV, ligated, and subsequently used as the template in PCR with primers based on the new S. epidermidis RNAIII sequence. DNA from S. simulans was cleaved with Sau3AI, ligated, and subsequently used as the template in PCR with primers based on the S. simulans RNAIII sequence.
All fragments to be sequenced were cloned into pGEM-T easy. DNA sequencing was performed by using the Taq Dye Deoxy Terminator Cycle Sequencing kit (Applied Biosystems), and the reaction mixtures were analyzed on an Applied Biosystems 373A DNA sequencer.
Construction of plasmids.
Plasmids used are listed in Table 1. All plasmid constructs were first propagated in E. coli DH5α and then transferred to the restriction-deficient S. aureus RN4220 (20) before they were introduced into S. aureus WA400. Competent E. coli cells were prepared and transformed by the method of Sambrook et al. (29). S. aureus strains were transformed by electroporation by the method of Schenk and Laddaga (30). E. coli transformants were selected on LB (Difco) plates containing 50 μg of ampicillin ml−1 and S. aureus transformants on NYE agar plates (29) containing 5 μg of tetracycline ml−1. Plasmid DNA was extracted by using the Qiagen plasmid mini kit (Qiagen Inc., Valencia, Calif.). Lysostaphin (100 μg ml−1) (Applied Microbiology Inc.) was used to lyse S. aureus cells.
TABLE 1.
Bacterial plasmids used in this study
| Plasmid | Relevant characteristics | Source |
|---|---|---|
| pSPT245 | Shuttle vector, pRN8054::pSP64 | 25 |
| pEX0122 | S. warneri RNAIII gene with S. aureus RNAIII promoter | This work |
| pEX0124 | S. epidermidis RNAIII gene with S. aureus RNAIII promoter | This work |
| pEX0125 | S. simulans RNAIII gene with S. aureus RNAIII promoter | This work |
| pEX0128 | S. aureus RNAIII gene with its own promoter | This work |
| pEX0129 | RNAIII hybrid, with the 5′ part of S. epidermidis RNAIII and the 3′ part of S. aureus RNAIII with S. aureus promoter | This work |
| pEX0130 | RNAIII hybrid, with the 5′ part of S. aureus RNAIII and the 3′ part of S. epidermidis RNAIII with S. aureus promoter | This work |
A 130-bp fragment containing the S. aureus RNAIII promoter region ending at position +17 was fused to the RNAIII gene fragments of S. epidermidis, S. simulans, and S. warneri, starting at position +18. The S. aureus promoter fragment was synthesized by PCR using primer 3 (nt 1656 to 1680) (18) and primer 20 (nt 1454 to 1474) (18). The RNAIII gene fragments were synthesized by using a primer complementary to primer 20 together with a downstream primer specific for the respective strains. After denaturation, the promoter fragment was allowed to anneal with each of the RNAIII gene fragments and subsequently elongated by TaqI polymerase to form a fusion fragment. These fusion fragments were then amplified by PCR and cloned into pGEM-T easy (Promega). From the resulting plasmid, the fusion genes were cut out with SphI and SacI and cloned in the shuttle vector pSPT245 (25) to generate pEX0122 (S. warneri), pEX0124 (S. epidermidis), and pEX0125 (S. simulans).
For a control plasmid, a 700-bp PCR fragment containing the S. aureus RNAIII gene including its promoter was cloned into pSPT245 to form pEX0128.
To generate S. aureus-S. epidermidis RNAIII hybrid genes, a 240-bp StyI-SacI fragment (5′ half of S. aureus RNAIII) in pEX0128 was substituted for the corresponding fragment of S. epidermidis (pEX0124), generating pEX0129. In the same manner, a 220-bp StyI-SacI fragment from pEX0124 (5′ half of S. epidermidis RNAIII gene) was substituted for pEX0128 to form pEX0130. Plasmid constructs were confirmed by DNA sequencing.
Northern blotting and primer extension analyses.
Total S. aureus RNA was prepared by extraction of lysostaphin-treated cells, with hot phenol as described previously (13). Concentration of RNA was determined spectrophotometrically as absorbance at 260 nm. Electrophoresis of RNA (10 μg of total RNA per lane), transfer to a Biodyne B nylon membrane (Pall Ultrafine Filtration Corp.), and hybridization were carried out as described previously (24). Internal fragments of the genes coding for alpha-toxin (nt 487 to 1930 [10]), serine protease (nt 435 to 1364 [5]), protein A (nt 815 to 1072 [22]), S. aureus RNAIII (nt 1095 to 1578 [18]), S. epidermidis RNAIII (nt 78 to 620 [this study]), S. simulans RNAIII (nt 80 to 640 [this study]), and S. warneri RNAIII (nt 80 to 748 [this study]) were amplified by PCR, radiolabeled with [α-32P]dCTP (Amersham) using a random prime labelling kit (Boehringer Mannheim Biochemicals), and used as probes. For a common probe for RNAIII, primer 28 (nt 1133 to 1156 [18]) was labeled with [γ-32P]ATP (Amersham), using polynucleotide kinase.
Primer extension analysis were carried out as described by Morfeldt et al. (25). The following primers were used for the indicated species: S. epidermidis, nt 125 to 146 (this study); S. simulans, nt 142 to 161 (this study); and S. warneri, nt 215 to 239 (this study). Radioactivity was detected by a radioisotope imaging system (PhosphorImager 445SI; Molecular Dynamics).
Sequence analysis.
The multiple alignment of the RNAIII gene sequences was done with the PileUp program, and the secondary structure predictions were done with the Squiggles program (energy minimization method of Zuker) (both programs from the Genetics Computer Group Inc.).
Nucleotide sequence accession numbers.
Sequence data have been submitted to the EMBL Nucleotide Sequence Database under the accession numbers AJ223774 (S. epidermidis hld gene), AJ223775 (S. simulans hld gene), and AJ223776 (S. warneri hld gene).
RESULTS
Identification and sequencing of the RNAIII gene in CoNS.
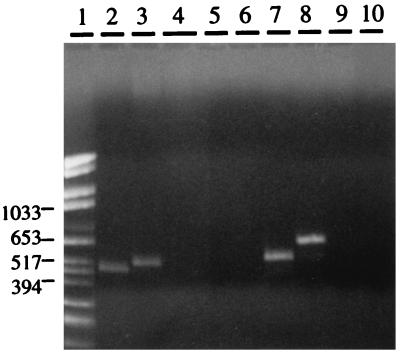
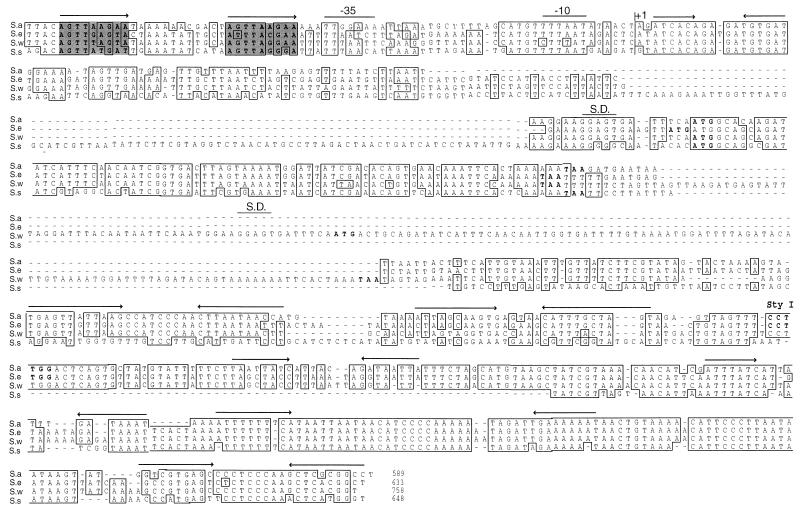
Seven different staphylococcal species (S. cohnii, S. epidermidis, S. haemolyticus, S. saprophyticus, S. simulans, S. warneri, and S. xylosus) were tested for the presence of sequences related to the S. aureus RNAIII gene by PCR. Internal RNAIII primers based on the S. aureus sequence were used. A major PCR product, ranging from 500 to 700 nt, was obtained only from S. epidermidis, S. simulans, and S. warneri (Fig. 1). The presumed internal RNAIII gene fragments generated from S. epidermidis, S. simulans, and S. warneri were cloned and sequenced. A high degree of identity to the S. aureus RNAIII gene was found in all cases, which confirmed that the amplified PCR products indeed were internal RNAIII gene fragments. In order to obtain flanking sequences, additional PCR and inverted PCR were carried out (see Materials and Methods). Based on the derived sequences, new primers were designed to amplify and clone the entire RNAIII determinants, which were then sequenced in both directions (Fig. 2).
FIG. 1.
Agarose gel analysis of PCR products, amplified with internal S. aureus RNAIII primers, from chromosomal DNAs of CoNS. Lanes: 1, size markers (in base pairs); 2, S. aureus; 3, S. cohnii; 4, S. epidermidis; 5, S. haemolyticus; 6, saprophyticus; 7, S. simulans; 8, S. warneri; 9, S. xylosus; 10, negative control.
FIG. 2.
Alignment of the RNAIII gene sequences from S. aureus (S.a) and three different CoNS species, S. epidermidis (S.e), S. warneri (S.w), and S. simulans (S.s). The alignment was done with the PileUp program from the Genetics Computer Group Inc., and the sequences were manipulated by introducing gaps (indicated by dashes) to fit transcription start points, the delta-lysin open reading frames, and secondary structure predictions. Positional sequence identity for at least three of the sequences (boxes) and conserved direct repeats (shaded boxes), which are important for regulation of RNAIII in S. aureus, are indicated. The transcriptional start site (+1) and the putative −10 and −35 promoter elements, direct and indirect repeats (arrows), Shine-Dalgarno sequence (S.D.), and the N-terminal methionine of the predicted delta-lysin open reading frames and stop codons (bold type) are indicated.
Transcription of the RNAIII gene in S. epidermidis, S. simulans, and S. warnerii.
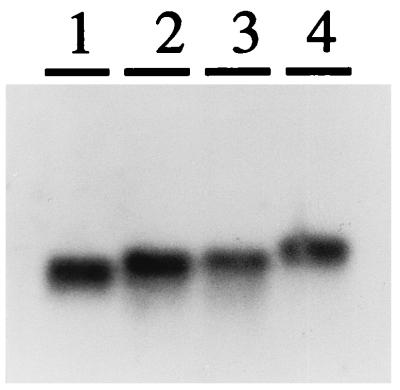
Northern blot analysis revealed that the RNAIII gene was expressed in all three strains (Fig. 3). The mobility of the transcripts corresponded to the lengths deduced from the DNA sequencing data and primer extension analysis. Upstream of the transcription start points (indicated in Fig. 2), putative −10 and −35 promoter elements were found, similar to those in S. aureus. The 3′ end of the molecules was identified by the striking sequence similarity to the terminator region of RNAIII in S. aureus. Based on these results, the estimated lengths of RNAIIIs are 560 nt for S. epidermidis, 573 nt for S. simulans, and 684 nt for S. warneri.
FIG. 3.
Northern blot analysis of RNAIII molecules from S. aureus, S. epidermidis, S. simulans, and S. warneri. Ten micrograms of total RNA from postexponential cells of each strain was loaded onto a 1.2% denaturing agarose gel. A mixture of DNA probes specific for RNAIII from S. aureus (lane 1), S. epidermidis (lane 2), S. simulans (lane 3), and S. warneri (lane 4) was used.
Immediately upstream of the −35 promoter element, a highly conserved direct repeat, earlier demonstrated to be important for the transcription of RNAIII in S. aureus (25), was found in all species. When CoNS RNAIII genes under the control of their own promoter, were introduced to S. aureus WA400, RNAIII was expressed mainly during late postexponential phase of growth (data not shown), indicating an agr-dependent regulation.
All three RNAIII genes contained open reading frames with high degrees of sequence identity to the S. aureus delta-lysin. As can be seen in Fig. 2, the predicted delta-lysin genes were located in the 5′ half of RNAIII but at different distances from the transcription start point. The predicted amino acid sequence of the S. epidermidis delta-lysin confirmed previous amino acid sequence data, except that an extra N-terminal methionine was found (23). In S. simulans delta-like protein, 19 of 26 amino acids were identical to those of S. aureus. Surprisingly, S. warneri RNAIII was predicted to encode two nonidentical copies of delta-like proteins, both 25 amino acids in length. These delta-lysin peptides differed in seven and five amino acid residues, respectively, compared to S. aureus delta-lysin. As can be seen in Fig. 4, the distribution of the charged residues was conserved between all predicted molecules, suggesting that they can form amphipatic α-helices, as has been described for S. aureus delta-lysin (8).
FIG. 4.
Predicted amino acid sequences of delta-lysin in S. epidermidis, S. simulans, and S. warneri compared with the amino acid sequence of S. aureus delta-lysin (14). Two delta-lysin genes were predicted in S. warneri (S. warneri-I and S. warneri-II). Charged residues are indicated by bold type.
Compared to S. aureus RNAIII, S. simulans has a 107-bp insertion upstream of the delta-lysin open reading frame and a deletion in the 3′ part of the gene. S. epidermidis and S. warneri both have small insertions of 25 bp upstream of their respective delta-lysin open reading frames. In addition, S. warneri has a 130-bp insertion, containing the second delta-lysin determinant. In spite of these differences, a very high degree of identity was seen for the RNAIII genes, especially in the first 50 and last 150 bp. However, computer analysis of the secondary structure revealed several conserved stem-loop structures of comparable energy throughout the molecule (Fig. 2).
Regulatory effect of RNAIII from different CoNS.
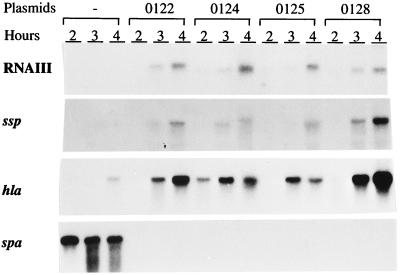
To test the ability of the RNAIII molecules from the CoNS to regulate transcription of virulence genes in S. aureus, plasmids containing the CoNS RNAIII genes were introduced into the RNAIII-deficient S. aureus strain, WA400. In order to ensure that the same level of RNAIII was expressed, the different RNAIII genes were put under the control of S. aureus RNAIII promoter. A similar plasmid with the S. aureus RNAIII gene was used as a control. The expression of RNAIII, alpha-toxin (hla), serine protease (ssp), and protein A (spa) mRNAs was analyzed by Northern blotting. To be able to compare the levels of RNAIII, a 24-nt primer recognizing a 100% conserved region was used as a probe. As can be seen in Fig. 5, comparable amounts of the different CoNS RNAIII molecules were produced by S. aureus WA400. All three CoNS RNAIII molecules stimulated the expression of the protease and alpha-toxin genes. However, the levels of transcripts were generally reduced compared to those seen in strain WA400 expressing S. aureus RNAIII. Transcription of the protein A gene was completely repressed in all cases. Taken together, these results show that RNAIII molecules from S. epidermidis, S. simulans, and S. warneri have the ability to regulate transcription of the alpha-toxin, serine protease, and protein A genes in S. aureus in the same manner as S. aureus RNAIII.
FIG. 5.
Northern blot analysis of RNAIII, alpha-toxin, serine protease, and protein A transcripts in strains WA400, WA400(pEX0128), WA400(pEX0124), WA400(pEX0125), and WA400(pEX0122) at different time points during growth. The pEX part of plasmid designations is not shown in the figure. Strain WA400 without plasmids (−) is the control. The same filter was hybridized with each of the specific probes.
Complementation with S. aureus-S. epidermidis hybrids.
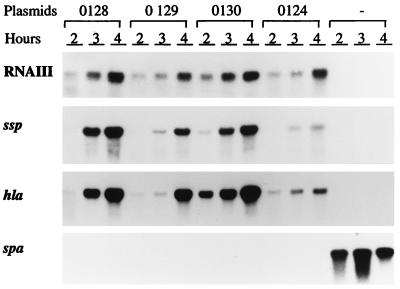
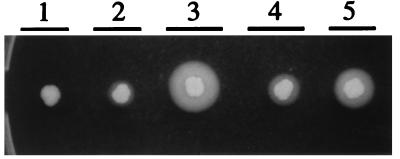
Though S. epidermidis RNAIII could stimulate the transcription of both serine protease and alpha-toxin genes, it was less efficient than wild-type S. aureus RNAIII. To test which part of RNAIII is most important for the regulatory function, hybrid molecules were created by using a conserved StyI site (Fig. 2), dividing the RNAIII gene into two parts of roughly the same length. A fusion between the 5′ half of S. aureus RNAIII and the 3′ half of S. epidermidis (pEX0130) increased the transcription of hla and ssp about fourfold compared to S. epidermidis wild-type RNAIII (pEX0124). However, the fusion molecule was slightly less efficient than wild-type S. aureus RNAIII in stimulating ssp expression (Fig. 6). The fusion between the 3′ half of S. epidermidis RNAIII and the 5′ half of S. aureus (pEX0129) also increased transcription of hla and ssp compared to pEX124 (S. epidermidis), though it was less efficient than pEX0130. The same differences were seen when zones of proteolysis on casein agar plates were compared (Fig. 7).
FIG. 6.
Northern blot analysis of RNAIII, alpha-toxin, serine protease, and protein A transcripts in strains WA400(pEX0128), WA400(pEX0129), WA400(pEX0130), WA400(pEX0124), and WA400 at different time points during growth. The pEX part of plasmid designations is not shown in the figure. Strain WA400 without plasmids (−) is the control. The same filter was hybridized with each of the specific probes.
FIG. 7.
Zones of proteolysis in different strains. The RNAIII-deficient S. aureus strain WA400 (zone 1) and the same strain expressing S. epidermidis RNAIII (pEX0124) (zone 2), S. aureus RNAIII (pEX0128) (zone 3), and S. aureus-S. epidermidis RNAIII hybrids pEX0129 (zone 4) and pEX0130 (zone 5) (see Table 1) were used.
These experiments indicate that both the 3′ and 5′ halves of RNAIII are important for the regulatory function and that the reduced ability of S. epidermidis RNAIII to stimulate transcription of ssp and hla must be due to differences in both the 5′ and 3′ halves of the molecule compared to S. aureus RNAIII.
DISCUSSION
In this study, we have identified RNAIII homologs in three different CoNS, i.e., S. epidermidis, S. warneri, and S. simulans. RNAIII molecules from these three species turned out to be very similar to that of S. aureus. The inability to detect an RNAIII gene in S. cohnii, S. xylosus, S. haemolyticus, and S. saprophyticus by the method used in this study does not unambiguously exclude the possibility that these species lack the gene. However, similar results were obtained by Donvito et al. (6) in a screening for RNAIII and delta-lysin in several CoNS species by PCR and Southern blotting, except that they did not obtain a positive signal in S. simulans. In addition, Vandenesch et al. (32) have identified RNAIII in S. lugdunensis. In contrast to the RNAIII molecules identified in this study, the S. lugdunensis RNAIII did not contain an open reading frame encoding delta-lysin. However, the remaining parts of S. lugdunensis RNAIII are very similar to the RNAIII molecules identified here, suggesting a common function.
RNAIII from all sequenced strains contained open reading frames encoding delta-lysin homologs with a high degree of sequence identity to the S. aureus delta-lysin. In particular, the distribution of the charged amino acid residues was conserved, suggesting that amphipatic α-helices can be formed and that they can function as pore-forming toxins, as described for the S. aureus delta-lysin (19). This is in agreement with the finding that delta-lysin-like activity is produced by many CoNS (9).
Northern blot analysis revealed that S. epidermidis, S. simulans, and S. warneri produced large amounts of RNAIII. Though the entire agr locus has not yet been identified, sequencing of the region upstream of the CoNS RNAIII determinants revealed a putative divergent promoter, similar to P2 of S. aureus, followed by the beginning of a tentative agrB gene (unpublished results). The finding that the CoNS RNAIII promoters were regulated in an agr-dependent way in S. aureus also supports the existence of an agr operon in some CoNS species. This was also supported by the observation that RNAIII in S. simulans was expressed only during the late exponential and postexponential phases of growth (unpublished results). Otto et al. (28) have recently sequenced the entire agr locus from an S. epidermidis strain, showing that it has the same architecture in S. aureus. Their sequence agreed completely with our data.
A number of big insertions and/or deletions have occurred mainly in the 5′ halves of the CoNS RNAIII molecules compared to that of S. aureus, while the 3′ halves seem more conserved. RNAIII molecules from S. aureus, S. epidermidis, and S. warneri seem to be the most closely related, while S. simulans RNAIII is less closely related, except for the first 50 and last 150 nt where all molecules are highly identical. It seems likely that these conserved regions are important for the regulatory function of RNAIII, as indicated by the ability of the CoNS RNAIII molecules to complement the RNAIII defect of S. aureus WA400.
The finding that the CoNS RNAIII repressed transcription of spa as efficiently as S. aureus RNAIII, while stimulation of hla and ssp transcription was impaired, suggests that these regulatory functions are independent. This is also indicated by the finding that some deletions in the S. aureus RNAIII gene affected transcription of spa but not transcription of hla and hlb and vice versa (27). It may also be concluded that the repressing activity should reside in those parts of the molecules with highly conserved sequences. However, conserved secondary structures in regions with less sequence identity may also be important for the regulatory function. On the other hand, since most of the predicted secondary structures seem to be conserved, the impaired stimulatory function of the CoNS RNAIII molecules compared to that of S. aureus RNAIII might be due to differences in the primary nucleotide sequence.
Slightly different effects were seen between the different RNAIII molecules. In particular, the kinetics of hla mRNA production was different with S. simulans RNAIII (pEX0125 in Fig. 5) compared to the other RNAIII species. This may be related to the fact that S. simulans RNAIII is the most distantly related to S. aureus RNAIII. Balaban and Novick (3) have presented evidence that S. aureus RNAIII alters between different conformations with different regulating activities during the growth cycle. Differences in the nucleotide sequence of the RNAIII molecule might influence the kinetics of these conformational changes and hence the regulation of hla transcription. It should be pointed out that maximum amounts of ssp mRNA were still obtained after 4 h with plasmid pEX0125, suggesting that slightly different mechanisms of regulation may operate.
The evidence that the S. epidermidis RNAIII molecule can be improved in its stimulatory function by substituting either the 5′ or 3′ half of the molecule for the S. aureus equivalent indicates that both parts are involved in the regulation. However, the hybrid RNAIII consisting of the 5′ part of S. aureus RNAIII and the 3′ part of S. epidermidis RNAIII was slightly more efficient than the inverse hybrid (Fig. 6 and 7), suggesting that the impaired stimulatory activity of S. epidermidis RNAIII was mainly due to sequence differences in the 5′ half of the molecule. Whether this means that independent regulatory domains are present or that the 5′ and 3′ parts of the molecule interact to create a functional structure is not known. Secondary structure predictions have indicated such an interaction (27), but these predictions must be demonstrated by biochemical methods.
ACKNOWLEDGMENTS
We thank Agneta Wahlquist for skillful technical assistance.
This work was supported in part by grant 4513 from the Swedish Medical Research Council and by scholarships to K.T. and E.M. from the Sigurd och Elsa Goljes Minne foundation and to K.T. from the Ragnhild och Einar Lundströms Minne, Karl Jeppssons Minne, and Emma och Erik Granes Minne foundations.
REFERENCES
- 1.Arvidson S. Hydrolysis of casein by three extracellular proteolytic enzymes from Staphylococcus aureus, strain V8. Acta Pathol Microbiol Scand Sect B. 1973;81:538–544. doi: 10.1111/j.1699-0463.1973.tb02239.x. [DOI] [PubMed] [Google Scholar]
- 2.Arvidson S, Janzon L, Löfdahl S, Morfeldt E. The exoprotein regulatory region (exp) of Staphylococcus aureus. In: Butler L O, Harwood C, Moseley B E B, editors. Genetic transformation and expression. Andover, Hants, United Kingdom: Intercept Ltd.; 1989. pp. 511–518. [Google Scholar]
- 3.Balaban N, Novick R P. Translation of RNAIII, the Staphylococcus aureus agr regulatory RNA molecule, can be activated by a 3′-end deletion. FEMS Microbiol Lett. 1995;133:155–161. doi: 10.1111/j.1574-6968.1995.tb07877.x. [DOI] [PubMed] [Google Scholar]
- 4.Björklind A, Arvidson S. Mutants of Staphylococcus aureus affected in the regulation of exoprotein synthesis. FEMS Microbiol Lett. 1980;7:202–206. [Google Scholar]
- 5.Carmona C, Gray G L. Nucleotide sequence of the serine protease gene of Staphylococcus aureus strain V8. Nucleic Acids Res. 1987;15:6757. doi: 10.1093/nar/15.16.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donvito B, Etienne J, Greenland T, Mouren C, Delorme V, Vandenesch F. Distribution of the synergistic haemolysis genes hld and slush with respect to agr in human staphylococci. FEMS Microbiol Lett. 1997;151:139–144. doi: 10.1111/j.1574-6968.1997.tb12562.x. [DOI] [PubMed] [Google Scholar]
- 7.Freer J H, Arbuthnott J P. Toxins of Staphylococcus aureus. Pharmacol Ther. 1983;19:55–106. doi: 10.1016/0163-7258(82)90042-0. [DOI] [PubMed] [Google Scholar]
- 8.Freer J H, Birkbeck T H. Possible conformation of delta-lysin, a membrane-active peptide of Staphylococcus aureus. J Theor Biol. 1982;94:535–540. doi: 10.1016/0022-5193(82)90299-5. [DOI] [PubMed] [Google Scholar]
- 9.Gemmel C G. Extracellular toxins and enzymes of coagulase-negative staphylococci. In: Eastmon C S F, Adlam C, editors. Staphylococci and staphylococcal infections. Vol. 2. London, United Kingdom: Academic Press, Inc. (London), Ltd.; 1983. pp. 809–827. [Google Scholar]
- 10.Gray G S, Kehoe M. Primary sequence of the alpha-toxin gene from Staphylococcus aureus Wood 46. Infect Immun. 1984;46:615–618. doi: 10.1128/iai.46.2.615-618.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 12.Heathly N G. A new method for the preparation of and some properties of staphylococcal delta-haemolysin. J Gen Microbiol. 1971;6:269–278. doi: 10.1099/00221287-69-2-269. [DOI] [PubMed] [Google Scholar]
- 13.Janzon L, Löfdahl S, Arvidson S. Evidence for a coordinate transcriptional control of alpha-toxin and protein A in Staphylococcus aureus. FEMS Microbiol Lett. 1986;33:193–198. [Google Scholar]
- 14.Janzon L, Löfdahl S, Arvidson S. Identification of the delta-lysin gene, hld, adjacent to the accessory gene regulator (agr) of Staphylococcus aureus. Mol Gen Genet. 1989;219:480–485. doi: 10.1007/BF00259623. [DOI] [PubMed] [Google Scholar]
- 15.Janzon L, Arvidson S. The role of the delta-lysin gene (hld) in regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus. EMBO J. 1990;9:1391–1399. doi: 10.1002/j.1460-2075.1990.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji G, Beavis R C, Novick R P. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276:2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 18.Kornblum J, Kreiswirth B, Projan S, Ross H, Novick R. agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In: Novick R P, editor. Molecular biology of staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 373–402. [Google Scholar]
- 19.Kreger A S, Kim K-S, Zaboretzky F, Bernheimer A W. Purification and properties of staphylococcal delta hemolysin. Infect Immun. 1971;3:449–465. doi: 10.1128/iai.3.3.449-465.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreiswirth B, Löfdahl S, Betley M J, O’Reilly M, Schleivert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 21.Lebeau C, Vandenesch F, Greenland T, Novick R P, Etienne J. Coagulase expression in Staphylococcus aureus is positively and negatively modulated by an agr-dependent mechanism. J Bacteriol. 1994;176:5534–5536. doi: 10.1128/jb.176.17.5534-5536.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Löfdahl S, Guss B, Uhlén M, Philipson L, Lindberg M. Gene for staphylococcal protein A. Proc Natl Acad Sci USA. 1983;80:697–701. doi: 10.1073/pnas.80.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKevitt A, Bjornson G, Mauracher C, Scheifele D. Amino acid sequence of deltalike toxin from Staphylococcus epidermidis. Infect Immun. 1990;58:1473–1475. doi: 10.1128/iai.58.5.1473-1475.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morfeldt E, Janzon L, Arvidson S, Löfdahl S. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol Gen Genet. 1988;211:435–440. doi: 10.1007/BF00425697. [DOI] [PubMed] [Google Scholar]
- 25.Morfeldt E, Tegmark K, Arvidson S. Transcriptional control of the agr-dependent virulence gene regulator, RNAIII, in Staphylococcus aureus. Mol Microbiol. 1996;21:1227–1237. doi: 10.1046/j.1365-2958.1996.751447.x. [DOI] [PubMed] [Google Scholar]
- 26.Novick R, Projan S, Kornblum J, Ross H F, Ji G, Kreiswirth B, Vandenesch F, Moghazeh S. The agr P2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol Gen Genet. 1995;248:446–458. doi: 10.1007/BF02191645. [DOI] [PubMed] [Google Scholar]
- 27.Novick R P, Ross H F, Projan S J, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otto M, Süssmuth R, Jung G, Götz F. Structure of the pheromone peptide of the Staphylococcus epidermidis agr system. FEBS Lett. 1998;424:89–94. doi: 10.1016/s0014-5793(98)00145-8. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Schenk S, Laddaga R A. Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol Lett. 1992;94:133–138. doi: 10.1016/0378-1097(92)90596-g. [DOI] [PubMed] [Google Scholar]
- 31.Vandenesch F, Kornblum J, Novick R P. A temporal signal, independent of agr, is required for hla but not spa transcription in Staphylococcus aureus. J Bacteriol. 1991;173:6313–6320. doi: 10.1128/jb.173.20.6313-6320.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandenesch F, Projan S, Kreiswirth B, Etienne J, Novick R P. agr-related sequences in Staphylococcus lugdunensis. FEMS Microbiol Lett. 1993;111:115–122. doi: 10.1111/j.1574-6968.1993.tb06370.x. [DOI] [PubMed] [Google Scholar]