Abstract
The subthalamic nucleus (STN) is a major neuromodulation target for the alleviation of neurological and neuropsychiatric symptoms using deep brain stimulation (DBS). STN-DBS is today applied as treatment in Parkinson´s disease, dystonia, essential tremor, and obsessive-compulsive disorder (OCD). STN-DBS also shows promise as a treatment for refractory Tourette syndrome. However, the internal organization of the STN has remained elusive and challenges researchers and clinicians: How can this small brain structure engage in the multitude of functions that renders it a key hub for therapeutic intervention of a variety of brain disorders ranging from motor to affective to cognitive? Based on recent gene expression studies of the STN, a comprehensive view of the anatomical and cellular organization, including revelations of spatio-molecular heterogeneity, is now possible to outline. In this review, we focus attention to the neurobiological architecture of the STN with specific emphasis on molecular patterns discovered within this complex brain area. Studies from human, non-human primate, and rodent brains now reveal anatomically defined distribution of specific molecular markers. Together their spatial patterns indicate a heterogeneous molecular architecture within the STN. Considering the translational capacity of targeting the STN in severe brain disorders, the addition of molecular profiling of the STN will allow for advancement in precision of clinical STN-based interventions.
Subject terms: Cellular neuroscience, Basal ganglia
The subthalamic nucleus is a highly evolutionary conserved brain region. Emerging studies reveal spatio-molecular heterogeneity leading to improved understanding of the organization of the STN and targeting for specific neurological disorders.
Introduction
The subthalamic nucleus (STN) is part of the extended basal ganglia and is a highly evolutionary conserved brain area identified in lampreys, rodents, cats, and primates1. The STN was first described in humans by French neurologist Jules Bernard Luys in 1865; with early literature references to body of Luys or corpus Luysii2. Its importance in motor function was later identified by James Martin in 1927, where a patient with damaged STN displayed abnormal involuntary movement (hemichorea); also subsequent cases of hemichorea showed similar STN pathology3. Ipsilateral STN lesions in non-human primate led to contralateral dyskinesias of the upper and lower limbs4, whereas lesion of the STN in non-human primate model of Parkinson’s disease (PD) reversed the motor deficits5. Later, deep brain stimulation (DBS) in the STN was demonstrated as a successful approach to reduce motor symptoms in PD patients6. In some cases, such STN-DBS was superior to pharmacological therapy even in PD patients with early motor complications7. Currently, STN-DBS is also applied as treatment of additional neurological and neuropsychiatric disorders, including obsessive-compulsive disorder (OCD)8–10 and dystonia11. STN is also a promising new target to treat refractory Tourette’s syndrome12–15. In addition, STN has been proposed as a target area for the treatment for drug addiction16–19. The successful targeting of STN in both motor and non-motor disorders is evidence of the complex function of the STN.
Parallel to symptom alleviation, some PD patients living with STN-DBS have reported adverse side-effects, including impact on cognitive, physiological, and limbic functions20. Both male and female PD patients report depression and weight gain while other symptoms show sex-differences. For example, sexual dysfunction and aggression were mainly reported by male patients21–25. However, further analysis of clinical reports will be required to identify the origin of adverse symptoms and to allow a segregation between PD-related symptoms and DBS-induced effects.
In addition to STN, also globus pallidus (GP) is an important target area for DBS electrodes in PD and dystonia26. Comparative reports of dystonia cases found that targeting of either STN or GP interna (GPi) is effective for dystonia. However, STN-DBS was superior for generalized dystonia and GPi-DBS was effective for truncal dystonia27,28. A comparison of adverse impact between brain regions indicated that targeting the STN relatively worsened the condition of apathy compared to GPi-DBS29. Retrospective studies comparing DBS in STN and GPi in PD show improvement on short-term motor symptoms, yet among STN-DBS treated patients there were incidence of suicide and impact on mood and quality of life30,31.
Due to established surgical protocols and successful outcomes improving debilitating neurological disorders, the STN is already a clinically important brain region in relation to motor, limbic and cognitive functions. In order to further refine treatments targeting the STN, better understanding of STN anatomy and its cellular and molecular composition should be of essence. For example, enhanced specificity and precision can be achieved by allowing such organizational knowledge improve the level of detail in stereotaxic brain maps implemented for surgical procedures in the STN. Further, any methodological developments towards cell-specific targeting of the STN might have the potential to reduce adverse side-effects and/or impact on non-motor symptoms.
Anatomy
Anatomically the STN is described as a compact nucleus located between the thalamus and midbrain, ventral to the zona incerta (ZI) and adjacent to the substantia nigra (SN), in close vicinity of fields of Forel H3. Its signature biconvex lens shape is similar across human, rat, cat, and monkey3,32. The STN has an oblique orientation with three anatomical axes that run along the rostrocaudal, mediolateral and dorsoventral axis33,34.
On average, there are 225,000 neurons per hemisphere in the human STN35–37. Magnetic resonance imaging (MRI) approaches report that the volume of the human STN is mainly between 50–80 mm3 with no difference between hemispheres or decrease with aging38,39. However other MRI techniques show a larger volume of ~155 mm3, within the range of 120 mm3 to 175 mm3,40. The variation in range could be contributed by different magnetic resonance imaging (MRI) techniques such as ultra-high field 7 tesla (T) and o3T MRI41. Moreover, the STN comprises a relatively small brain region and its boundaries are surrounded by dense fibres of the internal capsule, ansa lenticularis and lenticular fasciculus42.
MRI techniques show compartmentalization of the STN anatomical regions40. These have been referred to as motor, associative and limbic regions, forming the so called tripartite model of STN, correlating anatomy with function43,44. These STN compartments are also known in the literature as territories, domains, subdivisions, subareas. The STN compartments run through the rostrocaudal, mediolateral and dorsoventral axes42. The dorsolateral compartment is associated with sensorimotor function, the ventromedial (or central) compartment with cognitive/associative function, and the medial-most compartment with limbic function45. The limbic area of the STN is often referred to as the limbic tip, and it is directly associated with the para-STN towards which no specific anatomical border is apparent.
The anatomical subdivisions within the STN structure substantially overlap between functions46. It has been observed that, in monkeys, these compartments, particularly the associative and limbic, do not have well-defined boundaries, but are separated by functional gradients47. Emerging molecular marker and MRI studies in the STN challenge the tripartite model and the presence of three subdivisions of the STN44,48,49. A detailed analysis of publications from primate studies between 1925 to 2010 identified everything from zero to four subdivisions within the STN, and reported limited evidence to support the three subdivisions49. Further, in a recent report, six subparts were arbitrarily outlined (in cresyl violet stained tissue sections, i.e. not based on molecular markers); No 1 for the anteromedial part, No. 2 for the central part (further subdivided into centrodorsal (2d) and centroventral (2v), No 3 for lateral part (further subdivided into laterodorsal (3d) and lateroventral (3 v) and No 4 for posterolateral part50. Evidently, the internal organization of the STN is still under intense investigation, and more studies addressing the cellular, anatomical, structural organization from different aspects are likely to appear in literature in the near future.
A number of expression studies performed in human STN this millennium has identified new markers, rostro-caudal gradient expressions, and differences in neural densities along dorsal and ventral regions33,34,51,52. Together the rapidly expanding literature on STN organization supports further subdivisions within the STN which are defined by a combination of anatomy and spatial expression of molecular and/or cellular markers. The understanding of the internal organization of the STN is highly valuable towards clinical targeting of the STN, not least for refining treatment approaches. This includes the DBS technique which is dependent on anatomical specification to enable optimized implantation sites for electrode stimulation. Merging knowledge gained in recent studies may be an important step to develop an inclusive organization of the STN, including a common terminology to describe this internal organization.
Neural tracing and MRI studies of human and non-human primates confirm that the three proposed STN subdivisions have topologically organized connectivity to the basal ganglia (striatum and GP) and extended regions including substantia nigra (SN), pedunculopontine nucleus and ventral pallidum (VP)40,53 (Fig. 1A). In addition, the use of MRI has proven effective in demonstrating that motor network connectivity is predictive of effective subthalamic stimulation54,55. Human STN receives input from the orbitofrontal, prefrontal and limbic cortices as well as the motor cortex43,55–57.
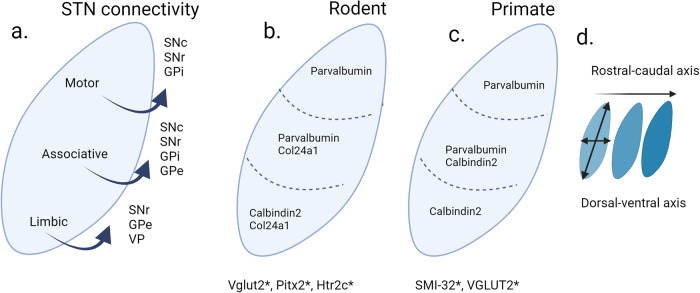
Fig. 1. The subthalamic nucleus (STN) outlined with connectivity and selected gene expression.
a Anatomical subdivisions are topologically organized with STN efferents based on published neural tracing studies by Carpenter et al., 1968, Parent et al., 1989, Nambu et al., 1996 and Karachi et al., 2004. b Selected gene expression (Parvalbumin, Calbindin 2, Col24a1: detected as mRNA or protein) in mouse (c) and primate based on Hardmann et al., 1997 (d). expression patterns are altered across rostral-caudal and the dorsal-ventral axis * indicates global STN gene expression. For details and full nomenclature, see the main text. Image created in Biorender.
In vivo diffusion weighted MRI technology to analyze the white matter connectivity of the STN from 12 healthy adults showed three distinct subregions defined by connectivity to cortical and subcortical regions40. In addition to the expected major dense cortical projections, overlapping networks between the motor and limbic connectivity were also identified40,46. Applying a similar structural connectivity-based parcellation protocol in 17 PD patients scheduled for STN-DBS surgery also showed three parcellations of the posterolateral motor region; and the limbic region located in the anteromedial part of the STN58. The convergence between these pathways may explain the impact on non-motor behaviors in PD patients treated with STN-DBS, and the importance of electrode placement within the STN for optimal motor improvements59.
PD patients are affected by several different non-motor symptoms that occur concurrently, or even precede, motor symptoms60. In addition to improving motor symptoms, DBS has been shown to be effective in relieving non-motor symptoms20,61–63. Further, improvement for some of these, including mood, apathy, attention, memory and sleep, has been shown to depend on the location of stimulation64.
On the other hand, adverse non-motor symptoms as side-effects are often reported from STN-DBS clinical patients25,59,65. For example, STN-DBS may induce hypomania, particularly when selected electrode contacts are located in the anteromedial part of the nucleus66, which is the region used with success to treat severe and resistant OCD8. These observations highlight the need for further understanding of the anatomical features of STN, and how this nucleus connects within the brain. Apart from the complex anatomy of STN, a striking heterogeneity in its transcriptomic landscape was recently revealed by spatio-molecular data originated from single-nuclei RNA-sequencing (snRNASeq) in mice67.
Various types of molecular, cellular, and anatomical heterogeneity, as well as connectivity, may be important to consider in the context of the beneficial effects of STN-DBS and also of adverse side-effects experienced as a consequence of this treatment. Alternative to anatomical targeting within the STN, cell-specific targeting is a futuristic approach26,68. Further, with rapid developments in neuromodulation technologies together with spatio-molecular mapping of STN, the potential of symptom-specific targeting with limited adverse impact is possible to foresee in the near future.
Overall, the STN shows a conserved anatomy across species with delineation of subregions which were initially proposed by functional domains1,69. However, the anatomical-functional organization of the STN remains to fully established. Anatomical tracing and MRI studies challenge the tripartite model. Below, we summarize cellular and molecular markers of the STN in primates and rodents, including neurotransmitter-related molecules and calcium-binding proteins as well as other, more recently described molecular markers within the STN. Thereafter, we address studies in which motor, associative and limbic functions of the STN have been addressed, followed by studies of the STN in neurological and neuropsychiatric disorders, including cellular changes and alterations in firing patterns.
Cellular markers of the STN
The STN is a major excitatory brain nucleus70. STN neurons primarily use glutamate as neurotransmitter. Beyond the glutamatergic identity, immuno-histological studies show intermingled neurotransmitter receptor identities of GABAergic, serotonergic, and dopamine receptors, indicative of complex afferent neurotransmission reaching the STN32,53,71–79. The fundamental cellular composition of the STN comes from gene expression analysis derived from mRNA and protein localization within the structure. The summary presented here includes studies from rodent, non-human primates and humans (data summarized below is also listed in Table 1).
Table 1.
Molecular marker expression in STN.
| Molecular marker (gene expression detected as mRNA or protein) | Abbreviation (mRNA in italics (mouse only), protein in capitals) | Species analyzed | Site of location (STN unless otherwise stated) (mRNA or protein) | Reference |
|---|---|---|---|---|
| Adenylate cyclase activating polypeptide 1 | Adcyap1 | Mouse | Central STN | Wallén-Mackenzie et al. 202067 |
| A2A adenosine receptor | ADORA2A | Human | Distributed in decreasing dorsal to ventral gradient | Emmi et al., 202297 |
| BAI1 associated protein 3 | Baiap3 | Mouse | pSTN and surrounding hypothalamic structures | Wallén-Mackenzie et al., 202067 |
| Calcium voltage-gated channel auxiliary subunit alpha2 delta 3 | Cacna2d3 | Mouse | Below detection or absent | Wallén-Mackenzie et al., 202067 |
| Calbindin 1 | CALB1 | Human |
Labelled on fibres Faint detection |
Augood et al., 199973 Munkle et al., 2000 |
| Calbindin 2 also known as Calretinin | CALB2 | Human | Intense immunoreactivity in ventral STN; both cell bodies and fibres. Highest detection in caudal aspect of STN. | Augood et al., 199973; Munkle et al., 2000; Wu et al., 2017; Alkemade et al., 201933, Bokulic et al., 2021 |
| Calbindin 2 | CALB2 | Monkey | Both cell bodies and fibres | Fortin and Parent 1996102 |
| Calbindin 2 | Calb2 | Mouse | Moderate in medial STN (STNa domain) | Wallén-Mackenzie et al., 202067 |
| Collagen type XXIV alpha 1 chain | Col24a1 | Mouse | Strong medal detection (STNa), sparser in central STN (STNb) | Wallén-Mackenzie et al., 202067 |
| Dopamine receptor 1 and 2 | D1R and D2R | Monkey | Presynaptic axons | Galvan et al., 201498 |
| Dopamine receptor 2 | D2R | Human | Distributed in decreasing gradient along ventromedial-to-dorsal axis | Hurd et al., 200176; Emmi et al., 202297 |
| Ferritin | FERR | Human | Distributed in oligodendrocytes within STN | Alkemade et al., 201933 |
| Fibroblast growth factor 11 | Fgf11 | Mouse | Throughout STN, low intensity | Wallén-Mackenzie et al., 202067 |
| Forkhead box protein P2 | FOXP2 | Human | Variable pattern within STN | Bokulic et al., 2021 |
| GABA-A receptor subunits | GABA-A (a1, a3, b2/3, and g2) | Human | STN principal neurons | Wu et al., 2017 |
| GABA-B receptor subunit | GABAB (R1 and R2) | Human | STN principal neurons | Wu et al., 2017 |
| GABA-A receptor subunit alpha 3 | GABRA3 | Human | Mainly neuronal staining and punctate fibre staining | Alkemade et al., 201933 |
| GABA-B receptor 1 | GABAB R1 | Monkey | Moderate distribution throughout the rostrocaudal extent of the STN | Charara et al., 2000 |
| GABA transporter 1 | GAT1 | Human | STN | Augood et al., 199973 |
| Glutamate decarboxylase 65/67 | GAD65/67 | Human | Parse positive neuronal staining, moderate fibre terminal staining (presynaptic boutons) in dorsolateral tip of STN | Wu et al., 2017; Alkemade et al., 201933 |
| Glutamate decarboxylase 1 | Gad1 | Mouse | Dorsal STN (STNds) and para-STN | Wallén-Mackenzie et al., 202067 |
| Glycine receptor alpha 3 | Glra3 | Mouse | Para-STN | Wallén-Mackenzie et al., 202067 |
| Glutamate receptor 1 and 2/3 |
GluR1 GluR2/3 |
Monkey | Cell bodies and synaptic clefts | Wang et al., 2000 |
| Metabotropic glutamate receptor subtype 1 and 5 |
mGluR1a mGluR5 |
Monkey Rat |
Strong labelling in neuropil and in postsynaptic STN neurons. |
Kuwajima et al., 200453 Awad et al., 200086 |
| N-methyl-D-aspartate receptor 1 | NMDAR1 | Monkey | Cell bodies and synaptic clefts | Wang et al., 2000 |
| Neuromedin b receptor | Nmbr | Mouse | Throughout STN. Low detection. | Wallén-Mackenzie et al., 202067 |
| Neurexophilin 1 | Nxph1 | Mouse | Throughout STN | Wallén-Mackenzie et al., 202067 |
| Neurexophilin 4 | Nxph4 | Mouse | Throughout STN except for dorsal strip (STNds) | Wallén-Mackenzie et al., 202067 |
| Neurofilament H | SMI-32 | Human | Uniformly detected | Wu et al., 2017; Alkemade et al., 201933 |
| Neuronal nitric oxide synthase | nNOS | Human | Most abundant neuronal marker in STN | Bokulic et al., 2021 |
| NK2 homeobox 1 | NKX2.1 | Human | Ventral regions in rostral and caudal plane. Uniform in central STN. | Bokulic et al., 2021 |
| Paired like Homeodomain 2 also known as pituitary homeobox 2 | Pitx2 | Mouse | Throughout STN |
Martin et al., 2004107 Skidmore et al., 2008109 Schweizer et al., 2014108 Schweizer et al., 2016; |
| Parvalbumin | PARV | Human | STN, primarily dorsolaterally/caudally. | Parent et al.,1996; Augood et al.,199973; Munkle et al., 2000; Alkemade et al., 201933, Bokulic et al., 2021 |
| Parvalbumin | PV | Mouse |
STN, dorsal and central (STNb,c domains). Note: In contrast to most other neurons, PV+ in STN neurons is mostly glutamatergic, (PV/Vglut2 colocalisation) |
Wallén-Mackenzie et al., 202067; Serra et al., 2023157 |
| Paired box protein 6 | PAX6 | Human | Variable pattern | Bokulic et al., 2021 |
| Potassium voltage-gated channel subfamily A regulatory beta subunit 3 | Kcnab3 | Mouse | Throughout STN. Co-localised with Pitx2 | Wallén-Mackenzie et al., 202067. |
| Serotonin | Serotonin / 5HT | Monkey | Highest density in medial and ventral STN | Mori et al.,198532 |
| Serotonin receptor subtype 2c | Htr2c | Mouse | Throughout STN; similar as Pitx2 and Vglut2 | Wallén-Mackenzie et al., 202067 |
| Serotonin transporter | SERT | Human | Passing fibres, more staining in anterior STN as compared to posterior STN | Parent et al., 2011, Alkemade et al., 201933 |
| Syntaxin binding protein 2 | Stxbp2 | Mouse | Moderate in STN | Wallén-Mackenzie et al., 202067 |
| Synaptophysin | SYN | Human | Punctate staining throughout | Alkemade et al., 201933 |
| Synaptoporin | Synpr | Mouse | Below detection or absent | Wallén-Mackenzie et al., 202067 |
| Tachykinin precursor 1 (encoded four products, including Substance P) | Tac1 | Mouse Monkey | Para-STN | Wallén-Mackenzie et al., 202067; Serra et al., 2023157 |
| Transferrin | TRANSF | Human | Oligodendrocytes and blood vessels in STN | Alkemade et al., 201933 |
| Tyrosine hydroxylase | TH | Human | None reported, passing fibres only | Hedreen 199974; Alkemade et al., 201933 |
| Vesicular glutamate transporter 1 | VGLUT1 | Human | Dense at STN borders and punctate in fibres | Alkemade et al., 201933 |
| Vesicular glutamate transporter 2 | VGLUT2 | Monkey | Throughout STN | Santin et al., 202350; Serra et al., 2023157 |
| Vesicular glutamate transporter 2 | Vglut2 | Mouse | Throughout STN, co-expressed with Pitx2 | Wallén-Mackenzie et al., 202067; Schweizer et al., 2014108, Serra et al., 2023157 |
*Italics denotes mRNA detection, mouse. #Terminology STN domains (STNa, STNb, STNc, STNds), see Fig. 2 (originally described in Wallén-Mackenzie et al. 67)
List of molecular markers in the STN. Gene expression detected as mRNA or protein and reported in human, monkey, rodent STN. For details, see main text.
Neurotransmitters
Efferent signaling from STN
Glutamate: Glutamatergic neurons are by far the most abundant cell type throughout the STN structure across species. In humans, the highest density of neurons positive for glutamatergic markers has been reported in the ventromedial part of the STN, with only a small population of GABAergic interneurons80. In mice, mRNA encoding the Vesicular glutamate transporter 2 (VGLUT2) is distributed throughout the STN67,81). A similar expression pattern of VGLUT2 mRNA was recently reported in macaque STN50.
Vesicular glutamate transporters (VGLUT1-3; encoded by the Slc17A7, Slc17A6, Slc17A8 genes, respectively) import glutamate into presynaptic vesicles and are the key cellular markers of glutamatergic excitatory neurotransmission. Since glutamate is an amino acid present in all cells, the presence of a VGLUT protein effectively means that the neuron in question can package and release glutamate from presynaptic vesicles upon depolarization and thus use its glutamate as neurotransmitter. Both VGLUT1 and VGLUT2 proteins have been identified as glutamate transporters in the membrane of pre-synaptic vesicles and are therefore considered as molecular markers defining the glutamatergic phenotype of neurons82–84. In terms of pre-synaptic vesicular transporters in neurons, distribution patterns of mRNA and protein will be strikingly different. While mRNA will be representing the cell soma of the encoding cell (and can be detected by in situ hybridization), the protein will be transported to the presynaptic terminal, and hence detected (by immunohistochemistry) at the synapse. Thus, in the mature neuron, the VGLUT protein will be found in a different brain area (in the pre-synpatic terminals synapsing on the target) than its corresponding mRNA (in the cell soma). This is particularly important to bear in mind when interpreting expression analysis of any vesicular transporter, including VGLUTs. Since VGLUT protein represents glutamatergic terminals, the presence of VGLUT protein in a structure thereby identifies incoming projections. Considering the STN of rodents and primates, the glutamatergic phenotype is molecularly identified by the presence of Vglut2 mRNA in the somata of STN neurons, while the corresponding VGLUT2 protein is found in their axonal terminals in target areas, including GP, VP, SN and pedunculopontine nucleus, as outlined above.
Afferent signaling to STN
Glutamate
In terms of glutamatergic afferent projections reaching the STN, analysis of human brain tissue has identified VGLUT1 protein, indicative of afferent terminals from the cortex (and possibly other excitatory brain areas positive for this subtype of VGLUT), localized to the dorso-caudal extent of the STN with punctate labelling of the fibers33. In monkey STN, AMPA receptor subunits GluR1, GluR2/3 and N-methyl-D-aspartate receptor 1 (NMDAR1) were detected85. Metabotropic glutamate receptor subtype 1 and 5 are located on STN neurons in both monkey and rats53,86.
GABA
GABAergic neurons, in STN considered interneurons70, have been reported to be significantly more numerous in the anteromedial STN than the rest of the STN structure in humans36,80. Glutamate decarboxylase (GAD65/67), a GABA marker, is sparsely detected on STN neurons with moderate fiber terminal staining (presynaptic boutons) in the dorsolateral tip of STN33. GABAA (a1, a3, b2/3, and g2) and GABAB (R1 and R2) receptor subunits are present on STN principal neurons. These GABAA receptor subunits are detected in a gradient along the dorsolateral to ventromedial axis in humans79. Further, GABA receptor a3 subunits (GABRA3) are localized in the anterior ventromedial region of the STN33. Anterograde tracing experiments in non-human primates with immunohistochemistry reveal major GABA afferents from GP and VP87–92.
Serotonin
Serotonin transporter (SERT) is present in passing fibers in rat, cat, monkey and human STN. In monkeys and humans, SERT was abundantly detected in medial parts of the STN32,33,50. In humans, 5’HT-immunoreactive fibers display a decreasing gradient along the mediolateral axis93. In rats, autoradiography studies show that SERT and serotonin receptor subtypes 5´HT1B and 5´HT2C are present in the STN. Notably, 5´HT1A and 5´HT2A receptors were not detected94. In mice, mRNA for 5´HT2C receptor was reported to be present along the entire STN structure, in a pattern similar to Vglut2 mRNA67.
Dopamine
The STN receives sparse dopaminergic projections from the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) as reported in monkeys95,96 and humans73. Dopamine receptor subtype 2 (D2R) is distributed in the human STN in decreasing gradient along a ventromedial-to-dorsal axis76,97. D2R is co-localised with adenosine receptor A2A within the dorsal and medial regions of the human STN97. In non-human primates, Dopamine receptor subtype 1 (D1R) and D2R are distributed on presynaptic axons and putative glutamatergic and GABAergic terminals98. In rats, D1R has high expression99. Further, firing rates of STN neurons are not altered by D2R stimulation, yet increased by selective stimulation of DRD1100.
In addition to different neurotransmitter receptors localized on STN neurons, also other types of molecular markers defining different cellular functions have been demonstrated in the STN, reviewed below.
Calcium-binding proteins
Calcium-binding proteins, specifically Parvalbumin (here abbreviated PARV), Calbindin (aka CALB1) and Calretinin (aka CALB2, Calbindin 2) are some of the major molecular markers examined in the STN in humans36,52,73,79. The distribution of these markers shows visible segmentation of PARV and CALB2 distribution in humans with approximately 50% of STN neurons positive for PARV and 50% CALB2-positive neurons101. These studies of the human STN also show that PARV is localized in the dorsal STN region and CALB2 in the ventral region of the STN, with some overlap or centrally33,36,52,73. This contrasting pattern of PARV and CALB2 has also been reported in monkeys. Similar to protein localization, their detection at the mRNA level shows similar distribution in the human STN73,102. This distinguishing molecular partition leads into three molecularly defined subdivisions (Parvalbumin+; Parvalbumin+/Calbindin2+; Calbindin2+) with distinctive anatomical localization (lateral-most; central; medial-most)36,47,103–105 (Fig. 1B–D).
Some functional features were supportive of the tripartite model of STN, in which the three anatomical subdivisions correlate with the three major STN functions; sensorimotor, associative and limbic44 (Fig. 3A). It has been proposed that PARV-positive STN neurons process motor functions whereas the CALB2-positive neurons are involved in limbic function. Moreover, this segmented localization of calcium-binding proteins suggests that differential calcium dynamics occurs along the STN73.
Fig. 3. Tripartite model of primate STN presented next to a recent model of molecular organization within mouse STN.
a Tripartite model of primate STN; motor, associative and limbic territories based on multiple anatomical-functional studies (references in main text). Drawing based on original illustration in Benarrouch, 2008; here updated with information of afferent and efferent projections as outlined by Baunez et al., 2016. Abbreviations: GPe, globus pallidus externa; GPi, globus pallidus interna; SNr, substantia nigra pars reticulata; SNc, substantia nigra pars compacta; PPN, pedunculopontine nucleus; VTA, ventral tegmental area. b Gene expression patterns spatially mapped in brain sections using fluorescent in situ hybridization (FISH) (original data reprinted in Fig. 2) allowed the outlining of molecularly profiled domains within mouse STN: STNa (ventromedial STN), STNb (central STN), STNc (dorsal STN), STNds (dorsal strip of STN showing GABA-positive, rather than glutamatergic, mRNA profile). Gene expression (mRNAs) present throughout the STN structure listed in the text box; unique gene expression patterns distinct for each domain listed under the domain name. c Selected mRNAs, their distribution and extent of co-localization with STN marker Pitx2, as described in the mouse STN. b, c: For mRNA full name, see main text. Illustrations based on original publication (Wallén-Mackenzie et al. 67; published under Open access).
Summarizing, the STN is an excitatory nucleus, with complex afferent interactions with other neurotransmitter systems. Moreover, the spatial distribution of calcium-binding proteins indicates differential functional capacity across the STN. Together, expression of neurotransmitter markers, including cognate receptors and calcium-binding proteins, reveals complex regulation of neural transmission within the STN.
Novel gene expressions detected within the STN of rodents
Similar to the human STN, the rodent STN shares the biconvex morphology and distribution of some cellular markers, including the distribution of Parvalbumin localized at the dorsal aspect and Calbindin 2 towards the ventral aspect of the STN33,36,52,67,73. However, there are also striking differences between rodent and primate, discussed further below.
The advantage of rodent studies is the availability of transgenics technology to label and manipulate specific genes and cells of interest within defined anatomical regions. Recent studies using fluorescent in situ hybridization (FISH) combining probes selective for several different markers show that Pitx2 mRNA, encoding the transcription factor Paired Like Homeodomain 2 (PITX2), is neatly localized within the STN and has overlapping expression with Vglut2 mRNA81. In fact, in the mouse STN, Pitx2 and Vglut2 mRNAs show 100% overlap106. Complete knock-out of the Pitx2 gene (Pitx2−/−) during embryonic development leads to abnormal development of the STN107. Two subsequent studies described the conditional knockout of Vglut2 in all Pitx2-positive STN neurons81,108. The implementation of Cre-driven mouse genetics achieved by crossing transgenic Pitx2-Cre recombinase mice109 with targeting mice carrying a floxed Vglut2 gene110, led to an overall reduction of 40% of Vglut2 expression levels in the STN108. Functionally, this conditional blunting of glutamatergic neurotransmission from the STN resulted in decreased post-synaptic currents, behavioral hyperlocomotion (further discussed in111) and decrease in sugar consumption with a significant effect on the midbrain dopamine system. These knockout studies thereby demonstrated opposite roles of STN glutamatergic neurons in motor compared to motivated behavior81,108.
Based on the complexity of functions mediated by the STN, it has been of particular interest to investigate if this structure is composed of molecularly defined neurons that may allow the dissociation of different behaviors. In the context of revealing transcriptional profiles within a cellular cluster or tissue, single-cell (sc) or single-nuclei (sn) RNA sequencing (RNASeq) technology is of particular importance112–115. In a recent study, application of snRNASeq in the Pitx2-Cre transgenic mouse line (described above) to direct selectivity to the STN was implemented. This resulted in the identification of six transcriptional clusters, all positive for Pitx2, and represented by strong expression of the glutamatergic marker Vglut2 (Slc17a6) and sparse expression of GABA markers67. From the abundance of genes representing different clusters, 16 mRNAs were selected for histological analysis using fluorescent in situ hybridization, FISH, to visualize the mRNA distribution in brain tissue sections covering the entire subthalamic area (included in Table 1, original data re-printed in Fig. 2; illustrated in Fig. 3). Spatial mapping of these 16 transcription products confirmed the distribution of Vglut2 and Pitx2 mRNAs throughout the mouse STN, and also identified a similar STN distribution of additional mRNAs. This included the serotonin receptor subtype 2c, 5´Htr2c, and Neurexophilin 1 mRNAs. Further, FISH analysis on serial sections allowed the definition of three major STN domains, based on clusters of combinatorial gene expression. For simplicity, these spatio-molecular domains were referred to as STNa, STNb, STNc, with STNa representing the medial-most domain; STNb, the central domain, and STNc, the lateral-most domain (Figs. 2, 3)67.
Fig. 2. Molecular heterogeneity of mouse STN as revealed by single nucleotide transcriptomics followed through with spatial mRNA mapping.
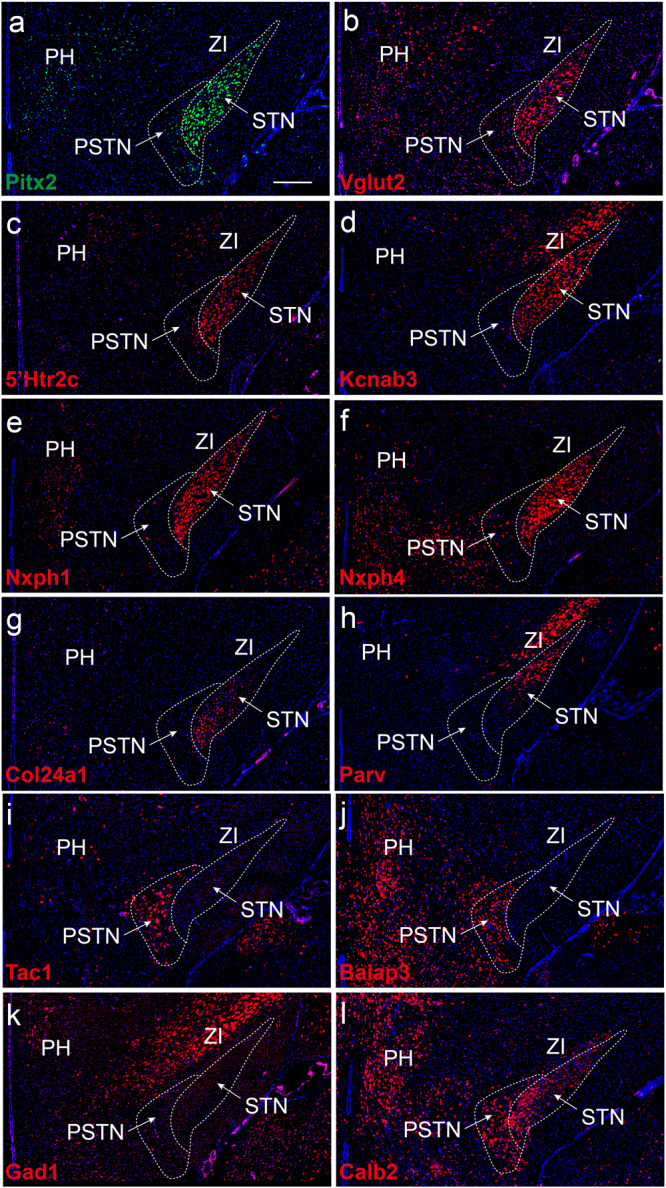
Serial mouse brain sections analyzed by fluorescent in situ hybridization (FISH) detects differential patterns and profiles of selected gene expression products; distribution within, and immediately surrounding, the STN structure: a Pitx2; b Vglut2; c Serotonin receptor subtype 2c, 5´Htr2c; d Kcnab3; e Nxph1; f Nxph4; g Col24a1; h PV i Tac1; j Baiap3; k Gad1; l Calb2. Note: Pitx2, Vglut2, Htr2c, Kcnab3, Nxph1 mRNAs (a–e) are present throughout the entire STN structure; Nxph4 mRNA is similar but excluded from dorsolateral-most part of STN; Col24a1 primarily in medially and centrally located STN neurons and excluded dorsally with opposite pattern of PV mRNA, primarily in dorsally and centrally located STN neurons while excluded medially (compare g and h); Tac1 and Baiap3 mRNAs absent from STN (i and j), but Tac1 strong in para-STN (PSTN), Baiap3 in PSTN and also surrounding hypothalamus (lateral and posterior hypothalamus, PH, LH); Gad1 (k; GABA marker) largely absent from STN and PSTN (in accordance with their excitatory phenotype) but present in GABAergic structure zona incerta (ZI), Calb2 mRNA stronger in medial STN and para-STN (PSTN) than dorsal STN (l). For mRNA full name, see the main text. See original publication for details (Wallén-Mackenzie et al. 67; published under Open access).
These three STN domains in the mouse were similar, but not identical, to the territories previously described for primate STN in terms of parvalbumin mRNA (for mouse here abbreviated PV) and Calbindin 2 (Calb2) mRNA such that PV mRNA was dominant laterally and decreased in a lateral-medial distribution, while Calb2 mRNA was most dominant medially. However, in the mouse STN, Calb2 was primarily detected in a spatially restricted cluster within the medial STNa domain, rather than distributed in a medial-lateral gradient as reported for the primate STN. This is a key difference between mice and primate. Interestingly, another molecular marker was identified in the mouse by snRNASeq which by histological analysis showed a similar distribution pattern as has been described for Calb2 in primate STN. This was Collagen Type XXIV Alpha 1 Chain (Col24a1) mRNA, which was distributed in an opposite gradient compared to PV mRNA. The largely opposite expression of PV and Col24a1 mRNAs was critical to the partitioning into three main domains in the mouse STN: STNa (Col24a1+/PV-), medial-most domain; STNb (Col24a1+/PV+), the central domain; STNc (Col24a1-/PV+), the lateral-most domain.
In addition to the three major spatio-molecular domains defined in the mouse STN, one small dorsal strip (termed STNds) was described, which differed in gene expression from the rest of the STN. For example, STNds was positive for Neurexophilin 1 but negative for Neurexophilin 4, while STNa, STNb, and STNc were all positive for both of these. Another distinguishing feature of STNds was positive labelling for Gad mRNA rather than Vglut2 mRNA, strongly indicating a GABAergic identity67. It remains to fully validate if STNds is a bona fide STN structure. One can imagine that stimulating electrodes placed in, or merely affecting, the STNds would give a strikingly rather different result than if they targeted the STNa, STNb, or STNc (Fig. 3B, C).
Further, histological mapping in serial brain sections showed that some markers identified in the snRNASeq experiment were not primarily expressed in the STN but were found to be more useful as markers of anatomically adjacent structures. This included Tac1 mRNA which was identified as a novel marker for the para-STN, the hypothalamic structure directly adjoined with the medial aspect of the STN (Fig. 3B)67.
The transcriptomics-based approach combined with spatial histological mapping in the subthalamic area enabled the identification of a range of molecular markers (gene expression patterns) that allowed dissociation of the STN structure into spatio-molecular defined domains and also the identification of molecularly defined subpopulations in adjacent areas. These anatomically neighbouring areas included the para-STN, and also zona incerta, and the mamillary and retro-mamillary nuclei (these latter structures not further reviewed here)67.
Co-expression studies of Col24a1 and Calb2 genes would be of interest to examine if they are localized in the same or different neurons within STNa domain. Knock-out of the Col24a1 gene in mice leads to increased grip strength (The International Mouse Phenotyping Consortium (IMPC)), indicative of motor function and deviating from the tripartite model that the dorsal region regulates motor control. Motor dysfunction in the STN may thus be related to absence Col24a1 function within the STN, of interest to address in future study. Further, it would be of interest if Col24a1-positive neurons are more correlated with limbic function of the STN, considering their presence in the ventro-medial tip of the mouse STN, the area which in primates is referred to as the limbic tip based on its projections to limbic brain areas such as VP, as described above. The implementation of the promoters for these spatially restricted expressed genes as drivers of Cre and Flp recombinases in viral-genetics and conditional knockout experiments holds promise for securing a higher level of resolution in studies of the anatomical-functional organization of the STN.
Functional analysis of neuronal STN populations in mice have demonstrated regulation of emotional, locomotor, reward, motivation, and arousal behavior. For example, optogenetic activation of Pitx2+ or Gabrr3+ neurons of the STN inhibited locomotion and increased repetitive self-grooming behavior in mice116,117 (discussed further below). Distinctive STN populations regulating different behaviors have also been demonstrated in human studies. Single neuron recording using micro-electrode recording in the STN of PD patients delineated two spatially distinct STN populations responding to valence and arousal118.
In summary, most protein localization studies were conducted in human or monkey brain tissue (Table 1). On the other hand, mRNA studies of the STN were mainly conducted in rodents, but also some in primates (Table 1). The major distinguishing markers of dorsal and ventral STN are Parvalbumin and Calbindin 2, respectively, that show similar (but not identical) distribution in human and rodent tissue. In addition, Col24a1 mRNA was recently identified in mouse STN as a marker for the medio-ventral and central parts of the STN.
Early studies in the 1900’s disputed whether the STN was a homogeneous structure with no subdivision or two subdivisions119,120, followed by the proposal of three subdivisions forming the tripartite organization of the STN42,121–123. However, a detailed summary of studies reported between 1925 and 2010 presented by Keuken et al., 2012 indicates a more complex organization of the STN49. This is supported by recent studies that have extended analysis across the STN revealing three-dimensional (3D) immunoreactivity of a number of molecular markers within the STN and showing gradient distribution patterns across rostral to caudal and dorsal to ventral plane within the STN in postmortem human brain33,34. The localization of gene expression and final protein products as outlined above have expanded the early proposals of cytoarchitecture to reveal a highly complex organization of the STN.
Gene expression studies in rodent studies reveal similar but not identical expression patterns to monkey and humans. The relatively highly conserved expression patterns allow neural modulation in rodents using cutting-edge technologies with high spatial selectivity and precision to then translate back into understanding STN organization in humans.
STN-regulated behaviors and STN-DBS side-effects
Most evidence of STN functions in behaviors other than motor function emerges indirectly from DBS in PD patients. While improving motor symptoms, some PD patients who underwent STN-DBS have reported weight gain, depression, development of addictive behaviors and other non-motor symptoms9,23,124–127. Due to the complex and progressive pathology of PD, along with pre-existing conditions and often in combination with pharmacological treatments, it is difficult to explicitly identify STN contributions in non-motor functions, and to specify if reported adverse effects are due to STN-DBS or due to the progression of the disease and/or pharmacological medications. Direct evidence of STN contributions come from animal models applying STN lesion, DBS, optogenetics and pharmacological manipulations, and that report altered movement, reward, feeding, addiction and impulsive behavior128–131. The major functions regulated by the STN are grouped by motor behavior, and associative and limbic functions. These in turn, are dependent on anatomical, molecular, cellular organizations alongside, afferent and efferent projections, forming distinct neurocircuitry.
Motor function
Activation of the STN is correlated with inhibition of movement via the indirect and hyperdirect pathways, both of which the STN is a critical component132,133. Studies of humans show that dorsolateral STN (dlSTN) mainly regulates motor functions, electrode placement for DBS in PD cases is targeted to the dorsolateral STN134,135. Pharmacological GABAergic inhibition of the STN in non-human primate model of PD further revealed mediolateral regionalisation within dlSTN where the medial region of dlSTN regulates upper limb and lateral dlSTN is associated to lower limbs136. Anterograde tracing studies demonstrated topographically organized cortical afferents to dlSTN, forming the hyperdirect pathway46.
The STN is part of the hyperdirect and indirect pathway of the basal ganglia motor circuitry. The indirect pathway works antagonistically to the direct pathway where the direct pathway promotes movement and the indirect pathway inhibits movement, exerting balanced control over movement137. Different populations of striatal neurons within the basal ganglia give rise to the direct and indirect pathways. Optogenetic activation under the control of DR1 or DR2 promoters leads to opposing results on movement. Activation of the direct pathway (DR1) increases movement whereas activation of indirect pathway (DR2) decreases movement, respectively133. The STN mainly receives inhibitory projections from globus pallidus (GP) externa (GPe) and extends glutamatergic projections to substantia nigra pars reticulata (SNr) and GPi138,139. In line with the relay of the indirect pathway, optogenetic inhibition of STN neurons promotes movement117,131,140,141 while optogenetic excitation of the STN inhibits movement, reducing specific locomotion tasks117,131. As described above, similar results have been observed upon conditional reduction of Vglut2 gene expression levels in STN in mice which induces hyperlocomotion without impacting on limbic and cognitive functions106,108. Curiously, optogenetic excitation of the STN did not only reduce motor tasks. Instead, some behaviors were increased, such as self-grooming and jumping behaviors which were initiated immediately upon photo-stimulation of the STN117. Together, these results demonstrate that STN neuronal activity is capable of bidirectional motor regulation.
Limbic function
The STN is an important mediator of reward-seeking behavior142–144. Significant weight gain has been reported by a number of PD patients following STN-DBS124. Potential mechanisms for shift in weight from clinical studies include decrease in energy expenditure127, changes in neuropeptide level (neuropeptide Y and ghrelin)145 and increase in food intake146. STN lesions in rodents show increase in motivation for food147,148. Real-time calcium imaging using fibre photometry in mice evoked enhanced activity of STN neurons during feeding behavior130. Increase in STN neural firing activity was specific for sweet food and standard rodent chow but not for bitter taste. The extent of neural activity was dependent on the valence and palatability of food. Bidirectional optogenetic manipulation during feeding behavior showed that inhibition of the STN led to increased food intake while activation of the STN decreased food intake, but only during the dark phase (period when nocturnal animals consume food)130.
Limbic functions with the STN are most likely influenced by input from the VP149. Neural tracer studies in monkeys reveal that the VP projects selectively to the ventral region of the STN, correlating to the limbic domain46,47. Similar connectivity is found in rats, where the dorsal lateral VP projects to the dorsal medial STN71,150–153. In a rodent model of alcohol addiction, the pathway from VP to the STN is active during relapse to alcohol-seeking, and inhibition of this pathway reduced relapse to alcohol-seeking without affecting locomotion149.
STN is involved in the motivation to respond for a variety of drugs of abuse including methamphetamine and cocaine18,142,148,154,155. STN-DBS decreased the effort for cocaine intake but increased food intake, demonstrating differential patterns of activity in the STN for drugs of abuse and natural rewards148. Low-frequency neuronal oscillations of theta and beta bands in the STN corelated with increased escalation of cocaine intake18. Moreover, DBS at low frequency of 30 Hz reduced compulsive cocaine-seeking in rats19. Administration of the neuropeptide oxytocin reduces relapse to Methamphetamine-seeking behavior156.
A recent study implemented Cre-driven optogenetics using Pitx2-Cre, Vglut2-Cre and PV-Cre to address the role of STN in emotional affect. It was shown that excitation of the whole STN using either Pitx2-Cre or Vglut2-Cre resulted in behavioral avoidance and cue-induced aversion. In contrast, PV-Cre, implemented to tease out the dorsal STN, did not give as the same potent aversive response. This result is a strong indicator that the PV-positive subpopulation of STN is not a primary driver of aversion mediated by the STN, but that STN aversion neurons remain to identify. The study also found an indirect pathway to the lateral habenula, a key hub for aversion and depression, via STN-projections to VP and GPi (called entopeduncular nucleus in rodents). In addition, this study demonstrated striking similarity in Pitx2, Vglut2, PV and Tac1 expression patterns between mouse and macaque monkey and identified a clear boundary between STN and para-STN defined by a mutually exclusive expression of Pitx2 (STN) and Tac1 (para-STN). Excitation of STN caused aversive learning, while similar excitation of para-STN did not results in a conditioned response. This study shows that the implementation of spatially selective promoter activities holds promise towards anatomical-functional decoding of the STN157.
Overall, changes in STN neural oscillation, activity and connectivity from the VP and neuropeptides such as neuropeptide oxytocin have been demonstrated to regulate limbic functions impacted in feeding and substance use disorders. Together, these findings provide insight towards refined approaches to target limbic dysfunctions. STN has been identified as a target for DBS treatment of addiction158,159. Further characterization of subpopulations of STN neurons engaged in specific limbic functions will be important.
Cognitive function
STN contributions in cognition have been demonstrated in action control, decision-making, attention, executive functions and verbal learning and memory160,161. The STN is strongly associated with cognitive behavior in monkey and humans, specifically in action selection between correct and incorrect behaviors162–165. Cortical afferents to the ventromedial STN region, the associative area of the STN in accordance with the tripartite model, have been characterized in primates and rats166–168. STN-DBS studies in clinical PD have identified a decline in cognitive activity in working memory, response inhibition and verbal functions when comparing these abilities pre-operative and post-operative DBS169–173. In OCD cases, STN-DBS impacts on decision-making and response inhibition163,174. Animal models with STN lesions show impaired response inhibition175,176 and increase in impulsivity177. Optogenetic inhibition of medial prefrontal control projections to STN impaired spatial working memory but not anxiety178. Changes in THETA oscillations in the STN regulates response inhibition179,180. Cognitive functions in the STN are dependent on oscillatory dynamics in the STN and inputs from the cortex178,181.
Recent studies clearly demonstrate the capacity of STN to regulate motor control emphasizing its essential role of the STN in motor regulation. Yet the specific role of STN in motivational and cognition requires further studies. In motivational behavior, the changes in STN neural oscillations and connectivity to the VP are established. However, the contributions of dopamine inputs in STN in motivational behavior is an important area that remains to be investigated, especially in the realm of motivational dysfunction in PD patients.
STN-related neurological disorders
The STN has been implicated in several neurological disorders, including PD, progressive supranuclear palsy (PSP), argyrophilic grain disease (AGD), Huntington’s disease, spinocerebellar ataxia 3 and 7, and dentatorubral-pallidoluysian atrophy (DRPLA)36,182,183. Apart from PD, in which no degeneration of STN neurons have been identified, the pathology of all other STN disorders includes neuronal loss and astrocytic gliosis in the STN51. In the case of spinocerebellar ataxia type 3, brownish discoloration of the STN is also a feature32,184–186. In Huntington’s chorea disease, the STN undergoes 25% reduction in volume and number of STN neurons183. PSP is a degenerative disorder that effects the STN and is often misdiagnosed as PD due to the PSP and PD diseases displaying similar motor symptoms182. While the STN of PSP patients undergoes neurodegeneration with significant loss of neurons, neurofibrillary tangles and gliosis, the STN of PD patients remain anatomically intact36,184.
PD is the second most common neurodegenerative disorder187. Lesion of the STN has been used as a treatment for PD in the past, providing therapeutic effects on motor symptoms188,189. STN-DBS provides a safer and more effective alternative, as it is a reversible and adjustable treatment. Despite the pivotal role of the STN in PD symptoms and treatment, our knowledge of its pathology during PD is limited. It has been established that the STN of PD patients is hyperactive with continuous abnormal ‘bursting’ which fires at a higher rate42,190. This hyperactivity not only contributes to the motor symptoms experienced in PD but shows correlation to the severity of motor deficits as well191,192. In PD, a loss of dopamine-producing neurons of the SNc results in decreased dopaminergic activity in the basal ganglia, causing dysfunction of the basal ganglia pathways. It is hypothesized that this results in excessive activity of the STN causing abnormal activation of the indirect pathway. This increased STN activity increases involuntary movements and decreased the ability to execute intended movement, characterized by the motor symptoms seen in PD patients191.
Cellular changes in STN-related disorders
The STN is a major treatment target for advanced stage PD, treatment resistant OCD, essential tremor and dystonia8,123,193–197. Efficacy and tolerability of DBS in Tourette’s syndrome is currently in trials for treatment of severely affected and otherwise treatment-resistant patients. However, there are no direct links to cellular or anatomical pathology of the STN in either disorder. Instead, interventions aim at targeting altered firing activity of STN neurons. In PD patients, the dysregulation in STN neural firing is correlated to the motor deficit severity, rather than changes in STN anatomy198.
Alterations in STN neurotransmission were proposed to be driven by GABA inputs from the GP and dopamine inputs from the SNc199. Post-mortem analysis has shown decreased density of GABAB receptors in the caudate putamen and GPe in PD patients compared to controls200. GABAA receptors mediate fast and GABAB mediate slow inhibitory responses in the STN. GABAA and GABAB receptor subunits are differentially localized in the human STN where GABAA was identified as more intensely distributed in the ventromedial region of the STN and GABAB subunits were detected throughout the STN79. Further analysis of GABA receptor subunits in the STN of PD cases should be an area of further investigation.
Among molecular markers in the STN reported in rodent, monkeys and humans (listed in Table 1), very few have been examined in STN-related disorders. However, it has been shown that the segmentation of PARV and CALB2 immunoreactivity in control post-mortem human brain tissues and PD cases were unchanged36, leaving the idea that the STN is anatomically intact in PD cases. One study has reported the presence of Lewy bodies in the STN of PD cases201. This finding suggests that instead of cell loss, there may be cellular dysfunction of STN neurons in PD.
The cellular pathologies identified within the STN tend to be disease-specific with either cell loss, aberrant neural firing pattens or changes at the protein level. Only a handful of cellular markers have been examined in STN. However, recent expression studies in healthy human STN have identified spatial expression of a range of proteins in the STN to the examined in disease cases.
Future directions
The molecular organization of the STN is strikingly heterogeneous. This heterogeneity, of which more is likely yet to be revealed, provides an increased understanding towards the neurobiological underpinnings of the roles played by STN in motor, limbic and cognitive regulation. While we are likely only at the doorstep of understanding the complexity of this clinically crucial brain area, the association of STN heterogeneity with the regulation of distinct behaviors will be significant throughout the fields of physiology, motivation, movement, cognition, psychology and more. For example, future therapeutic approaches such as optogenetic stimulation which target specific cell types or hybrid application of electric and optogenetic stimulation can refine current DBS approaches to reduce adverse or off-target effects202.
This review focused on summarizing current knowledge of long-established and newly described molecular and cellular markers within the STN. Future directions include firstly to functionally characterize such markers in the context of the different roles played by the STN, and secondly to assess their putative regulation in STN-related diseases. Further studies are required to extend current knowledge of the internal architecture of the STN and its plethora of roles in neurocircuitry and behavioral control. The examination of molecular profiles within the STN in normal brain physiology and in neurological disorders will aid towards a better understanding of STN´s role in health and disease, and add information towards more precise brain maps, which in turn can help towards refined treatment approaches.
Acknowledgements
Preparation of this manuscript was supported by grants to A.A.P. from the National Health and Medical Research Council of Australia (APP1160412) and to Å.W-M. from the Swedish Research Council (Vetenskapsrådet), Swedish Brain Foundation (Hjärnfonden), Swedish Parkinson Foundation (Parkinsonfonden), Hållsten Research Foundation and Åhlénstiftelsen. We thank Alessia Ricci, Eleonora Rubino and Gian Pietro Serra in MackenzieLab for constructive feedback, Alessia Ricci for illustrations in Fig. 3b, c and Sylvie Dumas, Oramacell, Paris, France, for Fig. 2 (all based on original publication Wallén-Mackenzie et al. 67; published under Open access).
Author contributions
A.A.P: conceptual design, acquisition, analysis, interpretation and review of reported data, figure preparation, writing–original draft, editing and revision; Å.W.M: conceptual design, acquisition, analysis, interpretation and review of reported data, figure preparation, writing––original draft, editing and revision.
Peer review
Peer review information
Communications Biology thanks Anneke Alkemade and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Christoph Anacker and Manuel Breuer.
Competing interests
The authors declare no competing interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Asheeta A. Prasad, Email: asheeta.prasad@sydney.edu.au
Åsa Wallén-Mackenzie, Email: asa.mackenzie@ebc.uu.se.
References
- 1.Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson B, Grillner S. Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Curr. Biol. 2011;21:1081–1091. doi: 10.1016/j.cub.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 2.John MP. The subthalamic nucleus and Jules Bernard Luys (1828–97) J. Neurol., Neurosurg. Psychiatry. 2001;71:783. doi: 10.1136/jnnp.71.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parent A. Jules Bernard Luys and the subthalamic nucleus. Mov. Disord. 2002;17:181–185. doi: 10.1002/mds.1251. [DOI] [PubMed] [Google Scholar]
- 4.Hamada I, DeLong MR. Excitotoxic acid lesions of the primate subthalamic nucleus result in reduced pallidal neuronal activity during active holding. J. Neurophysiol. 1992;68:1859–1866. doi: 10.1152/jn.1992.68.5.1859. [DOI] [PubMed] [Google Scholar]
- 5.Bergman H, Wichmann T, DeLong MR. Reversal of experimental Parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 6.Limousin P, Foltynie T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat. Rev. Neurol. 2019;15:234–242. doi: 10.1038/s41582-019-0145-9. [DOI] [PubMed] [Google Scholar]
- 7.Schuepbach WM, et al. Neurostimulation for Parkinson’s disease with early motor complications. N. Engl. J. Med. 2013;368:610–622. doi: 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- 8.Mallet L, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N. Engl. J. Med. 2008;359:2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- 9.Chabardes S, et al. Deep brain stimulation for obsessive-compulsive disorder: subthalamic nucleus target. World Neurosurg. 2013;80:S31 e31–S31 e38. doi: 10.1016/j.wneu.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Guzick A, et al. Improving long term patient outcomes from deep brain stimulation for treatment-refractory obsessive-compulsive disorder. Expert Rev. Neurother. 2020;20:95–107. doi: 10.1080/14737175.2020.1694409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tambirajoo, K., Furlanetti, L., Samuel, M. & Ashkan, K. Subthalamic nucleus deep brain stimulation in post-infarct dystonia. Stereotac. Funct. Neurosurg., 1-13 10.1159/000509317 (2020). [DOI] [PubMed]
- 12.Baldermann JC, et al. Deep brain stimulation for tourette-syndrome: a systematic review and meta-analysis. Brain Stimul. 2016;9:296–304. doi: 10.1016/j.brs.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Dai L, et al. Subthalamic deep brain stimulation for refractory Gilles de la Tourette’s syndrome: clinical outcome and functional connectivity. J. Neurol. 2022;269:6116–6126. doi: 10.1007/s00415-022-11266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vissani M, et al. Spatio-temporal structure of single neuron subthalamic activity identifies DBS target for anesthetized Tourette syndrome patients. J. Neural Eng. 2019;16:066011. doi: 10.1088/1741-2552/ab37b4. [DOI] [PubMed] [Google Scholar]
- 15.Gunduz A, Okun MS. A review and update on Tourette Syndrome: Where Is the field headed? Curr. Neurol. Neurosci. Rep. 2016;16:37. doi: 10.1007/s11910-016-0633-x. [DOI] [PubMed] [Google Scholar]
- 16.Bentzley BS, Aston-Jones G. Inhibiting subthalamic nucleus decreases cocaine demand and relapse: therapeutic potential. Addict. Biol. 2017;22:946–957. doi: 10.1111/adb.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelloux Y, Baunez C. [Surgical strategy to rescue the addicts] Med Sci. 2015;31:674–679. doi: 10.1051/medsci/20153106022. [DOI] [PubMed] [Google Scholar]
- 18.Pelloux Y, et al. Subthalamic nucleus high frequency stimulation prevents and reverses escalated cocaine use. Mol. Psychiatry. 2018;23:2266–2276. doi: 10.1038/s41380-018-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degoulet, M., Tiran-Cappello, A., Combrisson, E., Baunez, C. & Pelloux, Y. Subthalamic low-frequency oscillations predict vulnerability to cocaine addiction. Proc. Natl Acad. Sci. USA11810.1073/pnas.2024121118 (2021). [DOI] [PMC free article] [PubMed]
- 20.Jost ST, et al. Gender gap in deep brain stimulation for Parkinson’s disease. NPJ Parkinsons Dis. 2022;8:47. doi: 10.1038/s41531-022-00305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houeto JL, et al. Behavioural disorders, Parkinson’s disease and subthalamic stimulation. J. Neurol. Neurosurg. Psychiatry. 2002;72:701–707. doi: 10.1136/jnnp.72.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sensi M, et al. Explosive-aggressive behavior related to bilateral subthalamic stimulation. Parkinsonism Relat. Disord. 2004;10:247–251. doi: 10.1016/j.parkreldis.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Romito LM, Contarino FM, Albanese A. Transient gender-related effects in Parkinson’s disease patients with subthalamic stimulation. J. Neurol. 2010;257:603–608. doi: 10.1007/s00415-009-5381-2. [DOI] [PubMed] [Google Scholar]
- 24.Romito LM, et al. Transient mania with hypersexuality after surgery for high frequency stimulation of the subthalamic nucleus in Parkinson’s disease. Mov. Disord. 2002;17:1371–1374. doi: 10.1002/mds.10265. [DOI] [PubMed] [Google Scholar]
- 25.Romito LM, et al. Long-term follow up of subthalamic nucleus stimulation in Parkinson’s disease. Neurology. 2002;58:1546–1550. doi: 10.1212/WNL.58.10.1546. [DOI] [PubMed] [Google Scholar]
- 26.Spix TA, et al. Population-specific neuromodulation prolongs therapeutic benefits of deep brain stimulation. Science. 2021;374:201–206. doi: 10.1126/science.abi7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin, S. et al. Globus pallidus internus versus subthalamic nucleus deep brain stimulation for isolated dystonia: A 3-year follow-up. Eur. J. Neurol. 10.1111/ene.15895 (2023). [DOI] [PubMed]
- 28.Fan S, et al. Differential effects of subthalamic nucleus and globus pallidus internus deep brain stimulation on motor subtypes in Parkinson’s disease. World Neurosurg. 2022;164:e245–e255. doi: 10.1016/j.wneu.2022.04.084. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S, et al. Apathy following bilateral deep brain stimulation of subthalamic nucleus and globus Pallidus Internus in Parkinson’s disease: a meta-analysis. Parkinsons Dis. 2022;2022:4204564. doi: 10.1155/2022/4204564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Ghazal N, et al. The effects of deep brain stimulation on mood and quality of life in Parkinson’s disease: a systematic review and meta-analysis. Cureus. 2023;15:e44177. doi: 10.7759/cureus.44177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mainardi, M. et al. Deep brain stimulation of globus pallidus internus and subthalamic nucleus in Parkinson’s disease: a multicenter, retrospective study of efficacy and safety. Neurol. Sci.10.1007/s10072-023-06999-z (2023). [DOI] [PMC free article] [PubMed]
- 32.Mori S, Takino T, Yamada H, Sano Y. Immunohistochemical demonstration of serotonin nerve fibers in the subthalamic nucleus of the rat, cat and monkey. Neurosci. Lett. 1985;62:305–309. doi: 10.1016/0304-3940(85)90566-X. [DOI] [PubMed] [Google Scholar]
- 33.Alkemade A, et al. The functional microscopic neuroanatomy of the human subthalamic nucleus. Brain Struct. Funct. 2019;224:3213–3227. doi: 10.1007/s00429-019-01960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bokulić E, et al. The stereological analysis and spatial distribution of neurons in the human subthalamic nucleus. Front. Neuroanat. 2021;15:749390. doi: 10.3389/fnana.2021.749390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciriello J, Rosas-Arellano MP, Solano-Flores LP, de Oliveira CVR. Identification of neurons containing orexin-B (hypocretin-2) immunoreactivity in limbic structures. Brain Res. 2003;967:123–131. doi: 10.1016/S0006-8993(02)04233-6. [DOI] [PubMed] [Google Scholar]
- 36.Hardman CD, Halliday GM, McRitchie DA, Morris JG. The subthalamic nucleus in Parkinson’s disease and progressive supranuclear palsy. J. Neuropathol. Exp. Neurol. 1997;56:132–142. doi: 10.1097/00005072-199702000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Emmi, A., Antonini, A., Macchi, V., Porzionato, A. & De Caro, R. Anatomy and connectivity of the subthalamic nucleus in humans and non-human primates. Front. Neuroanat.1410.3389/fnana.2020.00013 (2020). [DOI] [PMC free article] [PubMed]
- 38.Alkemade A, et al. The Amsterdam Ultra-high field adult lifespan database (AHEAD): A freely available multimodal 7 Tesla submillimeter magnetic resonance imaging database. NeuroImage. 2020;221:117200. doi: 10.1016/j.neuroimage.2020.117200. [DOI] [PubMed] [Google Scholar]
- 39.Keuken MC, et al. Ultra-high 7T MRI of structural age-related changes of the subthalamic nucleus. J. Neurosci. 2013;33:4896–4900. doi: 10.1523/JNEUROSCI.3241-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambert C, et al. Confirmation of functional zones within the human subthalamic nucleus: Patterns of connectivity and sub-parcellation using diffusion weighted imaging. Neuroimage. 2012;60:83–94. doi: 10.1016/j.neuroimage.2011.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isaacs BR, et al. 3 versus 7 Tesla magnetic resonance imaging for parcellations of subcortical brain structures in clinical settings. PLoS One. 2020;15:e0236208. doi: 10.1371/journal.pone.0236208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM. The subthalamic nucleus in the context of movement disorders. Brain. 2004;127:4–20. doi: 10.1093/brain/awh029. [DOI] [PubMed] [Google Scholar]
- 43.Accolla EA, et al. Brain tissue properties differentiate between motor and limbic basal ganglia circuits. Hum. Brain Mapp. 2014;35:5083–5092. doi: 10.1002/hbm.22533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alkemade A, Schnitzler A, Forstmann BU. Topographic organization of the human and non-human primate subthalamic nucleus. Brain Struct. Funct. 2015;220:3075–3086. doi: 10.1007/s00429-015-1047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Temel Y, et al. Cognitive and limbic effects of deep brain stimulation in preclinical studies. Front Biosci. 2009;14:1891–1901. doi: 10.2741/3349. [DOI] [PubMed] [Google Scholar]
- 46.Haynes WI, Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J. Neurosci. 2013;33:4804–4814. doi: 10.1523/JNEUROSCI.4674-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karachi C, et al. The pallidosubthalamic projection: an anatomical substrate for nonmotor functions of the subthalamic nucleus in primates. Mov. Disord. 2005;20:172–180. doi: 10.1002/mds.20302. [DOI] [PubMed] [Google Scholar]
- 48.Forstmann BU, Keuken MC, Alkemade A. The next step for imaging the subthalamic nucleus. Brain. 2016;139:e69–e69. doi: 10.1093/brain/aww245. [DOI] [PubMed] [Google Scholar]
- 49.Keuken, M. et al. Are there three subdivisions in the primate subthalamic nucleus? Front. Neuroanat.610.3389/fnana.2012.00014 (2012). [DOI] [PMC free article] [PubMed]
- 50.Santin, M. d. N. et al. Anatomical characterisation of three different psychosurgical targets in the subthalamic area: from the basal ganglia to the limbic system. Brain Struct. Funct.10.1007/s00429-023-02691-2 (2023). [DOI] [PubMed]
- 51.Mazumder S, Bahar AY, Shepherd CE, Prasad AA. Post-mortem brain histological examination in the substantia nigra and subthalamic nucleus in Parkinson’s disease following deep brain stimulation. Front Neurosci. 2022;16:948523. doi: 10.3389/fnins.2022.948523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Münkle MC, Waldvogel HJ, Faull RLM. The distribution of calbindin, calretinin and parvalbumin immunoreactivity in the human thalamus. J. Chem. Neuroanat. 2000;19:155–173. doi: 10.1016/S0891-0618(00)00060-0. [DOI] [PubMed] [Google Scholar]
- 53.Kuwajima M, Hall RA, Aiba A, Smith Y. Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the monkey subthalamic nucleus. J. Comp. Neurol. 2004;474:589–602. doi: 10.1002/cne.20158. [DOI] [PubMed] [Google Scholar]
- 54.Accolla EA, et al. Brain networks modulated by subthalamic nucleus deep brain stimulation. Brain. 2016;139:2503–2515. doi: 10.1093/brain/aww182. [DOI] [PubMed] [Google Scholar]
- 55.Akram H, et al. Subthalamic deep brain stimulation sweet spots and hyperdirect cortical connectivity in Parkinson’s disease. Neuroimage. 2017;158:332–345. doi: 10.1016/j.neuroimage.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brunenberg EJ, et al. Structural and resting state functional connectivity of the subthalamic nucleus: identification of motor STN parts and the hyperdirect pathway. PLoS One. 2012;7:e39061. doi: 10.1371/journal.pone.0039061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avecillas-Chasin JM, Rascón-Ramírez F, Barcia JA. Tractographical model of the cortico-basal ganglia and corticothalamic connections: Improving our understanding of deep brain stimulation. Clin. Anat. 2016;29:481–492. doi: 10.1002/ca.22689. [DOI] [PubMed] [Google Scholar]
- 58.Plantinga BR, et al. Individualized parcellation of the subthalamic nucleus in patients with Parkinson’s disease with 7T MRI. Neuroimage. 2018;168:403–411. doi: 10.1016/j.neuroimage.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosley PE, Akram H. Neuropsychiatric effects of subthalamic deep brain stimulation. Handb. Clin. Neurol. 2021;180:417–431. doi: 10.1016/B978-0-12-820107-7.00026-4. [DOI] [PubMed] [Google Scholar]
- 60.LeWitt PA, Chaudhuri KR. Unmet needs in Parkinson disease: Motor and non-motor. Parkinsonism Relat. Disord. 2020;80:S7–s12. doi: 10.1016/j.parkreldis.2020.09.024. [DOI] [PubMed] [Google Scholar]
- 61.Castelli L, et al. Sexual well being in parkinsonian patients after deep brain stimulation of the subthalamic nucleus. J. Neurol. Neurosurg. Psychiatry. 2004;75:1260–1264. doi: 10.1136/jnnp.2003.034579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eghlidos Z, Rahimian Z, Vadiee G, Jahangiri S. Effects of subthalamic deep brain stimulation on non-motor symptoms of Parkinson’s disease: A meta-analysis. Acta Neurol. Scand. 2022;146:115–125. doi: 10.1111/ane.13652. [DOI] [PubMed] [Google Scholar]
- 63.Kurtis MM, Rajah T, Delgado LF, Dafsari HS. The effect of deep brain stimulation on the non-motor symptoms of Parkinson’s disease: a critical review of the current evidence. Npj Parkinson’s Dis. 2017;3:16024. doi: 10.1038/npjparkd.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dafsari, H. S. et al. EuroInf 2: Subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov. Disord.010.1002/mds.27626. [DOI] [PubMed]
- 65.Volkmann J, Daniels C, Witt K. Neuropsychiatric effects of subthalamic neurostimulation in Parkinson disease. Nat. Rev. Neurol. 2010;6:487–498. doi: 10.1038/nrneurol.2010.111. [DOI] [PubMed] [Google Scholar]
- 66.Kim JS, et al. Hypomania induced by subthalamic nucleus stimulation in a Parkinson’s disease patient: does it suggest a dysfunction of the limbic circuit? J. Mov. Disord. 2012;5:14–17. doi: 10.14802/jmd.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wallén-Mackenzie Å, et al. Spatio-molecular domains identified in the mouse subthalamic nucleus and neighboring glutamatergic and GABAergic brain structures. Commun. Biol. 2020;3:338. doi: 10.1038/s42003-020-1028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pouliopoulos AN, et al. Non-invasive optogenetics with ultrasound-mediated gene delivery and red-light excitation. Brain Stimul. 2022;15:927–941. doi: 10.1016/j.brs.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stephenson-Jones M, Ericsson J, Robertson B, Grillner S. Evolution of the basal ganglia: dual-output pathways conserved throughout vertebrate phylogeny. J. Comp. Neurol. 2012;520:2957–2973. doi: 10.1002/cne.23087. [DOI] [PubMed] [Google Scholar]
- 70.Lévesque JC, Parent A. GABAergic interneurons in human subthalamic nucleus. Mov. Disord. 2005;20:574–584. doi: 10.1002/mds.20374. [DOI] [PubMed] [Google Scholar]
- 71.Haber SN, Groenewegen HJ, Grove EA, Nauta WJ. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J. Comp. Neurol. 1985;235:322–335. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- 72.Kultas-Ilinsky K, Leontiev V, Whiting PJ. Expression of 10 GABA(A) receptor subunit messenger RNAs in the motor-related thalamic nuclei and basal ganglia of Macaca mulatta studied with in situ hybridization histochemistry. Neuroscience. 1998;85:179–204. doi: 10.1016/S0306-4522(97)00634-9. [DOI] [PubMed] [Google Scholar]
- 73.Augood SJ, Waldvogel HJ, Münkle MC, Faull RLM, Emson PC. Localization of calcium-binding proteins and GABA transporter (GAT-1) messenger RNA in the human subthalamic nucleus. Neuroscience. 1999;88:521–534. doi: 10.1016/S0306-4522(98)00226-7. [DOI] [PubMed] [Google Scholar]
- 74.Hedreen JC. Tyrosine hydroxylase-immunoreactive elements in the human globus pallidus and subthalamic nucleus. J. Comp. Neurol. 1999;409:400–410. doi: 10.1002/(SICI)1096-9861(19990705)409:3<400::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 75.Charara A, Heilman TC, Levey AI, Smith Y. Pre- and postsynaptic localization of GABA(B) receptors in the basal ganglia in monkeys. Neuroscience. 2000;95:127–140. doi: 10.1016/S0306-4522(99)00409-1. [DOI] [PubMed] [Google Scholar]
- 76.Hurd YL, Suzuki M, Sedvall GC. D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J. Chem. Neuroanat. 2001;22:127–137. doi: 10.1016/S0891-0618(01)00122-3. [DOI] [PubMed] [Google Scholar]
- 77.Sato F, Lavallée P, Lévesque M, Parent A. Single-axon tracing study of neurons of the external segment of the globus pallidus in primate. J. Comp. Neurol. 2000;417:17–31. doi: 10.1002/(SICI)1096-9861(20000131)417:1<17::AID-CNE2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 78.Parent M, Wallman MJ, Gagnon D, Parent A. Serotonin innervation of basal ganglia in monkeys and humans. J. Chem. Neuroanat. 2011;41:256–265. doi: 10.1016/j.jchemneu.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 79.Wu XH, Song JJ, Faull RLM, Waldvogel HJ. GABA(A) and GABA(B) receptor subunit localization on neurochemically identified neurons of the human subthalamic nucleus. J. Comp. Neurol. 2018;526:803–823. doi: 10.1002/cne.24368. [DOI] [PubMed] [Google Scholar]
- 80.Lévesque M, Parent A. The striatofugal fiber system in primates: a reevaluation of its organization based on single-axon tracing studies. Proc. Natl Acad. Sci. USA. 2005;102:11888–11893. doi: 10.1073/pnas.0502710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schweizer, N. et al. Reduced Vglut2/Slc17a6 gene expression levels throughout the mouse subthalamic nucleus cause cell loss and structural disorganization followed by increased motor activity and decreased sugar consumption. eNeuro310.1523/eneuro.0264-16.2016 (2016). [DOI] [PMC free article] [PubMed]
- 82.Trudeau LE, El Mestikawy S. Glutamate Cotransmission in Cholinergic, GABAergic and Monoamine systems: contrasts and commonalities. Front. Neural Circuits. 2018;12:113. doi: 10.3389/fncir.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.El Mestikawy S, Wallén-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat. Rev. Neurosci. 2011;12:204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- 84.Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu. Rev. Physiol. 2012;74:225–243. doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X-S, Ong W-Y, Lee H-K, Huganir RL. A light and electron microscopic study of glutamate receptors in the monkey subthalamic nucleus. J. Neurocytol. 2000;29:743–754. doi: 10.1023/A:1010990404833. [DOI] [PubMed] [Google Scholar]
- 86.Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of Metabotropic Glutamate Receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J. Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bevan, M. D. & Bolam, J. P. Cholinergic, GABAergic, and glutamate-enriched inputs from the mesopontine tegmentum to the subthalamic nucleus in the rat. J. Neurosci.15, 7105–7120 (1995). [DOI] [PMC free article] [PubMed]
- 88.Carpenter MB, Jayaraman A. Subthalamic nucleus of the monkey: connections and immunocytochemical features of afferents. J. Hirnforsch. 1990;31:653–668. [PubMed] [Google Scholar]
- 89.Joel D, Weiner I. The connections of the primate subthalamic nucleus: indirect pathways and the open-interconnected scheme of basal ganglia-thalamocortical circuitry. Brain Res Brain Res Rev. 1997;23:62–78. doi: 10.1016/S0165-0173(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 90.Carpenter MB, Baton RR, 3rd, Carleton SC, Keller JT. Interconnections and organization of pallidal and subthalamic nucleus neurons in the monkey. J. Comp. Neurol. 1981;197:579–603. doi: 10.1002/cne.901970404. [DOI] [PubMed] [Google Scholar]
- 91.Oertel WH, Nitsch C, Mugnaini E. Immunocytochemical demonstration of the GABA-ergic neurons in rat globus pallidus and nucleus entopeduncularis and their GABA-ergic innervation. Adv. Neurol. 1984;40:91–98. [PubMed] [Google Scholar]
- 92.Oertel WH, Mugnaini E. Immunocytochemical studies of GABAergic neurons in rat basal ganglia and their relations to other neuronal systems. Neurosci. Lett. 1984;47:233–238. doi: 10.1016/0304-3940(84)90519-6. [DOI] [PubMed] [Google Scholar]
- 93.Wallman MJ, Gagnon D, Parent M. Serotonin innervation of human basal ganglia. Eur. J. Neurosci. 2011;33:1519–1532. doi: 10.1111/j.1460-9568.2011.07621.x. [DOI] [PubMed] [Google Scholar]
- 94.Reznitsky M, Plenge P, Hay-Schmidt A. Serotonergic projections from the raphe nuclei to the subthalamic nucleus; a retrograde- and anterograde neuronal tracing study. Neurosci. Lett. 2016;612:172–177. doi: 10.1016/j.neulet.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 95.Lavoie B, Smith Y, Parent A. Dopaminergic innervation of the basal ganglia in the squirrel monkey as revealed by tyrosine hydroxylase immunohistochemistry. J. Comp. Neurol. 1989;289:36–52. doi: 10.1002/cne.902890104. [DOI] [PubMed] [Google Scholar]
- 96.François C, et al. Dopaminergic innervation of the subthalamic nucleus in the normal state, in MPTP-treated monkeys, and in Parkinson’s disease patients. J. Comp. Neurol. 2000;425:121–129. doi: 10.1002/1096-9861(20000911)425:1<121::AID-CNE10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 97.Emmi A, et al. Topography and distribution of adenosine A(2A) and dopamine D(2) receptors in the human Subthalamic Nucleus. Front. Neurosci. 2022;16:945574. doi: 10.3389/fnins.2022.945574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Galvan A, et al. Localization and function of dopamine receptors in the subthalamic nucleus of normal and parkinsonian monkeys. J. Neurophysiol. 2014;112:467–479. doi: 10.1152/jn.00849.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Savasta M, Dubois A, Scatton B. Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H]SCH 23390. Brain Res. 1986;375:291–301. doi: 10.1016/0006-8993(86)90749-3. [DOI] [PubMed] [Google Scholar]
- 100.Kreiss DS, Mastropietro CW, Rawji SS, Walters JR. The response of subthalamic nucleus neurons to dopamine receptor stimulation in a rodent model of Parkinson’s disease. J. Neurosci. 1997;17:6807–6819. doi: 10.1523/JNEUROSCI.17-17-06807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hardman CD, McRitchie DA, Halliday GM, Cartwright HR, Morris JG. Substantia nigra pars reticulata neurons in Parkinson’s disease. Neurodegeneration. 1996;5:49–55. doi: 10.1006/neur.1996.0007. [DOI] [PubMed] [Google Scholar]
- 102.Parent A, Fortin M, Côté PY, Cicchetti F. Calcium-binding proteins in primate basal ganglia. Neurosci. Res. 1996;25:309–334. doi: 10.1016/0168-0102(96)01065-6. [DOI] [PubMed] [Google Scholar]
- 103.Nambu A, Takada M, Inase M, Tokuno H. Dual somatotopical representations in the primate subthalamic nucleus: evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplementary motor area. J. Neurosci. 1996;16:2671–2683. doi: 10.1523/JNEUROSCI.16-08-02671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Parent A, Smith Y, Filion M, Dumas J. Distinct afferents to internal and external pallidal segments in the squirrel monkey. Neurosci. Lett. 1989;96:140–144. doi: 10.1016/0304-3940(89)90047-5. [DOI] [PubMed] [Google Scholar]
- 105.Carpenter MB, Fraser RA, Shriver JE. The organization of pallidosubthalamic fibers in the monkey. Brain Res. 1968;11:522–559. doi: 10.1016/0006-8993(68)90145-5. [DOI] [PubMed] [Google Scholar]
- 106.Schweizer N, et al. Reduced <em>Vglut2/Slc17a6</em> Gene Expression Levels throughout the Mouse Subthalamic Nucleus Cause Cell Loss and Structural Disorganization Followed by Increased Motor Activity and Decreased Sugar Consumption. eneuro. 2016;3:ENEURO.0264-0216.2016. doi: 10.1523/ENEURO.0264-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martin DM, et al. PITX2 is required for normal development of neurons in the mouse subthalamic nucleus and midbrain. Dev. Biol. 2004;267:93–108. doi: 10.1016/j.ydbio.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 108.Schweizer N, et al. Limiting glutamate transmission in a Vglut2-expressing subpopulation of the subthalamic nucleus is sufficient to cause hyperlocomotion. Proc. Natl Acad. Sci. USA. 2014;111:7837–7842. doi: 10.1073/pnas.1323499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Skidmore JM, Cramer JD, Martin JF, Martin DM. Cre fate mapping reveals lineage specific defects in neuronal migration with loss of Pitx2 function in the developing mouse hypothalamus and subthalamic nucleus. Mol. Cell Neurosci. 2008;37:696–707. doi: 10.1016/j.mcn.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wallén-Mackenzie A, et al. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J. Neurosci. 2006;26:12294–12307. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pupe S, Schweizer N, Wallén-Mackenzie Å. Reply to Konsolaki and Skaliora: Habituation, hyperlocomotion, and “genuine hyperlocomotion”. Proc. Natl Acad. Sci. USA. 2015;112:E5. doi: 10.1073/pnas.1417574112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heiman M, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Picelli S, et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods. 2013;10:1096–1098. doi: 10.1038/nmeth.2639. [DOI] [PubMed] [Google Scholar]
- 114.Stuart T, et al. Comprehensive Integration of Single cell data. Cell. 2019;177:1888–1902.e1821. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parolari L, Schneeberger M, Heintz N, Friedman JM. Functional analysis of distinct populations of subthalamic nucleus neurons on Parkinson’s disease and OCD-like behaviors in mice. Mol. Psychiatry. 2021;26:7029–7046. doi: 10.1038/s41380-021-01162-6. [DOI] [PubMed] [Google Scholar]
- 117.Guillaumin A, Serra GP, Georges F, Wallén-Mackenzie Å. Experimental investigation into the role of the subthalamic nucleus (STN) in motor control using optogenetics in mice. Brain Res. 2021;1755:147226. doi: 10.1016/j.brainres.2020.147226. [DOI] [PubMed] [Google Scholar]
- 118.Sieger T, et al. Distinct populations of neurons respond to emotional valence and arousal in the human subthalamic nucleus. Proc. Natl Acad. Sci. USA. 2015;112:3116–3121. doi: 10.1073/pnas.1410709112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rafols JA, Fox CA. The neurons in the primate subthalamic nucleus: a Golgi and electron microscopic study. J. Comp. Neurol. 1976;168:75–111. doi: 10.1002/cne.901680105. [DOI] [PubMed] [Google Scholar]
- 120.Kuzemenský J. Comparison of the cytoarchitectonic structure of the subthalamic nucleus in certain mammals. Folia Morphol. 1976;24:129–140. [PubMed] [Google Scholar]
- 121.DeLong MR, Crutcher MD, Georgopoulos AP. Primate globus pallidus and subthalamic nucleus: functional organization. J. Neurophysiol. 1985;53:530–543. doi: 10.1152/jn.1985.53.2.530. [DOI] [PubMed] [Google Scholar]
- 122.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res. Brain Res. Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-D. [DOI] [PubMed] [Google Scholar]
- 123.Krack P, Hariz MI, Baunez C, Guridi J, Obeso JA. Deep brain stimulation: from neurology to psychiatry? Trends Neurosci. 2010;33:474–484. doi: 10.1016/j.tins.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 124.Novakova L, et al. Hormonal regulators of food intake and weight gain in Parkinson’s disease after subthalamic nucleus stimulation. Neuro Endocrinol. Lett. 2011;32:437–441. [PubMed] [Google Scholar]
- 125.Appleby BS, Duggan PS, Regenberg A, Rabins PV. Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: A meta-analysis of ten years’ experience. Mov. Disord. 2007;22:1722–1728. doi: 10.1002/mds.21551. [DOI] [PubMed] [Google Scholar]
- 126.Péron J, Frühholz S, Vérin M, Grandjean D. Subthalamic nucleus: a key structure for emotional component synchronization in humans. Neurosci. Biobehav. Rev. 2013;37:358–373. doi: 10.1016/j.neubiorev.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 127.Montaurier C, et al. Mechanisms of body weight gain in patients with Parkinson’s disease after subthalamic stimulation. Brain. 2007;130:1808–1818. doi: 10.1093/brain/awm113. [DOI] [PubMed] [Google Scholar]
- 128.Vachez YM, Creed MC. Deep brain stimulation of the subthalamic nucleus modulates reward-related behavior: a systematic review. Front Hum. Neurosci. 2020;14:578564. doi: 10.3389/fnhum.2020.578564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Magnard R, et al. What can rodent models tell us about apathy and associated neuropsychiatric symptoms in Parkinson’s disease? Transl. Psychiatry. 2016;6:e753. doi: 10.1038/tp.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu, H. et al. Internal States Influence The Representation And Modulation Of Food Intake By Subthalamic Neurons. Neurosci Bull10.1007/s12264-020-00533-3 (2020). [DOI] [PMC free article] [PubMed]
- 131.Xie C, Power J, Prasad AA. Bidirectional optogenetic modulation of the subthalamic nucleus in a rodent model of Parkinson’s disease. Front. Neurosci. 2022;16:848821. doi: 10.3389/fnins.2022.848821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of Parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kravitz AV, et al. Regulation of Parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Richardson RM, Ostrem JL, Starr PA. Surgical repositioning of misplaced subthalamic electrodes in Parkinson’s disease: location of effective and ineffective leads. Stereotact. Funct. Neurosurg. 2009;87:297–303. doi: 10.1159/000230692. [DOI] [PubMed] [Google Scholar]
- 135.van den Munckhof P, Bot M, Schuurman PR. Targeting of the subthalamic nucleus in patients with Parkinson’s disease undergoing deep brain stimulation surgery. Neurol. Ther. 2021;10:61–73. doi: 10.1007/s40120-021-00233-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J. Neurophysiol. 1994;72:521–530. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- 137.Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–139. doi: 10.1016/0166-2236(92)90355-C. [DOI] [PubMed] [Google Scholar]
- 138.Kang, Y. & Kitai, S. T. Electrophysiological properties of pedunculopontine neurons and their postsynaptic responses following stimulation of substantia nigra reticulata. Brain Res.53510.1016/0006-8993(90)91826-3 (1990). [DOI] [PubMed]
- 139.Van Der Kooy D, Hattori T. Single subthalamic nucleus neurons project to both the globus pallidus and substantia nigra in rat. J. Comp. Neurol. 1980;192:751–768. doi: 10.1002/cne.901920409. [DOI] [PubMed] [Google Scholar]
- 140.Pamukcu A, et al. Parvalbumin+ and Npas1+ Pallidal Neurons Have Distinct Circuit Topology and Function. J. Neurosci. 2020;40:7855–7876. doi: 10.1523/JNEUROSCI.0361-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Heston, J. et al. Activation of subthalamic nucleus stop circuit disrupts cognitive performance. eNeuro710.1523/eneuro.0159-20.2020 (2020). [DOI] [PMC free article] [PubMed]
- 142.Lardeux S, Paleressompoulle D, Pernaud R, Cador M, Baunez C. Different populations of subthalamic neurons encode cocaine vs. sucrose reward and predict future error. J. Neurophysiol. 2013;110:1497–1510. doi: 10.1152/jn.00160.2013. [DOI] [PubMed] [Google Scholar]
- 143.Espinosa-Parrilla JF, Baunez C, Apicella P. Linking reward processing to behavioral output: motor and motivational integration in the primate subthalamic nucleus. Front. Comput. Neurosci. 2013;7:175. doi: 10.3389/fncom.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Espinosa-Parrilla JF, Baunez C, Apicella P. Modulation of neuronal activity by reward identity in the monkey subthalamic nucleus. Eur. J. Neurosci. 2015;42:1705–1717. doi: 10.1111/ejn.12938. [DOI] [PubMed] [Google Scholar]
- 145.Markaki E, et al. The role of ghrelin, neuropeptide Y and leptin peptides in weight gain after deep brain stimulation for Parkinson’s disease. Stereotact. Funct. Neurosurg. 2012;90:104–112. doi: 10.1159/000335045. [DOI] [PubMed] [Google Scholar]
- 146.Rieu I, et al. Body weight gain and deep brain stimulation. J. Neurol. Sci. 2011;310:267–270. doi: 10.1016/j.jns.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 147.Baunez C, Amalric M, Robbins TW. Enhanced food-related motivation after bilateral lesions of the subthalamic nucleus. J. Neurosci. 2002;22:562–568. doi: 10.1523/JNEUROSCI.22-02-00562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baunez C, Dias C, Cador M, Amalric M. The subthalamic nucleus exerts opposite control on cocaine and ‘natural’ rewards. Nat. Neurosci. 2005;8:484–489. doi: 10.1038/nn1429. [DOI] [PubMed] [Google Scholar]
- 149.Prasad AA, McNally GP. Ventral Pallidum output pathways in context-induced reinstatement of alcohol seeking. J. Neurosci. 2016;36:11716–11726. doi: 10.1523/JNEUROSCI.2580-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zahm DS. The ventral striatopallidal parts of the basal ganglia in the rat—II. Compartmentation of ventral pallidal efferents. Neuroscience. 1989;30:33–50. doi: 10.1016/0306-4522(89)90351-5. [DOI] [PubMed] [Google Scholar]
- 151.Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–142. doi: 10.1016/0306-4522(93)90115-V. [DOI] [PubMed] [Google Scholar]
- 152.Groenewegen HJ, Berendse HW. Connections of the subthalamic nucleus with ventral striatopallidal parts of the basal ganglia in the rat. J. Comp. Neurol. 1990;294:607–622. doi: 10.1002/cne.902940408. [DOI] [PubMed] [Google Scholar]
- 153.Bevan MD, Clarke NP, Bolam JP. Synaptic integration of functionally diverse pallidal information in the entopeduncular nucleus and subthalamic nucleus in the rat. J. Neurosci. 1997;17:308–324. doi: 10.1523/JNEUROSCI.17-01-00308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Baracz SJ, Cornish JL. Oxytocin modulates dopamine-mediated reward in the rat subthalamic nucleus. Horm. Behav. 2013;63:370–375. doi: 10.1016/j.yhbeh.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 155.Rouaud T, et al. Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proc. Natl Acad. Sci. USA. 2010;107:1196–1200. doi: 10.1073/pnas.0908189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Baracz SJ, et al. Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference. Behav. Brain Res. 2012;228:185–193. doi: 10.1016/j.bbr.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 157.Serra GP, et al. A role for the subthalamic nucleus in aversive learning. Cell Rep. 2023;42:113328. doi: 10.1016/j.celrep.2023.113328. [DOI] [PubMed] [Google Scholar]
- 158.Baunez C. A few examples of the contribution of animal research in rodents for clinical application of deep brain stimulation. Prog. Brain Res. 2011;194:105–116. doi: 10.1016/B978-0-444-53815-4.00013-3. [DOI] [PubMed] [Google Scholar]
- 159.Pelloux, Y. & Baunez, C. Deep brain stimulation for addiction: why the subthalamic nucleus should be favored. Curr. Opin. Neurobiol.2310.1016/j.conb.2013.02.016 (2013). [DOI] [PubMed]
- 160.Temel Y, Blokland A, Steinbusch HWM, Visser-Vandewalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog. Neurobiol. 2005;76:393–413. doi: 10.1016/j.pneurobio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 161.Weintraub DB, Zaghloul KA. The role of the subthalamic nucleus in cognition. Rev. Neurosci. 2013;24:125–138. doi: 10.1515/revneuro-2012-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J. Neurosci. 2008;28:7209–7218. doi: 10.1523/JNEUROSCI.0487-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bastin J, et al. Inhibitory control and error monitoring by human subthalamic neurons. Transl. Psychiatry. 2014;4:e439. doi: 10.1038/tp.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mosher CP, Mamelak AN, Malekmohammadi M, Pouratian N, Rutishauser U. Distinct roles of dorsal and ventral subthalamic neurons in action selection and cancellation. Neuron. 2021;109:869–881.e866. doi: 10.1016/j.neuron.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pasquereau, B. & Turner, R. S. A selective role for ventromedial subthalamic nucleus in inhibitory control. Elife610.7554/eLife.31627 (2017). [DOI] [PMC free article] [PubMed]
- 166.Canteras NS, Shammah-Lagnado SJ, Silva BA, Ricardo JA. Afferent connections of the subthalamic nucleus: a combined retrograde and anterograde horseradish peroxidase study in the rat. Brain Res. 1990;513:43–59. doi: 10.1016/0006-8993(90)91087-W. [DOI] [PubMed] [Google Scholar]
- 167.Kita T, Osten P, Kita H. Rat subthalamic nucleus and zona incerta share extensively overlapped representations of cortical functional territories. J. Comp. Neurol. 2014;522:4043–4056. doi: 10.1002/cne.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat. Neurosci. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- 169.Alegret M, et al. Effects of bilateral subthalamic stimulation on cognitive function in Parkinson disease. Arch. Neurol. 2001;58:1223–1227. doi: 10.1001/archneur.58.8.1223. [DOI] [PubMed] [Google Scholar]
- 170.Moretti R, et al. Cognitive changes following subthalamic nucleus stimulation in two patients with Parkinson disease. Percept. Mot. Skills. 2002;95:477–486. doi: 10.2466/pms.2002.95.2.477. [DOI] [PubMed] [Google Scholar]
- 171.Patel NK, et al. Unilateral subthalamotomy in the treatment of Parkinson’s disease. Brain. 2003;126:1136–1145. doi: 10.1093/brain/awg111. [DOI] [PubMed] [Google Scholar]
- 172.Morrison CE, et al. Neuropsychological functioning following bilateral subthalamic nucleus stimulation in Parkinson’s disease. Arch. Clin. Neuropsychol. 2004;19:165–181. doi: 10.1016/S0887-6177(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 173.Hershey T, et al. Stimulation of STN impairs aspects of cognitive control in PD. Neurology. 2004;62:1110–1114. doi: 10.1212/01.WNL.0000118202.19098.10. [DOI] [PubMed] [Google Scholar]
- 174.Burbaud P, et al. Neuronal activity correlated with checking behaviour in the subthalamic nucleus of patients with obsessive-compulsive disorder. Brain. 2013;136:304–317. doi: 10.1093/brain/aws306. [DOI] [PubMed] [Google Scholar]
- 175.Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci. Biobehav. Rev. 2010;34:50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Eagle DM, et al. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb. Cortex. 2008;18:178–188. doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- 177.Wiener M, Magaro CM, Matell MS. Accurate timing but increased impulsivity following excitotoxic lesions of the subthalamic nucleus. Neurosci. Lett. 2008;440:176–180. doi: 10.1016/j.neulet.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 178.Heikenfeld C, et al. Prefrontal-subthalamic pathway supports action selection in a spatial working memory task. Sci. Rep. 2020;10:10497. doi: 10.1038/s41598-020-67185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zavala, B. A., Jang, A. I. & Zaghloul, K. A. Human subthalamic nucleus activity during non-motor decision making. Elife610.7554/eLife.31007 (2017). [DOI] [PMC free article] [PubMed]
- 180.Zavala B, et al. Subthalamic nucleus local field potential activity during the Eriksen flanker task reveals a novel role for theta phase during conflict monitoring. J. Neurosci. 2013;33:14758–14766. doi: 10.1523/JNEUROSCI.1036-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zavala B, et al. Human subthalamic nucleus-medial frontal cortex theta phase coherence is involved in conflict and error related cortical monitoring. Neuroimage. 2016;137:178–187. doi: 10.1016/j.neuroimage.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009;8:270–279. doi: 10.1016/S1474-4422(09)70042-0. [DOI] [PubMed] [Google Scholar]
- 183.Lange H, Thörner G, Hopf A, Schröder KF. Morphometric studies of the neuropathological changes in choreatic diseases. J. Neurol. Sci. 1976;28:401–425. doi: 10.1016/0022-510X(76)90114-3. [DOI] [PubMed] [Google Scholar]
- 184.Mattila P, Togo T, Dickson DW. The subthalamic nucleus has neurofibrillary tangles in argyrophilic grain disease and advanced Alzheimer’s disease. Neurosci. Lett. 2002;320:81–85. doi: 10.1016/S0304-3940(02)00006-X. [DOI] [PubMed] [Google Scholar]
- 185.Togo T, Dickson DW. Ballooned neurons in progressive supranuclear palsy are usually due to concurrent argyrophilic grain disease. Acta Neuropathol. 2002;104:53–56. doi: 10.1007/s00401-002-0520-1. [DOI] [PubMed] [Google Scholar]
- 186.Yamada M, Sato T, Tsuji S, Takahashi H. CAG repeat disorder models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:71–86. doi: 10.1007/s00401-007-0287-5. [DOI] [PubMed] [Google Scholar]
- 187.Bergman H, Deuschl G. Pathophysiology of Parkinson’s disease: From clinical neurology to basic neuroscience and back. Mov. Disord. 2002;17:S28–S40. doi: 10.1002/mds.10140. [DOI] [PubMed] [Google Scholar]
- 188.Limousin P, et al. Effect of Parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95. doi: 10.1016/S0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 189.Benazzouz A, et al. Implication of the subthalamic nucleus in the pathophysiology and pathogenesis of Parkinson’s disease. Cell Transpl. 2000;9:215–221. doi: 10.1177/096368970000900207. [DOI] [PubMed] [Google Scholar]
- 190.Moro, E. et al. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain13310.1093/brain/awp261 (2010). [DOI] [PubMed]
- 191.Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J. Neurosci. 2000;20:7766–7775. doi: 10.1523/JNEUROSCI.20-20-07766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Di Giulio I, et al. Chronic subthalamic nucleus Stimulation in Parkinson’s disease: optimal frequency for gait depends on stimulation site and axial symptoms. Front Neurol. 2019;10:29. doi: 10.3389/fneur.2019.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Denys, D. & Mantione, M. in Progress in Brain Research Vol. Volume 175 (eds Elly M. Hol Inge Huitenga Jan Wijnholds Arthur B. Bergen Gerald J. Boer Joost Verhaagen & F. Swaab Dick) 419–427 (Elsevier, 2009).
- 194.Mosley PE, et al. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder should be an accepted therapy in Australia. Aust. N. Z. J. Psychiatry. 2022;56:430–436. doi: 10.1177/00048674211031482. [DOI] [PubMed] [Google Scholar]
- 195.Mosley PE, et al. A randomised, double-blind, sham-controlled trial of deep brain stimulation of the bed nucleus of the stria terminalis for treatment-resistant obsessive-compulsive disorder. Transl. Psychiatry. 2021;11:190. doi: 10.1038/s41398-021-01307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tsuboi T, et al. A pooled meta-analysis of GPi and STN deep brain stimulation outcomes for cervical dystonia. J. Neurol. 2020;267:1278–1290. doi: 10.1007/s00415-020-09703-9. [DOI] [PubMed] [Google Scholar]
- 197.Wong JK, et al. STN vs. GPi deep brain stimulation for tremor suppression in Parkinson disease: A systematic review and meta-analysis. Parkinsonism Relat. Disord. 2019;58:56–62. doi: 10.1016/j.parkreldis.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Georgiades MJ, et al. Hitting the brakes: pathological subthalamic nucleus activity in Parkinson’s disease gait freezing. Brain. 2019;142:3906–3916. doi: 10.1093/brain/awz325. [DOI] [PubMed] [Google Scholar]
- 199.Brichta L, Greengard P, Flajolet M. Advances in the pharmacological treatment of Parkinson’s disease: targeting neurotransmitter systems. Trends Neurosci. 2013;36:543–554. doi: 10.1016/j.tins.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 200.Calon F, et al. Changes of GABA receptors and dopamine turnover in the postmortem brains of Parkinsonians with levodopa-induced motor complications. Mov. Disord. 2003;18:241–253. doi: 10.1002/mds.10343. [DOI] [PubMed] [Google Scholar]
- 201.Ohama E, Ikuta F. Parkinson’s disease: distribution of Lewy bodies and monoamine neuron system. Acta Neuropathol. 1976;34:311–319. doi: 10.1007/BF00696560. [DOI] [PubMed] [Google Scholar]
- 202.Thompson AC, et al. Hybrid optogenetic and electrical stimulation for greater spatial resolution and temporal fidelity of cochlear activation. J. Neural Eng. 2020;17:056046. doi: 10.1088/1741-2552/abbff0. [DOI] [PubMed] [Google Scholar]