Abstract
The secondary metabolite hydrogen cyanide (HCN) is produced by Pseudomonas fluorescens from glycine, essentially under microaerophilic conditions. The genetic basis of HCN synthesis in P. fluorescens CHA0 was investigated. The contiguous structural genes hcnABC encoding HCN synthase were expressed from the T7 promoter in Escherichia coli, resulting in HCN production in this bacterium. Analysis of the nucleotide sequence of the hcnABC genes showed that each HCN synthase subunit was similar to known enzymes involved in hydrogen transfer, i.e., to formate dehydrogenase (for HcnA) or amino acid oxidases (for HcnB and HcnC). These similarities and the presence of flavin adenine dinucleotide- or NAD(P)-binding motifs in HcnB and HcnC suggest that HCN synthase may act as a dehydrogenase in the reaction leading from glycine to HCN and CO2. The hcnA promoter was mapped by primer extension; the −40 sequence (TTGGC … .ATCAA) resembled the consensus FNR (fumarate and nitrate reductase regulator) binding sequence (TTGAT … .ATCAA). The gene encoding the FNR-like protein ANR (anaerobic regulator) was cloned from P. fluorescens CHA0 and sequenced. ANR of strain CHA0 was most similar to ANR of P. aeruginosa and CydR of Azotobacter vinelandii. An anr mutant of P. fluorescens (CHA21) produced little HCN and was unable to express an hcnA-lacZ translational fusion, whereas in wild-type strain CHA0, microaerophilic conditions strongly favored the expression of the hcnA-lacZ fusion. Mutant CHA21 as well as an hcn deletion mutant were impaired in their capacity to suppress black root rot of tobacco, a disease caused by Thielaviopsis basicola, under gnotobiotic conditions. This effect was most pronounced in water-saturated artificial soil, where the anr mutant had lost about 30% of disease suppression ability, compared with wild-type strain CHA0. These results show that the anaerobic regulator ANR is required for cyanide synthesis in the strictly aerobic strain CHA0 and suggest that ANR-mediated cyanogenesis contributes to the suppression of black root rot.
Cyanide is a secondary metabolite produced by some gram-negative bacteria, such as Pseudomonas fluorescens, P. aeruginosa, and Chromobacterium violaceum (1, 5, 26). Hydrogen cyanide (HCN) and CO2 are formed stoichiometrically from glycine (6, 58) in a poorly understood oxidative reaction catalyzed by HCN synthase (4, 7). This enzyme or enzyme complex appears to be membrane bound (61). In extracts, HCN synthase of a Pseudomonas sp. oxidizes glycine in the presence of artificial electron acceptors, e.g., phenazine methosulfate (58). Flavin adenine dinucleotide (FAD) stimulates this reaction (59), whereas pyrrolnitrin, an inhibitor of many flavin enzymes, and o-phenanthroline, an iron chelator, strongly inhibit cyanide formation in vitro (58). HCN synthase is very sensitive to molecular oxygen and has been purified only partially from a Pseudomonas sp. and P. aeruginosa (4, 60). Nothing is known about the molecular structure of the enzyme.
In vivo, the four electrons produced by the HCN synthase reaction are transferred to oxygen, probably by components of the respiratory electron transport chain (4). In P. aeruginosa, no HCN is produced under fully anaerobic conditions when nitrate is the terminal electron acceptor (5). Optimal expression of HCN synthase occurs during the transition from the exponential to the stationary phase (9) and at low oxygen levels (8). Two regulatory proteins involved in these induction processes in P. aeruginosa have been identified: GacA and ANR (38, 66). The global activator GacA, a response regulator of a two-component system, positively controls the synthesis of HCN, other secondary metabolites, and exoenzymes by a cell-density-dependent mechanism (29, 38). The FNR-like anaerobic regulator ANR is required for the induction of HCN synthase, the arginine deiminase pathway, and the entire denitrification pathway (64, 66). P. aeruginosa mutants affected in either gacA or anr produce very little HCN (38, 66).
P. fluorescens CHA0 is an aerobic, root-colonizing biocontrol bacterium that protects several plants from root diseases caused by soilborne fungi (42, 52). HCN production by strain CHA0 contributes to the suppression of black root rot of tobacco, a disease caused by Thielaviopsis basicola, under gnotobiotic conditions (53). GacA-negative mutants of strain CHA0, which are pleiotropically defective in the synthesis of HCN, antibiotics, and exoenzymes, have lost the ability to protect tobacco from black root rot (29, 39). We previously isolated HCN biosynthetic genes from strain CHA0 and demonstrated their expression in other pseudomonads, with a concomitant improvement in biocontrol ability (15, 53). When the hcn structural genes are inactivated by insertion of a resistance cassette, strain CHA0 loses part of its ability to suppress black root rot. This defect can be restored by complementation with a plasmid carrying the hcn genes (53). Here we show that the hcn genes are organized as an hcnABC cluster which appears to be sufficient to encode HCN synthase. We also characterize the P. fluorescens anr gene, whose function is essential for the expression of the hcnABC cluster at low oxygen concentrations. Finally, we assess the importance of anr-dependent regulation for biocontrol by strain CHA0.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids are listed in Table 1. Strains of Escherichia coli and P. aeruginosa were routinely grown on nutrient agar (NA) plates and in nutrient yeast broth (NYB) with aeration at 37°C (18). Anaerobic growth and gas production of E. coli were assessed as described previously (17). For determination of HCN production by E. coli, strains were cultivated in a medium [LB(2x)-M9-MMC] containing, per liter, the following: tryptone (Oxoid), 20 g; yeast extract, 10 g; glucose, 4 g; glycine, 0.75 g; l-methionine, 1.5 g; NaCl, 2.5 g; NH4Cl, 1 g; KH2PO4, 3 g; Na2HPO4, 6 g; and FeCl3, 0.5 g. Minimal medium M9 (40) supplemented with 0.5% Methionine Assay Medium (Difco) was used for protein expression in P. aeruginosa ADD1976. P. fluorescens cells were routinely cultivated in NYB or on NA at 30°C. To measure HCN production by P. fluorescens, strains were grown under oxygen-limited conditions in tightly closed 120-ml bottles containing a synthetic minimal medium (MMC) described by Castric (5). In hcnA′-′lacZ expression experiments, MMC was also used in Erlenmeyer flasks, with shaking (180 rpm) to provide good aeration. For the determination of arginine deiminase activity and for the experiment measuring competition between strains CHA0 and CHA21, yeast extract-arginine (YEA) medium (49) was used. Antimicrobial compounds, when required, were added to the growth media at the following concentrations: ampicillin, 100 μg/ml (for E. coli); carbenicillin, 200 μg/ml (for E. coli); kanamycin sulfate, 25 μg/ml; HgCl2, 20 μg/ml; and tetracycline hydrochloride, 25 μg/ml (for E. coli) or 125 μg/ml (for P. fluorescens). 5-Bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) was incorporated into solid media to monitor β-galactosidase expression (40).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or phenotype | Reference or source |
|---|---|---|
| Strains | ||
| P. fluorescens | ||
| CHA0 | Wild type | 52 |
| CHA5 | hcnB::Ω-Hg | 53 |
| CHA21 | anr::Ω-Km | This study (Fig. 4) |
| CHA77 | ΔhcnABC | This study (Fig. 1) |
| P3 | Wild type | 53 |
| P. aeruginosa | ||
| PAO1 | Wild type | ATCC 15692 |
| ADD1976 | Chromosomal insertion of T7 pol lacIq | 2 |
| E. coli | ||
| BL21(DE3) | hsdS gal; chromosomal insertion of T7 pol | 44 |
| DH5α | recA1 endA1 hsdR17 supE4 gyrA96 relA1 Δ(lacZYA-argF)U169 (φ80dlacZΔM15) | 40 |
| JRG1728 | Δ(lacZYA)X74 galU galK Δ(ara-leu) rpsL Δ(tyrR-fnr-trg) | 17; J. R. Guest |
| RU4420 | thi-1 endA1 hsdR17 supE44 trp::Tn1725 | 47 |
| Plasmids | ||
| pBluescript II KS+ | Cloning vector; ColE1 replicon; Apr | Stratagene |
| pEB16 | Broad-host-range vector; pBR322-pRO1600 replicon; RK2 Mob; Apr Cbr | 2 |
| pHP45Ω-Km | ColE1 replicon; Apr Kmr | 14 |
| pMMB67EH | RSF1010 replicon; Apr | 16 |
| pNM482 | ColE1 replicon; Apr ′lacZ | 34 |
| pVK100 | Broad-host-range vector; IncP replicon; RK2 Mob; Tcr Kmr | 25 |
| pME497 | Mobilizing plasmid; IncP replicon; Tra; Apr | 54 |
| pME3013 | pVK100 with an 8-kb HindIII genomic fragment of P. fluorescens CHA0 containing the hcnABC genes for HCN biosynthesis | 53 |
| pME3071 | pMMB67 with a 5-kb XhoI-HindIII genomic fragment of CHA0 containing hcnABC′ | This study (Fig. 1) |
| pME3087 | Suicide vector; ColE1 replicon; RK2 Mob; Tcr | 52 |
| pME3205 | pVK100 with a 3.8-kb SalI-HindIII fragment of pME3071 containing the hcnABC′ genes behind the kanamycin promoter | This study (Fig. 1) |
| pME3206 | pVK100 with a 3.6-kb MaeI-HindIII fragment of pME3071 containing the hcnABC′ genes behind the kanamycin promoter | This study (Fig. 1) |
| pME3209 | pEB16 with a 1.3-kb BamHI-HindIII fragment of pME3071 containing the hcnC′ gene behind the T7 promoter | This study (Fig. 1) |
| pME3210 | pEB16 with a 3.6-kb MaeI-HindIII fragment of pME3071 containing the hcnABC′ genes behind the T7 promoter | This study (Fig. 1) |
| pME3212 | pEB16 with a 3.65-kb MaeI-StuI fragment of pME3071 containing the hcnABC genes behind the T7 promoter | This study (Fig. 1) |
| pME3219 | pME6010 with a 410-bp hcnA promoter fragment and an hcnA′-′lacZ translational fusion at the PstI site in hcnA | This study |
| pME3580 | pKT240 with a 1.3-kb SacII genomic fragment of P. aeruginosa PAO1 containing the anr gene | 17 |
| pME3812 | pVK100 with a 19-kb HindIII genomic fragment of P. fluorescens CHA0 containing the anr gene | This study |
| pME3815 | pBluescript II KS+ with a 2.5-kb PstI fragment of pME3812 containing the anr gene | This study (Fig. 4) |
| pME3816 | pME3087 ΔEcoRIa with a 4.7-kb fragment containing anr::Ω-Km | This study (Fig. 4) |
| pME3817 | pVK100 with a 1.6-kb BamHI-HindIII fragment of pME3818 | This study (Fig. 4) |
| pME3818 | pME3815 with a deletion of the 0.9-kb EcoRV fragment | This study (Fig. 4) |
| pME6010 | pACYC177-pVS1 shuttle vector; Tcr | S. Heeb (20a) |
ΔEcoRI signifies that the EcoRI site of pME3087 was deleted.
DNA manipulations and sequencing.
Small-scale preparations of plasmid DNA from E. coli and P. fluorescens were carried out by the CTAB method (12) for ColE1-based plasmids and pMMB67 or by the alkaline lysis method (40) for other plasmids. Large-scale preparations of plasmid DNA were carried out with Qiagen Tips (Qiagen Inc.). Restriction enzyme digestions, DNA fragment isolation from low-melting-point agarose gels, ligation, and agarose gel electrophoresis were performed according to standard procedures (40). Chromosomal DNA of P. fluorescens was isolated as described by Gamper et al. (18). Transformation of E. coli and P. aeruginosa strains with plasmid DNA was done by the standard CaCl2 procedure (40). Progressive deletions with nuclease Bal 31 were performed according to the instructions of the supplier (Boehringer Mannheim Biochemicals). For nucleotide sequence determination of the hcn genes, DNA fragments were cloned into M13mp18 and M13mp19 phages (63), and single-stranded DNA was sequenced by the dideoxy chain termination method with [α-32P]dATP, 7-deaza-dGTP, and Sequenase version 2.0 (United States Biochemical Corp.). PCR was carried out with plasmid DNA (0.5 μg) carrying the hcn genes as the template. Two oligonucleotide primers were used. Primer 1 anneals to ORF0 upstream of the hcn promoter (5′-GCTCGAATTCCTGCGTCATTACTCTT-3′) and contains an EcoRI restriction site (underlined) at the 5′ end. Primer 2 anneals to the ribosome-binding site upstream of the ATG at position 238 (see Fig. 2) (5′-CATGCAAGCTTCATCCGTGAAAAATGAATG-3′) and contains a HindIII restriction site (underlined) at the 5′ end. For the amplification reactions, the thermostable DNA polymerase PRIME ZYME (Biometra) was used. Thermal cycling (12 cycles) consisted of denaturation at 95°C for 1 min, primer annealing at 56°C for 1 min, and elongation at 72°C for 1.5 min. The PCR fragment obtained with primers 1 and 2 contained an artificial HindIII site, to which a ′lacZ fragment was fused as previously described (34). The unique PstI site in the hcnA gene was used to create a second ′lacZ translational fusion. The vector for these fusions was pME6010, a pACYC177-pVS1 shuttle vector (20a).
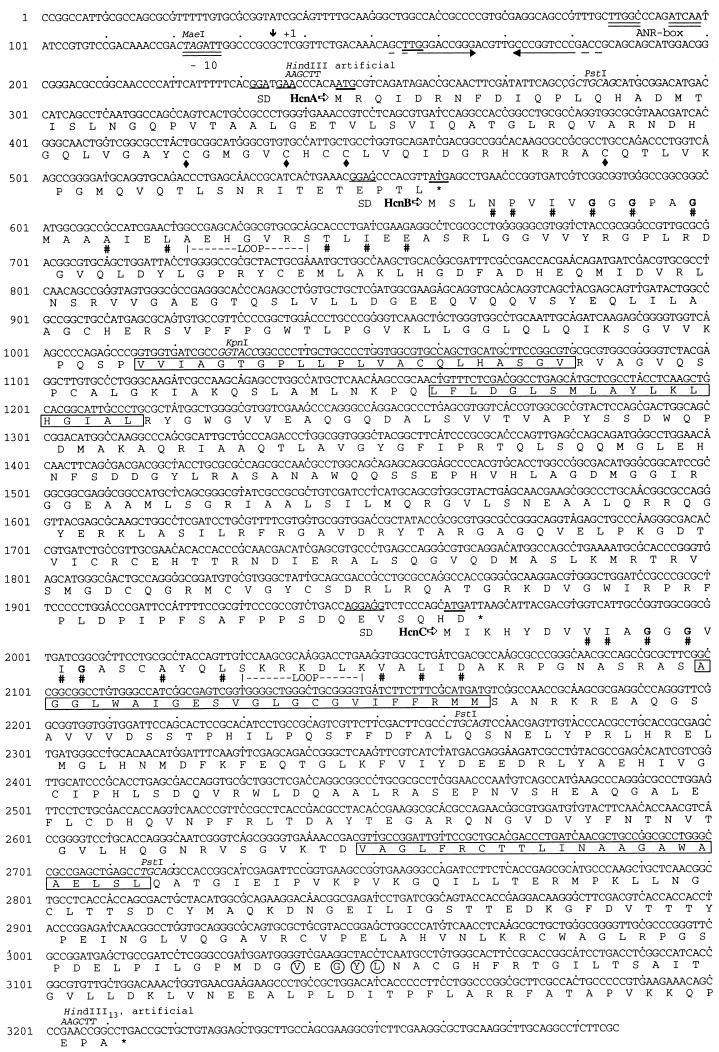
FIG. 2.
Nucleotide sequence of the hcn gene cluster of P. fluorescens CHA0 and deduced amino acid sequence of its protein products. The putative start codons and potential ribosome-binding site (SD) are underlined. The transcription start site is marked with +1. Double underlining below the sequence indicates a sequence (ANR-box) homologous to the E. coli FNR-binding site and the −10 region of the hcn promoter. Facing arrows indicate inverted repeats. Four cysteine residues in HcnA discussed as potential binding sites for an Fe—S cluster are indicated by diamonds. Eleven amino acid residues within HcnB and HcnC that define an ADP-binding motif, including three conserved glycine residues (boldface) (56), are marked by number signs, and the variable loop is indicated. Predicted transmembrane segments in HcnB and HcnC are boxed. Four amino acid residues in the C-terminal region of HcnC which are identical to the E. coli Pfl region containing the free glycyl radical in activated Pfl are circled. Introduction of artificial HindIII sites at the 5′ end of the hcnA gene and at the 3′ end of the truncated hcnC′ gene is described in the text.
The anr nucleotide sequence was determined by Genome Express (Grenoble, France). Nucleotide and deduced amino acid sequences were analyzed with the programs GAP (whole-length sequence alignments), PILEUP (multiple alignments), and BLAST by use of the Genetics Computer Group package (version 8).
Bacterial matings.
Triparental matings of P. fluorescens recipients with E. coli containing a mobilizable plasmid (pVK100 or pME3087) and with E. coli containing a mobilizing plasmid (pME497) were performed as previously described (52).
Tn1725 mutagenesis.
E. coli RU4420 (47) harboring pME3013 was spread on NA plates containing a chloramphenicol (0 to 1,000 μg/ml) gradient. Highly resistant colonies were purified on NA supplemented with chloramphenicol (500 μg/ml). They carried pME3013::Tn1725 derivatives.
Construction of P. fluorescens mutants by gene replacement.
For construction of the chromosomal hcn deletion mutant CHA77 (Fig. 1), a 2.4-kb PstI deletion was created within the hcn genes of pME3071. The hcn flanking sequences were cloned into the suicide vector pME3087, which carries a tetracycline resistance determinant (52). To obtain strain CHA21, in which the chromosomal anr gene is disrupted by the Ω-Km element (see Fig. 4), a chromosomal PstI fragment of pME3815 containing the anr gene was cloned into vector pME3087 with a deletion of its EcoRI site. The Ω-Km fragment of pHP45Ω was inserted into the unique EcoRI site within the anr gene. Suicide plasmids carrying either the hcn gene deletion or the anr gene disruption were mobilized by helper plasmid pME497 to wild-type strain CHA0 and chromosomally integrated, with selection for tetracycline resistance. Excision of the vector by a second crossover was carried out by enrichment for tetracycline-sensitive cells (64). Selection for kanamycin resistance ensured the presence of the Ω-Km insertion in strain CHA21. Both mutations were checked by Southern blotting (data not shown) and by testing of their HCN-negative phenotype.
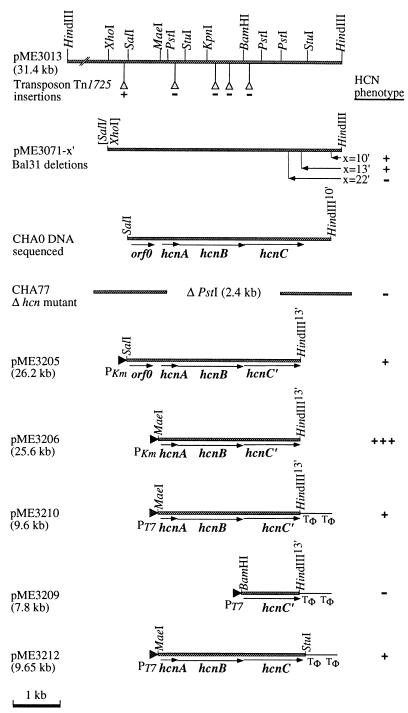
FIG. 1.
Recombinant plasmids carrying the hcnABC region from P. fluorescens CHA0. Symbols:  , genomic DNA from strain CHA0;
, genomic DNA from strain CHA0;  , sites of Tn1725 insertions; ▸, orientation of kanamycin resistance and T7 promoters (PKm and PT7, respectively) on vector plasmids; Tφ, transcription terminator; x, time of Bal 31 digestion (minutes) and extent of deletions created by Bal 31. The HCN phenotype was assessed by qualitative and quantitative tests for HCN production as follows: −, <5 μM HCN; +, wild-type levels of HCN; overproduction of HCN.
, sites of Tn1725 insertions; ▸, orientation of kanamycin resistance and T7 promoters (PKm and PT7, respectively) on vector plasmids; Tφ, transcription terminator; x, time of Bal 31 digestion (minutes) and extent of deletions created by Bal 31. The HCN phenotype was assessed by qualitative and quantitative tests for HCN production as follows: −, <5 μM HCN; +, wild-type levels of HCN; overproduction of HCN.
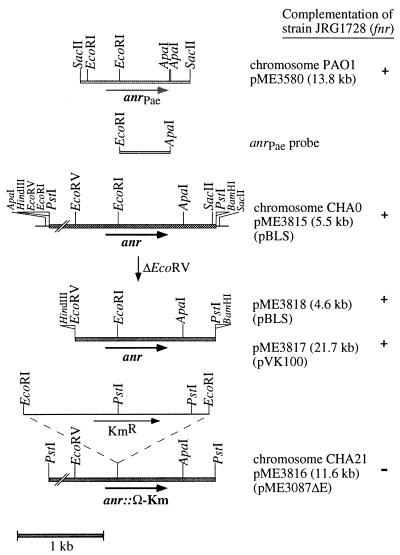
FIG. 4.
Cloning strategy for the anr gene of P. fluorescens CHA0 and mutant construction. Details are explained in the text. Plasmids are listed in Table 1. +, positive for complementation of the E. coli fnr mutant JRG1728; −, negative for complementation; anrPae, anr gene of P. aeruginosa.
Primer extension analysis.
Extraction of total RNA from P. fluorescens was performed according to the method reported by Kullik et al. (27). Primer extension reactions were carried out essentially as described previously (51). The oligonucleotides used as primers for cDNA synthesis, 5′-GGGGTTGCCGGCGTCCCGCCGTCCATGCTGC-3′ (HCN4; positions 218 to 188) and 5′-GCTGCTGCGGTCGGGACCGGGCAACGTCC-3′ (HCN5; positions 192 to 164), both annealed to the coding strand of hcnA (Fig. 2). The oligonucleotides (5 to 10 pmol each) were 5′ labeled with 10 U of T4 polynucleotide kinase (Pharmacia) and 20 μCi of [α-32P]dATP at 37°C for 30 min. Nonincorporated nucleotides were eliminated by passage over a Sephadex G-25 column. The primer elongation reaction was carried out at 43°C for 1 h with reverse transcriptase SuperScript (GIBCO BRL) and 7-deaza-dGTP. Unlabeled primers were used to generate a nucleotide sequence ladder upstream of the hcnA gene. Primer extension products were run in parallel with the sequencing reaction to map the transcription initiation site.
Colony hybridization.
A genomic library of P. fluorescens CHA0 cloned into pVK100 (53) was screened in E. coli with a probe containing most of the anr gene of P. aeruginosa (see Fig. 4). A 0.6-kb EcoRI-ApaI fragment of pME3580 (Table 1; see Fig. 4), which served as the probe, was excised from a low-melting-point agarose gel and labeled with [α-32P]dATP by the random-primer method (13). Colony hybridization with Hybond-N membranes was performed according to the protocol of the supplier (Amersham) and Perbal (37). Prehybridization, hybridization, and washing were carried out at 65°C (high-stringency conditions).
Protein expression.
The hcn genes of P. fluorescens were cloned under the control of the T7 promoter in pEB16, producing pME3209, pME3210, and pME3212 (Table 1 and Fig. 1). NYB cultures of P. aeruginosa ADD1976 (2) harboring any of these constructs were grown at 37°C until they reached an optical density at 600 nm of 0.8. Cells were centrifuged and resuspended in minimal medium M9 (40) supplemented with 0.5% Methionine Assay Medium, which contains all essential growth factors except methionine. After incubation at 37°C with shaking for 75 min, the chromosomal T7 RNA polymerase was induced by the addition of 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 1 h. Rifampin was added at 200 μg/ml and, after further incubation for 30 min, 10 μCi of l-[35S]methionine (Amersham) was mixed with the culture. After 50 min at 37°C, cells were harvested, washed in 50 mM glucose solution buffered with Tris-HCl (25 mM, pH 8.0), and lysed for 5 min at 100°C in sample buffer containing 62.5 mM Tris-HCl (pH 8.8), 2% (wt/vol) sodium dodecyl sulfate (SDS), 10% (vol/vol) glycerol, 5% (vol/vol) β-mercaptoethanol, and 0.005% bromophenol blue. Aliquots were electrophoresed on SDS–15% (wt/vol) polyacrylamide gels (28). Dried gels were autoradiographed with a screen at −80°C for 6 to 12 h.
Production of secondary metabolites.
HCN was quantified in P. fluorescens culture supernatants as described previously (19, 53). E. coli cultures were grown in closed 20-ml flasks containing 8 ml of LB(2x)-M9-MMC medium at 37°C with shaking. In this medium, HCN production by E. coli was high and cells were lysed at the end of growth, even when the T7 promoter had not been induced by IPTG. Since the expression of the hcn genes was toxic for E. coli, experiments were performed with freshly transformed cells, and carbenicillin (200 μg/ml) was used instead of ampicillin to prevent the loss of the hcn plasmid. After 24 h of incubation, HCN concentrations in the culture supernatants were determined by the method of Gewitz et al. (19). Strains growing on plates were tested qualitatively for HCN production by the indicator paper method (3). The antibiotics 2,4-diacetylphloroglucinol and pyoluteorin were quantified by established procedures (23).
Enzyme assays.
Arginine deiminase was measured in toluene-treated cells (32). β-Galactosidase specific activities were determined by the Miller method (40).
Gnotobiotic system.
Suppression of black root rot caused by T. basicola was determined with gnotobiotically grown tobacco plants as previously described (23, 53). Soil water content and soil water potential in the artificial soil of the gnotobiotic system were determined at the beginning of the experiments according to McInnes et al. (33).
Nucleotide sequence accession numbers.
The nucleotide sequences of the hcnABC and the anr genes reported here have been assigned GenBank accession numbers AF053760 and AF053611, respectively.
RESULTS
Nucleotide sequence analysis of the hcn genes.
A cyanide biosynthetic locus (hcn) of P. fluorescens CHA0 is carried by recombinant plasmid pME3013 (Fig. 1), previously described (53). To localize the hcn genes, we isolated Tn1725 insertions in pME3013 and generated progressive deletions with Bal 31 in a derivative of pME3013, pME3071 (Fig. 1). These constructs were mobilized into P. fluorescens P3, a strain that does not produce HCN naturally but will do so when carrying pME3013 (53). In this way, the HCN biosynthetic capacity was localized to a 3.8-kb fragment, which was inserted into the broad-host-range vector pVK100, giving pME3205 (Fig. 1).
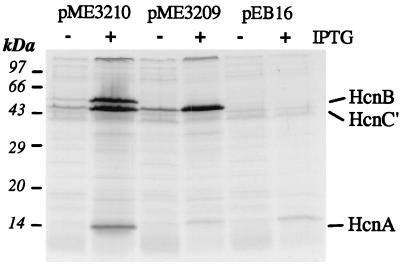
The nucleotide sequence of the 3.8-kb fragment was determined. Three contiguous open reading frames (ORFs), designated hcnABC, and the 3′ region of an additional ORF (ORF0) were found. ORF0 could be deleted in pME3206 (Fig. 1) without loss of cyanogenic capacity and was not analyzed further. The entire hcnABC region contained 66.2% G+C. The codon usage and the high G+C content at the third codon position (hcnA, 84%; hcnB, 89%; hcnC, 89%) were typical of Pseudomonas genes. The first ORF, hcnA, has two potential start codons, the ATG at position 245 and the upstream TTG at position 155 (Fig. 2). In order to determine the in vivo translation start site, we constructed two ′lacZ translational fusions, one at an artificial HindIII restriction site created by PCR at position 237 and the other at the natural PstI site located at position 282 (Fig. 2). In P. fluorescens CHA0, only the downstream hcnA′-′lacZ fusion, generated at the PstI site, gave measurable β-galactosidase activity (data not shown), indicating that in vivo translation of hcnA starts at the ATG codon. The molecular mass of the deduced HcnA polypeptide is 11,525 Da. The most probable ATG start codon of the second ORF, hcnB, overlaps the TGA stop codon of hcnA (Fig. 2). The expression of this ORF was verified with a lacZ translational fusion constructed at the unique KpnI site (Fig. 2) within hcnB (data not shown). The nucleotide sequence predicted a polypeptide of 50,647 Da for HcnB. For the third ORF, hcnC, the most likely ATG start codon is 2 bp upstream of the TAA stop codon of hcnB (Fig. 2). In the Bal 31-generated construct pME3071-10′, which gives an Hcn+ phenotype in strain P3, the distance from the TGA stop codon of hcnC to the 3′ end of the insert (marked by a HindIII linker) is 0.7 kb (Fig. 1). In the subsequent deletion construct pME3071-13′, the last three codons and the stop signal of hcnC were removed (Fig. 2) without affecting the Hcn+ phenotype conferred by this plasmid (Fig. 1). The deduced full-length HcnC polypeptide has a calculated molecular mass of 45,334 Da. The hcnABC′ segment derived from pME3071-13′ was inserted behind the T7 promoter in pME3210 (Fig. 1). In this context, the truncated HcnC protein (HcnC′) had a tail of a short peptide (KLGASRGSGS) resulting from the fusion of the truncated hcnC′ gene to the transcription terminator sequence of the vector. Thus, HcnC′ consists of a 46-kDa polypeptide. When the insert of pME3210 was expressed by T7 RNA polymerase in P. aeruginosa ADD1976, three polypeptides of approximately 12, 45, and 50 kDa were produced, corresponding to HcnA, HcnC′, and HcnB, respectively (Fig. 3). The hcnC′ construct pME3209 (Fig. 1) expressed in strain ADD1976 produced the expected 45-kDa band only (Fig. 3). Thus, the hcnABC gene products seen were in agreement with the nucleotide sequence data.
FIG. 3.
Expression of the proteins HcnA, HcnB, and HcnC′. The hcnABC′ genes were cloned in the T7 expression vector pEB16, giving plasmids pME3210 (hcnABC′) and pME3209 (hcnC′) (Fig. 1). Cultures of P. aeruginosa ADD1976 carrying either of these plasmids were induced with IPTG (2 mM) (+) or not induced (−). The proteins were labeled with [35S]methionine, separated by electrophoresis in an SDS–15% polyacrylamide gel, and visualized by autoradiography.
The hcnABC cluster encodes HCN synthase.
A 2.4-kb PstI deletion was created within the hcnABC genes and transferred to the chromosome of strain CHA0 by a double-crossover technique previously described (64). The Δhcn mutant CHA77 obtained did not produce measurable amounts of HCN, whereas the wild-type strain CHA0 and the complemented mutant CHA77/pME3013 did (Table 2). In E. coli, a bacterium that does not produce HCN naturally, the T7 expression construct pME3210 (hcnABC′) led to HCN production (Table 2) in the presence of low levels of T7 RNA polymerase (see Materials and Methods). The induction of T7 RNA polymerase was avoided to prevent host cell death by an HCN overdose. The T7 expression construct pME3212, containing the full-length hcnABC cluster (derived from pME3071-10′; Fig. 1), produced a similar amount of HCN (Table 2), indicating that the three C-terminal amino acid residues of HcnC (which are missing in HcnC′ carried by pME3210) are not essential for HCN synthase activity. Taken together, these results indicate that the hcnABC genes are the structural genes for HCN synthase. Their structural organization suggests that they form an operon.
TABLE 2.
HCN biosynthesis in P. fluorescens CHA0 and E. coli BL21 harboring hcnABC recombinant plasmids
| Strain | Relevant genotype | HCN productiona (μM) |
|---|---|---|
| CHA0 | hcnABC+ | 89 ± 5 |
| CHA77 | Δhcn | <5 |
| CHA77/pME3013 | hcnABC+ | 96 ± 32 |
| CHA77/pME3206 | PKm-hcnABC+ | 855 ± 90 |
| BL21/pEB16 | <5 | |
| BL21/pME3210 | PT7-hcnABC′ | 132 ± 25 |
| BL21/pME3212 | PT7-hcnABC+ | 102 ± 13 |
P. fluorescens was grown in MMC with O2 limitation and E. coli was grown in a rich, glucose-containing medium as indicated in Materials and Methods. Mean ± standard deviation values for triplicate experiments are given.
Similarities between the HcnABC polypeptides and dehydrogenases.
At the amino acid sequence level, HcnA has 34% identity with the α subunit of formate dehydrogenase from Moorella thermoacetica (Clostridium thermoaceticum; GenBank accession no. U73807). HcnA also has 31% identity with the HoxU subunit of hydrogenase from Anabaena variabilis (GenBank accession no. X79285) (41). A cluster of cysteine residues (Cys-X4-Cys-X2-Cys-Xn-Cys) in HcnA (Fig. 2) resembles a similar sequence motif in ferredoxins and may interact with a [2Fe—2S] center (46). HcnB and HcnC each have a typical FAD- or NAD(P)-binding motif, the ADP-binding βαβ fold (56), in their N-terminal parts (Fig. 2). HcnB is most similar to the SoxA subunit of sarcosine (N-methylglycine) oxidase from Corynebacterium sp. (32% identity in a stretch of 171 amino acid residues; GenBank accession no. Q46337) (10), to the OoxA subunit of octopine oxidase from Agrobacterium tumefaciens (30% identity; GenBank accession no. Z30328), and to the NoxA subunit of nopaline oxidase from the same organism (30% identity; GenBank accession no. Z30316) (65). HcnC is most similar to the DadA subunit of a putative d-amino acid oxidase from P. aeruginosa (31% identity; GenBank accession no. L48934) and to the homologous protein from E. coli (23% identity; GenBank accession no. P29011) (31). The SoxB subunit of sarcosine oxidase from Corynebacterium sp. (GenBank accession no. P40875) and HcnC also resemble each other (24% identity). The significance of these similarities is considered in the Discussion.
Mapping of the hcnA promoter.
RNA preparations from CHA0 cultures harvested at various growth phases were used for mapping the 5′ end of the hcnA transcript by primer extension (data not shown). The +1 site determined reveals a −10 sequence (TAGATT) and an FNR/ANR box which is centered around −41.5 (TTGGC… .ATCAA; Fig. 2) and which deviates in two positions from the consensus FNR recognition sequence (TTGAT… .ATCAA) (43, 57). The −41.5 location of an FNR/ANR box is typical of anaerobically inducible promoters that are controlled by FNR or FNR-like regulators (43). Since strain CHA0, like other pseudomonads, produces HCN optimally in oxygen-limited cultures (8, 53), the existence of an appropriately positioned FNR/ANR box in the hcnA promoter suggested that strain CHA0 may have an anr gene, which could positively control HCN production.
Cloning of the P. fluorescens anr gene.
An EcoRI-ApaI fragment carrying most of the P. aeruginosa anr gene (66) was used as a probe to screen a genomic library of P. fluorescens CHA0 established in cosmid pVK100 in E. coli (53). Three clones detected by colony hybridization each carried a pVK100 derivative with a common 19-kb HindIII insert. One recombinant cosmid, pME3812, was retained. It contained an internal 2.5-kb PstI fragment hybridizing to the anr gene of P. aeruginosa; the P. fluorescens origin of the 2.5-kb fragment was confirmed by Southern hybridization of P. fluorescens genomic DNA digested with PstI (data not shown). The 2.5-kb fragment was subcloned into pBluescript II KS+ resulting in pME3815. After removal of a 0.9-kb upstream segment by EcoRV digestion, pME3818 was obtained (Fig. 4). The E. coli fnr mutant JRG1728 was complemented for anaerobic gas production and anaerobic growth on glycerol-nitrate medium by plasmids pME3812, pME3815, and pME3818, indicating that they all contained a functional fnr homolog (Fig. 4). The anr gene of P. fluorescens was subcloned into broad-host-range vector pVK100. The recombinant plasmid obtained, pME3817, also complemented the fnr mutant of E. coli (Fig. 4). In these assays, pME3580 carrying the anr gene of P. aeruginosa (17) was included as a positive control.
The nucleotide sequence of the 1.6-kb insert of pME3818 revealed the anr gene of P. fluorescens CHA0. Its deduced product consists of 244 amino acid residues and has a calculated molecular mass of 27,155 Da. The G+C content of the anr gene is 61.5%.
Similarity among ANR of P. fluorescens, ANR of P. aeruginosa, and FNR of E. coli.
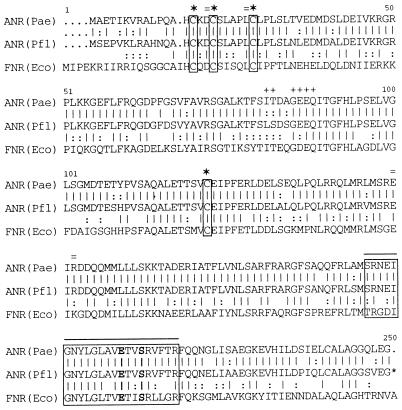
The deduced amino acid sequence of ANR of P. fluorescens (ANRPfl) shows overall identities of 88% (95% similarity) with the sequence of P. aeruginosa ANR (ANRPae) and of 53% (76% similarity) with the sequence of E. coli FNR (Fig. 5). In FNR, three N-terminal cysteine residues and one internal cysteine residue (Cys-20, Cys-23, Cys-29, and Cys-122) are essential for the function of the protein in response to anaerobiosis (43). These cysteine residues are assumed to bind a [4Fe—4S]2+ cluster which in vitro is converted to a [2Fe—2S]2+ cluster by oxygen, resulting in the inactivation of FNR (22, 48). In ANRPfl as well as in ANRPae (66), these cysteines are conserved, with exactly the same spacing as in FNR (Fig. 5). A domain involved in the interaction of FNR with RNA polymerase (Ile-81, Thr-82, Gly-85, Asp-86, Glu-87, and Gln-88) (43) is similar but is less strictly conserved in ANRPfl and in ANRPae (Fig. 5). FNR binds to its target DNA sequence in a dimeric state, which is favored by low oxygen availability (48). Four amino acids (Asp-22, Leu-28, Glu-150, and Asp-154) which appear to be involved in dimerization and stabilization of the Fe—S cluster (30) are strictly conserved in both ANRPfl and ANRPae (Fig. 5). Finally, the C-terminal helix-turn-helix DNA-binding motifs are identical in ANRPfl and ANRPae (Fig. 5).
FIG. 5.
Alignment of the deduced amino acid sequence of ANR from P. fluorescens (Pfl) with those of P. aeruginosa (Pae) ANR and E. coli (Eco) FNR. The sequences were aligned by use of the computer programs PILEUP and GAP. Identities are indicated by vertical lines, and similarities are indicated by colons. The following features of the E. coli FNR protein are highlighted: ★, cysteine residues required for activity; +, residues suggested to interact with RNA polymerase (43); and =, amino acid residues probably involved in dimerization (30). The predicted helix-turn-helix motif is boxed and marked by a solid line above the sequence. Two amino acid residues in this DNA-binding region (Glu-209 and Ser-212) which are essential for binding to the FNR box (43) are shown in boldface.
Construction and properties of a P. fluorescens anr mutant.
The anr gene of strain CHA0 was disrupted by insertion of an Ω-Km element and introduced into the CHA0 chromosome by use of the suicide vector pME3087 (see Materials and Methods), resulting in the anr mutant CHA21 (Fig. 4). The phenotypic properties of the mutant were compared to those of the wild-type strain CHA0 and the complemented mutant CHA21/pME3817 (Fig. 4). The anr mutant had a strongly reduced ability to synthesize HCN, as predicted, and contained noninduced levels of arginine deiminase, the first enzyme of an arginine catabolic ATP-generating pathway (Table 3).
TABLE 3.
Effect of an anr mutation on HCN production and arginine deiminase activity in P. fluorescens
| Strain | HCN productiona (μM) | Arginine deiminase sp actb (U/mg of protein)c |
|---|---|---|
| CHA0 | 89 ± 5 | 22 ± 3 |
| CHA21 | 6 ± 3 | 3 ± 1 |
| CHA21/pME3817 | 170 ± 44 | 29 ± 2 |
Mean ± standard deviation for four experiments in MMC.
Mean ± standard deviation for three experiments in YEA medium.
One unit of activity was defined as 1 μmol of citrulline produced per h.
In P. aeruginosa, the anr gene is essential for anaerobic growth on nitrate by denitrification and on arginine by the arginine deiminase pathway (17, 64, 66). Therefore, P. fluorescens CHA0 was tested for growth in an anaerobic jar (GasPak). However, this strain did not grow anaerobically on arginine (YEA plates), on nitrate (NA amended with 20 mM KNO3), or on nitrite (NA amended with 5 mM KNO2) at 30°C within 4 days. The control strain P. aeruginosa PAO1 grew on these media. Furthermore, P. fluorescens CHA0 did not produce gas in unshaken nitrate broth in 2 days. Thus, in these tests, strain CHA0 behaved as a strict aerobe, and no difference was apparent between the wild-type strain and the anr mutant.
Provided that the arginine deiminase pathway functions properly in P. fluorescens CHA0, the wild-type strain gains ATP from arginine but the anr mutant does not during oxygen limitation. In a competition experiment, strain CHA0 gradually displaced the anr mutant CHA21 when both strains were incubated in rich arginine (YEA) medium with limiting oxygen for 2 days (Table 4). Complementing plasmid pME3817 (anr+) largely protected the mutant (Table 4). Thus, the wild-type strain clearly benefits from anr function under such conditions.
TABLE 4.
Competition between the wild-type strain CHA0 and the anr mutant CHA21 (Kmr), with and without complementing plasmid pME3817, in YEA mediuma
| Incubation (h) | % Kmr colonies in mixtures of:
|
|
|---|---|---|
| CHA0 and CHA21b | CHA0 and CHA21/pME3817c | |
| 0 | 56 | 59 |
| 1 | 57 | 57 |
| 15 | 47 | 45 |
| 24 | 32 | 45 |
| 36 | 4 | 46 |
| 48 | 3 | 49 |
Pairs of competing strains were set up by mixing overnight cultures 1:1 (by volume) and diluting the mixtures 50-fold with fresh YEA medium (60 ml) in 120-ml flasks. Incubation of the tightly closed flasks at 30°C resulted in oxygen-limited stationary-phase cultures (approximately 109 cells/ml) after 10 to 15 h.
Mean values of triplicate experiments.
Mean values of duplicate experiments.
Regulation of hcn expression by oxygen limitation.
We confirmed that the ANR-dependent expression of the hcn genes was regulated by oxygen limitation in P. fluorescens. An hcnA′-′lacZ translational fusion in plasmid pME3219 (Table 1) was introduced into strains CHA0 and CHA21. On X-Gal plates, strain CHA0/pME3219 formed blue colonies, whereas strain CHA21/pME3219 remained white (corresponding to ≤5 Miller units of β-galactosidase), indicating that ANR is needed to drive the expression of the hcn genes. When strain CHA0/pME3219 was grown in MMC with good aeration, the hcnA′-′lacZ fusion gave low β-galactosidase activity (570 ± 20 Miller units). After growth under oxygen limitation, the same strain expressed an elevated level of β-galactosidase activity (18,000 ± 2,000 Miller units), demonstrating control by microaerophilic conditions.
Effect of an anr mutation on the ability of P. fluorescens to suppress black root rot of tobacco.
Suppression of root diseases by strain CHA0 depends to a large extent on antibiotic and HCN production (52). In vitro, the HCN-deficient anr mutant CHA21 produced the antibiotics 2,4-diacetylphloroglucinol (5.9 μg/ml) and pyoluteorin (15.5 μg/ml) in quantities that were comparable to those excreted by the wild-type strain CHA0 (7.2 and 9.9 μg/ml, respectively). Previously, we showed that, in a gnotobiotic system, the hcnB::Ω-Hg mutant CHA5, constructed by gene replacement, protects tobacco roots less effectively from the black root rot fungus T. basicola than does the wild-type strain CHA0. Moreover, hcn function and suppressive ability can be restored by complementation with pME3013 (53). We verified that the hcn deletion strain CHA77 gave reduced (84%) disease suppression in the tobacco-T. basicola system, in comparison with the wild-type strain CHA0 (disease suppression defined as 100%) and the complemented mutant CHA77/pME3013 (disease suppression, 110%), in terms of fresh plant weight (data not shown). The artificial soil used in these studies contained 23% water (soil water potential, −0.0028 MPa). The suppressive capacity of the anr mutant CHA21 in artificial soil containing 20% water (−0.004 MPa) was affected similarly (data not shown). In a wetter soil, containing 35% water (soil water potential, 0 MPa), under otherwise identical conditions, the anr mutant CHA21 afforded significantly reduced protection of tobacco, in terms of both plant weight and reduction of disease severity, in comparison with the wild-type strain CHA0 and the complemented mutant CHA21/pME3817 (Table 5). These data suggest that anr function can contribute to biocontrol, especially in soil with restricted oxygen availability.
TABLE 5.
Effect of an anr mutation in P. fluorescens CHA0 on the protection of tobacco against black root rot caused by T. basicola in a water-saturated soil under gnotobiotic conditions
| Microorganism addeda
|
Plant fresh wtb (mg) | Root fresh wtb (mg) | % Root surface infectedb,c | P. fluorescensb (log CFU/g of root) | |
|---|---|---|---|---|---|
| P. fluorescens | T. basicola | ||||
| None | − | 677 a | 224 a | 0 a | ND |
| CHA0 (wild type) | − | 681 a | 250 a | 0 a | 8.58 a |
| CHA21 (anr) | − | 616 a | 240 a | 0 a | 8.41 a |
| CHA21/pME3817 (anr+) | − | 621 a | 239 a | 0 a | 8.59 a |
| None | + | 151 d | 32 d | 83 d | ND |
| CHA0 (wild type) | + | 450 b | 152 b | 31 b | 8.98 a |
| CHA21 (anr) | + | 327 c | 97 c | 58 c | 9.13 a |
| CHA21/pME3817 (anr+) | + | 479 b | 175 b | 39 b | 8.80 a |
P. fluorescens strains and T. basicola were added at 107 CFU and 5 × 103 endoconidia per g of soil 3 and 2 days, respectively, before planting of a 5-week-old tobacco plant grown under sterile conditions. Water-saturated conditions (water content, 35%; water potential, 0 MPa) were induced 3 days after planting by adding sterile water to soil. Plants were harvested after 3 weeks.
Means within the same column followed by the same letter do not differ significantly at P = 0.05, according to Student’s t test. Data represent three individual repetitions of the same experimental setup, with eight replicates (flasks containing one tobacco plant) per treatment. ND, not detected.
Percentage of root surface darkened by the presence of chlamydospores of T. basicola (23).
DISCUSSION
Structure and function of HCN synthase.
The nucleotide sequence of HCN synthase, established here for the first time, supports one of several models proposed for bacterial HCN synthesis (26): the dehydrogenase model. According to this mechanism, glycine is first oxidized to iminoacetic acid [H-C(NH)-COOH]. Then, the C—C bond is split, with a concomitant second dehydrogenase reaction, which produces HCN and CO2 (58). The three HCN synthase subunits have similarities with known dehydrogenases: HcnA with a clostridial formate dehydrogenase and HcnB and HcnC with amino acid dehydrogenases (oxidases). All in all, the sequence comparisons strongly suggest that HCN synthase basically is an amino acid oxidase. By analogy with biochemically characterized amino acid oxidases (10, 35), it is predicted that HCN synthase is a flavoenzyme.
However, HCN synthase differs from d- and l-amino acid oxidases in an important aspect. The latter enzymes produce α-imino acids from their amino acid substrates; α-imino acids are rapidly hydrolyzed, giving the corresponding α-keto acids (20). In the HCN synthase reaction, however, the postulated intermediate iminoacetic acid does not appear to be converted hydrolytically to glyoxylic acid (26). Instead, enzyme-bound iminoacetic acid is assumed to be cleaved at the C—C bond.
This cleavage may have some similarity with the radical mechanism involved in the pyruvate-formate lyase (Pfl) reaction in E. coli (24). Cleavage of pyruvate is initiated by one-electron transfer from activated Pfl, which contains a free glycyl radical in the polypeptide chain. The glycine radical lies in a turn stretch (Val-Ser-Gly-Tyr) between two β strands in the C-terminal part of Pfl (55). The occurrence of a similar motif (Val-Glu-Gly-Tyr) forming a turn in the C-terminal region of HcnC (Fig. 2) may be fortuitous. However, a related sequence (Val-Cys-Gly-Tyr) is also found in anaerobic ribonucleotide reductase; at this site, a glycine radical is formed during activation of this enzyme (45). Molecular oxygen readily reacts with the glycine radical of Pfl, cleaving the polypeptide chain at this site (55). Perhaps the exquisite sensitivity of HCN synthase to oxygen (7, 60) could be explained by an analogous reaction.
Cleavage of iminoacetic acid by HCN synthase could produce HCN and formic acid, whose oxidation to CO2 might be catalyzed by the HcnA subunit. The four electrons removed from glycine by HCN synthase are probably transferred to a cyanide-insensitive terminal oxidase; the CioAB enzyme recently characterized for P. aeruginosa (11) is a good candidate. Two transmembrane segments are predicted for both HcnB and HcnC (Fig. 2), suggesting that HCN synthase is a membrane-bound enzyme. These features correlate with previous enzyme data. Using a partially purified HCN synthase preparation, Wissing (58, 59) obtained evidence for FAD as a cofactor and for an association of the enzyme with the cytoplasmic membrane.
ANR function in P. fluorescens.
For a wide variety of bacteria, more than 20 FNR homologs have been identified, most of which control gene expression in response to oxygen limitation (43, 50, 62). According to the phylogenetic tree of the FNR family proposed by Van Spanning et al. (50), ANRPfl belongs to group A, where it resembles most ANRPae (88% identity) and FnrA of P. stutzeri (84% identity) and is more distantly related to FNR in Enterobacteriaceae. The FNR-like regulator CydR of Azotobacter vinelandii (62) is also a close relative of ANRPfl (80% identity).
In P. aeruginosa PAO, the anr gene mediates anaerobic growth on nitrate and on arginine (17, 66). We recently studied the function of ANR as a transcriptional regulator in this bacterium (57). Promoters containing the FNR consensus box are induced about 20-fold by oxygen limitation, compared to the basal levels measured in well-aerated cells (57). In P. fluorescens CHA0, which does not grow anaerobically by denitrification or by arginine fermentation, the anr gene is nevertheless conserved and physiologically active. In particular, the FNR consensus promoter shows a similar ANR-dependent response to oxygen (21a). The wild-type strain CHA0 produces about 15 times more HCN than does the anr mutant strain CHA21 under conditions of oxygen limitation (Table 3). Oxygen limitation was shown to regulate the expression of an hcnA′-′lacZ translational fusion in strain CHA0. The level of β-galactosidase was 30 times higher in oxygen-limited cultures than in cells grown with good aeration. Moreover, the fusion was not expressed in the anr mutant. From the hcnA promoter sequence (Fig. 2) it can be predicted that ANR binds to the −40 region and thereby activates transcription.
Mutational inactivation of the anr gene reduced the biocontrol efficacy of P. fluorescens CHA0 in the tobacco-T. basicola system (Table 5). This effect is in agreement with previous work on the role of bacterial HCN in this plant-pathogen system (53). The anr function of strain CHA0 seemed to have a stronger positive effect on biocontrol in water-saturated soil than in more aerated soil, suggesting that ANR-dependent HCN production occurs predominantly in poorly aerated, water-soaked soils. The site(s) where GacA regulates HCN synthesis (29) has not yet been determined.
Although P. fluorescens CHA0 does not grow in the anaerobic atmosphere generated by the GasPak jar, it can adapt to microaerobic conditions by using ANR as a positive regulator. Natural habitats of P. fluorescens often contain little oxygen. In biofilms formed by P. fluorescens, the amount of oxygen available to cells in the lower layers is only a small percentage of the amount present at the surface (36). In the rhizosphere, oxygen consumption by roots and root-colonizing microorganisms can create a marked oxygen gradient. In a barley root model system, the innermost zone around the root, the rhizoplane, contains only 1 to 20% of the oxygen concentration that is present in the outer zones, lying 3 mm from the root surface (21). The oxygen concentration needed to activate ANR of P. fluorescens has not been determined. However, the fact that the anr gene can effectively restore an fnr mutant of E. coli suggests that ANR and FNR are functionally similar. Half-maximal induction of FNR-dependent promoters in E. coli occurs at about 5 μM O2, i.e., at about 2.5% air saturation (48). Thus, oxygen levels in the rhizoplane may be sufficiently low to allow the activation of ANR in P. fluorescens. ANR conferred a selective advantage on P. fluorescens cells in a nutrient-rich environment (Table 4). Another obligate aerobe, A. vinelandii, uses the FNR-like activator CydR for microaerobic growth (62). Thus, positive control of gene expression under microaerobic conditions may be quite common in aerobic bacteria having FNR-like regulators.
ACKNOWLEDGMENTS
We are indebted to L. Ritter-Hollenstein and P. Schmidli-Sacherer for help in early experiments. We thank F. Mascher for assistance with the determination of soil water potentials and S. Heeb for providing vector pME6010.
We gratefully acknowledge financial support from the Schweizerische Nationalfonds (projects 31-28570.90 and 31-32473.91) and from European project IMPACT 2 (BIO4CT960027).
The first and second authors contributed equally to this study.
REFERENCES
- 1.Askeland R A, Morrison S M. Cyanide production by Pseudomonas fluorescens and Pseudomonas aeruginosa. Appl Environ Microbiol. 1983;45:1802–1807. doi: 10.1128/aem.45.6.1802-1807.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunschwig E, Darzins A. A two-component T7 system for the overexpression of genes of Pseudomonas aeruginosa. Gene. 1992;111:35–41. doi: 10.1016/0378-1119(92)90600-t. [DOI] [PubMed] [Google Scholar]
- 3.Castric K F, Castric P A. Method for rapid detection of cyanogenic bacteria. Appl Environ Microbiol. 1983;45:701–702. doi: 10.1128/aem.45.2.701-702.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castric P. Influence of oxygen on the Pseudomonas aeruginosa hydrogen cyanide synthase. Curr Microbiol. 1994;29:19–21. [Google Scholar]
- 5.Castric P A. Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can J Microbiol. 1975;21:613–618. doi: 10.1139/m75-088. [DOI] [PubMed] [Google Scholar]
- 6.Castric P A. Glycine metabolism by Pseudomonas aeruginosa: hydrogen cyanide biosynthesis. J Bacteriol. 1977;130:826–831. doi: 10.1128/jb.130.2.826-831.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castric P A. The metabolism of hydrogen cyanide by bacteria. In: Vennesland B, Conn E E, Knowles C J, Westley J, Wissing F, editors. Cyanide in biology. London, England: Academic Press Ltd.; 1981. pp. 233–261. [Google Scholar]
- 8.Castric P A. Hydrogen cyanide production by Pseudomonas aeruginosa at reduced oxygen levels. Can J Microbiol. 1983;29:1344–1349. doi: 10.1139/m83-209. [DOI] [PubMed] [Google Scholar]
- 9.Castric P A, Ebert R F, Castric K F. The relationship between growth phase and cyanogenesis in Pseudomonas aeruginosa. Curr Microbiol. 1979;2:287–292. [Google Scholar]
- 10.Chlumsky L J, Zhang L, Jorns M S. Sequence analysis of sarcosine oxidase and nearby genes reveals homologies with key enzymes of folate one-carbon metabolism. J Biol Chem. 1995;270:18252–18259. doi: 10.1074/jbc.270.31.18252. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham L, Pitt M, Williams H D. The cioAB genes from Pseudomonas aeruginosa code for a novel cyanide-insensitive terminal oxidase related to the cytochrome bd quinol oxidases. Mol Microbiol. 1997;24:579–591. doi: 10.1046/j.1365-2958.1997.3561728.x. [DOI] [PubMed] [Google Scholar]
- 12.Del Sal G, Manfioletti G, Schneider C. A one-tube plasmid DNA mini-preparation suitable for sequencing. Nucleic Acids Res. 1988;16:9878. doi: 10.1093/nar/16.20.9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Addendum. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- 14.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of Gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 15.Flaishman M A, Eyal Z, Zilberstein A, Voisard C, Haas D. Suppression of Septoria tritici blotch and leaf rust of wheat by recombinant cyanide-producing strains of Pseudomonas putida. Mol Plant Microbe Interact. 1996;9:642–645. [Google Scholar]
- 16.Fürste J P, Pansegrau W, Frank R, Blöcker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 17.Galimand M, Gamper M, Zimmermann A, Haas D. Positive FNR-like control of anaerobic arginine degradation and nitrate respiration in Pseudomonas aeruginosa. J Bacteriol. 1991;173:1598–1606. doi: 10.1128/jb.173.5.1598-1606.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamper M, Ganter B, Polito M R, Haas D. RNA processing modulates the expression of the arcDABC operon in Pseudomonas aeruginosa. J Mol Biol. 1992;226:943–957. doi: 10.1016/0022-2836(92)91044-p. [DOI] [PubMed] [Google Scholar]
- 19.Gewitz H-S, Pistorius E K, Voss H, Vennesland B. Cyanide formation in preparations from Chlorella vulgaris Beijerinck: effect of sonication and amygdalin addition. Planta. 1976;131:145–148. doi: 10.1007/BF00389986. [DOI] [PubMed] [Google Scholar]
- 20.Hafner E W, Wellner D. Reactivity of the imino acids formed in the amino acid oxidase reaction. Biochemistry. 1979;18:411–417. doi: 10.1021/bi00570a004. [DOI] [PubMed] [Google Scholar]
- 20a.Heeb, S. Unpublished results.
- 21.Højberg O, Sørensen J. Microgradients of microbial oxygen consumption in a barley rhizosphere model system. Appl Environ Microbiol. 1993;59:431–437. doi: 10.1128/aem.59.2.431-437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Højberg, O., U. Schnider, H. Winteler, and D. Haas. Unpublished results.
- 22.Jordan P A, Thomson A J, Ralph E T, Guest J R, Green J. FNR is a direct oxygen sensor having a biphasic response curve. FEBS Lett. 1997;416:349–352. doi: 10.1016/s0014-5793(97)01219-2. [DOI] [PubMed] [Google Scholar]
- 23.Keel C, Schnider U, Maurhofer M, Voisard C, Laville J, Burger U, Wirthner P, Haas D, Défago G. Suppression of root diseases by Pseudomonas fluorescens CHA0: importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. Mol Plant Microbe Interact. 1992;5:4–13. [Google Scholar]
- 24.Knappe J, Sawers G. A radical-chemical route to acetyl-CoA: the anaerobically induced pyruvate formate-lyase system of Escherichia coli. FEMS Microbiol Rev. 1990;75:383–398. doi: 10.1111/j.1574-6968.1990.tb04108.x. [DOI] [PubMed] [Google Scholar]
- 25.Knauf V C, Nester E W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982;8:45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- 26.Knowles C J, Bunch A W. Microbial cyanide metabolism. Adv Microb Physiol. 1986;27:73–111. doi: 10.1016/s0065-2911(08)60304-5. [DOI] [PubMed] [Google Scholar]
- 27.Kullik I, Hennecke H, Fischer H-M. Inhibition of Bradyrhizobium japonicum nifA-dependent nif gene activation by oxygen occurs at the NifA protein level and is irreversible. Arch Microbiol. 1989;151:191–197. [Google Scholar]
- 28.Laemmli U K, Favre M. Maturation of the head of bacteriophage T4. J Mol Biol. 1973;80:575–579. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- 29.Laville J, Voisard C, Keel C, Maurhofer M, Défago G, Haas D. Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc Natl Acad Sci USA. 1992;89:1562–1566. doi: 10.1073/pnas.89.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazazzera B A, Beinert H, Khoroshilova N, Kennedy M C, Kiley P J. DNA binding and dimerization of the Fe—S-containing FNR protein from Escherichia coli are regulated by oxygen. J Biol Chem. 1996;271:2762–2768. doi: 10.1074/jbc.271.5.2762. [DOI] [PubMed] [Google Scholar]
- 31.Lobocka M, Hennig J, Wild J, Klopotowski T. Organization and expression of the Escherichia coli K-12 dad operon encoding the smaller subunit of d-amino acid dehydrogenase and the catabolic alanine racemase. J Bacteriol. 1994;176:1500–1510. doi: 10.1128/jb.176.5.1500-1510.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lüthi E, Mercenier A, Haas D. The arcABC operon required for fermentative growth of Pseudomonas aeruginosa on arginine: Tn5-751-assisted cloning and localization of structural genes. J Gen Microbiol. 1986;132:2667–2675. doi: 10.1099/00221287-132-10-2667. [DOI] [PubMed] [Google Scholar]
- 33.McInnes K J, Weaver R W, Savage M J. Soil water potential. In: Mickelson S H, editor. Methods of soil analysis, part 2. Madison, Wis: Soil Science Society of America; 1994. pp. 53–58. [Google Scholar]
- 34.Minton N P. Improved plasmid vectors for the isolation of translational lacZ fusions. Gene. 1984;31:269–273. doi: 10.1016/0378-1119(84)90220-8. [DOI] [PubMed] [Google Scholar]
- 35.Olsiewski P J, Kaczorowski G J, Walsh C T. Purification and properties of d-amino acid dehydrogenase, an inducible membrane-bound iron-sulfur flavoenzyme from Escherichia coli B. J Biol Chem. 1980;255:4487–4494. [PubMed] [Google Scholar]
- 36.Patel T D, Bott T R. Oxygen diffusion through a developing biofilm of Pseudomonas fluorescens. J Chem Tech Biotechnol. 1991;52:187–199. [Google Scholar]
- 37.Perbal D. A practical guide to molecular cloning. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1988. [Google Scholar]
- 38.Reimmann C, Beyeler M, Latifi A, Winteler H, Foglino M, Lazdunski A, Haas D. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol Microbiol. 1997;24:309–319. doi: 10.1046/j.1365-2958.1997.3291701.x. [DOI] [PubMed] [Google Scholar]
- 39.Sacherer P, Défago G, Haas D. Extracellular protease and phospholipase C are controlled by the global regulatory gene gacA in the biocontrol strain Pseudomonas fluorescens CHA0. FEMS Microbiol Lett. 1994;116:155–160. doi: 10.1111/j.1574-6968.1994.tb06694.x. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41.Schmitz O, Boison G, Hilscher R, Hundeshagen B, Zimmer W, Lottspeich F, Bothe H. Molecular biological analysis of a bidirectional hydrogenase from cyanobacteria. Eur J Biochem. 1995;233:266–276. doi: 10.1111/j.1432-1033.1995.266_1.x. [DOI] [PubMed] [Google Scholar]
- 42.Schnider U, Keel C, Blumer C, Troxler J, Défago G, Haas D. Amplification of the housekeeping sigma factor in Pseudomonas fluorescens CHA0 enhances antibiotic production and improves biocontrol abilities. J Bacteriol. 1995;177:5387–5392. doi: 10.1128/jb.177.18.5387-5392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spiro S. The FNR family of transcriptional regulators. Antonie Leeuwenhoek. 1994;66:23–36. doi: 10.1007/BF00871630. [DOI] [PubMed] [Google Scholar]
- 44.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 45.Sun X, Harder J, Krook M, Jörnvall H, Sjöberg B-M, Reichard P. A possible glycine radical in anaerobic ribonucleotide reductase from Escherichia coli: nucleotide sequence of the cloned nrdD gene. Proc Natl Acad Sci USA. 1993;90:577–581. doi: 10.1073/pnas.90.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ta D T, Vickery L E. Cloning, sequencing, and overexpression of a [2Fe-2S] ferredoxin gene from E. coli. J Biol Chem. 1992;267:11120–11125. [PubMed] [Google Scholar]
- 47.Ubben D, Schmitt R. Tn1725 derivatives for transposon mutagenesis, restriction mapping and nucleotide sequence analysis. Gene. 1986;41:145–152. doi: 10.1016/0378-1119(86)90093-4. [DOI] [PubMed] [Google Scholar]
- 48.Unden G, Schirawski J. The oxygen-responsive transcriptional regulator FNR of Escherichia coli: the search for signals and reactions. Mol Microbiol. 1997;25:205–210. doi: 10.1046/j.1365-2958.1997.4731841.x. [DOI] [PubMed] [Google Scholar]
- 49.Vander Wauven C, Piérard A, Kley-Raymann M, Haas D. Pseudomonas aeruginosa mutants affected in anaerobic growth on arginine: evidence for a four-gene cluster encoding the arginine deiminase pathway. J Bacteriol. 1984;160:928–934. doi: 10.1128/jb.160.3.928-934.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Spanning R J M, De Boer A P N, Reijunders W N M, Westerhoff H V, Stouthamer A H, Van Der Oost J. FnrP and NNR of Paracoccus denitrificans are both members of the FNR family of transcriptional activators but have distinct roles in respiratory adaption in response to oxygen limitation. Mol Microbiol. 1997;23:893–907. doi: 10.1046/j.1365-2958.1997.2801638.x. [DOI] [PubMed] [Google Scholar]
- 51.Vögtli M, Hütter R. Characterisation of the hydroxy-streptomycin phosphotransferase gene (sph) of Streptomyces glaucescens: nucleotide sequence and promoter analysis. Mol Gen Genet. 1987;208:195–203. doi: 10.1007/BF00330442. [DOI] [PubMed] [Google Scholar]
- 52.Voisard C, Bull C T, Keel C, Laville J, Maurhofer M, Schnider U, Défago G, Haas D. Biocontrol of root diseases by Pseudomonas fluorescens CHA0: current concepts and experimental approaches. In: O’Gara F, Dowling D N, Boesten B, editors. Molecular ecology of rhizosphere microorganisms. Weinheim, Germany: VCH; 1994. pp. 69–89. [Google Scholar]
- 53.Voisard C, Keel C, Haas D, Défago G. Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. EMBO J. 1989;8:351–358. doi: 10.1002/j.1460-2075.1989.tb03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voisard C, Rella M, Haas D. Conjugative transfer of plasmid RP1 to soil isolates of Pseudomonas fluorescens is facilitated by certain large RP1 deletions. FEMS Microbiol Lett. 1988;55:9–14. [Google Scholar]
- 55.Wagner A F V, Frey M, Neugebauer F A, Schäfer W, Knappe J. The free radical in pyruvate formate-lyase is located on glycine-734. Proc Natl Acad Sci USA. 1992;89:996–1000. doi: 10.1073/pnas.89.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wierenga R K, Terpstra P, Hol W G J. Prediction of the occurrence of the ADP-binding βαβ-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986;187:101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- 57.Winteler H V, Haas D. The homologous regulators ANR of Pseudomonas aeruginosa and FNR of Escherichia coli have overlapping but distinct specificities for anaerobically inducible promoters. Microbiology. 1996;142:685–693. doi: 10.1099/13500872-142-3-685. [DOI] [PubMed] [Google Scholar]
- 58.Wissing F. Cyanide formation from oxidation of glycine by a Pseudomonas species. J Bacteriol. 1974;117:1289–1294. doi: 10.1128/jb.117.3.1289-1294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wissing F. Cyanide production from glycine by a homogenate from a Pseudomonas species. J Bacteriol. 1975;121:695–699. doi: 10.1128/jb.121.2.695-699.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wissing F, Andersen K S. The enzymology of cyanide production from glycine by a Pseudomonas species. Solubilization of the enzyme. In: Vennesland B, Conn E E, Knowles C J, Westley J, Wissing F, editors. Cyanide in biology. London, England: Academic Press Ltd.; 1981. pp. 275–287. [Google Scholar]
- 61.Wissing F. Anaerobic column chromatography in the presence of detergents and its application to a bacterial HCN-producing enzyme. J Microbiol Methods. 1983;1:31–39. [Google Scholar]
- 62.Wu G, Hill S, Kelly M J S, Sawers G, Poole R K. The cydR gene product, required for regulation of cytochrome bd expression in the obligate aerobe Azotobacter vinelandii, is an Fnr-like protein. Microbiology. 1997;143:2197–2207. doi: 10.1099/00221287-143-7-2197. [DOI] [PubMed] [Google Scholar]
- 63.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 64.Ye R W, Haas D, Ka J-O, Krishnapillai V, Zimmermann A, Baird C, Tiedje J M. Anaerobic activation of the entire denitrification pathway in Pseudomonas aeruginosa requires Anr, an analog of Fnr. J Bacteriol. 1995;177:3606–3609. doi: 10.1128/jb.177.12.3606-3609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zanker H, Lurz G, Langridge U, Langridge P, Kreusch D, Schröder J. Octopine and nopaline oxidases from Ti plasmids of Agrobacterium tumefaciens: molecular analysis, relationship, and functional characterization. J Bacteriol. 1994;176:4511–4517. doi: 10.1128/jb.176.15.4511-4517.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zimmermann A, Reimmann C, Galimand M, Haas D. Anaerobic growth and cyanide synthesis of Pseudomonas aeruginosa depend on anr, a regulatory gene homologous with fnr of Escherichia coli. Mol Microbiol. 1991;5:1483–1490. doi: 10.1111/j.1365-2958.1991.tb00794.x. [DOI] [PubMed] [Google Scholar]