Abstract
Purpose
Erectile dysfunction (ED) is a common postoperative complication of pelvic surgery for which there is currently no effective treatment. This study investigated the therapeutic effects and potential mechanisms of adipose derived mesenchymal stem cells-derived mitochondria (ADSCs-mito) transplantation in a rat model of bilateral cavernous nerve injury (CNI) ED.
Materials and Methods
We isolated mitochondria from ADSCs and tested their quality. In vivo, twenty male Sprague Dawley rats were randomly divided into four groups: sham operation group and CNI groups that received intracavernous injection of either phosphate buffer solution, ADSCs-mito or ADSCs. Two weeks after therapy, the erectile function of the rats was evaluated and the penile tissues were harvested for histologic analysis and western blotting. In vitro, the apoptosis rate, reactive oxygen species (ROS), mitochondria derived active oxygen (mtROS) and adenosine triphosphate (ATP) levels were detected in corpus cavernosum smooth muscle cells (CCSMCs) after the incubation with ADSCs-mito. In addition, intercellular mitochondrial transfer was visualized by co-culture of ADSCs and CCSMCs.
Results
The ADSCs, ADSCs-mito and CCSMCs were isolated and identified successfully. ADSCs-mito transplantation notably restored the erectile function and smooth muscle content of CNI ED rats. Moreover, the levels of ROS, mtROS and cleaved-caspase 3 were reduced and the levels of superoxide dismutase and ATP were increased after ADSCs-mito transplantation. In CNI ED rats, the mitochondrial structure of cells in penile tissues was destroyed. ADSCs could transfer its own mitochondria to CCSMCs. Pre-treatment with ADSCs-mito could significantly decrease apoptosis rate, ROS levels and mtROS levels as well as restore the ATP level in CCSMCs.
Conclusions
ADSCs-mito transplantation significantly ameliorated ED induced by CNI, with similar potency to ADSCs treatment. The ADSCs-mito might exert their effects via anti-oxidative stress, anti-apoptosis and modulating energy metabolism of CCSMCs. Mitochondrial transplantation should be a promising therapeutic method for treating CNI ED in the future.
Keywords: Apoptosis, Erectile dysfunction, Mesenchymal stem cells, Mitochondria, Oxidative stress
INTRODUCTION
Prostate and rectal cancer are among the common cancers worldwide [1]. Pelvic surgery for prostate and rectal cancers often leads to sexual dysfunction [2,3], with an incidence of cavernous nerve injury induced erectile dysfunction (CNI ED) of more than 50% [4]. The main therapeutic approaches include phosphodiesterase type 5 inhibitors (PDE5Is), intracavernous injection therapy, and application of vacuum constriction devices, but these methods have achieved limited success [5,6]. New strategies for the treatment of CNI ED need to be investigated.
The pathophysiological mechanism underlying CNI ED has not been clarified. Apoptosis and oxidative stress often occur in CNI ED [7]. Moreover, proteins involved in energy metabolism and oxidative stress were found to be significantly modified after CNI [8]. Mitochondrial dysfunction has been shown to be associated with fibrosis [9]. There is growing evidence that mitochondrial dysfunction contributes to the development and progression of pulmonary fibrosis [10], renal fibrosis [11] and hepatic fibrosis [12]. A previous study found damage to the mitochondrial structure in the cavernous nerve and dorsal penile nerve after CN injury [13]. However, further research is needed to determine whether mitochondrial damage is involved in the pathological changes of corpus cavernosum (CC).
Mesenchymal stem cells (MSCs) have been used to treat various diseases and exert their therapeutic effects through different mechanisms including differentiation, cell fusion, paracrine secretion, and transfer of mitochondria [14]. The phenomenon of mitochondrial transfer has been observed in various tissues [15]. Mitochondrial transfer may be an important mechanism of MSCs exerting their repair effects. Adipose derived mesenchymal stem cells (ADSCs) have been used in the treatment of CNI ED with beneficial effects [16], although the underlying mechanism remains unclear and further research is required to determine whether the protective effects of ADSCs involve mitochondrial transfer. Furthermore, mitochondrial transplantation has shown promising results in the treatment of various diseases [17]. However, to our knowledge, the effects of mitochondrial transplantation on CNI ED have not been investigated to date.
In this study, we aimed to isolate ADSCs, ADSCs-derived mitochondria (ADSCs-mito), and corpus cavernosum smooth muscle cells (CCSMCs) and verify whether transplantation of ADSCs-mito could improve CNI ED and explore the mechanisms underlying its therapeutic effects. We hope that this research will contribute to the development of a novel approach for the treatment of CNI ED.
MATERIALS AND METHODS
1. Animals
Twenty male Sprague Dawley (SD) rats (8-week-old, 250 g) were randomly divided into four groups: Control, phosphate buffer solution (PBS), ADSCs-mito, and ADSCs group. Hence, we inserted the needle into the lateral cavernosum about 5 mm, withdrawn the needle slowly and made injection simultaneously. The construction methods of CNI model were described in the Supplementary Materials.
2. Ethics statement
The animal study protocols were approved by the Institutional Animal Care and Use Committee (IACUC) in South China Agricultural University (IACUC approval No.2021d134).
3. Cells isolation and the identification of ADSCs-mito
The primary ADSCs and CCSMCs were isolated from male SD rats (2-week-old, 100 g) following procedures described previously [18,19]. ADSCs were used for mitochondrial isolation by using a mitochondrial isolation kit (Solarbio) according to the instructions. JC-1 dye (Beyotime) and Mitotracker Red CMXRos (MTRC) (Invitrogen, Carlsbad, CA, USA) were used to identify the function of ADSCs-mito. Western blotting and transmission electron microscopy (TEM) were used to identify the purity and integrity of isolated ADSCs-mito.
4. ADSCs-mito uptake in vivo and in vitro
The ADSCs were incubated with 200 nM MTRC for 30 minutes before mitochondrial isolation, so the isolated ADSCs-mito was labeled with red signals. The CCSMCs were transfected with green fluorescent protein (GFP) to label with green signals. In vitro, CCSMCs were incubated with ADSCs-mito (50 µg/mL) at 37℃ for 24 hours. In vivo, the labeled ADSCs-mito (300 µg) was injected into the CC immediately after CNI and the frozen section of CC were prepared 24 hours postoperation. Smooth muscles were then immunostained with anti-alpha smooth muscle actin (anti-α-SMA) (1:200; Affinity) and the nuclei were stained with DAPI (Solarbio). The fluorescence signals were assessed by a fluorescence microscope (Olympus).
5. Co-culture of ADSCs and CCSMCs for mitochondrial transfer
To study mitochondrial transfer between cells, GFP-labeled CCSMCs were co-cultured with MTRC-labeled ADSCs. In direct co-culture condition, ADSCs and CCSMCs were mixed at a ratio of 1:1 and incubated for 24 hours. Similarly, in indirect condition, CCSMCs were co-cultured with ADSCs in a transwell plate for 24 hours. Finally, the cells were visualized under an inverted microscope (Olympus).
6. The detection of ADSCs-mito on functional improvement of CCSMCs in vitro
To study the effects of ADSCs-mito on CCSMCs, the CCSMCs were pre-treated with ADSCs-mito (50 µg/mL) for 24 hours and then treated with 600 µM H2O2 for 4 hours to induce cell injury. Specific assay kits were used to detect the levels of mitochondrial membrane potential (MMP), reactive oxygen species (ROS), adenosine triphosphate (ATP), mitochondria derived active oxygen (mtROS) and apoptosis rates (MMP assay kit with JC-1, ROS Assay Kit, Enhanced ATP Assay Kit: Beyotime; MitoSOX™ Red mitochondrial superoxide indicator: Invitrogen; Annexin V-FITC Apoptosis Detection Kit: Keygen Bio) in CCSMCs.
7. Transmission electron microscopy
Penile tissues were prepared following the methods previously described [13] for the ultrastructural analysis of the CC. The ultrathin sections were examined using a JEM-1400 electron microscope (JEOL).
8. Erectile function assessment
Two weeks after treatment, the intracavernous pressure (ICP) and the mean arterial pressure (MAP) of all rats were measured as described in our previous study [18]. BL-420s Biological Functional System (Taimeng Technology) was used to record the pressure curves. The ratios of the maximum ICP (ICPmax) and total ICP to MAP were used to evaluate erectile function.
9. The detection of superoxide and ATP levels in penile tissues
To detect the in situ superoxide levels in the CC, the frozen sections were incubated with dihydroethidium (10 µM; Beyotime) and MitoSOX (10 µM; Invitrogen). In addition, the proteins of the CC were obtained as soon as the animals were sacrificed, and the levels of superoxide dismutase (SOD) and ATP were measured by using a SOD test kit (Keygen Bio) and an enhanced ATP Assay Kit (Beyotime) following the manufacturer’s instructions.
10. Histological examination
The samples of CC were collected for immunofluorescence staining. The frozen sections were incubated with a primary antibody against anti-α-SMA (1:200; Affinity). Cy3-labeled goat anti-mouse IgG was used as secondary antibodies (1:500; Beyotime). Masson’s trichrome staining was performed to quantify the ratio of smooth muscle and collagen in the CC [18].
11. Western blotting
The CC proteins were collected for western blotting as described previously [18]. The membrane was incubated with primary antibodies against α-SMA (1:5,000; Affinity), cleaved-caspase3 (1:1,000; Affinity), GAPDH (1:1,000; Abmart) and β-actin (1:1,000; Affinity). After secondary body hybridization (1:5,000; Affinity), the bands were observed with an enhanced chemiluminescence substrate (Millipore Corp.).
12. Statistical analyses
The data were expressed as mean±standard error of the mean. All data obtained in this study were analyzed by GraphPad Prism version 9.0 (GraphPad). The differences among the groups were compared using analysis of variance and Newman-Keuls post-hoc analysis. Differences were considered significant at p<0.05.
RESULTS
1. Isolation and characterization of ADSCs and CCSMCs
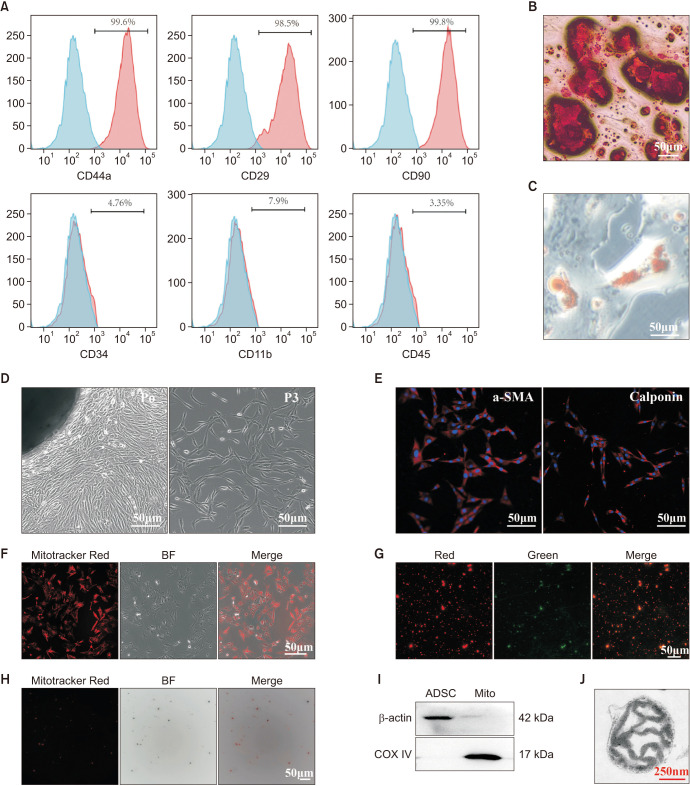
Primary ADSCs and CCSMCs were successfully isolated and cultured. The ADSCs expressed CD29, CD44a, and CD90, but not hematopoietic or endothelial markers such as CD11b, CD34, and CD45 (Fig. 1A). The ADSCs were positive for Alizarin Red staining after osteogenic induction (Fig. 1B) and Oil Red O staining after adipogenic induction (Fig. 1C). Similarly, primary CCSMCs could migrate from the CC and grow in a whirlpool-like pattern at passage 3 (Fig. 1D). The cultured cells were successfully identified by α-SMA and calponin (Fig. 1E). ADSCs and CCSMCs were isolated successfully.
Fig. 1. Characterization of ADSCs, CCSMCs and ADSCs-mito. (A) Flow cytometry showed that ADSCs were positive for mesenchymal stem cells markers (CD44a, CD29, CD90), but not for endothelial or hematopoietic markers (CD11b, CD34, CD45), blue means blank and red means positive percentage for the markers. (B) After induction, ADSCs showed typical osteocyte phenotype (Alizarin Red S staining). (C) After induction, ADSCs showed typical phenotypes of adipocytes (stained with Oil Red O), scale bar, 50 µm. (D) The CCSMCs migrated from penile tissues and grown in a whirlpool-like pattern after passaging, scale bar, 50 µm. (E) Identification of CCSMCs by immunofluorescence with anti-α-SMA and anti-calponin antibody, scale bar, 50 µm. (F) ADSCs were stained with a mitochondrial specific indicator, Mitotracker Red CMXRos. (G) ADSCs-mito was stained with Mitotracker Red CMXRos, scale bar, 50 µm. (H) ADSCs-mito was stained with JC-1, scale bar, 50 µm. (I) The purity of ADSCs-mito was confirmed by COX IV (a mitochondrial marker), ADSCs lysates were used as control. (J) Transmission electron microscopy of isolated ADSCs-mito, scale bar, 250 nm. ADSC: adipose derived mesenchymal stem cell, BF: bright field, CCSMCs: corpus cavernosum smooth muscle cells, ADSCs-mito: ADSCs-derived mitochondria, Mito: mitochondria, α-SMA: alpha smooth muscle actin.
2. Isolation and characterization of ADSCs-mito
Prior to ADSCs-mito transplantation, the quality of ADSC-mito was evaluated. After staining with MTRC, ADSCs showed obvious red fluorescence (Fig. 1F), indicating that ADSCs were suitable donor cells for mitochondrial isolation. After mitochondrial isolation, MTRC staining and JC-1 staining demonstrated that the isolated ADSC-mito were functional (Fig. 1G, 1H) as they had high membrane potential. The high expression of COX IV (mitochondrial maker) was observed in the isolated ADSCs-mito (Fig. 1I), indicating the ADSCs-mito was purified. TEM indicated that the morphology of the isolated ADSCs-mito was intact (Fig. 1J). The isolated ADSCs-mito was intact, functional, and purified.
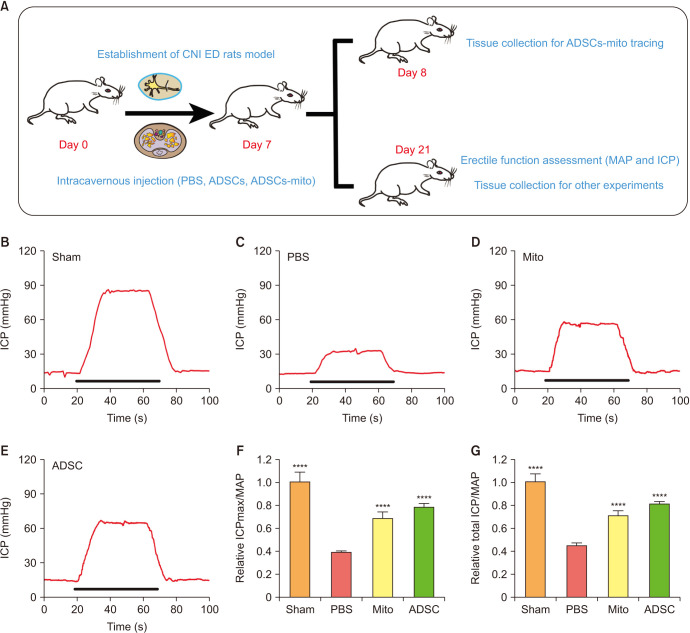
3. ADSCs-mito transplantation improved erectile function in CNI ED rats
We established a CNI ED rat model and assessed the therapeutical effects of ADSCs and ADSCs-mito as shown in the diagram (Fig. 2A). There was no significant difference in the MAP among the four groups (data not shown). The maximal ICP (ICPmax)/MAP and total ICP (area under the curve)/MAP in PBS group were decreased (Fig. 2B, 2C, 2F, 2G), although these increased after treatment with ADSCs or ADSCs-mito (Fig. 2D–2G), indicating that both ADSCs and ADSCs-mito transplantation may improve erectile function with comparable therapeutic effects.
Fig. 2. Transplantation of ADSCs-mito improved erectile function in CNI ED rats. (A) Diagram of study design and experimental flowchart. (B–E) Changes in ICP by electrical stimulation of the cavernous nerve in the sham, PBS, ADSCs-mito and ADSCs groups. (F, G) Maximum ICP to MAP ratio and total ICP to MAP ratio responses to electrostimulation in the four groups. Error bars: mean±standard deviation. ADSCs: adipose derived mesenchymal stem cells, ADSCs-mito: ADSCs-derived mitochondria, CNI ED: cavernous nerve injury induced erectile dysfunction, Mito: mitochondria, ICP: intracavernous pressure, MAP: mean arterial pressure, PBS: phosphate buffer solution. ****p<0.0001 comparison with PBS group.
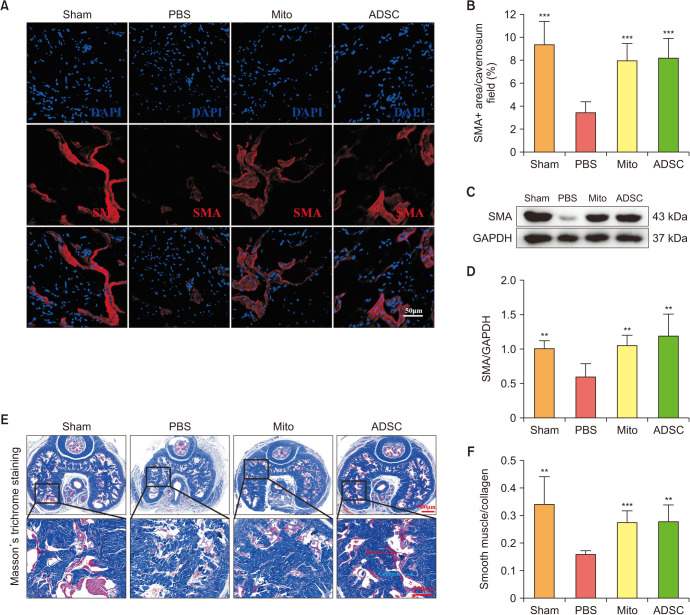
4. ADSCs-mito transplantation restored smooth muscle content in CC
After the evaluation of erectile function, the CC were collected for further analysis. After CNI, the expression of α-SMA decreased, although ADSCs or ADSCs-mito transplantation significantly restored α-SMA expression, indicating the recovery of smooth muscle content (Fig. 3A–3D). Similarly, ADSCs and ADSCs-mito could increase the ratio of smooth muscle to collagen in the CC too (Fig. 3E, 3F). Both ADSCs and ADSCs-mito could improve the smooth muscle content in the CC with no significant differences between the therapies.
Fig. 3. ADSCs-mito transplantation increased smooth muscle content in corpus cavernosum. (A) Immunofluorescence staining of SMA in penile tissues of sham, PBS, ADSCs-mito and ADSCs groups, scale bar, 50 µm. (B) Quantitative analysis of the SMA immunofluorescence positive area in the four groups. (C, D) The expression of SMA protein in penile tissues from the four groups was determined by western blotting. (E) Masson’s trichrome staining of the penile tissues used to assess the smooth muscle/collagen ratio in the four groups, smooth muscle and collagen were stained red and blue, respectively, scale bar, 500 µm and 200 µm. (F) Quantitative analysis of the Masson’s trichrome staining results. Error bars: mean±standard deviation. ADSCs: adipose derived mesenchymal stem cells, ADSCs-mito: ADSCs-derived mitochondria, Mito: mitochondria, SMA: smooth muscle actin, PBS: phosphate buffer solution. **p<0.01, ***p<0.001 comparison with PBS group.
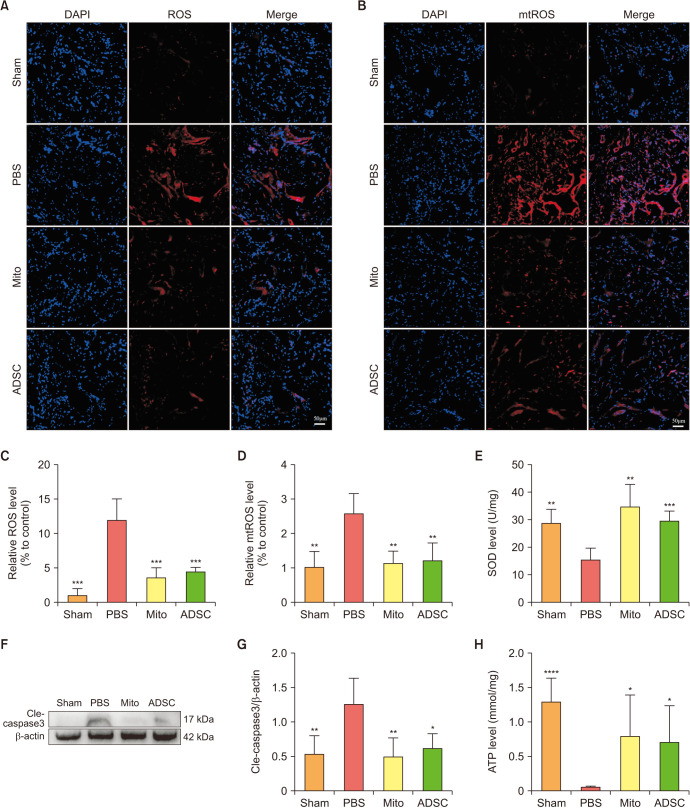
5. ADSCs-mito transplantation attenuated oxidative stress and enhanced anti-oxidant capacity in vivo
To explore oxidative stress levels in the CC, we investigated the fluorescence intensity of cellular and mitochondrial superoxide levels. The results showed that both of them were higher in the PBS group than those in the sham group, and significantly lower in both ADSCs and ADSCs-mito group (Fig. 4A–4D), indicating that ADSCs or ADSCs-mito transplantation could attenuate oxidative stress. Furthermore, after treatment with ADSCs or ADSCs-mito transplantation, the SOD levels were increased (Fig. 4E). These results show that ADSCs-mito was as effective as ADSCs in the treatment of anti-oxidative stress in CNI ED rats.
Fig. 4. ADSCs-mito transplantation attenuated oxidative stress and apoptosis in corpus cavernosum. (A, C) Immunofluorescent staining of ROS in penile tissue from the sham, PBS, ADSCs-mito, and ADSCs groups, scale bar, 50 µm. (B, D) Immunofluorescent staining of mtROS in penile tissue from the four groups, scale bar, 50 µm. (E) The superoxide dismutase levels in penile tissues were detected in the four groups. (F, G) The expression of cleaved-caspase 3 protein in penile tissues from the four groups was determined by western blot. (H) The adenosine triphosphate levels in penile tissues were detected in four groups. Error bars: mean±standard deviation. ADSCs: adipose derived mesenchymal stem cells, ADSCs-mito: ADSCs-derived mitochondria, Mito: mitochondria, ROS: reactive oxygen species, mtROS: mitochondria derived reactive oxygen species, PBS: phosphate buffer solution. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 comparison with the PBS group.
6. ADSCs-mito transplantation attenuated apoptosis and maintained ATP levels in vivo
To explore apoptosis levels in the CC, we investigated the activation of caspase 3. As shown in Fig. 4, the expression of cleaved-caspase3 was higher in the PBS group, although it was significantly decreased in both the ADSCs and ADSCs-mito groups (Fig. 4F, 4G), indicating the treatment with ADSCs and ADSCs-mito could protect CCSMCs from the caspase 3-dependent apoptosis pathway. After CNI, the ATP levels in CC were decreased, yet ADSCs or ADSCs-mito transplantation could restore ATP levels (Fig. 4H). Treatment with ADSCs and ADSCs-mito transplantation could attenuate apoptosis levels and maintain ATP levels, with no significant difference between the treatments.
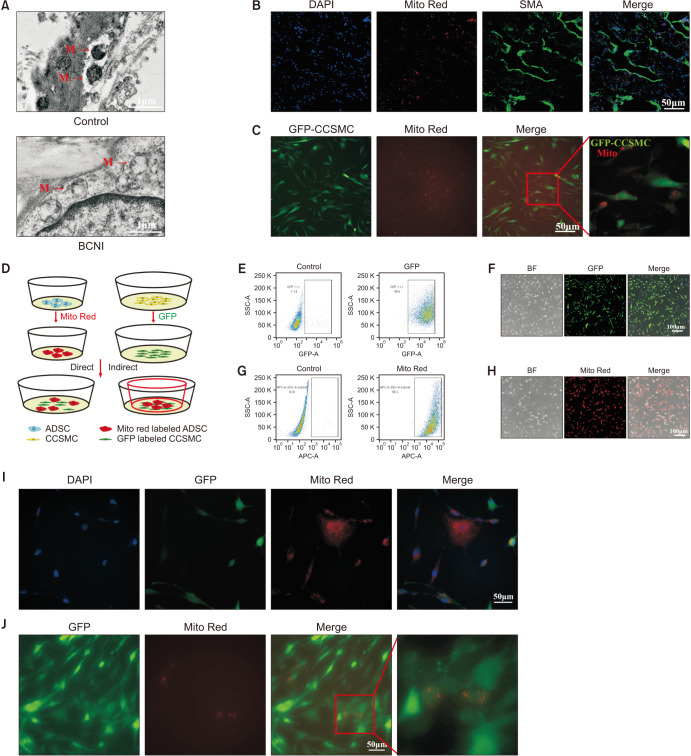
7. Mitochondrial structural damage occurred in CC of CNI ED rats
We used TEM to observe the ultrastructure of the CC. The mitochondrial structure of the CC in CNI ED rats changed significantly. We observed swelling of the mitochondria and the mitochondria matrix particles were disrupted significantly (Fig. 5A).
Fig. 5. Adipose derived mesenchymal stem cells (ADSCs) exerted therapeutic effects through mitochondria transfer. (A) Transmission electron microscopy of penile tissues in both sham rats and cavernous nerve injury (CNI) rats two weeks after the CNI erectile dysfunction (ED) rat models were established, scale bar, 1 µm. (B, C) ADSCs-derived mitochondria (ADSCs-mito) uptake in vivo and in vitro. (B) The internalization of isolated ADSCs-mito into penile tissues was detected in 24 hours after ADSCs-mito transplantation, scale bar, 50 µm. (C) ADSCs-mito was internalized into CCSMCs after co incubation for 24 hours, scale bar, 50 µm. (D) The schematic diagram of co-culture system of ADSCs and CCSMCs. (E, F) The primary CCSMCs were successfully transfected with GFP and shown as green cells, scale bar, 100 µm. (G, H) The ADSCs were successfully stained with specific mitochondrial probe Mitotracker Red (Mito Red) CMXRos and shown as red cells, scale bar, 100 µm. (I, J) Imaging of mitochondrial transfer from ADSCs to CCSMCs in both direct and indirect co-culture systems, scale bar, 50 µm. (I) In the direct co-culture system, ADSCs (red) were co-cultured with CCSMCs (green) for 24 hours, the presence of red signals in green cells indicated the transfer of ADSCs-mito. (J) The red signals were also detected in CCSMCs after indirect co-culture system by a transwell plate for 24 hours. SMA: smooth muscle actin, GFA: green fluorescent protein, BF: bright field, CCSMCs: corpus cavernosum smooth muscle cells, BCNI: bilateral cavernous nerve injury.
8. ADSCs-mito was internalized into target cells both in vitro and in vivo
The verify whether ADSCs-mito could be internalized into target cells, the ADSCs-mito was labeled with MTRC and tracked. In vivo, after 24 hours following mitochondrial transplantation, the ADSCs-mito internalization to the target cells was confirmed by emission of red fluorescent signals (Fig. 5B). Based on the histological assessments, the green CCSMCs containing red stained mitochondria within their cytoplasm (Fig. 5B). In vitro, the isolated ADSCs-mito could also been internalized into the green CCSMCs (Fig. 5C). These results indicated that the ADSCs-mito could be internalized into CCSMCs successfully both in vivo and in vitro.
9. Mitochondria could transfer from ADSCs to CCSMCs
To investigate whether ADSCs could transfer its mitochondria to CCSMCs, ADSCs and CCSMCs were labeled with red and green color, and then mixed in one plate in direct co-culture system, or in an indirect system (Fig. 5D). CCSMCs were transfected with GFP (Fig. 5E, 5F) and ADSCs were pre-stained with MTRC (Fig. 5G, 5H), almost all ADSCs and CCSMCs were labeled successfully. In direct co-culture condition, mitochondria were transferred from ADSCs to CCSMCs as evidenced by red fluorescence mitochondria located in the cytosol of the recipient green cells (Fig. 5I). Similarly, in indirect co-culture conditions, the fluorescence mitochondria were also found in green CCSMCs (Fig. 5J). These results revealed that mitochondria could transfer from ADSCs to CCSMCs.
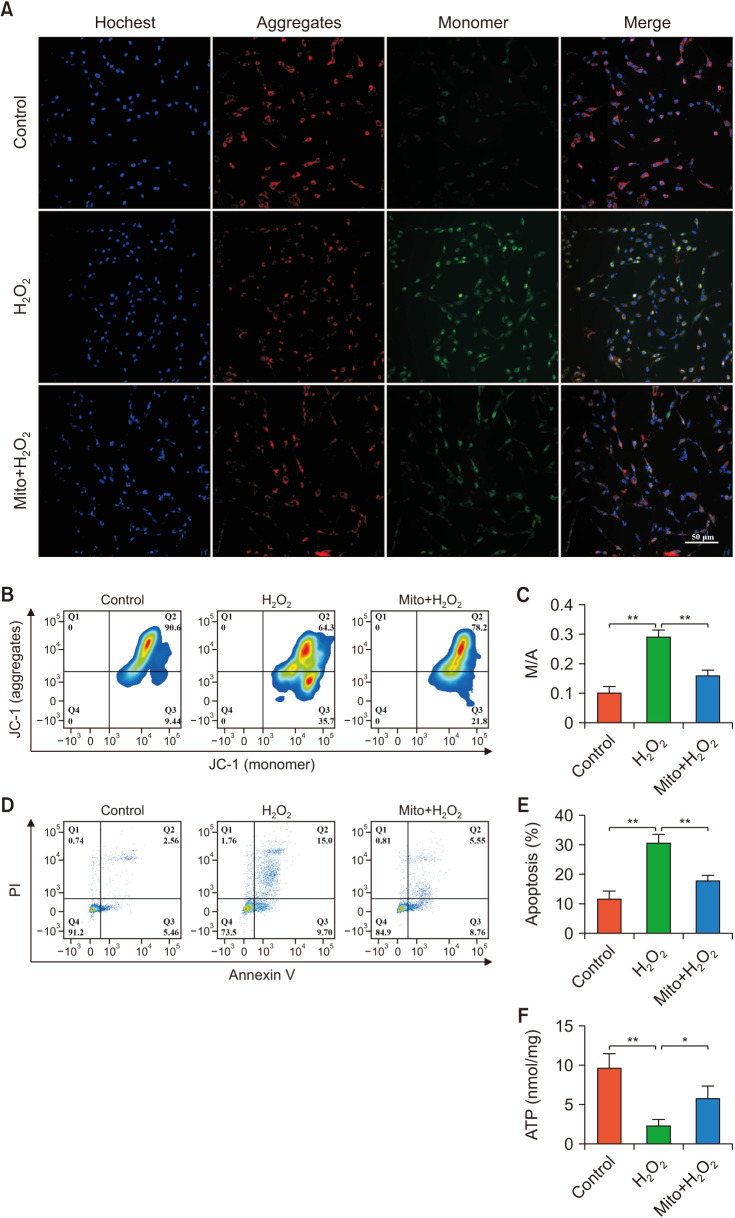
10. ADSCs-mito transplantation maintained MMP of CCSMCs in vitro
To examine whether ADSCs-mito could protect CCSMCs from H2O2-induced cell injury, CCSMCs were cultured in the absence or presence of ADSC-mito and treated with H2O2 to induce cell injury. First, we analyzed the MMP using immunofluorescence staining and flow cytometry. The cells in the control group were mainly red fluorescent, while the red fluorescence in the H2O2 group was significantly weakened, accompanied by a significant increase in green fluorescence, although ADSCs-mito pre-treatment could reverse these changes (Fig. 6A). Similarly, the results of flow cytometry showed that ADSCs-mito pre-treatment restored the MMP of CCSMCs (Fig. 6B, 6C). ADSCs-mito pretreatment is beneficial to maintain the MMP level of injured CCSMCs.
Fig. 6. ADSCs-derived mitochondria (ADSCs-mito) attenuated apoptosis in corpus cavernosum smooth muscle cells (CCSMCs). (A–C) The mitochondrial membrane potential of CCSMCs was maintained by pre-treatment with ADSCs-mito. (A) Images of JC-1 in CCSMCs from the control, H2O2, and Mito+H2O2 groups, aggregate form of the mitochondria (red), monomer form of the mitochondria (green), scale bar, 50 µm. (B) Flow cytometric analysis of JC-1 in the three groups. (C) Quantitative analysis of monomer to aggregate ratio results in the three groups. (D, E) Flow cytometric analysis of apoptosis in CCSMCs in the three groups. (F) The adenosine triphosphate levels in CCSMCs were detected in the three groups. Error bars: mean±standard deviation; scale bar, 50 µm. Mito: mitochondria, ADSCs: adipose derived mesenchymal stem cells. *p<0.05, **p<0.01.
11. ADSCs-mito transplantation enhanced anti-apoptosis ability and maintained ATP levels of CCSMCs in vitro
CCSMCs were stained with Annexin-V-propidium iodide double. After treatment with H2O2, the apoptosis rate of CCSMCs increased significantly, although ADSCs-mito pre-treatment could decrease the apoptosis rate (Fig. 6D, 6E). Therefore, the ADSCs-mito was capable of inhibiting apoptosis in CCSMCs. Similarly, ADSCs-mito pre-treatment could restore the ATP levels in CCSMCs (Fig. 6F), indicating that ADSCs-mito could prevent ATP depletion of injured CCSMCs.
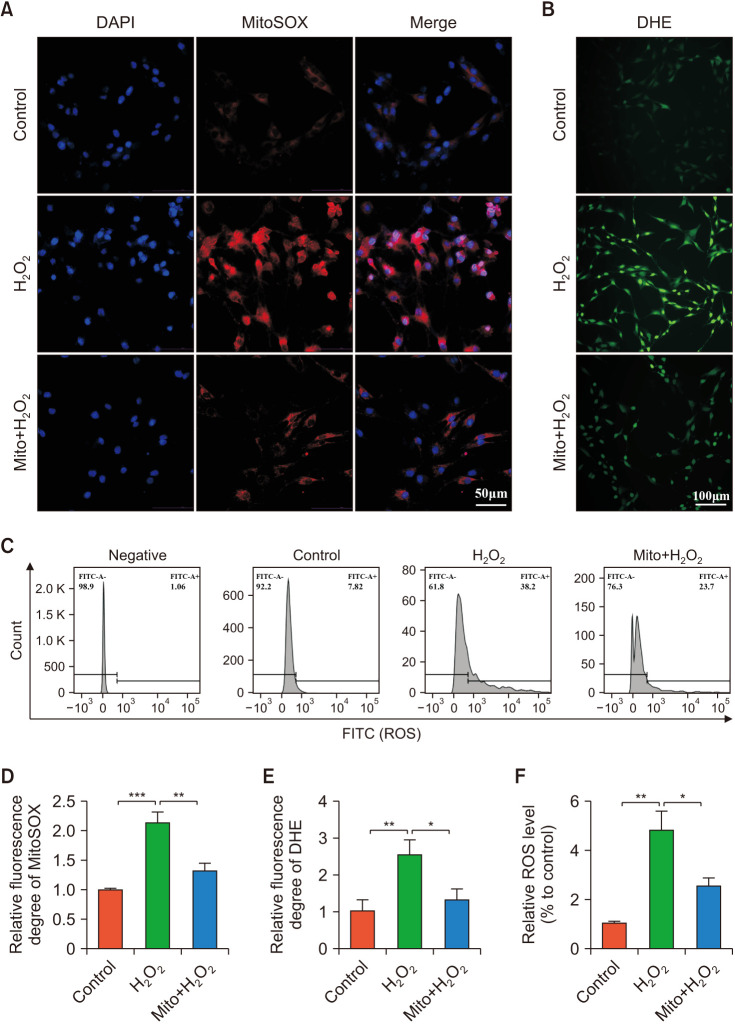
12. ADSCs-mito transplantation enhanced anti-oxidative stress ability of CCSMCs in vitro
The levels of cellular ROS and mtROS were detected by immunofluorescence staining and flow cytometry. After H2O2 treatment, both cellular ROS and mtROS increased significantly (Fig. 7). Compared to the control group, the cellular ROS in the H2O2-treated group increased significantly, the cellular ROS almost decreased to the pre-damage state after pre-treatment with ADSCs-mito (Fig. 7B, 7C, 7E, 7F). Similarly, ADSCs-mito pre-treatment could decrease the fluorescence intensities of mtROS in CCSMCs (Fig. 7A, 7D). ADSCs-mito transplantation was able to reduce oxidative stress in CCSMCs induced by H2O2 treatment.
Fig. 7. ADSCs-derived mitochondria (ADSCs-mito) attenuated oxidative stress in corpus cavernosum smooth muscle cells (CCSMCs). (A) Representative mitoSOX staining in the control, H2O2, and Mito+H2O2 groups, scale bar, 50 µm. (B) Representative DHE staining in each group, scale bar, 100 µm. (C) Flow cytometric analysis of ROS levels in each group. (D–F) Quantitative analysis of fluorescence intensity of mtROS and ROS in the three groups. Error bars: mean±standard deviation. ADSCs: adipose derived mesenchymal stem cells, Mito: mitochondria, ROS: reactive oxygen species, mtROS: mitochondria derived reactive oxygen species. *p<0.05, **p<0.01, ***p<0.001.
DISCUSSION
Sexual problems after pelvic surgery are common and multifactorial. CNI is coupled with penile hypoxia responses, apoptosis, oxidative stress, and fibrosis [5]. Further researches are required to elucidate the underlying pathophysiological mechanism of CNI ED. Stem cells therapy represents a promising option for the treatment of CNI ED [16], although its underlying mechanisms remain unclear. This study found that the mitochondrial structure of the CCSMCs was damaged and the mitochondria could transfer from ADSCs to CCSMCs. ADSCs may exert their therapeutic effects by mitochondrial transfer and ADSCs-mito transplantation may ameliorate CNI ED.
Mitochondrial dysfunction may be involved in a variety of diseases [20]. Studies have suggested that mitochondrial damage contributes to the pathogenesis of organic ED [21,22]. CNI results in serious damage to the structure of mitochondria in the cavernous nerve and dorsal penile nerve [23]. However, it is unclear whether mitochondrial dysfunction occurs in the CC after CNI. We found that the structure of the mitochondria in the CC was damaged, with mitochondrial swelling, broken mitochondrial membrane, irregular crista arrangement, and vacuolar structure. These results indicated an association between mitochondrial dysfunction and CNI ED.
Stem cells therapy may be a promising approach for ED therapy [24,25,26], although the specific repair mechanisms remain unclear. We found that ADSCs could transfer their mitochondria to CCSMCs in vitro. The phenomenon of mitochondrial transfer has been previously reported [27]. Following the first reports on intercellular mitochondria transfer, numerous studies have demonstrated that MSCs donate their healthy mitochondria to rescue the injured cell [15]. As mitochondrial damage may be involved in the pathophysiology of CNI ED, we postulated that ADSCs may exert their beneficial effects through mitochondrial transfer to CCSMCs. Our results show that ADSCs-mito transplantation was effective in ameliorating ED, with similar therapeutic potency to ADSCs, indicating that mitochondria may be involved in the mechanism underlying the beneficial effects of ADSCs transplantation therapy in CNI ED rats. In our previous study, we found that both MSCs and MSCs-derived exosomes therapy can improve CNI ED, and the latter showed a comparable effect to that observed in the MSCs treatment group [18]. This indicates that cell-free therapy (MSCs-derived exosomes therapy) has great potential in the treatment of CNI ED to replace cell therapy However, in this study, we focused on the mechanism of the therapeutic effect of ADSCs on CNI ED, and we found that ADSCs may play a role by transferring their mitochondria to CCSMCs. Understanding the mechanism underlying the therapeutic effect of MSCs in the treatment of ED will help its clinical transformation in the future.
In our study, the ADSCs-mito could be successfully internalized into CCSMCs and penile tissues, as previously reported [28]. Many beneficial effects of the isolated mitochondria have been reported in different injury models. In a nerve crush injury model, mitochondrial transplantation could prevent denervated muscle atrophy and augment nerve regeneration by reducing oxidative stress in denervated muscle [29]. After mitochondrial transplantation, the dysregulation of oxidative stress, apoptosis and MMP were attenuated in vitro, and inflammation was reduced in a tendinopathy rat model [28]. In our study, apoptosis and oxidative stress in CCSMCs were decreased after pre-treatment with ADSCs-mito. Similarly, ADSCs-mito could attenuate apoptosis and oxidative stress in CC of CNI ED rats. The anti-oxidative pathways may be triggered by the transplanted ADSCs-mito as the increase of SOD levels. Furthermore, ADSCs-mito could maintain the ATP content, which is consistent with previous report [30]. These findings indicate that the ADSCs-mito may act through many mechanisms including anti-oxidative stress, anti-apoptosis and regulating energy metabolism in CNI ED rat model.
CONCLUSIONS
To our knowledge, this is the first study to demonstrate the therapeutic effects of ADSCs-mito transplantation on CNI ED and that mitochondrial damage may be involved in the pathophysiological mechanism of CNI ED. Furthermore, this study found that ADSCs may exert their therapeutic effects by mitochondria transfer. Regarding the underlying mechanism for the therapeutic effects of ADSCs-mito, this may involve modulating oxidative stress, apoptosis, and energy metabolism. Mitochondrial transplantation appears to be a promising strategy for the treatment of CNI ED.
Acknowledgements
None.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This study was supported by grants from National Natural Science Foundation of China (81971378), Science and Technology Planning Project of Guangdong Province (2019B030316011) and Science and Technology Planning Project of Guangzhou City (201903010027).
- Conceptualization: JZ, HW.
- Data curation: JZ, PC.
- Formal analysis: JZ, PC.
- Funding acquisition: HW.
- Investigation: JZ, HW.
- Methodology: JZ, ZC, HW.
- Project administration: JZ, PC.
- Resources: HW.
- Software: HW.
- Supervision: ZC, WY.
- Validation: JZ, PC.
- Visualization: JZ, PC.
- Writing – original draft: JZ.
- Writing – review & editing: all authors.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.220233.
Cavernous nerve injury surgery
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Mercieca-Bebber R, Eggins R, Brown K, Gebski VJ, Brewer K, Lai L, et al. Patient-reported bowel, urinary, and sexual outcomes after laparoscopic-assisted resection or open resection for rectal cancer: the Australasian laparoscopic cancer of the rectum randomized clinical trial (ALaCart) Ann Surg. 2023;277:449–455. doi: 10.1097/SLA.0000000000005412. [DOI] [PubMed] [Google Scholar]
- 3.Ti Y, Yang M, Chen X, Zhang M, Xia J, Lv X, et al. Comparison of the therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells and adipose-derived stem cells on erectile dysfunction in a rat model of bilateral cavernous nerve injury. Front Bioeng Biotechnol. 2022;10:1019063. doi: 10.3389/fbioe.2022.1019063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell JD, Burnett AL. Neuroprotective and nerve regenerative approaches for treatment of erectile dysfunction after cavernous nerve injury. Int J Mol Sci. 2017;18:1794. doi: 10.3390/ijms18081794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magheli A, Burnett AL. Erectile dysfunction following prostatectomy: prevention and treatment. Nat Rev Urol. 2009;6:415–427. doi: 10.1038/nrurol.2009.126. [DOI] [PubMed] [Google Scholar]
- 6.Capogrosso P, Salonia A, Briganti A, Montorsi F. Postprostatectomy erectile dysfunction: a review. World J Mens Health. 2016;34:73–88. doi: 10.5534/wjmh.2016.34.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Meng XH, Zhang QJ, Wang YM, Chen C, Wang YC, et al. Losartan improves erectile function through suppression of corporal apoptosis and oxidative stress in rats with cavernous nerve injury. Asian J Androl. 2019;21:452–459. doi: 10.4103/aja.aja_8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Gao X, Pang J, Zhang Y, Wang K, Fang Y, et al. Proteomic analysis of rat penile tissue in a model of erectile dysfunction after radical prostatectomy. BJU Int. 2007;99:1500–1505. doi: 10.1111/j.1464-410X.2007.06775.x. [DOI] [PubMed] [Google Scholar]
- 9.Hsu CH, Roan JN, Fang SY, Chiu MH, Cheng TT, Huang CC, et al. Transplantation of viable mitochondria improves right ventricular performance and pulmonary artery remodeling in rats with pulmonary arterial hypertension. J Thorac Cardiovasc Surg. 2022;163:e361–e373. doi: 10.1016/j.jtcvs.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Bueno M, Calyeca J, Rojas M, Mora AL. Mitochondria dysfunction and metabolic reprogramming as drivers of idiopathic pulmonary fibrosis. Redox Biol. 2020;33:101509. doi: 10.1016/j.redox.2020.101509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miao J, Liu J, Niu J, Zhang Y, Shen W, Luo C, et al. Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell. 2019;18:e13004. doi: 10.1111/acel.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:14205–14218. doi: 10.3748/wjg.v20.i39.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye M, Zhao F, Ma K, Zhou K, Ma J, Fu H, et al. Enhanced effects of salidroside on erectile function and corpora cavernosa autophagy in a cavernous nerve injury rat model. Andrologia. 2021;53:e14044. doi: 10.1111/and.14044. [DOI] [PubMed] [Google Scholar]
- 14.Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7:125. doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomzikova MO, James V, Rizvanov AA. Mitochondria donation by mesenchymal stem cells: current understanding and mitochondria transplantation strategies. Front Cell Dev Biol. 2021;9:653322. doi: 10.3389/fcell.2021.653322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matz EL, Terlecki R, Zhang Y, Jackson J, Atala A. Stem cell therapy for erectile dysfunction. Sex Med Rev. 2019;7:321–328. doi: 10.1016/j.sxmr.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Gollihue JL, Rabchevsky AG. Prospects for therapeutic mitochondrial transplantation. Mitochondrion. 2017;35:70–79. doi: 10.1016/j.mito.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouyang X, Han X, Chen Z, Fang J, Huang X, Wei H. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. Stem Cell Res Ther. 2018;9:246. doi: 10.1186/s13287-018-1003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang W, Chen Z, Ma X, Ouyang X, Fang J, Wei H. Co-overexpression of VEGF and GDNF in adipose-derived stem cells optimizes therapeutic effect in neurogenic erectile dysfunction model. Cell Prolif. 2020;53:e12756. doi: 10.1111/cpr.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roushandeh AM, Kuwahara Y, Roudkenar MH. Mitochondrial transplantation as a potential and novel master key for treatment of various incurable diseases. Cytotechnology. 2019;71:647–663. doi: 10.1007/s10616-019-00302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang MG, Wang XJ, Shen ZJ, Gao PJ. Long-term oral administration of 5α-reductase inhibitor attenuates erectile function by inhibiting autophagy and promoting apoptosis of smooth muscle cells in corpus cavernosum of aged rats. Urology. 2013;82:743.e9–743.e15. doi: 10.1016/j.urology.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 22.Mostafa ME, Senbel AM, Mostafa T. Effect of chronic low-dose tadalafil on penile cavernous tissues in diabetic rats. Urology. 2013;81:1253–1259. doi: 10.1016/j.urology.2012.12.068. [DOI] [PubMed] [Google Scholar]
- 23.Wu YN, Liao CH, Chen KC, Chiang HS. Dual effect of chitosan activated platelet rich plasma (cPRP) improved erectile function after cavernous nerve injury. J Formos Med Assoc. 2022;121(1 Pt 1):14–24. doi: 10.1016/j.jfma.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Ryu JK, Kim DH, Song KM, Ryu DS, Kim SN, Shin DH, et al. Intracavernous delivery of clonal mesenchymal stem cells rescues erectile function in the streptozotocin-induced diabetic mouse. Andrology. 2016;4:172–184. doi: 10.1111/andr.12138. [DOI] [PubMed] [Google Scholar]
- 25.Ryu JK, Kim DH, Song KM, Yi T, Suh JK, Song SU. Intracavernous delivery of clonal mesenchymal stem cells restores erectile function in a mouse model of cavernous nerve injury. J Sex Med. 2014;11:411–423. doi: 10.1111/jsm.12380. [DOI] [PubMed] [Google Scholar]
- 26.You D, Jang MJ, Lee J, Jeong IG, Kim HS, Moon KH, et al. Periprostatic implantation of human bone marrow-derived mesenchymal stem cells potentiates recovery of erectile function by intracavernosal injection in a rat model of cavernous nerve injury. Urology. 2013;81:104–110. doi: 10.1016/j.urology.2012.08.046. [DOI] [PubMed] [Google Scholar]
- 27.Court AC, Le-Gatt A, Luz-Crawford P, Parra E, Aliaga-Tobar V, Batiz LF, et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020;21:e48052. doi: 10.15252/embr.201948052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JM, Hwang JW, Kim MJ, Jung SY, Kim KS, Ahn EH, et al. Mitochondrial transplantation modulates inflammation and apoptosis, alleviating tendinopathy both in vivo and in vitro. Antioxidants (Basel) 2021;10:696. doi: 10.3390/antiox10050696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheu ML, Shen CC, Tsou HK, Yang MY, Su HL, Sheehan J, et al. Dual regeneration of muscle and nerve by intramuscular infusion of mitochondria in a nerve crush injury model. Neurosurgery. 2021;89:E49–E59. doi: 10.1093/neuros/nyab105. [DOI] [PubMed] [Google Scholar]
- 30.Kim MJ, Hwang JW, Yun CK, Lee Y, Choi YS. Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function. Sci Rep. 2018;8:3330. doi: 10.1038/s41598-018-21539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cavernous nerve injury surgery
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.