Abstract
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a common and non-lethal urological condition with painful symptoms. The complexity of CP/CPPS’s pathogenesis and lack of efficient etiological diagnosis results in incomplete treatment and recurrent episodes, causing long-term mental and psychological suffering in patients. Recent findings indicate that the autonomic nervous system involves in CP/CPPS, including sensory, sympathetic, parasympathetic, and central nervous systems. Neuro-inflammation and sensitization of sensory nerves lead to persistent inflammation and pain. Sympathetic and parasympathetic alterations affect the cardiovascular and reproductive systems and the development of prostatitis. Central sensitization lowers pain thresholds and increases pelvic pain perception in chronic prostatitis. Therefore, this review summarized the detailed processes and mechanisms of the critical role of the autonomic nervous system in developing CP/CPPS. Furthermore, it describes the neurologically relevant substances and channels or receptors involved in this process, which provides new perspectives for new therapeutic approaches to CP/CPPS.
Keywords: Autonomic nervous system, Neuropeptides, Pelvic pain, Prostatitis
INTRODUCTION
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a chronic inflammatory disease of prostatic glands with dysuria and pain in pelvic areas. CP/CPSS may lead to tumors, affect fertility, and cause physical and mental stress to human life. Patients with CP/CPPS experience chronic pain in the pelvic, perineal, scrotal, rectal, testicular, penile, and lower back abdomen areas. This symptom is accompanied by obstructive or irritative voiding, erectile dysfunction, and psychosomatic disorders [1,2]. Prostatitis includes infectious and asymptomatic prostatitis and CP/CPPS [3]. CP/CPPS is the most prevalent, accounting for about 90%–95% of cases [4]. The pathogenesis of CP/CPPS is complex. There is no efficient etiological diagnosis method, which leads to ineffective treatment and reduplicated symptoms. Therefore, clarification of the pathogenesis of prostatitis is crucial in future prostatitis management.
Currently, the pathogenesis of CP/CPPS involves immunological, endocrine, and psychological etiologies and autonomic nervous systems [5]. The autonomic nervous system seems to be the link between them. The autonomic nervous system comprises sensory, sympathetic, and parasympathetic nerves and the central nervous system (CNS). Sensory nerves are related to neuro-inflammation. The persistence of chronic pelvic pain and its sensitization might also be responsible for recurrent conditions and prolonged pain in chronic prostatitis [6,7,8]. Research indicated that the autonomic nervous system regulates smooth muscles of the cardiovascular system and prostatic glandular secretion; therefore, the sympathetic and parasympathetic nerves are closely related to CP/CPPS [9].
Moreover, the cardiovascular system and male reproductive function are altered in CP/CPPS as well as patients with vasogenic impotence have autonomic dysfunction [10]. In addition, some studies elucidated that central sensitization also played an essential role in developing and maintaining chronic pain in the case of perpetual pain without inflammatory conditions [6]. Therefore, this review collated and described the specific processes and mechanisms of the autonomic nervous system in developing CP/CPPS. Furthermore, this review illustrates the neurologically-relevant substances and channels or receptors involved in this process.
GENERAL ANATOMY OF CELL COMPOSITION AND AUTONOMIC NERVES IN PROSTATES
1. Prostate function and cell composition
The prostate is the largest male auxiliary reproductive organ. The prostate secretes fluids that nourish and protects the sperm. During ejaculation, the prostate squeezes this fluid into the urethra and sperm and excretes them as semen.
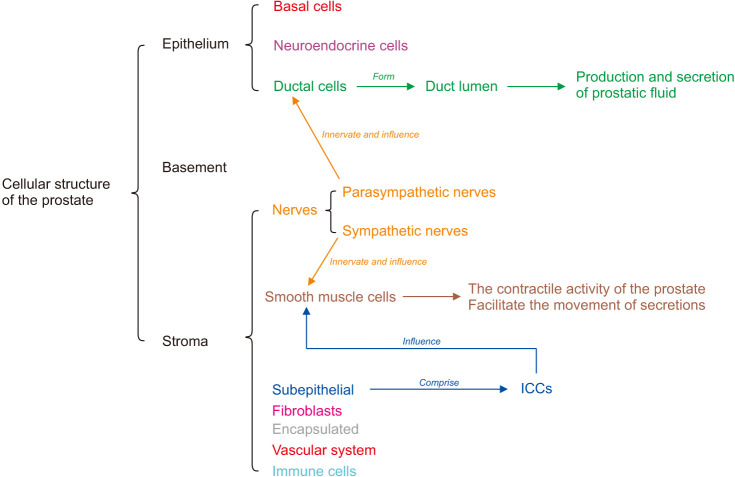
The cellular structure of the prostate tissue is divided into three parts: the epithelium, the basement membrane, and the stroma. The epithelium comprises basal, neuroendocrine, and ductal cells. The ductal cells form the duct lumen and are responsible for the prostate’s production and secretion of fluid that nourishes and protects the sperm. The parasympathetic nerves innervate the cells. The basement membrane comprises the extracellular matrix secreted by the basal epithelial cells. The stroma comprises subepithelial mesenchymal cells, smooth muscle cells, encapsulated cells, immune cells, fibroblasts, nerve, and vascular systems. The subepithelial interstitial cells are located near the basement membrane and between the bundles of smooth muscle cells, also known as interstitial cells of the prostate. In addition, interstitial cells of the Cajal (ICCs) are prostatic interstitial cells involved in the contractile activity of smooth muscle cells. These smooth muscle cells are involved in the prostrate’s contractile activity. They facilitate the movement of secretions from the ductal structures to the proximal urethra during ejaculation, which is innervated by sympathetic nerves (Fig. 1) [11,12,13,14,15,16,17].
Fig. 1. General anatomy of cell composition in prostates. The cellular structure of the prostate tissue is divided into three parts: the epithelium, the basement, and the stroma. The epithelium includes ductal cells, which form the lumen of duct production and participate in the secretion of fluid that nourishes and protects the sperm. The parasympathetic nerves innervate the duct cells. The basement membrane consists of the extracellular matrix secreted by epithelial cells. The stroma comprises smooth muscle cells, interstitial cells of the Cajal (ICCs), nerves, blood vessels, and immune cells. The smooth muscle cells are involved in the contractile activity of the prostate. They facilitate the movement of secretions from the ductal structures to the proximal urethra during ejaculation, which is innervated by sympathetic nerves.
2. Sensory nerve
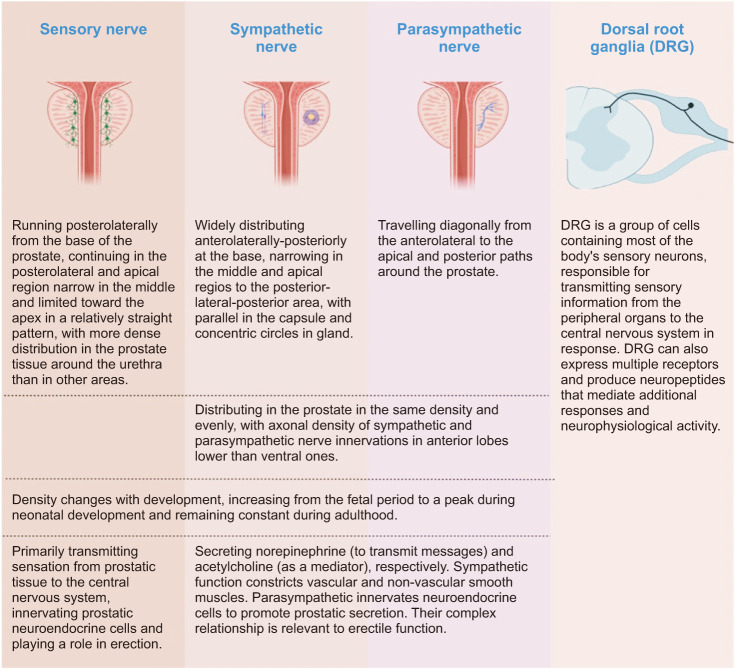
The sensory nerve primarily transmits sensation from prostatic tissue to the CNS. In addition, it innervates prostatic neuroendocrine cells and plays a role in erection [12,13]. The distribution of sensory nerves on the prostrate surface runs posterolaterally from the base of the prostate. It continues in the posterolateral and apical regions, narrow in the middle and limited toward the apex in a relatively straight pattern [13]. The distribution density in the prostate varies, with more dense distribution in the prostate tissue around the urethra than in other areas [12]. In addition, the density of sensory nerve growth changes according to the period of development, increasing during the fetal stage, peaking during neonatal development, and remaining constant during adulthood [12]. These sensory nerves are functionally connected to the spinal cord through the dorsal root ganglion (DRG) structure, traveling to the brain to produce sensations through information integration. The DRG is a group of cells containing most of the body’s sensory neurons. It is responsible for transmitting sensory information from the peripheral organs to the CNS in response [14]. DRG can also express multiple receptors and produce neuropeptides that mediate additional responses and neurophysiological activity [15].
Interestingly, the sensory nerves of the pelvic organs are generally accompanied by sympathetic and parasympathetic nerves, including prostates, in the sensory passway. These originate in the inferior hypogastric nerve (pelvic nerve) and connect to the spinal cord [16,17]. The spinal cord segments controlling prostate sensation are T13-L1 and L5-S2 [18]. Prostatic sensation conveys information accompanied by sympathetic and parasympathetic nerves from sensory nerves and DRG to T13-L1, L5-S2, and spinal cords, whereby the distribution and density of sensory nerves on the surface of the prostate are different (Fig. 2).
Fig. 2. Anatomy and function of autonomic nerves.
3. Sympathetic and parasympathetic nerves
The sympathetic and parasympathetic nerves secrete norepinephrine (to transmit messages) and acetylcholine (as a mediator), respectively. The sympathetic function constricts vascular and non-vascular smooth muscles. Additionally, the parasympathetic innervates neuroendocrine cells to promote prostatic secretion. Their complex relationship is relevant to erectile function. On the prostrate surface, the sympathetic and parasympathetic nerves are distributed differently. The sympathetic nerves are widely distributed anterolaterally-posteriorly at the base, narrowing in the middle and apical regions to the posterior-lateral-posterior area. In contrast, the parasympathetic nerves travel diagonally from the anterolateral to the apical and posterior paths around the prostate. Compared to sensory nerves, sympathetic and parasympathetic nerves are distributed in the prostate in the same density and evenly. However, their density changes with development, increasing from the fetal period to a peak during neonatal development and remaining constant during adulthood, similar to the sensory nerves [11,12,13].
Furthermore, according to a recent study, the axonal density of sympathetic and parasympathetic nerve innervations in anterior lobes is lower than ventral ones. The distribution of sympathetic nerves in the capsule is parallel. At the same time, the gland is concentric circles [19]. The sympathetic and parasympathetic nerves of the prostate are derived from the lower infra-abdominal plexus (pelvic plexus). The abdominal aortic plexus receives sympathetic nerves from the ganglia of the sympathetic trunks L1 and L2. Part of these trunks descends to receive nerves from the ganglia of the sympathetic trunks L3 and L4 to form the lower epigastric plexus. In turn, they receive nerves from the sacral ganglia of the sympathetic trunks and parasympathetic nerves from the sacral ganglia S2-4 on the way down, forming the lower infra-abdominal plexus (pelvic plexus).
In addition, the autonomic nerves of most pelvic organs are derived from the pelvic plexus, including the bladder, thus showing that the prostate and bladder are closely related and interact with each other. Prostatic sympathetic and parasympathetic innervation originate at the spinal cord levels of T13-L1 and L5-S2. These are based on the spinal cord’s innervated segments. In contrast, bladder sympathetic and parasympathetic innervation originate at the spinal cord levels of L1-2 and S2-4. They have a typical spinal cord segment, indicating that the prostate has a complex relationship with the bladder and the other organs of the pelvis [16,17].
In general, sympathetic and parasympathetic nerves are derived from the T13-L1 and L5-S2 spinal cords. They innervate and modulate the prostate. Their surficial distribution and density are distinguished, while the prostate has a complex relationship with the bladder and other pelvis organs, according to autonomic generation and distribution (Fig. 2).
AUTONOMIC NERVOUS SYSTEM REGULATION OF INFLAMMATION, PAIN, AND ORGAN DYSFUNCTION
The autonomic nervous system comprises autonomic nerves, their command center, and the CNS. The autonomic nerves are subdivided into sensory, sympathetic, and parasympathetic nerves, and the CNS is divided into the spinal cord’s lower centers and the brain’s higher centers. They are all involved in the production and development of inflammation, pain, and dysfunction of organs in CP/CPPS.
1. Sensory nerves
Sensory nerves are associated with neurogenetic inflammation and pain. Sensory nerves activate and release substance P (SP) and calcitonin gene-related peptide (CGRP) when nociceptors are stimulated in their terminals. CGRP- and SP-expressing nerves densely innervate vascular smooth muscles. Therefore, these substances will directly affect the vascular endothelial cells to participate in vascular effects, mediating the generation of neurogenetic inflammation.
SP is a key molecule in the neurogenic inflammatory response and interaction between the nervous and immune systems. It directly acts on vascular smooth muscle to increase vascular permeability, plasma extravasation, and edema and can activate mast cells. The activated mast cells can secrete relevant neuropeptides and promote the activation of sensory nerves. Therefore, the development of their vicious cycle mediates the occurrence and development of neurogenic inflammation. In addition, CGRP is a potent micro-vasodilator that contributes to most neurogenic vasodilation. It is also involved in the recruitment and activation of inflammatory cells [20,21].
These substances are synthesized by the DRG cell body and can be transported bi-directionally to the nerve endings to mediate the inflammatory reaction of the indisposed organ [22]. The convergent neurons in the DRG have dichotomous afferents; the cell bodies of sensory nerve fibers from two different organs are present in the same DRG. Therefore, it can be activated due to inflammation in one organ to produce these substances and transfer them to another organ, promoting the occurrence and development of neurogenic inflammation of cross-activation between organs [23,24]. In addition, sensory nerves play an essential role in the generation and development of pain. They become peripherally sensitized after repeating stimulation; a persistent state of response with a reduced threshold to pain stimuli leads to the generation, amplification, and maintenance of abnormal pain. In addition, developing a vicious cycle between sensory cells and mast cells contributes to the generation and development of pain behaviors [25,26]. Therefore, neurogenetic inflammation and pain are related to sensory nerves. After repeated stimulation, pro-inflammatory neurotransmitters (produced by DRG and the cell bodies of sensory nerves) and their transmission by their fibers to the nerve terminal contribute to the generation and development of inflammation. It causes cross-organs activation and peripheral sensitization, leading to persistent and abnormal pain.
2. Sympathetic and parasympathetic nerves
Sympathetic and parasympathetic nerves play an essential role in the generation and development of inflammation, pain, and dysfunctional organs. Little evidence exists regarding the relationship between parasympathetic nerves and prostatitis. However, research elucidates that they participate in the immune system and pain behaviors [27]. The latest research found that sympathetic nerves play a vital role in the prostatitis generation. The norepinephrine signal of the sympathetic nerve street can inhibit pro-inflammatory cytokines production and anti-inflammatory cytokines expression’s up-regulation. In addition, it can bind to the immune cell surface through the corresponding receptors, inhibit their function, and restore the homeostasis of the immune system [28]. Therefore, inhibition of β-adrenergic signaling leads to the up-regulation of pro-inflammatory genes, raising immune cell levels and exacerbating autoimmune responses [29]. Sympathetic denervation may lead to an autoimmune disorder to make prostatitis appears.
Additionally, sympathetic nerves are involved in pain development. Autonomic nerves innervate visceral smooth muscle, and pain may occur due to spasms of visceral smooth muscle. Several studies demonstrated that visceral smooth muscle spasms are caused by abnormalities in the sympathetic nerves that mediate them, leading to abdominal pain, chest pain, and urinary pain [30]. Therefore, the sympathetic nerve mediates the occurrence of immune abnormalities and may cause the abnormal spasm of smooth muscle to produce pain. Furthermore, the sympathetic and parasympathetic nerves are involved in the formation of cross-sensitization and dysfunction of organs. Some researchers suggested that the nerves’ high command in the autonomic nervous system activates or sensitizes so that the autonomic nervous of other organs also become activated and sensitive, leading to their abnormal activity. Abnormal organs can induce dysfunction of other organs through the ganglion in similar anatomical levels [31,32,33,34,35,36,37]. Overall, sympathetic and parasympathetic nerves play a vital role in developing prostatitis, chronic pain, cross-sensitization, and organ dysfunction. The symptoms of the urinary system are similar to CP/CPPS.
3. CNS
Central nervous sensitization contributes to the pathogenesis of pain and dysfunctional changes in organs. Central sensitization is characterized by extensive pain, multi-site hyperalgesia, and abnormal pain. Furthermore, it may be accompanied by abnormal organ function, cognitive impairment, depression, and other emotional problems. Chronic prostatitis can also cause generalized pelvic pain, scrotum, bladder, and other organ pain sensitivity. It can encourage ejaculation dysfunction, overactive bladder, cardiovascular problems, cognitive disorders, and depressive behavior. Therefore, central nervous sensitization plays an essential role in CP/CPPS.
Moreover, the activation of glial cells contributes to central sensitization. After persistent nociceptive stimulation, glial cells in the CNS activate and sensitize, increasing the transmission of pain signals to the spinal cord. It also leads to reducing pain thresholds, producing transmitters, and up-regulating associated receptors to sustain this reactive state, underlying the generation and development of persistent pain. The sympathetic and parasympathetic nerves’ command center core also becomes activated and sensitized, resulting in the abnormal activity of other organs. It is one of the pathogenesis of the generation and development of cross-sensitization organs [6,25,26]. The development and generation of pain and cross-sensitization of organs are closely associated with central sensitization.
AUTONOMIC NERVOUS SYSTEM DYSFUNCTION’S CLOSE RELATION WITH CP/CPPS
Several researchers have suggested that autonomic nervous system dysfunction is closely associated with CP/CPPS. Sensory nerves lead to persistent inflammation and pain. Sympathetic and parasympathetic alterations affect the dysfunction of organs and the development of prostatitis. Central sensitization decreases pain thresholds and increases pelvic pain perception in chronic prostatitis.
1. Sensory nerves
Sensory nerves play an essential role in transmitting nociception. Nociceptive receptors convert injurious stimuli into pain signals at the end of primary pain afferent nerve fibers. Furthermore, sensory nerve fibers transmit this signal through the DRG to the spinal cord and the brain. In chronic prostatitis, the primary sensory fibers activate and release neurogenic peptides after certain stimuli. Furthermore, they mediate neurogenetic inflammation and peripheral sensitization.
Peripheral sensitization plays an essential role in chronic pain development. When nociceptive receptors are repeatedly activated, sensory nerve fibers are altered, chemicals are released, and a series of associated receptors are up-regulated. It results in increased input, hyperexcitability in nociceptive receptors, and sensitivity of sensory nerve fibers to stimuli. Therefore, the pain threshold decreases, and the suprathreshold stimuli response enhances. This mechanism, called peripheral sensitization, is the leading cause of chronic pain. When it is not satisfactorily controlled or relieved by conventional analgesic drugs, it can seriously affect patients’ lives [7]. These abnormal pathways could manifest clinically as severe, persistent, and dysfunctional pain [8]. Peripheral sensitization might also be responsible for recurrent conditions and prolonged pain in chronic prostatitis. In addition, several alterations occur in the DRG, such as producing neurotransmitters and up-regulating channels or receptors in chronic prostatitis. These changes mediate the activation and sensitization of sensory nerves. Moreover, the DRG has a dichotomous function that might regulate the onset, progression, and duration of pelvic pain in chronic prostatitis (Fig. 3, 4).
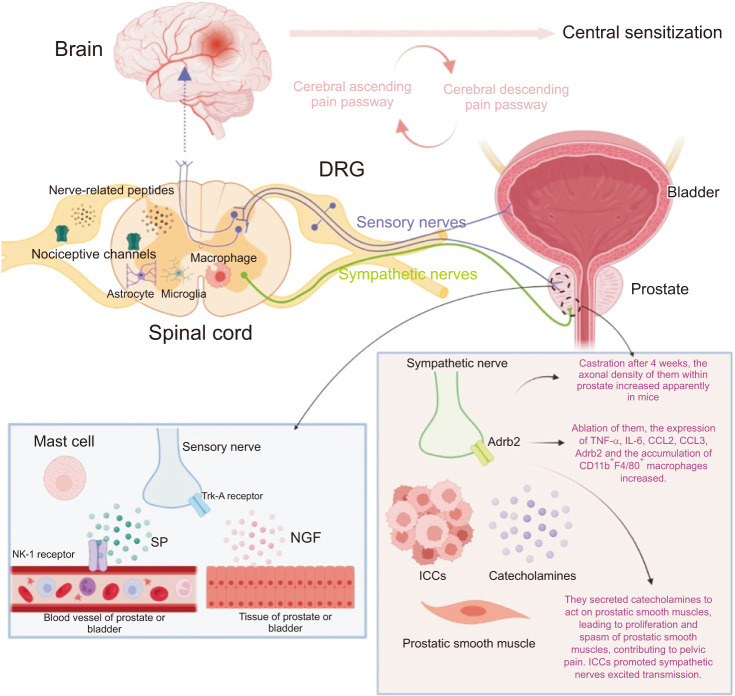
Fig. 3. Central sensitization includes the cerebrum and spinal cord. The alteration of brain structure and function and the disbalance of ascending and descending sensory passways contribute to central sensitization. The activation and sensitization of the spinal cord are caused by the activation of microglia and astrocyte, the accumulation of macrophages, and the up-regulation of nociceptive channels and nerve-related peptides. The sensory and sympathetic nerves accompany each other. Sensory nerves produce and release SP to mediate neurogenic inflammation and activate mast cells in the prostate and bladder after repeated stimuli. Sensory nerves also become peripheral sensitization becuse NGF band to Trk-A receptor produced by prostate or bladder. DRG is a part of the sensory nerve, consisting of cell bodies of sensory nerves and dichotomous function. DRG has increased expressions of nociceptive channels and nerve-related peptides. These contribute to peripheral activation, sensitization, and cross-sensitization of the prostate and bladder in CP/CPPS. Sympathetic nerves release catecholamines to promote the proliferation and spams of prostatic smooth muscle to contribute to pelvic pain. Furthermore, ICCs could enhance this reaction. The Adrb2 of sympathetic nerve contributes to prostatitis. Adrb2: antagonist of the belta2-adrenoceptor, CP/CPPS: chronic prostatitis/chronic pelvic pain syndrome, DRG: dorsal root ganglion, ICCs: interstitial cells of the Cajal, NGF: nerve growth factor, SP: substance P.
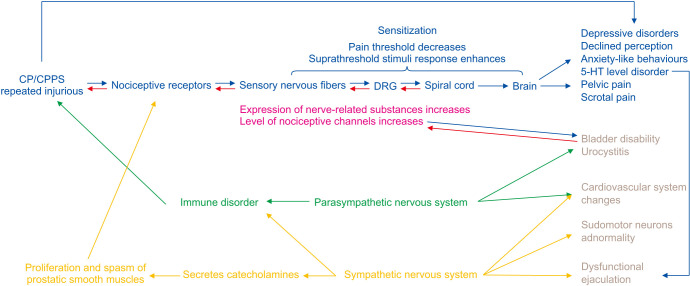
Fig. 4. Sensory nervous fibers activate and release neurogenic peptides to mediate neurogenetic inflammation and peripheral sensitization. DRG activates and sensitizes sensory nerves, producing neurotransmitters and up-regulation of channels or receptors. DRG has dichotomous neurons, which might regulate organ cross-sensitization. The activation and sensitization of parasympathetic nerves caused prostatitis, bladder disability and cardiovascular abnormality in CP/CPPS. Additionally, chronic prostatitis influences sympathetic nerves, and patients might present dysfunctional ejaculation, heart rate variability, skin resistance as well as pelvic pain. Chronic prostatitis could contribute to cerebral alterations and PVN, leading to a series of symptoms of CP/CPPS, including pelvic pain, depressive disorders, declined perception, anxiety-like behaviors, and dysfunctional ejaculation. CP/CPPS: chronic prostatitis/chronic pelvic pain syndrome, DRG: dorsal root ganglion, PVN: paraventricular nucleus.
1) C-fibers
Capsaicin-sensitive afferent fibers (C-fibers) are a kind of unmyelinated sensory fibers. These fibers innervate prostates and bladders and store and release SP in case of local stimulation [38,39,40]. Prostatitis could stimulate C-fibers and increases the SP level [41]. SP binds to vascular receptors neurokinin-1 (NK-1), increasing vascular vasodilation, permeability, and extravasation, resulting in neurogenic inflammation [20,42]. Once C-fibers are activated, nerve growth factor (NGF) level is elevated within prostates. In return, NGF combines with neuronal Trk-A receptors or TrkA/p75 NTR receptors to further promote their activation. Sensitive sensitized sensory nerves lead to neurogenic inflammation and prostatic pain NGF, which take part in the pathogenesis of CP/CPPS [43,44,45]. Spontaneous prostatitis developed in aged rats depicted changes in the density of sensory nervous innervations. It indicated that sensory nerves might play an essential role in the onset and development of chronic prostatitis [46]. Furthermore, there is an interaction effect between C-fibers and the pathogenesis of pelvic pain and chronic prostatitis.
2) DRG
DRG is a convergence of cell bodies of primary sensory neurons, which play a vital role in the pathogenesis of CP/CPPS. Stimulated by prostatitis, various neurological substances increase in DRG, including TRPV1 receptors [44,47,48], Trk-A receptors [47,48], and NGF-sensitive receptors [49]. These substances also include Granulocyte-macrophage colony-stimulating factor receptors (GM-CSFR) [50], chemokine (C-C motif) ligand2 (CCL2) [50,51], macrophage inflammatory protein-1α (CCL3) [50], NGF [47,48,50], GM-CS [50], CGRP [52], and SP [53]. These up-regulations contribute to sensory sensitization, which is the basis of CP/CPPS development. For example, TRPV1 receptors promote inflammatory hyperalgesia by decreasing the inflammatory pain threshold and amplifying the pain’s effect [45]. NGF-sensitive receptors are disposable to acquire nociceptive environmental signals and rapidly sensitize upon acute exposure to NGF during inflammatory pain [49]. In addition, sensory passways of pain are stimulated via a direct method in which GM-CSFR combines with GM-CS. Alternatively, they are indirectly stimulated by the induction of CCL2, CCL3, and NGF from GM-CS. These methods cause nociceptive sensitization to mediate development and perpetual pelvic pain in chronic prostatitis [50].
Some researchers suggest that DRG has an efferent function. CGRP, SP, and neurokinin A were created and reversely released to peripheral tissues. It is done via sensory fibers in the case of prostatic inflammation, mediating the development of inflammation [20,41]. In addition, these neuropeptides might promote a growing number of NGF within prostates and NGF connected with relative sensory nerve receptors to mediate the conveyance, generation, and development of pain to the brain [20,43].
DRG is a neuron with dichotomous fibers, CGRP, and SP they produce. DRG is emitted to other pelvic organs underlying the generation of neurogenetic inflammation so that messages of inflammatory pain from other pelvic organs are delivered to the CNS. It produces pain, but it is difficult to identify whether the prostate or other pelvic organs contribute to pelvic pain in chronic prostatitis [52]. Dysfunction of pelvic floor muscles might be associated with the perpetual pelvic pain of chronic prostatitis [54,55]. Since DRG has dichotomous functions, pelvic pain generated by dysfunction of pelvic floor muscles might be transmitted to the CNS through DRG. It leads to a disability to distinguish prostate problems from pelvic dysfunction [52]. In conclusion, a series of alterations were observed in the DRG in chronic prostatitis; thus, sensory passways become more sensitive and activated. DRG might mediate the onset, development, and duration of pelvic pain in chronic prostatitis.
Sensory nervous fibers activate and release neurogenic peptides to mediate neurogenetic inflammation and peripheral sensitization. In addition, DRG activates and sensitizes sensory nerves, producing neurotransmitters and up-regulation of channels or receptors. DRG has dichotomous neurons, which might regulate organ cross-sensitization. Sensory fibers play an essential role in the pathogenesis of CP/CPPS (Fig. 3, 4).
2. Sympathetic and parasympathetic nerves
Sympathetic and parasympathetic nerves develop prostatitis and persistent pelvic pain. It also causes cross-sensitization of organs, including the cardiovascular system, ejaculation, cutaneous sudomotor neurons, and bladders. ICCs, a kind of stromal cells located in the prostates, might assist sympathetic nerves in mediating the proliferation and spasm of prostatic smooth muscles leading to pelvic pain in chronic prostatitis.
1) Prostatic changes
Sympathetic and parasympathetic nerves might be associated with the hormone’s steady state. The axonal density of these nerves within the prostate increases in mice four weeks after castration, but the exact mechanism is unclear [19]. Sympathetic nerves contribute to prostatitis generation. A study found that mice disposed to CP/CPPS after ablation of sympathetic innervations were characterized by increased pro-inflammatory cytokines (TNFα and IL-6) and chemokines (CCL2 and CCL3). Similarly, TrkAfl/fl mice were disposable to spontaneous prostatitis as a special kind of mice with noticeable loss of sympathetic nerves. Sympathetic nerves play a significant part in the prostates’ steady state of the immune environment. Once these nerves are blocked, the immune disorder occurs, and autoimmunity leads to prostatitis development.
Furthermore, the expression of beita2-adrenoceptor (Adrb2) and the accumulation of CD11b+F4/80+ macrophages are highly expressed in the prostate without sympathetic nerves [19]. Therefore, it was necessary to study that sympathetic and parasympathetic nerves might be associated with hormones’ steady state, the immune, and the detailed mechanism. Prostatic sympathetic nerves play an important role in the generation and development of chronic prostatitis and contribute to pelvic pain. Catecholamines and innervation of sympathetic nerves cause the proliferation and spasm of prostatic smooth muscles, inducing pelvic pain. Sympathetic nerves secrete catecholamines to participate in physiological responses. They also contribute to the proliferation and spasm of prostatic smooth muscles through the secretion of catecholamines. It mediates pelvic pain in chronic prostatitis. In this process, ICCs promote sympathetic nerve excitement transmission involved in CP/CPPS. ICCs mediate sympathetic nervous signals in location and function sites.
Regarding position, ICCs were stromal cells located close between prostatic smooth muscle and sympathetic nerves [11,56]. Functionally, these interstitial cells own particular features, such as interaction with catecholamines via alpha1-adrenergic receptors, that require assistance from sympathetic nerves [56]. ICCs participate in the sympathetic modulation of prostatic smooth muscles and even regulate catecholamine level [57]. Sympathetic and parasympathetic nerves mediate prostatitis and pelvic pain generation and development in CP/CPPS (Fig. 3, 4).
2) Cross-sensitization of organs
Organ cross-sensitization might be the potential pathophysiology of CP/CPPS due to incomplete treatments for this disease and the negative impact on other organs’ functions. Prostatitis causes their autonomic nerves to become abnormal, affecting the cardiovascular system, ejaculation, cutaneous sudomotor neurons, and bladders.
The cardiovascular system is susceptible to alteration in CP/CPPS. Heart rate variability reportedly is lower in CP/CPPS men than in control, suggesting that chronic prostatitis influences sympathetic and parasympathetic nervous systems [37]. Furthermore, a report demonstrated that the sympathetic and parasympathetic activities of CP/CPPS men differed from control ones. During blood pressure measurements while lying down for 5 minutes and standing, parasympathetic activity hardly changed while sympathetic activity decreased in CP/CPPS patients, compared with decreased and increased, respectively, in control people [9]. Therefore, there might be differences in the sympathetic and parasympathetic nervous systems between CP/CPPS patients and normal healthy ones. These nervous dysfunctions might be one of the reasons for aggravating, developing, and incomplete cures of CP/CPPS.
CP/CPPS also influences ejaculation. CP/CPPS is a complicated disease with repeated pain, incomplete treatments, and ejaculation dysfunction, indicating that the sympathetic nervous system might inevitably function in the pathogenesis of chronic prostatitis. In addition, alteration of the paraventricular nucleus (PVN) in prostatitis is a reason for dysfunctional ejaculation [31,32]. PVN is the control center of sympathetic nerves of the spinal cords, and it is changed by prostatitis-derived interleukin-1β (IL-1β) via growing expression of N-methyl-D-aspartic acid (NMDA) receptors. Subsequently, sympathetic nerves of the spinal cords are disposable to activation and sensitization. Therefore, dysfunction of ejaculation appears in chronic prostatitis patients.
On the other hand, the disorders of 5-hydroxytryptamine (5-HT) levels in the CNS also contribute to dysfunctional ejaculation. Chronic prostatitis interrupts the balance of 5-HT levels in brains, decreasing 5-HT levels and increasing 5-HT1A receptors expression in the hippocampus to mediate dysfunctional ejaculation [18]. However, there is no clear explanation of their pathogenesis. Furthermore, sudomotor neuron abnormality is also present in prostatitis through sympathetic nervous system alteration. Dysfunctional cutaneous sudomotor neurons manifest skin resistance to electrical currents in CP/CPPS men, which was distinguished from normal ones [58]. Organ cross-sensitization can be found in prostatitis by altering sympathetic and parasympathetic nerves. CP/CPPS is a complicated disease accompanied by multiple organ dysfunction, indicating that these nervous systems might play an inevitable function in the pathogenesis of chronic prostatitis.
Bladder abnormality is associated with chronic prostatitis. There is a clinical and etiological crossover between CP/CPPS and painful bladder filling or urinary urgency [59]. The main symptoms of long-term bladder hyperexcitability are frequent and urgent urination, pain, and discomfort of the lower abdominal or perineum. These are similar to symptoms of CP/CPPS, indicating that bladder abnormality is relative to chronic prostatitis. It was found that chronic prostatitis excited its afferent nerves and increased transient receptor potential vanilloid1 (TRPV1) channel expression in L6-S1 DRG. It results in afferent neurons hyperexcitability, NGF up-regulation of bladders and prostates, and bladder abnormality [60]. Furthermore, it was observed in CP/CPPS that NGF was up-regulated, and the parasympathetic function of bladders was activated and sensitized following DRG excitement [41]. Prostatitis caused the up-regulation of NGF and activation of parasympathetic nerves of the bladder. It led to long-term bladder hyperexcitability, underlying one of the pathogenesis of pelvic pain in chronic prostatitis.
Additionally, dichotomized afferents involved in primary pelvic afferents, located in plenty of DRG, also play an important role in cross-sensitization of the prostate and bladder [34,61]. The bladder’s efferent nerves and afferent nerves change in chronic prostatitis [33,34,35,36]. Apart from DRG’s dichotomized function, prostates and bladders’ autonomic nerves are derived from the pelvic plexus and a common spinal cord segment. Long-term bladders hyperexcitability is observed in chronic prostatitis due to the bladder’s autonomic nerve activation and sensitization and the growing number of NGF in bladders. This condition might mediate the onset, development, and perpetual pain of CP/CPPS. Interestingly, urocystitis leads to prostatitis, which is caused by neurogenic inflammation in the bladder by stimulation of cross-visceral reflexes. Furthermore, adoptive macrophage migration inhibitory factor (MIF) in the bladder lumen could reduce prostatic inflammatory conditions, while the particular mechanism of these alterations is obvious [62].
In general, organ cross-sensitization might be novel pathophysiology of CP/CPPS. Chronic prostatitis manifests as long-term recurrent pain or discomfort in the pelvic area and paruria, which might be associated with long-term bladder hyperexcitability. It is caused by the activation and sensitization of sensory and parasympathetic nerves. Additionally, chronic prostatitis influences sympathetic nerves, and patients might present sexual abnormalities, such as dysfunctional ejaculation. Heart rate variability and skin resistance are also abnormal in CP/CPPS patients, confirming a close relationship between sympathetic/parasympathetic nerves and CP/CPPS (Fig. 4).
3. CNS changes’ close relation with CP/CPPS
The CNS integrates and processes nociceptive signals. These signals are transmitted from the periphery to produce nociception in the brain. Some studies confirmed that pain persisted. However, the inflammatory condition subsided, indicating that CNS sensitivity was crucial in developing and maintaining chronic pain [6]. Central sensitization is an increase in CNS responsiveness to normal or below-threshold stimuli. It exhibits abnormal excitation, which might be one of the pathogenesis of persistent pelvic pain in chronic prostatitis [6,7,44]. Various evidence elucidates spinal activation and sensitization in the spinal cords of chronic prostatitis to mediate pain sensitization, chemokines and channels up-regulation, and microglial cells and astrocytes activation. Cerebral sensitization also contributes to full-blown multisensory hypersensitivity, including pelvic muscles, prostate and scrotal threshold, underlying generation, development, and maintenance of abnormal pain in chronic prostatitis [59].
The production of persistent abnormal pain in chronic prostatitis is also caused by cerebral ascending and descending pain passways. The CNS processing of nociceptive signals is also modulated by ascending and descending pain-modulatory system. This system is a network that modulates the facilitation and inhibition of pain [63]. Therefore, abnormalities in these passways lower pain thresholds and increase trauma signals and pain levels in chronic prostatitis. Furthermore, activated PVN also influences dysfunctional ejaculation of CP/CPPS.
1) Low-level CNS
Spinal cord levels L6 and S1 with c-fos positive expression after rat prostates suffered from acute chemical stimulations. The skin appeared to have neurogenic inflammation innervated by identical cord levels. It suggested that spinal cords received stimulated messages conveyed from afferent nerves and then became activated and sensitive, leading to neurogenic inflammation in peripheral organs [41,64]. The activated and sensitive spinal cord might be the main culprit of prostatitis development. Various alterations in the spinal cords of chronic prostatitis mediate prostatitis generation and development. These include expressions of CCL2 [51,53], TRPV1 channels [44], 5-HT 1A receptors [18], and acid-sensing ion channel 1a (ASIC1a) channels [65]. Up-regulation in the spinal cords of chronic prostatitis contributes to central activation and sensitization. It decreases the inflammatory pain threshold, amplifies inflammatory hyperalgesia, and promotes the development of long-term pain in chronic prostatitis. The up-regulation of ASIC1a channels mediates pelvic pain and leads to a growing number of pro-inflammatory cytokines TNF-α, IL-2, IL-6, and IFN-γ. It promotes the generation and development of inflammation. These channels work together with NMDA receptors, resulting in central sensitization as a basis for pain hypersensitivity in CP/CPPS [65].
Furthermore, microglial cells and astrocytes in the spinal cords also play an essential role in generating and developing CP/CPPS. Activated microglia-induced central activation and sensitization are involved in chronic pain maintenance [7]. By contrast, their inhibitors reduce pain by approximately 20% in the experimental autoimmune prostatitis (EAP) models [66]. Microglial cells play an essential role in the generation and development of resistant pain. Furthermore, microglial cells also produce various chemokines and inflammatory cytokines, mediating neurogenic inflammation or further promoting central activation and sensitization [66].
On the other hand, astrocytes contribute to developing and maintaining chronic pain by responding to peripheral prostatitis stimuli [7]. Some studies found that astrocyte activation rose to the later stages of carrageenan-induced prostatitis pain five weeks after carrageenan injection. This observation indicated that astrocytes contribute to the development of pain in the later period. Therefore, astrocytes play an important part in the resistant pain of chronic abacterial prostatitis [67,68]. Various evidence shows the activation and sensitization of spinal cords in chronic prostatitis. It includes the up-regulation of chemokines and channels and the activation of microglial cells and astrocytes. Central sensitization consistently boosts spinal neurons of nociceptive progress, generation, development, and perpetual existence of pain [69]. Therefore, sensitization of spinal cords plays an important role in developing and maintaining chronic pain (Fig. 3, 4).
2) High-level CNS
Pelvic pain, scrotal pain, and dysfunctional ejaculation could be induced by alternations of cerebral structures and functions in CP/CPPS, including cortex, brainstem, thalamus, and PVN. Pelvic pain is a common symptom in the CP/CPPS, resulting from abnormalities of the cortex, brainstem, and thalamus. Brain structure and function are important factors to consider in long-term pain of chronic prostatitis, as cerebral cortex morphology facilitates or inhibits pain maintenance. Alterations in the medial areas of the motor and sensory cortex in chronic pelvic pain dominate the sense and motor of pelvic muscles. The cerebral cortex is altered in chronic prostatitis, leading to hypersensitivity in sensory areas that innervates the pelvic muscles and prostate tissue. It results in the generation, development, and maintenance of generalized pelvic pain, underlying a vital function in the pathogenesis of CP/CPPS [59].
Furthermore, the anterior cingulate cortex (ACC), insular cortex (INC), right medial prefrontal cortex (mPFC), brainstem, and right thalamus participate in the generation and development of pelvic pain in CP/CPPS. Both cerebral ascending and descending pain passways before the formation of pain perception in the cerebral cortex participated in the modulation of sensory transmission. ACC, INC, and mPFC play an essential role in descending pain passways, while the brainstem and right thalamus participate in ascending pain passways. However, ACC, INC, and mPFC are substantially inhibited in chronic prostatitis, so they fail to descend pain to cause long-term pelvic pain. By contrast, the brainstem and right thalamus are perceived to be abnormal in CP/CPPS patients compared to the control ones, so pain might be exaggerated to transmit to the cortex. Ascending and descending pain passways appear abnormal in CP/CPPS patients, leading to sensitization and amplification of pain due to decreased pain threshold, increased trauma signals, and algesthesis degree [70]. Abnormal scrotal pain and dysfunctional ejaculation were also disposable in chronic prostatitis. It was found that higher levels of IL-1β and IL-6 were measured in the thalamus and brain cortex in chronic prostatitis, resulting in their activation and sensitization.
Furthermore, the scrotal pain threshold decreased, contributing to the generation, development, and maintenance of abnormal scrotal pain [71]. Dysfunctional ejaculation, one of the symptoms of CP/CPPS, is caused by activated PVN located in the cerebrums, along with the up-regulation of NMDA receptors [31,32]. PVN is the central monitor of sympathetic nerves, and ejaculation is innervated and regulated by them. Consequently, activated PVN contributes to abnormal ejaculation in CP/CPPS.
Cerebral cortex sensitization and abnormal cerebral ascending and descending pain passways generate, develop, and maintain pelvic and scrotal pain in chronic prostatitis. In addition, activated PVN influences dysfunctional ejaculation of CP/CPPS. The high-level CNS is closely related to the pathogenesis of CP/CPPS. The autonomic nervous system becomes activated and sensitive to contribute to the generation and development of persistent pelvic pain and cross-sensitization of organs. In addition, the autonomic nerve also mediates neurogenetic inflammation or prostatitis.
Cross-sensitization plays an essential role in the pelvic pain of CP/CPPS. This report summarized three important points. First, DRG has dichotomous afferents, and the cell bodies of sensory nerve fibers from two different organs are present in the same DRG. Similarly, the sensory nerve cell bodies of the prostate and bladder are in the same DRG. If one has abnormal stimulation or inflammation, its sensory nerves get activated. The cell bodies of these activated sensory nerves produce and release nerve-related peptides and up-regulate the expression of nociceptive channels. Consequently, other inactive cell bodies in the same DRG anatomy space become activated through paracrine or autocrine. Activated sensory nerves have an efferent function. Therefore, these neuropeptides are transported from DRG to nerve endings, arriving at peripheral organs that are innervated or spinal cords. This mechanism insults injury to organs that are already inflamed, leading to neurogenic inflammation in the organs that were normal through dichotomous DRG and mediating peripheral (sensory nerves and organs) and central (spinal cords) sensitization. Second, spinal sensitization participates in organ cross-sensitization. Common or adjacent spinal cords could interact with each other. The prostate and bladder have the same and adjacent spinal cord segments. Therefore, a close relationship exists between the bladder and the prostate. Third, cerebral sensitization contributes to the generation and development of cross-sensitization of organs. Cerebral sensitization contributes to generalized pain, including prostate, pelvic muscles, and scrotal areas. Additionally, the central centers that direct sympathetic and parasympathetic nerves influence organ functions if activated and sensitive. It is how prostatitis could affect the cardiovascular system, ejaculatory function, skin reactions, and bladder activity [23,24,72].
CP/CPPS INFLUENCE ON THE CNS
CP/CPPS influences spinal cords, where there are various alterations, including up-regulation of cyclooxygenase-2 (COX-2), NGF, brain-derived neurotrophic factor (BDNF) [44], CCL2 [51,53], Trk-A channels, TRPV1 channels [44], TRPV1 channels [44], 5-HT 1A receptors [18], and ASIC1a channels [65]. Besides, CP/CPPS influences brain function and structures. ACC, INC, mPFC, and brain stem are perceived as alterations in CP/CPPS [59,70,73,74]. The caudate cortex, the lateral column of the periaqueductal gray, and the thalamus are strongly activated after intraprostatic capsaicin [44]. These cerebral areas are closely related to pain, and their positive BOLD signals are significantly increased, particularly the caudate cortex. Altered connections in the frontoparietal control network are measured in CP/CPPS patients, which might control pelvic pain and lower urinary tract symptoms [75]. Besides pain, depressive disorders and anxiety-like behaviors are influenced by CP/CPPS [18,74,76]. Some researchers suggested prostatitis contributes to activated cerebral microglia and pro-inflammatory cytokine production. It leads to the generation and development of depressive behaviors [76].
On the other hand, chronic prostatitis causes 5-HT levels to decrease and 5-HT1A receptors’ expression to increase in the hippocampus to mediate the generation of depressive behaviors [18]. Furthermore, cerebral microglial cell motivation and microglial synaptic contacts are also influenced by chronic prostatitis. Therefore, the declined perceptions and structural neuroplasticity were observed in CP/CPPS [77]. In addition, the PVN located in the brain also changed. The number of IL-6 and IL-1β increases in the thalamus and brain cortex in chronic prostatitis, leading to dysfunctional ejaculation and central sensitization [31,32,71]. Chronic prostatitis could contribute to cerebral alterations, including brain cortex, brain stem, thalamus, PVN, and connections in the frontoparietal control network. It indicated that chronic prostatitis affects brain structures and functions, leading to a series of symptoms of CP/CPPS, including pelvic pain, depressive disorders, declined perception, anxiety-like behaviors, and dysfunctional ejaculation (Fig. 4).
NERVE-RELATED SUBSTANCES IN CP/CPPS
In CP/CPPS, there were a series of substances involved in the generation and development of pain conditions in chronic prostatitis, including CGRP, SP, BDNF, NGF, MIF, GM-CSF, connexin-43 (Cx43), IL-1β, COX-2, prostaglandin E2 (PGE2), CCL3, CCL2, CXCL1, 5-HT, serotonin transporter (SERT), mast cells, and macrophages.
1. CGRP
CGRP is a member of the calcitonin peptide family and a potent vasodilator that mediates neurogenetic inflammation. It is produced and located in the C-sensory fibers, DRG, and CNS. Functionally, it participates in nociceptive signals transmission, neuro-inflammation, peripheral and central sensitization, and abnormal pain development [78]. Activated and sensitive C-fibers release CGRP to mediate neurogenic inflammation [6]. DRG neurons increase the CGRP expression and mediate hyperalgesia responses, peripheral sensitization, and neurogenetic pain development [79]. Stimulated by chronic prostatitis, sensory fibers activate and convey signs to DRG to measure the growing number of CGRP.
DRG releases CGRP to peripheral tissues, including prostates and other pelvic organs, promoting the generation and development of neurogenetic inflammation in these regions [52,80]. CGRP is also found in the spinal cords. It regulates astrocyte activation, processes pain, contributes to central sensitization, and is associated with developing and maintaining neuropathic pain [81]. In the chronic prostatitis models, increased CGRP release is observed in L5-S2 spinal cords. It indicates that chronic prostatitis excites the sensory nerves and activates an equivalent level of spinal cords [80]. In general, the alteration of CGRP is concomitant with chronic prostatitis. Consequently, DRG and spinal cords appear to have elevated levels of CGRP, which might play an important role in the generation, development, and persistence of pelvic pain in chronic prostatitis.
2. SP
SP is a neurotransmitter and a protein in nature. Its main receptor is the NK-1, located primarily in neurons in the brain, spinal cord, and vascular endothelium [21,82]. In response to repeated stimulation, nociceptive receptors transmit these signals to sensory nerve fibers and DRG, activate them, and produce large amounts of CGRP and SP. These substances are released to nerve terminals and the CNS [22]. SP is a key molecule in the neurogenic inflammatory response and critical interaction between the nervous and immune systems. Peripherally-released SP causes vasodilation and increases permeability and plasma protein leakage by acting on NK-1 receptors expressed in the vascular endothelium [21,22,82]. They also activate lymphocytes, and mast cells or macrophages, promoting neuro-inflammation and sensitizing sensory nerves to nociception [21,26,82]. In addition, SP could lead to the development of central sensitization [26,83].
SP also contributes to the pathogenesis of CP/CPPS. Sensory fibers, DRG, and spinal cords are disposable to an elevated expression of SP in the EAP models [53,80,84]. Activated DRG produces and releases SP to the tissues’ sensory fibers, terminal innervates, and spinal cords. Peripheral SP-induced neurogenetic inflammation, oxidation reaction in prostates, and SP of spinal cords increase central sensitization to pelvic nociception [84]. SP promotes a neurogenic inflammatory response in the prostates and mediates pain transmission from sensory nerves to the central system, which might be a novel pathogenesis of persistent, refractory, and repeating pelvic pain in chronic prostatitis [85].
3. NGF and BDNF
BDNF and NGF are neurotrophic factors (NTs) comprising p75 receptor (p75R) and tropomyosin receptor kinase A (TrkA). BDNF, a kind of soluble polypeptide, is synthesized by DRG and transported anterograde to the spinal cord [86]. In the prostatitis models, its level is increased in the spinal cords [66]. BDNF might play an important role in central sensitization by mediating the pelvic pain pathogenesis in chronic prostatitis. In contrast, NGF is a neurotrophic protein produced in mast cells that are stored in granules with two receptors, induced sensitization of nociception, and enhanced neuronal density in peripheral tissues. NGF stimulates the expression of inflammatory factors and affects ion channels, receptors, and damage-related peptides. It also induces persistent mechanical hypersensitivity, cooperates with mast cells, and increases excitability and sensitization of sensory and sympathetic nerves [87]. Evidence confirms that NGF is closely associated with CP/CPPS.
In the CP/CPPS patients, up-regulation of NGF is measured in prostatic fluid. It had been observed in EAP models [47,48,88,89], and the alteration of NGF is closely related to the severity of CPPS patients [90]. Prostatitis stimulates excitement of their afferent sensory nerves, which reversely stimulate prostates. It leads to the growing expression of NGF in the prostates. It causes neurogenic inflammation in prostates and sensitizes their afferent nerves to nociception. Prostatitis promotes NGF up-regulation in bladders, resulting in erethism and abnormal bladder excitement. It also produces pelvic pain, and the symptoms are similar to those of CP/CPPS [35,44,60,91]. NGF plays an important role in chronic prostatitis onset and perpetual pain [88]. Prostatitis-induced up-regulation of NGF levels in prostates and bladders leads to the development of neurogenetic inflammation in prostates, the occurrence of abnormal activation in bladders, and the sensitization of afferent sensory nerves.
4. Mast cell
Mast cells are immune cells that play an important role in the immune system. They interact with sensory nerve fibers and influence each other. Locally, mast cells accumulate around nerves, providing a solid basis for involvement in signal transduction at neuroimmune synapses and playing a pivotal role in pain transmission and peripheral sensitization [92]. Functionally, interactions and influences between mast cells and sensory nerve fibers mediate the generation, development, and maintenance of nociception. Stimulated sensory nerve fibers release neurotransmitters that activate mast cells, causing mast cells to express various receptors and neuropeptides, including NGF and trypsin-like enzymes. This mechanism mediates the promotion of peripheral sensory nerve sensitization. Sensitized nerves release large amounts of neurotransmitters that interact with mast cell surface receptors. They activate mast cell degranulation one more time to release nociceptive substances. It results in a vicious cycle of mast cell and injury receptor activation underlying persistent neurogenic inflammation and pain [7,93,94]. A similar mechanism is observed in the CP/CPPS.
In CP/CPPS patients, elevated trypsin-like enzymes were detected in prostate secretions and urine from CP/CPPS patients [1,95,96]. Mast cell degranulation was increased in prostate secretions and biopsies [95]. In the prostates of the CP/CPPS model, the number of mast cells, degranulated ones, and the levels of trypsin and NGF were significantly increased in prostates [94]. A mouse model of EAP was presented with abnormal pelvic mechanical pain associated with increased mast cell infiltration and activation [48,91,97,98]. The sensory nerves innervating prostates were activated in response to the stimulation of prostatitis. Their terminal secreted SP and NGF, along with NGF interacting with NGF receptors (TrkA) on the surface of the mast cell membrane. Subsequently, these mast cells degranulated and released inflammatory factors, including trypsin-like enzymes and more NGF and SP. These trypsin-like enzymes acted on the neurons via protease-activated receptor2 (PAR2) to induce neuronal excitability. It increased peripheral sensitization, neuronal excitability, pelvic hyperalgesia, and chronic pain in chronic prostatitis patients [1,7,96,97]. Reducing the number of total and degranulated mast cells in the prostate could relieve pelvic pain, which was more efficient in clarifying mast cells that play an essential role in the generation and development of pelvic pain in chronic prostatitis [89,94].
5. Macrophage
A macrophage is a type of white blood cell in the immune system that engulfs and digests pathogens, mediating inflammatory responses. In addition, it plays an important role in peripheral and central sensitization and the generation and development of chronic pain. Macrophages migrate and infiltrate in the DRG and spinal cords, leading to peripheral and central sensitization, contributing to the pathological process of inflammatory pain, and the generation and development of chronic pain [99]. Macrophages also play an essential role in CP/CPPS. Infiltration of macrophages into the DRG increases in the EAP to take part in the early phase of peripheral sensitization. In addition, macrophage infiltration was also observed in the lumbosacral (LS) region of the spinal cord (L6-S2) in EAP mice. It closely interacted with activated and increased microglia and macrophages in the dorsal horn of the spinal cord to induce and maintain pelvic pain in chronic prostatitis [51].
6. MIF
MIF is a pro-inflammatory cytokine involved in the inflammatory response [100], which might contribute to prostatitis pathogenesis. Trichomonas vaginalis macrophage migration inhibitory factor (TvMIF) could enhance RWPE-1 cell activate inflammatory factors, taking part in the development and proliferation of prostatitis [101]. It is also present in nerve cells and mediates changes in the nervous system [102]. Changes in MIF levels could hyperactivate nociception in the DRG and consistently promote pain [103]. In addition, the expressions of MIF and MIF receptor CD74 in formalin-induced inflammatory nociception were up-regulated in the dorsal horn of the spinal cord. It might lead to central sensitization mediating the sensitivity to inflammatory pain [104]. This condition could also be seen in the CP/CPPS. Formalin reduced L6/S1 LS spinal cord, DRG, and up-regulated MIF expression in prostatitis. The afferent nerves in these areas also innervated bladders, activating their C-fiber afferents to release SP. This substance induced neurogenic inflammation and released MIF into the bladder lumen [62,105]. MIF compensates and persists temporary neurogenetic inflammation induced by SP. In addition, elevated MIF expression of spinal cords mediates hyperreflexia and perpetual pain in bladders [106,107]. Therefore, prostatitis causes abnormal bladder, paruria, and pelvic pain. It has similar symptoms of CP/CPPS underlying the generation, development, and persistence of pelvic pain in chronic prostatitis.
The inflammation alteration in the bladder could also influence the inflammatory progress of prostatitis. Intraluminal MIF inhibitors alleviate neurogenetic inflammation in the bladder induced by SP and relieve inflammatory response of the prostate [62,105]. Up-regulation of MIF level mediates and persists neurogenetic inflammation induced by SP. In addition, elevated MIF expression of L6/S1 spinal cords might play a vital role in the relationship between prostates and bladders.
7. GM-CSF and Cx43
GM-CSF is a cytokine that interacts with inflammatory cells, mediating autoimmune and chronic inflammatory diseases. It also plays an important role in the pain process, and a high level of it indicates extensive pain, increasing sensitization to mechanical stimulation and directly reacting with neurons [108]. In addition, GM-CSF takes part in CP/CPPS.
GM-CSF mRNA is significantly increased in the prostatic fluid from CP/CPPS patients. In mice induced with EAP, GM-CSF mRNA increases more significantly in the prostates and coagulation glands’ ventral and dorsal lobes than control. Furthermore, an increase in GM-CSF receptors was measured in prostates and DRG. Notably, GM-CSF-deficient mice demonstrated less tissue inflammation, less chronic pelvic pain, and significantly reduced CCL2, CCL3, and NGF mRNA expression in prostate tissue in the EAP. These phenomena suggest that GM-CSF is essential for maintaining chronic pelvic pain in the EAP. It encourages nociceptive sensitization to mediate development and perpetual pelvic pain in chronic prostatitis [50].
Cx43, a kind of connexon, forms gap junctions to build wide networks among astrocytes in the CNS, influencing chronic pain diseases [109]. Cx43 is expressed in the astrocytes and significantly increases in the dorsal spinal cords of capsaicin-related prostatitis after five weeks, modulating production and releasing CXCL1, and underlying the latter development and persistent pain of chronic prostatitis [67]. Cx43 up-regulation in astrocytes of spinal cords persists after spinal cord or nerve injury [6], and the expression of Cx43 increases in the spinal cords in the later phase of EAP. These two observations indicate that Cx43 might mediate the latter stage of pelvic pain in chronic prostatitis and contribute to persistent pain and chronic prostatitis development.
8. IL-1β
IL-1β is a member of the interleukin-1 cytokine family, involved in the inflammatory response. Its elevated expression is observed in prostate secretions expressed by patients with CP/CPPS [110]. An increasing number of IL-1β is seen in the serum and prostates of EAP models [31,111,112,113]. Interestingly, IL-1β is involved in the inflammatory response to chronic prostatitis and mediation of pain development. It increases spinal excitability and modulated pain sensitivity at the spinal cord level [114,115]. In addition, spinal astrocytes produce IL-1β, which activates astrocytes by binding to the IL-1 receptor (IL-1R), contributing to central sensitization and leading to long-term maintenance of chronic pain [116]. In the models of prostatitis, the expression of IL-1β is up-regulated in the spinal cords [67]. CGRP promotes IL-1β production from spinal glial cells and CP/CPPS spinal cords and is susceptible to the up-regulation of CGRP expression. Therefore, CGRP promotes the growing expression of IL-1β in the spinal cords in CP/CPPS to increase pain sensitization [81]. In addition, IL-1β significantly increases and plays an important role in the brain to mediate central system excitation and sensitization [114]. Higher levels of IL-1β were measured in the thalamus and cortex of CP/CPPS animals, resulting in scrotal pain threshold decreasing significantly and susceptibility to seizures underlying central sensitization [71]. Furthermore, IL-1β also plays an important role in NMDA receptor activity [114]. Prostatic IL-1β enters the CNS via the blood-brain barrier, inducing up-regulation of NMDA receptors, making the sympathetic nervous system sensitive, and promoting abnormal ejaculation in CP/CPPS [31].
9. PGE2 and COX-2
COX-2, prostaglandin-endoperoxide synthase, is a kind of enzyme participating in the synthesis of PGE2. PGE2 binds to their receptors (EP1-4) and acts as a pro-inflammatory mediator. This process induces peripheral and central inflammatory pain sensitization and sensitizes peripheral nociception. This behavior contributes to the development and maintenance of inflammatory nociceptive sensitization [114]. In the chronic prostatitis models, the serous PGE2 was increased significantly [117]. In addition, prostates of CP/CPPS depicted an increased expression of COX-2 and PGE2 [118,119,120]. Inflammatory responses could enhance their expression of them in the prostatic tissues.
On the other hand, prostatitis promotes IL-1β levels, acting as a mediator of inflammatory hyperalgesia. It could induce up-regulation of COX-2 and stimulate the subsequent release of COX-2 [118]. Up-regulation of COX-2 was observed in prostatitis, promoting mass production of PGE2. It resulted in peripheral and central inflammatory pain sensitization, decreased pain threshold of prostates, and induced abnormality of bladders by cross-sensitization of organs. This mechanism promotes the generation, development, and persistence of pelvic pain in chronic prostatitis. Down-regulation of COX-2 overexpression could reduce PGE2 levels, increase the pain threshold, and alleviate chronic pelvic pain in chronic prostatitis [118,119]. COX-2 and PGE2 increase the excitability and sensitivity of nociceptors and peripheral and central inflammatory pain sensitization.
Moreover, PGE2 also mediates the cross-sensitization of organs between prostates and bladders. Prostatitis causes activation of DRG due to dichotomous afferent sensory neurons between prostates and bladders. It leads to up-regulation and interaction of PGE2, EP4, and TRPV1 in the bladder mucosa. Therefore, the bladder afferent nerve becomes sensitive, contributing to abnormal activity of bladders and pelvic pain in chronic prostatitis [121].
10. CCL3, CCL2, and C-X-C motif ligand 1 (CXCL1)
Chemokines are a family of small cellular factors or signaling proteins secreted by cells to induce the directional movement of leukocytes and other cells. They are essential in inflammation, central sensitization, and chronic pain. Among them, chemokine C-C motif ligand and C-X-C motif ligand are members of the subfamily. CCL3 and CCL2 belong to the chemokine C-C motif ligand, while CXCL1 is the C-X-C motif ligand.
CCL3, also known as macrophage inflammatory protein 1-alpha (MIP-1-alpha), is a cytokine involved in the acute inflammatory state in the recruitment and activation of polymorphonuclear leukocytes. It also plays an important role in neurogenetic pain by binding to its receptors CCR1 and CCR5 [122]. Furthermore, CCL3 plays an important role in CP/CPPS.
CCL3 levels are increased in seminal plasma, EPS, and urine of CP/CPPS patients after prostate massage [1,123]. In addition, CCL3 is increased in chronic abacterial prostatitis models’ prostates and spinal cords [50,66,95]. Significantly increased CCL3 levels in the spinal cords mediate communication between neurons and glial cells, leading to macrophage migration and microglia activation. It results in central sensitization, nociceptive hyperalgesia, and persistent mechanical abnormalities in pain. It is involved in neuropathic pain development [99,124]. However, CCL3-deficient mice exhibit resistance to the maintenance of pelvic pain. Injecting CCR1 and CCR5 inhibitors into the spinal cords reduced symptoms of persistent mechanical aberrant pain, indicating that CCL3 is an important mediator of CP/CPPS [99,125]. CCL3 is increased in the spinal cords of the chronic abacterial prostatitis model. It mediates central sensitization and persistent pelvic pain of chronic prostatitis.
CCL2 is a secreted protein that acts as a pro-inflammatory mediator, recruiting immune cells to damaged tissues and mediating inflammation development. It also mediates peripheral and central sensitization to underlying pain pathogenesis of chronic persistent pain [126]. Similarly, CCL2 also plays an essential role in CP/CPPS.
High levels of CCL2 could be measured in the prostatic fluid of CP/CPPS patients [123]. In the EAP models, CCL2 is up-regulated in the prostates, spinal cords, and DRG [50,51,95,127]. Astrocytes release nociceptive factors that lead to central sensitization, encouraging chronic pain development, severity, and persistence [6,128]. Therefore, astrocytes and neurons in the spinal cord are activated and perceived in elevated CCL2 expression in the EAP model. It might promote central sensitization to pain and mediate chronic pelvic pain of chronic prostatitis [51].
In addition, CCL2 is released from activated glial cells of the DRG in a calcium-dependent manner and could promote increased Ca2+ ion influx through binding to its receptor CCR2 in the DRG. This mechanism promotes pain signaling and synaptic plasticity in excitatory neurons, leading to central sensitization and pain sensitivity. CCR2 antagonists also alleviate and inhibit pain development, suggesting that CCL2 in the DRG mediated key mechanisms of chronic inflammatory pain [126]. Therefore, high expression of CCL2 in the DRG is observed in the EAP model, and its role as a macrophage recruitment factor promotes macrophage infiltration in the DRG. It might mediate the early events of EAP-related peripheral sensitization. Up-regulation of CCL2 is seen in the spinal cords and DRG, promoting macrophage infiltration in these regions. It also encourages pain-signaling delivery and synaptic plasticity in excitatory neurons, leading to peripheral and central sensitization. It contributes to the generation and development of pelvic pain in chronic prostatitis.
CXCL1 is a small peptide belonging to the CXC chemokine family, and CXCR is its receptor, mediating inflammatory responses. CXCL1 modulates neuronal excitability by altering ionic currents and channels under neuro-inflammatory conditions. Therefore, it plays an essential role in central sensitization and chronic pain [6,124]. Conversely, CXCL1 inhibition in spinal astrocytes effectively attenuates chronic pain in rats [129]. CXCL1 up-regulation is disposable in the prostatic tissues and dorsal spinal cords of chronic prostatitis models [67,130]. However, intraspinal administration of CXCL1 antibodies and CXCR2 antagonists alleviates mechanical pain hypersensitivity in chronic prostatitis. This effect indicates that CXCL1 plays an important role in central sensitization, underlying the generation, development, and persistence of chronic pelvic pain in prostatitis.
11. 5-HT and SERT
5-HT is a monoamine neurotransmitter synthesized and stored in the CNS. 5-HT is associated with decreased sexual function and inhibits ejaculation by tonic inhibition mediated by 5-HT1B and 5-HT2 receptors [131]. Its absence is associated with depression. SERT, a highly selective SERT protein located in the presynaptic membrane, removes 5-HT from the synaptic gap, terminating its action and recycling it in a sodium-dependent manner [132,133]. However, some researchers elucidated that there is efficient evidence to prove the intimate relationship between 5-HT and depression [133]. 5-HT also contributes to CP/CPPS. Compared to controls, EAP rats are disposed to typical depressive behaviors and impaired sexual function, with a significant decrease of 5-HT expression in the hippocampus and spinal cords (T13-L1, L5-S2). However, it is an elevated level of SERT in the hippocampus and spinal cords (L5-S2) by contrast [18]. The decreased 5-HT in the CNS contributes to depressive behaviors and sexual dysfunction in EAP rats. Even the growing expression of SERT in these regions reduces 5-HT levels, aggravating these symptoms. Therefore, depression and sexual disorders are often observed in CP/CPPS patients and might be influenced by decreased levels of 5-HT in CNS.
In general, a growing level of nerve-related substances in chronic prostatitis participates in neurogenic inflammation, peripheral and central sensitization, and sympathetic nervous alteration. An increased expression of SP in chronic prostatitis promotes a neurogenic inflammatory response and MIF-mediated and persistent neurogenetic inflammation induced by it. In addition, the number of BDNF, GM-CSF, COX-2, PGE2, mast cells, and macrophages increases in CP/CPPS, contributing to peripheral and central sensitization to decrease pain threshold and amplify the pain effect. Up-regulation of CGRP, NGF, IL-1β, CCL3, CCL2, CXCL1, and Cx43 is perceived in chronic prostatitis. It mediates neurogenetic inflammation and peripheral and central sensitization. Notably, Cx43 could regulate the production and release of CXCL1 in the chronic prostatitis condition. 5-HT mediates CNS alteration, decreases its number, and contributes to depressive behavior and sexual dysfunction. The growing expression of SERT in these regions further reduces 5-HT levels to aggravate these symptoms. Interestingly, IL-1β balance is broken in chronic prostatitis, making the sympathetic nervous system sensitive and contributing to abnormal ejaculation in CP/CPPS.
NOCICEPTIVE CHANNELS
A series of nociceptive receptors or channels generate and develop pain conditions in chronic prostatitis in CP/CPPS. These include PAR2, TRPV1 channel, TrkA, ASIC1a, GM-CSFR, 5-HT1A, and 5-HT2C receptors, P2X2, P2X3, and P2X4 receptors, CCR2, CXCR2, CXCR3 and CXCR4, TREK1, and TREK2 channels, and NMDAR.
1. PAR2
PAR is a subfamily of G protein-coupled receptors (GPCR) and is categorized into four types: proteinase-activated receptors 1-4 (PAR1-4) [134]. PAR2 is activated by trypsin-like proteases that cleave its N-terminal structural domain and initiate changes in intracellular calcium uptake and MAPK/ERK pathways Its activation causes direct pain by stimulating the release of P substances and CGRP or by sensitizing TRPV1 receptors in peripheral afferent nerves [134]. This activation mediates neurogenic inflammation and central transmission of pain in peripheral tissues or indirectly by promoting the release of pro-nociceptive factors after its activation [134]. PAR2 also contributes to CP/CPPS.
Compared with controls, the expression of murine protease homolog MMCP-6 is increased in EAP, and PAR2 expression is increased fourfold in prostate stroma and epithelium. In addition, these phenomena result in increased pro-inflammatory gene expression, elevated intracellular Ca2+ levels in the DRG, and sustained p-ERK1/2 expression. Interestingly, alleviating pelvic pain could be observed in the PAR2-knockout mice and after being administered with PAR2-neutralizing antibodies [135]. Therefore, PAR2 is important in developing pelvic pain in chronic prostatitis.
2. TRPV1 channel
TRP channels are a superfamily of non-selective cation-permeable channels that are divided into six subfamilies, of which vanilloid (TRPV) comprises four members, TRPV1-4. TRPV1 is widely involved in natural responses such as pain and itching. In response to various stimuli, such as toxic heat (>43℃), capsaicin, or acidic conditions, TRPV1 channels open to facilitate calcium entry to mediate sensory pain transmission and play an essential role in inflammatory hyperalgesia. These channels decrease the inflammatory pain threshold and amplify the effect of pain [136,137]. TRPV1 channel also plays an essential part in the chronic pain of CP/CPPS.
TRPV1 expression is up-regulated in prostate tissue, DRG, and spinal cord in EAP [44,47]. Alleviating pelvic pain can be perceived in the TRPV1-knockout mice and after being administered with TRPV1 antagonists in prostatitis [48]. Moreover, plenty of TRPV1 channels promote intracellular calcium overload, leading to disharmony contraction of smooth muscle and impairment secretion of the epithelial cell to produce CPPS. In addition, TRPV1 channels might take part in cross-sensitization between bladders and prostates. In chronic prostatitis, bladders are disposed to elevated expression of TRPV1, leading to dysfunction of bladders, whereby the symptoms are similar to CP/CPPS [35,47,138]. Prostatitis might increase TRPV1 expressions in L6-S1 DRG and innervate sensory nerves of pelvic organs, including bladders. This behavior results in afferent neurons hyperexcitability and NGF up-regulation of bladders. It encourages bladder abnormality and plays an important role in the pathogenesis of CP/CPPS [60]. In general, TRPV1 channels are increased in the prostates, bladders, DRG, and spinal cords of prostatitis. It results in afferent nervous pain sensitization to amplify the effect of pain and abnormal activity of bladders by cross-sensitization between bladders and prostates. It also contributes to the generation and development of pelvic pain in chronic prostatitis.
3. TrkA
The Trk receptor family comprises three transmembrane receptors TrkA, TrkB, and TrkC. TrkA is a high-affinity catalytic receptor for NGF and has been proven to be a promising target for chronic pain development [139]. The binding of NGF to TrkA activates intracellular signaling systems that transmit signals to the nucleus for transport to DRG cell bodies, thereby activating downstream signaling systems that mediate pain development. Meanwhile, NGF-TrkA-activated downstream signaling pathways could also reduce TRPV1 opening thresholds and up-regulate TRPV1 expression [45,140]. The effect of NGF-TrkA contributes to nociceptive sensory neurons and inhibition of NGF-TrkA interactions, effectively alleviating inflammation and chronic pain [141].
Similarly, NGF-TrkA mediates the development of chronic pelvic pain in CP/CPPS. Three days after intraprostatic capsaicin injection, Trk-A and TRPV1 expressions were up-regulated in the L6 DRG and the spinal cord compared to controls [44,47]. Therefore, the effect of NGF-TrkA is effectively observed in CP/CPPS. NGF-TrkA promotes the generation and development of pain, up-regulates TRPV1, and encourages one of the pathogenesis of pelvic pain in chronic prostatitis.
4. ASIC1a
ASIC family consists of six isoforms (ASIC1a, 1b, 2a, 2b, 3, and 4) encoded by four genes. ASIC1a, located in the peripheral and spinal cord neurons, is conformationally altered and opens permeable channels to Na+ and Ca2+ after being activated by extracellular H+. This mechanism mediates pain behaviors associated with peripheral inflammation and is critical in regulating pain sensitivity [142]. ASIC1a is significantly up-regulated in the CP model, and Ca2+ concentration and p-p38 expression are increased in the dorsal horn neurons of the spinal cord. Furthermore, p-p38 was the active form of p38/MAPK. ASIC1a antagonists could alleviate the phenomena mentioned above [65]. ASIC1a channels in spinal dorsal horn neurons of EAP mice are activated in an inflammatory environment. Their opening increases Ca2+ concentrations, activating the downstream p38/MAPK signaling pathway to mediate pain behaviors associated with peripheral inflammation. It also contributes to central sensitization in the pelvic pain hypersensitivity response. It is one of the pathogenesis of persistent pelvic pain in prostatitis patients.
5. GM-CSFR
GM-CSFR consists of two subunits: the specific A subunit and the nonspecific βC subunit, which are mediated by interaction with GM-CSF nociceptive sensitization [143]. GM-CSFR could be expressed in prostate tissue and DRG. It interacts with GM-CSF, maintains chronic pelvic pain in the EAP with nociceptive sensitization, and mediates the development of perpetual pelvic pain in chronic prostatitis [50].
6. 5-HT1A and 5-HT2C receptors
5-HT receptors are G protein-coupled in the central and peripheral nervous systems and can be divided into seven families (5-HT1–7) with 14 subtypes. 5-HT1A receptors up-regulation is involved in depression-like behavior in rats [144]. Similarly, overactivity of the 5-HT2C receptors might lead to depression [145]. In addition, these two receptors have been associated with sexual dysfunction [146]. Interestingly, mice with overexpression of 5-HT1A receptors and reduced central serotonin levels have a higher response to painful stimuli. It indicated that central 5-HT1A mediates central sensitization and amplifies the nociception effect [144]. The expression of 5-HT1A and 5-HT2C receptors was significantly elevated in both hippocampal and spinal L5-S2 spinal segments in the EAP rats. These rats appeared to have depressive behavior and sexual dysfunction [18]. Therefore, the central 5-HT1A and 5-HT2C receptor expressions are significantly elevated. They mediate depression, ejaculatory dysfunction, and central sensitization to nociception in EAP rats.
7. P2X2, P2X3, and P2X4 receptors
The P2X purinoceptors are a family of ATP-gated, non-selective, and cation channel-type receptors comprising seven subtypes (P2X1–P2X7). Three subunits form a receptor channel, and the ATP binding site is located among them. ATP binding causes the subunits to bend and separate to open the central channel, facilitating the influx of extracellular cations into the cell, thereby depolarizing the cell membrane [147]. P2X3R and P2X2R expressions in DRG-mediated nociceptive hyperalgesia, mechanically-abnormal pain in peripheral tissue inflammation, and P2X4R expression in glial cells cause mechanically abnormal pain [148]. Interestingly, P2X2, P2X3, and P2X4 receptors are also associated with chronic pain in prostatitis. Compared with control, P2X4R expression in the spinal cord of EAP mice was increased approximately threefold [66]. In addition, P2X2R and P2X3R were significantly up-regulated in the DRG of thoracolumbar (TL) and LS [91]. In general, prostatitis might promote elevated P2X3R and P2X2R in DRG and P2X4R expressions in glial cells, and ATP was perceived to be emission. Stimulating P2X2, P2X3, and P2X4 receptors by releasing ATP facilitates the influx of extracellular cations into the cell and depolarizes the cell membrane. This mechanism contributes to peripheral and central sensitization to amplify the effect of nociception. It mediates the generation, development, and persistent pelvic pain in chronic prostatitis.
8. CCR2, CXCR2, CXCR3, and CXCR4
Chemokines are divided into four major subfamilies: CXC, CC, CX3C, and C. CXC and CC’s corresponding receptors are CC chemokine and CXC chemokine, and G protein-related receptors, which are divided into 11 subtypes (CCR1–11) and six types (CXCR1–6), respectively. Additionally, CCR2, CXCR2, CXCR3, and CXCR4 are related to the occurrence and development of pain. CCR2 blockade reduces the excitability of sensory neurons and the sensitivity of pain perception, preventing the release of SP from sensory nerves to the periphery. SP could induce neurological inflammation. Therefore, CCR2 is important in inducing peripheral inflammation and sensitizing injury [149]. CXCR2, CXCR3, and CXCR4 signaling pathways contribute to the development and maintenance of pain through the central sensitization mechanism. Their inhibitors obstruct spinal microglia activation, reduce pain sensitivity transmission, and play a primary role in anti-abnormal pain [150].
CCR2, CXCR2, CXCR3, and CXCR4 also contribute to pelvic pain in chronic prostatitis. CCR2 is crucial to chronic pelvic pain development because EAP-induced pain was reportedly relieved in mice with anti- CCR2 treatment [95]. In addition, the spinal cord CXCR2 receptor is involved in developing chronic pain. The intrathecal injection of the CXCR2 antagonist significantly reduces lower abdominal mechanical abnormal pain for more than 1 hour [67]. Furthermore, CXCR3 and CXCR4 induce inflammatory cell infiltration in the prostate and mediate the development of pelvic pain of prostatitis. CXCR3 is highly expressed in human prostate tissues with inflammation. It is correlated with the severity of inflammation, promoting the infiltration of macrophages and the secretion of proinflammatory mediators by interacting with CXCL10. In addition, CXCR4 promotes T-cell infiltration in the prostate by CXCL12, which leads to the generation and development of chronic prostatitis. Pelvic pain in chronic prostatitis was alleviated after the administration of inhibitors of CXCR3 and CXCR4, indicating that these two receptors contributed to the generation and development of the pelvic chain in chronic prostatitis [151,152,153]. Therefore, CCR2, CXCR2, CXCR3, and CXCR4 are essential in generating and developing persistent pelvic pain in chronic prostatitis. In addition, CCR2 plays an important role in peripheral inflammation, and CXCR3 and CXCR4 induced inflammatory cell infiltration in the prostate, mediating the inflammatory process of chronic prostatitis.
9. TWIK-related K+ (TREK) 1/2 channel
Potassium channels are involved in cell polarization and depolarization. They are essential for controlling cell excitability and are divided into three families: voltage-gated (Kv), inwardly rectifying (Kir), and tandem pore domain (K2P). The two-pore potassium (K2P) family consists of 15 members, including TREK channels. TREK channel comprises TREK1, TREK2, and TWIK-related arachidonic acid-sensitive K+ (TRAAK) channels. TREK1 and TREK2 channels contribute to the homeostasis of cells, but their inhibitions could cause depolarization [154]. Similarly, stimulation of TREK1 and TREK2 channels reduces the excitability of the sensory neurons, alleviating mechanical abnormal pain and inflammation by stabilizing the situation of cells [155]. Up-regulation of TREK-1 and TREK-2 channels is observed in the DRG of TL and LS in the chronic prostatitis models [91]. Increasing TREK1 and TREK2 channels compared to other substances promotes cell polarization. If this fails, the nerve cells appear excited and sensitized to pain, mediating the generation and development of pelvic pain in chronic prostatitis.
10. NMDAR
Up-regulation of NMDAR enhances synaptic efficacy in the CNS and leads to Ca2+ influx, activation, initiation, and maintenance of intracellular signaling pathways for central sensitization [6]. NMDAR mediates central sensitization in chronic prostatitis. NMDAR expression is increased in the cerebral PVN of EAP rats. Paraventricular nuclei are control centers of sympathetic nerves of the spinal cords. Up-regulation of NMDAR contributes to the activation and sensitization of sympathetic nerves innervating ejaculation. Therefore, its dysfunction appears in chronic prostatitis, and its symptoms are similar to CP/CPPS symptoms [31,32].
Generally, a growing level of nociceptive channels or receptors participates in neurogenic inflammation, peripheral and central sensitizations, and sympathetic nervous alteration in chronic prostatitis. The expressions of CCR2, CXCR3, CXCR4, PAR2, NGF-TrkA, and ASIC1a channels increase in chronic prostatitis. They mediate the generation and development of neurogenic or peripheral inflammation. In addition to the channels, CXCR2, TRPV1 channels, GM-CSFR, NMDAR, 5-HT1A, and 5-HT2C receptors, P2X2, P2X3, and P2X4 contribute to peripheral or central sensitization to decrease the pain threshold and produce abnormal pain. The changes in the CNS are related to the generation and development of pain and affect depression and artery dysfunction by up-regulating 5-HT1A and 5-HT2C receptors expression. In addition, the sympathetic nervous system also changes. NMDAR affects the sympathetic nervous system, changing men’s sexual functions. Although TREK1 and TREK2 channels contrast with the above channels or receptors that promote cell polarization, they stabilize the cellular membrane and decrease cellular excitability, reducing pain signals’ transmission through neural pathways.
CONCLUSION AND PROSPECT
This review focused on the close relationship between the autonomic nervous system and CP/CPPS. Chronic pelvic pain in CP/CPPS exists in the pelvic and perineal regions and other areas. It is often present with urinary abnormalities, erectile dysfunction, cognitive impairment, and mental status abnormalities, indicating that anomalies of the autonomic nervous system likely mediate the onset and development of pelvic pain in chronic prostatitis. Sensory nerve secretion of neuropeptides-mediated neuro-inflammation is involved in developing prostatitis and its sensitization. These decrease the pain thresholds and increase the transmission of nociceptive signals in afferent pathways. Sympathetic and parasympathetic nerves play an essential role in the generation and development of CP/CPPS. Their abnormalities lead to normally or abnormally exciting functions of the cardiovascular system, sexual organs, and bladder. Sympathetic denervation also contributes to prostrate inflammation. Central system sensitization could lead to widespread pain, reduced nociceptive thresholds, and persistent abnormal nociception in prostatitis.
Moreover, prostatitis affects the centers that dominate the sympathetic and parasympathetic nerves, making the other organs be observed as abnormalities. Additionally, chronic prostatitis influences brain structures and functions, resulting in the appearance of depressive behavior and perceptual disturbances. During these processes, several substances, such as neuropeptides and cells, and associated channels or receptors are involved.
Primary bladder pain syndrome is also characterized by overactivation of sensory nerves and increased neuronal plasticity, resulting in the classic pain symptoms seen in this pathology. Antibiotics do not effectively relieve these pain symptoms, but which can be successfully relieved by turning off neurogenic inflammation and preventing uncontrolled mast cell activation [156]. Therefore, we can use the autonomic nervous system as the entry point to create effective drugs for CP/CPPS.
However, more research needs to be done to study the relationship between the autonomic nervous system and the prostate and address the problem of recurrent and incomplete treatment of CP/CPPS to relieve patients’ physical and mental suffering.
Nerve pain has been studied in the context of neurological disorders than prostatitis. More investigation needs to be conducted on the autonomic nervous system, neuropeptides, and channels in CP/CPPS by reviewing the studies on neurogenic pain, inflammation, and prostatitis. Therefore, in this review, specific complex processes and mechanisms of the autonomic nervous system in developing CP/CPPS were collated and described. Furthermore, the neural-related substances and channels or receptors involved in this process were described, providing new perspectives for new therapeutic approaches to CP/CPPS.
Acknowledgements
All figures are created with BioRender.com.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81860456); The Jiangxi Natural Science Foundation (No. 20202BABL206031).
- Conceptualization: HH, HL, JZ.
- Writing–original draft: HH, HL, JZ.
- Writing–review & editing: all the authors.
References
- 1.Breser ML, Salazar FC, Rivero VE, Motrich RD. Immunological mechanisms underlying chronic pelvic pain and prostate inflammation in chronic pelvic pain syndrome. Front Immunol. 2017;8:898. doi: 10.3389/fimmu.2017.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee G. Chronic prostatitis: a possible cause of hematospermia. World J Mens Health. 2015;33:103–108. doi: 10.5534/wjmh.2015.33.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krieger JN, Nyberg L, Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–237. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 4.Habermacher GM, Chason JT, Schaeffer AJ. Prostatitis/chronic pelvic pain syndrome. Annu Rev Med. 2006;57:195–206. doi: 10.1146/annurev.med.57.011205.135654. [DOI] [PubMed] [Google Scholar]
- 5.Pena VN, Engel N, Gabrielson AT, Rabinowitz MJ, Herati AS. Diagnostic and management strategies for patients with chronic prostatitis and chronic pelvic pain syndrome. Drugs Aging. 2021;38:845–886. doi: 10.1007/s40266-021-00890-2. [DOI] [PubMed] [Google Scholar]
- 6.Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology. 2018;129:343–366. doi: 10.1097/ALN.0000000000002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang JX, Wang HF, Chen JZ, Li HY, Hu JC, Yu AA, et al. Potential neuroimmune interaction in chronic pain: a review on immune cells in peripheral and central sensitization. Front Pain Res (Lausanne) 2022;3:946846. doi: 10.3389/fpain.2022.946846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah N, Padalia D. In: StatPearls. Abai B, Abu-Ghosh A, Acharya AB, Acharya U, Adhia SG, Sedeh PA, editors. Treasure Island (FL): StatPearls Publishing; 2022. Intrathecal delivery system. [Google Scholar]
- 9.Yilmaz U, Liu YW, Berger RE, Yang CC. Autonomic nervous system changes in men with chronic pelvic pain syndrome. J Urol. 2007;177:2170–2174. doi: 10.1016/j.juro.2007.01.144. discussion 2174. [DOI] [PubMed] [Google Scholar]
- 10.Jazayeri M, Kazemi B, Aminsharifi A, Ashraf A, Naseri M, Nasseri A, et al. Sympathetic skin response in patients with vascular erectile dysfunction. World J Mens Health. 2014;32:36–42. doi: 10.5534/wjmh.2014.32.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakrabarty B, Lee S, Exintaris B. Generation and regulation of spontaneous contractions in the prostate. Adv Exp Med Biol. 2019;1124:195–215. doi: 10.1007/978-981-13-5895-1_8. [DOI] [PubMed] [Google Scholar]
- 12.Turco AE, Cadena MT, Zhang HL, Sandhu JK, Oakes SR, Chathurvedula T, et al. A temporal and spatial map of axons in developing mouse prostate. Histochem Cell Biol. 2019;152:35–45. doi: 10.1007/s00418-019-01784-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sievert KD, Hennenlotter J, Dillenburg T, Toomey P, Wöllner J, Zweers P, et al. Extended periprostatic nerve distributions on the prostate surface confirmed using diffusion tensor imaging. BJU Int. 2019;123:995–1004. doi: 10.1111/bju.14508. [DOI] [PubMed] [Google Scholar]
- 14.Ahimsadasan N, Reddy V, Khan Suheb MZ, Kumar A. In: Stat-Pearls. Abai B, Abu-Ghosh A, Acharya AB, Acharya U, Adhia SG, Sedeh PA, editors. Treasure Island (FL): StatPearls Publishing; 2022. Neuroanatomy, dorsal root ganglion. [PubMed] [Google Scholar]
- 15.Haberberger RV, Barry C, Dominguez N, Matusica D. Human dorsal root ganglia. Front Cell Neurosci. 2019;13:271. doi: 10.3389/fncel.2019.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malladi P, Simeoni S, Panicker JN. The role of pelvic neurophysiology testing in the assessment of patients with voiding dysfunction. Curr Bladder Dysfunct Rep. 2020;15:229–239. [Google Scholar]
- 17.Yoham AL, Bordoni B. In: StatPearls. Abai B, Abu-Ghosh A, Acharya AB, Acharya U, Adhia SG, Sedeh PA, editors. Treasure Island (FL): StatPearls Publishing; 2022. Anatomy, abdomen and pelvis, inferior hypogastric plexus. [PubMed] [Google Scholar]
- 18.Zhang Y, Li X, Zhou K, Zhou M, Xia K, Xu Y, et al. Influence of experimental autoimmune prostatitis on sexual function and the anti-inflammatory efficacy of celecoxib in a rat model. Front Immunol. 2020;11:574212. doi: 10.3389/fimmu.2020.574212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu H, Cui Y, Yang J, Cao Y. Loss of the sympathetic signal produces sterile inflammation of the prostate. Front Mol Neurosci. 2022;15:855376. doi: 10.3389/fnmol.2022.855376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birder LA, Kullmann FA. Role of neurogenic inflammation in local communication in the visceral mucosa. Semin Immunopathol. 2018;40:261–279. doi: 10.1007/s00281-018-0674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graefe SB, Mohiuddin SS. In: StatPearls. Abai B, Abu-Ghosh A, Acharya AB, Acharya U, Adhia SG, Sedeh PA, editors. Treasure Island (FL): StatPearls Publishing; 2022. Biochemistry, substance P. [Google Scholar]
- 22.Yaksh TL, Di Nardo A. Complexity of systems and actions underlying neurogenic inflammation. Semin Immunopathol. 2018;40:225–228. doi: 10.1007/s00281-018-0683-z. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Dong X, Yang Z, Zhao J, Lu Q, Zhu J, et al. Inhibition of CXCR4 in spinal cord and DRG with AMD3100 attenuates colon-bladder cross-organ sensitization. Drug Des Devel Ther. 2022;16:67–81. doi: 10.2147/DDDT.S336242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao LY, Tiwari N. Spinal neuron-glia-immune interaction in cross-organ sensitization. Am J Physiol Gastrointest Liver Physiol. 2020;319:G748–G760. doi: 10.1152/ajpgi.00323.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seifert O, Baerwald C. Interaction of pain and chronic inflammation. Z Rheumatol. 2021;80:205–213. doi: 10.1007/s00393-020-00951-8. [DOI] [PubMed] [Google Scholar]
- 26.Seidel MF, Hügle T, Morlion B, Koltzenburg M, Chapman V, MaassenVanDenBrink A, et al. Neurogenic inflammation as a novel treatment target for chronic pain syndromes. Exp Neurol. 2022;356:114108. doi: 10.1016/j.expneurol.2022.114108. [DOI] [PubMed] [Google Scholar]
- 27.Aranow C, Atish-Fregoso Y, Lesser M, Mackay M, Anderson E, Chavan S, et al. Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial. Ann Rheum Dis. 2021;80:203–208. doi: 10.1136/annrheumdis-2020-217872. Erratum in: Ann Rheum Dis 2021;80:e82. [DOI] [PubMed] [Google Scholar]
- 28.Udit S, Blake K, Chiu IM. Somatosensory and autonomic neuronal regulation of the immune response. Nat Rev Neurosci. 2022;23:157–171. doi: 10.1038/s41583-021-00555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallesh S, Ten Hove AS, Schneider R, Schneiker B, Efferz P, Kalff JC, et al. Sympathetic innervation modulates mucosal immune homeostasis and epithelial host defense. Cells. 2022;11:2606. doi: 10.3390/cells11162606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darweish E, Marzouk HM, Fayez YM, Eissa MS. Advanced spectrophotometric resolution techniques for a spectrally overlapping spasmolytic binary mixture along with dichloroaniline as a toxic impurity: application to content uniformity testing. J AOAC Int. 2022;106:14–25. doi: 10.1093/jaoacint/qsac092. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Luan JC, Chen JH, Zhang QJ, Xue JX, Wang YM, et al. Prostate-derived IL-1β upregulates expression of NMDA receptor in the paraventricular nucleus and shortens ejaculation latency in rats with experimental autoimmune prostatitis. Asian J Androl. 2022;24:213–218. doi: 10.4103/aja202142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Zhang QJ, Zhang JY, Wang YM, Zhu GQ, Song NH, et al. Upregulated expression of NMDA receptor in the paraventricular nucleus shortens ejaculation latency in rats with experimental autoimmune prostatitis. Andrology. 2021;9:352–360. doi: 10.1111/andr.12879. [DOI] [PubMed] [Google Scholar]
- 33.Ni J, Mizoguchi S, Bernardi K, Suzuki T, Kurobe M, Takaoka E, et al. Long-lasting bladder overactivity and bladder afferent hyperexcitability in rats with chemically-induced prostatic inflammation. Prostate. 2019;79:872–879. doi: 10.1002/pros.23794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Funahashi Y, Takahashi R, Mizoguchi S, Suzuki T, Takaoka E, Ni J, et al. Bladder overactivity and afferent hyperexcitability induced by prostate-to-bladder cross-sensitization in rats with prostatic inflammation. J Physiol. 2019;597:2063–2078. doi: 10.1113/JP277452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz ES, La JH, Young EE, Feng B, Joyce S, Gebhart GF. Chronic prostatitis induces bladder hypersensitivity and sensitizes bladder afferents in the mouse. J Urol. 2016;196:892–901. doi: 10.1016/j.juro.2016.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aydogdu O, Gocun PU, Aronsson P, Carlsson T, Winder M. Prostate-to-bladder cross-sensitization in a model of zymosan-induced chronic pelvic pain syndrome in rats. Prostate. 2021;81:252–260. doi: 10.1002/pros.24101. [DOI] [PubMed] [Google Scholar]
- 37.Cho DS, Choi JB, Kim YS, Joo KJ, Kim SH, Kim JC, et al. Heart rate variability in assessment of autonomic dysfunction in patients with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2011;78:1369–1372. doi: 10.1016/j.urology.2011.07.1379. [DOI] [PubMed] [Google Scholar]
- 38.Sharkey KA, Williams RG, Schultzberg M, Dockray GJ. Sensory substance P-innervation of the urinary bladder: possible site of action of capsaicin in causing urine retention in rats. Neuroscience. 1983;10:861–868. doi: 10.1016/0306-4522(83)90223-3. [DOI] [PubMed] [Google Scholar]
- 39.Ventura S, Lau WA, Buljubasich S, Pennefather JN. Species differences in the actions of sensory neuropeptides on contractility of the smooth muscle of the rat and guinea-pig prostate. Clin Exp Pharmacol Physiol. 2000;27:917–921. doi: 10.1046/j.1440-1681.2000.03361.x. [DOI] [PubMed] [Google Scholar]
- 40.Chuang YC, Yoshimura N, Wu M, Huang CC, Chiang PH, Tyagi P, et al. Intraprostatic capsaicin injection as a novel model for nonbacterial prostatitis and effects of botulinum toxin A. Eur Urol. 2007;51:1119–1127. doi: 10.1016/j.eururo.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 41.Park JS, Jin MH, Hong CH. Neurologic mechanisms underlying voiding dysfunction due to prostatitis in a rat model of nonbacterial prostatic inflammation. Int Neurourol J. 2018;22:90–98. doi: 10.5213/inj.1836124.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 43.Wang HJ, Tyagi P, Chen YM, Chancellor MB, Chuang YC. Low energy shock wave therapy inhibits inflammatory molecules and suppresses prostatic pain and hypersensitivity in a capsaicin induced prostatitis model in rats. Int J Mol Sci. 2019;20:4777. doi: 10.3390/ijms20194777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang HJ, Su CH, Chen YM, Yu CC, Chuang YC. Molecular effects of low-intensity shock wave therapy on L6 dorsal root ganglion/spinal cord and blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) changes in capsaicin-induced prostatitis rat models. Int J Mol Sci. 2022;23:4716. doi: 10.3390/ijms23094716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stratiievska A, Nelson S, Senning EN, Lautz JD, Smith SE, Gordon SE. Reciprocal regulation among TRPV1 channels and phosphoinositide 3-kinase in response to nerve growth factor. Elife. 2018;7:e38869. doi: 10.7554/eLife.38869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keith IM, Jin J, Neal D, Jr, Teunissen BD, Moon TD. Cell relationship in a Wistar rat model of spontaneous prostatitis. J Urol. 2001;166:323–328. [PubMed] [Google Scholar]
- 47.Zhang J, Yi QT, Gong M, Zhang YQ, Liu D, Zhu RJ. Upregulation of TRPV1 in spinal dorsal root ganglion by activating NGF-TrkA pathway contributes to pelvic organ cross-sensitisation in rats with experimental autoimmune prostatitis. Andrologia. 2019;51:e13302. doi: 10.1111/and.13302. [DOI] [PubMed] [Google Scholar]
- 48.Roman K, Hall C, Schaeffer AJ, Thumbikat P. TRPV1 in experimental autoimmune prostatitis. Prostate. 2020;80:28–37. doi: 10.1002/pros.23913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawrence GW, Zurawski TH, Dolly JO. Ca2+ signalling induced by NGF identifies a subset of capsaicin-excitable neurons displaying enhanced chemo-nociception in dorsal root ganglion explants from adult pirt-GCaMP3 mouse. Int J Mol Sci. 2021;22:2589. doi: 10.3390/ijms22052589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Li Y, Liu Q, Wu Z, Cui J, Zhu K, et al. Role of GM-CSF in a mouse model of experimental autoimmune prostatitis. Am J Physiol Renal Physiol. 2019;317:F23–F29. doi: 10.1152/ajprenal.00013.2018. [DOI] [PubMed] [Google Scholar]
- 51.Liu Z, Murphy SF, Wong L, Schaeffer AJ, Thumbikat P. Neuronal/astrocytic expression of chemokine (C-C motif) ligand 2 is associated with monocyte/macrophage recruitment in male chronic pelvic pain. Pain. 2020;161:2581–2591. doi: 10.1097/j.pain.0000000000001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y, Song B, Jin XY, Xiong EQ, Zhang JH. Possible mechanism of referred pain in the perineum and pelvis associated with the prostate in rats. J Urol. 2005;174:2405–2408. doi: 10.1097/01.ju.0000180421.90260.65. [DOI] [PubMed] [Google Scholar]
- 53.Chen H, Xie Y, Deng C, Zhang C, Lv L, Yao J, et al. The anti-inflammatory and antioxidative effects of ningmitai capsule in the experimental autoimmune prostatitis rat model. Evid Based Complement Alternat Med. 2020;2020:5847806. doi: 10.1155/2020/5847806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cornel EB, van Haarst EP, Schaarsberg RW, Geels J. The effect of biofeedback physical therapy in men with chronic pelvic pain syndrome type III. Eur Urol. 2005;47:607–611. doi: 10.1016/j.eururo.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Anderson RU, Wise D, Sawyer T, Glowe P, Orenberg EK. 6-Day intensive treatment protocol for refractory chronic prostatitis/chronic pelvic pain syndrome using myofascial release and paradoxical relaxation training. J Urol. 2011;185:1294–1299. doi: 10.1016/j.juro.2010.11.076. [DOI] [PubMed] [Google Scholar]
- 56.Wang JP, Ding GF, Wang QZ. Interstitial cells of Cajal mediate excitatory sympathetic neurotransmission in guinea pig prostate. Cell Tissue Res. 2013;352:479–486. doi: 10.1007/s00441-013-1572-3. [DOI] [PubMed] [Google Scholar]
- 57.Xu Z, Zou C, Guo M, Bian H, Zhao W, Wang J. Metastasis-associated protein 1 (MTA1) regulates the catecholamine production homeostasis via transcriptional repression of aromatic l-amino acid decarboxylase (Aadc) in the interstitial cells of Cajal of mouse prostate. Biochem Biophys Res Commun. 2020;528:732–739. doi: 10.1016/j.bbrc.2020.05.125. [DOI] [PubMed] [Google Scholar]
- 58.Eslahi A, Farpour H, Hosseini A, Ahmed F, Chowdhury U, Nikbakht HA. Evaluation of the sympathetic skin response in men with chronic prostatitis: a case-control study. Res Rep Urol. 2020;12:239–245. doi: 10.2147/RRU.S253101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clemens JQ, Mullins C, Ackerman AL, Bavendam T, van Bokhoven A, Ellingson BM, et al. MAPP Research Network Study Group. Urologic chronic pelvic pain syndrome: insights from the MAPP Research Network. Nat Rev Urol. 2019;16:187–200. doi: 10.1038/s41585-018-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Igarashi T, Tyagi P, Mizoguchi S, Saito T, Furuta A, Suzuki Y, et al. Therapeutic effects of nerve growth factor-targeting therapy on bladder overactivity in rats with prostatic inflammation. Prostate. 2021;81:1303–1309. doi: 10.1002/pros.24227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee S, Yang G, Xiang W, Bushman W. Retrograde double-labeling demonstrates convergent afferent innervation of the prostate and bladder. Prostate. 2016;76:767–775. doi: 10.1002/pros.23170. [DOI] [PubMed] [Google Scholar]
- 62.Meyer-Siegler KL, Vera PL. Intraluminal antibodies to macrophage migration inhibitory factor decrease substance P induced inflammatory changes in the rat bladder and prostate. J Urol. 2004;172(4 Pt 1):1504–1509. doi: 10.1097/01.ju.0000140213.54457.97. [DOI] [PubMed] [Google Scholar]
- 63.De Ridder D, Vanneste S, Smith M, Adhia D. Pain and the triple network model. Front Neurol. 2022;13:757241. doi: 10.3389/fneur.2022.757241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishigooka M, Zermann DH, Doggweiler R, Schmidt RA. Similarity of distributions of spinal c-Fos and plasma extravasation after acute chemical irritation of the bladder and the prostate. J Urol. 2000;164:1751–1756. [PubMed] [Google Scholar]
- 65.Fan S, Hao ZY, Zhang L, Zhou J, Zhang YF, Tai S, et al. ASIC1a contributes to the symptom of pain in a rat model of chronic prostatitis. Asian J Androl. 2018;20:300–305. doi: 10.4103/aja.aja_55_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong L, Done JD, Schaeffer AJ, Thumbikat P. Experimental autoimmune prostatitis induces microglial activation in the spinal cord. Prostate. 2015;75:50–59. doi: 10.1002/pros.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deng GC, Lu M, Zhao YY, Yuan Y, Chen G. Activated spinal astrocytes contribute to the later phase of carrageenan-induced prostatitis pain. J Neuroinflammation. 2019;16:189. doi: 10.1186/s12974-019-1584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang C, Li D. Effects of electroacupuncture on alleviating prostatodynia and inflammation in rats with chronic nonbacterial prostatitis. J Pain Res. 2021;14:2757–2765. doi: 10.2147/JPR.S321119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mendell LM. The path to discovery of windup and central sensitization. Front Pain Res (Lausanne) 2022;3:833104. doi: 10.3389/fpain.2022.833104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin Y, Bai Y, Liu P, Yang X, Qin W, Gu J, et al. Alterations in regional homogeneity of resting-state cerebral activity in patients with chronic prostatitis/chronic pelvic pain syndrome. PLoS One. 2017;12:e0184896. doi: 10.1371/journal.pone.0184896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Šutulović N, Grubač Ž, Šuvakov S, Jovanović Đ, Puškaš N, Macut Đ, et al. Chronic prostatitis/chronic pelvic pain syndrome increases susceptibility to seizures in rats and alters brain levels of IL-1β and IL-6. Epilepsy Res. 2019;153:19–27. doi: 10.1016/j.eplepsyres.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 72.Ge P, Ren J, Harrington AM, Grundy L, Castro J, Brierley SM, et al. Linaclotide treatment reduces endometriosis-associated vaginal hyperalgesia and mechanical allodynia through viscerovisceral cross-talk. Pain. 2019;160:2566–2579. doi: 10.1097/j.pain.0000000000001657. [DOI] [PubMed] [Google Scholar]
- 73.Ge S, Hu Q, Guo Y, Xu K, Xia G, Sun C. Potential alterations of functional connectivity analysis in the patients with chronic prostatitis/chronic pelvic pain syndrome. Neural Plast. 2021;2021:6690414. doi: 10.1155/2021/6690414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Šutulović N, Grubač Ž, Šuvakov S, Jerotić D, Puškaš N, Macut D, et al. Experimental chronic prostatitis/chronic pelvic pain syndrome increases anxiety-like behavior: the role of brain oxidative stress, serum corticosterone, and hippocampal parvalbumin-positive interneurons. Oxid Med Cell Longev. 2021;2021:6687493. doi: 10.1155/2021/6687493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang X, Chen J, Liu S, Gong Q, Liu T, Lu C, et al. Impaired frontal-parietal control network in chronic prostatitis/chronic pelvic pain syndrome revealed by graph theoretical analysis: a DTI study. Eur J Neurosci. 2021;53:1060–1071. doi: 10.1111/ejn.14962. [DOI] [PubMed] [Google Scholar]
- 76.Du HX, Chen XG, Zhang L, Liu Y, Zhan CS, Chen J, et al. Microglial activation and neurobiological alterations in experimental autoimmune prostatitis-induced depressive-like behavior in mice. Neuropsychiatr Dis Treat. 2019;15:2231–2245. doi: 10.2147/NDT.S211288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Du H, Chen X, Zhang L, Liu Y, Zhan C, Chen J, et al. Experimental autoimmune prostatitis induces learning-memory impairment and structural neuroplastic changes in mice. Cell Mol Neurobiol. 2020;40:99–111. doi: 10.1007/s10571-019-00723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anderson MB, Das S, Miller KE. Subcellular localization of neuronal nuclei (NeuN) antigen in size and calcitonin gene-related peptide (CGRP) populations of dorsal root ganglion (DRG) neurons during acute peripheral inflammation. Neurosci Lett. 2021;760:135974. doi: 10.1016/j.neulet.2021.135974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiang Q, Tao JS, Li JJ, Tian RB, Li XH. Changes in dorsal root ganglion CGRP expression in mouse pinch nerve injury model: modulation by somatostatin type-2 receptor. J Chem Neuroanat. 2022;121:102086. doi: 10.1016/j.jchemneu.2022.102086. [DOI] [PubMed] [Google Scholar]
- 80.Tang W, Song B, Zhou ZS, Lu GS. Intrathecal administration of resiniferatoxin produces analgesia against prostatodynia in rats. Chin Med J (Engl) 2007;120:1616–1621. [PubMed] [Google Scholar]
- 81.Sun C, An Q, Li R, Chen S, Gu X, An S, et al. Calcitonin gene-related peptide induces the histone H3 lysine 9 acetylation in astrocytes associated with neuroinflammation in rats with neuropathic pain. CNS Neurosci Ther. 2021;27:1409–1424. doi: 10.1111/cns.13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sorkin LS, Eddinger KA, Woller SA, Yaksh TL. Origins of antidromic activity in sensory afferent fibers and neurogenic inflammation. Semin Immunopathol. 2018;40:237–247. doi: 10.1007/s00281-017-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsuda M, Huh Y, Ji RR. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth. 2019;33:131–139. doi: 10.1007/s00540-018-2579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chiang BJ, Kuo HC, Liao CH. Can botulinum toxin A still have a role in treatment of lower urinary tract symptoms/benign prostatic hyperplasia through inhibition of chronic prostatic inflammation? Toxins (Basel) 2019;11:547. doi: 10.3390/toxins11090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seidel MF, Netzer C, Chobaz V, Hügle T, Geurts J. Localization of nerve growth factor expression to structurally damaged cartilaginous tissues in human lumbar facet joint osteoarthritis. Front Immunol. 2022;13:783076. doi: 10.3389/fimmu.2022.783076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao S, Wang F, Wang L, Xu Y, Lv L, Duan W, et al. Involvement of the BDNF-TrkB-KCC2 pathway in neuropathic pain after brachial plexus avulsion. Brain Behav. 2022;12:e2464. doi: 10.1002/brb3.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barker PA, Mantyh P, Arendt-Nielsen L, Viktrup L, Tive L. Nerve growth factor signaling and its contribution to pain. J Pain Res. 2020;13:1223–1241. doi: 10.2147/JPR.S247472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller LJ, Fischer KA, Goralnick SJ, Litt M, Burleson JA, Albertsen P, et al. Nerve growth factor and chronic prostatitis/chronic pelvic pain syndrome. Urology. 2002;59:603–608. doi: 10.1016/s0090-4295(01)01597-7. [DOI] [PubMed] [Google Scholar]
- 89.Done JD, Rudick CN, Quick ML, Schaeffer AJ, Thumbikat P. Role of mast cells in male chronic pelvic pain. J Urol. 2012;187:1473–1482. doi: 10.1016/j.juro.2011.11.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watanabe T, Inoue M, Sasaki K, Araki M, Uehara S, Monden K, et al. Nerve growth factor level in the prostatic fluid of patients with chronic prostatitis/chronic pelvic pain syndrome is correlated with symptom severity and response to treatment. BJU Int. 2011;108:248–251. doi: 10.1111/j.1464-410X.2010.09716.x. [DOI] [PubMed] [Google Scholar]
- 91.Schwartz ES, Xie A, La JH, Gebhart GF. Nociceptive and inflammatory mediator upregulation in a mouse model of chronic prostatitis. Pain. 2015;156:1537–1544. doi: 10.1097/j.pain.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron. 2019;101:412–420.e3. doi: 10.1016/j.neuron.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu H, Shi X, Li X, Zou J, Zhou C, Liu W, et al. Neurotransmitter and neuropeptide regulation of mast cell function: a systematic review. J Neuroinflammation. 2020;17:356. doi: 10.1186/s12974-020-02029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song Z, Jin C, Bian Z, Liang C. Extracorporeal shock wave therapy decreases the number of total and degranulated mast cells and alleviates pelvic pain in a rat model of prostatitis. Mol Cell Biochem. 2021;476:1905–1913. doi: 10.1007/s11010-020-04009-w. [DOI] [PubMed] [Google Scholar]
- 95.Quick ML, Mukherjee S, Rudick CN, Done JD, Schaeffer AJ, Thumbikat P. CCL2 and CCL3 are essential mediators of pelvic pain in experimental autoimmune prostatitis. Am J Physiol Regul Integr Comp Physiol. 2012;303:R580–R589. doi: 10.1152/ajpregu.00240.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pattabiraman G, Liu Z, Paul M, Schaeffer AJ, Thumbikat P. mMCP7, a mouse ortholog of δ tryptase, mediates pelvic tactile allodynia in a model of chronic pelvic pain. Front Pain Res (Lausanne) 2022;2:805136. doi: 10.3389/fpain.2021.805136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin D, Zhang M, Luo C, Wei P, Cui K, Chen Z. Targeting ferroptosis attenuates inflammation, fibrosis, and mast cell activation in chronic prostatitis. J Immunol Res. 2022;2022:6833867. doi: 10.1155/2022/6833867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roman K, Murphy SF, Done JD, McKenna KE, Schaeffer AJ, Thumbikat P. Role of PAR2 in the development of lower urinary tract dysfunction. J Urol. 2016;196:588–598. doi: 10.1016/j.juro.2016.01.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang G, Tian C, Liang T, Chi H, Wu A, Li J, et al. The analgesic properties of Yu-Xue-Bi tablets in the inflammatory pain mice: by the inhibition of CCL3-mediated macrophage transmigration into the spinal cord. J Ethnopharmacol. 2022;289:115051. doi: 10.1016/j.jep.2022.115051. [DOI] [PubMed] [Google Scholar]
- 100.Sumaiya K, Langford D, Natarajaseenivasan K, Shanmughapriya S. Macrophage migration inhibitory factor (MIF): a multifaceted cytokine regulated by genetic and physiological strategies. Pharmacol Ther. 2022;233:108024. doi: 10.1016/j.pharmthera.2021.108024. [DOI] [PubMed] [Google Scholar]
- 101.Lin L, Xia S, Zhang W, Chen S. Influence of Trichomonas vaginalis macrophage migration inhibitory factor on the proliferation activity of prostate epithelial cell line and its preliminary mechanism. Andrologia. 2022;54:e14397. doi: 10.1111/and.14397. [DOI] [PubMed] [Google Scholar]
- 102.Leyton-Jaimes MF, Kahn J, Israelson A. Macrophage migration inhibitory factor: a multifaceted cytokine implicated in multiple neurological diseases. Exp Neurol. 2018;301(Pt B):83–91. doi: 10.1016/j.expneurol.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 103.Bavencoffe AG, Spence EA, Zhu MY, Garza-Carbajal A, Chu KE, Bloom OE, et al. Macrophage migration inhibitory factor (MIF) makes complex contributions to pain-related hyperactivity of nociceptors after spinal cord injury. J Neurosci. 2022;42:5463–5480. doi: 10.1523/JNEUROSCI.1133-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang F, Shen X, Guo X, Peng Y, Liu Y, Xu S, et al. Spinal macrophage migration inhibitory factor contributes to the pathogenesis of inflammatory hyperalgesia in rats. Pain. 2010;148:275–283. doi: 10.1016/j.pain.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 105.Vera PL, Meyer-Siegler KL. Inflammation of the rat prostate evokes release of macrophage migration inhibitory factor in the bladder: evidence for a viscerovisceral reflex. J Urol. 2004;172(6 Pt 1):2440–2445. doi: 10.1097/01.ju.0000138055.01611.13. [DOI] [PubMed] [Google Scholar]
- 106.Ma F, Meyer-Siegler KL, Leng L, Bucala R, Vera PL. Spinal macrophage migration inhibitory factor and high mobility group box 1 mediate persistent bladder pain. Neurosci Lett. 2019;699:54–58. doi: 10.1016/j.neulet.2019.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma F, Kouzoukas DE, Meyer-Siegler KL, Hunt DE, Leng L, Bucala R, et al. MIF mediates bladder pain, not inflammation, in cyclophosphamide cystitis. Cytokine X. 2019;1:100003. doi: 10.1016/j.cytox.2019.100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karshikoff B, Martucci KT, Mackey S. Relationship between blood cytokine levels, psychological comorbidity, and widespreadness of pain in chronic pelvic pain. Front Psychiatry. 2021;12:651083. doi: 10.3389/fpsyt.2021.651083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xing L, Yang T, Cui S, Chen G. Connexin hemichannels in astrocytes: role in CNS disorders. Front Mol Neurosci. 2019;12:23. doi: 10.3389/fnmol.2019.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ashok A, Keener R, Rubenstein M, Stookey S, Bajpai S, Hicks J, et al. Consequences of interleukin 1β-triggered chronic inflammation in the mouse prostate gland: altered architecture associated with prolonged CD4+ infiltration mimics human proliferative inflammatory atrophy. Prostate. 2019;79:732–745. doi: 10.1002/pros.23784. [DOI] [PubMed] [Google Scholar]
- 111.Feng B, Dong Z, Wang Y, Yan G, Yang E, Cheng H, et al. Li-ESWT treatment reduces inflammation, oxidative stress, and pain via the PI3K/AKT/FOXO1 pathway in autoimmune prostatitis rat models. Andrology. 2021;9:1593–1602. doi: 10.1111/andr.13027. [DOI] [PubMed] [Google Scholar]
- 112.Chen J, Zhang LG, Du HX, Zhan CS, Liu Y, Zhang M, et al. Melatonin attenuates prostatic inflammation and pelvic pain via Sirt1-dependent inhibition of the NLRP3 inflammasome in an EAP mouse model. Prostate. 2021;81:1179–1190. doi: 10.1002/pros.24214. [DOI] [PubMed] [Google Scholar]
- 113.Liu H, Zhu X, Cao X, Chi A, Dai J, Wang Z, et al. IL-1β-primed mesenchymal stromal cells exert enhanced therapeutic effects to alleviate chronic prostatitis/chronic pelvic pain syndrome through systemic immunity. Stem Cell Res Ther. 2021;12:514. doi: 10.1186/s13287-021-02579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Solorza J, Oliva CA, Castillo K, Amestica G, Maldifassi MC, López-Cortés XA, et al. Effects of interleukin-1β in glycinergic transmission at the central amygdala. Front Pharmacol. 2021;12:613105. doi: 10.3389/fphar.2021.613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Neves AF, Farias FH, de Magalhães SF, Araldi D, Pagliusi M, Jr, Tambeli CH, et al. Peripheral inflammatory hyperalgesia depends on P2X7 receptors in satellite glial cells. Front Physiol. 2020;11:473. doi: 10.3389/fphys.2020.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gajtkó A, Bakk E, Hegedűs K, Ducza L, Holló K. IL-1β induced cytokine expression by spinal astrocytes can play a role in the maintenance of chronic inflammatory pain. Front Physiol. 2020;11:543331. doi: 10.3389/fphys.2020.543331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu C, Cheng K, Wu XL, Tai HY, Chai YM, Yang ZW, et al. Expression profiling of L5-S2 spinal cord dorsal horn in a rat model of chronic pelvic pain syndrome uncovers potential mechanism of electroacupuncture mediated inflammation and pain responses. J Pain Res. 2022;15:2067–2084. doi: 10.2147/JPR.S364972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu X, Cheng K, Xu C, Liu S, Sun Q, Yang Z, et al. Mechanism of acupuncture and moxibustion on chronic prostatitis/chronic pelvic pain syndrome: a narrative review of animal studies. Pain Res Manag. 2021;2021:2678242. doi: 10.1155/2021/2678242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peng X, Guo H, Yuan J, Chen Y, Xia Y, Wang L, et al. Extracellular vesicles released from hiPSC-derived MSCs attenuate chronic prostatitis/chronic pelvic pain syndrome in rats by immunoregulation. Stem Cell Res Ther. 2021;12:198. doi: 10.1186/s13287-021-02269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jeon SH, Zhu GQ, Kwon EB, Lee KW, Cho HJ, Ha US, et al. Extracorporeal shock wave therapy decreases COX-2 by inhibiting TLR4-NFκB pathway in a prostatitis rat model. Prostate. 2019;79:1498–1504. doi: 10.1002/pros.23880. [DOI] [PubMed] [Google Scholar]
- 121.Mizoguchi S, Wolf-Johnson AS, Ni J, Mori K, Suzuki T, Takaoka E, et al. The role of prostaglandin and E series prostaglandin receptor type 4 receptors in the development of bladder overactivity in a rat model of chemically induced prostatic inflammation. BJU Int. 2019;124:883–891. doi: 10.1111/bju.14845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang BC, Liu T, Gao YJ. Chemokines in chronic pain: cellular and molecular mechanisms and therapeutic potential. Pharmacol Ther. 2020;212:107581. doi: 10.1016/j.pharmthera.2020.107581. [DOI] [PubMed] [Google Scholar]
- 123.Desireddi NV, Campbell PL, Stern JA, Sobkoviak R, Chuai S, Shahrara S, et al. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha as possible biomarkers for the chronic pelvic pain syndrome. J Urol. 2008;179:1857–1861. doi: 10.1016/j.juro.2008.01.028. discussion 1861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mert T, Metin TO, Sahin E, Yaman S, Sahin M. Neuroprotective and anti-neuropathic actions of pulsed magnetic fields with low frequencies in rats with chronic peripheral neuropathic pain. Brain Res Bull. 2021;177:273–281. doi: 10.1016/j.brainresbull.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 125.Liu Y, Mikrani R, Xie D, Wazir J, Shrestha S, Ullah R, et al. Chronic prostatitis/chronic pelvic pain syndrome and prostate cancer: study of immune cells and cytokines. Fundam Clin Pharmacol. 2020;34:160–172. doi: 10.1111/fcp.12517. [DOI] [PubMed] [Google Scholar]
- 126.Du S, Wu S, Feng X, Wang B, Xia S, Liang L, et al. A nerve injury-specific long noncoding RNA promotes neuropathic pain by increasing Ccl2 expression. J Clin Invest. 2022;132:e153563. doi: 10.1172/JCI153563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu X, Fan S, Zheng M, Chen J, Zhang J, Li H. The mediation of interleukin-17 and chemokine ligand 2 in pelvic pain of experimental autoimmune prostatitis. Exp Ther Med. 2017;14:51–58. doi: 10.3892/etm.2017.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Long JY, Wang XJ, Li XY, Kong XH, Yang G, Zhang D, et al. Spinal microglia and astrocytes: two key players in chronic visceral pain pathogenesis. Neurochem Res. 2022;47:545–551. doi: 10.1007/s11064-021-03486-9. [DOI] [PubMed] [Google Scholar]
- 129.Ni H, Xu M, Xie K, Fei Y, Deng H, He Q, et al. Liquiritin alleviates pain through inhibiting CXCL1/CXCR2 signaling pathway in bone cancer pain rat. Front Pharmacol. 2020;11:436. doi: 10.3389/fphar.2020.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang C, Chen J, Wang H, Chen J, Zheng MJ, Chen XG, et al. IL-17 exacerbates experimental autoimmune prostatitis via CXCL1/CXCL2-mediated neutrophil infiltration. Andrologia. 2022;54:e14455. doi: 10.1111/and.14455. [DOI] [PubMed] [Google Scholar]
- 131.Estrada-Reyes R, Dorantes-Barrón AM, Arrieta-Báez D, Gómez-Patiño MB, Bernal-Trujillo A, Castro-García M, et al. Piper auritum Kunth (Piperaceae) improves the sexual performance of sluggish male rats through enhancing ejaculation. J Ethnopharmacol. 2019;231:453–463. doi: 10.1016/j.jep.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 132.Bakshi A, Tadi P. In: StatPearls. Abai B, Abu-Ghosh A, Acharya AB, Acharya U, Adhia SG, Sedeh PA, editors. Treasure Island (FL): StatPearls Publishing; 2022. Biochemistry, serotonin. [Google Scholar]
- 133.Moncrieff J, Cooper RE, Stockmann T, Amendola S, Hengartner MP, Horowitz MA. The serotonin theory of depression: a systematic umbrella review of the evidence. Mol Psychiatry. 2022 doi: 10.1038/s41380-022-01661-0. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heuberger DM, Schuepbach RA. Protease-activated receptors (PARs): mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb J. 2019;17:4. doi: 10.1186/s12959-019-0194-8. Erratum in: Thromb J 2019;17:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roman K, Done JD, Schaeffer AJ, Murphy SF, Thumbikat P. Tryptase-PAR2 axis in experimental autoimmune prostatitis, a model for chronic pelvic pain syndrome. Pain. 2014;155:1328–1338. doi: 10.1016/j.pain.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Diver MM, Lin King JV, Julius D, Cheng Y. Sensory TRP channels in three dimensions. Annu Rev Biochem. 2022;91:629–649. doi: 10.1146/annurev-biochem-032620-105738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li F, Wang F. TRPV1 in pain and itch. Adv Exp Med Biol. 2021;1349:249–273. doi: 10.1007/978-981-16-4254-8_12. [DOI] [PubMed] [Google Scholar]
- 138.Andersson KE, Gratzke C, Hedlund P. The role of the transient receptor potential (TRP) superfamily of cation-selective channels in the management of the overactive bladder. BJU Int. 2010;106:1114–1127. doi: 10.1111/j.1464-410X.2010.09650.x. [DOI] [PubMed] [Google Scholar]
- 139.Wu X, Li Q, Wan S, Zhang J. Molecular dynamics simulation and free energy calculation studies of the binding mechanism of allosteric inhibitors with TrkA kinase. J Biomol Struct Dyn. 2021;39:202–208. doi: 10.1080/07391102.2019.1708798. [DOI] [PubMed] [Google Scholar]
- 140.Hirose M, Kuroda Y, Murata E. NGF/TrkA signaling as a therapeutic target for pain. Pain Pract. 2016;16:175–182. doi: 10.1111/papr.12342. [DOI] [PubMed] [Google Scholar]
- 141.Malerba F, Bruni Ercole B, Florio R, Cattaneo A. A quantitative bioassay to determine the inhibitory potency of NGF-TrkA antagonists. SLAS Discov. 2021;26:823–830. doi: 10.1177/24725552211000672. [DOI] [PubMed] [Google Scholar]
- 142.Wang Y, Hu X, Sun Y, Huang Y. The role of ASIC1a in inflammatory immune diseases: a potential therapeutic target. Front Pharmacol. 2022;13:942209. doi: 10.3389/fphar.2022.942209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Broughton SE, Hercus TR, Nero TL, Dottore M, McClure BJ, Dhagat U, et al. Conformational changes in the GM-CSF receptor suggest a molecular mechanism for affinity conversion and receptor signaling. Structure. 2016;24:1271–1281. doi: 10.1016/j.str.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 144.Haleem DJ. Targeting serotonin1A receptors for treating chronic pain and depression. Curr Neuropharmacol. 2019;17:1098–1108. doi: 10.2174/1570159X17666190811161807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ślifirski G, Król M, Turło J. 5-HT receptors and the development of new antidepressants. Int J Mol Sci. 2021;22:9015. doi: 10.3390/ijms22169015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Roaiah MF, Elkhayat YI, Rashed LA, GamalEl Din SF, El Guindi AM, Soliman IF, et al. 5HT-1A receptor polymorphism effects ejaculatory function in Egyptian patients with lifelong premature ejaculation. Rev Int Androl. 2019;17:138–142. doi: 10.1016/j.androl.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 147.Sheng D, Hattori M. Recent progress in the structural biology of P2X receptors. Proteins. 2022;90:1779–1785. doi: 10.1002/prot.26302. [DOI] [PubMed] [Google Scholar]
- 148.Inoue K. The role of ATP receptors in pain signaling. Neurochem Res. 2022;47:2454–2468. doi: 10.1007/s11064-021-03516-6. [DOI] [PubMed] [Google Scholar]
- 149.Dansereau MA, Midavaine É, Bégin-Lavallée V, Belkouch M, Beaudet N, Longpré JM, et al. Mechanistic insights into the role of the chemokine CCL2/CCR2 axis in dorsal root ganglia to peripheral inflammation and pain hypersensitivity. J Neuroinflammation. 2021;18:79. doi: 10.1186/s12974-021-02125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Piotrowska A, Ciapała K, Pawlik K, Kwiatkowski K, Rojewska E, Mika J. Comparison of the effects of chemokine receptors CXCR2 and CXCR3 pharmacological modulation in neuropathic pain model-in vivo and in vitro study. Int J Mol Sci. 2021;22:11074. doi: 10.3390/ijms222011074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang M, Liu Y, Chen J, Chen L, Zhang L, Chen X, et al. Targeting CXCL12/CXCR4 signaling with AMD3100 might selectively suppress CXCR4+ T-cell chemotaxis leading to the alleviation of chronic prostatitis. J Inflamm Res. 2022;15:2551–2566. doi: 10.2147/JIR.S352336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hua X, Zhang J, Ge S, Liu H, Du H, Niu Q, et al. CXCR3 antagonist AMG487 ameliorates experimental autoimmune prostatitis by diminishing Th1 cell differentiation and inhibiting macrophage M1 phenotypic activation. Prostate. 2022;82:1223–1236. doi: 10.1002/pros.24395. [DOI] [PubMed] [Google Scholar]
- 153.Hua X, Ge S, Zhang M, Mo F, Zhang L, Zhang J, et al. Pathogenic roles of CXCL10 in experimental autoimmune prostatitis by modulating macrophage chemotaxis and cytokine secretion. Front Immunol. 2021;12:706027. doi: 10.3389/fimmu.2021.706027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ávalos Prado P, Chassot AA, Landra-Willm A, Sandoz G. Regulation of two-pore-domain potassium TREK channels and their involvement in pain perception and migraine. Neurosci Lett. 2022;773:136494. doi: 10.1016/j.neulet.2022.136494. [DOI] [PubMed] [Google Scholar]
- 155.Qiu Y, Huang L, Fu J, Han C, Fang J, Liao P, et al. TREK channel family activator with a well-defined structure-activation relationship for pain and neurogenic inflammation. J Med Chem. 2020;63:3665–3677. doi: 10.1021/acs.jmedchem.9b02163. [DOI] [PubMed] [Google Scholar]
- 156.Trama F, Illiano E, Marchesi A, Brancorsini S, Crocetto F, Pandolfo SD, et al. Use of intravesical injections of platelet-rich plasma for the treatment of bladder pain syndrome: a comprehensive literature review. Antibiotics (Basel) 2021;10:1194. doi: 10.3390/antibiotics10101194. [DOI] [PMC free article] [PubMed] [Google Scholar]