Abstract
Purpose
Sperm DNA fragmentation (SDF) is a functional sperm abnormality that can impact reproductive potential, for which four assays have been described in the recently published sixth edition of the WHO laboratory manual for the examination and processing of human semen. The purpose of this study was to examine the global practices related to the use of SDF assays and investigate the barriers and limitations that clinicians face in incorporating these tests into their practice.
Materials and Methods
Clinicians managing male infertility were invited to complete an online survey on practices related to SDF diagnostic and treatment approaches. Their responses related to the technical aspects of SDF testing, current professional society guidelines, and the literature were used to generate expert recommendations via the Delphi method. Finally, challenges related to SDF that the clinicians encounter in their daily practice were captured.
Results
The survey was completed by 436 reproductive clinicians. Overall, terminal deoxynucleotidyl transferase deoxyuridine triphosphate Nick-End Labeling (TUNEL) is the most commonly used assay chosen by 28.6%, followed by the sperm chromatin structure assay (24.1%), and the sperm chromatin dispersion (19.1%). The choice of the assay was largely influenced by availability (70% of respondents). A threshold of 30% was the most selected cut-off value for elevated SDF by 33.7% of clinicians. Of respondents, 53.6% recommend SDF testing after 3 to 5 days of abstinence. Although 75.3% believe SDF testing can provide an explanation for many unknown causes of infertility, the main limiting factors selected by respondents are a lack of professional society guideline recommendations (62.7%) and an absence of globally accepted references for SDF interpretation (50.3%).
Conclusions
This study represents the largest global survey on the technical aspects of SDF testing as well as the barriers encountered by clinicians. Unified global recommendations regarding clinician implementation and standard laboratory interpretation of SDF testing are crucial.
Keywords: Delphi method; Diagnostic test; DNA fragmentation; Infertility, male; Survey
INTRODUCTION
Spermatozoa are highly differentiated cells in which DNA integrity is essential for successful fertilization and subsequent embryo development. Although the oocyte is capable of partially repairing sperm DNA damage [1], prevention of damage by defense mechanisms (such as chromatin condensation) is crucial, as the mature sperm is unable to repair DNA damage once the damage has occurred [2]. Damage to the sperm genome may be in the form of sperm DNA fragmentation (SDF), which includes single- and double-stranded DNA breaks (SSBs and DSBs, respectively), that may be attributed to extrinsic (e.g., heat, smoking, pollutants, and drugs), intrinsic (e.g., defective germ cell maturation and abortive apoptosis), or mixed factors [3,4]. Oxidative stress (OS) is considered a major underlying mechanism leading to SDF.
High levels of SDF have been observed in subjects with unexplained or idiopathic male infertility (UMI and IMI, respectively) and in conditions well known to be associated with infertility, such as varicocele [3,5]. According to recent reports, high SDF values are also associated with recurrent pregnancy loss (RPL), with a positive correlation between SDF and the age of patients and the number of miscarriages [6]. The impact of SDF on assisted reproductive technology (ART) outcome is also documented. A meta-analysis by Simon et al [7], which included 41 studies with a total of 8,068 ART cycles (3,734 in-vitro fertilization [IVF], 2,282 intracytoplasmic sperm injection [ICSI], and 2,052 mixed IVF+ICSI), reported a significant effect of sperm DNA damage on clinical pregnancy.
During male fertility evaluation, the diagnostic capacity of the conventional semen analysis is limited by a lack of information on the functional status of spermatozoa, which is related to their true fertilization potential [8]. SDF may represent a better predictor than conventional seminal parameters in terms of male infertility and ART success, as demonstrated by one study that used an alkaline comet assay to measure SDF [9].
To address the need for advanced sperm testing, SDF was recently introduced in the sixth edition of the World Health Organization (WHO) laboratory manual for the examination and processing of human semen and was included in the section “Extended Semen Examination” [10]. However, the manual does not provide precise guidance on the method to be used and the lack of a definite cut-off in the literature makes it necessary to use laboratory-specific reference limits [11]. The standardization of this parameter including testing conditions and cut-off values remains a necessity. Furthermore, it is important to identify the limitations to testing that clinicians face worldwide which may prevent them from ordering this test when necessary. Therefore, the aims of this study are as follows:
1) To investigate the global practices related to the technical aspects of SDF testing.
2) To summarize and present professional society guidelines related to the technical aspects of SDF testing and compare them to our findings.
3) To provide expert recommendations on the technical aspects of SDF testing based on global practices, society guidelines, and available evidence in the literature.
4) To investigate the barriers and limitations of SDF testing, as well as the opinions of reproductive specialists worldwide, and to identify areas that need improvement and have the potential for future advancement.
MATERIALS AND METHODS
This was a cross-sectional online survey constructed and disseminated in accordance with the CHERRIES checklist criteria [12]. The survey was administered by the Global Andrology Forum [13] and was targeted at reproductive clinicians across the globe. The complete survey captured demographic variables, practices related to indications of SDF testing, management of elevated SDF, technical aspects of SDF testing, and the barriers and limitations that clinicians encounter that hinder the implementation of this parameter into their practice. The complete methodology along with the CHERRIES checklist are included in Supplement File 1. The complete survey is included in Supplement File 2.
This article presents the responses related to the technical aspects of SDF testing (questions 29–32) as well as the barriers and limitations to incorporating SDF testing into clinical practice (questions 61–64). The complete responses to these sections are provided in Supplement File 3. In addition, professional society guidelines were screened for recommendations related to the technical aspects of SDF testing. These included guidelines of the American Urological Association/American Society for Reproductive Medicine (AUA/ASRM) [14,15], the European Association of Urology (EAU) [16,17], the European Society of Human Reproduction and Embryology (ESHRE) [18], the European Academy of Andrology (EAA) [19], the Italian Society of Andrology and Sexual Medicine (SIAMS) [20], and the German Society of Gynecology and Obstetrics (DGGG), the Austrian Society of Gynecology and Obstetrics (OEGGG), and the Swiss Society of Gynecology and Obstetrics (SGGG) [21]. Lastly, expert recommendations regarding the technical aspects of SDF testing were devised and a consensus was reached using the Delphi technique [22].
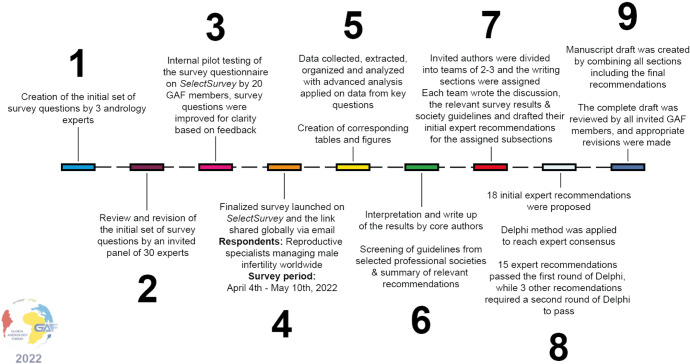
Fig. 1 summarizes the survey methodology.
Fig. 1. Complete survey methodology. The complete survey consisted of 64 questions on SDF clinical practices divided into five sections: demographics, indications for SDF testing, technical aspects of SDF testing, management of elevated SDF, and barriers in incorporating SDF into clinical practice. A total of 18 recommendations were made as follows: seven for indications for SDF testing, ten for management of infertile men with elevated SDF, and one for technical aspects of SDF testing. Passing criteria for the Delphi method was set at >80% scoring the recommendation ≥7 in agreement. SDF: sperm DNA fragmentation, GAF: global andrology forum.
1. Ethics statement
Submission of the survey was voluntary to all invited participants. Informed consent was described and obtained from all participants who agreed to submit the survey. No participant identifiers were shared with any third party. No patient information was elicited from any participant. No patients were involved in this study.
RESULTS, GUIDELINES, DISCUSSION, AND EXPERT RECOMMENDATIONS
1. Participant demographics
A total of 436 responses representing the practices of clinicians from 55 countries were included in the final analysis. The majority (60.1%) of respondents were urologists or reproductive urologists/andrologists, followed by ART specialists (16.1%). Most (69.4%) had more than 5 years of experience in managing male infertility. The complete demographic data are found in Supplement File 4.
2. Technical aspects of sperm DNA fragmentation testing
1) Results
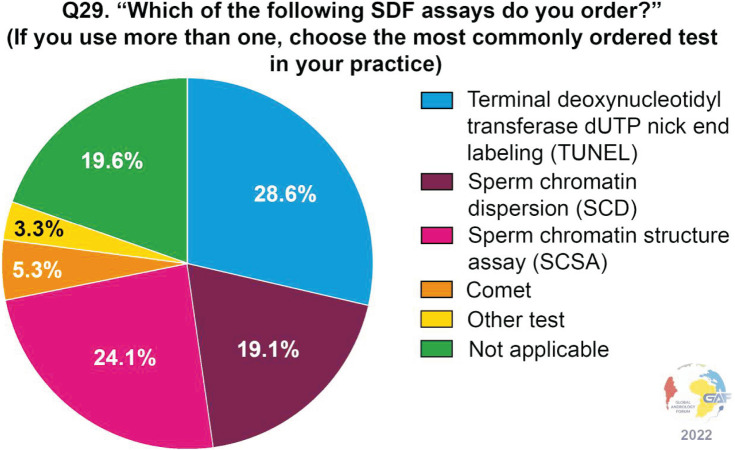
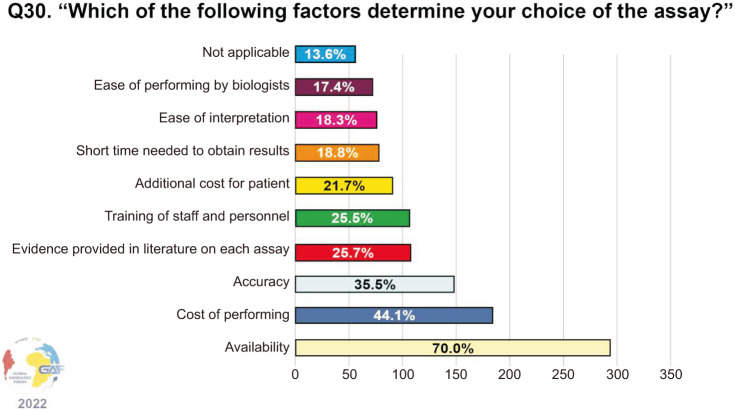
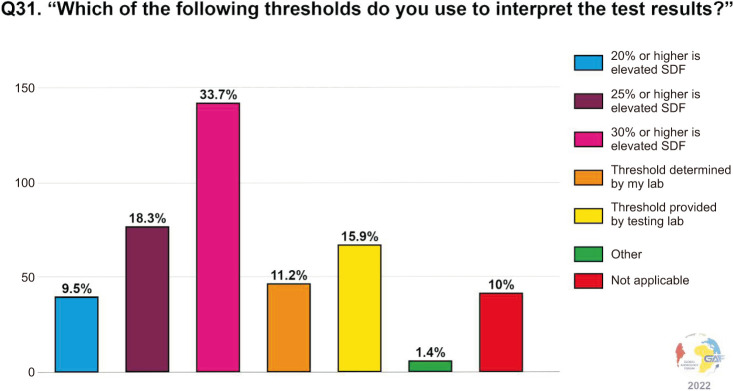
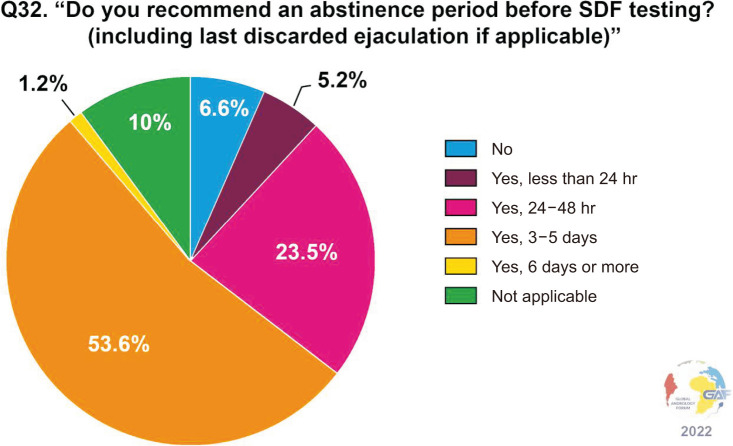
When asked which assays are ordered for measuring SDF, the most frequently used assay by clinicians responding to the survey was terminal deoxynucleotidyl transferase deoxyuridine triphosphate Nick-End Labeling (TUNEL) (28.6%). Less common were the sperm chromatin structure assay (SCSA) and sperm chromatin dispersion (SCD) assays (24.1% and 19.1%, respectively). Conversely, only a minority of respondents used the comet assay (5.3%). Notably, nearly one-fifth of respondents (19.6%) selected the answer “not applicable.” The above data are shown in Fig. 2. The main factors determining the choice of the assay are availability (70.0%), cost (44.1%), and accuracy (35.5%) (Fig. 3). When asked about the thresholds used for the interpretation of the results, the cut-off most commonly considered as significant is 30% (33.7%), followed by 25% (18.3%), and 20% (9.5%) (Fig. 4). Concerning time of abstinence before testing, more than half (53.6%) of respondents recommend 3–5 days, while 23.5% consider a shorter period of 24–48 hours to be more suitable (Fig. 5).
Fig. 2. SDF assays ordered by the respondents. SDF: sperm DNA fragmentation.
Fig. 3. Factors determining the choice of SDF assay. Respondents were allowed to select more than one answer. The percentage for each answer was calculated by dividing the number of respondents who had selected it by the total number of respondents who had answered this question (n=420). SDF: sperm DNA fragmentation.
Fig. 4. Thresholds used for interpretation by the respondents. SDF: sperm DNA fragmentation.
Fig. 5. Recommended abstinence period before SDF testing. SDF: sperm DNA fragmentation.
2) Society guidelines
The AUA/ASRM guidelines do not comment on any particular SDF testing methods, however, they do acknowledge the inconsistencies in SDF results due to the various assays used, non-standardized protocols, and ambiguous result interpretation [14,15].
The EAU guidelines briefly discuss the four assays for SDF (TUNEL, comet, SCD, and SCSA), but also acknowledge the controversy and unreliability regarding their use [16]. Their discussion on the various testing assays including the advantages and limitations of each are further expanded upon in the “Clinical Consultation Guide on the Indications for Performing SDF Testing in Men with Infertility”, but still no definitive recommendations are provided regarding the use or interpretation of these assays [17].
The ESHRE guidelines do not mention SDF testing assays [18]. EAA guidelines list the following examples when discussing the assessment of sperm DNA integrity: SCSA, alkaline comet, and TUNEL [19]. The position statement from SIAMS describes the four tests for the assessment of SDF (TUNEL, comet, SCD, and SCSA), along with the advantages and disadvantages of each, while no specific technique or cut-off values are recommended [20]. There is no mention of SDF testing methods in the guidelines of the DGGG, OEGGG, and SGGG [21].
3) Discussion
(1) Types of assays
The 6th edition of the WHO manual details the laboratory techniques for performing four SDF assays: the TUNEL assay, the single cell gel electrophoresis assay (also known as the comet assay), the SCD assay, and acridine orange flow cytometry (AO-FCM) (also known as the SCSA) [10]. The cut-off values are specific to each test and the manual states that appropriate thresholds of the tests should be determined and validated by the performing laboratory.
The TUNEL assay is a direct method of detecting SSBs and DSBs by incorporating deoxynucleotides (usually deoxyuridine triphosphate) coupled to a fluorescent marker into the sites of breaks. This technique can be used with fluorescence microscopy or flow cytometry, allowing the analysis of thousands of cells [10,23]. TUNEL is sensitive, reliable, with minimal inter-observer variability, and can be performed on few sperm (in situ), but requires inter-laboratory standardization, and the use of expensive equipment and personnel training [3,24].
The comet assay is another direct method to evaluate SDF based on the differential migration of DNA fragments under the influence of an electric field [10,25]. One unique feature of the comet assay is its ability to distinguish between SSBs and DSBs, depending on the pH of the medium used. An alkaline comet detects both types, while a neutral comet detects DSBs [23,26]. The comet assay can be performed in very low sperm counts, is sensitive and reproducible, but requires an experienced observer, and suffers from high inter-observer variability as well as variable protocols and thresholds [3,24,27].
The SCD assay is an indirect method for measuring SDF in that it relies on the susceptibility of chromatin to denaturation after the action of an acid treatment, which occurs more when there is fragmented DNA [28]. It is a simple assay with the advantage that it does not require the use of fluorescence or any specialized equipment, and in fact, there are commercial kits available as well [3,24]. The reliability of SCD has been commended with high intra-individual agreement [29]. However, it has been criticized for high inter-observer variability [27].
The SCSA is a well-described and commonly utilized test with a standardized protocol for estimating DNA fragmentation index [30]. It is an indirect assay that relies on the different fluorescence of acridine orange dye depending on its binding to denatured or intact DNA. The use of flow cytometry allows the simultaneous examination of a large number of cells [3,24]. Despite it being highly standardized and reproducible, expensive equipment and personnel training are required which may limit its use [3,24].
Consistent with the results of this survey, the TUNEL assay is reported to be the most commonly utilized [31]. However, it is important to emphasize that the choice of SDF assay strongly depends on availability, the quality of laboratories and their trained biologists, as well as the direct and indirect costs related to the reagents and run-time for each assay.
(2) Cut-off values
A lack of standard cut-off values has led the WHO 6th edition to recommend every laboratory to establish its reference values based on predictive values for fertility outcomes [11]. Different studies have reported on the sensitivities and specificities of using different SDF thresholds, with marked variability [3]. A recent meta-analysis by Santi et al [32] indicated that an SDF cut-off of 20% can differentiate between fertile and infertile men with 79% sensitivity and 86% specificity. This universal cut-off has also been cited by Agarwal et al [3] in their guidelines for clinicians. Esteves et al [33] also recommended this cut-off when using TUNEL, SCSA, and SCD, but cited a higher value of 26% for alkaline comet assay. Nevertheless, the definition of a reliable cut-off should be interpreted cautiously due to the multifactorial character of a couple’s infertility.
(3) Abstinence period
Most scientific literature has reported that time of abstinence is related to SDF, with a prolonged abstinence period considered a risk factor for higher SDF [34]. The most accepted hypothesis is that a longer epididymal transit subjects spermatozoa to higher OS and increases acid denaturation of chromatin [35]. To allow reliable interpretation and comparison of SDF assays results, confounding factors such as abstinence time before testing should be standardized. More than half the respondents recommend 3 to 5 days, a period similar to that of the conventional semen analysis, while almost a quarter recommend a shorter duration. In their clinical practice recommendations, Esteves et al [33] recommended a period of 2 to 5 days, as well as a fixed period of ejaculatory abstinence when performing repeat SDF tests to monitor management success.
4) Expert recommendations
The method to test for SDF should take into consideration the availability of resources, personnel, and laboratory complexity of the tests. The recommended assays are TUNEL, comet, SCD, and SCSA. There is a lack of standard cut-off values, and every laboratory might be able to establish its own reference values based on predictive values for fertility outcomes. Decreased abstinence period is associated with lower SDF, therefore SDF testing is recommended with an abstinence period of fewer than five days.
3. Barriers and limitations in incorporating SDF testing into clinical practice
1) Results
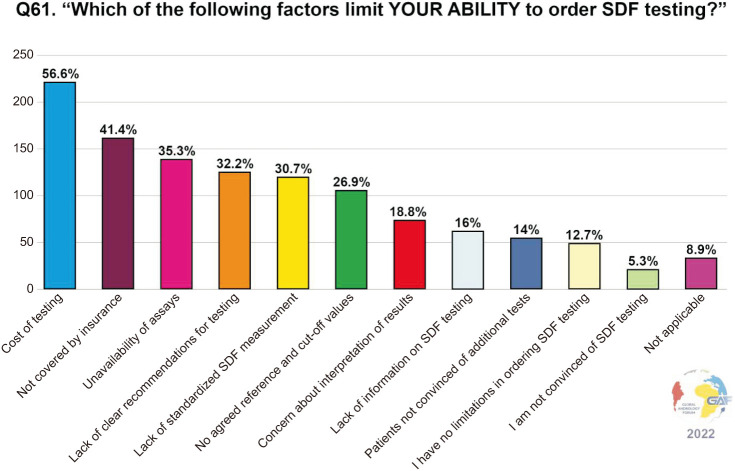
When asked about the factors that limit a clinician’s ability to order SDF in their practice, 56.6% of the participants selected “cost” as a limiting factor, followed by testing not being covered by insurance (41.4%). Other limiting factors were the unavailability of assays as reported by 35.3%, lack of clear recommendations for testing by 32.2%, and lack of standardized SDF measurement by 30.7%. Interestingly, 14.0% reported that their patients are not convinced of additional tests. The limiting factors to ordering SDF testing are summarized in Fig. 6.
Fig. 6. Factors that limit the ability of responding practitioners to order SDF testing. Respondents were allowed to select more than one answer. The percentage for each answer was calculated by dividing the number of respondents who had selected it by the total number of respondents who had answered this question (n=394). SDF: sperm DNA fragmentation.
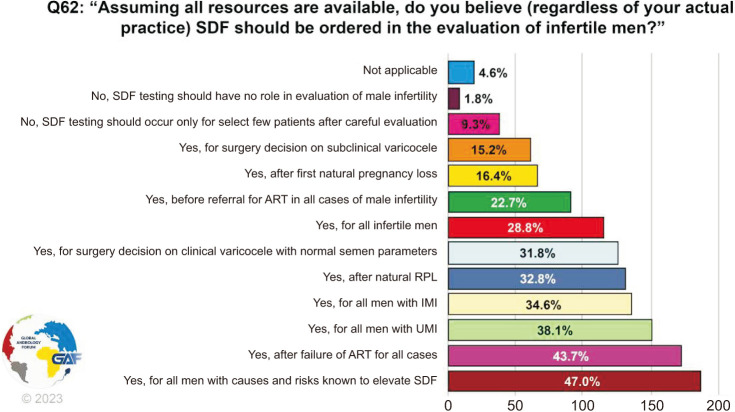
When asked about their opinion on when SDF testing should be ordered in the evaluation of infertile men, answers were variable with the three most common scenarios being: for all men with causes and risks known to elevate SDF (47.0%), after the failure of ART for all cases (43.7%), and for all men with UMI (38.1%) (Fig. 7). Only 9.3% felt that SDF testing should occur only for a select few patients after careful evaluation, while 28.8% believe it should be ordered for all infertile men.
Fig. 7. Opinion on SDF evaluation for infertile men. The percentage for each answer was calculated by dividing the number of respondents who had selected it by the total number of respondents who had answered this question (n=396). SDF: sperm DNA fragmentation, ART: assisted reproductive technology, RPL: recurrent pregnancy loss, IMI: idiopathic male infertility, UMI: unexplained male infertility.
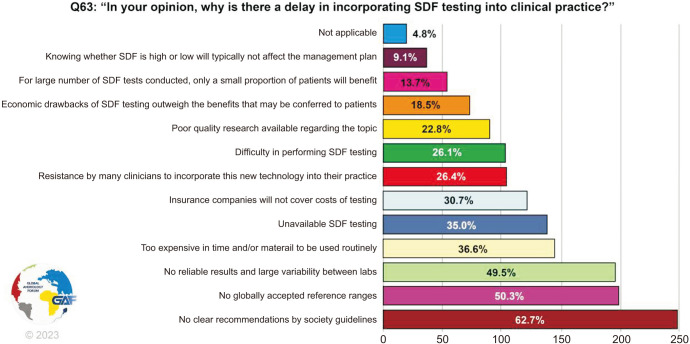
Respondents were then asked why they think there is a delay in incorporating SDF testing into clinical practice, with almost 62.7% choosing lack of recommendations by society guidelines as a reason, followed by no globally accepted reference ranges (50.3%), and lack of reliable results with variability between labs (49.5%) (Fig. 8).
Fig. 8. Reasons for delay in incorporating SDF into clinical practice. The percentage for each answer was calculated by dividing the number of respondents who had selected it by the total number of respondents who had answered this question (n=394). SDF: sperm DNA fragmentation.
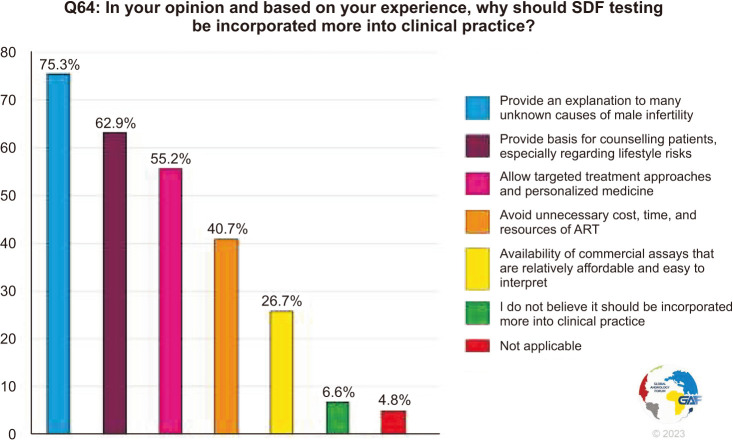
Finally, the participants were asked to choose reasons why they feel SDF should be incorporated more into clinical practice based on their experience. The majority (75.3%) chose that it can provide an explanation to many unknown causes of male infertility, followed by providing a basis to counsel patients (69.2%) and allow personalized therapeutic approaches (55.2%), while 6.6% believe it should not be utilized more (Fig. 9).
Fig. 9. Reasons why SDF should be incorporated more into clinical practice. The percentage for each answer was calculated by dividing the number of respondents who had selected it by the total number of respondents who had answered this question (n=393). SDF: sperm DNA fragmentation, ART: assisted reproductive technology.
2) Discussion
From our survey, the two major factors limiting the clinical utilization of SDF testing are financial factors and technical factors in terms of standardization. The global coverage of our survey can influence the barriers faced by clinicians in each country, especially when there are disparities in the allocation of healthcare costs. This has limited our ability to generalize the financial limitations faced by some of the respondents. On the other hand, a lack of globally standardized recommendations is further stressed upon by this survey. The responses strongly highlight the need to establish standardized testing methods, thresholds, and techniques, as well as the clinical application of testing in terms of indications for testing and management of infertile men with elevated SDF.
Generally, the indications for SDF testing include UMI and IMI, RPL, clinical varicocele, presence of known risk factors, recurrent ART failure, and in some cases ART planning [36]. Many different treatment strategies also exist for managing infertile men with elevated SDF. These include management of underlying causes such as repair of clinical varicocele and antibiotics for genital tract infections, weight loss, avoidance of risk factors, empiric antioxidants, and reduction in ejaculatory abstinence [37]. Sperm selection techniques and testicular sperm have also been shown to reduce SDF levels and can be used in ART.
As pregnancy is affected by many controlled and uncontrolled factors, the predictive value of SDF alone on reproduction is limited, however, recent research has provided us with a better understanding of situations where SDF testing is indicated. This is demonstrated in our survey which shows respondents advocating for SDF testing in infertile men with known risks or conditions, after ART failure, those with unexplained or idiopathic infertility, and couples experiencing RPL. Only less than 10% do not support SDF testing in these scenarios. Finally, the majority of clinicians feel that SDF testing will uncover an underlying etiology for male infertility, and will allow personalized medicine with targeted therapy and individualized patient-based counseling.
LIMITATIONS OF THE PRESENT STUDY
This survey was limited by its open nature and inability to capture response rates. To ensure worldwide representation of reproductive experts, the survey was disseminated in different forms. Furthermore, many demographic variables were captured by the survey and revealed heterogeneity in the respondents’ countries, specialization, experience, and practice. Although this diversity strengthens our survey in terms of accurate representation of practitioners who manage male infertility, we were unable to conduct stratified analysis to capture the difference in practice based on potential confounding demographic factors. This is especially true when discussing technical tests which have different costs and availability in different regions, as well as clinical barriers and limitations that may differ due to economic, social, or administrative factors.
CONCLUSIONS
This study discusses the technical aspects of SDF testing as well as the barriers and limitations that face a large number of clinicians, represented by over 400 clinicians from 55 countries. In general, TUNEL is the most commonly used assay, closely followed by SCSA and SCD. Prolonged abstinence before testing is not recommended and should be less than 5 days. Unfortunately, there is no universal cut-off value for SDF interpretation and different clinicians use different ways to interpret it. The current recommendations are that each laboratory should determine and validate its thresholds. Future research should focus on establishing a gold standard test with clear cut-off points for measuring and interpreting SDF levels.
The most common reason cited by the respondents, for their perception as to why there is a delay in incorporating SDF into clinical practice, is a lack of clear recommendations by professional society guidelines. This also explains the marked heterogeneity in responses of clinicians to various questions of our survey, further reinforcing the need for universal recommendations by the professional societies related to all aspects of SDF in the evaluation and management of infertile men, including technical aspects and cut-off points.
Acknowledgements
The authors are thankful to the following societies for promoting this our online survey through the efforts of their members listed below:
1. AK Andrologie und Sexuelle Funktionsstörungen as part of the Österreichische Gesellschaft für Urologie und Andrologie (Germar-Michael Pinggera, MD, Austria).
2. Algerian Association of Urology (Nazim Gherabi, MD, Algeria).
3. Andrology Working Group, Society of Urologic Surgery in Turkey (Gökhan Çeker, MD, Turkey; Oğuzhan Kahraman, MD, Turkey; Erman Ceyhan, MD, Turkey).
4. Egyptian Society for Sexual Medicine & Surgery (Ahmed El-Sakka, MD, Egypt).
5. Egyptian Society of Andrology (Taymour Mostafa, MD, Egypt).
6. Indonesian Society of Andrological Urology (Gede Wirya Kusuma Duarsa, PhD, Indonesia).
7. Indonesian Urological Association (Ponco Birowo, MD, PhD Indonesia; Gede Wirya Kusuma Duarsa, PhD, Indonesia; Fahmi Bahar, MD, Indonesia).
8. Italian Society of Andrology and Sexual Medicine (Aldo E. Calogero, MD, Italy).
9. Italian Society of Human Reproduction (Carlo Trotta, MD, Italy; Giovanni M. Colpi, MD, Italy; Lucia Rocco, PhD, Italy).
10. Italian Society of Urology (Gian Maria Busetto, MD, PhD, Italy).
11. Lebanese Society of Urology (Mohamad Moussa, MD, Lebanon).
12. Malaysian Society of Andrology and the Study of the Aging Male (Christopher Ho, MD, Malaysia; Kay Seong, NGOO, MD, Malaysia).
13. Malaysian Urological Association (Teng Aik Ong, MD, Malaysia).
14. Mediterranean Society for Reproductive Medicine (Hassan Sallam, MD, PhD, Egypt).
15. Middle East Society for Sexual Medicine (Amr El Meliegy, MD, Egypt).
16. Romanian Association for Sexual Medicine (Catalina Zenoaga-Barbarosie, MSc, Romania).
17. Saudi Andrology Group (Naif Alhathal, MD, Saudi Arabia).
18. Society for Men's Health Singapore (King Chien; Joe Lee, MD, Singapore).
19. Society of Egyptian Fellows and Members of the Royal College of Obstetricians and Gynecologists (Hassan Sallam MD, PhD, Egypt).
20. Turkish Association of Urology (Arif Kalkanli, MD, Turkey; Ateş Kadıoğlu, MD, Turkey).
21. Vietnam Society of Sexual Medicine (Ho Vinh Phuoc Nguyen, MD; Quang Nguyen, MD, PhD; Quang Tien Long Tran, MD; Tan V. Le, MD).
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: None.
- Conceptualization: AA.
- Data curation: SK, AMH.
- Formal analysis: AMH.
- Methodology: AA, RS, AF, AMH.
- Project administration: AA, RS, AF.
- Supervision: AA, RS.
- Writing – original draft: AF, GS, PK, RAG, FB, MG, TT, GIR, DD.
- Writing – review & editing: all the authors.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.230076.
Complete methodology with CHERRIES checklist
Raw data
Complete demographic data
References
- 1.Meseguer M, Santiso R, Garrido N, Garcia-Herrero S, Remohi J, Fernandez JL. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril. 2011;95:124–128. doi: 10.1016/j.fertnstert.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Marin C, Gosalvez J, Roy R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci. 2012;13:14026–14052. doi: 10.3390/ijms131114026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal A, Majzoub A, Baskaran S, Panner Selvam MK, Cho CL, Henkel R, et al. Sperm DNA fragmentation: a new guideline for clinicians. World J Mens Health. 2020;38:412–471. doi: 10.5534/wjmh.200128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A, Farkouh A, Parekh N, Zini A, Arafa M, Kandil H, et al. Sperm DNA fragmentation: a critical assessment of clinical practice guidelines. World J Mens Health. 2022;40:30–37. doi: 10.5534/wjmh.210056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol. 2012;9:678–690. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 6.Carlini T, Paoli D, Pelloni M, Faja F, Dal Lago A, Lombardo F, et al. Sperm DNA fragmentation in Italian couples with recurrent pregnancy loss. Reprod Biomed Online. 2017;34:58–65. doi: 10.1016/j.rbmo.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Simon L, Zini A, Dyachenko A, Ciampi A, Carrell DT. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J Androl. 2017;19:80–90. doi: 10.4103/1008-682X.182822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbăroşie C, Agarwal A, Henkel R. Diagnostic value of advanced semen analysis in evaluation of male infertility. Andrologia. 2021;53:e13625. doi: 10.1111/and.13625. [DOI] [PubMed] [Google Scholar]
- 9.Simon L, Lutton D, McManus J, Lewis SE. Sperm DNA damage measured by the alkaline Comet assay as an independent predictor of male infertility and in vitro fertilization success. Fertil Steril. 2011;95:652–657. doi: 10.1016/j.fertnstert.2010.08.019. Erratum in: Fertil Steril 2012;97:1479. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) WHO laboratory manual for the examination and processing of human semen. 6th ed. Geneva: WHO; 2021. [Google Scholar]
- 11.Boitrelle F, Shah R, Saleh R, Henkel R, Kandil H, Chung E, et al. The sixth edition of the WHO manual for human semen analysis: a critical review and SWOT analysis. Life (Basel) 2021;11:1368. doi: 10.3390/life11121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of Internet e-surveys (CHERRIES) J Med Internet Res. 2004;6:e34. doi: 10.2196/jmir.6.3.e34. Erratum in: J Med Internet Res 2012;14:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal A, Saleh R, Boitrelle F, Cannarella R, Hamoda TAA, Durairajanayagam D, et al. The global andrology forum (GAF): a world-wide, innovative, online initiative to bridge the gaps in research and clinical practice of male infertility and sexual health. World J Mens Health. 2022;40:537–542. doi: 10.5534/wjmh.220127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. Fertil Steril. 2021;115:54–61. doi: 10.1016/j.fertnstert.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part II. Fertil Steril. 2021;115:62–69. doi: 10.1016/j.fertnstert.2020.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. EAU Working Group on Male Sexual and Reproductive Health. European Association of Urology guidelines on sexual and reproductive health-2021 update: male sexual dysfunction. Eur Urol. 2021;80:333–357. doi: 10.1016/j.eururo.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Tharakan T, Bettocchi C, Carvalho J, Corona G, Jones TH, Kadioglu A, et al. EAU Working Panel on Male Sexual Reproductive Health. European Association of Urology guidelines panel on male sexual and reproductive health: a clinical consultation guide on the indications for performing sperm DNA fragmentation testing in men with infertility and testicular sperm extraction in nonazoospermic men. Eur Urol Focus. 2022;8:339–350. doi: 10.1016/j.euf.2020.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018;2018:hoy004. doi: 10.1093/hropen/hoy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colpi GM, Francavilla S, Haidl G, Link K, Behre HM, Goulis DG, et al. European Academy of Andrology guideline Management of oligo-astheno-teratozoospermia. Andrology. 2018;6:513–524. doi: 10.1111/andr.12502. [DOI] [PubMed] [Google Scholar]
- 20.Ferlin A, Calogero AE, Krausz C, Lombardo F, Paoli D, Rago R, et al. Management of male factor infertility: position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS): endorsing organization: Italian Society of Embryology, Reproduction, and Research (SIERR) J Endocrinol Invest. 2022;45:1085–1113. doi: 10.1007/s40618-022-01741-6. [DOI] [PubMed] [Google Scholar]
- 21.Toth B, Baston-Büst DM, Behre HM, Bielfeld A, Bohlmann M, Bühling K, et al. Diagnosis and treatment before assisted reproductive treatments. Guideline of the DGGG, OEGGG and SGGG (S2k level, AWMF register number 015-085, February 2019) - part 2, hemostaseology, andrology, genetics and history of malignant disease. Geburtshilfe Frauenheilkd. 2019;79:1293–1308. doi: 10.1055/a-1017-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Villiers MR, de Villiers PJ, Kent AP. The Delphi technique in health sciences education research. Med Teach. 2005;27:639–643. doi: 10.1080/13611260500069947. [DOI] [PubMed] [Google Scholar]
- 23.Sharma R, Iovine C, Agarwal A, Henkel R. TUNEL assay-standardized method for testing sperm DNA fragmentation. Andrologia. 2021;53:e13738. doi: 10.1111/and.13738. [DOI] [PubMed] [Google Scholar]
- 24.Farkouh A, Salvio G, Kuroda S, Saleh R, Vogiatzi P, Agarwal A. Sperm DNA integrity and male infertility: a narrative review and guide for the reproductive physicians. Transl Androl Urol. 2022;11:1023–1044. doi: 10.21037/tau-22-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortés-Gutiérrez EI, López-Fernández C, Fernández JL, Dávila-Rodríguez MI, Johnston SD, Gosálvez J. Interpreting sperm DNA damage in a diverse range of mammalian sperm by means of the two-tailed comet assay. Front Genet. 2014;5:404. doi: 10.3389/fgene.2014.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutta S, Henkel R, Agarwal A. Comparative analysis of tests used to assess sperm chromatin integrity and DNA fragmentation. Andrologia. 2021;53:e13718. doi: 10.1111/and.13718. [DOI] [PubMed] [Google Scholar]
- 27.Cho CL, Agarwal A, Majzoub A, Esteves SC. Clinical utility of sperm DNA fragmentation testing: concise practice recommendations. Transl Androl Urol. 2017;6(Suppl 4):S366–S373. doi: 10.21037/tau.2017.07.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59–66. [PubMed] [Google Scholar]
- 29.Esteves SC, López-Fernández C, Martínez MG, Silva EA, Gosálvez J. Reliability of the sperm chromatin dispersion assay to evaluate sperm deoxyribonucleic acid damage in men with infertility. Fertil Steril. 2022;117:64–73. doi: 10.1016/j.fertnstert.2021.08.045. [DOI] [PubMed] [Google Scholar]
- 30.Evenson DP. Sperm chromatin structure assay (SCSA®) for fertility assessment. Curr Protoc. 2022;2:e508. doi: 10.1002/cpz1.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baskaran S, Agarwal A, Panner Selvam MK, Finelli R, Robert KA, Iovine C, et al. Tracking research trends and hotspots in sperm DNA fragmentation testing for the evaluation of male infertility: a scientometric analysis. Reprod Biol Endocrinol. 2019;17:110. doi: 10.1186/s12958-019-0550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santi D, Spaggiari G, Simoni M. Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management - meta-analyses. Reprod Biomed Online. 2018;37:315–326. doi: 10.1016/j.rbmo.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 33.Esteves SC, Zini A, Coward RM, Evenson DP, Gosálvez J, Lewis SEM, et al. Sperm DNA fragmentation testing: summary evidence and clinical practice recommendations. Andrologia. 2021;53:e13874. doi: 10.1111/and.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sokol P, Drakopoulos P, Polyzos NP. The effect of ejaculatory abstinence interval on sperm parameters and clinical outcome of ART. A systematic review of the literature. J Clin Med. 2021;10:3213. doi: 10.3390/jcm10153213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pons I, Cercas R, Villas C, Braña C, Fernández-Shaw S. One abstinence day decreases sperm DNA fragmentation in 90 % of selected patients. J Assist Reprod Genet. 2013;30:1211–1218. doi: 10.1007/s10815-013-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal A, Farkouh A, Saleh R, Abdel-Meguid Hamoda TA, Harraz AM, Kavoussi P, et al. Global Andrology Forum. Controversy and consensus on indications for sperm DNA fragmentation testing in male infertility: a global survey, current guidelines, and expert recommendations. World J Mens Health. 2023;41:575–602. doi: 10.5534/wjmh.220282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farkouh A, Agarwal A, Hamoda TAA, Kavoussi P, Saleh R, Zini A, et al. Global Andrology Forum. Controversy and consensus on the management of elevated sperm DNA fragmentation in male infertility: a global survey, current guidelines, and expert recommendations. World J Mens Health. 2023 doi: 10.5534/wjmh.230008. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete methodology with CHERRIES checklist
Raw data
Complete demographic data