Abstract
Alcaligenes eutrophus H16 produces a soluble hydrogenase (SH) and a membrane-bound hydrogenase (MBH) which catalyze the oxidation of H2, supplying the organism with energy for autotrophic growth. The promoters of the structural genes for the SH and the MBH, PSH and PMBH, respectively, were identified by means of the primer extension technique. Both promoters were active in vivo under hydrogenase-derepressing conditions but directed only low levels of transcription under conditions which repressed hydrogenase synthesis. The cellular pools of SH and MBH transcripts under the different growth conditions correlated with the activities of the respective promoters. Also, an immediate and drastic increase in transcript pool levels occurred upon derepression of the hydrogenase system. Both promoters were dependent on the minor sigma factor ς54 and on the hydrogenase regulator HoxA in vivo. PSH was stronger than PMBH under both heterotrophic and autotrophic growth conditions. The two promoters were induced at approximately the same rates upon derepression of the hydrogenase system in diauxic cultures. The response regulator HoxA mediated low-level activation of PSH and PMBH in a heterologous system.
Alcaligenes eutrophus H16 is a gram-negative, strictly respiratory bacterium with a facultatively lithoautotrophic lifestyle. The organism grows on a wide range of sugars and organic acids. In the absence of such substrates, it can utilize H2 and CO2 as the sole sources of energy and carbon, respectively (reviewed in references 6, 17, and 18). Two biochemically and cytologically distinct enzymes catalyze the oxidation of molecular hydrogen in A. eutrophus. A heterodimeric membrane-bound hydrogenase (MBH) couples hydrogen oxidation to electron transport phosphorylation in a membrane-bound respiratory complex (42). The MBH is attached to the periplasmic surface of the inner membrane (14, 23). This enzyme is representative of a widespread type of [NiFe] hydrogenase, examples of which have been found in many different groups of gram-negative bacteria (17). The other hydrogenase of A. eutrophus is a heterotetrameric soluble hydrogenase (SH). Like the MBH, it is a nickel metalloenzyme (21). The SH couples hydrogen oxidation to NAD reduction, supplying the cell with reducing power under lithoautotrophic conditions (44). Homologous enzymes have been found in both gram-negative and gram-positive bacteria (26).
Both the SH and the MBH undergo a complex maturation process requiring an ensemble of specialized accessory proteins (4, 10, 29, 30, 35, 48). The enzymes themselves and their respective accessory proteins are encoded in neighboring gene clusters on the 450-kb endogenous megaplasmid pHG1 (11, 29, 48, 51). Altogether, 31 hydrogenase-related genes have been identified to date. Specialized maturation proteases (4, 48), metal-center-assembly proteins (10), a type b cytochrome (3), and a high-affinity nickel transporter (15) are among the products of the hydrogenase gene cluster.
Although similar hydrogenases are found in other lithoautotrophs, the pattern of hydrogenase regulation in A. eutrophus H16 is exceptional. Even in the closest relatives, such as A. hydrogenophilus, hydrogenase expression is strictly H2 dependent (33). In contrast, A. eutrophus H16 synthesizes both hydrogenases not only in the presence of H2 but also during growth on poor carbon sources. Hydrogenase synthesis is blocked during growth on preferentially utilized carbon sources, such as succinate and pyruvate. Thus, it appears that the signal which triggers derepression of the hydrogenase system is not a particular substrate but rather is a physiological cue related to the energy status of the cell (19, 22).
Early studies showed that the expression of the hydrogenases of A. eutrophus H16 is coordinate, implying the existence of a central regulatory function or functions (20). Indeed, subsequent genetic analysis led to the identification of a locus, designated hoxA, encoding a factor required for the synthesis of both hydrogenases (41). Sequencing revealed that the hoxA gene product is a member of the NtrC group of the superfamily of transcriptional activators (12). Somewhat later, a gene encoding the cognate histidine kinase was discovered (18). However, the product of this gene is inactive (18, 32). Among the hydrogenase null mutants was a strain with a defect in a chromosomal locus. The defective gene product turned out to be an rpoN homolog, indicating that the expression of at least some of the hydrogenase genes is dependent on the minor sigma factor ς54 (39, 53).
The finding that an NtrC-like activator is required for hydrogenase synthesis in A. eutrophus H16 suggests that key genes of the hydrogenase system are regulated at the level of transcription. The present study, focusing on the promoters controlling the genes for the catalytic subunits of the SH (hoxFUYH) and the MBH (hoxKG), provides the first decisive evidence for this assumption. We identified the probable promoter sites by means of primer extension mapping. Plasmid-borne reporter constructs and a quantitative assay for hydrogenase transcripts were used to demonstrate that the expression of the hydrogenase genes is regulated at the level of promoter activity and to monitor the course of induction. We show that the activities of the SH and the MBH promoters in vivo are dependent on HoxA and ς54. Furthermore, a heterologous expression system was used to measure the HoxA-mediated activation of the SH and the MBH promoters in the absence of other A. eutrophus gene products.
MATERIALS AND METHODS
Strains and plasmids.
Bacterial strains and plasmids are listed in Table 1. A. eutrophus H16 is the wild-type strain harboring the endogenous megaplasmid pHG1. Strains HF09 (20) and HF18 (41) are derivatives of H16. Escherichia coli S17-1 (46) served as a donor in conjugative transfers.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| Alcaligenes eutrophus | ||
| H16 | MBH+ SH+ | DSM428; ATCC 17699 |
| HF18 | H16 hoxA18; MBH− SH− | 20 |
| HF09 | H16 rpoN09; MBH− SH− | 41 |
| Escherichia coli | ||
| S17-1 | Tra+recA pro thi hsdR chr::RP4-2 | 46 |
| JM109 | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB) e14− (F′ lacIqlacZΔM15 proAB traD36) | 56 |
| Plasmids | ||
| pTrc99B | Apr; trc promoter; lacIq | 1 |
| pEDY305 | Tcr RK2 ori Mob+; promoterless lacZ gene | E. Schwartz and B. Friedrich |
| pGE53 | 7.0-kb SalI fragment of the MBH locus in pVK101 | 29 |
| pCH128 | Derivative of pSUP202 carrying hoxF | 13 |
| pCH179 | 4.9-kb PstI-SalI fragment of pGE53 in pTZ18R | C. Kortlüke and B. Friedrich |
| pCH182 | Derivative of pTZ19R carrying hoxK | 4 |
| pCH185 | 2.0-kb PstI-EcoRI fragment of pCH179 in pTZ18R | This study |
| pCH220 | Derivative of pTZ19R carrying hoxA | 12 |
| pCH292 | Derivative of pTZ18R carrying hoxF | 57 |
| pCH618 | Derivative of pTrc99B with hoxA under the control of the trc promoter | This study |
| pGE319 | 572-bp PstI-FspI fragment of pCH182 in pEDY305 | This study |
| pGE320 | 811-bp BssHI-NruI fragment of pCH128 in pEDY305 | O. Lenz and B. Friedrich |
Tra, transfer of mobilizable plasmids; ori, origin of replication; Mob, ability to be mobilized.
Plasmid pGE319 is a derivative of the mobilizable, broad-host-range promoter assay vector pEDY305 carrying the hoxK promoter region inserted upstream of the promoterless lacZ gene. This plasmid was generated by inserting a 572-bp PstI-FspI fragment of pCH182 between the PstI and SrfI sites of pEDY305. The corresponding fragment for the hoxS promoter region, pGE320, contained an 811-bp BssHI-NruI fragment of pCH128 between the AscI and SrfI sites of the pEDY305 polylinker.
A plasmid for the controlled expression of the regulator protein HoxA was constructed from vector plasmid pTrc99B (1). The synthetic oligonucleotides BF366 (5′-CATGTCTGACAAGCAGGCCACTGTTCTTGTCG-3′) and BF367 (5′-TCGACGACAAGAACAGTGGCCTGCTTGTCAGA-3′) were hybridized by heating at 70°C for 10 min. The hybrid (containing the first 11 codons of hoxA) was ligated into NcoI-SalI-cut pTrc99B. The resulting plasmid was cut with HindIII, end polished, recut with SalI, dephosphorylated, and ligated to a 1,615-bp SalI-PvuII fragment of plasmid pCH220 carrying the corresponding 3′ portion of hoxA. This process resulted in a plasmid, pCH618, with reconstituted hoxA under the control of the trc promoter. The region of this plasmid containing the synthetic DNA was verified by sequencing.
Media and growth conditions.
Strains of A. eutrophus were grown in modified Luria broth containing 0.25% sodium chloride and 0.4% fructose or in mineral salts medium as described previously (12). Synthetic media for heterotrophic growth contained 0.4% fructose (FN medium), 0.4% succinate (SN medium), or 0.2% fructose and 0.2% glycerol (FGN medium). FGN medium contained 1 μM NiCl2 in place of standard trace elements mixture SL6 (12). Lithoautotrophic cultures were grown in mineral salts medium under an atmosphere of hydrogen, carbon dioxide, and oxygen (8:1:1, vol/vol/vol). Strains of E. coli were grown in Luria-Bertani medium or in M9 medium containing glycerol (36). Solid media contained 1.5% agar. Antibiotics were added as appropriate (for A. eutrophus: kanamycin, 350 μg/ml; tetracycline, 15 μg/ml; for E. coli: kanamycin, 25 μg/ml; tetracycline, 15 μg/ml; ampicillin, 100 μg/ml). For induction of trc promoter-driven expression constructs, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to cultures to a final concentration of 1 mM.
Conjugative plasmid transfer.
Mobilizable plasmids were transferred from E. coli S17-1 to A. eutrophus by a spot mating technique (46). Transconjugants were selected on FN medium plates containing the appropriate antibiotics.
DNA techniques.
Standard DNA techniques were used in this study (2). Large-scale isolation of plasmid DNA was carried out by the alkaline lysis procedure followed by ethidium bromide-cesium chloride gradient centrifugation. Smaller amounts of plasmid DNA were isolated with QIAGEN tip-20 columns (QIAGEN Inc.) according to the manufacturer’s instructions. DNA fragments used in plasmid constructions were isolated from agarose gels with the GlassMAX spin column system (Life Technologies, Inc.).
Isolation of RNA.
Total cellular RNA was isolated from 2-ml samples of cell suspension by a hot-phenol method (24).
Primer extension analysis.
5′ Ends of in vivo mRNAs were mapped by a primer extension protocol (24). The synthetic oligonucleotide BF153 (5′-GTATGTCGATCAGCCGTGTACGG-3′) was used as a specific primer for SH transcripts. BF174 (5′-TTCAGGAAACTTCGTCGCGA-3′) and BF185 (5′-TGCCTGCGCATGACTTCATA-3′) were used for mapping MBH transcripts. Primer extension reaction mixtures included 10 μg of RNA, 0.2 pmol of 32P-labelled oligonucleotide, and 200 U of Moloney murine leukemia virus reverse transcriptase (Life Technologies). Following phenol-chloroform extraction and ethanol precipitation, extension products were separated in 6% sequencing gels together with sequence ladders of the corresponding regions and detected by autoradiography. For quantitative transcript determinations, the radioactivity of excised bands was determined with a Canberra-Packard 1600TR liquid scintillation counter.
RNase protection assays.
Riboprobes were synthesized with a MAXIscript kit (Ambion, Inc.) and 32P-labelled UTP (800 Ci/mmol; Dupont NEN). NdeI-linearized plasmid pCH185 and DdeI-linearized plasmid pCH292 served as templates for the generation of the MBH- and SH-specific probes, respectively. The sizes of the riboprobes were 204 and 225 nucleotides, respectively. The efficiency of incorporation of the radioactive label was monitored by trichloroacetic acid precipitation. Subsequently, the in vitro transcripts were purified by two rounds of ethanol precipitation. Total RNA (5 to 20 μg) was added to 30 μl of hybridization buffer (40 mM piperazine-N,N′-bis(2-ethanesulfonic acid [PIPES] [pH 6.4], 0.4 M NaCl, and 1 mM EDTA in a 1:4 (vol/vol) mixture of water-deionized formamide) containing 105 to 106 cpm of the appropriate riboprobe. After an initial denaturation step (5 min at 85°C), hybridization proceeded for at least 8 h at 45°C. RNase digestion cocktail (10 mM Tris-HCl [pH 7.5], 300 mM NaCl, 5 mM EDTA, 40 μg of RNase A per ml, 2 μg of RNase T1 per ml) (350 μl) was added, and the mixture was incubated for 30 min at 30°C. Treatment with proteinase K (10 μl of 20% [wt/vol] sodium dodecyl sulfate [SDS], 2.5 μl of proteinase K (20 mg/ml); 37°C for 15 min) was followed by phenol extraction and precipitation in the presence of 10 μg of yeast tRNA. The pellet was dissolved in 3 to 5 μl of formamide loading buffer (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol FF), and the mixture was applied to a 6% sequencing gel. In vitro transcripts of known lengths served as size standards. Quantitation of the protected fragments was done either by counting the radioactivity of excised bands with a Canberra-Packard 1600TR liquid scintillation counter or by analyzing scanned images obtained with a Molecular Dynamics 445 SI storage PhosphorImager by use of IPLab Gel software (Signal Analytics).
Enzyme assays.
For enzyme assays, independent single colonies were picked from plates and inoculated into liquid media. Precultures were incubated for 15 to 20 h at 35°C. Since the hydrogenase system is repressed at temperatures above 33°C, this step ensured that the cells were uniformly devoid of hydrogenase at the beginning of an experiment. SH (hydrogen:NAD+ oxidoreductase; EC 1.12.1.2) activity was assayed by spectrophotometric determination of H2-dependent NAD reduction in detergent-treated cells (16). MBH (ferredoxin:H+ oxidoreductase; EC 1.18.99.1) activity was determined by measurement of H2-dependent methylene blue reduction in isolated membranes (42). One unit of hydrogenase activity was the amount of enzyme which catalyzed the formation of 1 μmol of product per min. β-Galactosidase was assayed as described previously (57), and the activity (in units) was calculated according to Miller (36) except that cell optical density was measured at 436 nm (OD436). Unless otherwise indicated, enzyme activities were assayed in mid-log-phase cells, i.e., cells grown to optical densities of 3 in SN medium, 5 in FN medium, 7 in FGN medium, and 1 under lithoautotrophic conditions. Protein determinations were done according to the method of Lowry et al. (34).
RESULTS
Primer extension mapping.
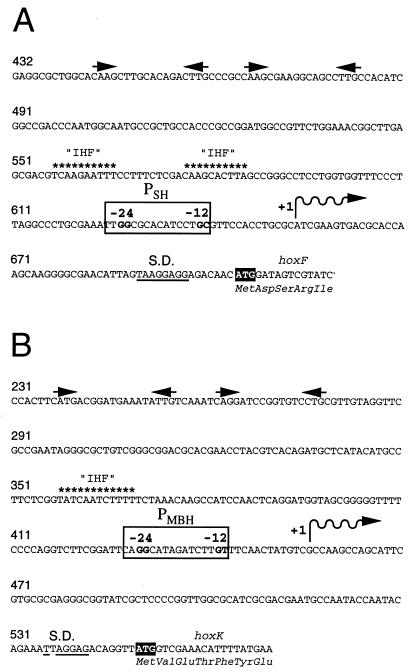
Previous complementation studies with cloned DNA fragments carrying the SH and the MBH loci identified the 5′ boundaries of the upstream sequences sufficient for high-level expression of the two enzymes. Sequencing showed that the complementing segments extended 259 bp upstream of the initiation codon of hoxF and 544 bp upstream of the initiation codon of hoxK, respectively (29, 51). In order to further localize the respective promoters, the 5′ ends of in vivo transcripts of the two enzymes were mapped by means of the primer extension technique. For the SH, we detected a single extension product with a 5′ terminus corresponding to bp 654 of the published sequence (51 bp upstream of the ATG of hoxF) (Fig. 1A). This finding confirms the previously published result (51) and suggests that the SH transcript starts at this site. The sequence 5′-TTGGCGCACATCCTGC-3′, located a short distance upstream, is a likely candidate for the SH promoter (PSH). Primer extension analysis of MBH transcripts gave unique signals for the two primers used. The sizes of the extension products indicated a common 5′ end corresponding to bp 456 of the published sequence (94 bp upstream of the ATG of hoxK), suggesting a transcription start site at this position (Fig. 1B). Located 13 bp upstream is a sequence resembling a −24/−12 promoter: 5′-CAGGCATAGATCTTGT-3′. This sequence (PMBH) differs from the canonical sequence for RpoN-dependent promoters (49) at the second position of the second conserved dinucleotide (T instead of C). However, a similar sequence has been reported for the promoter of the psp operon (55).
FIG. 1.
Nucleotide sequences of the PSH (A) and the PMBH (B) regions. The start codons of hoxF and hoxK are highlighted, and the deduced N-terminal amino acid sequences are given below the nucleotide sequences. Shine-Dalgarno (S.D.) homologies are underlined. The 5′ ends of in vivo mRNAs determined by primer extension are indicated by wavy lines. The putative PSH and PMBH are boxed, and the conserved dinucleotides of the −24/−12 motifs are emphasized. Sequence elements resembling the consensus E. coli IHF binding site (9, 25) are indicated by asterisks. Palindromic sequence motifs are indicated by pairs of arrows above the sequence. Numbering is based on respective sequence publications (29, 51). A “G” was added to the sequence of the hoxF upstream region between positions 472 and 473 to correct a sequence error. The GenBank entry (accession no. 55230) has been amended accordingly.
Expression of the genes for the SH and the MBH enzymes is regulated at the transcriptional level.
In order to study the activity of PSH and PMBH in vivo, we inserted fragments carrying the respective regions upstream of the promoterless lacZ gene in low-copy vector plasmid pEDY305 and monitored the β-galactosidase activities produced by the resulting plasmids in cells of A. eutrophus H16 growing under hydrogenase-repressing and hydrogenase-inducing conditions. Succinate is a preferred substrate for A. eutrophus H16 and supports rapid growth. Both hydrogenases are tightly repressed in cultures grown on succinate (19). Succinate-grown cells harboring the indicator plasmids contained only small amounts of β-galactosidase, indicating very weak transcription from the two promoters (Fig. 2A and B). Under autotrophic conditions with H2 as the energy source, the organism relied on the hydrogenases to generate energy, and both enzymes were synthesized at high levels (Fig. 2E and F). The test-plasmid-harboring strains produced high levels of β-galactosidase during growth on H2, indicating strong transcription from PSH and PMBH (Fig. 2A and B). The hydrogenases are also derepressed to various degrees during growth on suboptimal carbon sources, i.e., carbon sources which support lower growth rates than does succinate. Cells grown on fructose, for instance, produce intermediate levels of SH and MBH (19). The test-plasmid-harboring strains produced intermediate levels of β-galactosidase when cultivated on fructose (Fig. 2A and B). A. eutrophus H16 grows very slowly on glycerol and synthesizes large quantities of active SH and MBH (19). We also measured β-galactosidase activities in test strains grown on glycerol. For convenience, the latter experiments were done with diauxic cultures supplemented with a mixture of fructose and glycerol. High levels of β-galactosidase were present in the test strains following the transition to glycerol (Fig. 2A and B). Taken together, these results reveal a pattern of transcription approximately reflecting the patterns of SH and MBH activities (Fig. 2E and F). We conclude that transcriptional control is the major component in the regulation of these enzymes.
FIG. 2.
Comparison of promoter activities, transcript pool sizes, and hydrogenase activities for the SH and the MBH. Single colonies of A. eutrophus H16 or of A. eutrophus H16 harboring the indicator plasmids pGE319 and pGE320 were picked, inoculated into fructose medium, and grown for 15 to 20 h at 35°C. Fresh media were seeded from the precultures to an OD436 of 0.1, and the cultures were grown to the mid-log phase. Samples were taken for determination of β-galactosidase or hydrogenase activity or for preparation of RNA. For heterotrophic cultures, the carbon and energy sources were succinate (SN medium), fructose (FN medium), and a mixture of fructose and glycerol (FGN medium). Autotrophic cultures (H2/CO2) were treated with a mixture of hydrogen, carbon dioxide, and oxygen gases. Specific activities of the hydrogenase enzymes are given as micromoles of product formed per minute per milligram of protein. The values for transcript abundance for the lithoautotrophic cultures were arbitrarily taken as 100%. Bars represent the means of five independent determinations. Except in the case of the transcript determinations, standard errors were too small for graphic representation. Rel., relative.
Cellular levels of the SH and the MBH transcripts reflect the activities of PSH and PMBH.
The experiments with the promoter assay plasmids reported above showed that the differential activity of PSH and PMBH is the basis of the derepression of the hydrogenase enzymes. In this test system, however, differences in translational efficiency can skew promoter activity measurements. Therefore, quantitative comparisons are necessarily limited to values obtained under identical conditions. We therefore assayed a second transcriptional parameter, transcript abundance, by the RNase protection technique. A. eutrophus was grown under the standard repressing and derepressing conditions described above, and samples were taken from mid-log-phase cultures for the isolation of total cellular RNA. In RNA from succinate-grown cells, SH and MBH transcripts were not detectable (Fig. 2C and D). High levels of both transcripts were detected in glycerol- and H2-grown cells. Cells grown on fructose contained intermediate amounts of SH and MBH transcripts. Thus, the patterns of transcript abundance for the SH and the MBH mRNAs reflected the profiles of apparent PSH and PMBH activities, confirming the transcriptional regulation of the hydrogenase genes.
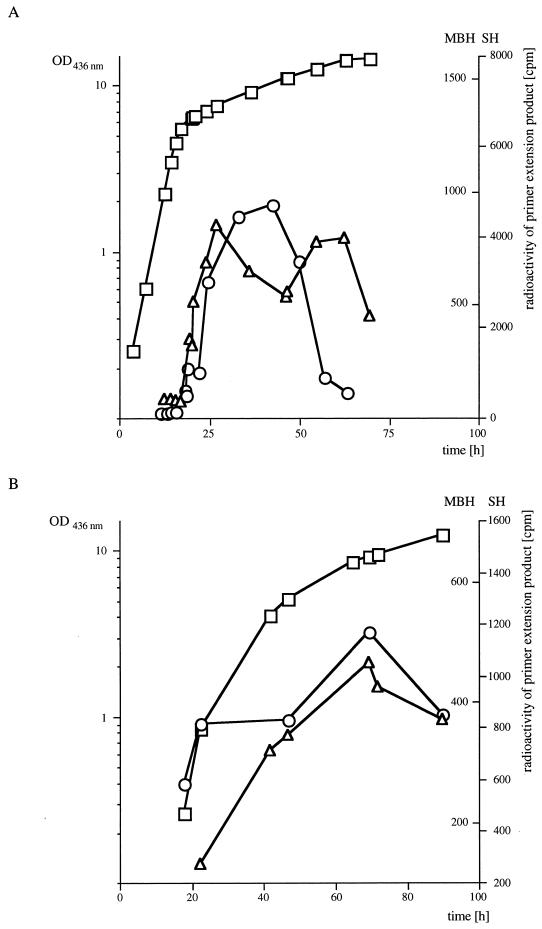
SH and MBH transcript levels increase abruptly upon derepression.
We used quantitative primer extension assays to monitor changes in the SH and the MBH transcript pools in A. eutrophus during diauxic growth on a mixture of fructose and glycerol and during lithoautotrophic growth on H2 (Fig. 3A and B). Diauxic and lithoautotrophic cultures were seeded with fructose-grown cells. In the diauxic cultures, constant transcript levels were found during late exponential growth on fructose. After this substrate was exhausted, the cells entered a lag phase before resuming growth (not visible at the scale of the graph in Fig. 3A). An increase in hydrogenase transcript levels began shortly after the onset of this lag phase and continued for 10 to 20 h. After this period, transcript pool levels began declining. Transfer from a heterotrophic culture with a preferred substrate, such as fructose, to lithoautotrophic growth conditions also led to an increase in transcript levels (Fig. 3B). This increase continued during exponential growth. The mRNA levels peaked at the onset of the stationary phase (at an OD436 of 9), whereupon a rapid decline set in. It appears that in both the lithoautotrophic and the diauxic cultures, transcription is triggered in response to exhaustion of a preferentially utilized substrate.
FIG. 3.
Kinetics of SH and MBH transcript pools following derepression of the hydrogenase system. (A) Relative transcript abundance in diauxic fructose-glycerol-grown cells. (B) Relative transcript abundance in lithoautotrophic cells. Cultures were grown at 30°C and sampled at various intervals. After determination of the OD436, total cellular RNA was isolated from 2-ml samples, and 10 μg each of the resulting RNA samples was added to primer extension reactions containing the specific 32P-labeled primers. After separation in 6% sequencing gels, the radioactive bands were excised and counted in a liquid scintillation counter. Radioactivity (in counts per minute) and cell density (OD436) were plotted against time. Symbols: ○, radioactivity of extension products obtained with the SH-specific primer; ▵ radioactivity of extension products obtained with the MBH-specific primer; □ cell density.
Transcription from PSH and PMBH is dependent on ς54 and the hydrogenase regulator HoxA.
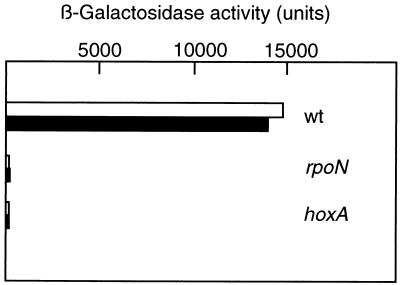
Genetic studies revealed that ς54 and the positive regulator HoxA are absolutely required for hydrogenase synthesis (12, 39). In order to directly test the requirement for these gene products for the transcriptional activities of PSH and PMBH, we introduced indicator plasmids into A. eutrophus HF09 and HF18 (Table 1), which are null mutants for rpoN and hoxA, respectively. Comparison of the β-galactosidase activities produced by the mutant and wild-type strains under hydrogenase-derepressing conditions indicated that the promoter activities were marginal in both the ς54- and the HoxA-deficient backgrounds (Fig. 4). RNase protection assays with RNAs from strains HF09 and HF18 failed to detect SH and MBH transcripts (data not shown). Taken together, these data show that PSH and PMBH require both gene products for activation.
FIG. 4.
Activities of PSH and PMBH in rpoN and hoxA mutants. A. eutrophus H16 and transconjugants harboring plasmids pGE319 (open bars) and pGE320 (closed bars) were grown on FGN medium to an OD436 of 8. Samples were assayed for β-galactosidase activity (see the legend to Fig. 2 for details). Bars represent the means of five independent determinations. Standard errors were too small for graphic representation. wt, wild type.
Induction of PSH and induction of PMBH proceed at similar rates.
Promoter activity measurements with the two test-plasmid-harboring strains showed that β-galactosidase activities remained constant during the course of logarithmic growth on succinate, fructose, and H2, as was expected when the rates of transcription, translation, transcript decay, and enzyme degradation were in equilibrium (data not shown). In contrast, transcriptional measurements in cells grown on glycerol revealed prolonged induction kinetics (data not shown). Thus, under the latter conditions, singular measurements at arbitrary time points do not provide a meaningful measure for quantitating relative promoter strength. In this situation, the rate of induction of a promoter is a more reliable representation of its transcriptional activity. Therefore, we monitored the increase in β-galactosidase activity during the initial phase of logarithmic growth on glycerol and plotted the data points against cell density (Fig. 5). Both promoters showed linear induction kinetics. The induction of PSH and the induction of PMBH proceeded at the same rates during growth on glycerol.
FIG. 5.
Induction rates for PSH and PMBH during exponential growth on glycerol. A. eutrophus H16 harboring plasmids pGE320 (open circles) and pGE319 (closed circles) was grown on FGN medium. Samples were taken at 30-min intervals from the mid-log-phase cultures and assayed for β-galactosidase activity (see the legend to Fig. 2 for details). Linear regression plots show β-galactosidase activities as a function of OD436. Representative results from two experiments are shown.
HoxA activates transcription from PSH and PMBH in a heterologous system.
HoxA is a response regulator-type transcriptional activator (12). Regulatory proteins of this class are typically paired with sensory proteins of the histidine kinase family. Interaction between the two alters the phosphorylation status of the regulator, thereby altering its capacity to activate cognate promoters (54). Some response regulators are also capable of activating transcription in the absence of their specific kinase (27, 40). In order to confirm the role of HoxA in the activation of PSH and PMBH and to determine whether other gene products unique to A. eutrophus are required, we introduced a plasmid-borne copy of hoxA under the control of a regulatable promoter into E. coli strains carrying indicator plasmids and measured β-galactosidase activities after the induction of HoxA (Fig. 6). The β-galactosidase levels of the induced cultures increased over the course of the experiment, whereas the levels of the uninduced controls remained constant. The β-galactosidase activities of the induced cultures were relatively low; nevertheless, the difference between the induced and the uninduced cultures was significant. Thus, HoxA mediates weak activation of cognate promoters in a heterologous system. The low level of transcription suggests that additional regulatory components specific to A. eutrophus are necessary for efficient activation.
FIG. 6.
HoxA-mediated activation of PSH and PMBH in E. coli. E. coli JM109(pCH618/pGE320) and E. coli JM109(pCH618/pGE319) were inoculated into fresh minimal medium containing glycerol and grown at 30°C to an OD600 of 0.3. The cultures were then split, and one duplicate of each was induced by the addition of IPTG to a final concentration of 1 mM (0 h). Samples were taken immediately and thereafter at 2-h intervals for 8 h and assayed for β-galactosidase activity. Final samples were taken at 18 h. Symbols: ○ and • induced cultures; ▿ and ▾ uninduced cultures; • and ▾, JM109(pCH618/pGE320); ○ and ▿, JM109(pCH618/pGE319). The experiment was done twice, with comparable results.
DISCUSSION
Previous studies suggested that the expression of the SH and the MBH genes in A. eutrophus is controlled by a transcriptional mechanism similar to the glnAp2 paradigm (54). The above results provide extensive evidence in support of this hypothesis. Two independent lines of evidence confirm transcriptional control. First, in vivo assays of promoter activity revealed high levels of transcription under hydrogenase-derepressing conditions. Second, determination of the relative abundances of the SH and the MBH transcripts by a physical method showed an approximate correlation between transcript pools and the respective hydrogenase activities. The latter method permitted a direct quantitative comparison between data collected under different growth conditions for a given transcript. It should be noted that, under our experimental conditions, a quantitative comparison of the SH and the MBH mRNA pools relative to each other was not possible. On the other hand, the promoter activity data allowed conclusions to be drawn about the relative strengths of PSH and PMBH. The activity of PSH was higher under all conditions except growth on glycerol. The correlation between the two data sets suggests that differential transcript stability does not play a major role in the regulation of SH and MBH expression.
The hydrogenase activities reflect the transcriptional data, with an obvious exception. The activity of the SH in lithoautotrophically grown cells was disproportionately low. This result may have been due to the inactivation of the enzyme in the presence of O2 and electron donors, e.g., H2 (43). This inactivation has been shown to take place in vivo and may be caused by superoxide radicals produced by the hydrogenase itself.
The kinetics of the transcript pools of cells growing on glycerol and on H2 revealed a dramatic increase in SH and MBH mRNAs upon derepression. On the whole, these kinetics resembled the kinetics of enzyme activities for the two hydrogenases. Furthermore, the increase in transcript levels was coordinate, as was the appearance of the enzyme activities (19). This finding suggests that derepression is synchronized by a common mechanism. Our data showing that both PSH and PMBH are HoxA dependent indicate that HoxA is the synchronizing agent. Earlier studies on carbon- and oxygen-limited continuous cultures revealed a link between the derepression of hydrogenase synthesis and limitation of energy (19, 22). The transcriptional data presented here are compatible with the physiological findings. Transcript levels began rising during the lag phase after the exhaustion of fructose in the fructose-glycerol cultures or during the initial lag phase in the lithoautotrophic cultures.
Primer extension analysis identified putative transcription start points upstream of hoxF and hoxK. Although data of this type are notoriously subject to artifacts due to, e.g., transcriptase stalling, transcript degradation, and promiscuous priming, they are an invaluable aid in identifying promoters. Sequence elements resembling the −24/−12 consensus sequence of ς54-dependent promoters are located just upstream of the putative transcription start points. The presumptive SH promoter, 5′-TTGGCGCACATCCTGC-3′, contains the typical GG-N10-GC motif. The candidate for the MBH promoter, 5′-CAGGCATAGATCTTGT-3′, contains a T at the −12 position. This exception is rare but has been documented in at least one case (55). The finding that transcription from the SH and the MBH upstream regions is ς54 dependent supports the assignment of these sequences as the SH and the MBH promoters. RNA polymerase bound to an ς54-dependent promoter requires a transcriptional activator to form an open complex. Our data show that the activities of both PSH and PMBH are absolutely dependent on the NtrC homolog HoxA.
Studies of the glnAp2 promoter led to a molecular model for transcriptional activation (31). Key features of this model are the binding of an activator protein at a specific site upstream of a ς54-dependent promoter and looping of the DNA to permit direct contact of the activator and the polymerase (47). The spacing of the regulatory sites is important. Typically, the binding site for the activator lies between −120 and −160 relative to the transcription start site (8). In the hoxF upstream region, tandem palindromes consisting of the motif 5′-CAAG-N10-CTTG-3′ are centered at −159 and −202. Deletion analysis showed that this region contains a signal essential for high-level SH expression (57). A similar sequence motif is found in the hoxK upstream region. This motif consists of the sequence elements 5′-CATG-N11-ATTG-3′ and 5′-CAGG-N9-CTTG-3′ centered at −187 and −210, respectively. These distances are atypical but are within the range of published values (8). For the nifF promoter, for instance, the NifA binding site is located between −250 and −270 (37).
An important feature of the glnAp2 activation mechanism is the participation of the DNA-bending protein integration host factor (IHF) (28). IHF binds to the DNA at a site between the promoter and the activator binding site and facilitates DNA looping. E. coli IHF binds to fragments containing the hoxF promoter in vitro (57). It remains to be shown whether an A. eutrophus IHF homolog plays a role in the activation of the hoxF and/or hoxK promoters.
Among the best-studied hydrogen-oxidizing bacteria are Rhodobacter capsulatus, Bradyrhizobium japonicum, and Rhizobium leguminosarum. The genes encoding the dimeric hydrogenases of these organisms have been identified, and the controlling promoters have been characterized. These studies revealed remarkable similarities. In all three cases, the hydrogenase genes are transcribed from ς54-dependent promoters under the control of NtrC-like transcriptional activators (7, 38, 52). Furthermore, in vivo and/or in vitro data document the role of IHF-like proteins in promoter activation (5, 7, 50).
Experiments with a heterologous system revealed that, outside the context of the A. eutrophus cell, HoxA is capable of mediating only weak activation of its cognate promoters PSH and PMBH. This low-level transcription may be an indication that other regulatory components and/or modification of HoxA are required for full activation.
This study represents the first stage of a systematic investigation of the regulation of the hydrogenase genes. A detailed molecular analysis of the two promoters identified here is now under way. Screening of the hydrogenase gene cluster has, to date, identified four additional promoters, which are presently being characterized (45). The ultimate goal of these studies is to understand the workings of a complex multigene system.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft through SFB 344 and by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Amann E, Ochs B, Abel K-J. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69:301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1987. [Google Scholar]
- 3.Bernhard M, Benelli B, Hochkoeppler A, Zanoni D, Friedrich B. The membrane-bound hydrogenase (MBH) of Alcaligenes eutrophus H16: functional and structural role of the cytochrome b subunit. Eur J Biochem. 1997;248:179–186. doi: 10.1111/j.1432-1033.1997.00179.x. [DOI] [PubMed] [Google Scholar]
- 4.Bernhard M, Schwartz E, Rietdorf J, Friedrich B. The Alcaligenes eutrophus membrane-bound hydrogenase gene locus encodes functions involved in maturation and electron transport coupling. J Bacteriol. 1996;178:4522–4529. doi: 10.1128/jb.178.15.4522-4529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black L K, Maier R J. IHF- and RpoN-dependent regulation of hydrogenase expression in Bradyrhizobium japonicum. Mol Microbiol. 1995;16:405–413. doi: 10.1111/j.1365-2958.1995.tb02406.x. [DOI] [PubMed] [Google Scholar]
- 6.Bowien B, Bednarski R, Kusian B, Windhövel U, Freter A, Schäferjohann J, Schäferjohann J-G. Genetic regulation of CO2 assimilation in chemoautotrophs. In: Murrell J C, Kelly D P, editors. Microbial growth on C1 compounds. Andover, United Kingdom: Intercept; 1993. pp. 481–491. [Google Scholar]
- 7.Brito B, Martínez M, Fernández D, Rey L, Cabrera E, Palacios J M, Imperial J, Ruiz-Argüeso T. Hydrogenase genes from Rhizobium leguminosarum bv. viciae are controlled by the nitrogen fixation regulatory protein NifA. Proc Natl Acad Sci USA. 1997;94:6019–6024. doi: 10.1073/pnas.94.12.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collado-Vides J, Magasanik B, Gralla J D. Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev. 1991;55:371–394. doi: 10.1128/mr.55.3.371-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig N L, Nash H A. Escherichia coli integration host factor binds to specific sites in DNA. Cell. 1984;39:707–716. doi: 10.1016/0092-8674(84)90478-1. [DOI] [PubMed] [Google Scholar]
- 10.Dernedde J, Eitinger T, Patenge N, Friedrich B. hyp gene products in Alcaligenes eutrophus are part of a hydrogenase-maturation system. Eur J Biochem. 1996;235:351–358. doi: 10.1111/j.1432-1033.1996.00351.x. [DOI] [PubMed] [Google Scholar]
- 11.Dernedde J, Eitinger M, Friedrich B. Analysis of a pleiotropic gene region involved in formation of catalytically active hydrogenase in Alcaligenes eutrophus H16. Arch Microbiol. 1993;159:545–553. doi: 10.1007/BF00249034. [DOI] [PubMed] [Google Scholar]
- 12.Eberz G, Friedrich B. Three trans-acting functions control hydrogenase expression in Alcaligenes eutrophus. J Bacteriol. 1991;173:1845–1854. doi: 10.1128/jb.173.6.1845-1854.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberz G, Hogrefe C, Kortlüke C, Kamienski A, Friedrich B. Molecular cloning of structural and regulatory hydrogenase genes (hox) of Alcaligenes eutrophus H16. J Bacteriol. 1986;168:636–641. doi: 10.1128/jb.168.2.636-641.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eismann K, Milejnek K, Zipprich D, Hoppert M, Geberding H, Mayer F. Antigenic determinants of the membrane-bound hydrogenase in Alcaligenes eutrophus are exposed towards the periplasm. J Bacteriol. 1995;177:6309–6312. doi: 10.1128/jb.177.21.6309-6312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eitinger T, Friedrich B. Cloning, nucleotide sequence, and heterologous expression of a high-affinity nickel transport gene from Alcaligenes eutrophus. J Biol Chem. 1991;266:3222–3227. [PubMed] [Google Scholar]
- 16.Friedrich B, Heine E, Fink A, Friedrich C G. Nickel requirement for hydrogenase formation in Alcaligenes eutrophus. J Bacteriol. 1981;145:1144–1149. doi: 10.1128/jb.145.3.1144-1149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedrich B, Schwartz E. Molecular biology of hydrogen utilization in aerobic chemolithotrophs. Annu Rev Microbiol. 1993;47:351–383. doi: 10.1146/annurev.mi.47.100193.002031. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich B, Bernhard M, Dernedde J, Eitinger T, Lenz O, Massanz C, Schwartz E. Hydrogen oxidation by Alcaligenes. In: Lindstrom M E, Tabita F R, editors. Microbial growth on C1 compounds. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 110–117. [Google Scholar]
- 19.Friedrich C G. Derepression of hydrogenase during limitation of electron donors and derepression of ribulosebisphosphate carboxylase during carbon limitation of Alcaligenes eutrophus. J Bacteriol. 1982;149:203–210. doi: 10.1128/jb.149.1.203-210.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedrich C G, Bowien B, Friedrich B. Formate and oxalate metabolism in Alcaligenes eutrophus. J Gen Microbiol. 1979;115:185–192. [Google Scholar]
- 21.Friedrich C G, Schneider K, Friedrich B. Nickel in the catalytically active hydrogenase of Alcaligenes eutrophus. J Bacteriol. 1982;152:42–48. doi: 10.1128/jb.152.1.42-48.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedrich C G, Friedrich B, Bowien B. Formation of enzymes of autotrophic metabolism during heterotrophic growth of Alcaligenes eutrophus. J Gen Microbiol. 1981;122:69–78. [Google Scholar]
- 23.Gerberding H, Mayer F. Localization of the membrane-bound hydrogenase in Alcaligenes eutrophus by electron microscopic immunocytochemistry. FEMS Microbiol Lett. 1989;50:265–270. doi: 10.1016/0378-1097(89)90500-4. [DOI] [PubMed] [Google Scholar]
- 24.Gerischer U, Dürre P. mRNA analysis of the adc gene region of Clostridium acetobutylicum during the shift to solventogenesis. J Bacteriol. 1992;174:426–433. doi: 10.1128/jb.174.2.426-433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goosen N, van de Putte P. The regulation of transcription initiation by integration host factor. Mol Microbiol. 1995;16:1–7. doi: 10.1111/j.1365-2958.1995.tb02386.x. [DOI] [PubMed] [Google Scholar]
- 26.Grzeszik C, Roß K, Schneider K, Reh M, Schlegel H G. Location, catalytic activity, and subunit composition of NAD-reducing hydrogenases of some Alcaligenes strains and Rhodococcus opacus MR22. Arch Microbiol. 1997;167:172–176. [PubMed] [Google Scholar]
- 27.Hertig C, Li R Y, Louarn A-M, Garnerone A-M, David M, Batut J, Kahn D, Boistard P. Rhizobium meliloti regulatory gene fixJ activates transcription of R. meliloti nifA and fixK genes in Escherichia coli. J Bacteriol. 1989;171:1736–1738. doi: 10.1128/jb.171.3.1736-1738.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoover T R, Santero E, Porter S, Kustu S. The integration host factor stimulates interaction of RNA polymerase with NTRC, the transcriptional activator for nitrogen fixation operons. Cell. 1990;63:11–22. doi: 10.1016/0092-8674(90)90284-l. [DOI] [PubMed] [Google Scholar]
- 29.Kortlüke C, Horstmann K, Schwartz E, Rohde M, Binsack R, Friedrich B. A gene complex coding for the membrane-bound hydrogenase of Alcaligenes eutrophus H16. J Bacteriol. 1992;174:6277–6289. doi: 10.1128/jb.174.19.6277-6289.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kortlüke C, Friedrich B. Maturation of membrane-bound hydrogenase of Alcaligenes eutrophus H16. J Bacteriol. 1992;174:6290–6293. doi: 10.1128/jb.174.19.6290-6293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kustu S, North A K, Weiss D S. Prokaryotic transcriptional enhancers and enhancer-binding proteins. Trends Biochem Sci. 1991;16:397–402. doi: 10.1016/0968-0004(91)90163-p. [DOI] [PubMed] [Google Scholar]
- 32.Lenz, O., and B. Friedrich. Unpublished data.
- 33.Lenz O, Strack A, Tran-Betcke A, Friedrich B. A hydrogen-sensing system in transcriptional regulation of hydrogenase gene expression in Alcaligenes species. J Bacteriol. 1997;179:1655–1663. doi: 10.1128/jb.179.5.1655-1663.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 35.Massanz C, Fernandez V M, Friedrich B. C-terminal extension of the H2-activating subunit, HoxH, directs maturation of the NAD-reducing hydrogenase in Alcaligenes eutrophus. Eur J Biochem. 1997;245:441–448. doi: 10.1111/j.1432-1033.1997.t01-3-00441.x. [DOI] [PubMed] [Google Scholar]
- 36.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 37.Minchin S D, Austin S, Dixon R A. The role of activator binding sites in transcriptional control of the divergently transcribed nifF and nifLA promoters from Klebsiella pneumoniae. Mol Microbiol. 1988;2:433–442. doi: 10.1111/j.1365-2958.1988.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 38.Richaud P, Colbeau A, Toussaint B, Vignais P M. Identification and sequence analysis of the hupR1 gene, which encodes a response regulator of the NtrC family required for hydrogenase expression in Rhodobacter capsulatus. J Bacteriol. 1991;173:5928–5932. doi: 10.1128/jb.173.18.5928-5932.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Römermann D, Warrelmann J, Bender R A, Friedrich B. An rpoN-like gene of Alcaligenes eutrophus and Pseudomonas facilis controls the expression of diverse metabolic pathways, including hydrogen oxidation. J Bacteriol. 1989;171:1093–1099. doi: 10.1128/jb.171.2.1093-1099.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roy C R, Miller J F, Falkow S. The bvgA gene of Bordetella pertussis encodes a transcriptional activator required for coordinate regulation of several virulence genes. J Bacteriol. 1989;171:6338–6344. doi: 10.1128/jb.171.11.6338-6344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schink B, Schlegel H G. Mutants of Alcaligenes eutrophus defective in autotrophic metabolism. Arch Microbiol. 1978;117:123–129. doi: 10.1007/BF00402299. [DOI] [PubMed] [Google Scholar]
- 42.Schink B, Schlegel H G. The membrane-bound hydrogenase of Alcaligenes eutrophus. Solubilization, purification and biochemical properties. Biochim Biophys Acta. 1979;567:315–324. doi: 10.1016/0005-2744(79)90117-7. [DOI] [PubMed] [Google Scholar]
- 43.Schlesier M, Friedrich B. In vivo inactivation of soluble hydrogenase of Alcaligenes eutrophus. Arch Microbiol. 1981;129:150–153. doi: 10.1007/BF00455352. [DOI] [PubMed] [Google Scholar]
- 44.Schneider K, Schlegel H G. Purification and properties of the soluble hydrogenase from Alcaligenes eutrophus H16. Biochim Biophys Acta. 1976;452:66–80. doi: 10.1016/0005-2744(76)90058-9. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz, E., and B. Friedrich. Unpublished data.
- 46.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 47.Su W, Porter S C, Kustu S, Echols H. DNA-looping and enhancer activity: association between DNA-bound NtrC and RNA polymerase at the glnA promoter. Proc Natl Acad Sci USA. 1990;87:5504–5508. doi: 10.1073/pnas.87.14.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thiemermann S, Dernedde J, Bernhard M, Schroeder W, Massanz C, Friedrich B. Carboxyl-terminal processing of the cytoplasmic NAD-reducing hydrogenase of Alcaligenes eutrophus requires the hoxW gene product. J Bacteriol. 1996;178:2368–2374. doi: 10.1128/jb.178.8.2368-2374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thöny B, Hennecke H. The −24/−12 promoter comes of age. FEMS Microbiol Rev. 1989;63:341–358. doi: 10.1016/0168-6445(89)90028-4. [DOI] [PubMed] [Google Scholar]
- 50.Toussaint B, Bosc C, Richaud P, Colbeau A, Vignais P M. A mutation in a Rhodobacter capsulatus gene encoding an integration host factor-like protein impairs in vivo hydrogenase expression. Proc Natl Acad Sci USA. 1991;88:10749–10753. doi: 10.1073/pnas.88.23.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tran-Betcke A, Warnecke U, Böcker C, Zaborosch C, Friedrich B. Cloning and nucleotide sequence of the genes for the subunits of the NAD-reducing hydrogenase of Alcaligenes eutrophus H16. J Bacteriol. 1990;172:2920–2929. doi: 10.1128/jb.172.6.2920-2929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Soom C, de Wilde P, Vanderleyden J. HoxA is a transcriptional regulator for expression of the hup structural genes in free-living Bradyrhizobium japonicum. Mol Microbiol. 1997;23:967–977. doi: 10.1046/j.1365-2958.1997.2781648.x. [DOI] [PubMed] [Google Scholar]
- 53.Warrelmann J, Eitinger M, Schwartz E, Römermann D, Friedrich B. Nucleotide sequence of the rpoN (hno) gene region of Alcaligenes eutrophus: evidence for a conserved gene cluster. Arch Microbiol. 1992;158:107–114. doi: 10.1007/BF00245213. [DOI] [PubMed] [Google Scholar]
- 54.Wedel A, Kustu S. The bacterial enhancer-binding protein NTRC is a molecular machine: ATP hydrolysis is coupled to transcriptional activation. Genes Dev. 1995;9:2042–2052. doi: 10.1101/gad.9.16.2042. [DOI] [PubMed] [Google Scholar]
- 55.Weiner L, Brisette J L, Model P. Stress-induced expression of the Escherichia coli phage shock protein operon is dependent on ς54 and modulated by positive and negative feedback mechanisms. Genes Dev. 1991;5:1912–1923. doi: 10.1101/gad.5.10.1912. [DOI] [PubMed] [Google Scholar]
- 56.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 57.Zimmer D, Schwartz E, Tran-Betcke A, Gewinner P, Friedrich B. Temperature tolerance of hydrogenase expression in Alcaligenes eutrophus is conferred by a single amino acid exchange in the transcriptional activator HoxA. J Bacteriol. 1995;177:2373–2380. doi: 10.1128/jb.177.9.2373-2380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]