Abstract
Objective
Nowadays, more than 90% of people over 50 years suffer from intervertebral disc degeneration (IDD), but there are exist no ideal drugs. The aim of this study is to identify a new drug for IDD.
Methods
An approved small molecular drug library including 2040 small molecular compounds was used here. We found that taurocholic acid sodium hydrate (NAT) could induce chondrogenesis and osteogenesis in mesenchymal stem cells (MSCs). Then, an in vivo mouse model of IDD was established and the coccygeal discs transcriptome analysis and surface plasmon resonance analysis (SPR) integrated with liquid chromatography–tandem mass spectrometry assay (LC‐MS) were performed in this study to study the therapy effect and target proteins of NAT for IDD. Micro‐CT was used to evaluate the cancellous bone. The expression of osteogenic (OCN, RNX2), chondrogenic (COL2A1, SOX9), and the target related (ERK1/2, p‐ERK1/2) proteins were detected. The alkaline phosphatase staining was performed to estimate osteogenic differentiation. Blood routine and blood biochemistry indexes were analyzed for the safety of NAT.
Results
The results showed that NAT could induce chondrogenesis and osteogenesis in MSCs. Further experiments confirmed NAT could ameliorate the secondary osteoporosis and delay the development of IDD in mice. Transcriptome analysis identified 128 common genes and eight Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways for NAT. SPR‐LC–MS assay detected 57 target proteins for NAT, including MAPK3 (mitogen‐activated protein kinase 3), also known as ERK1 (extracellular regulated protein kinase 1). Further verification experiment confirmed that NAT significantly reduced the expression of ERK1/2 phosphorylation.
Conclusion
NAT would induce chondrogenesis and osteogenesis of MSCs, ameliorate the secondary osteoporosis and delay the progression of IDD in mice by targeting MAPK3.Furthermore, MAPK3, especially the phosphorylation of MAPK3, would be a potential therapeutic target for IDD treatment.
Keywords: Chondrogenesis, Intervertebral Disc Degeneration (IDD), Mesenchymal Stem Cells (MSCs), Osteogenesis, Taurocholic Acid Sodium Salt Hydrate (NAT)
The primary finding of this study was the identification of a novel repurposing drug, taurocholic acid sodium hydrate (NAT), which would induce chondrogenesis and osteogenesis of mesenchymal stem cells (MSCs), ameliorate the secondary osteoporosis and delay the progression of intervertebral disc degeneration (IDD) in mice by targeting MAPK3 (mitogen‐activated protein kinase 3). Furthermore, MAPK3, especially the phosphorylation of MAPK3, as a relevant drug target, would be a potential therapeutic agent in in osteogenic differentiation for IDD treatment.
Introduction
A global burden of disease study in 2019 showed that low back pain (LBP) was one of the top 10 causes of disability‐adjusted life‐years, especially in the groups for 10–24 years and 25–49 years. 1 Intervertebral disc disease (IVD) and Intervertebral disc degeneration (IDD), as the leading and major cause of LBP, affects more than 90% of people over 50 years of age. 2 , 3 , 4 It is the largest avascular structure in the human body and receives all nutrients from the bone marrow of adjacent vertebral bodies via the only nutrition channel of endplate cartilage (CEP). 5 In IDD, CEP degeneration results in the blockage of the nutrition channel in the endplate, leading to the degeneration of nucleus pulposus (NP) and annulus fibrosis (AF), or even secondary osteoporosis. 5 Previous studies showed that mesenchymal stem cells (MSCs) in the adjacent vertebral body could migrate to the NP physiologically through the nutrition channel to maintain the IVD environment balance. Therefore, the identification and the differentiation of stem/progenitor cells in the IVD have inspired a novel treatment for IDD. 6 , 7
Drug repurposing, the process of identifying and developing new uses for approved or investigational drugs, owns multiple advantages over the synthesis of a new drug. Most important of all, the repurposed drugs may reveal new targets and pathways which would be further explored. 8 , 9 Recently, chemical compounds with molecular weight (MW) below 1000Da have gained increasing attention as powerful tools for tissue regeneration. 10 Due to the inherently avascular, high osmotic pressure harsh environment of IVD, and dense fibrous ring envelops limiting the entry of cells and compounds in large quantities, 11 we hypothesize that the small molecular drug would be an ideal drug to IDD therapy.
In this study, we aim to identify a repurposed drug for IDD. We have found that taurocholic acid sodium hydrate (NAT), previously used as cholagogue and choleretic, could induce chondrogenesis and osteogenesis of MSCs. Based on this finding, the purpose of the study is briefly as follows: (i) the therapeutic effect of NAT in treating with IDD; (ii) the molecular mechanisms of NAT in IDD treatment: and (iii) the identification and verification of target proteins for NAT.
Methods
Ethics Approval
This work was approved by Nanfang Hospital Animal Ethic Committee of Southern Medical University (NFYY‐2020‐1031).
Screening of the Approved Small Molecular Drug Library
The approved drug library is a collection of 2040 small molecular compounds from TargetMol company, which comes from 25 different functional components (Fig. 1A), were used here. For primary screening, the HUC‐MSCs were cultured in 48‐well plates, and the concentration of 2040 drugs with 0.1 μM and 1 μM were added and co‐cultured. The positive control of insulin‐transferrin‐selenium (ITS, Beyotime, Shanghai, China), known as the cartilage inducing media, and the negative control of dimethyl sulfoxide (DMSO, Sigma, Saint Louis, MO, USA) were also added to 48‐well plates. After 7 days, toluidine blue (TB, Solarbio, Beijing, China) was used to dye the cells. For the secondary screening, the protein expression of COL2A1 was detected after being cultured by the selected small molecular drugs. Further used NAT was purchased from Shanghai Topscience Co. Ltd. (T1138. CAS 345909‐26‐4, Shanghai, China).
Fig. 1.
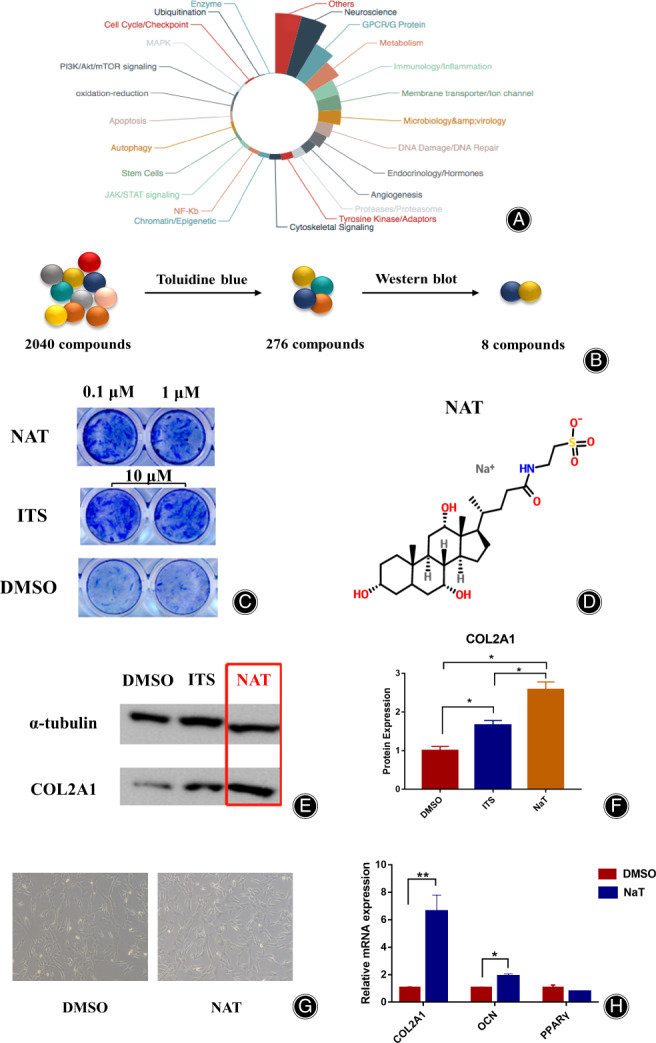
NAT was identified as a small molecular drug for IDD. (A) The 2040 small molecular drug library. (B)The process of the screening system by using TB staining and Western blot verification. (C) The results of TB staining in NAT (0.1 μM and 1 μM), ITS (10 μM) and DMSO groups. (D) The molecular structure of NAT. (E) The COL2A1 protein expression results among DMSO, ITS, and NAT groups. (F) The protein expression of COL2A1 in DMSO, ITS and NAT groups. (G) The microscope of HUC‐MSCs between DMSO and NAT (1 μM) after 3 days intervention (50×). (H) The mRNA expression of COL2A1, OCN, and PPARγ between DMSO and NAT groups. *p < 0.05,**p < 0.01.
Finally, eight candidates were identified (Fig. 1B). Among these, NAT could induce the HUC‐MSCs into chondrocytes remarkably. The TB results showed that both 0.1 μM and 1 μM concentration of NAT had strengthened induction, and showed similarity compared to ITS group (Fig. 1C). The protein expression of COL2A1 in NAT group was the strongest among the three groups (Fig. 1E). We also found that not only the mRNA expression of COL2A1 was up‐regulated in NAT group (p = 1.60 × 10−3), but also OCN, which is secreted by osteoblasts and regulates bone remodeling, was up‐regulated in NAT group (p = 2.44 × 10−2) (Fig. 1F). However, the mRNA expression of PPARγ, known as a regulator of adipocyte differentiation, showed no obvious difference between the two groups.
Culturation and Characteristic of HUC‐MSCs
Human umbilical cord mesenchymal stem cells (HUC‐MSCs) were stained with PE‐labeled monoclonal antibodies of CD34, CD90, CD105, CD44 and HLA‐DR to identify the purity of their surface antigens by flow cytometric (FCM, Becton, Dickinson Co., NJ, USA) after 7 days culturation both in normal and NAT (1 μM) medium. 12 In this study, the passage of the 6th to 12th generation was used.
Induction of IDD Model and NAT Intragastric Administration
A total of 24, 7‐week‐old male wild‐type C57BL/6 mice were obtained from Liaoning Changsheng Biotechnology Company (Lianyungang, China). The mice were given 7 days to adapt to the new housing and husbandry environment before further experiments. The mice were randomly divided into four groups, the negative control group (NC, n = 6), the IDD positive group (IDD_dH2O group, dH2O, n = 6), the IDD low dosage treatment group (IDD_NAT1 group, NAT 2 mg/kg, n = 6), and the IDD high dosage treatment group (IDD_NAT2 group, NAT 20 mg/kg, n = 6). For the IDD experiment groups, the mice were anesthetized under repeated general isoflurane gas, then their tail IVDs were injured with a 26G needle marked 1.5 mm inserted depth under an operating microscope, approximately the vertical distance from the skin to the centre of the NP. 13 , 14 The needle stayed in the IVD for about 30 s and was pulled out rotationally. In this study, the coccygeal discs between the fourth and fifth coccygeal vertebrae (Co4/5) and the sixth and seventh coccygeal vertebrae (Co6/7) in experiment groups were located under X‐ray and injured by the needle. After that, the mice were fed with the standard diet. Also, the mice in IDD_dH2O, IDD_NAT1 and IDD_NAT2 groups were given 220 μL of dH2O, 220 μL of 2 mg/kg NAT and 220 μL of 20 mg/kg NAT respectively twice a week for 8 weeks. After that, the mice were anesthetized under repeated general isoflurane gas, then the heart blood were collected by cardiac puncture. In this study, the Co4/5 discs were obtained for micro‐CT and histological analysis, and Co6/7 discs were obtained for transcriptome analysis. The intervertebral disc degeneration was assessed by a disc degeneration assessment scoring system. 13
Micro‐CT Scan
The Co4/5 discs were obtained and fixed in 4% formaldehyde for 24 h, each of them was scanned by a high‐resolution Micro‐CT system (μCT 80, Scanc Medical, AG, Zurich, Switzerland) with an isotropic voxel size of 12 μm (55kV, 145 μA, integration time 300 ms, averaged two times).
The trabecular bone volume per total volume (BV/TV), the trabecular number (Tb.N), the trabecular thickness (Tb.Th), and the trabecular separation (Tb.Sp) for 23 layers (276 μm) upon the subchondral bone, were analyzed to evaluate the cancellous bone. Additionally, the initial digital imaging and communications in medicine (DICOM) data of the mice were loaded into Mimics 20.0 software for image processing and three‐dimension (3D) modeling of coccygeal vertebrae.
RT‐PCR
Total RNA was isolated according to TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse‐transcribed to cDNA by Evo M‐MLVRT Premix (AG11706, Accurate Biotechnology, Shanghai, China). Real‐time PCR was performed with the Premix Pro Taq HS qPCR Kit (AG11701, Accurate Biotechnology, Changsha, China). The cycle of threshold (CT) of each sample was averaged and normalized to GAPDH. The triplicate results were analyzed by comparative CT equation (2−△△CT).
Western Blot
Cells were lysed with Radioimmunoprecipitation assay (RIPA, Solarbio, Beijing, China) lysis buffer, separated by the SDS‐polyacrylamidgelectrophoresis (SDS‐PAGE, Epizyme, Shanghai, China), transferred to polyvinylidenefluoride (PVDF, Millipore, Boston, MA, USA) membranes. The PVDF membranes were blocked in protein‐free rapid blocking buffer (Epizyme, PS108P, Shanghai, China) for 20–30 min, and incubated with primary antibodies at 4°C overnight and secondary antibodies for 1 h at room temperature. eBlot (Touch imager S, Shanghai, China) was used to analyze the protein bands. Proteins were analyzed with antibodies recognizing COL2A1 (Abcam, 1:1000, Cambridge, UK), OCN (Santa, 1:500, Santa Anna, TX, USA), GAPDH (Proteintech, 1:6000, Chicago, IL, USA), ERK1/2 (Cell signaling, 1:1000, Danvers, MA, USA), p‐ERK1/2 (Cell signaling, 1:1000), SOX9 (Affinity, 1:2000, Cincinnati, OH, USA), RUNX2 (Abcam, 1:1000).
Immunohistochemical (IHC) and Multiplexed Immunohistochemistry (mIHC)
The discs were embedded into decalcification in 10% EDTA for 4 weeks at 4°C. Then, the samples were embedded in paraffin and 5 μm sections were used for IHC and mIHC. Endogenous peroxidase was inhibited by 3% H2O2 (Boster, Pleasanton, CA, USA) for 15 min and nonspecific background was blocked by goat serum (Boster). The IHC protocol was performed as previously described. 15 Briefly, Rabbit SP reagent (18112A11, ZSGB‐Bio., Beijing, China) was used following the manual. Primary antibodies of COL2A1 (1:200, Bioss, Beijing, China) and OCN (1:100, Affinity, Melbourne, FL, USA) were used here. For mIHC, the slides were incubated with primary antibodies for 30 min at 37°C, then treated with anti‐rabbit horseradish peroxidase‐conjugated (HRP, ZSGB‐Bio.) secondary antibody for 10 min. After that, tyramide signal amplification (TSA) was performed using a four color multiple fluorescent immunohistochemical staining kit (Absin, Shanghai, China) following the manufacturer's instructions. The same process was repeated for the following antibodies/fluorescent dyes. Primary antibodies contained COL2A1 (1:400, Bioss, Beijing, China), OCN (1:400, Affinity, Melbourne, USA), and ERK1/2 (Cell signaling, 1:300, Danvers, MA, USA). Each slide was then treated with one drop of mounting medium with DAPI (abcam, ab104139, Cambridge, UK). Images were captured using the ECLIPSE Ti2‐E Imaging System (Nikon, Tokyo, Japan).
Detection of Blood Routine Indexes and Biochemical Indicators
To verify the safety of NAT in vivo. The peripheral blood was used to detect the blood routine indexes and biochemical indicators from the four groups. The blood routine tests were performed on the Mindray BC‐2600Vet Auto Hematology Analyzer (Mindray, Shenzhen, China), and the blood biochemical tests were using an IDEXX Catalyst Dx Chemistry Analyzer (IDEXX, Westbrook, ME, USA).
The Alkaline Phosphatase (ALP) Staining
For estimating osteogenic differentiation, the ALP staining was used here. The slides were stained with an ALP staining kit (Beyotime, Shanghai, China) according to the manufacturer's protocol.
Analysis of Coccygeal Discs Transcriptome after NAT Treatment
For the analysis of coccygeal discs transcriptome after NAT treatment, the Co6/7 discs were isolated carefully (n = 3 for each group). Then, total RNA was isolated using the Trizol Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). The sequence was performed on a Hiseq platform (Illumina NovaSeq 6000, San Diego, CA, USA) by Shanghai Personal Biotechnology Cp. Ltd.
Identification and Quantification of NAT Target Proteins
For detecting the direct target proteins of NAT in HUC‐MSCs, the SPR‐LC–MS assay was designed. NAT at a concentration of 100 mM was formulated with 50% DMSO. The chemically modified label‐free photo‐cross‐linker sensor chips were provided by Betterways Inc. (Guangzhou, China). The sensor chip is capable of immobilizing small chemical molecules (MW > 60Da) with no chemical label linked onto the molecular. For array spotting, a BioDot™ AD‐1520 Array Printer (BIODOT Inc., Irvine, CA, USA) was employed to print samples and controls on the surface. Then, the flow cell chambers were assembled onto the chip surface, and the chips were inserted and tested in the SPR instrument one after another. In order to monitor the enrichment process of the target protein, we performed a real‐time surface plasmon resonance experiment using bScreen LB 991 Label‐free Microarray System (Berthold Technologies, Bad Wildbad, Germany). An LC–MS experiment is used to identify the types and relative abundance of proteins captured on the surface of the chip. Differential expression ratios for proteins were obtained using Mascot software (Matrix Science 2.4).
By screening the direct targets of NAT to HUC‐MSCs, a total of 57 target proteins were identified (Fig. 2A, Supplementary File S1). The protein interaction network showed that they were mainly clustered in three groups (Fig. 2B). The first clustering mainly contained the Chondrogenesis and Repulsive guidance molecule (strength 2.24, FDR = 1.20 × 10−3), BMP signaling pathway (strength 1.96, FDR = 1.42 × 10−5) (Fig. 2B, red color). The second clustering mainly contained the Tripeptide Transmembrane Transporter Activity, Urate Salt Excretion (strength 2.87, FDR = 3.98 × 10−8), Canalicular Bile Acid Transport (strength 2.75, FDR = 1.80 × 10−5) (Fig. 2B, blue color). The third clustering included Bile Acid Secretion (strength 3.02, FDR = 1.27 × 10−8), Canalicular Bile Acid Transport (strength 2.72, FDR = 3.50 × 10−3) (Fig. 2B, green color). Combining the above results, we finally chose MAPK3, also named ERK1, as a target protein of NAT for further analysis. MAPK3 is the top target of NAT, whose score is 1058.15, and it belongs to the first red clustering, indicating its potential participant in chondrogenesis and osteogenesis. Notably, MAPK3 was identified in most of the enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, including MAPK signaling pathway (Fig. 2D).
Fig. 2.
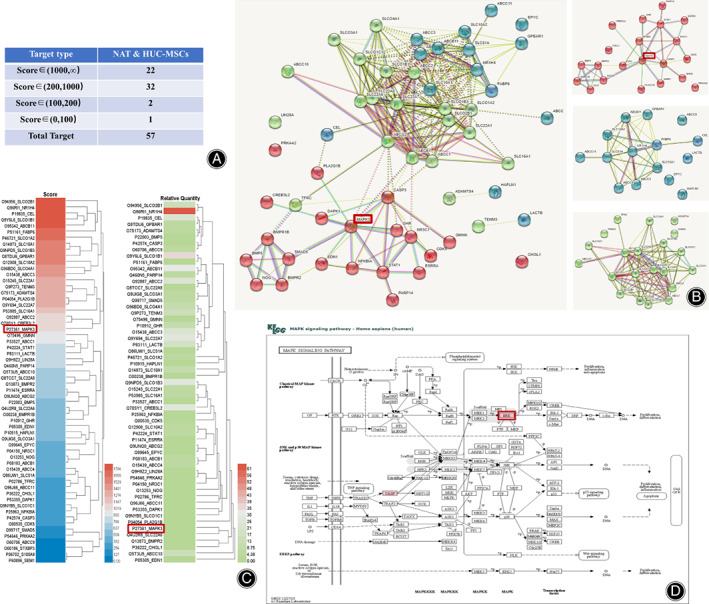
Identification of MAPK3 as the target protein of NAT in HUC‐MSCs. (A) The table of the 57 total target proteins of NAT identified by SPR‐HPLC‐MS assay. (B) The protein–protein interaction networks of the 57 target proteins. (C) The score and relative quantity of the 57 target proteins. (D) The target protein (MAPK3, red color) of NAT identified in MAPK signaling pathway.
Inhibition of MAPK3 Expression in HUC‐MSCs
To validate whether MAPK3 is the target protein of NAT in HUC‐MSCs. U0126 (10 μM, Cell Signaling, Danvers, MA, USA), an ERK1/2 inhibitor, was used to intervene HUC‐MSCs for 0.5 h and 1 h every day to compared with NAT induction. After 14 days of intervention, the proteins were extracted for further detection.
Functional Enrichment and Annotation Analysis
KEGG pathway enrichment analyses of the identified significant genes by mRNA sequencing analysis were performed by an R package and functional mapping and annotation (FUMA) software. 16
Statistical Analysis
All data were expressed as mean ± SD and analyzed by GraphPad Prism 7.0 software. A one‐way analysis of variance (ANOVA) and independent samples t‐test were used for comparisons of the results. The threshold was defined as p < 0.05.
Results
The Surface Antigens of HUC‐MSCs
FCM results showed that the CD105 (92.1%), CD44 (98.3%) is positive, and HLA‐DR (0.15%), CD34 (0.026%) is negative in NAT group, as normal as other stem cells. But CD90 (58.5%) showed reduction in NAT group, which may be related to osteogenic differentiation (Supplementary File S2). 17
Identification of NAT in Vivo
As shown in Fig. 3A, the disc height index (DHI) showed that IDD_dH2O group had markedly decreased (p = 3.00 × 10−4) (Fig. 3B). The results of the disc histological grade have shown that both IDD_dH2O (p = 2.00 × 10−3) and IDD_NAT2 (p = 9.00 × 10−4) group had high grade compared to NC group, while IDD_NAT1 group showed no significant differences (p = 5.71 × 10−2) (Fig. 3C). The IHC results indicated that the expression of COL2A1 in IDD_dH2Ogroup was down‐regulated obviously (p < 1.00 × 10−4) (Fig. 3D,E). The expression of OCN showed that IDD_dH2O group was down‐regulated (p = 1.84 × 10−2), while both IDD_NAT1(p = 4.90 × 10−3) and IDD_NAT2 (p = 2.22 × 10−2) showed up‐regulated trend (Fig. 3D,F). The ALP staining showed that IDD_NAT1 group remarkedly upregulated when compared to the other three groups (Fig. 3G,H). The osteogenic differentiation in IDD_dH2O group (p = 3.42 × 10−2) and IDD_NAT2 group (p = 3.60 × 10−3)was declined remarkably (Fig. 3G,H).
Fig. 3.
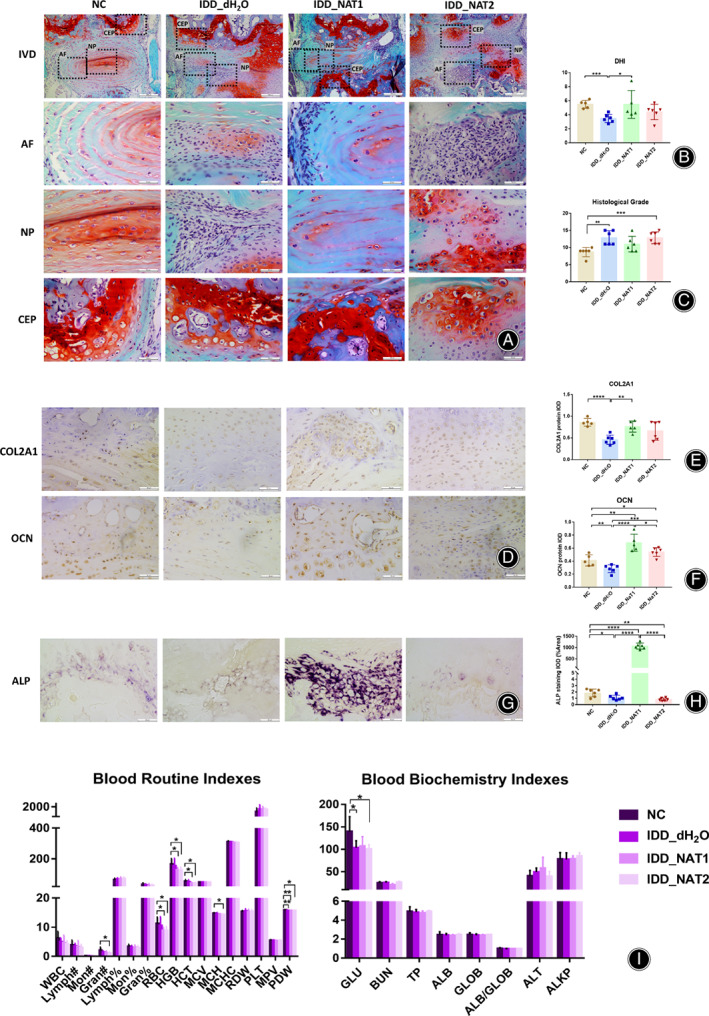
NAT induced chondrogenesis and osteogenesis in vivo. (A) The safranine O‐fast green staining in IVD samples among the four groups, NC group, IDD_H2O group, IDD_NAT1group (2 mg/kg), and IDD_NAT2 group (20 mg/kg)after treatment for 8w, n = 6 for each group, scale bars is 200 μm in IVD and 50 μm in AF, NP, and CEP.IVD: intervertebral disc; AF: anulus fibrosus; NP, nucleus pulposus; CEP: endplate cartilage. (B) The DHI results of the four groups. C) The histological grade results of the four groups. (D) The IHC staining of COL2A1 and OCN in the four groups (n = 6), scale bar is 50 μm. (E) The IHC results of COL2A1 protein expression. (F) The IHC results of OCN protein expression. (G) The ALP staining of the four groups. Scale bar is 50 μm in ALP staining. ALP: alkaline phosphatase. (H) The ALP staining results of the four groups. (I) The results of the blood routine indexes and blood biochemistry indexes in the four groups.ALB, albumin; ALB/GLOB, albumin/globulin ratio; ALKP, alkaline phosphatase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; GLOB, globulin; GLU, glucose; Gran#, granulocyte count; Gran%, granulocyte ratio; HCT, hematocrit; HGB, hemoglobin; Lymph#, lymphocyte count; Lymph%, lymphocyte ratio; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; Mon#, monocyte count; Mon%, monocyte ratio; MPV, mean platelet volume; PDW, platelet distribution width; PLT, platelet count; RBC, red blood cell count; RDW, red blood cell volume distribution width; TP, total protein; WBC, white blood cell count; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Among blood routine indexes, the IDD_NAT2 group had a lower level of granulocyte count (Gran, p = 4.05 × 10−2), red blood cell count (RBC, p = 2.67 × 10−2), hemoglobin (HGB, p = 2.14 × 10−2), hematocrit (HCT, p = 2.55 × 10−2), mean corpuscular hemoglobin (MCH, p = 1.03 × 10−2), and platelet distribution width (PDW, p = 3.83 × 10−2). IDD_NAT1 group had a lower level of RBC (p = 2.88 × 10−2), HGB (p = 2.50 × 10−2), HCT (p = 2.87 × 10−2), and PDW (p = 3.60 × 10−3). IDD_dH2O group had a lower level of PDW (p = 2.80 × 10−3) (Fig. 3I). The decrease of Gran in IDD_NAT2 group in routine blood examination indicated aplastic anemia, infection, leukemia or drug treatment. And the decrease of RBC, HGB, HCT, MCH, PDW in the groups in routine blood examination indicated the cause of anemia.
Among blood biochemical indexes, a lower level of glucose (GLU) was detected in IDD_ dH2O group (p= 3.87 × 10−2) and IDD_NAT2 group (p = 3.40 × 10−2). There was no other statistical difference among the four groups in other indexes (Fig. 3I).
Micro‐CT scan was performed to further detect the vertebral subchondral bone (Fig. 4). The BV/TV (p= 3.50 × 10−3, Fig. 4B) and Tb.N (p = 3.59 × 10−2, Fig. 4D) results of IDD_dH2O group were significantly declined, while the Tb.Sp results of IDD_dH2O group were ascended obviously (p = 1.50 × 10−2, Fig. 4E). The volume of Co4/5 inIDD_dH2O group was significantly decreased (p = 3.37 × 10−2) (Fig. 4F), and the two NAT groups showed no significant differences (Fig. 4G), indicating NAT may ameliorate the severe of secondary osteoporosis in IDD.
Fig. 4.
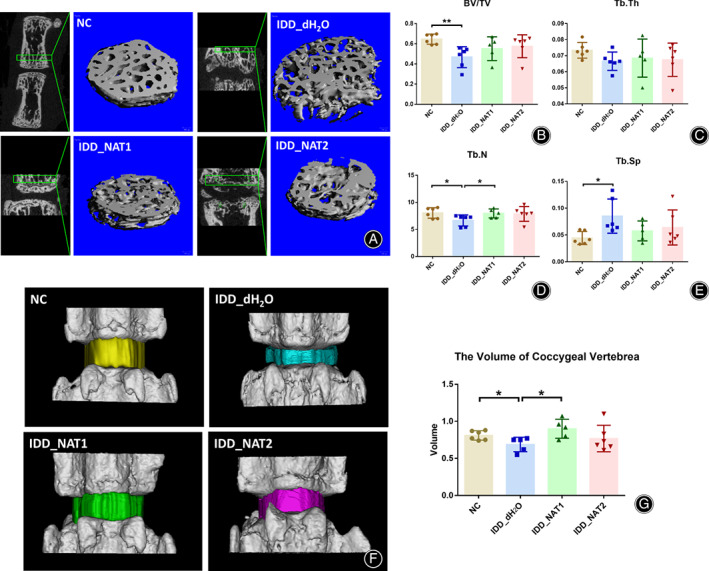
NAT attenuated the secondary osteoporosis of IDD and delayed the development of IDD. (A) Quantitative micro‐CT analysis of Co4/5 discs for 23 layers (276 μm) upon the subchondral bone, n = 6 for each group. (B)The results of trabecular bone volume per total volume(BV/TV). (C)The results of trabecular thickness (Tb.Th). (D) The results of trabecular number (Tb.N). (E) The results of trabecular separation (Tb.Sp). (b) Three‐dimensional models for Co4/5 in the four groups. (F) The 3D models of coccygeal vertebrae were analyzed by Mimics 20.0 software. (G) The volume of coccygeal vertebrae in the four groups.*p < 0.05, **p < 0.01.
The Results of Coccygeal Discs Transcriptome after NAT Treatment
Totally, 190 significant genes were identified in IDD_NAT1 group and 233 genes were identified in IDD_NAT2 group compared to NC group (Supplementary File S3). Venn diagram showed there are 128 common genes in both the two NAT groups (P adj <0.05) (Fig. 5A), including Coch (P NAT1 = 1.51 × 10−14, P NAT2 = 3.86 × 10−17), Igfnl (P NAT1 = 5.80 × 10−13, P NAT2 = 1.47 × 10−13), Mmp13 (P NAT1 = 4.31 × 10−7, P NAT2 = 5.50 × 10−7), (Supplementary File S3).
Fig. 5.
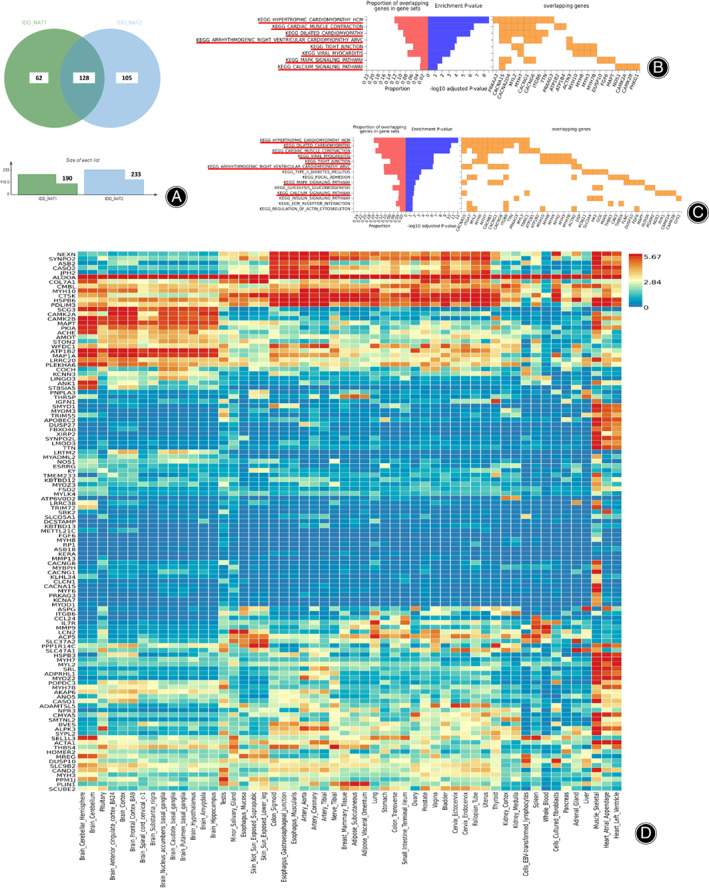
Coccygeal vertebrae transcriptome determined by RNA sequencing. (A) The Venn diagram of the common genes between IDD_NAT1 group and IDD_NAT2 group (P adj <0.05). (B) KEGG pathway analysis for differentially expression genes identified by IDD_NAT1 group. (C) KEGG pathway analysis for differentially expression genes identified by IDD_NAT2 group. (D) Heatmap of the 128 common genes between IDD_NAT1 group and IDD_NAT2 group. N = 3 for each group.
Gene enrichment analysis identified 8 significant KEGG pathways in IDD_NAT1 group, and 14 KEGG pathways in IDD_NAT2 group (P adj <0.05). Interestingly, the eight KEGG pathways identified in IDD_NAT1 group were also the top pathways in IDD_NAT2 group, mainly including Hypertrophic cardiomyopathy HCM (P NAT1 = 1.08 × 10−11, P NAT2 = 4.50 × 10−15), Tight junction (P NAT1 = 4.20 × 10−6, P NAT2 = 1.02 × 10−8), MAPK signaling pathway (P NAT1 = 3.64 × 10−4, P NAT2 = 2.65 × 10−4), etc.(Fig. 5B,C).
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation / Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA009764) that will be publicly accessible at https://ngdc.cncb.ac.cn/gsa after October 1, 2023. 18 , 19
Identification of MAPK3 as the Target of NAT
To further detect the role of MAPK3 in NAT (N) treatment, U0126 (U), an ERK1/2 inhibitor, was used here. As a result, the protein expression of COL2A1 up‐regulated in the group of N + U0.5 h (p = 2.38 × 10−2), and showed the ascend trend in NAT group (Fig. 6A,B). OCN ascended obviously in NAT group (p = 6.10 × 10−3), N + U 0.5 h (p = 1.00 × 10−4), and C + U 0.5 h (p = 3.33 × 10−2) (Fig. 6A,D). RUNX2 declined obviously in C + U 1 h compared to N + U 0.5 h (p = 2.89 × 10−2) and N + U 1 h (p = 3.47 × 10−2) (Fig. 6A,E). For the expression of P‐ERK1/2, the three NAT groups, including NAT (p = 2.00 × 10−2), N + U 0.5 h (p = 1.79 × 10−2) and N + U 1 h (p = 2.89 × 10−2) were showed significantly declining (Fig. 6A,F), but the total expression of ERK1/2 were showed no difference (Fig. 6A,G). To detect the relationship between NAT and MAPK3 in vivo, the mIHC of the Co4/5 was also performed in the four groups of IDD mouse model (Fig. 6H). As is shown, in IDD_dH2O group, the expression of ERK1/2 was significantly enhanced (p = 2.89 × 10−2, Fig. 6I), and both COL2A1 (p = 3.32 × 10−2, Fig. 6J) and OCN (p = 1.00 × 10−3, Fig. 6K) were declined remarkedly, which were in coincidence with the IHC results before (Fig. 2D–F). The NAT groups showed the trend to alleviate the descending of COL2A1 and OCN, and the ascending of ERK1/2 in the process of IDD (Fig. 6H–K).
Fig. 6.
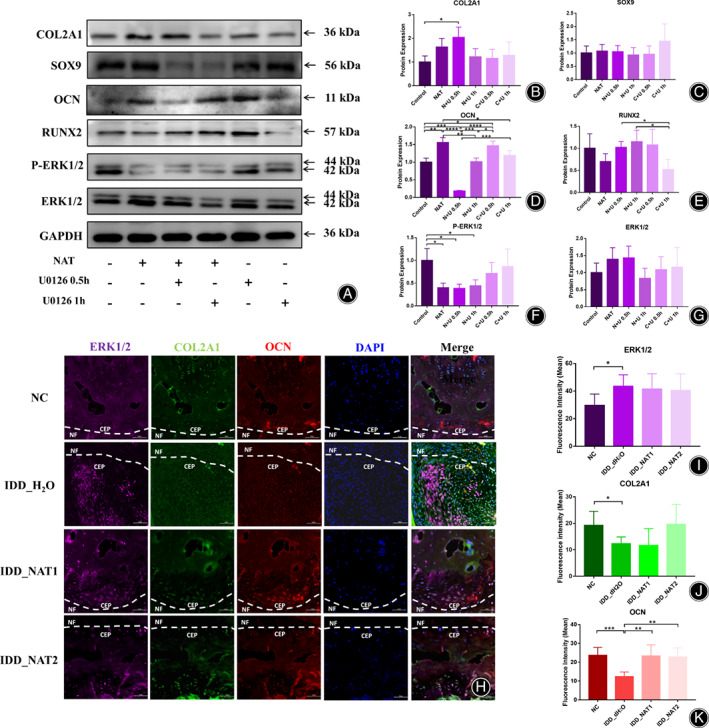
Verification of MAPK3 in vitro and in vivo. (A) The protein expression of COL2A1, SOX9, OCN, RUNX2, P‐ERK1/2, ERK1/2 after NAT with or without U0126 (10 μM for 0.5 h and 1 h) intervention for 14 days. Full‐length blots are presented in Supplementary File S4. (B) The results of COL2A1 protein expression in the six groups. (C) The results of SOX9 protein expression in the six groups. (D) The results of OCN protein expression in the six groups. (E) The results of RUNX2 protein expression in the six groups. (F) The results of phosphorylation ERK1/2 protein expression in the six groups. (G) The results of total ERK1/2 protein expression in the six groups. (H) Immunofluorescence staining of the subchondral bone of Co4/5 after NAT treatment for 8w in the four groups. (I) The fluorescence intensity resultsofERK1/2 in the four groups. (J) The fluorescence intensity results of COL2A1 in the four groups. (K) The fluorescence intensity results of OCN in the four groups. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
IDD has been considered as the main cause of LBP, and affects the quality of life even causing disability. With its high prevalence, it brings a heavy economic burden worldwide. 20 In this study, we have found a novel repurposing drug, NAT, for IDD treatment, which could induce chondrogenesis and osteogenesis and delay the degeneration of IVD.
Main Findings of this Study
The findings of this study mainly included: (i) a small molecular drug, NAT, was found to induce chondrogenesis and osteogenesis of HUC‐MSCs; (ii) an IDD mice model was established, and NAT could ameliorate the secondary osteoporosis and delay the degeneration of IVD; (iii) 128 genes, eight KEGG pathways and 57 target proteins were identified for NAT; and (iv) one of the target proteins, MAPK3, especially its phosphorylation, was verified to participate in IDD treatment.
The Repurposing Drug of NAT
NAT is an anionic bile acid detergent synthesized from cholic acid and shows a choleretic activity promoting the secretion of bile from the liver. Previously, it is used as a cholagogue and choleretic in the emulsification of lipids and fats. 21 Recently, some other applications from bile acid family have been studied in osteoarthritis. Arai et al. found that ursodeoxycholic acid, a hydrophilic bile acid, which has antioxidant and anti‐inflammatory activities, could induce bone regeneration via stimulating the osteogenic differentiation of MSCs. 22 Additionally, tauroursodeoxycholic acid was identified as a potential candidate for osteoarthritis by its chondroprotective. 23 , 24 In this study, we found a small molecular compound of bile acid family, NAT, which could enhance expression of COL2A1 and OCN both in vivo and in vitro, ameliorate the secondary osteoporosis, and delay the development of IDD. Moreover, we confirmed that a low dose of NAT showed major impact on osteoblasts differentiation.
The Phenotype Detection of HUC‐MSCs after NAT Treatment
MSCs, which reside in bone marrow and many other tissues, have been found in healthy and diseased cartilage and have the potential for cartilage repair. 25 The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy proposes have defined the criteria for human MSC in 2006, including plastic‐adherent in standard culture conditions, expressing CD105, CD73, CD90 and lack expression of CD45, CD34, CD14, HLA‐DR, etc., and the ability to differentiate to osteoblasts, adipocytes and chondroblasts in vitro. 26 In this study, HUC‐MSCs expressed CD105, CD44 and lack expression of CD34, HLA‐DR after NAT intervention, which are in accordance with the criteria of human MSC. However, the expression of CD90was reduced in NAT intervened HUC‐MSCs, which maybe enhance the differentiation of osteogenesis and adipogenesis in MSCs. 17
The Transcriptome Analysis of Coccygeal Vertebrae
To further detect the molecular mechanism of NAT, the transcriptome analysis was performed. As a result, 128 common genes and eight common KEGG pathways were identified for NAT. Among which, MAPK signaling pathway are involved in the molecular mechanism of NAT treatment. Mitogen‐activated protein (MAP) kinases, also known as extracellular signaling‐regulated kinases (ERKs), act in a signaling cascade and regulate various cellular functions, such as cell proliferation, cell migration, differentiation, apoptosis, autophagy, and senescence. 27 It has been known that MAPK signaling pathway, a negative regulator of bone growth, plays an important role in the regulation of chondrocyte differentiation. 28 Furthermore, MAPK signaling pathway is closely related to various pathological processes involved in IDD, such as mediating ECM metabolic imbalance, promoting cell apoptosis, inhibiting cell proliferation, or participating in the oxidative stress regulation. 27 Therefore, it is highly believed that NAT would produce a treating effect on IDD via MAPK signaling pathway.
Identification of MAPK3 as the Target Marker of NAT
To explore the direct target of NAT in MSCs, an SPR‐LC–MS assay was designed and found that NAT could mainly target 57 proteins in HUC‐MSCs, including MAPK3. MAPK3, also known as ERK1, is a member of the MAP kinase family. It is a member of the MAP kinase family and activated by upstream kinases before translocating to the nucleus and phosphorylating nuclear targets. The in vitro study showed that NAT could reduce the phosphorylation of ERK1/2 proteins more remarkably. In vivo study also confirmed that the expression of ERK1/2 showed higher expression in IDD_H2O group, but the ascending expression of ERK1/2 would be alleviated in the two NAT groups. It has been known that ERK phosphorylated in osteoblasts. 29 Moreover, ERK1/2 is involved in the mechanisms mediating the increased osteoblast proliferation, differentiation and the expression of ALP. 30 Activation of ERK1/2 via phosphorylation has been identified to regulate the differentiation of mesenchymal stem cells towards the osteogenesis. 31 Further confirmed that phosphorylation of ERK1/2 would be the downstream of NAT. To our knowledge, MAPK signaling pathway has been studied as a potential target for the treatment of IDD. 27 Therefore, NAT, which could target MAPK3 and regulate MAPK signaling pathway, may become a novel repurposing drug for IDD treatment strategy.
Strengths and Limitations
The primary strength of our study lies in the identification of small molecular drugs for IDD from an approved drug library, which could lead to time and cost savings. Another strength of this study is the SPR‐LC–MS assay. SPR analysis is a novel bioanalytical tool to analyze the interaction between proteins, DNA, enzymes, and other biomolecules, and LC–MS is a novel protein analytical approach to protein identification. The integration of the two approaches, SPR‐LC‐MS assay, has shown great potential in target identification. 32 The limitation of this study is that this study is the first step of the repurposing drug of NAT for IDD, and the interaction between NAT and MAPK3 in osteogenesis and chondrogenesis of MSCs needs further research.
Conclusion
In summary, the primary finding of this study was the identification of a novel repurposing drug, NAT, which would induce chondrogenesis and osteogenesis of MSCs, ameliorate the secondary osteoporosis and delay the progression of IDD in mice by targeting MAPK3. Furthermore, MAPK3, especially the phosphorylation of MAPK3, as a relevant drug target, would be a potential therapeutic agent in osteogenic differentiation for IDD treatment.
Conflict of Interest
The authors have no conflicts of interest to declare.
Ethics Statement
This work was approved by Nanfang Hospital Animal Ethic Committee of Southern Medical University (NFYY‐2020‐1031).
Author Contributions
Zhongmin Zhang and Xiaochun Bai contributed to conception and design, and drafting and revision of the paper for important intellectual content. Jiajia Xu contributed to drafting and revision of the paper for important intellectual content. Ping Li, Zesen Chen and Keyu Meng contributed to statistical analysis, and drafting and revision of the paper for important intellectual content. Yanlin Chen, Xin Xiang, Xiuhua Wu, Zhiping Huang, Ruijun Lai, Peng Li and Zhongming Lai contributed to drafting and revision of the paper for important intellectual content. Xiang Ao, Zhongyuan Liu and Kaifan Yang contributed to drafting and revision of the paper for important intellectual content. All authors approved the final approval of manuscript.
Supporting information
File S1. The 57 target proteins of NAT identified in HUC‐MSCs.
File S2. The phenotype detection of HUC‐MSCs.
File S3. The significant genes identified by coccygeal vertebrae transcriptome analysis. 2_1 Significant genes identified between IDD_NAT1 group and NC group. 2_2 Significant genes identified between IDD_NAT2 group and NC group. 2_3 The 128 common genes identified between IDD_NAT1 group and IDD_NAT2.
File S4. Full length uncropped original western blots. The full length uncropped original western blot of COL2A1 (a), SOX9 (b), OCN (c), RUNX2 (d), ERK1/2 (e), p‐ERK1/2 (f), and GAPDH (g).
Acknowledgements
This work was supported by National Key R&D Program of China (Grant No.2022YFC2502901), National Natural Science Foundation of China (Grant No. 82072520 and 82272527), and the Presidential Foundation of NanFang Hospital (Grant No.2021B022). HUC‐MSCs were kindly provided by Ruijun Lai from Southern Medical University. We would like to thank all study participants.
Contributor Information
Xiaochun Bai, Email: baixc15@smu.edu.cn.
Zhongmin Zhang, Email: nfzzm@163.com.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA009764) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa after July 1st, 2023.
References
- 1. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Vol 396. Lancet: GBD 2019 Diseases and Injuries Collaborators; 2020. p. 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vedicherla S, Buckley CT. In vitro extracellular matrix accumulation of nasal and articular chondrocytes for intervertebral disc repair. Tissue Cell. 2017;49:503–513. [DOI] [PubMed] [Google Scholar]
- 3. Song Y, Lu S, Geng W, Feng X, Luo R, Li G, et al. Mitochondrial quality control in intervertebral disc degeneration. Exp Mol Med. 2021;53:1124–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li G, Zhang W, Liang H, Yang C. Epigenetic regulation in intervertebral disc degeneration. Trends Mol Med. 2022;28:803–805. [DOI] [PubMed] [Google Scholar]
- 5. Xie L, Chen Z, Liu M, Huang W, Zou F, Ma X, et al. MSC‐derived exosomes protect vertebral endplate chondrocytes against apoptosis and calcification via the miR‐31‐5p/ATF6 Axis. Mol Ther. Nucleic Acids. 2020;22:601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Y, Li Y, Nan LP, Wang F, Zhou SF, Feng XM, et al. Insights of stem cell‐based endogenous repair of intervertebral disc degeneration. World J Stem Cells. 2020;12:266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liang H, Luo R, Li G, Zhang W, Song Y, Yang C. The proteolysis of ECM in intervertebral disc degeneration. Int J Mol Sci. 2022;23:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41–58. [DOI] [PubMed] [Google Scholar]
- 9. Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3:673–683. [DOI] [PubMed] [Google Scholar]
- 10. Chen Y, Sun H, Yao X, Yu Y, Tian T, Xu W, et al. Pharmaceutical therapeutics for articular regeneration and restoration: state‐of‐the‐art technology for screening small molecular drugs. Cell Mol Life Sci. 2021;78:8127–8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu S, Xing H, Zhang J, Zhu Z, Yin Y, Zhang N, et al. Mesenchymal stem cell‐derived extracellular vesicles: immunomodulatory effects and potential applications in intervertebral disc degeneration. Stem Cells Int. 2022;2022:7538025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu J, Chen J, Liu B, Yang C, Xie D, Zheng X, et al. Acellular spinal cord scaffold seeded with mesenchymal stem cells promotes long‐distance axon regeneration and functional recovery in spinal cord injured rats. J Neurol Sci. 2013;325:127–136. [DOI] [PubMed] [Google Scholar]
- 13. Han B, Zhu K, Li FC, Xiao YX, Feng J, Shi ZL, et al. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine. 2008;33:1925–1934. [DOI] [PubMed] [Google Scholar]
- 14. Tian Z, Ma X, Yasen M, Mauck RL, Qin L, Shofer FS, et al. Intervertebral disc degeneration in a percutaneous mouse tail injury model. Am J Phys Med Rehabil. 2018;97:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li P, Ning Y, Guo X, Wen Y, Cheng B, Ma M, et al. Integrating transcriptome‐wide study and mRNA expression profiles yields novel insights into the biological mechanism of chondropathies. Arthritis Res Ther. 2019;21:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li P, Cheng S, Wen Y, Cheng B, Liu L, Wu X, et al. Identifying candidate genes associated with sporadic amyotrophic lateral sclerosis via integrative analysis of transcriptome‐wide association study and messenger RNA expression profile. Cell Mol Neurobiol. 2023;43:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moraes DA, Sibov TT, Pavon LF, Alvim PQ, Bonadio RS, Da Silva JR, et al. A reduction in CD90 (THY‐1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res Ther. 2016;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen T, Chen X, Zhang S, Zhu J, Tang B, Wang A, et al. The genome sequence archive family: toward explosive data growth and diverse data types. Genomics Proteomics Bioinformatics. 2021;19:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Database Resources of the National Genomics Data Center . China National Center for bioinformation in 2022. Nucleic Acids Res. 2022;50:D27–D38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kasamkattil J, Gryadunova A, Martin I, Barbero A, Schären S, Krupkova O, et al. Spheroid‐based tissue engineering strategies for regeneration of the intervertebral disc. Int J Mol Sci. 2022;23:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anwer MS. Cellular regulation of hepatic bile acid transport in health and cholestasis. Hepatology. 2004;39:581–590. [DOI] [PubMed] [Google Scholar]
- 22. Arai Y, Park H, Park S, Kim D, Baek I, Jeong L, et al. Bile acid‐based dual‐functional prodrug nanoparticles for bone regeneration through hydrogen peroxide scavenging and osteogenic differentiation of mesenchymal stem cells. J Control Release. 2020;328:596–607. [DOI] [PubMed] [Google Scholar]
- 23. Arai Y, Choi B, Kim BJ, Rim W, Park S, Park H, et al. Tauroursodeoxycholic acid (TUDCA) counters osteoarthritis by regulating intracellular cholesterol levels and membrane fluidity of degenerated chondrocytes. Biomater Sci. 2019;7:3178–3189. [DOI] [PubMed] [Google Scholar]
- 24. Kusaczuk M, Naumowicz M, Krętowski R, Cukierman B, Cechowska‐Pasko M. Molecular and cellular effects of chemical chaperone‐TUDCA on ER‐stressed NHAC‐kn human articular chondrocytes cultured in normoxic and hypoxic conditions. Molecules. 2021;26:878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson K, Zhu S, Tremblay MS, Payette JN, Wang J, Bouchez LC, et al. A stem cell‐based approach to cartilage repair. Science. 2012;336:717–721. [DOI] [PubMed] [Google Scholar]
- 26. Dominici M, Le Blanc K, Mueller I, Slaper‐Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 27. Zhang HJ, Liao HY, Bai DY, Wang ZQ, Xie XW. MAPK /ERK signaling pathway: a potential target for the treatment of intervertebral disc degeneration. Biomed Pharmacother. 2021;143:112170. [DOI] [PubMed] [Google Scholar]
- 28. Murakami S, Balmes G, McKinney S, Zhang Z, Givol D, de Crombrugghe B. Constitutive activation of MEK1 in chondrocytes causes Stat1‐independent achondroplasia‐like dwarfism and rescues the Fgfr3‐deficient mouse phenotype. Genes Dev. 2004;18:290–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lavoie H, Gagnon J, Therrien M. ERK signalling: a master regulator of cell behaviour, life and fate. Nat Rev Mol Cell Biol. 2020;21:607–632. [DOI] [PubMed] [Google Scholar]
- 30. Jackson RA, Kumarasuriyar A, Nurcombe V, Cool SM. Long‐term loading inhibits ERK1/2 phosphorylation and increases FGFR3 expression in MC3T3‐E1 osteoblast cells. J Cell Physiol. 2006;209:894–904. [DOI] [PubMed] [Google Scholar]
- 31. Jansen JH, Weyts FA, Westbroek I, Jahr H, Chiba H, Pols HA, et al. Stretch‐induced phosphorylation of ERK1/2 depends on differentiation stage of osteoblasts. J Cell Biochem. 2004;93:542–551. [DOI] [PubMed] [Google Scholar]
- 32. Gu C, Yin Z, Nie H, Liu Y, Yang J, Huang G, et al. Identification of berberine as a novel drug for the treatment of multiple myeloma via targeting UHRF1. BMC Biol. 2020;18:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. The 57 target proteins of NAT identified in HUC‐MSCs.
File S2. The phenotype detection of HUC‐MSCs.
File S3. The significant genes identified by coccygeal vertebrae transcriptome analysis. 2_1 Significant genes identified between IDD_NAT1 group and NC group. 2_2 Significant genes identified between IDD_NAT2 group and NC group. 2_3 The 128 common genes identified between IDD_NAT1 group and IDD_NAT2.
File S4. Full length uncropped original western blots. The full length uncropped original western blot of COL2A1 (a), SOX9 (b), OCN (c), RUNX2 (d), ERK1/2 (e), p‐ERK1/2 (f), and GAPDH (g).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA009764) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa after July 1st, 2023.


