Abstract
Objective
To analyse the factors influencing clinical pregnancy outcome of in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) in older women, and to establish a risk prediction model.
Methods
A total of 425 patients receiving IVF/ICSI from March 2018 to March 2020 were divided into pregnancy group (n=194) and non-pregnancy group (n=231). The factors affecting the outcomes of IVF/ICSI were explored by univariate and multivariate logistic regression analyses. A nomogram prediction model was constructed.
Results
The two groups had significantly different age, body mass index, dysmenorrhea, parity, times of full-term births, history of cesarean section, basal follicle stimulating hormone, basal antral follicle count (AFC), number of high-quality embryos, and basal estradiol, luteinizing hormone and endometrial thickness on the day of human chorionic gonadotropin (HCG) administration (P<0.05). Age ≥40 years old, dysmenorrhea, history of cesarean section, basal AFC<9, number of high-quality embryos <4, and endometrial thickness on the day of HCG administration <11 mm led to IVF/ICSI failure. The established model exhibited high calibration and discrimination degrees in predicting the outcome of IVF/ICSI.
Conclusion
The risk prediction model for the pregnancy outcome of IVF/ICSI in older women helps evaluate the fertility probability and risk, providing references for formulating reasonable assisted reproduction plans.
Keywords: Older pregnant woman, in vitro fertilization/intracytoplasmic sperm injection, pregnancy outcome, risk factor, nomogram prediction model
Introduction
As the society develops, more women have chosen to marry and to give birth late1. However, older pregnant women are usually in a state of decreased ovarian reserve function, and the incidence rate of infertility rises with aging2. The incidence rate of infertility in women aged 35-39 years old is 30%, while that in women aged 39-44 years old increases to 64%3. The success rate of natural pregnancy greatly reduces in older pregnant women, so assisted reproductive technology is needed to meet their fertility demand4. In vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) is an important method for treating infertility5. The proportion of older pregnant women hoping to get pregnant through IVF/ICSI is gradually increasing. However, the clinical pregnancy outcome is still unsatisfactory6. In the process of assisted reproduction, older pregnant women have small numbers of retrieved oocytes and transferable embryos, poor quality of transferable embryos, low clinical pregnancy rate and high abortion rate, resulting in the failure of operation7. The embryo implantation rate drops gradually in older pregnant women at a speed of 2.7% per year8. In the field of reproductive medicine, therefore, elevating the clinical pregnancy success rate of older pregnant women receiving IVF/ICSI remains rather difficult and thus needs extensive research9.
So far, the factors leading to IVF/ICSI failure in older pregnant women have not been fully elucidated. Thereby motivated, the factors affecting the clinical pregnancy outcome of IVF/ICSI in older women were investigated herein, aiming to provide valuable references for formulating assisted reproduction plans for older pregnant women and to increase the clinical pregnancy success rate.
Materials and methods
General data
A total of 425 patients receiving IVF/ICSI in our hospital from March 2018 to March 2020 were divided into pregnancy group (n=194) and non-pregnancy group (n=231). In the pregnancy group, the women were aged 36-43 years old, with an average age of (39.18±1.85) years old. In the non-pregnancy group, the women were aged 37-45 years old, with a mean of (40.64 ±2.12) years old. This study was reviewed and approved by the medical ethics committee of our hospital.
The inclusion criteria involved: (1) patients meeting the relevant ethical principles in the Code of Human assisted Reproductive Technology10, (2) those aged >35 years old, (3) those meeting the relevant indications of IVF/ICSI, (4) those receiving fresh cycle embryo transfer (ET), and (5) those who and whose family members were informed of this study.
The exclusion criteria were as follows: (1) those who or whose partners suffered from sexually transmitted diseases, acute infections of the genitourinary system or severe mental diseases, (2) those who or whose partners had serious bad habits such as taking drugs, (3) those with abnormal development of the reproductive tract or uterus, (4) those suffering from infertile diseases or hereditary diseases stipulated in the Mother and Infant Health Care Law of the People's Republic of China, or (5) those who or whose partners were exposed to teratogenic drugs or poisons or rays and in the active period.
Collection of general data
The general data of patients were collected through electronic medical records, including age, height, weight, body mass index (BMI), duration of infertility, type of infertility (primary or secondary infertility), age of menarche, dysmenorrhea, parity, times of full-term births, history of cesarean section, basal follicle stimulating hormone (FSH), basal luteinizing hormone (LH), basal estradiol (E2), basal testosterone (T), and basal antral follicle count (AFC).
Observation indices
The insemination method (IVF or ICSI), numbers of oocytes retrieved, high-quality oocytes, high-quality embryos and transferred embryos, E2, LH, progesterone (P), endometrial thickness on the day of human chorionic gonadotrophin (HCG) administration, and application duration and total dosage of gonadotropin (Gn) were recorded.
Evaluation criteria for embryo quality11: (1) The quality of embryos at cleavage stage was assessed and classified into 4 grades according to the number, uniformity and proportion of blastomeres: Grade I: Blastomeres in a uniform size, with no obvious DNA fragments. Grade II: Blastomeres in a slightly uniform size, with a DNA fragmentation rate <20%. Grade III: Blastomeres in an obviously non-uniform size, with a DNA fragmentation rate of 20-50%. Grade IV: A DNA fragmentation rate >50%. Embryos with double pronuclei at 1 d after fertilization and 7-9 cells at 3 d after fertilization and at morphological grade I-II were defined as high-quality embryos at cleavage stage. (2) The quality of embryos at blastocyst stage was evaluated and divided into 6 phases according to the size of blastocyst cavity and the degree of hatching. Among them, blastocysts at >phase 3 were scored according to inner cell mass and trophoblast cells, which were divided into grades A, B and C. Embryos with blastocyst morphological score ≥3 BB (i.e. the size and hatching degree of blastocyst cavity were at phase 3, and the inner cell mass and trophoblast cells were scored grade B) at 5 d after fertilization and blastocyst morphological score ≥4 BB 6 d after fertilization were defined as high-quality embryos at the blastocyst stage.
Clinical pregnancy was determined as follows: The level of serum β-HCG was detected 14 d after ET, and the patients with positive results were examined by ultrasound 28-35 d after ET. Clinical pregnancy was confirmed in patients with gestational sac and fetal heart and fetal buds in the gestational sac.
Construction and validation of nomogram prediction model
Multivariate logistic regression analysis was used to analyse the factors affecting the clinical pregnancy outcome of IVF/ICSI in older women, and the variables with significant differences were assigned. The 425 older pregnant women were randomly divided into training set (n=340) and validation set (n=85) at a ratio of 4:1. The training set was used to construct the nomogram prediction model, while the validation set was utilized for the internal validation of the nomogram prediction model. The nomogram prediction model was established using R software (R 3.3.2) and the rms software package. The calibration of the nomogram prediction model was evaluated using the calibration curve, and the discrimination of the nomogram prediction model was evaluated by the receiver operating characteristic (ROC) curve.
Statistical analysis
SPSS19.0 software was utilized for statistical analysis, and GraphPad Prism 5.0 software was employed for plotting. The numerical data were expressed as percentage, and chi-square test was performed for intergroup comparison. The measurement data were expressed as mean ± standard deviation, and independent t-test was conducted for intergroup comparison. P<0.05 indicated that difference was statistically significant.
Results
General data and IVF/ICSI status
Significant differences were observed in age, BMI, dysmenorrhea, parity, times of full-term births, history of cesarean section, basal FSH and basal AFC between the two groups (P<0.05). Nevertheless, there were no significant differences in other general data between the two groups (P>0.05) (Table 1).
Table 1.
General data
| Group | Pregnancy group (n=194) | Non-pregnancy group (n=231) | t/χ2 | P |
| Age (year) | 39.18±1.85 | 40.64±2.12 | 7.491 | 0.000 |
| BMI (kg/m2) | 23.25±2.64 | 22.34±2.58 | 3.584 | 0.000 |
| Duration of infertility (year) | 3.34±2.02 | 3.05±1.95 | 1.502 | 0.134 |
| Type of infertility [n (%)] | 0.236 | 0.627 | ||
| Primary infertility | 31 (15.98) | 33 (14.29) | / | / |
| Secondary infertility | 163 (84.02) | 198 (85.71) | / | / |
| Age of menarche (year) | 13.61±1.68 | 13.48±1.47 | 0.851 | 0.395 |
| Dysmenorrhea [n (%)] | 5.596 | 0.018 | ||
| Yes | 3 (1.55) | 14 (6.06) | / | / |
| No | 191 (98.45) | 217 (93.94) | / | / |
| Parity (time) | 0.60±0.52 | 0.73±0.53 | 2.540 | 0.011 |
| Times of full-term births (time) | 0.53±0.49 | 0.66±0.50 | 2.694 | 0.007 |
| History of cesarean section [n (%)] | 9.582 | 0.002 | ||
| Yes | 19 (9.79) | 48 (20.78) | / | / |
| No | 175 (90.21) | 183 (79.22) | / | / |
| Basal FSH (U/L) | 7.82±3.21 | 9.45±4.06 | 4.528 | 0.000 |
| Basal LH (U/L) | 4.85±2.13 | 4.64±2.08 | 1.025 | 0.306 |
| Basal E2 (pmol/L) | 206.73±51.64 | 212.85±53.49 | 1.194 | 0.233 |
| Basal T (nmol/L) | 1.11±0.72 | 1.05±0.69 | 0.875 | 0.382 |
| Basal AFC | 10.13±4.72 | 7.84±4.15 | 5.321 | 0.000 |
IVF/ICSI status
Significant differences were found in the number of high-quality embryos, and E2, LH and endometrial thickness on the day of HCG administration between the two groups (P<0.05). However, no significant differences were observed in the insemination method, number of oocytes retrieved, high-quality oocytes, transferred embryos, P on the day of HCG administration, and application duration and total dosage of Gn between the two groups (P>0.05) (Table 2).
Table 2.
IVF/ICSI status
| Group | Pregnancy group (n=194) | Non-pregnancy group (n=231) | t | P |
| Insemination method [n (%)] | 0.717 | 0.397 | ||
| IVF | 160 (82.47) | 183 (79.22) | / | / |
| ICSI | 34 (17.53) | 48 (20.78) | / | / |
| Number of oocytes retrieved | 7.47±3.65 | 6.98±3.42 | 1.427 | 0.154 |
| Number of high-quality oocytes | 6.39±3.48 | 5.78±3.12 | 1.904 | 0.058 |
| Number of high-quality embryos | 4.48±2.23 | 3.74±2.08 | 3.535 | 0.000 |
| Number of transferred embryos | 1.86±0.31 | 1.90±0.29 | 1.372 | 0.171 |
| E2 on the day of HCG administration (nmol/L) | 126.37±27.31 | 110.26±25.83 | 6.239 | 0.000 |
| LH on the day of HCG administration (U/L) | 2.13±1.05 | 2.90±1.28 | 6.697 | 0.000 |
| P on the day of HCG administration (nmol/L) | 3.85±1.12 | 3.96±1.23 | 0.956 | 0.339 |
| Endometrial thickness on the day of HCG administration (mm) | 11.53±2.42 | 10.73±2.38 | 3.425 | 0.001 |
| Application duration of Gn (d) | 10.12±2.31 | 9.82±2.06 | 1.415 | 0.158 |
| Total dosage of Gn (IU) | 2347.65±683.74 | 2328.45±675.82 | 0.290 | 0.772 |
Univariate and multivariate logistic regression analysis results of factors affecting pregnancy outcome
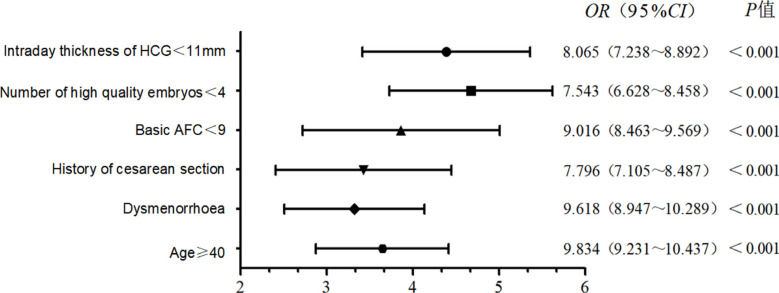
Age ≥40 years old odds ratio (OR): 3.876, 95% confidence interval (CI): 3.023-4.729, P=0.005), dysmenorrhea (OR: 3.586, 95% CI: 2.736-4.436, P=0.012), history of cesarean section (OR: 3.648, 95% CI: 2.837-4.459, P=0.010), basal AFC <9 (OR: 4.0758, 95% CI: 2.9875.169, P=0.001), number of high-quality embryos <4 (OR: 4.876, 95% CI: 4.023-5.729, P<0.001) and endometrial thickness on the day of HCG administration <11 mm (OR: 4.583, 95% CI: 3.674-5.519, P<0.001) were associated with the clinical pregnancy outcome of IVF/ICSI in older women (Table 3).
Table 3.
Univariate logistic regression analysis results of factors affecting pregnancy outcome
| Item | β | S.E. | Wald χ2 | P | OR (95% CI) |
| Age ≥40 years old | 1.731 | 1.239 | 3.859 | 0.005 | 3.876 (3.023~4.729) |
| Dysmenorrhea | 1.648 | 1.421 | 5.438 | 0.012 | 3.586 (2.736~4.436) |
| History of cesarean section | 1.259 | 1.764 | 3.462 | 0.010 | 3.648 (2.837~4.459) |
| Basal AFC <9 | 1.583 | 1.587 | 4.231 | 0.001 | 4.078 (2.987~5.169) |
| Number of high-quality embryos <4 | 1.374 | 1.321 | 3.745 | 0.000 | 4.876 (4.023~5.729) |
| Endometrial thickness on the day of HCG administration <11 mm | 1.682 | 1.425 | 2.647 | 0.000 | 4.583 (3.674~5.519) |
Using the indices associated with the clinical pregnancy outcome of IVF/ICSI in older women in univariate analysis as independent variables, and the pregnancy outcome (pregnancy=0 and non-pregnancy=1) as the dependent variable, multivariate logistic regression analysis was employed to analyse the factors affecting the pregnancy outcome. Age ≥40 years old, dysmenorrhea, history of cesarean section, basal AFC <9, number of high-quality embryos <4, and endometrial thickness on the day of HCG administration <11 mm were all risk factors for the clinical pregnancy failure of IVF/ICSI in older pregnant women (Figure 1).
Figure 1.
Multivariate logistic regression analysis results of factors affecting pregnancy outcome
Construction and evaluation of nomogram prediction model
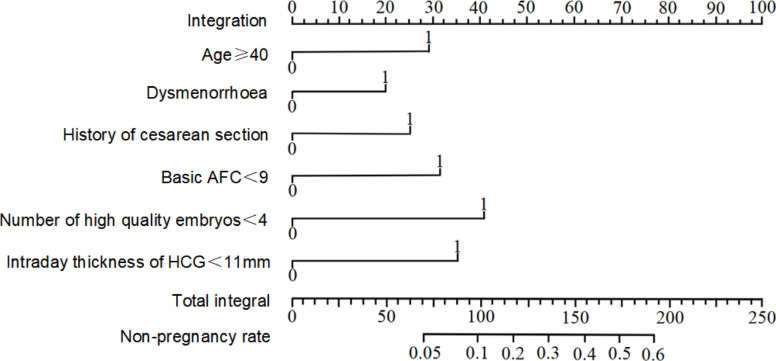
According to the results of multivariate regression analysis, a nomogram model was constructed to predict the clinical pregnancy failure of IVF/ICSI in older pregnant women. The scores for age ≥40 years old, dysmenorrhea, history of cesarean section, basal AFC <9, number of high-quality embryos <4, and endometrial thickness on the day of HCG administration <11 mm was 20, 25, 32, 42 and 35 points, respectively. The total score was 181 points, based on which the corresponding clinical pregnancy failure rate of IVF/ICSI in older pregnant women was 54.35% (Figure 2).
Figure 2.
Construction of nomogram prediction model
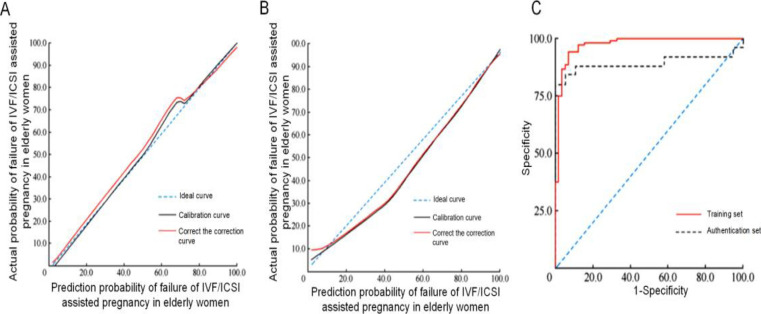
In the calibration curve of the training set, when the predicted probability was <50%, the difference between actual and predicted probabilities was small. When the predicted probability was 50-70%, the actual probability was slightly lower than the predicted one. When the predicted probability was >70%, the calibration curve did not deviate from the ideal one, showing a high degree of calibration. In the calibration curve of the validation set, the calibration curve and corrected one deviated from the ideal curve. When the predicted probability was 40%, the difference between actual and predicted probabilities was the largest (10%). The areas under the ROC curves predicted by the nomogram prediction model of training and validation sets were 0.926 (95% CI: 0.885-0.967, P<0.001) and 0.884 (95% CI: 0.852-0.916, P<0.001), respectively. The sensitivities were 91.64% and 82.73%, and the specificities was 94.25% and 88.72%, respectively, showing high a degree of discrimination (Figure 3).
Figure 3.
Evaluation of nomogram prediction model. A: Calibration curve in the nomogram prediction model of training set, B: Calibration curve in the nomogram prediction model of validation set, C: ROC curves of training set and validation set
Discussion
Due to the rapid development of society, the infertility rate of women has increased annually12. After the age of 35, the fertility of women declines yearly with aging, and the pregnancy rate of 45-year-old women is only 10%13. IVF/ICSI has become a crucial assisted reproduction method14. Nevertheless, the success rate is still far lower than the normal pregnancy rate in healthy people. In particular, the success rate of assisted reproduction remarkably decreases in older pregnant women15. The clinical pregnancy success rate and live birth rate of IVF/ICSI in women over 40 years old are obviously lower than those of young infertile patients16.
Lu et al. explored the factors affecting the clinical pregnancy outcome of female long-term IVF/ICSI, and found that older age was the risk factor leading to the failure of assisted reproduction in older pregnant women17. The number, quality and ovarian reserve function of oocytes decrease with increasing female age, and the response capability of ovary to ovulation induction drugs declines, so both oocyte retrieval rate and transferable embryos reduce, thus lowering the success rate of IVF/ICSI. Pelvic endometriosis, as a common cause of dysmenorrhea in women, has an incidence rate of up to 35-50% in infertility patients with dysmenorrhea. Endometriosis leads to poor oocyte quality, ovulation disorder, tubal adhesion and poor endometrial capacitance, thereby increasing the failure rate of IVF/ICSI in patients with infertility and dysmenorrhea. In this study, history of cesarean section was a factor leading to the failure of IVF/ICSI in older pregnant women. Women undergoing cesarean section have the risks such as scar diverticulum, scar pregnancy and uterine rupture. Patounakis et al. found that history of cesarean section increased the difficulty of ET, and the average time of ET was prolonged by 30 s18. Zhang et al. revealed that basal AFC was a factor influencing the clinical pregnancy outcome of fresh cycle IVF-ET in older women19. Basal AFC, which is a vital index to reflect the ovarian reserve function, has high predictive value for ovarian response. Palmsten et al. found that the pregnancy rate in women ≥38 years old receiving IVF-ET was dramatically lower than that of women <38 years old, the number of high-quality embryos was evidently smaller than that of women <38 years old, and the number of high-quality embryos was related to the pregnancy rate of older pregnant women20. Moreover, Chen et al. reported that endometrial thickness on the day of HCG administration was a factor affecting the clinical pregnancy outcome of female IVF-ET/ICSI21. Endometrial thickness can reflect the endometrial receptivity which is an important factor influencing the pregnancy outcome.
In this study, age ≥40 years old, dysmenorrhea, history of cesarean section, basal AFC <9, number of high-quality embryos <4, and endometrial thickness on the day of HCG administration <11 mm were all risk factors leading to the clinical pregnancy failure of IVF/ICSI in older pregnant women. The nomogram model for predicting the pregnancy outcome of assisted reproduction in older pregnant women had high degrees of calibration and discrimination, showing a high predictive value. In clinical practice, attention should be paid to the above risk factors. Older pregnant women should receive assisted reproduction treatment as soon as possible, and reasonable assisted reproduction plans should be formulated to raise the clinical pregnancy rate.
In conclusion, analysing factors affecting the clinical pregnancy outcome of IVF/ICSI in older women and constructing a risk prediction model are conducive to the evaluation of patients' fertility probability and risk, providing references for formulating reasonable assisted reproduction plans for older pregnant women.
Acknowledgment
This study was financially supported by Scientific Research Project of Health Commission of Hunan Province (No. B2019032).
Conflict of interest
There is no conflict of interest.
References
- 1.Abdallah A, Shawki H, Abdel-Rasheed M, et al. Role of 3-D Transvaginal Ultrasonography in Women Undergoing in Vitro Fertilization/Intra-cytoplasmic Sperm Injection. Ultrasound Med Biol. 2020;46:1424–1427. doi: 10.1016/j.ultrasmedbio.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Dai X, Wang Y, Yang H, et al. AMH has no role in predicting oocyte quality in women with advanced age undergoing IVF/ICSI cycles. Sci Rep. 2020;10:19750–19758. doi: 10.1038/s41598-020-76543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Wolff M, Schwartz AK, Bitterlich N, et al. Only women's age and the duration of infertility are the prognostic factors for the success rate of natural cycle IVF. Arch Gynecol Obstet. 2019;299:883–889. doi: 10.1007/s00404-018-5034-8. [DOI] [PubMed] [Google Scholar]
- 4.Attali E, Yogev Y. The impact of advanced maternal age on pregnancy outcome. Best Practice & Research. Clinical Obstetrics & Gynaecology. 2021 Jan 1;70:2–9. doi: 10.1016/j.bpobgyn.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Alshehre SM, Narice BF, Fenwick MA, et al. The impact of endometrioma on in vitro fertilisation/intra-cytoplasmic injection IVF/ICSI reproductive outcomes: a systematic review and meta-analysis. Arch Gynecol Obstet. 2020;303:3–16. doi: 10.1007/s00404-020-05796-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim EH, Lee J, Lee SA, Jung YW. Impact of Maternal Age on Singleton Pregnancy Outcomes in Primiparous Women in South Korea. Journal of Clinical Medicine. 2022 Feb 12;11(4):969. doi: 10.3390/jcm11040969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leijdekkers JA, van Tilborg TC, Torrance HL, et al. Do female age and body weight modify the effect of individualized FSH dosing in IVF/ICSI treatment? A secondary analysis of the OPTIMIST trial. Acta Obstet Gynecol Scand. 2019;98:1332–1340. doi: 10.1111/aogs.13664. [DOI] [PubMed] [Google Scholar]
- 8.Yin H, Jiang H, He R, et al. Cumulative live birth rate of advanced-age women more than 40 with or without poor ovarian response. Taiwan J Obstet Gynecol. 2019;58:201–205. doi: 10.1016/j.tjog.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Yang BY, Sun Y, Qian Z, Xaverius PK, Aaron HE, Zhao X, Zhang Z, Liu R, Dong GH, Yin C. Non-linear relationship of maternal age with risk of spontaneous abortion: a case-control study in the China Birth Cohort. Frontiers in Public Health. 2022. p. 2167. [DOI] [PMC free article] [PubMed]
- 10.Ministry of Health, People's Republic of China, author. Code of Human assisted Reproductive Technology. Chin J Reprod Health. 2004;15:446–451. [Google Scholar]
- 11.Lv H, Li X, Du J, et al. Effect of endometrial thickness and embryo quality on live-birth rate of fresh IVF/ICSI cycles: a retrospective cohort study. Reprod Biol Endocrinol. 2020;218:89–98. doi: 10.1186/s12958-020-00636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackens S, Santos-Ribeiro S, Racca A, et al. The proliferative phase endometrium in IVF/ICSI: an in-cycle molecular analysis predictive of the outcome following fresh embryo transfer. Hum Reprod. 2020;35:130–144. doi: 10.1093/humrep/dez218. [DOI] [PubMed] [Google Scholar]
- 13.Brandt JS, Cruz Ithier MA, Rosen T, et al. Advanced paternal age, infertility, and reproductive risks: A review of the literature. Prenat Diagn. 2019;39:81–87. doi: 10.1002/pd.5402. [DOI] [PubMed] [Google Scholar]
- 14.Mohammad EH, Abou El Serour AG, Mohamed EAH, et al. Efficacy of growth hormone supplementation with ultrashort GnRH antagonist in IVF/ICSI for poor responders; randomized controlled trial. Taiwan J Obstet Gynecol. 2021;60:51–55. doi: 10.1016/j.tjog.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 15.McPherson NO, Zander-Fox D, Vincent AD, et al. Combined advanced parental age has an additive negative effect on live birth rates—data from 4057 first IVF/ICSI cycles. J Assist Reprod Genet. 2018;35:279–287. doi: 10.1007/s10815-017-1054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao Q, Liang Y, Shao Y, et al. Advanced glycation end product concentrations in follicular fluid of women undergoing IVF/ICSI with a GnRH agonist protocol. Reprod Biomed Online. 2018;36:20–25. doi: 10.1016/j.rbmo.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Lu HQ, He YJ. Impact of female age and number of oocytes retrieved on clinical outcome of IVF/ICSI with long protocol of controlled ovarian hyperstimulation. J Reprod Med. 2019;28:1017–1026. [Google Scholar]
- 18.Patounakis G, Ozcan MC, Chason RJ, et al. Impact of a prior cesarean deliveryon embryo transfer: aprospecive study. Fertil Steril. 2016;106:311–316. doi: 10.1016/j.fertnstert.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C, Liu S, Liu G, et al. β-Edorphin predict pregnancy outcome of PCOS and DOR women after IVF-ET. Arch Gynecol Obstet. 2021;303:1207–1216. doi: 10.1007/s00404-020-05899-3. [DOI] [PubMed] [Google Scholar]
- 20.Palmsten K, Homer MV, Zhang Y, et al. In vitro fertilization, interpregnancy interval, and risk of adverse perinatal outcomes. Fertil Steril. 2018;109:840–848. doi: 10.1016/j.fertnstert.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Zheng JB, Wang DM, et al. Association between Vascular Endothelial Growth Factor and Clinical Outcomes of IVF-ET/ICSI. J Coll Physicians Surg Pak. 2019;29:19–23. doi: 10.29271/jcpsp.2019.01.19. [DOI] [PubMed] [Google Scholar]