Abstract
This is the first English translation of the work Periodic phenomena in the sleep in children, published in 1926 in the Journal Novoe v refleksologii i fiziologii nervnoi sistemy (Vol. 2, pp. 338–345) by Maria Denisova and Nicholai Figurin; it is the first study to report data on what is currently termed rapid eye movement (REM) sleep. The authors acquired continuous quantitative respiration data, as well as, eye and body movements during sleep in children for up to 6 hours, and discovered several novel features of sleep cycles in healthy infants from birth to about 1 year of age. First, the study reports cyclical periods of increased respiration and eye and body movements, with rapid ocular movements visible under relaxed eyelids (separation: 0.5–1 mm). These observations suggest atonia of REM sleep. Second, the length of the complete cycle (alternating active and quiet sleep phases or states) is about 50 minutes, an estimate that is consistent with later work. Third, the study identifies infant-specific ordering of sleep states, with the active phase beginning after sleep onset, followed by the quiescence phase. Importantly, these published data on sleep cycles precede all published studies related to the state now termed REM sleep by about 30 years (i.e. publishing in Science and in the Journal of Applied Physiology in the 1950s by Eugene Aserinski and Nathaniel Kleitman). In the historical commentary accompanying this translation, the findings of those later works are carefully compared to the original data on respiration and ocular and body motility cycles during sleep in infants, first reported and published by Denisova and Figurin (1926).
Keywords: sleep in infants, rapid eye movements, stages of sleep in infants, movements in infants
Graphical Abstract
Graphical Abstract.
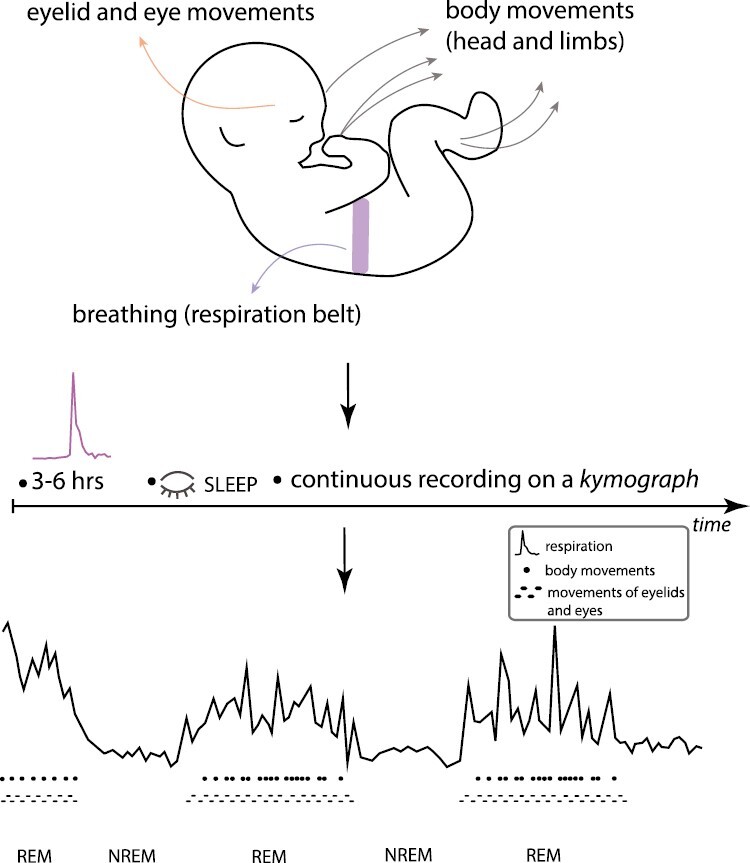
Statement of Significance.
Translator’s note on the historical significance of the original work: This is an English translation of the first report in the worldwide literature on respiration and motility cycles during sleep in infants in the 1920’s, as part of a series of studies on sensorimotor movements in infant sleep and child development [Denisova, M. P. and Figurin, N. L. (1926). Periodic phenomena in the sleep of children. Novoe v refleksologii i fiziologii nervnoi sistemy, 2, 338-345]. The author (KD) translated this text after extensive research into the history of infant sleep vis à vis sensorimotor brain development. The author discovered that this study investigates, using quantitative techniques, hypotheses related to infant sleep states. This study is the first to report data on what is currently termed active sleep or rapid eye movements (REM) sleep, thus preceding all published studies related to REM by other labs by about 30 years. Heretofore, these ideas were thought to be developed much later, in the 1950’s. Thus, the comprehensive commentary accompanying this translation considers several noteworthy comparisons between Denisova and Figurin’s (1926) study and later reports by Aserinsky and Kleitman (1955; 1953). In addition, biographical details of the original researchers and the Institute at which they worked are also presented for interested readers. Importantly, this significant study on infant sleep cycles has never been translated for Western audiences. Great care was applied to establish and map the meaning of original words used to terminology that would be readily recognizable by sleep researchers today, while also striving to retain the original nuances that conveyed the spirit and efforts of the authors, including unique turns of speech describing the research methods and procedures, approaches, and novel observations on infant sleep. For clarity, long sentences have been split into two separate sentences. There are several instances where Latin was used in the original text; these were retained. Occasions necessitating translator’s clarifications for terms or words used are indicated as such by square brackets.
Data on Respiration and Eye and Body Motility Cycles During Sleep in Infants in the Translated 1926 Study by Denisova and Figurin
As part of an extensive sensorimotor and cognitive brain research program on child development, Denisova and Figurin (1926) reported, initially at a conference in 1925 and then via a publication in 1926, groundbreaking data on respiration and motility cycles during sleep in infants [Denisova, M. P. and Figurin, N. L. (1926). Периодические явления во сне у детей (Periodic phenomena in the sleep of children). Novoe v refleksologii i fiziologii nervnoi sistemy (Beiträge zur reflexologie und physiologie des nervensystems; News in reflexology and physiology of the nervous system), 2, 338–345].
To put these 1926 data in context, the current paper is organized as follows. The methods and main findings of Denisova and Figurin are summarized, followed by a commentary comparing the work by Aserinsky and Kleitman (1955; 1953). Thereafter, historical context is provided, with biographical details of the researchers performing the 1926 study. The final section contains the English translation of the original study.
Denisova and Figurin’s study quantitatively investigated breathing patterns during sleep in relation to eye and body movements during sleep, in healthy, typically developing infants from birth to about 1 year of age, in several sets of experiments. Participants were male (M) and female (F) infants ages 1, 2, 4, 5, and 8 months and 1 year; longitudinal data from one child (Zoya; F) were also acquired (at 1 and 4 months). All measures were acquired from infants in the 24-hour boarding nursery affiliated with Infant Division of the Institute (i.e. the usual “home” environment of the children) and from several older children from private families.
Quantitative recordings of breathing/respiration were acquired using a pneumograph (a respiration belt), with data recorded by means of the kymograph, an instrument used for recording time series. Specifically, breathing data from the pneumograph were transmitted to the kymograph, and recorded as time series tracings on a slowly moving tape of the kymograph. Ocular and body movements were observed and recorded on kymograph’s tape simultaneously with the breathing trace (see summary Figure 1). Data were collected continuously, for as long as possible, from 3 to 6 hours mostly during nighttime (after the 10 pm feeding) or during daytime (after 11 am). Other events of interest (exogenous or endogenous) detailed below were recorded on the kymograph’s tape manually, as they occurred. For example, the authors examined stimulus-evoked responses in order to study the depth of sleep during distinct phases, using an acoustic stimulus consisting of 5 turns of a rattle-like noisemaker (which produced sound of constant intensity), as well as the role of turning over an infant while sleeping (change in position) on sleep cycles. Additional studies examined the impact of urination on sleep cycle patterns. In some infants, pulse measures were acquired through the fontanel. Data were also presented from older children and adults.
Figure 1.
Respiration and eye and body motility cycles during sleep in infants were first reported in 1926 by M. Denisova and N. Figurin. The top of the schematic overviews the methods and devices used in the study, while the bottom presents overall findings of distinct, alternating phases during sleep, currently termed active sleep (or rapid eye movement, REM) and quiet sleep (NREM). (This summary illustration is made by K. Denisova for this commentary.)
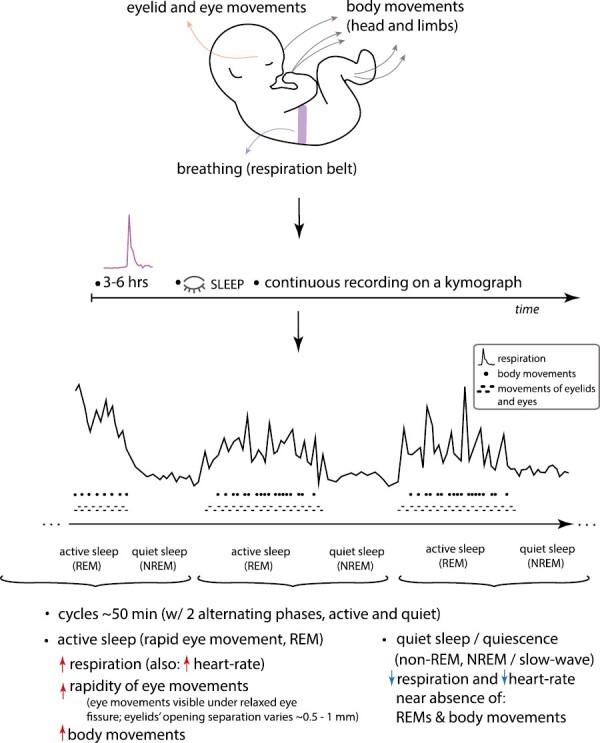
The main findings revealed for the first time the existence of periodic, cyclic fluctuations during infant sleep. The cycles (about 50 minutes in duration) consisted of periods of rapid breathing accompanied by heightened motility (increased rapidity of body, head, and eye movements), and of alternating periods of sparse, quiet breathing and few movements. The rapidity of ocular and body motility was found to be tightly associated with the sleep phase characterized by rapid breathing; fewer movements occurred in the sleep phase characterized by quiet breathing. Importantly, Denisova and Figurin reported that during the rapid breathing phase, eye movements were visible through relaxed opening between the eyelids (i.e. the palpebral fissure) with a variable gap between 0.5 and 1 mm, as well as beneath closed eyelids, moving from side-to-side and up-and-down. This set of facts suggests atonia and thereby represents the circumstances of the sleep state that is currently termed REM sleep. In contrast, during the quiet or sparse breathing phase, the eyes are immobile beneath tightly closed eyelids.
Importantly, the onset of sleep begins with the rapid breathing and ocular and body movement phase, followed by the quiet sleep phase. These periodic fluctuations were robust starting around 2 months after birth. Within the age range studied, the data indicate prolonged quiet sleep state with increasing age, and reduced periods of rapid sleep state with increasing age.
Moreover, in investigating the roles of different stimuli on sleep cycles, the authors found that the effect of an exogenous stimulus (a “noise-maker”) depends on the depth of sleep. While the reaction was limited during sparse breathing state, this stimulus elicited a strong reaction (e.g. waking up, crying) during the period of increased breathing rapidity, suggesting that the depth of sleep also has fluctuations. There was no effect of turning over the infant (i.e. changing the infant’s position) on the periodicity of sleep cycles of the child. The occurrence of urination did not alter sleep cycles. Furthermore, the authors reported that more rapid pulse was observed during the rapid breathing sleep state.
Denisova and Figurin used specific words including “rapid,” “frequent” and “quiet,” “sparse” to describe the alternating sleep phases shown by their 1926 data. The full translation of the study and Figures are included in the last section herein; several excerpts are below:
“These curves make very clear this phenomenon of periodic change. That is, more or less constant and infrequent breathing suddenly changes for rapid and very inconsistent.” (pp. 339-340 in the original).
“. . . there is a certain relationship between the changes of breathing during sleep and movements” (p. 339 in the original).
“. . . the periodic change in the rapidity of breathing is not an isolated phenomenon, rather it is accompanied by several others.” (p. 341 in the original).
“. . . it becomes clear that there is an important connection between these phenomena.” (p. 341 in the original).
“During the periods of increased rapidity of breathing . . . there is a concentration of movements . . . and definitely the movements of eyelids . . .” (p. 341 in the original).
“[eye] movements are apparent . . . in relaxed palpebral fissure [eye opening], so that between the eyelids sometimes appears variable gap, 0.5 to 1 mm” (p. 341 in the original).
“During periods of sparse [quiet] breathing, . . . the eyes are immobile under tightly closed eyelids” (p. 341 in the original).
The 1926 data on distinct sleep states, which are differentially quantified by increases (vs. decreases) in rapidity of respiration and eye and bodily motility, precede any other data known to relate these phenomena, in particular, data on the REM that appear during the rapid sleep phase, currently termed active sleep or REM sleep.
Key Comparisons of the Translated 1926 Study With the 1955 and 1953 Papers of Aserinsky and Kleitman
As there are several noteworthy comparisons between Denisova and Figurin’s (1926) study and later reports by Aserinsky and Kleitman (1955; 1953), these are presented in detail in the next few sections. Before proceeding, important methodological details of both studies are presented, as follows.
Methodological details: Aserinsky and Kleitman (1955; infant study)
Aserinsky and Kleitman (1955) studied sleep in infants from 1 to 7 months (from a total N = 14, including 4 infants with complete motility cycles). Existing behavioral data from several other infants were also analyzed. For the main study, measures were acquired in the home of the family. The study acquired quantitative recordings of body movements using a spring, with data recorded on tape (the type of apparatus used was not specified). To measure movement, a mechanical leverage device that inscribed a mark on a slowly moving tape was used. For a few observations, infants’ body motility was recorded electronically by a clock, which registered the duration of each movement in seconds. No additional events were recorded quantitatively. Eye movements were tracked by close viewing (direct observation). The authors also measured inter-feeding intervals, as they were interested to know if time between feedings is a function of the motility cycle.
Methodological details: Aserinsky and Kleitman (1953; adult study)
Aserinsky and Kleitman’s (1953) sleep study involved adult participants (N = 20). Eye movements were recorded by electrooculograms (EOGs), with the potentials collected via a Grass Electroencephalograph with EOG channels. For comparison to the EOG data, a monopolar frontal electrocortical recording was also acquired (EEG). For several participants, the presence of ocular movements was verified by direct observation, and under different illumination conditions. For two participants, videography was acquired under varying illumination conditions. Videography served to validate findings from other recordings, in particular, regarding the synchronicity of eye movements. A set of experiments focused on investigating whether eye motility was associated with dreaming; 10 participants were awakened during periods of eye activity and also after a period of quiescence. Eleven participants had undergone a continuous overnight recording (mean duration: 7 hours) in order to estimate the number of motility periods per night. Another set of experiments (N = 14) included calculations of respiratory rate (calculated for a minimum of ½ minute during eye motility periods, as well as 15 minutes before and after).
Comparisons to Aserinsky and Kleitman’s (1955) infant sleep study
Differentiation of sleep into two phases: active sleep and quiet sleep.
Denisova and Figurin (1926) reported data on two alternating phases during sleep in infants. One phase is characterized by the rapidity of breathing, rapidity of eye movements (including eye movements that were visible under relaxed eyelids with the eye-opening separated by 0.5 to 1 mm, suggesting atonia of REM sleep), and rapidity of body movements. The other phase is characterized by an almost complete absence of these phenomena. These observations parallel findings in Aserinsky and Kleitman (1955). Specifically, the close relationship between heightened motility (rapid ocular activity and body movement) during sleep was evident mainly in one of the phases; this phase alternated with the phase characterized by the relative absence of these movements (Aserinsky and Kleitman (1955)). Note the similarity between plotted curves showing alternating cycles of sleep phases observed by Denisova and Figurin (1926) (curves 2–5 shown in Figures 3 and 4) relative to the curves of sleep phases reported and shown in Figure 1 in Aserinsky and Kleitman (1955).
Figure 3.
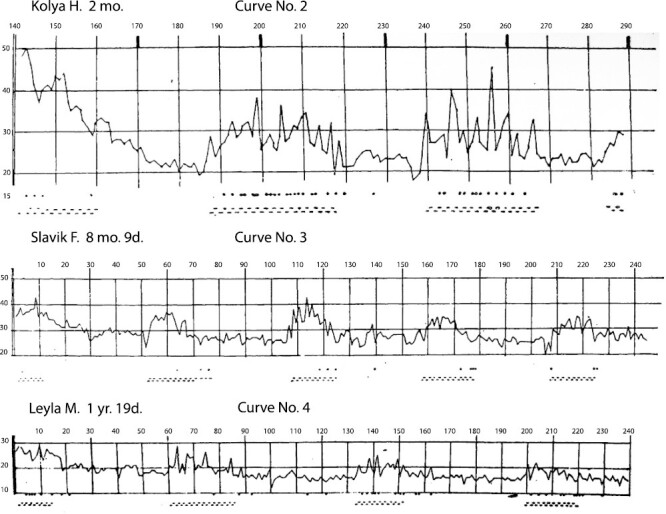
Curve No.2 (Name: Kolya H. [boy]; Age: 2 months). Curve No.3 (Name: Slavik F. [boy]; Age: 8 months 9 days). Curve No.4 (Name: Leyla M. [girl]; Age: 1 year 19 days). Figure is from [Denisova, M. P. and Figurin, N. L. (1926). Periodic phenomena in the sleep of children. Novoe v refleksologii i fiziologii nervnoi sistemy, 2, 338–345]. This material is provided solely for scientific and educational uses.
Figure 4.
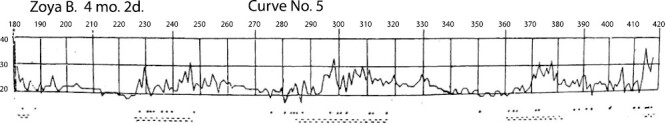
Curve No.5 (Name: Zoya B. (girl); Age: 4 months 2 days). Figure is from [Denisova, M. P. and Figurin, N. L. (1926). Periodic phenomena in the sleep of children. Novoe v refleksologii i fiziologii nervnoi sistemy, 2, 338–345]. This material is provided solely for scientific and educational uses.
Total cycle length: about 50 minutes (slightly less than 1 hour).
Denisova and Figurin (1926) observed a cycle length of about 50 minutes. Similarly, the mean length of the motility cycle measured by direct observation was reported at 54.2 minutes by Aserinsky and Kleitman (1955).
Unique ordering of sleep phases in infants: rapid eye movement phase is at the onset of infant sleep.
Denisova and Figurin (1926) reported that the phase characterized by rapid movements and rapid breathing begins first, followed by the quiescence phase. Similarly, examination of Figure 1 in Aserinsky and Kleitman (1955) indicates that the active sleep phase (heightened ocular activity and body movements) begins after initial sleep onset, thereafter alternating with the phase lacking ocular movements and characterized by reduced body movements. Thus, both infant studies report similar, infant-unique ordering of the two cycles after sleep onset (in contrast, in adults, the NREM phase begins soon after sleep onset).
Comparisons to Aserinsky and Kleitman (1953) adult sleep study
There are a few relevant comparisons between Denisova and Figurin’s (1926) infant study and Aserinsky and Kleitman’s (1953) study of adults.
Increased frequency of respiration in the active sleep phase.
Denisova and Figurin (1926) detected greater breathing rate (in addition to increased eye movements) during periods of increased rapidity of eye movements, that is, during active or rapid eye movement sleep phase. (In Denisova and Figurin’s study, respiration was measured quantitatively using a pneumograph.) These data are echoed in later findings by Aserinsky and Kleitman (1953) who observed higher respiration rate during eye motility, in contrast to respiration during quiescence, although the methodology for measuring respiration/breathing was not specified.
Pulse is more rapid during active sleep phase.
Denisova and Figurin (1926) measured pulse through fontanel in some infants. The authors reported higher pulse during periods of increased rapidity of eye movements during infant sleep. Similarly, Aserinsky and Kleitman (1953) mentioned some results of additional ongoing experiments that indicate that heart-rate is also “probably higher” in the presence of these eye movements.
Existence of multiple cycles/phases of high and low motility throughout the night.
Denisova and Figurin (1926) observed multiple alternating periods of high and low motility during sleep throughout the night. Aserinsky and Kleitman (1953) reported the existence of several periods of eye motility in adult partcipants during a continuous overnight recording (mean duration: 7 hours; duration in Denisova and Figurin’s study ranged between 3 and 6 hours). The periods of eye motility alternate with periods of quiescence (“often as many as 3 cycles, and sometimes a 4th one, during the course of the night”), consistent with the observations reported in 1926 by Denisova and Figurin during infant sleep.
Hunger a driver of high motility periods?.
Finally, Aserinsky and Kleitman (1953) noted their interest in the role of hunger related to high levels of central nervous system’s (CNS) irritability, as potentially driving periods of high motility during sleep, for future work. This factor (hunger) was already ruled out in the previous literature as a cause for movements associated with the active sleep phase, including literature published mainly in German on this topic and discussed in Denisova and Figurin (1926) (note: infants in Denisova and Figurin’s study (1926) were recorded after feeding).
Influence of 1926 data in next steps for sleep research
The significant contribution of the 1926 data are establishing the existence of two unique sleep states using quantitative methods, thus paving the way for future researchers to characterize each state (and detect additional states) with finer and increasingly more precise techniques including electrocortical recordings. Indeed, Denisova and Figurin’s (1926) study is cited as reference #1 in Aserinsky and Kleitman (1955; infant study), suggesting that Aserinsky and Kleitman were familiar with this early work. The original discoveries of infant sleep cycles by Denisova and Figurin (1926) including the active sleep state characterized by rapid breathing and ocular and body movements (currently termed REM sleep), the estimated duration of the sleep cycle (subsuming both active and quiet phases), and the unique ordering of the sleep states in infant sleep, provided the seminal basis for investigating sleep.
Biographical Details of the Authors of the Translated Infant Sleep Study and the Institute in Which They Worked
Maria Petrovna Denisova (b. 1898) and Nicholai Lvovich Figurin (b. 1896) were researchers and later collaborators at the Institute for Brain Research in Leningrad, Soviet Union. Dr. Maria Denisova graduated from the Women’s Medical Institute of Petrograd (Leningrad/St. Petersburg) in the early 1920’s and by around 1922 was conducting research on early child development in the Infant Division of the Institute for Brain Research. At the time, Nicholai Figurin was an assistant to Dr. Denisova; Dr. Figurin was a medical doctor who was also on staff at the Institute. Both Denisova and Figurin worked in the Infant Division, headed by Nicholai Matveevich Shchelovanov. All three were trainees of Vladimir Mikhailovich Behterev.
The Institute was founded by V.M. Behterev himself on May 17, 1918, and commenced work on October 1, 1918. The mission of the Institute was to advance research on brain and psychological function and included several research divisions, many of which were applied to help address specific challenges in society. For example, results of empirical studies on early child development helped inform early child education and interventions. By 1924, there were several divisions including the Division of Brain Morphology, Division of Reflexology, Infant (pedological) Division (where Denisova developed an infant sleep and child development research program), Labor (work) Physiology Division, and Scientific-Industrial Division. As an aside, it is not surprising that sleep was the focus of one of the Divisions of the new Brain Institute, since already by 19th century, sleep research had a prominent place in St. Petersburg, advanced by M.M. Manaseina, a female sleep scientist, and thereafter in the 20th century by Behterev, Pavlov, and later Bikov.
The Institute was well-funded and equipped with the latest technology. Discretionary funds supported staff travel, scientific visits to conferences, and active collaborations with scientists in other countries. Importantly, V.M. Behterev established a number of initiatives, including a regular meeting of the Scientific Society for Reflexology, Neurology, and Biophysics. More broadly, V.M. Behterev’s and his trainees’ research on human brain, psychological functioning, and the role of environmental factors on cognition (including his discovery of association reflex in human participants) can be contrasted to animal-based research by his main competitor, Nobel Laureate Ivan Petrovich Pavlov, who carried out reflexological experiments and surgeries in dogs and who discovered conditioned reflex.
On December 24, 1927, V.M. Behterev died at the first All-Union Conference of Neuropathologists and Psychiatrists in Moscow. At that time, he was the Head of the Psycho-neurological Academy, which subsumed his Institute for Brain Research. After his death, the Institute was renamed in 1929 in his honor as the V.M. Behterev Institute for Brain Research. The new Head of the Institute in 1929 became Victor Petrovich Osipov. In the 1930’s the Division where Denisova and Figurin worked continued to be headed by N.M. Shchelovanov, but was now called the Developmental Division of Genetic Reflexology and contained several laboratories; children from birth to 1 year were studied in the Clinic of Pedology and Neuropathology of Infancy. Furthermore, in 1931 the Division, Clinic, and its staff were transferred to Moscow and became part of the Institute for Scientific Research of Maternal and Infant Care. That Institute has foundational roots in the 18th century and is currently subsumed under the auspices of the Academy of Medical Sciences.
During the course of their career, Denisova and Figurin produced many research studies focusing on newborn and infant sleep, newborn, infant, and toddler reflexes, early cognition and perception (e.g. color and object perception), and child education, publishing in Journals edited by Behterev, Blonsky and Vygotsky. Notably, as part of their series of studies into sensorimotor movements in infant sleep and child development, they reported at a conference and then published in 1926 original quantitative data on respiration and eye and body motility cycles during sleep in infants, becoming the first report in the worldwide literature to produce and publish data on sleep states and in particular on what is now termed rapid eye movements (REM) sleep state. Their several monographs include a compendium of their research in early child development, published in 1949. Fundamentally, their body of research established the importance of sensitive periods in a child’s development and cognitive functioning, in particular, the role of sleep in development, starting from the first few months of life.
References
Archives of The National Library of Russia (1, Ostrovskogo Square, St. Petersburg)
Aserinsky, E. and Kleitman, N. (1953). Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science, 118(3062), 273–274. doi:10.1126/science.118.3062.273
Aserinsky, E. and Kleitman, N. (1955). A motility cycle in sleeping infants as manifested by ocular and gross bodily activity. J Appl Physiol, 8(1), 11–18. doi:10.1152/jappl.1955.8.1.11
Denisova, M. P. and Figurin, N. L. (1926). Периодические явления во сне у детей (Periodic phenomena in the sleep of children). Novoe v refleksologii i fiziologii nervnoi sistemy (Beiträge zur reflexologie und physiologie des nervensystems; News in reflexology and physiology of the nervous system), 2, 338–345.
Figurin, N. L. and Denisova, M. P. (1949). Этапы развития поведения детей в возрасте от рождения до одного года(Stages of behavioral development in children from birth to one year). Editors: N. M. Shchelovanov and N.M. Aksarina. Volume 3. State Publisher of Medical Literature (Medgiz), Moscow.
Kovalzon, B.M., Korostovzeva, L.C., Ruzkova, E.M., and Sviryaev, U.V. Somnology in Petersburg. Human Physiology, 2016, Volume 42, No 2, pp. 134–136. DOI: 10.7868/S0131164616010124
Shamrey V.K., Chudinovskih A.G., and Manizer N. M. “V.P. Osipov—leader of the V.M. Behterev’s Brain Institute.” Medical Psychology in Russia: electronic scientific Journal. 2016. No. 4(39) (electronic resource) URL: http://mprj.ru (date of retrieval: 10.10.2022).
Vygotsky, L.S. (2021). L.S. Vygotsky’s Pedological Works. Volume 2. The Problem of Age. Perspectives in Cultural-Historical Research 10. Translated by David Kellogg and Nikolai Veresov. Springer Verlag, Singapore.
English Translation of the Original Research Study Published in 1926 First Reporting Data on Respiration and Eye and Body Motility Cycles During Sleep in Infants by Maria P. Denisova and Nicholai L. Figurin [Denisova, M. P. and Figurin, N. L. (1926). Периодические явления во сне у детей (Periodic phenomena in the sleep of children). Novoe v refleksologii i fiziologii nervnoi sistemy (Beiträge zur reflexologie und physiologie des nervensystems; News in reflexology and physiology of the nervous system), 2, 338-345]
Journal: Novoe v refleksologii i fiziologii nervnoi sistemy (Beiträge zur reflexologie und physiologie des nervensystems; News in reflexology and physiology of the nervous system)
Volume: 2 (Dedicated to Academic V.M. Behterev)
Publisher: State Psycho-Neurological Academy and State Reflexological Institute for Brain Research
Place: Leningrad
Year: 1926
Pages: 338–345
Authors: by Drs. Maria P. Denisova and Nicholai L. Figurin
Affiliation: From The Infant Division at the Institute for Brain Research, Leningrad. Chair N. M. Shchelovanov
Title: Периодические явления во сне у детей (Periodic phenomena in sleep in children)1
1: Presented at the III National Pediatric Conference, June 1925, in Leningrad.
A child, especially a newborn, is strikingly different from an adult, and not only morphologically, but also with respect to functional particularities, for example, in the degree of working ability and coordination of the various systems of inner organs. The investigation of the many age-related particularities of a developing organism is one of the most important questions of genetic investigation in child research, with not only theoretical, but also practical significance.
One of these particularities we have encountered while investigating the nature of sleep in newborns.
Among other topics of related interest in our investigation, our research also involved the study of breathing during sleep in connection with the motility picture [motor activities] during sleep. Experiments were set up as follows. To register and record breathing [respiration], we used tracing of breathing made on a kymograph [a graphical device for capturing physiological processes as a time series] with an ink quill coupled with Lemanovsky pneumograph [a respiration belt]. To register movements during sleep we had to resort (due to the absence of more objective methods) to a parallel, continuous observational recording, on a special time-marked net, where the moment of movement onset and its content was recorded (i.e. what is moving, and how).
The pneumograph was tied around the stomach of the infant while awake, and the infant fell asleep with the respiration belt. The time of experimentation was largely nighttime, after 10 pm at night (after feeding), or daytime—after 11 am in the morning.
During nighttime experiments, there remained minimal ambient lighting. The child always was isolated, both from the other children and from the kymograph (the latter was placed in a different room); there was observed complete silence, and all potential distractions were minimized and removed. The recording lasted as long as possible, from 3 to 6 hours.
The children usually fell asleep at the regular bedtime (despite the presence of the respiration belt on the stomach) and the overall picture of sleep did not differ during these recordings, according to our data, relative to regular sleep as it occurs outside of experiments with respiration recordings.
Our participants were infants from the Infant Division between 0 and 1 years, healthy and normally developing, as well as, several slightly older children from private families.
Because of relative constancy in the children’s cohort housed at the Infant Division unit, we have an opportunity for longitudinal recordings. In a single child, we can repeat our measures a few days apart, as well as with a delay of 2–3 and more months, in order to record age-related changes.
When analyzing recorded data, we traced composite curves, where we juxtaposed the breathing picture (according to the pneumograph recording) with the motility picture of sleep (according to the observational recording). First of all, we calculated the number of breathing waves per minute according to the pneumograph curve, where a marker recorded data every ½ minute, and produced a curve of the frequency of breathing per minute for the duration of the entire sleep recording.
The movements of the newborn during sleep, contrary to widely held opinion, are quite numerous and variable. We distinguish between general movements—those encompassing many muscle groups (for example, stretching, etc.), and local—head turns, a bend of one’s hand. Besides these, we particularly emphasize the movements of the eyelids and eyeballs under the lids, which for the most part appear in isolation, but sometimes at the same time with other movements.
As it turns out the depth of breathing (according to the height of the wave peaks on the pneumograph curve) is not constant during sleep, and there are—on the juxtaposed curve—marks also of fluctuation of the depth of breathing. As such, we acquired complex diagrams, where were combined all data from the single experiment—on the breathing and on the movements.
Investigating these diagrams, we saw that, first, there is a certain relationship between the changes in breathing during sleep and movements, and second, the overall picture of sleep fluctuates depending on the age of the child.
According to our data, there are approximately 3 periods, during which the picture of sleep is distinct: first—from birth to 1–2 months of age; second—from 1 to 2 months to 2 years; and third—from 2 years of age.
Curve number No.1 is characteristic of the first period; it is based on one session with a girl 1 month 15 days (Figure 2).
Figure 2.
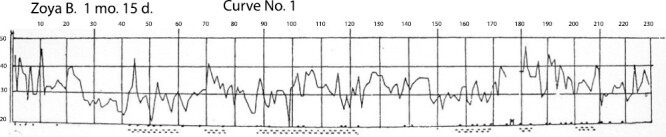
Curve No.1 (Name: Zoya B. [girl]; Age: 1 month 15 days). Explanation for curve No. 1: the values from 20 to 50 on the vertical axis indicate the number of breaths per minute. The values at the top indicate minutes from sleep onset. Points (.) = general movements. Dashes (::) = movements of eyelids and eyes. M = urination. Figure is from [Denisova, M. P. and Figurin, N. L. (1926). Periodic phenomena in the sleep of children. Novoe v refleksologii i fiziologii nervnoi sistemy, 2, 338–345]. This material is provided solely for scientific and educational uses.
Characteristic here are jerky fluctuations in the frequency [rapidity] of breathing from minute to minute (33-43-30) and the frequency and the abundance of general movements. There is a lack of consistency in the patterns of changes in rapidity of breathing (sometimes observed was a somewhat overall downfall of the rapidity of breathing towards the end of the sleep, which is absent in this curve). Regarding the motility picture, both the general movements and movements of eyelids and eyeballs are chaotically spread out and do not correspond to landmarks on the breathing curve. The depth of breaths rapidly fluctuates at this age, also without a marked connection with other phenomena in the newborn during sleep. (The curves presented do not illustrate the depth of breaths.)
Thus, this chaotic overall picture of sleep characterizes the first age period—before 1–2 months of age.
Thereafter the picture changes. The curves No.No. 2, 3, 4, and 5 indicate that after the initial downfall of the rapidity of breathing during 40–60 minutes after sleep onset, breathing begins periodic fluctuations between greater and lesser rapidity (Figure 3).
These curves make very clear this phenomenon of periodic change. That is, more or less constant and infrequent breathing suddenly changes for rapid and very inconsistent. What seems to occur is a return to the type of breathing observed at a much younger age with its sudden fluctuations in the rapidity of breathing, and then, in a little while, breathing returns to a previous phase. Specifically, once again it is becoming less frequent and more consistent, then again beginning to increase in rapidity, etc. until waking, that is, often on the order of 6–7 hours.
A change in these phases may be sometimes perfectly rhythmic, that is, the intervals of time from the beginning of one period of increasing rapidity until the next one are equal or almost equal.
More often these intervals fluctuate in duration around 50 minutes, but, similarly to the height and duration of the period of increased rapidity, vary depending on the individual as well as on age.
Curve No.2 (2-month infant) shows shorter periods of sparse and quiet breathing, relative to periods of increased rapidity of breathing. In an 8-month, 9-day infant (curve No.3) there is already predominance of sparse breathing, and in a girl aged 1 year 19 days (curve No. 4) the periods of increased rapidity of breathing are still clearly visible, but the height of them is already insignificant relative to the younger ages and the periods of sparse [quiet] breathing are significantly longer.
Curve No.5 shows results from the same child whose results are also presented in Curve No.1, only at later age, at 4 months and 2 days (Figure 4).
Here one observes already clearly demarcated periodicity in the rapidity of breathing, a pattern absent in the first curve (No.1). Moreover, this curve illustrates our proposal that periodic phenomena during sleep occur both at the beginning of sleep (other curves), as well as, at the end of sleep. Here the recording continued for 7 hours, with this curve representing the last hours of sleep and the wavelike character is expressed until waking.
However, the periodic change in the rapidity of breathing is not an isolated phenomenon, rather it is accompanied by several others.
At the same time with the increasing rapidity of breathing there commences as well the deepening of breathing. Curve No. 6 illustrates the moment of onset of deeper breathing, which coincides with the onset of rapidity of breathing (Figure 5).
Figure 5.

Curve No.6 (Name: Zhenya; Age: 4 months). Pneumographic curve at the onset of period with increased rapidity of breathing. Figure is from [Denisova, M. P. and Figurin, N. L. (1926). Periodic phenomena in the sleep of children. Novoe v refleksologii i fiziologii nervnoi sistemy, 2, 338–345]. This material is provided solely for scientific and educational uses.
Further, with decreasing rapidity of breathing, the depth of breathing also decreases.
Thereafter, combining represented graphically on our curves the rapidity of breathing over several hours of sleep with the motility picture during the same time, it becomes clear that there is an important connection between these phenomena. During the periods of increased rapidity of breathing, there is a concentration of movements, both general as well as local, and definitely the movements of eyelids. The latter (i.e. movements surrounding the eye apparatus), are always appearing during the phase of increasing rapidity of breathing, even when movements of other kinds are infrequent or absent. This pattern holds regardless of light levels: at dusk as well as during the day and in bright electric light. These movements are apparent first of all in relaxed palpebral fissure [eye opening], so that between the eyelids sometimes appears variable gap, 0.5 to 1 mm. Furthermore, sometimes the eyes open for a second, and there appear blinking movements. At the same time, the eyeballs underneath the eyelids could also move from side to side and down. During the periods of sparse breathing, a typical picture is that the eyes are immobile under tightly closed eyelids.
We tried to determine the status of pupils during different periods of sleep, but could not get clear results. The width of the pupil easily could change in a reflexive manner with forced opening of the eyelids. Also, it is very difficult to immediately detect the pupil when the eyeballs are lifted upwards.
The coincidence of a number of general movements with the phase of increased rapidity is clear from examining the curves.
It remains to be added, that this entire collection of phenomena related to the increased rapidity of breathing, more often begins precisely with the increases in rapidity and with increases in rapidity and movements of eyelids (in 60% of cases), and less often increases in rapidity precede or with its onset coincides another movement.
The status of pulse we needed to determine using direct listening and counting. During the periods of increased rapidity of breathing, we observed some increases in the rapidity of pulse (by 10-20 beats per minute). However, we could not examine this more precisely due to technical difficulties related to longer-term recording of pulsation of fontanel in an infant.
Using a certain noisemaker during different periods of sleep (during increased rapidity of breathing and during sparse breathing), we ascertained that the so-called depth of sleep in infants also has wave-like fluctuations. Thus, five turns of a noise-maker (which produces a sound of constant intensity) during sleep in a child during the period of increased rapidity of breathing provoke overall reaction, including full awakening with screams for 1–2 minutes. During sparse periods of breathing, reaction is limited to weak manifestations (does not lead to screams and turning to the sound). From chance observations, we also have data showing that, during the period of increased rapidity of breathing, the child can awaken from the slightest disturbance, while during the period of sparse breathing, it can be quite difficult to awaken the child even with strong and varied stimuli.
It is necessary also to say that the daytime sleep of the child at this age (until 2 years of age) has exactly the same character, as nighttime sleep, that is, we observe the same periodicity.
This type of wave-like flow of sleep with the periodic changes of various phases of breathing and in the motility picture, observed throughout the entire sleep, we found in all children without exception ages 1–2 months until 2 years of age. After 2 years, the child develops a more permanent rapidity of breathing, similar to adults. Curve No.7, based on the results from a 4-year-old child, does not contain periodicity neither in rapidity of breathing nor in appearance of movements; the movements of eyelids and eyeballs are almost nonexistent (Figure 6).
Figure 6.
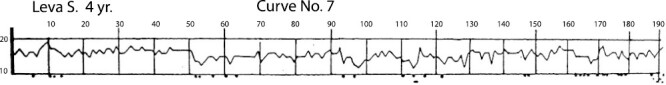
Curve No.7 (Name: Leva S. [boy]; Age: 4 years). Figure is from [Denisova, M. P. and Figurin, N. L. (1926). Periodic phenomena in the sleep of children. Novoe v refleksologii i fiziologii nervnoi sistemy, 2, 338-345]. This material is provided solely for scientific and educational uses.
However, lately, we have recorded curves indicating that children older than 2 years also have periodicity of the same type, only with weakly expressed increases in the rapidity of breathing and for a short time, with the period of sparse breathing lasting about 2 hours. In adults (four individuals tested) we did not observe uninterrupted sleep during testing greater than 3–4 hours, during which periodic phenomena in clear form were not seen.
Our data speak only about periodic phenomena during sleep; it cannot be excluded that there is a possibility that also during waking time there is a similar phenomenon, for instance in the sphere of breathing. But, it is difficult to make conclusive statements about this possibility, using our method. In children, because of the abundance of movements during wakefulness, which corrupts the breathing curve, while in adults, because of impossibility to remove cortical influences on the breathing center.
Thus, according to our data, although the picture of sleep during the child’s first period (before 1–2 months) is more or less chaotic and rich in movements, during the second period there is already periodic change in movements with increasing rapidity in breathing and full rest with slowed breathing, and only in the third period, after second year of life, sleep with predominance of rest is established.
The greatest interest in terms of analysis is the second period (from 2 months to 2 years) with the wave-like picture of sleep.
On its own, the combination of these phenomena, appearing periodically—that is, increasing rapidity of breathing, movements of eyelids and eyeballs, and general movements (stretching, turning, etc.)—is more or less clear. One needs to recall that the reticular formation (in which so-called breathing centers are located), is linked via the dorsal longitudinal fasciculus with the centers of eye-movement muscles, as well as linked with nucleus n. facialis.
Here, investigating the reasons for onset of the phase of increasing rapidity of breathing, etc. against the background of sparse breathing, we ruled out first of all the proposition about the influence of periodic filling of the bladder. That is because, while urination may be observed during sleep in children, and specifically only during the phase of increasing rapidity of sleep; however, as is evident from presented curves, urination often does not occur at all during sleep, despite well-established periodicity (Curves No.No.2, 3, and 4).
Furthermore, among the inner [endogenous] disturbances (since the outside [exogenous] disturbances were ruled out), we considered the fact that, long periods of lying in one pose may influence local blood circulation, etc., and evoke the so-called “reflex of position change,” that is, general and local movements and, by default, all other phenomena. To determine this, we carried out several tests. The results from one of them are indicated on Curves No.No.8 and 9, as follows (Figure 7).
Figure 7.
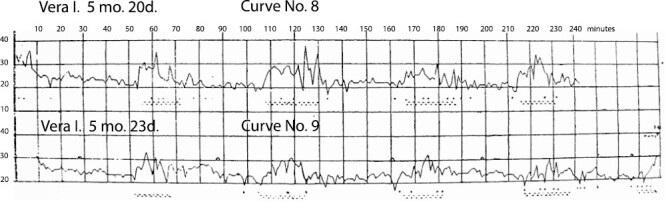
Curve No.8 (Name: Vera I. [girl]; Age: 5 months 20 days). Curve No.9 (shows data from the same child, a few days later) (Name: Vera I.; Age: 5 months 23 days). Explanation for curve No.9: symbol “^” marks the minutes (shown at ~28, 88, 160, and 198 minutes) when the child was turned over to the other side. Figure is from [Denisova, M. P. and Figurin, N. L. (1926). Periodic phenomena in the sleep of children. Novoe v refleksologii i fiziologii nervnoi sistemy, 2, 338–345]. This material is provided solely for scientific and educational uses.
Having determined the type of periodicity of sleep in a girl 5 months of age (this child turned out to have very proper rhythmicity, Curve No. 8), after 2 days we tested her again, but now we turned her over ourselves from one side to another during the sparse breathing phase. We reasoned that, if our proposed factor, that is, position, has any role in the changing of phases, then the phase of increasing rapidity of breathing, etc. must—after the change of position—be delayed for some amount relative to the control recording. However, this did not happen. The test curve with position change (No.9) can be “superimposed” on the control curve (No. 8). Conversely, these curves serve to illustrate the constancy of the individual periodicity of sleep in a child.
Periodic functioning of the entire digestive system according to Boldirev, observed in animals and humans, and present also in children (presented by Dr. Volovik at the third Pediatric Congress) does not coincide in terms of rhythm with our periodicity. There, it is on the order of 3–4 hours, while here, it is 50–60 minutes. Moreover, Boldirev’s periodicity appears during the state of hunger, whereas we, for the most part, worked with children who were just fed.
Despite this, we cannot completely rule out the link between our periodicity and Boldirev’s periodicity, because of (as of yet) incomplete factual knowledge about this matter. And if it turns out that this link exists, then this would constitute considerable interest. That is because in this case, Boldirev’s periodicity will be subsumed under a more general periodicity (in a vegetative sphere?) that in the area of digestive tract is obscured with filling up of the stomach, while in the rest of the domains (for instance, in breathing) appears invariably. Besides Boldirev’s periodicity, the literature contains suggestions for other phenomena in a human organism (and observed mainly during sleep), that also constitute a type of periodicity, although improper.
For instance, A. Peiper, who studied skin galvanic reflex in children, found that “in sleep . . . there could occur improper fluctuations of the innervation of sweat glands (undulations). These occur already in newborn children.” Thereafter he relates these undulations with waves of the third order (Wellen III Ordnung) in pletismogrammes, observed by S. Mayer, Mosso, K. Mays, Weber, and others. These fluctuations of the average line of pulse curve, independent of breathing and dreaming, may or may not occur in the same person on different days.
Besides this, Rählmann and Witkowski described occasionally occurring during sleep slow uncoordinated eye movements. A. Peiper summarizes all of this and says: “Waves of the 3rd order, uncoordinated eye movements and undulations—very similar phenomena. Perhaps, during sleep activity of the higher centers is slowed down, and due to this lower centers are working improperly and independently from each other.”
For now, the periodicity of these phenomena is completely unclear, the degree to which it is proper, whether there is any association among the separate phenomena and so forth. For these reasons, we cannot say, what is the relationship between these undulations, etc. vis à vis the periodic phenomena during sleep in children described by us. In any case, we did not find in the literature guidance as to these facts presented by us.
There are many research studies with children of a breast-feeding age, but most of the researchers examined only the depth of sleep under various conditions. With this, according to data from Czerny, the curve of the depth of sleep in an infant in general is not different from the curve of an adult (i.e. this curve goes up during the first hour up to a maximum, then slowly decreases, in the morning again there is a slight increase and then rapid decline before waking).
Karger, investigating movement picture of sleep with the aid of a special instrument Nägeli, worked mostly with older children who were ill, and did not observe periodicity in the occurrence of movements.
Cramaussel, having long ago recorded breathing in a child of breast-feeding age, also does not mention anything about periodicity, and he did not provide curves on rapidity of breathing. From his data, it can be stated only that, similar to our data, in a child up to 1 and ½ months of age breathing is very uneven in depth and disordered.
G.E. Shumkov has the following statement: “The breathing curve is a sensitive reagent not only to outside disturbances, but also to sleep, the state or depth of which is reflected on the form of the curve and the sensitivity of it to outside disturbances.”
We think that described by us the occurrence of periodicity of sleep in children may be explained only along with explanations regarding other types of periodicity in the developing organism.
At the third congress of pediatric physicians in Leningrad, Professor Speransky presented a fact that he found regarding periodic and lawful fluctuations in the number of leukocytes in the blood of children of a breast-feeding age. This periodicity was especially pronounced also during sleep. Professor Speransky believes that this phenomenon is “related to the state of the vegetative nervous system” (theses).
In our case, considering the periodicity in the rapidity of breathing and other phenomena during sleep, it may be possible for the time being, hypothetically, to conceive of the situation as follows. Specifically, this is an age-related peculiarity related to insufficient coordination of the work of sympathetic and parasympathetic systems. In particular, it is possible, that during the first- and second- months of life, there is a prevalence of the sympathetic system (which is phylogenically older), and thereafter comes time, after 2 months of age, when the parasympathetic and sympathetic systems take turns with regard to superiority of one over another, and from 2 years of age, they become mutually balanced.
Whether this is so or not so will be shown in the future.
References
1) Boldirev. Dissertation. St. Petersburg.
2) E. Cramaussel. Sommeil d’un petit enfant. Arch. de Psychol. 1911-v. 10-11, and 1912-v.12
3) Czerny. Jahrbuch f. Kinderheilk. 1892. Bd. 33 and 1896, Bd. 41.
4) P. Karger. Ueber den Schlaf des Kindes. Berlin 1925.
5) K. Mays. Ueber die Bewegungen des menschlichen Gehirns. Virchow’s Archiv 1882. 88.
6) Mosso. Ueber den Kreislauf des Blutes im menschlich. Gehirn. Leipzig. 1881.
7) A. Peiper. Untersuchungen ueber den galvanisch. Hautreflex in Kindesalter. Jahrb. f. Kinderh. 1924. Bd. 107.
8) Rählmann und Witkowski. Arch. für Anatomie und Physiologie. 1877 and 1878.
9) E. Weber. Einfluss psychischer Zustände auf den Körper. Berlin. 1910.
10) G.E. Shumkov. Psychology News. 1908. Vol. 1 and 2.
Funding
The Denisova lab gratefully acknowledges support from the Simons Foundation Autism Research Initiative (SFARI) under Award Number 614242 (PI: Kristina Denisova), National Institute of Mental Health of the National Institutes of Health under Award Number R01MH121605 (PI: Kristina Denisova), and faculty start-up funds from the City University of New York, Queens College. The content is solely the responsibility of the translating author (KD) and does not necessarily represent the official views of the National Institutes of Health. The sponsors had no role in study design, in the collection, analysis, and interpretation of data, in the writing of this report, and in the decision to submit the manuscript for publication. The author declares no conflicts of interest, either financial or nonfinancial, related to this work.
Disclosure Statement
Financial Disclosure Statement: The translator and author of the commentary (KD) declares no conflicts of interest for this work of translation. The Denisova lab gratefully acknowledges support from the Simons Foundation Autism Research Initiative (SFARI) under Award Number 62646 (PI: Kristina Denisova) and National Institute of Mental Health of the National Institutes of Health under Award Number R01MH121605 (PI: Kristina Denisova), and faculty start-up funds from the City University of New York, Queens College. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. The sponsors had no role in study design, in the collection, analysis, and interpretation of data, in the writing of this report, and in the decision to submit the manuscript for publication.
Nonfinancial Disclosure Statement: The translator and author of the commentary (KD) declares no conflicts of interest for this work of translation. This work was carried out in the absence of any relationships which could be perceived as a potential conflict of interest. The author knows of no familial relationship.


