Abstract
Bovine trypanosomosis, caused by Trypanosoma vivax, is a disease that originated in Africa and currently affects cattle in several South American countries, including almost all Brazilian states. Despite the reports on T. vivax infection in southern Brazil, data on its circulation status is currently unavailable. In this study, we aimed to detect anti-Trypanosoma spp. IgG antibodies in cattle from Rio Grande do Sul and suggest areas with T. vivax transmission risk. A total of 691 serum samples from cattle in the intermediate regions of Rio Grande do Sul were analyzed using indirect immunofluorescence assay (IFA). The overall seroprevalence of anti-Trypanosoma antibodies in cattle was 24.6% (170/691). The detection rate ranged from 0-37.3%, with a high prevalence in the intermediate regions of Ijuí (37.3%), Uruguaiana (30.7%), and Passo Fundo (28.9%). Thus, these regions were suggested as possible bovine trypanosomosis risk areas due to the high seroprevalence. This is the first serological study to determine Trypanosoma spp. infection status in cattle from Rio Grande do Sul, providing data on the epidemiology of trypanosomosis in the state.
Keywords: Trypanosoma vivax, serological diagnosis, epidemiology
Resumo
A tripanossomose bovina, causada por Trypanosoma vivax, é uma doença que teve origem na África, e atualmente, afeta bovinos em diversos países da América do Sul, incluindo quase todos os estados brasileiros. Apesar dos relatos de infecção por T. vivax, no sul do Brasil, dados sobre seu status de circulação não estão disponíveis atualmente. Neste estudo, o objetivo foi detectar anticorpos IgG anti-Trypanosoma spp. em bovinos do Rio Grande do Sul e sugerir possíveis áreas de risco de transmissão de T. vivax. Foi analisado um total de 691 amostras de soro de bovinos, das regiões intermediárias do Rio Grande do Sul, usando-se o ensaio de imunofluorescência indireta (IFA). A soroprevalência geral de anticorpos anti-Trypanosoma em bovinos foi de 24,6% (170/691). A taxa de detecção variou de 0 a 37,3%, com uma alta prevalência nas regiões intermediárias de Ijuí (37,3%), Uruguaiana (30,7%) e Passo Fundo (28,9%). Portanto, essas regiões foram sugeridas como possíveis áreas de risco para a tripanossomose bovina, devido à alta soroprevalência. Este é o primeiro estudo sorológico a determinar o status de infecção por Trypanosoma spp., em bovinos do Rio Grande do Sul, fornecendo dados sobre a epidemiologia da tripanossomose no estado.
Palavras-chave: Trypanosoma vivax, diagnóstico sorológico, epidemiologia
Introduction
Trypanosomes are flagellate protozoans belonging to the family Trypanosomatidae and genus Trypanosoma. Various parasitical species belonging to Trypanosoma, such as Trypanosoma brucei, Trypanosoma congolense, Trypanosoma evansi, and Trypanosoma vivax, cause trypanosomosis, a livestock disease prevalent in Africa, Latin America, and Asia (Stevens & Brisse, 2004). In South America, T. vivax is the most important causative agent of trypanosomosis in cattle, causing losses in the cattle industry. Other species, such as non-pathogenic T. theileri and T. evansi, also infect cattle but rarely cause diseases (Radostitis et al., 2007). Economic losses in Brazilian cattle-rearing are associated with animal mortality following outbreaks, and indirect interferences of subclinical and undiagnosed infections that reduce weight gain and milk production as well as cause abortion, infertility, and other reproductive disorders, thus implicating livestock productivity (Otte et al., 1994; Seidl et al., 1999; Jones & Dávila, 2001).
T. vivax was introduced in Latin America through infected cattle imported from Africa and has spread across several countries (Jones & Dávila, 2001). T. vivax-mediated bovine trypanosomosis was first reported in the Amazon region of Brazil: in buffalos from Pará (Shaw & Lainson, 1972) and cattle from Amapá (Serra-Freire, 1981). Subsequently, infections in cattle herds occurred with increasing frequency, with reported T. vivax infection in other states in the North (Linhares et al., 2006), Northeast (Batista et al., 2007; Guerra et al., 2013; Lopes et al., 2018), Midwest (Silva et al., 1996; Osório et al., 2008), Southeast (Carvalho et al., 2012; Cadioli et al., 2012), and South Brazil (Silva et al., 2009). Currently, T. vivax is considered endemic in some Pantanal and Amazon rainforest regions.
African dissemination of T. vivax includes cyclical transmission by Glossina spp., with T. vivax developing in their digestive tract. Since Glossina spp. is absent in South America, the major transmission methods include non-cyclical or mechanical transmission by blood-sucking flies (Otte & Abuabara, 1991), such as tabanids (horseflies) and Stomoxys calcitrans (stable flies). Although not confirmed, cyclical transmission may involve one or more vector species (Otte & Abuabara, 1991). Furthermore, iatrogenic transmission through fomites and transplacental infections have also been described (Cadioli et al., 2012). T. vivax infects various domestic and wild species, including sheep, goats, horses, and cervids, which can act as important reservoirs (Dávila et al., 2003).
In South America, T. vivax infection manifests variable virulence and pathogenicity levels (Gardiner & Mahmoud, 1992), causing non-specific clinical signs, such as severe anemia, weight loss, edema, immunosuppression, and reproductive failure. Some acute cases can develop various neurological disorders that eventually cause the death of the affected animals (Gonzatti et al., 2014). Asymptomatic and chronic infections are common in cattle from endemic regions and can be reactivated by nutritional and physical stress, concomitant disease, pregnancy, and lactation (Garcia et al., 2016). No single clinical sign is pathognomonic and the disease may simulate many other infections (Büscher, 2014); therefore, it is easily overlooked or confused with other diseases.
In southern Brazil, despite reports of T. vivax infection in cattle (Silva et al., 2009) and naturally infected horses (Silva et al., 2011), no data on pathogen circulation status in Rio Grande do Sul (RS) have been recorded. RS is geographically located between territories that have characteristics that may favor the spread of the disease. The region of Argentina, which borders Brazil, has numerous cattle herds and the culture of practicing rodeos, in addition, there are several reports of animal trafficking to Brazil. This can provide a risk factor for the introduction of diseases to brazilian heards. Thus, understanding the actual T. vivax circulation in RS is important. This study aimed to detect anti-Trypanosoma spp. antibodies in cattle in the regions of RS and elucidate the trypanosomosis transmission risk areas in the region under study.
Materials and Methods
A total of 691 serum samples were obtained from the sera bank of Laboratório de Doenças Parasitárias of Universidade Federal de Santa Maria (LADOPAR/UFSM) from 2020-2022. Samples were from taurine (Bos taurus), zebu (Bos indicus), and crossbreed cattle in the intermediate regions of RS: Uruguaiana, Santa Maria, Porto Alegre, Passo Fundo, Ijuí, Caxias do Sul, Santa Cruz do Sul, and Pelotas (IBGE, 2017). The total number of samples was determined based on statistical analysis using the Epi Info (v7.2.5) system according to the cattle population of RS (IBGE, 2022). The confidence interval was 95%, with 3% standard error.
Indirect immunofluorescence assay (IFA) described by Camargo (1966) was used to detect IgG antibodies against Trypanosoma spp. with some modifications. Briefly, IFA was performed using a microscopic strain infected with T. vivax trypomastigotes. The serum samples were diluted in phosphate buffered saline (pH 7.2) to 1:80 (Cuglovici et al., 2010). Commercial fluorescein-labeled anti-bovine IgG© (Rabbit Anti-Bovine IgG FITC®, F7884, Sigma Bio Sciences, St. Louis, Missouri, USA) secondary antibody was diluted to 1:400. The samples were incubated with both the primary and secondary antibodies for 50 min at 37 °C in a dark and humid chamber. Confirmed positive and negative serum samples diluted to 1:80 were used as controls. The slides were observed at 400× magnification under a fluorescence microscope (Optiphase INV403F). The samples were considered reactive when the trypomastigotes revealed total fluorescence, showing binding between the FITC-labeled secondary antibody and the antigen–antibody complex. Serum that did not show fluorescence was considered non-reactive.
We conducted an exploratory data analysis of the samples. The investigation involved calculating the absolute frequencies and prevalence of cattle trypanosomosis. Additionally, contingency tables were constructed, considering each herd's intermediate region and the cattle breeding. To assess the presence of significant differences in the prevalence of these variables, we performed the Fisher’s exact test, considering 5% as the significance level.
Results
Anti-Trypanosoma spp. IgG was detected in 24.6% (170/691) cattle serum samples. The detection rate in the study region was 0-63.6% (Table 1). A total of 24 herds were sampled, of which 9 and 5 were dairy cattle and beef cattle, respectively, and 10 herds did not report this information. At least one positive sample was detected in 21 herds (89.6%). The detection frequency in the positive herds ranged from 4.2-63.6%. The highest detection frequencies were observed in three dairy herds from Passo Fundo (63.6%, 7/11; 46.1%, 6/13; and 45.4%, 10/22), one herd from Porto Alegre (39.1%, 9/23), and one herd from Pelotas (39.3%, 22/56) (Table 1).
Table 1. Absolute frequencies and prevalences by herd for the serological detection of Trypanosoma spp. in cattle serum samples collected from the Rio Grande do Sul intermediate regions.
| Herd Identifier | Trypanosomosis | Total | Prevalence (%) | Intermediate Region | Cattle breeding | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| 1 | 0 | 19 | 19 | 0.0 | Caxias do Sul | Not informed |
| 2 | 0 | 7 | 7 | 0.0 | Ijuí | Not informed |
| 3 | 0 | 7 | 7 | 0.0 | Ijuí | Not informed |
| 4 | 31 | 38 | 69 | 44.9 | Ijuí | Dairy Cattle |
| 5 | 4 | 35 | 39 | 10.3 | Passo Fundo | Dairy Cattle |
| 6 | 6 | 7 | 13 | 46.1 | Passo Fundo | Dairy Cattle |
| 7 | 7 | 4 | 11 | 63.6 | Passo Fundo | Dairy Cattle |
| 8 | 4 | 9 | 13 | 30.8 | Passo Fundo | Dairy Cattle |
| 9 | 10 | 12 | 22 | 45.5 | Passo Fundo | Dairy Cattle |
| 10 | 9 | 31 | 40 | 22.5 | Passo Fundo | Dairy Cattle |
| 11 | 22 | 34 | 56 | 39.3 | Pelotas | Not informed |
| 12 | 1 | 23 | 24 | 4.2 | Pelotas | Not informed |
| 13 | 3 | 24 | 27 | 11.1 | Pelotas | Not informed |
| 14 | 9 | 14 | 23 | 39.1 | Porto Alegre | Not informed |
| 15 | 5 | 52 | 57 | 8.8 | Porto Alegre | Not informed |
| 16 | 5 | 14 | 19 | 26.3 | Santa Cruz do Sul | Dairy Cattle |
| 17 | 2 | 16 | 18 | 11.1 | Santa Cruz do Sul | Not informed |
| 18 | 2 | 6 | 8 | 25.0 | Santa Cruz do Sul | Not informed |
| 19 | 4 | 65 | 69 | 5.8 | Santa Maria | Dairy Cattle |
| 20 | 3 | 9 | 12 | 25.0 | Uruguaiana | Beef Cattle |
| 21 | 3 | 5 | 8 | 37.5 | Uruguaiana | Beef Cattle |
| 22 | 3 | 16 | 19 | 15.8 | Uruguaiana | Beef Cattle |
| 23 | 1 | 8 | 9 | 11.1 | Uruguaiana | Beef Cattle |
| 24 | 36 | 66 | 102 | 35.3 | Uruguaiana | Beef Cattle |
| Total | 170 | 521 | 691 | 24.6 | ||
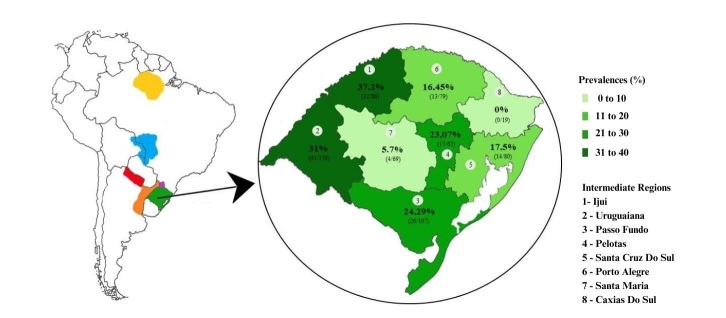
Regarding the intermediate regions, the higher prevalences were found in Ijuí (37.3%), Uruguaiana (30.7%), and Passo Fundo (28.9%), respectively (Table 2 and Figure 1). The lowest prevalence was observed in Pelotas (24.3%), Santa Cruz do Sul (23.0%), Porto Alegre (17.5%), Santa Maria (5.7%), and Caxias do Sul (0%) (Table 2 and Figure 1). When calculating prevalence ratio using Santa Maria as reference, we verify that the prevalence is 6.4 times higher in Ijuí than in herds from this region. In addition, besides Caxias do Sul, which did not present any prevalent case, all the regions showed a prevalence more than three times higher than Santa Maria. The Fisher's exact test corroborates this result since it returns a p-value smaller than 0.001, leading to the rejection of the hypothesis that the prevalences are equal in all intermediate regions at the significance level of 5%. The Cattle breeding comparison also leads to significant differences (p-value=0.006 in the Fisher's exact test). It means that bovine trypanosomiasis differs within the categories of cattle breeding. The prevalence ratio shows that bovine trypanosomiasis in beef cattle is 1.7 times higher than in cattle with unspecified breeding. For dairy cattle, the prevalence ratio indicates that its prevalence is 1.5 times higher than in cattle with unspecified breeding.
Table 2. Contingency tables and prevalences by intermediate region and cattle breeding.
| Variable | Trypanosomosis | Total | Prevalence (%) | Prevalence ratio | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Intermediate Region | |||||
| Caxias Do Sul | 0 | 19 | 19 | 0.0 | 0.0 |
| Ijuí | 31 | 52 | 83 | 37.3 | 6.4 |
| Passo Fundo | 40 | 98 | 138 | 29.0 | 5.0 |
| Pelotas | 26 | 81 | 107 | 24.3 | 4.2 |
| Porto Alegre | 14 | 66 | 80 | 17.5 | 3.0 |
| Santa Cruz Do Sul | 9 | 36 | 45 | 20.0 | 3.4 |
| Santa Maria | 4 | 65 | 69 | 5.8 | 1.0 |
| Uruguaiana | 46 | 104 | 150 | 30.7 | 5.3 |
| Cattle breeding | |||||
| Beef Cattle | 46 | 104 | 150 | 30.7 | 1.7 |
| Dairy Cattle | 80 | 215 | 295 | 27.1 | 1.2 |
| Not informed | 44 | 202 | 246 | 17.9 | 1.0 |
Figure 1. Map of Rio Grande do Sul (right) showing Trypanosoma spp. seroprevalence in cattle according to the intermediate regions. (Left) Map showing T. vivax occurrence in South America: upper Rio Paraguay basin, including portions of the Brazilian, Bolivian, and Paraguayan Pantanal regions (blue); Alto Paraná, Amambay, Canindeyú, and Concepción regions of Paraguay (pink); western Santa Catarina (purple); Formosa region of Argentina (red); Mesopotamia Argentina (orange); and Rio Grande do Sul (green).
Discussion
Trypanosomosis, caused by T. vivax, is considered a non-endemic disease in cattle in southern Brazil. Currently, this species is only considered endemic in some Pantanal and Amazon rainforest regions. However, it sporadically infects various species throughout southern Brazil regions. T. vivax infects cattle and horses with clinical signs in the central cities of RS (Silva et al., 2009, 2011). Nevertheless, since serological studies have not been performed using serum samples collected in RS, no data on cattle trypanosomosis in RS is available. This is the first bovine serological study on anti-Trypanosoma spp. antibody detection and frequency among all regions of RS, highlighting the importance of widespread disease-causing pathogen analysis for the differential diagnosis of agents that cause hemolytic, neurological, or reproductive disorders.
Though RS is not a T. vivax endemic region, the presence of anti-Trypanosoma spp. antibodies over almost all intermediate regions (7/8) indicated that trypanosomes have spread throughout the cattle from RS and may be associated with symptomatic or asymptomatic infections in beef and dairy herds. According to these findings, there is a risk of trypanosomosis outbreaks in the state, mainly in the cities of intermediate regions with high seroprevalence detection. The presence of vectors and favorable conditions for their development (mainly S. calcitrans), food restriction, inadequate transport conditions, and thermal stress in dairy cattle herds is associated with the needle-sharing practice during oxytocin application in lactating cows; these factors, among other, favor disease epidemiology in cattle herds in Brazil (Cadioli et al., 2012).
The detection rate in RS (24.6%) is lower than that in endemic areas such as the Pantanal and Amazon rainforests (93.1-98.5%; Guedes et al., 2008; De Mello et al., 2019), but higher than that in enzootic unstable areas for T. vivax (11.90-15.99%; Guerra et al., 2013). However, the seroprevalence results can vary according to the study region, herd, seasonality, and cattle breeding management type.
The difference in the prevalence of antibodies against anti-Trypanosoma spp. in different regions in RS support defining areas for trypanosomosis transmission risk. The western intermediate regions of Uruguaiana and Ijuí had the highest serological prevalence, with over 30% detection frequency.
Several circumstances contribute to trypanosome dissemination over a region. The western RS is characterized by a high cattle population with proprieties that have animal trafficking characteristics. The national and international live livestock trade represents a serious risk factor; moreover, the introduction of animals infected with subclinical or chronic trypanosomosis is the main risk factor for T. vivax dissemination from endemic to non-endemic regions. Further studies on naturally infected herds have demonstrated that T. vivax induces chronic asymptomatic infection in cattle from endemic areas (Ventura et al., 2001; Dávila et al., 2003). Another concerning observation in animal trade is the occurrence of horses as T. vivax reservoirs, as observed in Paraguayan regions that border southwest Brazil and Argentina (Suganuma et al., 2022). In both animal species, obligatory T. vivax-mediated trypanosomosis diagnosis is not required for international or national trade.
Territorial proximity facilitates pathogen dispersion from different locations, which is an epidemiologically important factor because western RS borders Santa Catarina state and Argentina, where T. vivax was detected in cattle (Paoletta et al., 2018; Silva et al., 2022). Some serological studies using symptomatic cattle from the western region of Santa Catarina detected 39.0% (57/146) seropositive animals, suggesting T. vivax existence in these areas. In the Argentine territory, T. vivax outbreaks have been described in cattle from Formosa and Mesopotamia Argentina. The agro-ecological conditions of these neighboring regions are similar to those of western RS, with potential risk of dispersion among the Pampa biome regions that border Brazil (Abdala et al., 2021; Monzon et al., 2013; Paoletta et al., 2018). Moreover, the main intermediate regions (Ijuí, Uruguaiana, and Passo Fundo) where anti-Trypanosoma spp. antibodies were detected in this study, border these locations in Santa Catarina and Argentina. Thus, it is important to suggest the intermediate regions of Ijuí, Uruguaiana, and Passo Fundo as risk areas for trypanosomosis in cattle from RS.
Immunological cross-reactivity between trypanosome species may occur, mainly between T. vivax and T. evansi, which share many common antigens (Uzcanga et al., 2004). Their coexistence in southern Brazil makes precise interpretation of serological tests difficult, necessitating the use of molecular tools to determine the species involved. Furthermore, the new species discovered in Argentina may also be present in Brazilian herds and must be considered without knowing its influence on immunological tests. Therefore, antibodies should be associated with specific species with caution; the antibodies found in this study are not considered T. vivax-specific.
Despite the high detection rate in cattle from the same herd, anti-Trypanosoma spp. IgG antibodies were observed in many dairy herds from Passo Fundo, Porto Alegre, and Pelotas. Rainy periods and/or high humidity contribute to developing mechanical vectors in the environment, as well as improving the possibility of increasing the infection rate of cattle from the same herd. This phenomenon was also observed by Cuglovici et al. (2010), where seroprevalence ratios in dairy cattle herds from Igarapé and Minas Gerais were 7.4-48.0%, with the highest incidence was correlated with an increased S. calcitrans population during rainy seasons. Thereby, S. calcitrans can corroborate trypanosomosis epidemiology in RS, as it is a widely distributed ectoparasite in RS.
In summary, detecting antibodies against Trypanosoma spp. in cattle from RS provides evidence that these herds have been exposed to these pathogens. Further studies on trypanosomosis epidemiology should attempt to conclusively link the pathogens to animal production losses. Owing to the possibility of cross-reactions with other trypanosomes in serological methods, combined parasitological and molecular methods would provide a clear understanding of the specific species infecting the seropositive animals and the epizootiological situation of the trypanosomes present in cattle herds from RS.
Conclusion
This is the first serological study of trypanosomosis in cattle from RS. This study detected antibodies against Trypanosoma spp. in cattle from almost all intermediate regions, thus suggesting a possible risk of trypanosomosis outbreaks, mainly in animals from the western RS. The intermediate regions from Ijuí, Uruguaiana, and Passo Fundo were suggested as risk areas for bovine trypanosomosis due to high seroprevalence. These findings highlight the need for an effective surveillance program to diagnose and prevent trypanosomosis spread, thus reducing disease impact on livestock productivity, which may otherwise be overlooked and underdiagnosed in RS.
Acknowledgements
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Personnel (CAPES) - Financial code 001 and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the financial support.
Footnotes
How to cite: Samoel GVA, Fernandes FD, Roman IJ, Rodrigues BT, Miletti LC, Bräunig P, et al. Detection of anti-Trypanosoma spp. antibodies in cattle from southern Brazil. Braz J Vet Parasitol 2024; 33(1): e013723. https://doi.org/10.1590/S1984-29612024002
Ethics declaration: Not applicable
References
- Abdala AA, Larriestra AJ, Signorini M. Estimación de pérdidas económicas causadas por Trypanosoma vivax en un rodeo lechero de Argentina. Rev Vet. 2021;31(2):115–119. doi: 10.30972/vet.3124728. [DOI] [Google Scholar]
- Batista JS, Riet-Correa F, Teixeira MMG, Madruga CR, Simões SDV, Maia TF. Trypanosomiasis by Trypanosoma vivax in cattle in the Brazilian semiarid: description of an outbreak and lesions in the nervous system. Vet Parasitol. 2007;143(2):174–181. doi: 10.1016/j.vetpar.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Büscher P. In: Trypanosomes and Trypanosomiasis. Magez S, Radwanska M, editors. Vienna: Springer; 2014. Diagnosis of African trypanosomiasis. pp. 189–216. [DOI] [Google Scholar]
- Cadioli FA, Barnabé PA, Machado RZ, Teixeira MCA, André MR, Sampaio PH, et al. First report of Trypanosoma vivax outbreak in dairy cattle in São Paulo state, Brazil. Rev Bras Parasitol Vet. 2012;21(2):118–124. doi: 10.1590/S1984-29612012000200009. [DOI] [PubMed] [Google Scholar]
- Camargo ME. Fluorescent antibody test for the serodiagnosis of American tripanosomiasis: technical modification employing preserved culture forms of Trypanosoma cruzi in a slide test. Rev Inst Med Trop São Paulo. 1966;8(5):227–235. [PubMed] [Google Scholar]
- Carvalho AHO, Silva FA, Jr, Daher DO, Rocha CMBM, Guimarães AM. Efeito do sistema de produção de leite sobre a estabilidade enzoótica para Anaplasma marginale e Babesia bovis em bezerras na região do Campo das Vertentes de Minas Gerais, Brasil. Semina: Ciênc Agrár. 2012;33(1):323–332. doi: 10.5433/1679-0359.2012v33n1p323. [DOI] [Google Scholar]
- Cuglovici DA, Bartholomeu DC, Reis-Cunha JL, Carvalho AU, Ribeiro MFB. Epidemiologic aspects of an outbreak of Trypanosoma vivax in a dairy cattle herd in Minas Gerais state, Brazil. Vet Parasitol. 2010;169(3-4):320–326. doi: 10.1016/j.vetpar.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Dávila AMR, Herrera HM, Schlebinger T, Souza SS, Traub-Cseko YM. Using PCR for unraveling the cryptic epizootiology of livestock trypanosomosis in the Pantanal, Brazil. Vet Parasitol. 2003;117(1-2):1–13. doi: 10.1016/j.vetpar.2003.08.002. [DOI] [PubMed] [Google Scholar]
- De Mello VVC, Souza Ramos IA, Herrera HM, Mendes NS, Calchi AC, Campos JBV, et al. Occurrence and genetic diversity of hemoplasmas in beef cattle from the Brazilian Pantanal, an endemic area for bovine trypanosomiasis in South America. Comp Immunol Microbiol Infect Dis. 2019;66:101337. doi: 10.1016/j.cimid.2019.101337. [DOI] [PubMed] [Google Scholar]
- Garcia HA, Ramírez OJ, Rodrigues CMF, Sánchez RG, Bethencourt AM, Pérez GDM, et al. Trypanosoma vivax in water buffalo of the Venezuelan llanos: an unusual outbreak of wasting disease in an endemic area of typically asymptomatic infections. Vet Parasitol. 2016;230:49–55. doi: 10.1016/j.vetpar.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Gardiner PR, Mahmoud MM. In: Parasitic protozoa. 2nd. Kreier JP, Baker JR, editors. London: Academic Press; 1992. Salivarian trypanosomes causing disease in livestock outside Sub-Saharan Africa. pp. 277–313. [Google Scholar]
- Gonzatti MI, González-Baradat B, Aso PM, Reyna-Bello A. In: Trypanosomes and Trypanosomiasis. Magez S, Radwanska M, editors. Vienna: Springer; 2014. Trypanosoma (Duttonella) vivax and Typanosomosis in Latin America: Secadera/Huequera/Cacho Hueco. pp. 261–285. [DOI] [Google Scholar]
- Guedes DS, Jr, Araújo FR, Silva FJM, Rangel CP, Barbosa JD, No, Fonseca AH. Frequency of antibodies to Babesia bigemina, B. bovis, Anaplasma marginale, Trypanosoma vivax and Borrelia burdgorferi in cattle from the northeastern region of the state of Pará, Brazil. Rev Bras Parasitol Vet. 2008;17(2):105–109. doi: 10.1590/S1984-29612008000200008. [DOI] [PubMed] [Google Scholar]
- Guerra NR, Monteiro MFM, Sandes HMM, Cruz NLN, Ramos CAN, Santana VLA, et al. Detecção de anticorpos IgG anti-Trypanosoma vivax em bovinos através do teste de imunofluorescência indireta. Pesq Vet Bras. 2013;33(12):1423–1426. doi: 10.1590/S0100-736X2013001200005. [DOI] [Google Scholar]
- IBGE . Regiões geográficas do Brasil. Rio de Janeiro: IBGE; 2022. [cited 2022 Mar 3]. Instituto Brasileiro de Geografia e Estatística. online. Available from: https://www.ibge.gov.br/apps/regioes_geograficas/#/home/ [Google Scholar]
- IBGE . Triênio 2018-2020. Rio de Janeiro: IBGE; 2017. [cited 2022 Sep 16]. Instituto Brasileiro de Geografia e Estatística. online. Available from: https://www.ibge.gov.br/pt/inicio.html . [Google Scholar]
- Jones TW, Dávila AMR. Trypanosoma vivax – out of Africa. Trends Parasitol. 2001;17(2):99–101. doi: 10.1016/S1471-4922(00)01777-3. [DOI] [PubMed] [Google Scholar]
- Linhares GFC, Dias FC, Fo, Fernandes PR, Duarte SC. Tripanossomíase em bovinos no município de Formoso do Araguaia, Tocantins (relato de caso) Cienc Anim Bras. 2006;7(4):455–460. doi: 10.5216/cab.v7i4.876. [DOI] [Google Scholar]
- Lopes STP, Prado BS, Martins GHC, Beserra HEA, Sousa MAC, Fo, Evangelista LSM, et al. Trypanosoma vivax em bovino leiteiro. Acta Sci Vet. 2018;46(Suppl. 1):287. [Google Scholar]
- Monzon CM, Mancebo OA, Giménez JN, Russo AM. Evolución de la Trypanosomosis bovina por Trypanosoma vivax en Formosa (Argentina). Años 2007-2012 y su potencial dispersión en el país. Rev Ibero Latin Parasitol. 2013;72(1):38–44. [Google Scholar]
- Osório ALAR, Madruga CR, Desquenes M, Soares CO, Ribeiro LRR, Da Costa CG. Trypanosoma (Dutonella) vivax: its biology, epidemiology, pathogenesis, and introduction in the New World - a review. Mem Inst Oswaldo Cruz. 2008;103(1):1–13. doi: 10.1590/S0074-02762008000100001. [DOI] [PubMed] [Google Scholar]
- Otte MJ, Abuabara JY, Wells EA. Trypanosoma vivax in Colombia: epidemiology and production losses. Trop Anim Health Prod. 1994;26(3):146–156. doi: 10.1007/BF02241071. [DOI] [PubMed] [Google Scholar]
- Otte MJ, Abuabara JY. Transmission of South-American Trypanosoma vivax by the neotropical horsefly Tabanus nebulosus. Acta Trop. 1991;49(1):73–76. doi: 10.1016/0001-706X(91)90033-G. [DOI] [PubMed] [Google Scholar]
- Paoletta MS, López Arias L, de la Fourniere S, Guillemi EC, Luciani C, Sarmiento NF, et al. Epidemiology of Babesia, Anaplasma and Trypanosoma species using a new expanded reverse line blot hybridization assay. Ticks Tick Borne Dis. 2018;9(2):155–163. doi: 10.1016/j.ttbdis.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Radostitis OM, Gay CC, Hinchcliff KW, Constable PD. Veterinary medicine: a textbook of the diseases of cattle, sheep, goats, pigs and horses. 10th. Edinburg: Elsevier Saunders; 2007. [Google Scholar]
- Seidl A, Dávila AMR, Silva RAMS. Estimated financial impact of Trypanosoma vivax on the Brazilian Pantanal and Bolivian Lowlands. Mem Inst Oswaldo Cruz. 1999;94(2):269–272. doi: 10.1590/S0074-02761999000200027. [DOI] [PubMed] [Google Scholar]
- Serra-Freire NM. Oiapoque-outro foco de Trypanosoma vivax no Brasil. Rev Bras Med Vet. 1981;4:30–31. [Google Scholar]
- Shaw JJ, Lainson R. Trypanosoma vivax in Brazil. Ann Trop Med Parasitol. 1972;66(1):25–32. doi: 10.1080/00034983.1972.11686794. [DOI] [PubMed] [Google Scholar]
- Silva AS, Costa MM, Polenz MF, Polenz CH, Teixeira MMG, Lopes STA, et al. Primeiro registro de Trypanosoma vivax em bovinos no Estado do Rio Grande do Sul, Brasil. Cienc Rural. 2009;39(8):2550–2554. doi: 10.1590/S0103-84782009005000189. [DOI] [Google Scholar]
- Silva AS, Molosse VL, Deolindo GL, Cecere BG, Vitt MG, Nascimento LFN, et al. Trypanosoma vivax infection in dairy cattle: parasitological and serological diagnosis and its relationship with the percentage of red blood cells. Microb Pathog. 2022;166:105495. doi: 10.1016/j.micpath.2022.105495. [DOI] [PubMed] [Google Scholar]
- Silva AS, Perez HAG, Costa MM, França RT, Gasperi D, Zanette RA, et al. Horses naturally infected by Trypanosoma vivax in southern Brazil. Parasitol Res. 2011;108(1):23–30. doi: 10.1007/s00436-010-2036-2. [DOI] [PubMed] [Google Scholar]
- Silva RAMS, Silva JA, Schneider RC, Freitas J, Mesquita D, Mesquita T, et al. Outbreak of trypanosomiasis due to Trypanosoma vivax (Ziemann, 1905) in bovines of the Pantanal, Brazil. Mem Inst Oswaldo Cruz. 1996;91(5):561–562. doi: 10.1590/S0074-02761996000500005. [DOI] [PubMed] [Google Scholar]
- Stevens J, Brisse S. In: The Trypanosomiases. Maudlin I, Holmes PH, Miles MA, editors. Wallingford: CABI Publishing; 2004. Systematics of trypanosomes of medical and veterinary importance. pp. 1–23. [DOI] [Google Scholar]
- Suganuma K, Kayano M, Kida K, Gröhn YT, Miura R, Ohari Y, et al. Genetic and seasonal variations of Trypanosoma theileri and the association of Trypanosoma theileri infection with dairy cattle productivity in Northern Japan. Parasitol Int. 2022;86:102476. doi: 10.1016/j.parint.2021.102476. [DOI] [PubMed] [Google Scholar]
- Uzcanga GL, Perrone T, Noda JA, Pérez-Pazos J, Medina R, Hoebeke J, et al. Variant surface glycoprotein from Trypanosoma evansi is partially responsible for the cross-reaction between Trypanosoma evansi and Trypanosoma vivax. Biochemistry. 2004;43(3):595–606. doi: 10.1021/bi0301946. [DOI] [PubMed] [Google Scholar]
- Ventura RM, Paiva F, Silva RAMS, Takeda GF, Buck GA, Teixeira MM. Trypanosoma vivax: characterization of the spliced-leader gene of a Brazilian stock and species-specific detection by PCR amplification of an intergenic spacer sequence. Exp Parasitol. 2001;99(1):37–48. doi: 10.1006/expr.2001.4641. [DOI] [PubMed] [Google Scholar]