Abstract
Background:
Adjuvant radiotherapy (RT) impacts survival after surgery for young children (age < 3 years) diagnosed with intracranial ependymoma. Conformal photon RT promised to spare normal tissue and was introduced more than 25 years ago to improve outcomes for these vulnerable patients. Long-term results for those first treated with conformal methods provide valuable information and serve as a comparison against newer methods.
Methods:
Between 1997 and 2018, 101 patients were treated when age < 3.1 years with conformal and intensity-modulated photon therapy after definitive surgery for intracranial ependymoma. The median time age at RT was 2.1 years and the time from diagnosis to RT start 10 weeks. The extent of resection was gross-total in 82% and 38% underwent more than one attempt at resection. The total prescribed dose was 54–59.4 Gy at 1.8 Gy per fraction.
Results:
The 10-year event-free and overall survivals were 58.5% ± 5.0% and 72.6% ± 4.5%, respectively, with a median follow-up of 18.4 years (range, 4.2–23.3 years). Tumor progression occurred in 34 patients with a median time of 1.6 years. Death occurred in 34 patients from ependymoma (n=24), secondary malignancy (n=6), necrosis (n=2), shunt failure (n=1), and anaphylactic reaction (n=1). Twenty-three patients developed a secondary tumor including 6 cases of fatal high-grade glioma. Of the surviving cohort and those age 18 years or older, 98% obtained a high school diploma, 64% had a current driver’s license, 89% were students or employed full or part time, 32% were living independently, and 70% received higher education or training.
Conclusion:
Long-term results of children treated using photon conformal radiation therapy after surgery demonstrate that adjuvant RT resulted in long-term disease control and functional independence. These results point to the need for new treatment strategies to improve tumor control and provide investigators hope that newer RT methods will further reduce complications.
Introduction
Ependymoma is a neuroepithelial tumor that arises from the ependymal lining of the ventricular system and spinal canal. The peak incidence of pediatric ependymoma is reported to occur between 0 and 3 years of age and most commonly arises within the posterior fossa, although it may also arise in the supratentorial brain or spine.1 Ependymoma accounts for approximately 10% of all pediatric brain tumors, making it the third most common central nervous system (CNS) malignancy in children.2 The mainstay of treatment in nonmetastatic patients includes aggressive local therapy with maximal safe resection followed by adjuvant radiotherapy (RT). The use of chemotherapy in the treatment of pediatric ependymoma is not clearly defined and is under consideration given the suggestion that adjuvant chemotherapy may impact event-free and overall survival.3 Chemotherapy has also been used in very young patients to delay or avoid RT. Previous evidence suggests a strong correlation between extent of surgical resection and survival; with overall survival (OS) after gross total resection (GTR) and adjuvant RT estimated between 63–93%, while patients who underwent less than a GTR followed by adjuvant RT achieved an OS between 22–56%.4–14 Merchant et al. reported a significantly lower risk of all-cause mortality after GTR as compared to subtotal resection (STR)/near-total resection (NTR).4 GTR is often highly morbid in the setting of infiltrative tumors, particularly those located within the posterior fossa due to the close proximity of intricate neurovascular structures.
Focal radiotherapy has been established as the standard adjuvant therapy modality, even after GTR in most ependymoma patients. One challenge in the management of this disease is nearly half of pediatric ependymomas are diagnosed before age 3. It has been well-established that CNS RT is associated with increased risk for long-term morbidity in young children.15 Attempts at avoiding or delaying RT by utilizing adjuvant chemotherapy or observation have failed to yield adequate disease control. In 2007, Grundy et al. reported 42% freedom from RT at 5 years in children less than 3 years of age who were treated with maximal resection followed by adjuvant chemotherapy. RT was permitted at the time of radiographic evidence of disease progression. Ultimately, 47% of patients required RT with median age at irradiation of 3.6 years.16 There is limited data to suggest that a selected group of pediatric ependymoma patients who have completely resected, supratentorial tumors with classic histology may do well after GTR alone, however, this applies to a minority of cases.17,18
Over the last several three decades, advances in diagnostic and treatment techniques have improved long term outcomes in this population. High-resolution MRI may allow for better disease delineation and facilitate earlier diagnosis, while MRI-guided surgery can allow for greater extent of resection and decreased operative morbidity. Conformal radiotherapy techniques, including proton therapy and intensity, modulated radiation therapy, may reduce local and neuraxis failure while minimizing dose to surrounding healthy tissues by increasing dose conformality around the target.4,5,19–21 Given the propensity for pediatric ependymoma to arise in the posterior fossa, tight dose conformality is vital to preserving critical brainstem and cerebellar function.4 As diagnostic and therapeutic advancements translate into better disease control and improved survival, there is a need for long-term data to inform the longitudinal survivorship needs of very young patients ependymoma patients. Five prospective trials included patients 3 years and younger and have reported outcomes over a median follow-up of 3–8 years, with overall survival after definitive surgery and RT approximately 80%.4,5,18,21,22 We designed a study to identify the adulthood functional and physical complications that may arise in survivors of ependymoma treated at a very young age.
Methods
Patients
A retrospective review was performed that included 101 patients with non-metastatic intracranial ependymoma treated with surgery and adjuvant photon conformal or intensity-modulated radiotherapy beginning August 14, 1997, to July 11, 2018 (median date, December 16, 2002), at a single institution. The study was limited to those with localized tumors aged less than 3.1 years when starting radiotherapy (median age, 2.1 years, range 0.9–3.1 years). Dates of diagnosis ranged from February 22, 1996, to December 6, 2017 (median date, May 11, 2002). Gross total resection was performed in 83 (82%), near-total resection 7 (7%), and subtotal resection 11 (11%) based on criteria previously described.18 All patients had histologically confirmed ependymoma based on expert neuropathology review23 and were evaluated according to the 2000 WHO grading system.24 There were 71 anaplastic tumors and 30 classic tumors. Tumor location was infratentorial (n=90) or supratentorial (n=11). No patients with primary spinal cord tumors were included. There were 46 females and 55 males. Patient race/ethnicity was recorded as White (n=80), Black (n=9), Asian (n=4), Hispanic (n=4), and Other (n=4).
Eight-six patients were treated on sequential phase II protocols4,18,25,26 and 15 patients were treated according to a non-protocol treatment plan that included similar RT guidelines and informed consent process. The prospective protocols, in order of their activation status, included RT1 (NCT00187226, n=61), ACNS0121 (NCT00027846, n=14), SJYC07 (NCT00602667, n=10), ACNS0831 (NCT01096368, n=1). Reasons for choosing a non-protocol treatment plan for the remaining 15 patients included patient/guardian preference, treating physician discretion, lack of an available open trial. Given the treatment era, molecular characterization of the cohort was not possible for all patients. Amongst the 90 infratentorial cases, 74 were classified as PFA subtype, 2 were classified as PFB subtype, and the remaining 14 were not classified. The current retrospective study was approved by the institutional review board as involving only minimal risk to human subjects. The study was exempt from full review and did not require consent from the subjects included in the report.
Surgery
Multiple operations were permitted for maximal safe resection. Surgery was permitted to be performed at an outside institution. Gross total resection was defined as no evidence of residual tumor intraoperatively and no evidence of residual tumor on postoperative imaging. Near-total resection was defined as minimal residual disease evident by the surgeon or on post-operative imaging. Subtotal resection included tumor debulking and substantial residual disease noted by the surgeon or on post-operative imaging measuring ≥ 0.5 cm. The number of resections prior to RT was 1 (62), 2 (30), 3 (5), and 4 (4). The extent of resection at the start of radiation was GTR for 83 patients, NTR for 7 patients, and STR for 11 patients. A shunt was required in 52 patients.
Radiation and Chemotherapy
All patients were treated with conformal photon therapy using planning and treatment guidelines previously described.4 Prior chemotherapy was permitted but the previous course of intracranial irradiation was exclusionary. The gross tumor volume (GTV) was defined as the postoperative tumor bed and gross disease seen on MRI. The clinical target volume (CTV) was a 0.5 (n=22) −1.0 (n=79) cm anatomical expansion on the GTV to account for subclinical disease. The planning target volume (PTV) was a 0.3–0.5 cm geometric expansion on the CTV to account for setup and image registration uncertainty. The prescribed was dose 54 (n=28 patients) or 59.4 Gy (n=73 patients) delivered at 1.8 Gy per day. The median time from diagnosis to delivery of the first fraction was 10.3 weeks (range 3.3–109.1). Patients who did not receive chemotherapy started with a median of 9.0 weeks (range 9.7–3.3), those who received chemotherapy had a time interval of 28.4 weeks (9.1–109.1 weeks). All patients completed their radiotherapy course as intended. The total prescribed dose was limited to 54 Gy after gross-total resection for patients aged < 18 months (n=16), those enrolled on a protocol that included 4 cycles of induction chemotherapy (n=8), and physician discretion (n=4). There were 32 patients, including 22 survivors, who received chemotherapy before (n=30), after (n=1) or before and after (n=1) radiation therapy. Nineteen received chemotherapy according to a non-protocol treatment plan using agents previously recommended to delay or avoid irradiation.27 The median duration was 6 months (range 1–15 months). For those enrolled on a protocol,18,25,26,28 the median duration of was 5 months, range 1–6 months).
Functional Assessment
Follow-up included clinical evaluation and MRI brain every 3 months for the first 2 years (1997–2002) or every 4 months for the first 3 years (2003–07), then every 6 months up to 5 years, and then annually. Spinal MRI was performed annually. The individual protocols included prospective assessment of neurologic,29 endocrine,30 and cognitive31 function with severe complications graded according to the Common Toxicity Criteria of the time. None of the patients were lost to follow-up. Functional outcome was evaluated using six metrics that have been validated and used in previous work.32–37 These include the use of an Individualized Education Program (IEP) or a 504 plan (yes, no), driver’s license (yes, no), high school diploma (yes, no), higher education (yes: graduated from, currently attending, or attempted university, associates or vocational studies; no: none), employment status (unemployed: unemployed or disabled; employed: working full or part-time; student) and living situation (independent: living alone, with a partner or roommate(s); dependent: living with parents or guardian). The latter five metrics were only assessed in survivors over the age of 18 (n=44).
Statistical Methods
Fisher’s exact test was used to compare patient demographic and treatment characteristics. We evaluated overall and progression-free survival (PFS); incidence, pattern, and timing of recurrence; incidence of secondary malignancy; surgical number and extent; shunt status; age at RT; RT dose; tumor location and histology; race; gender; pre-RT chemotherapy, the incidence of various neurologic toxicities; functional outcome. The Kaplan-Meier method was used to estimate OS and PFS. Overall survival (OS) was defined as the interval from the date of delivery of the first fraction of RT to the date of death or last contact. Event-free survival (EFS) was defined as the interval from the date of delivery of the first fraction of RT to the date of disease progression or recurrence note on imaging, date of secondary neoplasm, death from any cause, or date of last imaging, whichever occurred first. Patients who were alive at follow-up without progression were censored. All the statistical testing and graphs were created through GraphPad Prism 9 software for MacOS. Most of the protocols included baseline and serial clinical evaluations for up to 5 years after treatment initiation, those enrolled on RT1 were followed systematically for 10 years. All survivors had follow-ups within the last 2 years.
Results
Disease Control and Major Complications
With a median follow-up of 18.43 years (range, 4.2–23.3 years), only 8 patients followed for less than 10 years, there were 67 survivors with a current age of 20.8 years median (range, 7.5–26.8 years) at last contact. Event-free survival estimates at 10 and 20 years were 58.5% ± 5.0% and 57.3% ± 5.1%, respectively. Overall survival estimates at 10 and 20 years were 72.6% ± 4.5% and 63.7% ± 5.1%, respectively. (Figure 1) Thirty-four patients died from tumor progression (n=24), secondary malignancy (n=6), radiation necrosis (n=2), CSF shunt failure (n=1), or an unrelated anaphylactic reaction (n=1). (Table 1)
Figure 1.
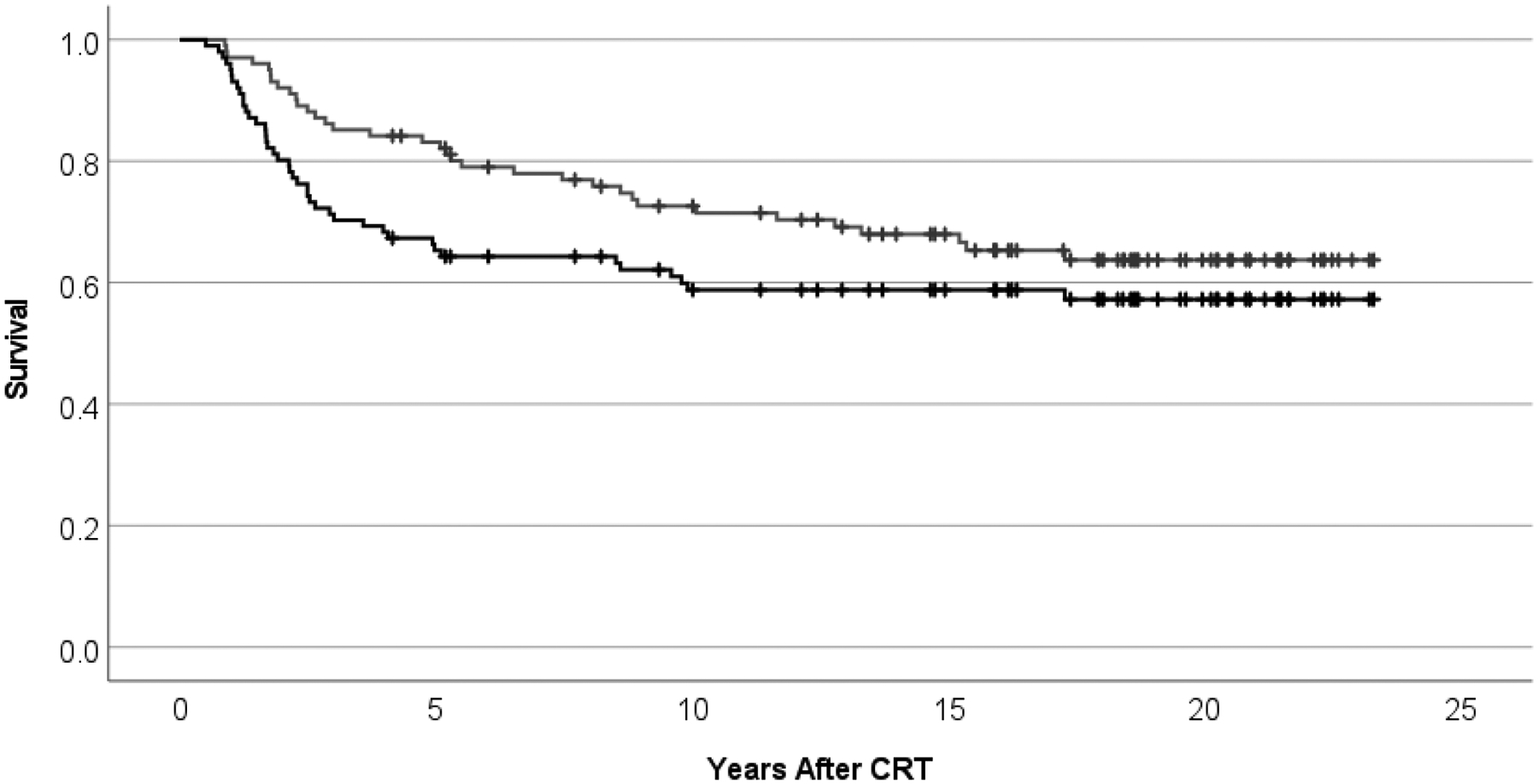
Event-free (lower, black) and overall (upper, gray) survival after conformal photon radiation therapy for ependymoma age < 3 years.
Table 1.
Clinical variables and outcomes for 101 young children with intracranial ependymoma.
| Number | Time | |
|---|---|---|
| Age at RT | ||
| Diagnosis | 1.68 years (0.01–3.02 years) | |
| Radiation Therapy | 2.12 years (0.89–3.10 years) | |
| Last Follow-up | 20.81 years (7.46–26.84 years) | |
| Sex | ||
| M | 55 | |
| F | 46 | |
| Race/Ethnicity | ||
| Asian | 4 | |
| Black | 9 | |
| Hispanic | 4 | |
| Other | 4 | |
| White | 80 | |
| Extent of Resection | ||
| Gross-total Resection | 83 | |
| Near-total Resection | 7 | |
| Sub-total Resection | 11 | |
| Clinical Target Volume Margin | ||
| 0.5cm | 22 | |
| 1.0cm | 79 | |
| Total Dose | ||
| 54.0 Gy | 28 | |
| 59.4 Gy | 73 | |
| Tumor Location | ||
| Infratentorial | 90 | |
| Supratentorial | 11 | |
| Grade | ||
| Classic | 30 | |
| Anaplastic | 71 | |
| Shunt | ||
| None | 49 | |
| Yes | 52 | |
| Cause of Death | ||
| Tumor Progression | 24 | |
| High-grade Glioma | 6 | |
| Necrosis | 2 | |
| Shunt Failure | 1 | |
| Anaphylaxis | 1 | |
| Pattern of Failure | ||
| Local | 17 | 2.02 years (0.86–9.46 years) |
| Distant | 11 | 1.70 years (0.39–4.48 years) |
| Local and Distant | 6 | 0.89 years (0.63–2.90 years) |
| Secondary Tumor | ||
| Benign Thyroid Nodule | 5 | 14.4 years (4.00–16.19 years) † |
| Meningiomatosis | 1 | 4.92 years |
| Cholesteatoma | 1 | 13.01 years |
| Low-grade glioma | 1 | 2.80 years |
| High-grade Glioma | 6 | 9.79 years (4.05–14.53 years) ‡ |
| Meningioma | 7 | 14.25 years (6.19–18.39 years) |
| Papillary Thyroid Cancer | 3 | 9.16 years (6.84–11.32 years) |
| Vestibular Schwannoma | 1 | 11.14 years |
Two patients who developed thyroid nodules in addition to meningioma are included.
Two re-irradiated patients who developed high-grade glioma 8.39 and 14.53 years after their initial treatment with radiation therapy are included.
Disease progression was experienced by 34 patients. The median time to progression was 1.59 years (range, 0.4–9.5 years). The pattern of failure was local (n=17), distant (n=11), and both local and distant (n=6), with pathologic confirmation in all cases. Two of 7 progression events during the first year of follow-up were local. The two progression events that occurred after the year five follow-up timepoint were local. (Table 1)
Ten of the 17 patients with local progression were re-irradiated and 5 died of tumor progression. The 5 surviving patients achieved durable tumor control, median 13.28 years (range, 10.4–15.5 years). None of the 6 patients with local and distant progression were re-irradiated, all died of tumor progression. Seven of the 11 patients with distant progression were re-irradiated and only 2 survived (2.8 and 17.2 years): 3 died from tumor progression and 2 died from secondary tumors 6.4 and 13.4 years after initial tumor progression. One patient with distant tumor progression was salvaged with surgery and chemotherapy (13.4 years).
Factors that might affect event-free and overall survival were evaluated including sex, age, race, diagnosis to RT start interval, primary tumor location, tumor grade, extent of resection, surgery number, pre-RT chemotherapy, radiation dose (54 Gy vs 59.4 Gy) and shunt. We noted that 94% of survivors had a GTR/NTR, whereas 79% of patients who died had a GTR/NTR. Those treated with GTR/NTR had a higher rate of progression-free (P=0.006) and overall survival (P=0.002). The 10-year estimates were 69.3% ± 4.9% GTR/NTR vs. 32.7% ± 15.0% STR for progression-free survival and 77.1% ± 4.5% GTR/NTR vs. 36.4% ± 14.5% STR for overall survival.
Twenty-three patients were found to have 25 secondary tumors: high-grade glioma (n=6), meningioma (n=5), meningioma + benign thyroid nodules (n=2), papillary thyroid cancer (n=3), benign thyroid nodules (n=3), and single cases of low-grade glioma, vestibular schwannoma, cholesteatoma, and meningiomatosis. (Table 1) Surgical treatment of meningioma was undertaken for 5 cases. High-grade glioma was uniformly fatal and included 2 patients who were long-term survivors of recurrent ependymoma treated with a second course of irradiation. Surgical and radiotherapy treatment of papillary thyroid cancer was successful in all cases. Thyroid nodules were pathologically confirmed in all but one case. The attribution of RT to the development of supratentorial low-grade glioma was uncertain given the infratentorial location of the affected patient’s primary tumor. The low-grade glioma was resected without complications and there was no additional treatment. Incidence rate estimates at 10 and 20 years were 16.9% ± 4.3% and 33.8% ± 5.9% for the development of any neoplasm, 2.7% ± 1.9% and 12.5% ± 4.6% for meningioma, and 6.5% ± 3.2% and 6.5% ± 3.2% for high-grade glioma. (Figure 2)
Figure 2.
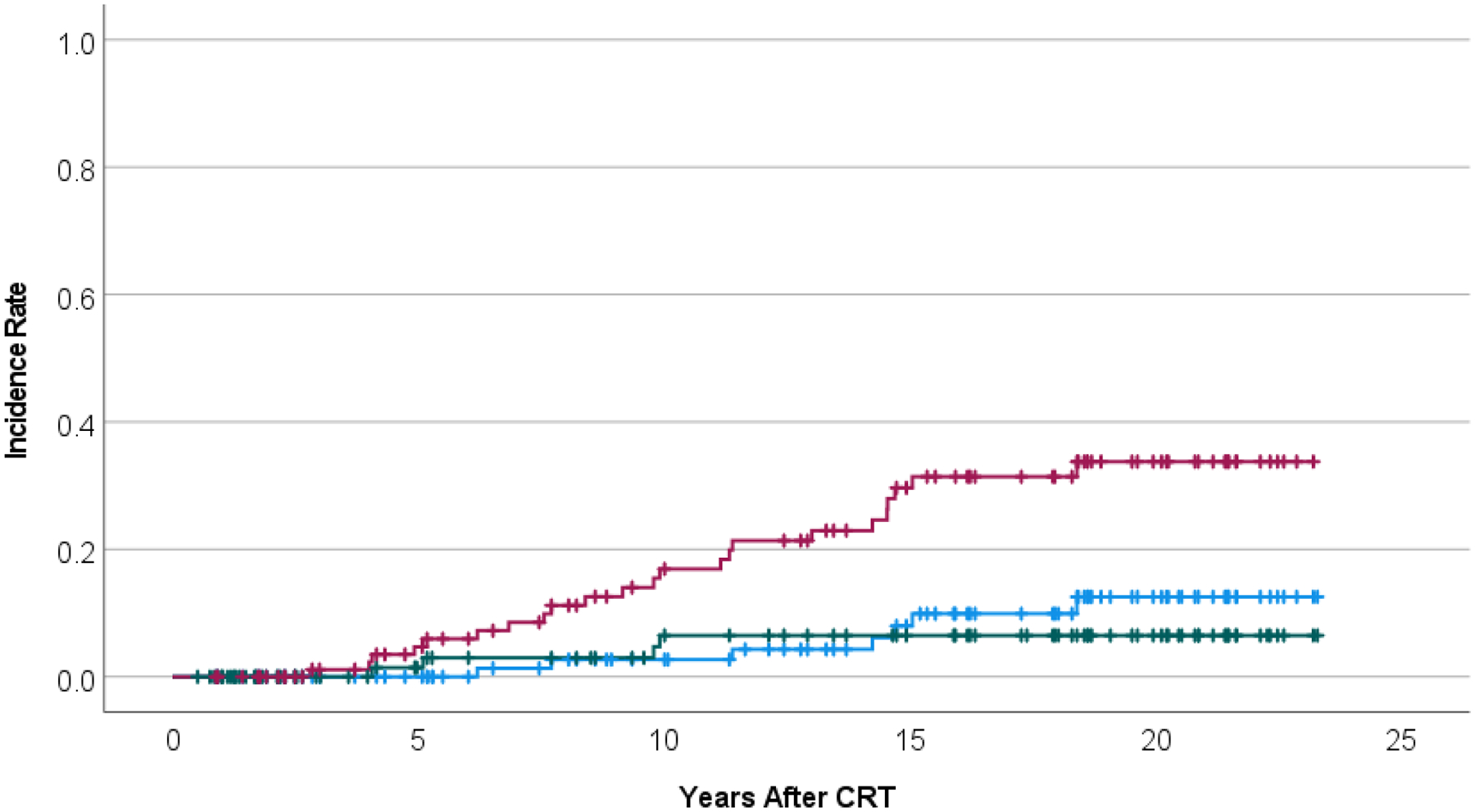
Incidence rate for the development of any neoplasm (upper curve, red), meningioma (middle curve, blue), or high-grade glioma (lower, green) after conformal photon radiation therapy for ependymoma, age < 3 years.
Vasculopathy and Seizure Disorders
The following information was obtained from the 9 supratentorial and 58 infratentorial tumor location survivors. Notable complications and imaging findings included a subcortical vascular lesion in 1 of 9 supratentorial patients. Six patients with infratentorial tumors had vascular lesions and separately, there were 3 patients who had ischemic strokes. One infratentorial patient with a cavernoma was treated with surgery and one infratentorial patient with ischemic stroke underwent bilateral revascularization surgery. Seizure disorders were reported for 9 patients after treatment including 7 of 9 with supratentorial tumor location (χ2, p-value is < .00001.
Neurology, Audiology, and Endocrinology
The following information was obtained from the 9 supratentorial and 58 infratentorial tumor location survivors. Supratentorial – there were no permanent cranial nerve deficits, 3 cases of hemiparesis, 2 cases of autism, and one patient with significant depressive disorder. Three had bilateral hearing loss (1 was prescribed hearing aids), 1 had unilateral hearing loss, and 5 were normal. Endocrine deficiencies (growth hormone deficiency and central hypothyroidism) that required treatment were observed in one patient with supratentorial primary (temporal lobe) tumor location. Infratentorial – single (n=5) or multiple cranial nerve deficits (13) were reported long-term. Five patients had ataxia, 2 had weakness, and 4 reported hemi-paresis. Two infratentorial patients had autism. There was one patient who was tracheostomy dependent, and this patient and two others had permanent gastrostomy for enteral feeding and required a walker. (Table 2)
Table 2.
Chronic conditions reported in 67 long-term survivors of childhood ependymoma treated with photon radiation therapy under the age of 3 years.
| Infratentorial (n=58) | Supratentorial (n=9) | |
|---|---|---|
| Seizure | ||
| None | 56 | 2 |
| Yes | 2 | 7 |
| Cranial Nerve | ||
| None | 40 | 9 |
| Single | 5 | |
| Multiple | 13 | |
| Any Deficits | 18 | |
| Motor/Coordination | ||
| None | 47 | 6 |
| Ataxia | 5 | |
| Weakness | 2 | |
| Hemiparesis | 4 | 3 |
| Any Deficits | 11 | 3 |
| Major/Developmental | ||
| Autism | 2 | 2 |
| Depression | 2 | 1 |
| Gastrostomy/Walker | 3 | |
| Any Deficits | 7 | 2 |
| Hearing Loss | ||
| None | 18 | 5 |
| Unilateral | 21 | 1 |
| Bilateral | 8 | 2 |
| Bilateral with Hearing Aids | 11 | 1 |
| Any Deficits | 40 | 4 |
| Endocrine | ||
| None | 31 | 8 |
| GHD | 11 | 0 |
| GHD, TSHD | 1 | 1 |
| GHD, TSHD, ACTHD | 2 | |
| GHD, TSHD, ACTHD, LH/FSHD | 1 | |
| GHD, TSHD, LH/FSHD | 1 | |
| GHD, TSHD, CPP | 1 | |
| GHD, LH/FSHD | 2 | |
| TSHD | 4 | |
| TSHD 2° PTC | 3 | |
| CPP | 1 | |
| Any Deficits | 27 | 1 |
| ≥ 2 Deficits | 8 |
Legend: GHD, growth hormone deficiency; TSHD, thyroid stimulating hormone deficiency; ACTHD, adrenocorticotropin hormone deficiency; LH/FSHD, luteinizing and follicle stimulating hormone deficiency; PTC, papillary thyroid carcinoma; CPP, central precocious puberty.
Functional and Social Attainment
Of the surviving cohort, 87% required an IEP or 504 plan. Among survivors aged 18 and older, 98% obtained a high school diploma, 64% reported having a current driver’s license, 89% were students or employed full or part time, 32% (upper age of the surviving cohort was 26.84 years) indicated they were living independently, and 70% received some level of higher education. (Table 3)
Table 3.
Social achievement and functional outcomes.
| Infratentorial (n=58) | Supratentorial (n=9) | |
|---|---|---|
| Driver’s License | ||
| < 18 years | 20† | 3 |
| None | 11 | 5 |
| Yes | 27 | 1 |
| IEP/504 | ||
| None | 8 | 1 |
| Yes | 50 | 8 |
| High School Diploma | ||
| < 18 years | 20 | 3 |
| None | 1 | 0 |
| Yes | 37 | 6 |
| Higher Education or Training | ||
| < 18 years | 20 | 3 |
| None | 9 | 4 |
| Attending | 14 | 1 |
| Yes | 15 | 1 |
| Employment Status | ||
| < 18 years | 20 | 3 |
| Full time | 13 | 2 |
| Part time | 3 | 1 |
| Part time + Disability | 1 | |
| Part time + Student | 9 | |
| Student | 8 | 2 |
| Unemployed | 3 | |
| Disability | 2 | 1 |
| Living Situation | ||
| < 18 years | 20 | 3 |
| Dependent w/Parent(s) | 24 | 5 |
| Assisted Living | 1 | |
| Independent | 14‡ | 0 |
3 patients in the group age < 18 years have a driver’s license
Independent = alone (n=2), roommates (n=4), partner (n=8)
Discussion
The purpose of this investigation was to clarify the long-term impact of adjuvant conformal radiotherapy using photons to achieve tumor control in young children with intracranial ependymoma and to understand the long-term consequences of aggressive surgery and radiotherapy administered to children under the age of 3 years. Historically, efforts have been made to delay irradiation in this age group because of concern for neurocognitive deficits and other complications given their potential vulnerability. Past reports suggest that survival is relatively poor for young children diagnosed with ependymoma. This has been attributed to more aggressive tumor subtypes, advanced disease at presentation, age-related fragility, or vulnerability to treatment-related morbidity and mortality. An earlier study examined 89 ependymoma patients aged 3 years or younger who were treated with maximal resection followed by alternating myelosuppressive and non-myelosuppressive chemotherapy for 1 year. The authors reported 5-year event-free and overall survivals of 42% and 63%, respectively. The median delay to RT was 20 months and the median age at RT was 3.6 years. As reported, nearly half of the patients ultimately required radiotherapy.16
Over the last several decades, efforts have been made to investigate the safety of immediate radiotherapy following definitive surgery for very young patients, in hopes of minimizing therapy and improving outcomes.38 Conformal radiotherapy techniques promise to improve the toxicity profile by better sparing normal tissue and, therefore, cognitive function.31
Newer methods of radiotherapy, including proton therapy, have been implemented to further reduce collateral doses to normal tissues, As reported in a series of 179 patients 21 years of age or younger who underwent maximal safe resection followed by adjuvant PRT. The mean radiation dose for enrollees 3 years of age or younger was 54 Gy, while older patients received 59.4 Gy. The investigators reported an impressive 3-year progression-free survival (PFS) rate of 76%, local control (LC) 86%, and overall survival (OS) 90%. Importantly, the rate of ≥ grade 2 brainstem toxicity at 3 years was 5.5% and the rate of hormone deficiency was 7.3%.22 A retrospective review of patients treated with PRT or IMRT after definitive surgery revealed discrepant outcomes after photon vs proton therapy, with PFS and OS 60% and 81% for IMRT vs 82% and 97% with PBT, respectively.15 While there is currently no prospective comparison of conformal photon and proton therapy, we do have evidence that adjuvant radiotherapy is well-tolerated and confers disease control and survival benefit in the adjuvant setting.1,4,5,18,21,22
Several findings from this photon series with long-term follow-up were in keeping with short-term follow-up from a large proton series.39 The patterns of failure were similar, and a total dose of 54 Gy resulted in acceptable outcomes. Distant failure as a component of failure is an established outcome in those treated with focal radiotherapy, and accepting 54 Gy, at least for the youngest patients, might increase the acceptance of radiotherapy for young children and simplify treatment guidelines.
Long-term functional independence is a major concern of pediatric ependymoma survivors and their families. Our findings suggest that while survivors are likely to need individualized educational assistance, they are highly likely to obtain a high school diploma and go on to receive higher education. We found that 61% of survivors obtained a driver’s license, indicating a high level of functional independence in adulthood. This is consistent with the rate of obtaining a driver’s license reported among the general United States adult population.40 Moreover, we note that catastrophic neurologic deficits (including vasculopathy and persistent seizure disorder) in survivors were rare. In the years to follow their treatment, these patients are at increased risk for secondary malignancy, most of which are highly curable. It is possible that with the use of proton therapy this cumulative incidence of secondary tumors and secondary high-grade glioma will be lower for the normal tissue sparing properties of conformal therapy are substantial. It is safe to say that based on beam delivery characteristics alone, the incidence of thyroid nodules and papillary thyroid cancer will be lower. There is also hope that the incidence of hearing loss will be lower when the dose to the cochleae may be safely reduced. The same may be true about the incidence of endocrinopathy although the incidence is low, to begin with and patients often present with hormone deficiencies including those diagnosed with ependymoma.
There are a few weaknesses and a number of unique strengths in this report. Our institution accepts patients for treatment regardless of their ability to pay provided they are eligible for an active protocol or live within the catchment area of our treatment center or 8 regional affiliates. All patients are offered follow-up for 10 years or until the age of 18 years, whichever is longer, and those who experience tumor progression prior to achieving the age of majority are offered salvage therapy and additional follow-up. Patients are followed by their primary oncologist and may be transferred for follow-up to the survivorship program and the after completion of therapy clinic during the time intervals outlined. Once the patient achieves alumni status they are followed in their community and may have trouble with access to care, resources, and the encouragement to achieve compliance associated with the primary treatment team. As feasible, patients are regularly contacted to update survival status and to exchange information about major outcomes including tumor progression, secondary tumor development, and severe medical complications. None of the patients in this series were lost to follow-up and the chosen outcomes, medical and social/functional, were largely those that would have been affected by pre-existing conditions, the primary tumor, and the acute, subacute, and early late effects of treatment presenting during the follow-up intervals outlined above. The length of follow-up for such a young cohort of irradiated patients is unprecedented. Nevertheless, even with the magnitude of the follow-up, the young age at treatment leaves several patients in the adolescent and young adult age range to which some of our chosen endpoints cannot be applied. This is further impacted by including several patients who were treated during the past 5–10 years when most with the same diagnosis were offered proton therapy. Our goal here was to include the largest possible data set of photon patients and to recognize the importance of pre-existing deficits and early complications in these vulnerable patients.
The disease control and survivorship outcomes presented in this report are favorable or comparable to previously reported outcomes in this population after surgery and chemotherapy and provide insight into what can be expected in the decades that follow treatment for ependymoma in a very young child.16 When disease progression occurs, it is most likely to be local and surgery followed by re-irradiation can be curative. Close follow-up at a multidisciplinary care center is vital to optimizing functional and physical outcomes. Based upon the findings presented here, the authors conclude that surgery followed by immediate conformal radiotherapy is a safe and effective treatment option in ependymoma patients under the age of 3. This report should serve as a benchmark against which adjuvant proton therapy regimens could be compared in this patient population.
Funding:
This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC), Cancer Center Support Grant No. CA21765-23 from the National Cancer Institute, and by Research Project Grant No. RPG-99-252-01-CCE from the American Cancer Society.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest concerning the materials or methods used in this study or the findings reported in this paper.
Data Availability Statement:
All data generated or analyzed during this study are included in the published article.
References
- 1.Purdy E, Johnston DL, Bartels U, et al. Ependymoma in children under the age of 3 years: a report from the Canadian Pediatric Brain Tumour Consortium. J Neurooncol. 2014;117(2):359–364. [DOI] [PubMed] [Google Scholar]
- 2.Villar RC, Merchant TE. Chapter 9: Ependymoma. In: Radiation Oncology for Pediatric CNS Tumors by Anita Mahajan; Paulino Arnold C. Cham, Switzerland: Springer International Publishing; 2018:165–187. [Google Scholar]
- 3.Smith A, Onar-Thomas A, Ellison D, et al. EPEN-54. ACNS0831, PHASE III RANDOMIZED TRIAL OF POST-RADIATION CHEMOTHERAPY IN PATIENTS WITH NEWLY DIAGNOSED EPENDYMOMA AGES 1 TO 21 YEARS. Neuro-Oncology. 2020;22(Supplement_3):iii318–iii319. [Google Scholar]
- 4.Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22(15):3156–3162. [DOI] [PubMed] [Google Scholar]
- 6.Foreman NK, Love S, Thorne R. Intracranial ependymomas: analysis of prognostic factors in a population-based series. Pediatr Neurosurg. 1996;24(3):119–125. [DOI] [PubMed] [Google Scholar]
- 7.Pollack IF, Gerszten PC, Martinez AJ, et al. Intracranial ependymomas of childhood: long-term outcome and prognostic factors. Neurosurgery. 1995;37(4):655–666; discussion 666–657. [DOI] [PubMed] [Google Scholar]
- 8.Robertson PL, Zeltzer PM, Boyett JM, et al. Survival and prognostic factors following radiation therapy and chemotherapy for ependymomas in children: a report of the Children’s Cancer Group. J Neurosurg. 1998;88(4):695–703. [DOI] [PubMed] [Google Scholar]
- 9.Rousseau P, Habrand JL, Sarrazin D, et al. Treatment of intracranial ependymomas of children: review of a 15-year experience. Int J Radiat Oncol Biol Phys. 1994;28(2):381–386. [DOI] [PubMed] [Google Scholar]
- 10.Perilongo G, Massimino M, Sotti G, et al. Analyses of prognostic factors in a retrospective review of 92 children with ependymoma: Italian Pediatric Neuro-oncology Group. Med Pediatr Oncol. 1997;29(2):79–85. [DOI] [PubMed] [Google Scholar]
- 11.Nazar GB, Hoffman HJ, Becker LE, Jenkin D, Humphreys RP, Hendrick EB. Infratentorial ependymomas in childhood: prognostic factors and treatment. J Neurosurg. 1990;72(3):408–417. [DOI] [PubMed] [Google Scholar]
- 12.Healey EA, Barnes PD, Kupsky WJ, et al. The prognostic significance of postoperative residual tumor in ependymoma. Neurosurgery. 1991;28(5):666–671; discussion 671–662. [DOI] [PubMed] [Google Scholar]
- 13.Vitanza NA, Partap S. Pediatric Ependymoma. J Child Neurol. 2016;31(12):1354–1366. [DOI] [PubMed] [Google Scholar]
- 14.Snider CA, Yang K, Mack SC, et al. Impact of radiation therapy and extent of resection for ependymoma in young children: A population-based study. Pediatr Blood Cancer. 2018;65(3). [DOI] [PubMed] [Google Scholar]
- 15.Sato M, Gunther JR, Mahajan A, et al. Progression-free survival of children with localized ependymoma treated with intensity-modulated radiation therapy or proton-beam radiation therapy. Cancer. 2017;123(13):2570–2578. [DOI] [PubMed] [Google Scholar]
- 16.Grundy RG, Wilne SA, Weston CL, et al. Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol. 2007;8(8):696–705. [DOI] [PubMed] [Google Scholar]
- 17.Palma L, Celli P, Mariottini A, Zalaffi A, Schettini G. The importance of surgery in supratentorial ependymomas. Long-term survival in a series of 23 cases. Childs Nerv Syst. 2000;16(3):170–175. [DOI] [PubMed] [Google Scholar]
- 18.Merchant TE, Bendel AE, Sabin ND, et al. Conformal Radiation Therapy for Pediatric Ependymoma, Chemotherapy for Incompletely Resected Ependymoma, and Observation for Completely Resected, Supratentorial Ependymoma. J Clin Oncol. 2019;37(12):974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDonald SM, Safai S, Trofimov A, et al. Proton radiotherapy for childhood ependymoma: initial clinical outcomes and dose comparisons. Int J Radiat Oncol Biol Phys. 2008;71(4):979–986. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder TM, Chintagumpala M, Okcu MF, et al. Intensity-modulated radiation therapy in childhood ependymoma. Int J Radiat Oncol Biol Phys. 2008;71(4):987–993. [DOI] [PubMed] [Google Scholar]
- 21.Massimino M, Gandola L, Giangaspero F, et al. Hyperfractionated radiotherapy and chemotherapy for childhood ependymoma: final results of the first prospective AIEOP (Associazione Italiana di Ematologia-Oncologia Pediatrica) study. Int J Radiat Oncol Biol Phys. 2004;58(5):1336–1345. [DOI] [PubMed] [Google Scholar]
- 22.Indelicato DJ, Bradley JA, Rotondo RL, et al. Outcomes following proton therapy for pediatric ependymoma. Acta Oncol. 2018;57(5):644–648. [DOI] [PubMed] [Google Scholar]
- 23.Godfraind C, Kaczmarska JM, Kocak M, et al. Distinct disease-risk groups in pediatric supratentorial and posterior fossa ependymomas. Acta Neuropathol. 2012;124(2):247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleihues P, Sobin LH. World Health Organization classification of tumors. Cancer. 2000;88(12):2887–2887. [DOI] [PubMed] [Google Scholar]
- 25.Smith A. Maintenance Chemotherapy or Observation Following Induction Chemotherapy and Radiation Therapy in Treating Patients With Newly Diagnosed Ependymoma. ClinicalTrials.gov Identifier: NCT01096368. https://clinicaltrials.gov/ct2/show/NCT01096368. In:2010. [Google Scholar]
- 26.Upadhyaya SA, Robinson GW, Onar-Thomas A, et al. Molecular grouping and outcomes of young children with newly diagnosed ependymoma treated on the multi-institutional SJYC07 trial. Neuro Oncol. 2019;21(10):1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328(24):1725–1731. [DOI] [PubMed] [Google Scholar]
- 28.Cohen BH, Geyer JR, Miller DC, et al. Pilot Study of Intensive Chemotherapy With Peripheral Hematopoietic Cell Support for Children Less Than 3 Years of Age With Malignant Brain Tumors, the CCG-99703 Phase I/II Study. A Report From the Children’s Oncology Group. Pediatr Neurol. 2015;53(1):31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merchant TE, Chitti RM, Li C, Xiong X, Sanford RA, Khan RB. Factors associated with neurological recovery of brainstem function following postoperative conformal radiation therapy for infratentorial ependymoma. Int J Radiat Oncol Biol Phys. 2010;76(2):496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Iersel L, van Santen HM, Potter B, et al. Clinical impact of hypothalamic-pituitary disorders after conformal radiation therapy for pediatric low-grade glioma or ependymoma. Pediatr Blood Cancer. 2020;67(12):e28723. [DOI] [PubMed] [Google Scholar]
- 31.Conklin HM, Li C, Xiong X, Ogg RJ, Merchant TE. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol. 2008;26(24):3965–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunin-Batson A, Kadan-Lottick N, Zhu L, et al. Predictors of independent living status in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2011;57(7):1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koch SV, Kejs AM, Engholm G, Moller H, Johansen C, Schmiegelow K. Leaving home after cancer in childhood: a measure of social independence in early adulthood. Pediatr Blood Cancer. 2006;47(1):61–70. [DOI] [PubMed] [Google Scholar]
- 34.Khan F, Amatya B. Factors associated with long-term functional outcomes, psychological sequelae and quality of life in persons after primary brain tumour. J Neurooncol. 2013;111(3):355–366. [DOI] [PubMed] [Google Scholar]
- 35.Corrigan JD, Cuthbert JP, Harrison-Felix C, et al. US population estimates of health and social outcomes 5 years after rehabilitation for traumatic brain injury. J Head Trauma Rehabil. 2014;29(6):E1–9. [DOI] [PubMed] [Google Scholar]
- 36.Brinkman TM, Ness KK, Li Z, et al. Attainment of Functional and Social Independence in Adult Survivors of Pediatric CNS Tumors: A Report From the St Jude Lifetime Cohort Study. J Clin Oncol. 2018;36(27):2762–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ustun T. Measuring Health and Disability: Manual for Who Disability Assessment Schedule Whodas 2.0 . In. Geneva: World Health Organization; 2010. [Google Scholar]
- 38.Merchant TE, Zhu Y, Thompson SJ, Sontag MR, Heideman RL, Kun LE. Preliminary results from a Phase II trail of conformal radiation therapy for pediatric patients with localised low-grade astrocytoma and ependymoma. Int J Radiat Oncol Biol Phys. 2002;52(2):325–332. [DOI] [PubMed] [Google Scholar]
- 39.Peters S, Merta J, Schmidt L, et al. Evaluation of dose, volume, and outcome in children with localized, intracranial ependymoma treated with proton therapy within the prospective KiProReg Study. Neuro Oncol. 2022;24(7):1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Federal Highway Administration, Highway Statistics 2018. Washington, DC: Department of Transportation (US);2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the published article.


