Abstract
The open reading frame yqgR (now termed glcK), which had been sequenced as part of the genome project, encodes a glucose kinase of Bacillus subtilis. A 1.1-kb DNA fragment containing glcK complemented an Escherichia coli strain deficient in glucose kinase activity. Insertional mutagenesis of glcK resulted in a complete inactivation of glucose kinase activity in crude protein extracts, indicating that B. subtilis contains one major glucose kinase. The glcK gene encodes a 321-residue protein with a molecular mass of 33.5 kDa. The glucose kinase was overexpressed as a fusion protein to a six-His affinity tag and purified to homogeneity. The enzyme had Km values for ATP and glucose of 0.77 and 0.24 mM, respectively, and a Vmax of 93 μmol min−1 mg−1. A B. subtilis strain deficient for glucose kinase grew at the same rate on different carbon sources tested, including disaccharides such as maltose, trehalose, and sucrose.
Glucose utilization in Bacillus subtilis is dependent on the uptake of the sugar by the glucose phosphoenolpyruvate-dependent phosphotransferase system (PTS), resulting in cytoplasmic glucose-6-phosphate. One would expect that glucose kinase is not necessary for glucose utilization. However, unphosphorylated glucose may accumulate in the cytoplasm from disaccharide hydrolysis, as we have previously proposed for the trehalose system. After transport by a specific trehalose PTS (15, 27), phosphorylated trehalose-6-phosphate is hydrolyzed into glucose-6-phosphate and glucose by the phospho-α-1,1-glucosidase TreA (12, 15). Recently, we found additional evidence that cytoplasmic unphosphorylated glucose can be obtained by maltose or isomaltose metabolism (28). Therefore, we have postulated the existence of a glucose kinase necessary for the phosphorylation of the glucose released after disaccharide hydrolysis (12). Previous studies provided evidence of a glucose kinase in B. subtilis (8, 9). For Escherichia coli, as well, the existence of internal glucose has been concluded (21), which may originate from UDP-glucose in analogy to the formation of free galactose from UDP-galactose (37) or by a sugar phosphate transferase (31).
Expression of glucose kinase.
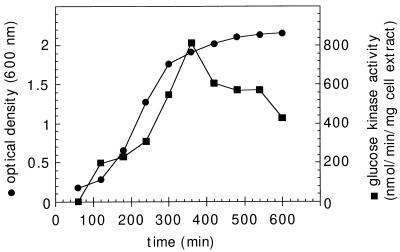
In order to investigate glucose kinase expression, the enzymatic activity of glucose kinase was determined in crude protein extracts from wild-type cells lysed by sonication with a Branson sonifier (8 times, 30 s each time, 40 W) at 4°C. Cell debris was eliminated by centrifugation, and protein concentrations of the resulting supernatants were determined by the method of Bradford (26). Specific glucose kinase activity was determined in a coupled enzyme assay by the method of Seno and Charter (30) (in a solution consisting of 50 mM Tris-HCl [pH 7.0], 20 mM glucose, 25 mM MgCl2, 0.5 mM NADP, 1 mM ATP, and 0.8 U of glucose-6-phosphate dehydrogenase). Glucose-6-phosphate dehydrogenase activity was assayed by monitoring the change in the optical density at 340 nm at 25°C with NADP as a cofactor. GlcK activity was analyzed in cells in different growth phases and in different media. As presented in Fig. 1, the activity reached a maximum at the end of the vegetative growth period. After the cells entered the stationary phase, the enzymatic activity slowly decreased. This latter phenomenon could be explained by protein instability or by reduced glcK expression when strains enter the stationary growth phase. This result suggests that the gene is under the control of the vegetative ςA-dependent promoter (14). An identical expression profile has been reported for the trehalose system (15). It has been suggested that glucose kinase contributes to disaccharide metabolism. Therefore, similar expression profiles can be expected.
FIG. 1.
Expression of glucose kinase activity during exponential growth and the early stationary phase. Wild-type B. subtilis cells were grown in Luria broth. The corresponding optical density at 600 nm of the culture is indicated. Aliquots were harvested at the indicated times, and glucose kinase activity was determined as described in the text and expressed in nanomoles of NADP reduced minute−1 milligram of protein−1 of cell extracts.
Glucose kinase activity has also been tested in cells grown in minimal medium C (22) containing several different sugars as the sole carbon source (20 mM each), such as glucose, fructose, trehalose, maltose, or sucrose. In all these cases, the glucose kinase expression profile resembles the profile shown in Fig. 1 (data not shown). This result can be interpreted as a constitutive expression of the type one would expect for housekeeping genes. Similar results were recently reported for the expression of glucokinase of E. coli (21). However, we cannot exclude the possibility that regulation is affected on the transcriptional or posttranscriptional level under conditions we have not tested so far.
Cloning of the glcK gene.
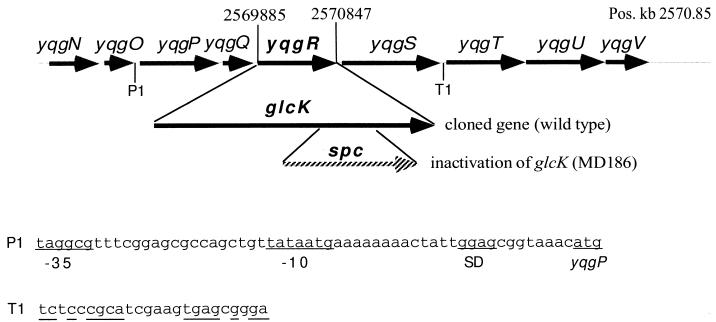
In a data bank search for potential open reading frames encoding a putative glucose kinase, one open reading frame (yqgR) from the B. subtilis genome sequencing project was identified (see Fig. 2) (17). The corresponding region from position 2569849 to 2570922 of the B. subtilis genome (17) was cloned by PCR amplification (23) using the following pair of oligonucleotides: 5′-CGGTCTAGATTTTTATAAAGGCTA and 5′-CCCAAGCTTCTCATTAACACATCAA, introducing a XbaI site 5′ to glcK and a HindIII site 3′ to glcK. The 1,073-bp DNA fragment thereby obtained was ligated behind the strong constitutive degQ36 promoter to mediate expression in E. coli (6). The resulting plasmid, pMD492, was able to complement an E. coli strain, UE26, (see Table 1) deficient for glucose kinase on MacConkey agar base (Difco) indicator plates (see Table 2) supplemented with 55 mM glucose and 1 mM fucose (1), which is supported by a detectable glucose kinase activity in crude protein extract (see Table 2). However, the presence of pMD492 in E. coli UE26 does not lead to higher enzymatic activity than that determined for the wild type.
FIG. 2.
Map of the glcK (yqgR) locus presenting the protein and RNA coding regions at kilobase position 2570.85 on the B. subtilis genome (17). The locations, orientations, and sizes of glcK and the flanking open reading frames (gray arrows) are indicated by arrows. The 1.1-kb DNA fragment containing the glcK gene whose nucleotide sequence has been determined is presented below. The insertion of a spc cassette between two NaeI restriction sites leading to inactivation of glcK in strain MD186 is depicted below the map. P1 and T1 indicate a potential promoter and terminator, respectively. The corresponding sequences are presented below, showing a potential ςA-dependent promoter with −35 and −10 regions in front of the ribosome binding site (Shine-Dalgarno sequence [SD]) of yqgP (start codon is underlined) and a palindrome of the suggested terminator T1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Reference or source |
|---|---|---|
| Strains | ||
| Bacillus subtilis | ||
| 168 | trpC2 (wild type) | BGSC,a 1A1 |
| MD186 | trpC2 glck::spc | This workb |
| Escherichia coli | ||
| JM101 | supE thi Δ(lac-proAB) F′ [traD36 proAB+ lacIqlacZΔM15] | 20 |
| RB791 | F′ [lacIqL8] hsdR+ hsdM | 4 |
| UE26 | F−ptsG2 ptsM1 glk7 rpsL150 | 3, 5 |
| Plasmids | ||
| pDG1726 | pSB119 derivative containing the spc antibiotic cassette | 13 |
| pMD402 | pUC18 derivative containing degS under degQ36 promoter control | 6 |
| pMD492 | pMD402 derivative containing glcK under degQ36 promoter control | This workb |
| pMD495 | pMD492 derivative carrying glcK::spc | This workb |
| pMD496 | pQE9 derivative carrying glcK fused in frame to the His tag-coding region | This workb |
| pQE9 | Expression vector for His tag fusions under T5 promoter control | 24 |
BGSC, Bacillus Genetic Stock Center, Ohio State University, Columbus, Ohio.
For detailed description of construction, see text.
TABLE 2.
Glucose kinase activity in E. coli and B. subtilis with glucose kinase or deficient for glucose kinase activity
| Strain | Appearance on glucose MacConkey agar supplemented with fucose | Glucose kinase activity in cell extracts (nmol of NADP reduced min−1 mg of protein−1)a |
|---|---|---|
| E. coli JM101 | Red | 245 |
| E. coli UE26 | White | 15 |
| E. coli UE26/pUC18 | White | 18 |
| E. coli UE26/pMD492 | Red | 293 |
| E. coli UE26/pMD495 | White | 16 |
| B. subtilis 168 | NDb | 340 |
| B. subtilis MD186 | ND | –c |
Cells were harvested at an optical density at 600 nm of 0.8.
ND, not determined.
–, no detectable glucose kinase activity was observed.
Nucleotide sequence analysis of glcK and protein sequence comparison.
DNA sequences were determined by the cycle sequencing technique and ABI PRISM 310 genetic analyzer following the recommendations of the manufacturer (Perkin-Elmer Corp., Foster City, Calif.). We used primers 3′ and 5′ from the insertion (primers 1201 and 1211; New England Biolabs, Beverly, Mass.) and two internal glcK primers 5′-CATAATCAACCACCTCAAGGGC and 5′-CCATCTGGAGACAGAAACCG. The sequences overlap each other and covered the entire cloned DNA fragment of pMD492. The nucleotide sequence showed that glcK is identical to yqgR (17) with one exception, a nucleotide change from T to G at position 2570339, which does not alter the amino acid sequence (position numbers according to the genome sequencing project [17]). Sequence analysis revealed one complete open reading frame of 963 bp, starting with an ATG initiation codon at nucleotide position 2569885 and ending with a TAA codon at position 2570847 (Fig. 2). The ATG codon is predicted at an appropriate distance by the putative ribosome binding site AAGG (35).
As discussed above, the expression profile of glucose kinase indicates that transcription is under ςA promoter control. Therefore, we looked for potential ςA-dependent promoters upstream of glcK. The closest upstream sequence similar to ςA promoters found (marked P1 in Fig. 2), which fits the −10 region and matches the −35 region of the ςA consensus sequence (14) but has three changes is located 26 bp upstream of the suggested yqgP start codon. A similar search was carried out for rho-independent terminators. As depicted in Fig. 2, a putative terminator (T1), based on sequence analysis prediction as previously described (17), is located downstream of the yqgS open reading frame. These data obtained from sequence analysis suggest that glcK may be located in an operon with two upstream open reading frames (yqgP and yqgQ) and one downstream (yqgS) open reading frame. Further studies must be carried out to prove the potential organization of a glucose kinase operon.
In the B. subtilis genome sequencing project, the putative yqgR gene was classified as a transcriptional repressor of xylose-utilizing enzymes, since it showed a homology score of 26.5% identity for 298 amino acids to the NagC protein of E. coli (17). Indeed, the glucose kinase belongs to the so-called ROK protein family (regulators, open reading frames, and kinases) to which NagC, xylose repressors from several bacilli, and glucose kinases from several organisms belong (33). However, the highest scores of amino acid identities have been found for glucose kinases (data not shown). Furthermore, the sequence analysis revealed no apparent α-helix–turn–α-helix motif for DNA binding in GlcK. Glucose kinases contain a typical AIDLGGT motif, which has been identified as an ATP binding site (2). A remarkable similarity in the nucleotide binding core has been reported by Flaherty et al. (7) for yeast hexokinase and the ATP binding regions of 70-kDa heat shock proteins. This N-terminal ATP-binding motif is highly conserved in glucose kinases and hexokinases (29) and is also present in GlcK of B. subtilis. All in all, the sequence analysis obtained strong evidence that GlcK is the glucose kinase.
Inactivation of glcK.
To identify the glcK gene product and to demonstrate the physiological role of GlcK in B. subtilis, the glcK gene was inactivated on the chromosome. For that purpose, a 432-bp NaeI DNA fragment of plasmid pMD492 was exchanged for a 1,158-bp HincII-EcoRV DNA fragment of plasmid pDG1726 (13), carrying the spc gene encoding spectinomycin resistance (Table 1 and Fig. 2). Recombinants were selected in E. coli on Luria broth plates supplemented with ampicillin (100 μg/ml) and spectinomycin (100 μg/ml). The construct pMD495 was verified by restriction analysis to determine the correct resulting size of the plasmid and the orientation of spc. This plasmid is no longer able to reconstitute the glucose kinase phenotype in E. coli UE26 (Table 2). Inactivation of the glucose kinase gene in B. subtilis was achieved by transformation of linearized plasmid pMD495 in wild-type B. subtilis (Table 1) by a one-step procedure (16), followed by selection on Luria broth supplemented with spectinomycin (100 μg/ml). The strain was verified by Southern blot hybridization, showing the correct insertion by homologous double-crossover recombination (data not shown). Analysis of the resulting strain MD186 (Table 1) for glucose kinase activity showed its complete loss (Table 2). The inactivation, together with the data from complementation with plasmid pMD492 in E. coli UE26, clearly identifies glcK as the gene coding for glucose kinase of B. subtilis.
In a glkA mutant of Staphylococcus xylosus (glkA is the homolog of glcK of B. subtilis), 75% of the wild-type level of glucose kinase activity persists (36). Therefore, GlkA obviously constitutes only a minor glucose kinase in S. xylosus. In B. subtilis, glcK::spc mutants exhibit no significant additional glucose kinase activity, indicating that during vegetative growth B. subtilis contains only one major glucose kinase.
Overproduction and purification of glucose kinase.
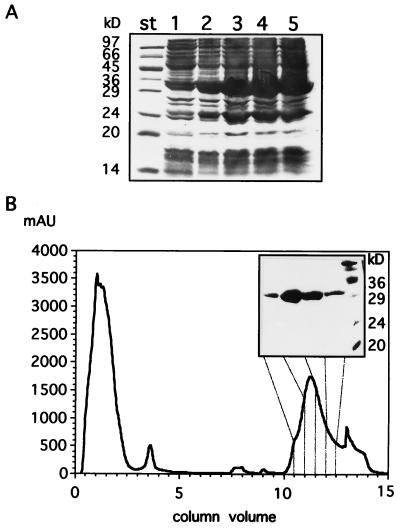
Overproduction of glucose kinase was achieved by constructing plasmid pMD496 (Table 1). To construct pMD496, the glcK gene was amplified by PCR, using plasmid pMD492 as a template and the following pair of oligonucleotides: 5′-CGCGGATCCATGGACGAGATATGGTTTGCG and 5′-CCCAAGCTTCTCATTAACACATCAA, introducing a BamHI site 5′ to glcK and a HindIII site 3′ to glcK. The resulting 1,037-bp DNA fragment was ligated to the BamHI and HindIII cloning sites of plasmid pQE9 (24), yielding plasmid pMD496. In this plasmid glcK is fused N terminally in frame to the six-His tag-coding region of plasmid pQE9, leading to expression under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible T5 promoter in E. coli RB791 (4, 24). The plasmid-encoded protein has a 12-residue N-terminal extension including the affinity tag (underlined): Met-Arg-Gly-Ser-His-His-His-His-His-His-Gly-Ser-Met… Overproduction of glucose kinase was achieved in E. coli RB791/pMD496 after IPTG (1 mM) induction in Luria broth medium (containing 100 μg of ampicillin per ml) for at least 1 h, as shown by an intense protein band migrating at the expected molecular mass of 34 kDa on a sodium dodecyl sulfate–12% polyacrylamide gel (SDS-12%PAG) (18) (Fig. 3A). For protein purification, the cells were harvested after 4 h of induction with IPTG. After sonication of the cells and centrifugation, overproduced GlcK was present in the supernatant (data not shown). GlcK was purified to homogeneity in a Ni2+ affinity gel chromatography (24), using the ÄKTA purifier system (Fig. 3B). During protein purification, we analyzed the total and specific GlcK activity from each overexpression and purification step of GlcK protein (data not shown). The enrichment of GlcK activity was calculated to be 75-fold. A 100-ml culture yielded about 5 mg of pure protein.
FIG. 3.
Overproduction and purification of glucose kinase. (A) Glucose kinase was overproduced as described in the text and analyzed on an SDS-12%PAG. Aliquots were taken from uninduced cells (lane 1) and IPTG-induced cells after 1 h (lane 2), 2 h (lane 3), 3 h (lane 4), 4 h (lane 5). The positions of molecular mass standards (st) are indicated to the left of the gel. (B) Purification of glucose kinase was performed by loading a Ni2+ HiTrap chelating column with crude cell extracts from harvested cells after 4 h of IPTG induction as depicted in panel A. The elution profile in panel B was monitored as presented by the protein absorption at 280 nm (in milliabsorption units) correlated with the column volume (1 ml). (Insert) Four selected fractions from the elution fractions as indicated by the gray lines are presented on an SDS-12%PAG, showing purification of GlcK. Molecular size standards (rightmost lane) with the indicated masses (in kilodaltons) are indicated to the right of the inserted gel.
Properties and kinetic parameters of glucose kinase.
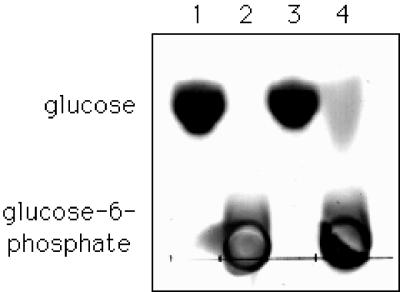
The standard glucose kinase activity assay with 3 μg of purified GlcK per ml was used to test enzyme properties and kinetic constants. Without ATP, no significant glucose kinase activity can be observed (data not shown). Replacement of ATP by ADP also revealed no detectable activity. As mentioned before, GlcK contains an N-terminal, conserved typical AIDLGGT motif, which has been identified as an ATP binding site (2). We analyzed the ability of purified GlcK to bind ATP by native gel electrophoresis with vertical gel slabs containing 12% acrylamide. Prior loading, glucose kinase and 10 μCi of [α-32P]ATP diluted with unlabelled ATP to yield a final concentration of 2.5 μM were incubated in 50 mM Tris-HCl (pH 7.5)–10 mM MgCl2 for 10 min at 20°C. The quantification of an autoradiogram after native gel electrophoresis in a phosphorimager showed that ATP binding depends linearly on the amount of GlcK in the reaction mixture (data not shown). In order to distinguish whether GlcK is a hexokinase or a specific glucose kinase, we tested its ability to phosphorylate several hexoses. Purified glucose kinase (10 μl; 0.5 mg/ml) in a buffer containing 50 mM Tris-HCl (pH 7.5) and 10 mM MgCl2, supplemented with a 50 mM concentration of either glucose, fructose, galactose, or mannose, in the absence or presence of 50 mM ATP, was incubated at 30°C for 20 min. The same reaction mixture without protein served as a control. The resulting products were analyzed on silica-coated thin-layer chromatography plates (Merck, Darmstadt, Germany), developed, and visualized as described previously (21). As shown in Fig. 4, GlcK phosphorylates glucose in the presence of ATP, whereas no phosphorylation of fructose, galactose, or mannose could be detected under the same conditions (data not shown). Thus, all these data together clearly identify GlcK as an ATP-dependent glucose kinase. Since we could demonstrate that GlcK is the B. subtilis glucose kinase, we determined the Michaelis-Menten kinetic parameters in a glucose kinase assay (30). GlcK had Km values for ATP and glucose of 0.77 and 0.24 mM, respectively, and a Vmax of 93 μmol min−1 mg−1.
FIG. 4.
Phosphorylation of glucose by glucose kinase GlcK. Ten-microliter samples of each reaction mixture were spotted onto thin-layer chromatography plates (see text). In addition to the buffer contents, the mixtures contain glucose (lane 1), glucose-6-phosphate (lane 2), glucose and glucose kinase (lane 3), and glucose, ATP, and glucose kinase (lane 4).
Several parameters which influence the enzymatic activity of glucose kinase have been tested. In order to determine the optimal reaction temperature, the assay has been carried out at various temperatures ranging from 15 to 42°C. The enzyme has an optimal reaction temperature of 32°C, leading to an about twofold-higher activity compared to the standard procedure at room temperature. For determination of temperature stability, glucose kinase was preincubated for 20 min at various temperatures ranging from 20 to 70°C, followed by a standard glucose kinase reaction assay at 25°C. Heat treatment for 20 min showed half-maximal activity at 52°C and that the enzyme is stable up to 40°C. No detectable enzymatic activity has been observed at temperatures exceeding 60°C.
Metabolic function of the glucose kinase.
The B. subtilis glcK mutant MD186 (Table 1 and Fig. 2) was tested for its ability to grow on glucose and fructose and the disaccharides maltose, sucrose, and trehalose, respectively, as the sole carbon source in comparison to the wild type. Surprisingly, this strain showed no significant effect on the generation times, although it has defective glucose kinase (data not shown). If glucose kinase participates in disaccharide metabolism, another mechanism(s) must be postulated to compensate for the glucose kinase-minus mutation. It has been suggested that glucose can enter the pentose shunt via gluconate and phosphorylation to gluconate-6-phosphate (8). In that case, a functional glucose kinase is not necessary for metabolism of cytoplasmic unphosphorylated glucose. This reaction could, in principle, be achieved by the glucose dehydrogenase encoded by gdh (19). However, glucose dehydrogenase is produced specifically during sporulation after the forespore has been formed (10), and no enzyme activity or cross-reacting material to antiserum against purified glucose dehydrogenase was detected during vegetative growth (34). Therefore, the possibility that glucose dehydrogenase plays a role in disaccharide utilization, especially during vegetative growth, can be excluded. A second possibility is based on the observation that glucose in its unphosphorylated form is exported in E. coli (25). If this were also the case in B. subtilis, glucose could reenter the cell by the glucose PTS.
The question of whether GlcK also plays a direct metabolic role in glucose utilization cannot be answered at present. There is evidence of an additional glucose utilization system besides the glucose PTS. A glucose enzyme II (ptsG)-minus strain of B. subtilis is still able to grow on glucose as the sole carbon and energy source but with 1.8 to 2.5-fold-reduced generation times, depending on the constructs used (11). A ptsGHI mutation causing defective enzyme IIGlc, HPr, and enzyme I completely abolishes growth on glucose (11). This has been interpreted as an indication that B. subtilis contains an additional glucose uptake system, which is also a PTS or is regulated via the PTS. The existence of two glucose transport systems has been described for the related organism Bacillus licheniformis (32): one is a PTS, and the other operates by an alternative mechanism. If such an alternative glucose uptake system is also present in B. subtilis, a glucose kinase might play a metabolic role.
Acknowledgments
We thank U. Ehmann for her interest in this work and for many helpful suggestions and K. Oliva for editing the manuscript. This work was carried out in the laboratories of W. Hillen, whose support is greatly appreciated.
Financial support was obtained from the Deutsche Forschungsgemeinschaft (Da248/2-2 and Da248/5-2), SFB473, and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Angell S, Schwarz E, Bibb M J. The glucose kinase of Streptomyces coelicolor A3(2): its nucleotide sequence, transcriptional analysis and role in glucose repression. Mol Microbiol. 1992;6:2833–2844. doi: 10.1111/j.1365-2958.1992.tb01463.x. [DOI] [PubMed] [Google Scholar]
- 2.Arora K K, Shenbagamurhi P, Fanciulli M, Pedersen P L. Glucose phosphorylation. J Biol Chem. 1990;265:5324–5328. [PubMed] [Google Scholar]
- 3.Boos W, Ehmann U, Forkl H, Klein W, Rimmele M, Postma P. Trehalose transport and metabolism in Escherichia coli. J Bacteriol. 1990;172:3450–3461. doi: 10.1128/jb.172.6.3450-3461.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brent R, Ptashne M. Mechanism of action of the lexA gene product. Proc Natl Acad Sci USA. 1981;78:4202–4208. doi: 10.1073/pnas.78.7.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis S J, Epstein W. Phosphorylation of d-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975;122:1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahl M K, Msadek T, Kunst K, Rapoport G. Mutational analysis of the Bacillus subtilis DegU regulator and its phosphorylation by the DegS protein kinase. J Bacteriol. 1991;173:2539–2547. doi: 10.1128/jb.173.8.2539-2547.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flaherty K M, DeLuca-Flaherty C, McKay D B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990;346:623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- 8.Fortnagel P. Glycolysis. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria; biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 171–180. [Google Scholar]
- 9.Freese E, Kofart W, Galliers E. Commitment to sporulation and induction of glucose-phosphoenolpyruvate transferase. Biochim Biophys Acta. 1970;222:265–289. doi: 10.1016/0304-4165(70)90115-7. [DOI] [PubMed] [Google Scholar]
- 10.Fujita Y R, Ramaley R, Freese E. Location and properties of glucose dehydrogenase in sporulating cells and spores of Bacillus subtilis. J Bacteriol. 1977;132:282–293. doi: 10.1128/jb.132.1.282-293.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzy-Tréboul G, de Waard J H, Zagorec M, Postma P W. The glucose permease of the phosphotransferase system of Bacillus subtilis: evidence for IIGlc and IIIGlc domains. Mol Microbiol. 1991;5:1241–1249. doi: 10.1111/j.1365-2958.1991.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 12.Gotsche S, Dahl M K. Purification and characterization of the phospho-α-1,1-glucosidase (TreA) of Bacillus subtilis. J Bacteriol. 1995;177:2721–2726. doi: 10.1128/jb.177.10.2721-2726.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guérout-Fleury A-M, Shazand K, Frandsen N, Stragier P. Antibiotic-resistance cassettes for Bacillus subtilis. Gene. 1995;167:335–336. doi: 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- 14.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helfert C, Gotsche S, Dahl M K. Cleavage of trehalose-phosphate in Bacillus subtilis is catalyzed by a phospho-α-(1,1)-glucosidase encoded by the treA gene. Mol Microbiol. 1995;16:111–120. doi: 10.1111/j.1365-2958.1995.tb02396.x. [DOI] [PubMed] [Google Scholar]
- 16.Kunst F, Msadek T, Rapoport G. Signal transduction network controlling degradative enzyme synthesis and competence in Bacillus subtilis. In: Piggot P J, Moran C P Jr, Youngman P, editors. Regulation of bacterial differentiation. Washington, D.C: American Society for Microbiology; 1994. pp. 1–20. [Google Scholar]
- 17.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Lampel K A, Uratani B, Chaudhry G R, Ramaley R F, Rudikoff S. Characterization of the developmentally regulated Bacillus subtilis glucose dehydrogenase gene. J Bacteriol. 1986;166:238–243. doi: 10.1128/jb.166.1.238-243.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messing J. A multipurpose cloning system based on single-stranded DNA bacteriophage M13. Recomb DNA Tech Bull. 1979;2:43–45. [Google Scholar]
- 21.Meyer D, Schneider-Fresenius C, Horlacher R, Peist R, Boos W. Molecular characterization of glucokinase from Escherichia coli K-12. J Bacteriol. 1997;179:1298–1306. doi: 10.1128/jb.179.4.1298-1306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Msadek T, Kunst F, Henner D, Klier A, Rapoport G, Dedonder R. Signal transduction pathway controlling synthesis of a class of degradative enzymes in Bacillus subtilis: expression of the regulatory genes and analysis of mutations in degS and degU. J Bacteriol. 1990;172:824–834. doi: 10.1128/jb.172.2.824-834.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullis K B, Faloona F A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 24.Qiagen. The QIAexpressionist. 3rd ed. Hilden, Germany: Qiagen Inc.; 1997. [Google Scholar]
- 25.Rimmele M, Boos W. Trehalose-6-phosphate hydrolase of Escherichia coli. J Bacteriol. 1994;176:5654–5664. doi: 10.1128/jb.176.18.5654-5664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez R L, Tait R C. Recombinant DNA techniques: an introduction. Reading, Mass: Addison-Wesley Publishing Co.; 1983. [Google Scholar]
- 27.Schöck F, Dahl M K. Analysis of DNA flanking the treA gene of Bacillus subtilis reveals genes encoding a putative specific enzyme IITre and a potential regulator of the trehalose operon. Gene. 1996;175:59–63. doi: 10.1016/0378-1119(96)00120-5. [DOI] [PubMed] [Google Scholar]
- 28.Schönert S, Buder T, Dahl M K. Identification and enzymatic characterization of the maltose-inducible α-glucosidase MalL (sucrase-isomaltase-maltase) of Bacillus subtilis. J Bacteriol. 1998;180:2574–2578. doi: 10.1128/jb.180.9.2574-2578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwab D A, Wilson J E. Complete amino acid sequence of the type III isozyme of rat hexokinase, deduced from the cloned cDNA. Arch Biochem Biophys. 1991;285:365–370. doi: 10.1016/0003-9861(91)90373-q. [DOI] [PubMed] [Google Scholar]
- 30.Seno E T, Charter K F. Glycerol catabolic enzymes and their regulation in wild type and mutant strains of Streptomyces coelicolor A3(2) J Gen Microbiol. 1983;129:1403–1413. doi: 10.1099/00221287-129-5-1403. [DOI] [PubMed] [Google Scholar]
- 31.Stevens-Clark J S, Theisen M C, Conklin K A, Smith R A. Phosphoramidates. VI. Purification and characterization of a phosphoryl transfer enzyme from Escherichia coli. J Biol Chem. 1968;243:4468–4473. [PubMed] [Google Scholar]
- 32.Tangney M, Priest F G, Mitchell W J. Two glucose transport systems in Bacillus licheniformis. J Bacteriol. 1993;175:2137–2142. doi: 10.1128/jb.175.7.2137-2142.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Titgemeyer F, Reizer J, Reizer A, Saier J M H. Evolutionary relationships between sugar kinases and transcriptional repressors in bacteria. Microbiology. 1994;140:2349–2354. doi: 10.1099/13500872-140-9-2349. [DOI] [PubMed] [Google Scholar]
- 34.Vasantha N, Uratani B, Ramalay R, Freese E. Isolation of a developmental gene of Bacillus subtilis and its expression in Escherichia coli. Proc Natl Acad Sci USA. 1983;80:785–789. doi: 10.1073/pnas.80.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vellanoweth R L. Translation and its regulation. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 699–711. [Google Scholar]
- 36.Wagner E, Marcandier S, Egeter O, Deutscher J, Götz F, Brückner R. Glucose kinase-dependent catabolite repression in Staphylococcus xylosus. J Bacteriol. 1995;177:6144–6152. doi: 10.1128/jb.177.21.6144-6152.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H C P. Role of galactose transport system in the establishment of endogenous induction of the galactose operon in Escherichia coli. J Mol Biol. 1967;24:213–223. doi: 10.1016/0022-2836(67)90327-0. [DOI] [PubMed] [Google Scholar]