Abstract
Background:
Odour discrimination and identification (DI) are markers associated with disability worsening and neuroaxonal damage in multiple sclerosis (MS).
Objective:
The main objective of this research is to investigate whether longitudinal change of DI predicts long-term MS disease course.
Methods:
This is a 6-year prospective longitudinal study on MS patients at the MS Clinic Innsbruck. Clinical, bi-annual visits assessed patients’ history and Expanded Disability Status Scale (EDSS) score. DI and cognitive function were assessed at baseline (BL), Year 1 (Y1), Year 2 (Y2) and Year 6 (Y6) by the ‘Sniffin’ Sticks’/Symbol Digit Modalities Test.
Results:
Around 92 of 139 patients were available for Y6 follow-up. Mean DI scores significantly decreased over time (BL = 27.8, Y1 = 27.5, Y2 = 26.3 and Y6 = 26.3; p < 0.001) and negatively correlated with patients’ age (rs = −0.120, p = 0.032) and disease duration (rs = −0.103, p = 0.041). Multivariable regression analyses revealed that lower absolute DI scores and larger DI score loss over time were associated with higher probability of EDSS worsening (per −1 point: hazard ratio (HR) = 1.40 (1.16–1.68) and 2.34 (1.27–4.21)), progression independent of relapse activity (PIRA) (HR = 1.49 (1.20–1.85) and 2.22 (1.33–3.31)) and cognitive deterioration (HR = 1.75 (1.35–2.27) and 4.29 (1.26–2.84)) at Y6, but not with time to first relapse.
Conclusion:
Odour DI is an irreversible marker of neuroaxonal damage, associated with PIRA, cognitive deterioration and EDSS worsening.
Keywords: Multiple sclerosis, biomarker, odour discrimination, identification, disability, cognition, PIRA
Introduction
Odour perception is a complex neurobiological process in which various neuroanatomical structures are involved. 1 Olfactory impairment, a common symptom in different neurological diseases like Alzheimer’s disease, Parkinson’s disease but also multiple sclerosis (MS),2–4 can occur at different stages of the olfactory perception. Generally, the impairment of peripheral functions, so-called ‘cable functions’, are distinguished from impairment of the more complex cortical functions, termed ‘processing functions’. 5 In short, the former include all stages of transmission of odour information, initiated by odour molecules inducing action potentials in the bipolar receptor cells located in the olfactory fila, 6 which project directly to the primary olfactory cortex. 1 The latter subsume encoding, valuating and assessing odours, where different central sites such as the piriform cortex, the amygdala or the orbitofrontal cortex are involved.1,7,8 Interestingly, these two domains are affected by different pathological processes, namely inflammation and neuroaxonal degeneration.9–13 The wide-spread concept of MS pathophysiology assumes a predominance of acute demyelinating inflammation in early disease stages giving way to a more microcompartimentalized inflammation resembling ‘neurodegeneration’ in later stages,14,15 although this concept is evolving towards a continuum with (often subclinical) neurodegeneration already present in earliest stages.16–18 Against this backdrop, it is of particular interest that impairment of aforementioned ‘processing functions’, that is, identifying and discriminating odours, is associated with severity of physical and cognitive disability as well as paraclinical markers of neuroaxonal damage, such as brain atrophy and retinal layer thinning,5,9,19–21 but not with the occurrence of relapses. Thus, odour discrimination and identification (DI) have been suggested as a biomarker of neuroaxonal damage in MS.5,9,20
To date, there is no long-term (up to 6 years) observational data on olfactory DI in MS patients.
Methods
Patients and definitions
We included 92 patients with relapsing MS (RMS) diagnosed according to the 2010 McDonald criteria aged between 18 and 65 years from a prospective observational study initiated in 2013. 21 Clinical study visits were conducted at least biannually for a minimum follow-up period of 6 years. Demographic data, neurological and treatment history including disease-modifying treatment (DMT) were obtained from each participant at every visit. Cognitive function was assessed at baseline (BL), Year 1 (Y1), Year 2 (Y2) and Year 6 (Y6) by the Symbol Digit Modalities Test (SDMT).
For the primary endpoint, time to first relapse was used. Secondary endpoints were defined as follows: time to Expanded Disability Status Scale (EDSS) worsening, time to cognitive deterioration and time to progression independent of relapse (PIRA). EDSS worsening was defined as a confirmed EDSS increase of ⩾1.5 points in patients with a BL score of 0, ⩾1.0 point in patients with a BL score of 1.0–5.5, or an increase of ⩾0.5 points in patients with a BL score of >5.5 sustained for at least 24 weeks as compared to BL. 22 Cognitive deterioration, assessed by the SDMT, was defined as a loss of ⩾4 points or a ⩾10% decrease in SDMT score as compared to BL. 23 PIRA was defined as either an EDSS worsening or cognitive deterioration during the observation period confirmed after 24 weeks with no relapse in the 30 days before or after the EDSS/SDMT worsening. 22
DMT was grouped as following: (1) ‘no DMT’ (N-DMT) defined as patients receiving no DMT at least 6 months prior to BL visit and during the whole observation period, (2) ‘moderate effective DMT’ (M-DMT) defined as patients receiving one or more DMT of either interferon beta preparations, glatiramer acetate, dimethylfumarate, or teriflunomide during the whole observation period, (3) ‘highly effective DMT’ (H-DMT) defined as patients receiving one or more DMT of either alemtuzumab, natalizumab, fingolimod/siponimod/ozanimod/ponesimod, ocrelizumab/ofatumumab/rituximab or cladribine during the whole observation period and (4) ‘ESC-DMT’ defined as patients in whom DMT was escalated either from no DMT to moderate effective DMT or from moderate effective DMT to highly effective DMT during the observation period.
Odour DI
Odour DI were assessed at BL and after 1 (Y1), 2 (Y2), and 6 (Y6) years using the respective subscores of the extended version of the Sniffin’ Sticks test (Burghart Medizintechnik, Wedel, Germany) according to the manufacturer’s instruction including change of testing sticks every 6 months.
The Sniffin’ Sticks is based on pen-like odour-dispensing devices. For testing odour discrimination, the subject is presented with 16 sets of three different pens in a randomized order and asked to discriminate between two pens containing the same odorant and one containing a different one. For testing odour identification, the ability of the subject to identify an odorant is assessed by testing 16 different odours from a single pen by a forced choice from four options. The maximum score for each test is 16 points and reflects optimal olfactory function. The two subscores were summed in a composite score (DI score), which provides better correlation to clinical variables than each subscore alone. 5 The maximum DI score is 32 points and reflects optimal odour DI, while lower scores are associated with impaired ability to identify and discriminate odours. The normative values are based on data from 3000 healthy subjects. 24 Olfactory testing was postponed for 4 weeks, if the patient had received corticosteroids within 4 weeks or if upper respiratory tract infections were present at the time of assessment.
Ethics
The study was approved by the ethics committee of the Medical University Innsbruck (approval number: AM3743-281/4.3), and all participants gave written informed consent before inclusion.
Statistics
Statistical analysis was performed using SPSS 26.0 (SPSS Inc, Chicago, IL, USA) and R-Statistical Software (Version 4.0.0). Univariate comparisons were done by chi-square-test, Mann–Whitney U test or independent t-test (with Welch’s correction in case of unequal variances between the groups) as appropriate. Correlation analyses were done by Spearman’s correlation coefficient (rs).
To investigate associations of change in DI score over the respective observation periods with occurrence of relapse/EDSS worsening/SDMT deterioration/PIRA during the observation period, mixed-effects linear regression analyses were used adjusting for age, sex, disease duration, EDSS, SDMT at BL and DMT status. Herein, the change of DI scores over time was used as dependent variable, while occurrence of relapse/EDSS worsening/SDMT deterioration/PIRA was used as independent one. To investigate the potential of DI scores as a predictor of clinical events, multivariable Cox regression analyses were run using relapse/EDSS worsening/SDMT deterioration/PIRA as the dependent variable and DI scores as a time-dependent covariate adjusting for age, sex, disease duration, EDSS, SDMT at BL, and DMT status as a time-varying covariate. 25 Missing values were handled by multiple (20 times) imputation using the missing not at random (MNAR) approach according to Rubin’s rules. 26 A two-sided p value < 0.05 was considered statistically significant.
Data availability statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the ethics committee of the Medical University Vienna.
Results
Of 139 MS patients included in the original cohort, 21 92 (66%) patients had follow-up of at least 6 (median: 6.2–7.5) years; 47 patients were lost to follow-up (40 without giving a reason, 3 refused olfactory follow-up testing, 4 declined to have onsite visits due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemia). Demographics and BL clinical characteristics of the study cohort are given in Table 1. Patients lost to follow-up did not significantly differ in any of the BL characteristics from the study cohort.
Table 1.
Demographic and baseline clinical characteristics.
| Number of patients | 92 |
|---|---|
| Females a | 74 (80.4) |
| Age b at baseline (years) | 35.7 (9.1) |
| MS disease duration c (years) | 5.3 (0.1–35.8) |
| EDSS at baseline c | 1.5 (0–6.0) |
| SDMT b | 53.7 (9.5) |
| DMT at baseline c | |
| No DMT | 35 (38.0) |
| Moderately effective | 43 (46.8) |
| Interferon beta preparations | 30 (32.6) |
| Glatiramer acetate | 13 (14.1) |
| Highly effective | 14 (15.2) |
| Natalizumab | 7 (7.6) |
| Fingolimod | 7 (7.6) |
MS: multiple sclerosis; DMT: disease-modifying therapy; EDSS: Expanded Disability Status Scale; SDMT: Symbol Digit Modalities Test.
Number (percentage).
Mean and standard deviation.
Median and range.
At last follow-up, an EDSS worsening had occurred in 49 (52.1%) patients after a mean of 3.1 years (SD 1.9), while cognitive deterioration was found in 38 (40.4%) after 2.6 years (SD 2.1) and PIRA in 27 (28.7%) after 3.4 years (SD 2.1). Sixty-three (67.0%) patients suffered relapse after a mean of 2.0 years (SD 1.7).
Mean DI score at BL was 27.8 points (SD 2.8) and significantly decreased over time reaching 27.5 (SD 3.0) at Y1, 27.2 (SD 3.2) at Y2 and 26.3 (SD 3.1) at Y6 (p < 0.001). Patients’ age (rs = −0.120, p = 0.032), disease duration (rs = −0.103, p = 0.041), BL EDSS (rs = −0.210, p = 0.045) and BL SDMT (rs = −0.282, p = 0.006) negatively correlated with DI scores at BL, while sex did not.
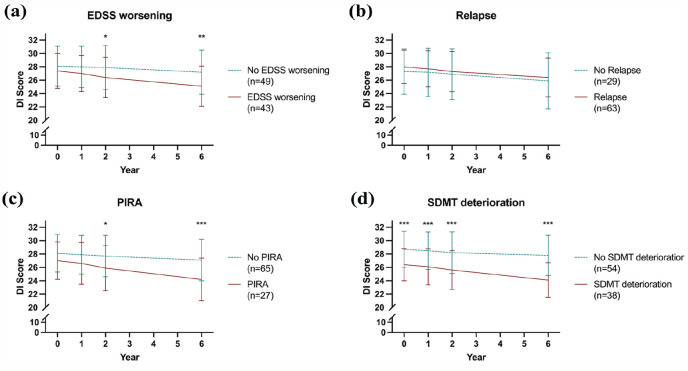
Patients suffering EDSS worsening during the observation period displayed significantly lower DI scores than patients without EDSS worsening at Y2 (26.4 vs 27.9, p = 0.027) and Y6 (25.1 vs 27.2, p = 0.002, Figure 1(a)), while there was no significant difference between patients with and without relapse (Figure 1(b)). Likewise, significantly lower DI scores were observed in patients with PIRA at Y2 (25.9 vs 27.7, p = 0.017) and Y6 (24.2 vs 27.1, p < 0.001, Figure 1(c)). Patients with SDMT deterioration showed significantly lower DI scores than those without already at BL (26.4 vs 28.7, p < 0.001) and at all following points of measurement (Y1: 26.1 vs 28.5, p < 0.001; Y2: 25.6 vs 28.2, p < 0.001; Y6: 24.1 vs 27.8, p < 0.001, Figure 1(d)).
Figure 1.
Longitudinal changes of DI scores according to primary and secondary outcomes.
DI: odour discrimination and identification; EDSS: Expanded Disability Status Scale; PIRA: progression independent of relapse activity; SDMT: Symbol Digit Modalities Test.
*p < 0.05, **p < 0.01, ***p < 0.001.
Decrease of DI scores over time (i.e. between BL and Y1, Y1 and Y2, Y2 and Y6) was larger in patients with EDSS worsening (−0.4 vs −0.1, p = 0.015; −0.7 vs −0.1, p < 0.001; and −1.1 vs −0.6, p < 0.001, respectively), PIRA (−0.4 vs −0.1, p = 0.010; −0.7 vs −0.2, p < 0.001; and −1.7 vs −0.6, p < 0.001) and SDMT deterioration (−0.4 vs −0.1, p = 0.012; −0.5 vs −0.3, p = 0.044; and −1.6 vs −0.5, p = 0.001), than in those without. In addition, mean decrease of DI scores over the entire follow-up period of 6 years was higher in patients with EDSS worsening (−1.2 vs −0.5, p < 0.001), PIRA (−1.4 vs −0.6, p < 0.001) and SDMT deterioration (−1.2 vs −0.5, p < 0.001), than in those without. DI scores and their changes did not differ in patients with or without relapses after BL. Detailed results of univariate analyses are displayed in Supplemental Table 1.
Mixed-effects linear regression models confirmed the associations between stronger DI score decrease during follow-up and EDSS worsening (−0.9, p < 0.001), PIRA (−1.1, p < 0.001), and SDMT deterioration (−1.0, p < 0.001), while relapse was again not associated with change of DI score (−0.2, p = 0.237).
Looking at DI scores as a predictor of clinical events, multivariable Cox regression models showed that lower DI scores at BL were independently associated with an increased risk of EDSS worsening (hazard ratio (HR) 1.19 per 1 point lower DI score, 95% confidence interval (CI) 1.01–1.39, p = 0.036), PIRA (HR 1.23, CI 1.01–1.50, p = 0.040) and cognitive deterioration (HR 1.44, CI 1.17–1.76, p < 0.001), but not with risk of relapse (Table 2).
Table 2.
Multivariable analyses for odour discrimination and identification as predictor of clinical events.
| Relapse | EDSS worsening | PIRA | SDMT deterioration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR a | 95% CI | p value | HR a | 95% CI | p value | HR a | 95% CI | p value | HR a | 95% CI | p value | |
| DI baseline b | ||||||||||||
| Per 1 point | 0.98 | 0.87–1.10 | 0.752 | 1.19 | 1.01–1.39 | 0.036 | 1.23 | 1.01–1.50 | 0.04 | 1.44 | 1.17–1.76 | <0.001 |
| DI loss over follow-up c | ||||||||||||
| Per 1 point | 1.21 | 0.83–1.25 | 0.792 | 2.32 | 1.25–4.25 | 0.025 | 2.46 | 1.31–3.28 | <0.001 | 4.11 | 1.26–2.73 | 0.004 |
| >1 Point | 1.15 | 0.64–2.14 | 0.731 | 2.56 | 1.05–5.69 | 0.048 | 2.41 | 1.43–3.82 | <0.001 | 4.71 | 1.68–13.6 | <0.001 |
| >2 Points | 0.9 | 0.49–1.72 | 0.698 | 3.51 | 1.43–6.31 | <0.001 | 3.02 | 1.67–5.39 | <0.001 | 6.72 | 1.89–24.2 | <0.001 |
| Adjusted for age, sex, disease duration, and DMT status | Adjusted for age, sex, disease duration, EDSS at baseline, and DMT status. | Adjusted for age, sex, disease duration, EDSS at baseline, and DMT status | Adjusting for age, sex, disease duration, SDMT at baseline, and DMT status | |||||||||
DMT: disease-modifying treatment; HR: hazard ratio; 95% CI: 95% confidence interval.
Values above/below 1 indicate higher/lower probability of relapse/EDSS worsening/PIRA/SDMT deterioration.
Calculated by multivariable Cox regression models using relapse/EDSS worsening/PIRA/SDMT deterioration as the dependent variable and baseline DI score as the independent variable.
Calculated by multivariable Cox regression models using relapse/EDSS worsening/PIRA/SDMT deterioration as the dependent variable and change in DI score as a time-dependent variable.
Bold faced values indicate p-value below the set level of statistical significance (<0.05).
Worsening of DI scores from BL at any timepoint during follow-up was also independently associated with a higher risk of EDSS worsening (HR 2.32 per 1 point decrease, CI 1.25–4.25, p = 0.025), PIRA (HR 2.46, CI 1.31–3.28, p < 0.001) and cognitive deterioration (HR 4.11, CI 1.26–2.37, p = 0.004), but again not with risk of relapse (Table 2).
Discussion
Distinct olfactory functions are impaired by different pathological processes contributing to the disability accumulation of MS patients. The rather complex ‘processing functions’ of discriminating and identifying odours are mainly affected by neuroaxonal degeneration.5,9 In this study, we provide long-term data of DI scores and clinical disease course of a prospective MS cohort, which substantiate this hypothesis.
First, the DI capability of MS patients is deteriorating in a slow, progressive, and irreversible manner, strongly resembling the course of neuroaxonal degeneration. Second, the degree and speed of this deterioration is associated with age and disease duration, but also with EDSS worsening, cognitive deterioration and PIRA. In contrast, we did not find any association between DI scores and relapse activity.
Deficits of olfactory function have been known as a common symptom in neurological diseases for a long time and were initially attributed primarily to classical neurodegenerative diseases such as Alzheimer’s disease or Parkinson’s disease.3,4,27 Alongside with an awakening research interest, however, olfactory dysfunction has been reported in various other neurological disorders, ranging from epilepsy, 28 over traumatic brain injury 29 to MS. 2 In MS research, early studies focused on prevalence of olfactory deficits in MS and the correlation to lesion load and location.2,30 More recently, it has been suggested that impairment of sub-domains of olfaction, namely olfactory threshold and DI, may be impaired by different pathophysiological processes, namely inflammation and degeneration.5,9,31 This may also be discussed in the context of the classical concept of MS pathology, of inflammatory processes dominating the early disease stages and degeneration dominating the later ones,14,15 which has recently been challenged by findings of inflammation being present until advanced disease stages and vice versa.16–18
The findings of our study are in line with the current literature and substantiate the role of olfactory DI as a marker of slow progression and disability accumulation. This disability accumulation is independent of relapse activity, meeting the criteria of PIRA 22 and includes both physical disability progression, indicated by EDSS worsening, as well as cognitive deterioration, measured by decreasing performance in SDMT testing. According to our data, the role of DI as a marker of neuroaxonal damage is constant over the entire follow-up time of 6 years and irreversible. Of note, the association of low DI scores with cognitive performance is manifest already at BL testing and getting more pronounced during follow-up testing. As both discriminating and identifying odours are complex cortical functions, DI scores could be seen as surrogate of cognitive testing. Furthermore, in our cohort, there was no association between the occurrence of relapses and DI impairment. This further substantiates that deficits in the different olfactory domains resemble the two pathological key drivers of MS inflammation and degeneration.5,9,31
Our data also demonstrate a negative correlation of age and disease duration with DI scores. Although decreasing olfactory ability has been reported repeatedly with increasing age,24,32 we believe that in our cohort, this decrease is mainly driven by accumulating axonal damage. Indeed, deficits in olfaction attributed to normal aging processes are classically more pronounced for olfactory threshold and manifest at a higher age. In healthy persons younger than 65 years, a relevant decrease of olfactory functions has been described in only 2%. Only thereafter, the proportion increases significantly. 32 However, the mean age of our cohort at BL is 35 years and therefore significantly lower. Hence, we attribute the worsening to neuroaxonal degeneration rather than to so-called ‘presbyosmia’, which probably occurs later.
Some limitations of this study must be acknowledged. Evidently, magnetic resonance imaging (MRI) data are lacking. Therefore, structural damages at any part of the complex olfactory system cannot be excluded for sure. Such structural damages, for instance caused by white matter lesions or other pathologies, could bias the findings of reduced DI scores. Furthermore, it has been reported that low DI scores are associated with brain atrophy of the olfactory system. 20 Due to missing MRI data, we cannot substantiate this finding, even though it is a promising direction for future studies. Inherent to the study design, there is some missing data, although these amount to less than 2% of clinical and olfactory data points. As prespecified in the study protocol, multiple imputation was used to account for missing data. To check for potential bias by multiple imputation, we conducted a sensitivity analyses including only patients with complete data points, which did not change any of the results significantly. In addition, our study represents a single-centre investigation of an MS cohort. Only a small number of investigators contributed to the acquisition of data. It has to be pointed out that this may have reduced a possible rater-dependent source of measurement errors, as well as the overall variability of DI scores. Nevertheless, in a real-world setting, a multi-centre approach, different test protocols and different investigators may contribute as potential source of bias.
In conclusion, with this study, we provide data on development of DI capability in MS patients over a long-term follow-up of 6 years. DI scores deteriorate in a slow, progressive, and irreversible manner, strongly resembling the course of neuroaxonal degeneration, where the degree and speed of this deterioration is associated with age and disease duration, but also with EDSS worsening, cognitive deterioration and PIRA.
Supplemental Material
Supplemental material, sj-docx-1-ilr-10.1177_13524585231201093 for Odour discrimination and identification as a biomarker of long-term disability worsening in multiple sclerosis by Klaus Berek, Harald Hegen, Michael Auer, Robert Barket, Franziska Di Pauli, Jakob Hocher, Nik Krajnc, Anne Zinganell, Florian Deisenhammer, Thomas Berger and Gabriel Bsteh in Multiple Sclerosis Journal
Footnotes
Author Contributions: Klaus Berek: patient recruitment, acquisition of data, statistical analysis and interpretation of data, drafting of manuscript.
Harald Hegen: study concept and design, patient recruitment, acquisition of data, statistical analysis and interpretation of data, drafting of manuscript.
Nik Krajnc: patient recruitment, acquisition of data, interpretation of data, critical revision of manuscript for intellectual content
Michael Auer: patient recruitment, acquisition of data, interpretation of data, critical revision of manuscript for intellectual content
Robert Barket: patient recruitment, acquisition of data, interpretation of data, critical revision of manuscript for intellectual content
Franziska Di Pauli: patient recruitment, acquisition of data, interpretation of data, critical revision of manuscript for intellectual content
Anne Zinganell: patient recruitment, acquisition of data, interpretation of data, critical revision of manuscript for intellectual content
Florian Deisenhammer: patient recruitment, acquisition of data, interpretation of data, critical revision of manuscript for intellectual content
Thomas Berger: patient recruitment, acquisition of data, interpretation of data, critical revision of manuscript for intellectual content
Gabriel Bsteh: study concept and design, patient recruitment, interpretation of data, statistical analysis and interpretation of data, critical revision of manuscript for intellectual content.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: K.B. has participated in meetings sponsored by and received travel funding or speaker honoraria from Roche, Teva, Merck, Biogen, Sanofi and Novartis. H.H. has participated in meetings sponsored by, received speaker honoraria or travel funding from Bayer, Biogen, Celgene, Janssen, Merck, Novartis, Sanofi-Genzyme, Siemens, Teva and received honoraria for acting as consultant for Biogen, Celgene, Novartis and Teva. He is associate editor of Frontiers in Neurology. M.A. received speaker honoraria and/or travel grants from Biogen, Merck, Novartis, Sanofi-Genzyme and Horizon Therapeutics. R.B. has participated in meetings sponsored by or received travel grants from, Novartis, Janssen-Cilag and Sanofi-Genzyme. Received honoraria from Janssen-Cilag.F.D.P. has participated in meetings sponsored by, received honoraria (lectures, advisory boards, consultations) or travel funding from Bayer, Biogen, Merck, Novartis, Sanofi-Genzyme, Teva, Celgene and Roche. N.K. has participated in meetings sponsored by, received speaker honoraria or travel funding from BMS/Celgene, Janssen-Cilag, Merck, Novartis, Roche and Sanofi-Genzyme and held a grant for a Multiple Sclerosis Clinical Training Fellowship Programme from the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). A.Z. has participated in meetings sponsored by, received speaking honoraria or travel funding from Biogen, Merck, Novartis, Sanofi-Genzyme and Teva. F.D. has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Alexion, Almirall, Biogen, Celgene, Merck, Novartis, Roche and Sanofi-Genzyme. His institution received scientific grants from Biogen and Sanofi-Genzyme.
T.B. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, Biologix, BMS/Celgene, Eisai, Janssen-Cilag, Jazz/GW, Horizon, MedDay, Merck, Novartis, Octapharma, Roche, Sandoz, Sanofi-Genzyme, UCB, Teva. His institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, BMS/Celgene, Merck, Novartis, Sanofi Aventis, Teva and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme, Teva. G.B.: has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene/BMS, Lilly, Merck, Novartis, Roche, Sanofi-Genzyme and Teva, and received honoraria for consulting Biogen, Celgene/BMS, Novartis, Roche, Sanofi-Genzyme and Teva. He has received unrestricted research grants from Celgene/BMS and Novartis.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Klaus Berek  https://orcid.org/0000-0003-2755-2043
https://orcid.org/0000-0003-2755-2043
Robert Barket  https://orcid.org/0000-0001-6909-6109
https://orcid.org/0000-0001-6909-6109
Nik Krajnc  https://orcid.org/0000-0002-4146-5870
https://orcid.org/0000-0002-4146-5870
Florian Deisenhammer  https://orcid.org/0000-0003-4541-8841
https://orcid.org/0000-0003-4541-8841
Thomas Berger  https://orcid.org/0000-0001-5626-1144
https://orcid.org/0000-0001-5626-1144
Gabriel Bsteh  https://orcid.org/0000-0002-0825-0851
https://orcid.org/0000-0002-0825-0851
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Klaus Berek, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Harald Hegen, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Michael Auer, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Robert Barket, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Franziska Di Pauli, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Jakob Hocher, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Nik Krajnc, Department of Neurology, Medical University of Vienna, Vienna, Austria; Comprehensive Center for Clinical Neurosciences & Mental Health, Medical University of Vienna, Vienna, Austria.
Anne Zinganell, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Florian Deisenhammer, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Thomas Berger, Department of Neurology, Medical University of Vienna, Vienna, Austria; Comprehensive Center for Clinical Neurosciences & Mental Health, Medical University of Vienna, Vienna, Austria.
Gabriel Bsteh, Department of Neurology, Medical University of Vienna, Vienna, Austria; Comprehensive Center for Clinical Neurosciences & Mental Health, Medical University of Vienna, Vienna, Austria.
References
- 1. Doty RL. The olfactory system and its disorders. Semin Neurol 2009; 29(1): 74–81. [DOI] [PubMed] [Google Scholar]
- 2. Doty RL, Li C, Mannon LJ, et al. Olfactory dysfunction in multiple sclerosis. N Engl J Med 1997; 336(26): 1918–1919. [DOI] [PubMed] [Google Scholar]
- 3. Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: A general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 1988; 38(8): 1237–1244. [DOI] [PubMed] [Google Scholar]
- 4. Doty RL, Reyes PF, Gregor T. Presence of both odor identification and detection deficits in Alzheimer’s disease. Brain Res Bull 1987; 18(5): 597–600. [DOI] [PubMed] [Google Scholar]
- 5. Bsteh G, Berek K, Hegen H, et al. Smelling multiple sclerosis: Different qualities of olfactory function reflect either inflammatory activity or neurodegeneration. Mult Scler 2020; 26(1): 57–68. [DOI] [PubMed] [Google Scholar]
- 6. Pevsner J, Hou V, Snowman AM, et al. Odorant-binding protein. Characterization of ligand binding. J Biol Chem 1990; 265(11): 6118–6125. [PubMed] [Google Scholar]
- 7. Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science (New York, NY) 2003; 301(5636): 1104–1107. [DOI] [PubMed] [Google Scholar]
- 8. Gottfried JA, Deichmann R, Winston JS, et al. Functional heterogeneity in human olfactory cortex: An event-related functional magnetic resonance imaging study. J Neurosci 2002; 22(24): 10819–10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bsteh G, Hegen H, Ladstätter F, et al. Change of olfactory function as a marker of inflammatory activity and disability progression in MS. Mult Scler 2019; 25(2): 267–274. [DOI] [PubMed] [Google Scholar]
- 10. Tanik N, Serin HI, Celikbilek A, et al. Olfactory bulb and olfactory sulcus depths are associated with disease duration and attack frequency in multiple sclerosis patients. J Neurol Sci 2015; 358(1–2): 304–307. [DOI] [PubMed] [Google Scholar]
- 11. Holinski F, Schmidt F, Dahlslett SB, et al. MRI study: Objective olfactory function and CNS pathologies in patients with multiple sclerosis. Eur Neurol 2014; 72(3–4): 157–162. [DOI] [PubMed] [Google Scholar]
- 12. Li LM, Yang LN, Zhang LJ, et al. Olfactory dysfunction in patients with multiple sclerosis. J Neurol Sci 2016; 365: 34–39. [DOI] [PubMed] [Google Scholar]
- 13. Schmidt FA, Goktas O, Harms L, et al. Structural correlates of taste and smell loss in encephalitis disseminata. PLoS ONE 2011; 6(5): e19702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lucchinetti CF, Popescu BF, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 2011; 365(23): 2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 2015; 14(2): 183–193. [DOI] [PubMed] [Google Scholar]
- 16. Kamma E, Lasisi W, Libner C, et al. Central nervous system macrophages in progressive multiple sclerosis: Relationship to neurodegeneration and therapeutics. J Neuroinflammation 2022; 19(1): 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Milo R, Korczyn AD, Manouchehri N, et al. The temporal and causal relationship between inflammation and neurodegeneration in multiple sclerosis. Mult Scler 2020; 26(8): 876–886. [DOI] [PubMed] [Google Scholar]
- 18. Rojas JI, Patrucco L, Míguez J, et al. Brain atrophy in radiologically isolated syndromes. J Neuroimaging 2015; 25(1): 68–71. [DOI] [PubMed] [Google Scholar]
- 19. Bsteh G, Berek K, Hegen H, et al. Serum neurofilament light levels correlate with change of olfactory function in multiple sclerosis. Mult Scler J Exp Transl Clin 2019; 5(4): 2055217319885987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bsteh G, Steiger R, Tuovinen N, et al. Impairment of odor discrimination and identification is associated with disability progression and gray matter atrophy of the olfactory system in MS. Mult Scler 2019: 1352458519838205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bsteh G, Hegen H, Teuchner B, et al. Peripapillary retinal nerve fibre layer as measured by optical coherence tomography is a prognostic biomarker not only for physical but also for cognitive disability progression in multiple sclerosis. Mult Scler 2019; 25(2): 196–203. [DOI] [PubMed] [Google Scholar]
- 22. Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol 2020; 77(9): 1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benedict RH, DeLuca J, Phillips G, et al. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler 2017; 23(5): 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hummel T, Kobal G, Gudziol H, et al. Normative data for the ‘Sniffin’ Sticks’ including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol 2007; 264(3): 237–243. [DOI] [PubMed] [Google Scholar]
- 25. Clark TG, Bradburn MJ, Love SB, et al. Survival analysis part IV: Further concepts and methods in survival analysis. Br J Cancer 2003; 89(5): 781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Research Council (US) Panel on Handling Missing Data in Clinical Trials. The prevention and treatment of missing data in clinical trials. Washington, DC: National Academies Press (US), 2010. [PubMed] [Google Scholar]
- 27. Ansari KA, Johnson A. Olfactory function in patients with Parkinson’s disease. J Chronic Dis 1975; 28(9): 493–497. [DOI] [PubMed] [Google Scholar]
- 28. Kohler CG, Moberg PJ, Gur RE, et al. Olfactory dysfunction in schizophrenia and temporal lobe epilepsy. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14(2): 83–88. [PubMed] [Google Scholar]
- 29. Doty RL, Yousem DM, Pham LT, et al. Olfactory dysfunction in patients with head trauma. Arch Neurol 1997; 54(9): 1131–1140. [DOI] [PubMed] [Google Scholar]
- 30. Doty RL, Li C, Mannon LJ, et al. Olfactory dysfunction in multiple sclerosis: Relation to longitudinal changes in plaque numbers in central olfactory structures. Neurology 1999; 53(4): 880–882. [DOI] [PubMed] [Google Scholar]
- 31. Lutterotti A, Vedovello M, Reindl M, et al. Olfactory threshold is impaired in early, active multiple sclerosis. Mult Scler 2011; 17(8): 964–969. [DOI] [PubMed] [Google Scholar]
- 32. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: A standardized microencapsulated test of olfactory function. Physiol Behav 1984; 32(3): 489–502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ilr-10.1177_13524585231201093 for Odour discrimination and identification as a biomarker of long-term disability worsening in multiple sclerosis by Klaus Berek, Harald Hegen, Michael Auer, Robert Barket, Franziska Di Pauli, Jakob Hocher, Nik Krajnc, Anne Zinganell, Florian Deisenhammer, Thomas Berger and Gabriel Bsteh in Multiple Sclerosis Journal
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the ethics committee of the Medical University Vienna.