Abstract
The 2.4-kb plasmid pAP1 from Arcanobacterium (Actinomyces) pyogenes had sequence similarity within the putative replication protein and double-stranded origin with the pIJ101/pJV1 family of plasmids. pJGS84, a derivative of pAP1 containing a kanamycin resistance gene, was able to replicate in Escherichia coli and Corynebacterium pseudotuberculosis, as well as in A. pyogenes. Detection of single-stranded DNA intermediates of pJGS84 replication suggested that this plasmid replicates by the rolling circle mechanism.
The gram-positive bacterium Arcanobacterium (Actinomyces) pyogenes (21), while commensal on the mucous membranes of many domestic animals, is also capable of causing a wide range of suppurative infections (6). A. pyogenes secretes a hemolytic exotoxin, pyolysin, and its gene, plo, was recently cloned and sequenced in our laboratory (3). However, further examination of the action of pyolysin in A. pyogenes pathogenesis has been hampered by the lack of techniques for the genetic manipulation of this organism. Recently, a method was developed for the introduction of plasmid DNA into A. pyogenes by electroporation using the corynebacterial vector pEP2 and RSF1010-based pJRD215 (10). This report describes the identification and sequencing of a native A. pyogenes plasmid, pAP1, its mode of replication, and its host range.
All of the Escherichia coli strains used in this study were grown on Luria-Bertani agar or in Luria-Bertani broth (Difco) supplemented with 100-μg/ml ampicillin or 50-μg/ml kanamycin (KM), when appropriate. A. pyogenes and Corynebacterium pseudotuberculosis strains were grown on brain heart infusion (Difco) agar supplemented with 5% sheep blood or in brain heart infusion broth supplemented with 5% fetal bovine serum. Media were additionally supplemented with 30-μg/ml KM when appropriate. A. pyogenes strains were grown in an atmosphere of 5% CO2.
Identification and molecular cloning of a native A. pyogenes plasmid, pAP1.
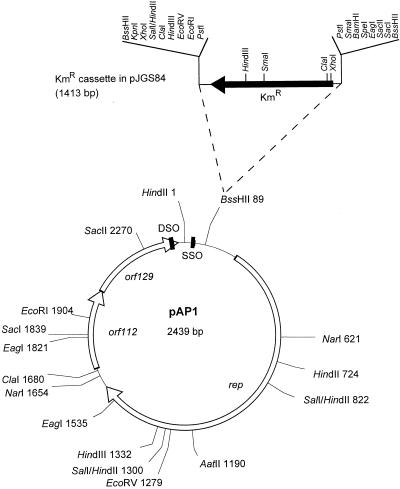
Plasmid DNA preparations were obtained from A. pyogenes BBR1 as previously described (10), and a plasmid band was observed (data not shown). This plasmid was native to strain BBR1 and was designated pAP1. Results of subsequent restriction endonuclease mapping of pAP1 indicated single sites for EcoRV and HindIII, and the calculated size of the plasmid was 2.4 kb. The unique HindIII site within pAP1 was utilized to clone the entire pAP1 sequence into HindIII-digested pBluescript SKII(+) (Stratagene). The resulting plasmids, pJGS65 and pJGS66, which contained the entire pAP1 sequence in either orientation, were used to construct a detailed restriction map of pAP1 (Fig. 1).
FIG. 1.
Schematic representation of pAP1. Restriction sites for appropriate enzymes are shown, followed by their positions in base pairs from the zero point at the top of the map, arbitrarily defined by a HindIII site. The three open reading frames, rep, orf112, and orf129, are indicated by open arrows. The positions of the putative DSO and SSO are indicated by the black rectangles. The Kmr gene inserted at the unique BssHII site to create pJGS84 is shown. All of the restriction sites shown on the map have been confirmed by actual digestion of pAP1 DNA.
pAP1 shows similarity to RCR plasmids.
The complete nucleotide sequences of both strands of pAP1 were determined by the Automated DNA Sequencing Service of the Laboratory of Molecular Systematics and Evolution at The University of Arizona by using a 373A DNA sequencer (Applied Biosystems Inc.). Sequence data were compiled by use of the Sequencher program (GeneCodes, Ann Arbor, Mich.). Salient features of the sequence are indicated on the restriction endonuclease map in Fig. 1. pAP1 is 2,439 bp long and contains three open reading frames, designated rep, orf112, and orf129. Protein database searches with the deduced products of orf112 and orf129 by using the BlastP algorithm (1) indicated no similarity to the sequences in the protein databases. However, the stop codon of orf112 overlaps the start codon of orf129 in a manner suggesting translational coupling.
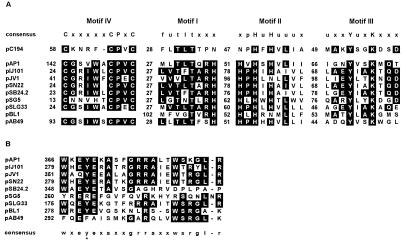
The precise translational start codon for the putative Rep protein is uncertain, as several ATG codons are in frame, but none of these have upstream sequences with strong similarity to consensus ribosome binding sites. If the first putative start codon is employed, the rep gene encodes a protein of 459 amino acids. The pAP1 Rep protein has amino acid similarity (15 to 23% identity, 27 to 39% similarity) to replication proteins from plasmids of the pIJ101/pJV1 family (9, 13), which replicate by rolling circle replication (RCR). This family, which shares some similarities with the pC194 family (13), includes Streptomyces plasmids pIJ101 (12), pJV1 (22, 23), pSN22 (11), pSB24.2 (4), pSLG33 (7), and pSG5 (17); brevibacterial plasmid pBL1 (8); and, putatively, Acinetobacter baumannii plasmid pAB49 (GenBank accession no. L77992). Analysis of the amino acid sequence of the pAP1 Rep protein indicated the presence of each of the three motifs (motifs I to III) universally associated with proteins that initiate RCR (9), suggesting that pAP1 replicates by this mechanism (Fig. 2A). RCR Rep proteins possess both the DNA nicking-closing and topoisomerase activities required for replication and ligation of the leading strand during RCR. Motif III contains a tyrosine residue that has been proposed to be involved in DNA linking (9) and is required for the activity of the pC194 Rep protein (18). Motif II contains conserved histidine residues which may act as ligands for the Mg2+ and Mn2+ ions which are required for Rep function (9). The pAP1 Rep protein also possesses an N-terminal cysteine-rich motif (motif IV) shared by the pC194 family of Rep proteins, as well as the transposase of IS91 (16, 17) (Fig. 2A). Mendiola and de la Cruz (16) have suggested that this motif, like motif II, is involved in heavy metal binding. Alignment of the Rep proteins of members or putative members of the pIJ101/pJV1 family allowed the identification of a novel C-terminal motif shared by these proteins (Fig. 2B). This motif overlaps a region which Fernandez-Gonzalez et al. (8) suggested may be an additional sequence involved in DNA linking, due to the presence of a conserved tyrosine in the sequences of pBL1, pIJ101, pJV1, and pSB24.2. However, neither the pSG5 nor the pAB49 Rep protein possesses the conserved tyrosine residue, although the pSG5 sequence has two tyrosines in the motif sequence. This motif also contains at least one conserved glutamic acid residue, and in some cases two. Glutamic acid residues within the pC194 Rep protein have been demonstrated to be important in the replication of this plasmid, possibly mediating the nucleophilic attack on the newly synthesized strand at the termination of synthesis of the leading strand (18).
FIG. 2.
Amino acid sequence motifs of the pAP1 Rep protein. (A) Identification of the three motifs universally conserved in RCR Rep proteins (9), as well as a cysteine-rich, putative heavy metal binding motif (motif IV) (5, 16, 17), within the amino acid sequence of the pAP1 Rep protein. These motifs are aligned with homologous regions of Rep proteins from pC194, pIJ101, pJV1, pSN22, pSB24.2, pSG5, pSLG33, pBL1, and pAB49. Numbers indicate the numbers of amino acids between motifs in each protein. Residues are boxed where five or more sequences have identical amino acids. (B) Alignment of a conserved C-terminal segment of pAP1 and Rep proteins of the pIJ101/pJV1 family. The tyrosine residue postulated by Fernandez-Gonzalez et al. (8) as a secondary site involved in DNA linking is indicated by the asterisk. Residues are boxed where five or more sequences have identical amino acids.
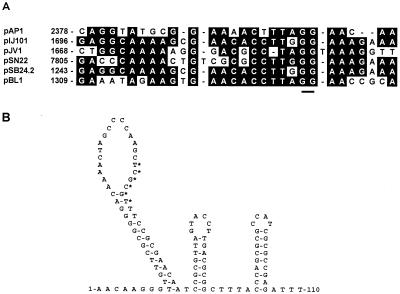
Within the nucleotide sequence of pAP1, there are sequences which resemble the double (DSO)- and single (SSO)-stranded origins of replication of RCR plasmids. The nick sites within the DSOs of pIJ101, pJV1, and pSN22 have been identified experimentally and are shown in Fig. 3A (22, 25), while the DSOs of pSB24.2 and pBL1 (8) have been inferred on the basis of similarity to this sequence. A sequence with strong similarity to the DSOs of these plasmids was identified within the 3′ end of orf129 (Fig. 3A). The GG dinucleotide at the nick site is totally conserved, and the nucleotides surrounding this dinucleotide are highly conserved in pAP1.
FIG. 3.
Origins of replication of pAP1. (A) Alignment of the putative DSO with the known DSOs of pIJ101, pJV1, and pSN22 and homologous regions from pSB24.2 and pBL1. The underlined GG dinucleotide indicates the position of the nick site (22, 25). Residues are boxed where four or more sequences have identical amino acids. (B) Secondary structure of the putative SSO of pAP1 predicted by using the mfold program, version 2.3 (Micheal Zuker, Washington University, St. Louis, Mo.). The hexanucleotide sequence TCGCGT, which is similar to the ssi consensus sequence TAGCGT defined by Zaman et al. (26), is indicated by the asterisks.
The nucleotide sequences of the SSOs of RCR plasmids are not well conserved. However, SSOs generally exist within a region of strong secondary structure located upstream of or within the coding sequence for the rep gene. In addition, there is conservation of a six-nucleotide sequence, TAGCGT, in many plasmids which is proposed to act as the site of second-strand initiation (ssi) (26). A sequence with a high level of secondary structure lies near the putative DSO of pAP1 and just upstream of the rep gene of pAP1 (Fig. 3B). The sequence TCGCGT, located within the putative pAP1 SSO, shares five of six nucleotides with the consensus ssi sequence and, like similar sequences within the SSOs of pIJ101 (26) and pBL1 (8), is located within the loop of a large hairpin structure.
Distribution of pAP1 among A. pyogenes isolates.
A total of 38 A. pyogenes isolates from veterinary diagnostic laboratories or personal culture collections were screened by colony hybridization (2) by using a digoxigenin-labeled pAP1 probe synthesized with a DIG DNA Labeling and Detection Kit (Boehringer Mannheim). Of the 38 isolates, 5 hybridized to the pAP1 probe; i.e., strains BBR1, JGS190, 424, 1992, and 3053. Plasmid enrichments were performed on all 38 isolates, and no additional plasmids, with the exception of pAP1, were isolated. Of the five strains that hybridized to the pAP1 probe, plasmid DNA could be reliably extracted from only two strains, BBR1 and JGS190. To resolve this anomaly, total genomic DNA was isolated from each of the five pAP1-hybridizing strains by the method of Pospiech and Neumann (19). This DNA was digested with EcoRI, which cuts the plasmid only once, or HindII, which digests pAP1 into four fragments, and subjected to Southern blotting (2) by using the pAP1 probe. The same pattern of hybridizing DNA fragments was obtained from each of the five strains in both EcoRI and HindII digests, indicating that the strains carry similar plasmids (data not shown).
The replication machinery of pAP1 is functional in E. coli.
To assess the ability of the pAP1 replicon to function in E. coli, the 1.4-kb BssHII cassette containing the Tn903 KM resistance (Kmr)-encoding gene, from pJM4 (15) was ligated with BssHII-digested pAP1. The unique BssHII site in pAP1 is located upstream of the rep gene (Fig. 1). This ligation was introduced into E. coli DH5α (Bethesda Research Laboratories), and Kmr recombinants were obtained after 48 of 72 h of incubation. Plasmid DNA extraction confirmed the presence of a pAP1 derivative carrying the Kmr gene, and this plasmid was designated pJGS84 (Fig. 1). Several attempts to introduce a Kmr or Emr cassette at either the ClaI site immediately 5′ of orf112, the EcoRI and SacI sites within orf112, or the SacII site within orf129 did not yield transformants. These results suggest that the orf112-orf129 region codes for a function essential in the replication or maintenance of pAP1.
Replication of pJGS84 in A. pyogenes.
To determine whether pJGS84 was truly capable of replicating in both E. coli and A. pyogenes, pJGS84 plasmid DNA extracted from DH5α was introduced into A. pyogenes BBR1 by the method of Jost et al. (10). Kmr transformants were obtained at a frequency of 5 × 104 CFU/μg of plasmid DNA, which is similar to the electroporation efficiencies obtained for other replicating plasmids (10). Examination of plasmid DNA from these transformants revealed the presence of pJGS84, but not pAP1. It is possible that pJGS84 recombined with pAP1, facilitating the maintenance of the Kmr gene. It is more likely that there was incompatibility between two plasmids carrying the pAP1 replicon, and thus, selection for pJGS84 resulted in the loss of pAP1. To determine whether pJGS84 was capable of independent replication in A. pyogenes, E. coli-replicated pJGS84 was introduced into A. pyogenes OX8, which lacks pAP1 and does not hybridize with pAP1 sequences. OX8 transformants containing pJGS84 were obtained at a frequency similar to those obtained with a control plasmid, pEP2 (20).
To test the ability of the pAP1 replicon to function in a gram-positive species other than A. pyogenes, pJGS84 was introduced into the related, but taxonomically distinct, bacterium C. pseudotuberculosis Whetten 1 essentially as previously described (24). Kmr transformants were obtained at a frequency of 2.4 × 106 CFU/μg of plasmid DNA. Autonomous replication of pJGS84 was confirmed by isolation and visualization of plasmid DNA from Kmr Whetten 1 transformants.
The ability of the pAP1 replicon to function in gram-negative, as well as gram-positive, bacteria provides an excellent basis for the construction of an E. coli-A. pyogenes shuttle vector, eliminating the need to introduce extraneous DNA in the form of a gram-negative replicon. The pAP1 replicon is one of only a few replicons with the ability to function in both gram-negative and gram-positive bacteria, and even fewer of these are of gram-positive origin. Like pAP1, the archetypal plasmid of this kind, pWV01, is a small, gram-positive, broad-host-range plasmid which replicates by RCR (14).
Stability of pJGS84 in A. pyogenes and E. coli.
Duplicate cultures of E. coli DH5α and A. pyogenes BBR1 harboring pJGS84 were grown for approximately 21 cell doublings in the presence or absence of KM, and the cultures were maintained in exponential growth throughout. Appropriate dilutions were plated onto selective and nonselective media to evaluate relative plasmid loss. pJGS84 was maintained in both E. coli and A. pyogenes in the presence of KM but was rapidly lost from E. coli hosts in the absence of selection, with an average loss of 94.5% after 21 cell doublings. No significant loss (<1%) of pJGS84 from BBR1 was observed in the absence of KM. The instability of pJGS84 in E. coli in the absence of selection suggests that this plasmid replicates inefficiently in this host. It is possible that prolonged maintenance in a gram-negative host leads to the acquisition of mutations which improve the maintenance of pJGS84 in these hosts but may potentially interfere with its replication in A. pyogenes. Conversely, the stability of pAP1 and derivatives in A. pyogenes in the absence of antibiotic selection will be of great benefit when attempting to complement mutations in situations in which antibiotic selection for the plasmid is difficult to maintain, e.g., in vivo. In fact, bacteria recovered from the liver and peritoneal fluid of mice infected with BBR1(pJGS84) all retained the plasmid, as measured by Kmr (data not shown).
Detection of single-stranded intermediates of replication from pJGS84.
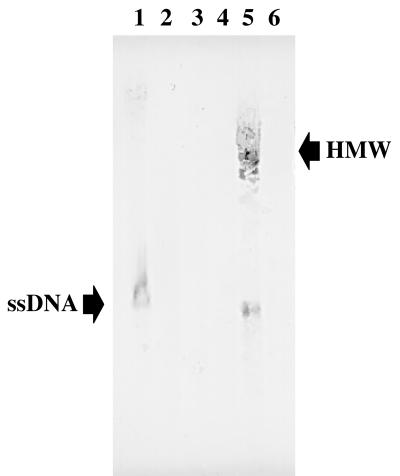
The similarity between pAP1 and members of the pIJ101/pJV1 family suggests that pAP1 replicates by RCR. To provide further support for this notion, genomic DNA was extracted from DH5α(pJGS84), BBR1, and BBR1(pJGS84). DNA samples were subjected to agarose gel electrophoresis following incubation with or without S1 nuclease (Promega) for 20 min at 37°C. DNA fragments were transferred to nitrocellulose without prior denaturation, and the resulting membrane was hybridized with digoxigenin-labeled, strand-specific riboprobes generated with a DIG RNA Labeling Kit (Boehringer) and T7 RNA polymerase from template plasmids pJGS65 (complementary to the clockwise strand in Fig. 1) and pJGS66 (complementary to the counterclockwise strand in Fig. 1). Hybridizing bands were observed only for pJGS84 when the pJGS66-derived riboprobe was used and only in the absence of treatment with S1 nuclease (Fig. 4), indicating that the generation of single-stranded DNA (ssDNA) intermediates from this plasmid is on the strand opposite to rep. This is unusual, as transcription of the rep gene and leading-strand synthesis proceed in the same direction in most RCR plasmids (13). However, in pSN2 and related plasmids, replication of the leading strand does appear to occur in the direction opposite to rep gene transcription (13). Synthesis of ssDNA in the counterclockwise orientation suggests that the putative SSO sequence is synthesized close to last during replication of the leading strand. ssDNA intermediates were observed for pJGS84 in both E. coli and A. pyogenes, but in BBR1, high-molecular-weight ssDNA was also observed, possibly ssDNA in the act of conversion to double-stranded DNA in a high-molecular-weight complex. No ssDNA intermediate was observed for pAP1 from BBR1. This result may reflect rapid conversion of the ssDNA intermediate of the native plasmid to a double-stranded plasmid in its native host. However, detection of ssDNA intermediates for pJGS84 strongly suggests that both pJGS84 and pAP1 replicate via a rolling circle mechanism.
FIG. 4.
Detection of ssDNA intermediates in the replication of pJGS84. Genomic DNA derived from E. coli DH5α(pJGS84) (lanes 1 and 2) and A. pyogenes BBR1 (lanes 3 and 4) and BBR1(pJGS84) (lanes 5 and 6) were hybridized with a pJGS66-derived riboprobe, with (lanes 2, 4, and 6) or without (lanes 1, 3, and 5) prior treatment with S1 nuclease. The positions of hybridizing ssDNAs are indicated. High-molecular-weight (HMW) hybridizing bands observed in BBR1(pJGS84) are also indicated. This is a digital image prepared in Adobe Photoshop 3.0 from a direct scan of the nylon membrane following colorimetric development.
The discovery and analysis of the native A. pyogenes plasmid, pAP1, described here have provided us with a strong basis for the rational construction of E. coli-A. pyogenes shuttle vectors, which will contribute significantly to the number of genetic tools available for the manipulation of this gram-positive pathogen. These studies, combined with the cloning of the pyolysin gene (3) and the description of an electroporation protocol (10), represent major steps forward in the study of A. pyogenes genetics and pathogenesis.
Nucleotide sequence accession number.
The nucleotide sequence data presented here were submitted to the GenBank database and assigned accession no. U83788.
Acknowledgments
This work was supported in part by USDA grant 97-35204-4750.
We thank Veronica Enriquez for technical assistance.
REFERENCES
- 1.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: Greene Publishing Associates and John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 3.Billington S J, Jost B H, Cuevas W A, Bright K R, Songer J G. The Arcanobacterium (Actinomyces) pyogenes hemolysin, pyolysin, is a novel member of the thiol-activated cytolysin family. J Bacteriol. 1997;179:6100–6106. doi: 10.1128/jb.179.19.6100-6106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolotin A P, Sorokin A V, Aleksandrov N N, Danilenko V N, Kozlov Y I. Nucleotide sequence of DNA of the actinomycete plasmid pSB24.2. Dokl Biochem. 1986;283:260–263. [PubMed] [Google Scholar]
- 5.Brasch M A, Cohen S N. Sequences essential for replication of plasmid pIJ101 in Streptomyces lividans. Plasmid. 1995;33:191–197. doi: 10.1006/plas.1995.1020. [DOI] [PubMed] [Google Scholar]
- 6.Carter G R, Chengappa M M. Essentials of veterinary bacteriology and mycology. 4th ed. Philadelphia, Pa: Lea & Febiger; 1991. [Google Scholar]
- 7.Felsberg J, Petricek M, Tichy P. Nucleotide sequence of the mini-plasmid pSLG33 from Streptomyces lavendulae-grasserius RIA746. Nucleic Acids Res. 1993;21:3582. doi: 10.1093/nar/21.15.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Gonzalez C, Cadenas R F, Noirot-Gros M F, Martin J F, Gil J A. Characterization of a region of plasmid pBL1 of Brevibacterium lactofermentum involved in replication via the rolling circle model. J Bacteriol. 1994;176:3154–3161. doi: 10.1128/jb.176.11.3154-3161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilyina T V, Koonin E V. Conserved sequence motifs in the initiator proteins for rolling circle DNA replication encoded by diverse replicons from eubacteria, eucaryotes and archaebacteria. Nucleic Acids Res. 1992;20:3279–3285. doi: 10.1093/nar/20.13.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jost B H, Billington S J, Songer J G. Electroporation-mediated transformation of Arcanobacterium (Actinomyces) pyogenes. Plasmid. 1997;38:135–140. doi: 10.1006/plas.1997.1299. [DOI] [PubMed] [Google Scholar]
- 11.Kataoka M, Kiyose Y M, Michisuji Y, Horiguchi T, Seki T, Yoshida T. Complete nucleotide sequence of the Streptomyces nigrifaciens plasmid, pSN22: genetic organization and correlation with genetic properties. Plasmid. 1994;32:55–69. doi: 10.1006/plas.1994.1044. [DOI] [PubMed] [Google Scholar]
- 12.Kendall K J, Cohen S N. Complete nucleotide sequence of the Streptomyces lividans plasmid pIJ101 and correlation of the sequence with genetic properties. J Bacteriol. 1988;170:4634–4651. doi: 10.1128/jb.170.10.4634-4651.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan S A. Rolling-circle replication of bacterial plasmids. Microbiol Mol Biol Rev. 1997;61:442–455. doi: 10.1128/mmbr.61.4.442-455.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leenhouts K, Tolner J B, Bron S, Kok J, Venema G, Seegers J F M L. Nucleotide sequence and characterization of the broad-host-range lactococcal plasmid pWV01. Plasmid. 1991;26:55–66. doi: 10.1016/0147-619x(91)90036-v. [DOI] [PubMed] [Google Scholar]
- 15.McNamara P J, Bradley G A, Songer J G. Targeted mutagenesis of the phospholipase D gene results in decreased virulence of Corynebacterium pseudotuberculosis. Mol Microbiol. 1994;12:921–930. doi: 10.1111/j.1365-2958.1994.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 16.Mendiola M V, de la Cruz F. IS91 transposase is related to the rolling-circle-type replication proteins of the pUB110 family of plasmids. Nucleic Acids Res. 1992;20:3521. doi: 10.1093/nar/20.13.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muth G, Farr M, Hartmann V, Wohlleben W. Streptomyces ghanaensis plasmid pSG5: nucleotide sequence analysis of the self transmissible minimal replicon and characterization of the replication mode. Plasmid. 1995;33:113–126. doi: 10.1006/plas.1995.1013. [DOI] [PubMed] [Google Scholar]
- 18.Noirot-Gros M F, Bidnenko V, Ehrlich S D. Active site of the replication protein of the rolling circle plasmid pC194. EMBO J. 1994;13:4412–4420. doi: 10.1002/j.1460-2075.1994.tb06761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pospiech A, Neumann B. A versatile quick-prep of genomic DNA from gram-positive bacteria. Trends Genet. 1995;11:217–218. doi: 10.1016/s0168-9525(00)89052-6. [DOI] [PubMed] [Google Scholar]
- 20.Radford A J, Hodgson A L. Construction and characterization of a Mycobacterium-Escherichia coli shuttle vector. Plasmid. 1991;25:149–153. doi: 10.1016/0147-619x(91)90029-v. [DOI] [PubMed] [Google Scholar]
- 21.Ramos C P, Foster G, Collins M D. Phylogenetic analysis of the genus Actinomyces based on 16S rRNA gene sequences: description of Arcanobacterium phocae sp. nov., Arcanobacterium bernardiae comb. nov., and Arcanobacterium pyogenes comb. nov. Int J Syst Bacteriol. 1997;47:46–53. doi: 10.1099/00207713-47-1-46. [DOI] [PubMed] [Google Scholar]
- 22.Servín-González L. Relationship between the replication functions of Streptomyces plasmids pJV1 and pIJ101. Plasmid. 1993;30:131–140. doi: 10.1006/plas.1993.1040. [DOI] [PubMed] [Google Scholar]
- 23.Servín-González L, Sampieri A I I I, Cabello J, Galván L, Juárez V, Castro C. Sequence and functional analysis of the Streptomyces phaeochromogenes plasmid pJV1 reveals a modular organization of Streptomyces plasmids that replicate by rolling circle. Microbiology. 1995;141:2499–2510. doi: 10.1099/13500872-141-10-2499. [DOI] [PubMed] [Google Scholar]
- 24.Songer J G, Hilwig R W, Leeming M N, Iandolo J J, Libby S J. Transformation of Corynebacterium pseudotuberculosis by electroporation. Am J Vet Res. 1991;52:1258–1261. [PubMed] [Google Scholar]
- 25.Suzuki I, Seki T, Yoshida T. Nucleotide sequence of a nicking site of the Streptomyces plasmid pSN22 replicating by the rolling circle mechanism. FEMS Microbiol Lett. 1997;150:283–288. doi: 10.1111/j.1574-6968.1997.tb10382.x. [DOI] [PubMed] [Google Scholar]
- 26.Zaman S, Radnedge L, Richards H, Ward J M. Analysis of the site for second-strand initiation during replication of the Streptomyces plasmid pIJ101. J Gen Microbiol. 1993;139:669–676. doi: 10.1099/00221287-139-4-669. [DOI] [PubMed] [Google Scholar]