Abstract
Purpose:
NCT03253744 was a phase I trial to identify the maximally tolerated dose (MTD) of image-guided, focal, salvage stereotactic body radiotherapy (SBRT) for patients with locally radiorecurrent prostate cancer. Additional objectives included biochemical control and imaging response.
Methods and Materials:
The trial design included three dose levels (DLs): 40Gy (DL1), 42.5Gy (DL2), and 45Gy (DL3) in 5 fractions delivered ≥48 hours apart. The prescription dose was delivered to the MRI and PSMA imaging-defined tumor volume. Dose escalation followed a 3+3 design with a 3-patient expansion at the MTD. Toxicities were scored until two years after completion of SBRT using CTCAE v5.0 criteria. Escalation was halted if two dose limiting toxicities occurred, defined as any persistent (>4 days) grade 3 toxicity occurring within the first 3 weeks after SBRT, and any grade 3 GU or grade 4 GI toxicity thereafter.
Results:
Between 08/2018 and 05/2022, 8 patients underwent salvage focal SBRT with a median follow-up of 35 months. No DLTs were observed on DL1. Two patients were enrolled in DL2 and experienced grade 3 GU toxicities, prompting de-escalation and expansion (n=6) at the MTD (DL1). The most common toxicities observed were G2+ GU toxicities, with only a single G2 GI toxicity and no G3+ GI toxicities. One patient experienced biochemical failure (PSA nadir + 2.0) at 33 months.
Conclusions:
The MTD for focal salvage SBRT for isolated intraprostatic radiorecurrence was 40Gy in 5 fractions producing a 100% 24-month bPFS, with one post-study failure at 33 months. The most frequent clinically significant toxicity was late grade 2+ GU toxicity.
INTRODUCTION
Advances in medical imaging, fusion biopsy techniques, and local salvage options with favorable toxicity profiles have led to increasing ability to localize the origin of biochemically recurrent prostate cancer after definitive radiotherapy, defined by an increase in PSA of 2.0 ng/mL above the post-treatment nadir. Imaging techniques such as MRI and PSMA-based PET/CT have been shown to have excellent sensitivity and specificity in the identification of local and distant recurrence of prostate cancer in the setting of biochemical recurrence [1], an advance that has enhanced the confidence in patient selection for local salvage therapies [2, 3].
Several forms of local salvage treatments have been reported, and re-irradiation with external beam radiation therapy (EBRT) is increasingly recognized as a viable option due to the favorable efficacy and toxicity profile [4]. Stereotactic body radiation therapy (SBRT) has become of widespread interest in the setting of re-irradiation due to its capacity to deliver highly conformal, ablative doses to tumors with reduced biologically equivalent dose exposure to normal tissues. The optimal dose regimen and treatment volume for salvage SBRT remains unknown. Focal salvage re-irradiation approaches may allow escalation of dose beyond that achievable with whole gland exposures, but the resulting efficacy and toxicity of this approach is uncertain. Herein, we report the results of the first phase I trial designed to define the maximum tolerable dose (MTD) for salvage, focal SBRT guided by MRI and PSMA-based PET/CT in patients with local recurrence after prior EBRT, which is termed radiorecurrence hereafter.
METHODS AND MATERIALS
NCT03253744 was a single-institution, phase I trial designed to identify the MTD of image-guided, focal, dose-escalated, salvage SBRT for isolated local radiorecurrence. The trial was conducted with approval from the institutional review board of the National Cancer Institute, and all study participants provided informed consent. The trial included two parallel cohorts. The first, reported here, consisted of participants with local recurrence after EBRT. The second included participants with local recurrence after brachytherapy and will be reported upon maturity.
Eligible patients had biochemically recurrent prostate (defined by the Phoenix criteria [5]) and biopsy-verified, intraprostatic recurrence with or without seminal vesicle invasion. Additional eligibility criteria included age ≥ 18 and ECOG performance status ≤ 1. Exclusion criteria included ongoing grade 3 or higher toxicities from prior EBRT, prior prostatectomy, distant metastases, or recurrence within one year of definitive EBRT. All patients underwent staging with a multiparametric MRI (mpMRI), 18F-NaF PET/CT or 99mTc-MDP bone scan, and 18F-DCFPyL PET/CT.
TREATMENT PROTOCOL
Treatment was delivered on one of three dose levels: 40Gy (DL1), 42.5Gy (DL2), and 45Gy (DL3) in 5 fractions delivered over 10–12 days (Supplemental Table 1). Patients were maintained on pharmacologic therapy for pre-existing urinary symptoms if previously prescribed (n = 5/8); however, no prophylactic medication was prescribed. The gross target volume (GTV) was defined using a combination of simulation CT, multiparametric MRI (mpMRI), 18F-DCFPyL PET/CT, and targeted biopsy mapping. The planning target volume (PTV) was a 3mm expansion beyond the GTV posteriorly and superiorly and 5mm in all other directions. ADT was not used with exception of a single instance due to a COVID 19-related delay in treatment. Radiation simulation, treatment planning, and delivery are detailed in the supplemental materials (Supplemental Table 2).
The specified dose was prescribed to the 100% isodose line and delivered with volumetric modulated arc therapy (VMAT). The V110% of the PTV (PTV receiving ≥110% of the prescription dose) was limited to <5%. Organs at risk (OARs) were contoured and the maximum dose (Dmax) was limited to 100%, 105%, and 105% of the prescription dose for the rectal wall outside of the PTV, bladder wall, and urethra, respectively (Supplemental Table 3). The urethra was delineated on the fused treatment planning MRI by a GU specialized radiologist in collaboration with the treating radiation oncologist. No foley catheter was used during simulation or treatment. As detailed in Supplemental Table 2, interfraction motion was managed with a pre-treatment cone beam CT (CBCT) and intrafraction motion was managed with a CBCT between each VMAT arc. All registrations were conducted via implanted fiducial markers.
ASSESSMENTS
Subjects were followed for 24 months after completion of SBRT with serial PSA measurements. Adverse events (AEs) were classified using the CTCAE v5.0 system and were scored weekly during treatment through 1 month, and thereafter at 3-month intervals for 24 months.
STATISTICAL DESIGN
The primary objective was to determine the MTD for mpMRI- and 18F-DCFPyL PET/CT-guided focal, salvage SBRT. Dose escalation followed a 3+3 design [6–8]. Dose limiting toxicities (DLTs) were defined as any of the following: (1) treatment delays of ≥1 week due to toxicity, (2) persistent (>4 days) grade (G) 3 or higher gastrointestinal (GI), genitourinary (GU) toxicity, (3) other in-field toxicity occurring during or within 3 weeks of treatment completion, or (4) ≥G3 GU or ≥G4 GI toxicities occurring thereafter. Secondary objectives included a characterization of biochemical progression free survival (bPFS). Exploratory objectives included descriptions of (1) dosimetric predictors of toxicity, (2) PSA kinetics, (3) baseline imaging (mpMRI and 18F-DCFPyL PET/CT), and (4) 6-month post-treatment imaging response. A full description of these objectives is included in the Supplemental Methods.
All radiologic assessments were made by dedicated, GU-specialized radiologists with expertise in 18F-DCFPyL PET/CT and prostate MRI. Paired Wilcoxon testing was conducted to compare baseline and 6-month post-treatment imaging with a p-value < 0.05 considered statistically significant. As these analyses were exploratory in nature, no adjustment for multiple comparisons was made.
RESULTS
Patient Characteristics
Eight patients who had experienced biochemical recurrence a median of 9.1 years (range: 6.3–16.4 years) after their initial course of EBRT±ADT were enrolled between 08/2018 and 05/2022. The median dose of prior EBRT was 76.5Gy (min-max: 72.0–79.2Gy) delivered in 1.8Gy-2.0Gy per fraction. Patient characteristics are summarized in Table 1. The median follow-up after SBRT reirradiation was 35 months.
Table 1.
Patient Characteristics at Initial Diagnosis and Salvage SBRT
| Characteristic | Number (%) / Median (min-max) |
|---|---|
| Demographics | |
| Race | - |
| White | 4 (50%) |
| Black | 4 (50%) |
| Ethnicity | - |
| Non-Hispanic | 8 (100%) |
| Initial Diagnosis | |
| Age | 63.8 (52.6–73.0) |
| PSA | 12.9 (4.7–33.4) |
| T-stage | - |
| T1c | 4 (50%) |
| T2a | 3 (37.5%) |
| T2c | 1 (12.5%) |
| Gleason Grade | 7 (6–9) |
| 3+3 = 6 | 3 (37.5%) |
| 3+4 = 7 | 3 (37.5%) |
| 4+5 = 9 | 2 (25%) |
| Risk Stratum | - |
| Low-Risk | 1 (12.5%) |
| Intermediate-Risk | 3 (37.5%) |
| High-Risk | 4 (50%) |
| EBRT Dose (Course 1) | 76.5 (72.0–79.2) |
| < 74 Gy | 1 (12.5%) |
| ≥ 74 Gy | 7 (87.5%) |
| EBRT Volume (Course 1) | - |
| Prostate | 1 (12.5%) |
| Prostate + SV | 2 (25%) |
| Prostate + SV + PLN | 5 (62.5%) |
| At Recurrence | |
| PSA | 2.92 (2.05–7.92) |
| Grade | 9 (8–10) |
| 4+4 = 8 | 2 (25%) |
| 4+5 = 9 | 4 (50%) |
| 5+5 = 10 | 1 (12.5%) |
| Adenocarcinoma with Treatment Effect | 1 (12.5%) |
Abbreviations: SBRT: Stereotactic Body Radiation Therapy; EBRT: External Beam Radiation Therapy; SV = seminal vesicles, PLN = pelvic lymph nodes
Primary Objective and Toxicity
The MTD was found to be 40Gy in 5 fractions (DL1). Two patients were treated on DL2, both of whom experienced self-limited G3 toxicities: hematuria of a 4-day duration and urge incontinence of a 7-week duration. Thus, accrual at DL2 was halted and accrual resumed at DL1. No DLTs occurred in the 6 patients accrued to DL1.
AEs possibly, probably, or definitely related to radiation are summarized in Table 2. Most common were G1 (n=6) and G2 (n=3) GU AEs experienced by patients treated on DL1. These included dysuria, cystitis, urgency, increased frequency, weak stream, and hematuria. In DL2, both patients experienced G3 events, hematuria and urge incontinence, at 9 weeks and 12.8 months post-treatment, respectively. Representations of the treatment plans of patients with G3 GU toxicity are shown in Supplemental Figure 5. The exploratory dosimetric analysis was limited due to sample size, however a trend towards higher bladder maximum dose, bladder D1cc and D5cc, and urethral Dmax was observed in patients experiencing G3+ GU toxicity.
Table 2.
CTCAE v5.0 adverse events by dose level in NCT03253744 adjudicated to be possibly, probably, or definitely related to radiation therapy.
| Adverse Event | G1 (n) | G2 (n) | G3 (n) | Latency (indexed from COT) | Duration (on trial) |
|---|---|---|---|---|---|
| Dose Level 1 (n=6) | |||||
| Total GU | 6 | 3 | 0 | ||
| Urinary Urgency | 1 | 1 | - | +3 wk, +1mo | 9 d, ≥23 mo |
| Urinary Frequency | - | 1 | - | +1 mo | ≥23 mo |
| Urinary Tract Pain | 2 | - | - | +5 mo, +1 d | 6 wk, 6 wk |
| Cystitis, noninfective | 2 | - | - | +8 mo, +5mo | 10 d, ≥19 mo |
| Slow urine flow* (Renal and urinary – other) | - | 1 | - | -3 d | 1 mo |
| Hematuria | 1 | +2.5 wk | 1 d | ||
| Total GI | 2 | 1 | 0 | ||
| Proctitis | - | 1 | - | +3.25 mo | 2.5 wk |
| Hemorrhoidal Hemorrhage | 1 | - | - | +9 mo | ≥15 mo |
| Flatulence | 1 | - | - | +3.25 mo | 2.5 wk |
| Total Sexual Function | 0 | 0 | 0 | ||
| Dose Level 2 (n=2) | |||||
| Total GU | 0 | 2 | 2 | ||
| Urinary Incontinence | - | - | 1 | +12.8 mo | 7.5 wk |
| Cystitis, noninfective | - | 1 | - | +6.75 mo | 6 mo |
| Urinary Tract Obstruction | - | 1 | - | +9 d | 2 wk |
| Hematuria | - | - | 1 | +9 wk | 4 d |
| Total GI | 0 | 0 | 0 | ||
| Total Sexual Function | 1 | 0 | 0 | ||
| Hematospermia (Ejaculation Disorder) | 1 | - | - | −3 d | 10 d |
| Dose Level 1 & 2 (n=8) | |||||
| Total GU | 6 | 5 | 2 | ||
| Total GI | 2 | 1 | 0 | ||
| Total Sexual Function | 1 | 0 | 0 | ||
Abbreviations: G: Grade; GU: Genitourinary; GI: Gastrointestinal; D: Day; Wk: Week; Mo: Month; COT: Completion of Treatment
Two GI AEs were observed, G1 hemorrhoidal bleeding with constipation (DL1), G1 flatulence and G2 self-limited proctitis (DL1). Both patients with GI toxicities underwent hydrogel spacer installation. The patient who developed G2 proctitis experienced rectal wall infiltration that delayed treatment until hydrogel resorption. This patient had the highest rectal D1cc (31.2Gy) and D2cc (25.3Gy).
Oncologic Outcomes
Patients were followed up for a median of 27 months (min-max: 6–43 months) after trial enrollment. The 12-month and 24-month bPFS were 100% and 87.5% (n = 7/8) of the patients remained biochemical progression-free at the time of their last follow-up. The one patient with biochemical failure (BF) experienced it at 33 months post treatment. Restaging showed an equivocal focus in the right fifth rib indeterminate for metastatic disease.
PSA
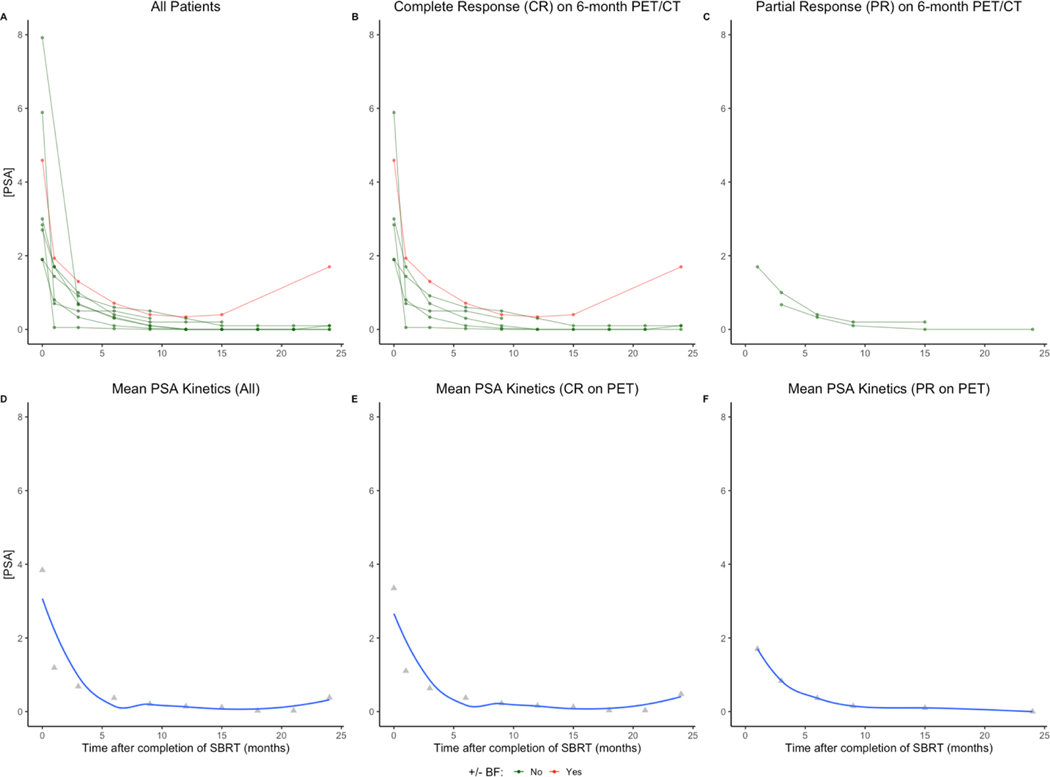
During the reported follow-up, 7 of 8 patients reached a PSA nadir. The mean PSA nadir was 0.09, with no transient rises (i.e., “PSA bounces”) observed. The median time to nadir was 12 months (95% confidence interval [CI]: 9 months – not reached). The single patient with BF after treatment had the highest nadir PSA (0.34). The mean time to nadir was numerically shorter for subjects who achieved only a partial response (PR) on 6-month post-treatment 18F-DCFPyL PET/CT (10.4 months) than those who achieved a CR (12.3 months). PSA kinetics are detailed in Figure 1A–C for the total population and for subgroups by PET response; the corresponding means for these subgroups are detailed in Figure 1D–F.
Figure 1.
Individual and mean PSA kinetics for the entire population (panels A and D, respectively), patients with a complete response (CR) on 6-month post-treatment 18F-DCFPyL PET/CT (panels B and E, respectively), and patients with a partial response (PR) on 6-month post-treatment 18F-DCFPyL PET/CT (panels C and F, respectively).
Abbreviations: BF: Biochemical failure
Treatment Volumes
Eleven intra-prostatic GTVs in the 8 patients were treated. One patient had a 5mm 18F-DCFPyL PET/CT detected periprostatic lymph node that was included in the target volume in addition to his intraprostatic recurrence. This patient remained disease free at last follow-up (36 months) and had mild toxicity from treatment (maximum G1). GTVs were most commonly in the peripheral zone (PZ, 73%), transitional zone (TZ, 27%) and posterior gland (82%). The majority (64%) of lesions involved more than one region (apex 55%, mid 82%, base 36%). The mean GTV was 3.7cc (min-max: 0.4–10.2cc), and mean PTV was 9.7cc (min-max: 3.3–25.9cc).
MRI Outcomes
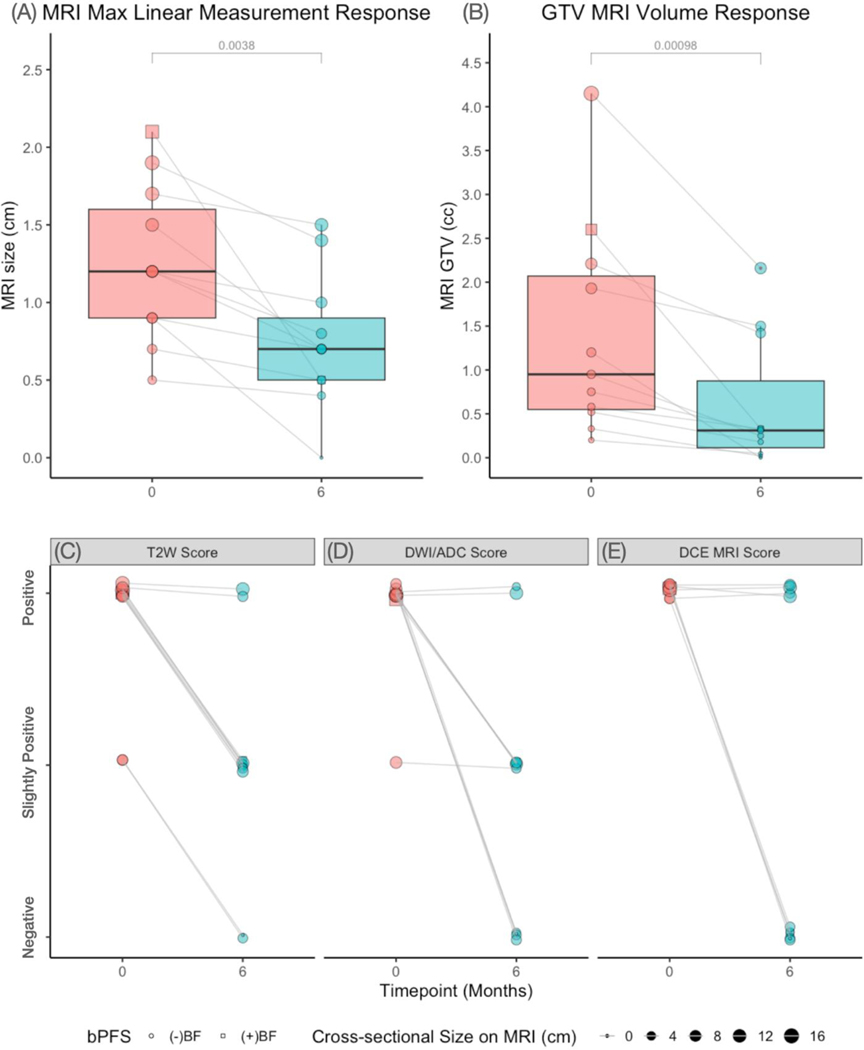
At baseline, the mean maximum axial dimension (MRIMax) was 1.3 cm (min-max: 0.5 −2.1cm) and mean GTVMRI was 1.3cc (min-max: 0.2–4.15cc). A significant reduction in both the MRIMax and GTVMRI decreased between pretreatment and 6 months after completion of treatment (p-values < 0.01; Figure 2A–B). Qualitative MRI analysis showed that the majority of lesions showed decreased imaging signatures on T2-weighted (81%, n=9/11), DWI/ADC (82%, n=8/11), and DCE imaging (50%, n=4/8) as shown in Figure 2C,2D, and 2E, respectively. Figure 3A and 3B depicts case examples of a complete and partial response, respectively. The single patient with biochemical failure (BF) in our study (Figure 2; box icon) showed both MRIMax response (23%) and qualitative response on T2-weighted, DWI/ADC, and DCE sequences on 6-month post-treatment MRI.
Figure 2.
MRI imaging response by (A) MRIMax (B) GTVMRI, (C) qualitative T2-weighted (T2W) image score, (D) qualitative DWI/ADC score, and (E) qualitative dynamic contrast enhancement (DCE) score. Qualitative scores were determined by a GU-specialized prostate radiology. A ternary scale (negative, slightly positive, and positive) was used for T2W and DWI/ADC images, whereas a binary score (negative or positive) was used for DCE imaging. The single patient with biochemical failure is shown with a box icon (☐).
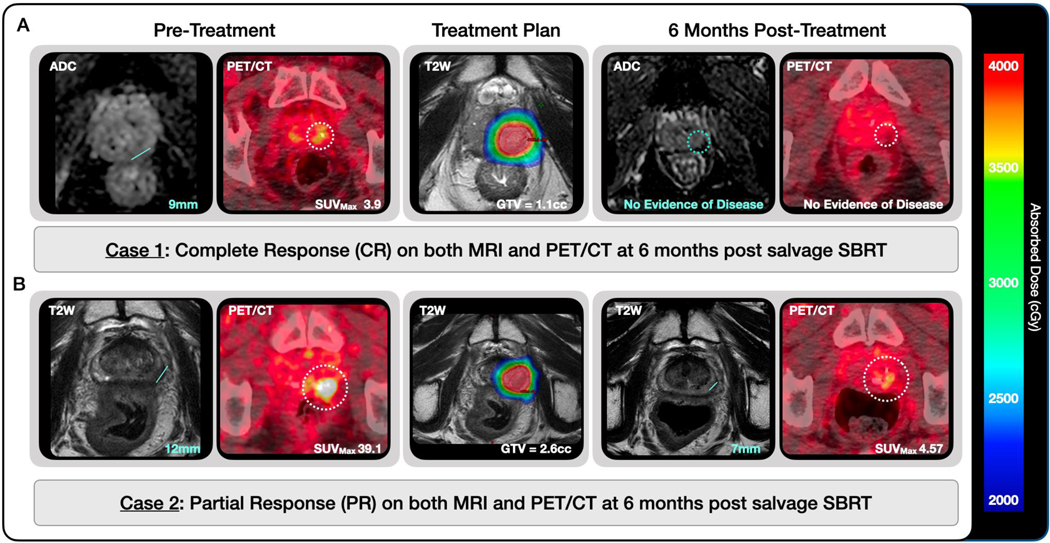
Figure 3.
Imaging response on 6-month MRI and 18F-DCFPyL PET/CT in (A) a patient with complete response on both imaging modalities and (B) a patient with evidence of persistent disease at 6 months post salvage SBRT. Maximum linear measurements (MRIMax) are shown with a line on MRIs and GTVPSMA are delineated with a dotted circle on PET/CTs. Treatment planning MRIs are shown with the treatment planning GTV described and delineated in red. The planned dose is shown in color wash from 20–40Gy as noted in the legend.
PET/CT Response
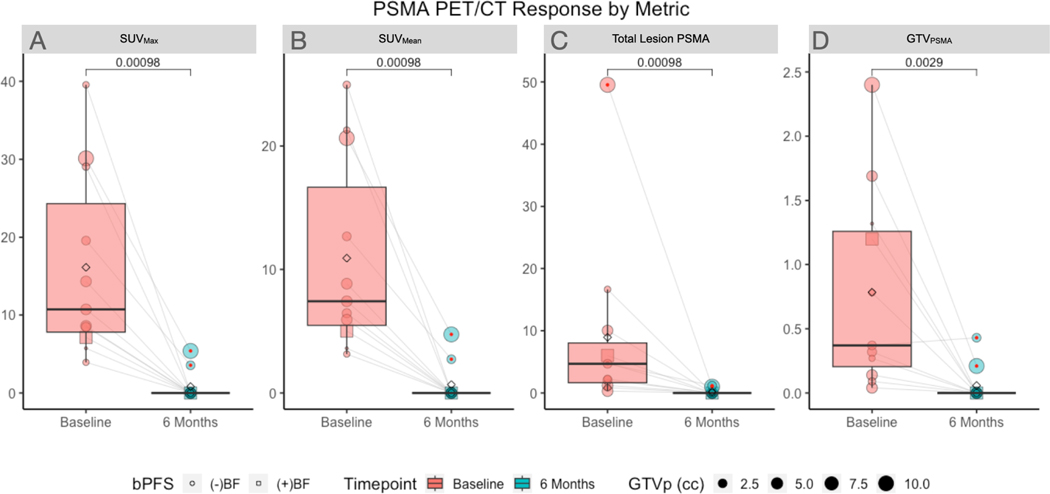
Amongst treated lesions there was a wide variability in SUVMax (median 10.7, min-max: 3.9–39.6; Figure 4A), and SUVMean (median: 7.4, min-max: 3.2 to 25.0; Figure 4B) at baseline. Similarly, the Total Lesion PSMA and GTVPSMA are summarized in Figure 4C–D. The distribution of each quantitative PET measurements was observed to have a significant reduction at 6 months from baseline (all p<0.01), as all but one lesion demonstrated response on all parameters (Figure 4A–D). This lesion was noted to have slight growth of the GTVPSMA at 6-month PET/CT (0.37 to 0.43cc) (Figure 4D).
Figure 4.
18F-DCFPyL PET/CT response at 6 months from baseline on (A) SUVMax, (B) SUVMean, (C) Total Lesion PSMA (SUVMean × GTVPSMA), and (D) GTVPSMA. The single patient with biochemical failure is shown with a box icon (☐). All lesions were noted to produce response on all parameters with exception of a single lesion which did not have GTVPSMA response in a patient who failed to achieve an undetectable PSA nadir.
Exploratory analyses demonstrated that there were significant differences in the SUVMax (p<0.01) independent of contour technique and SUVMean (p<0.01) for the 40% isocontour technique when AC images were compared to Q.Clear reconstructed images. This change, however, was not seen in the measurement of Total Lesion PSMA or GTVPSMA (all p>0.05; Supplemental Figure 3A). There were no significant differences observed by contour technique (all p>0.05; Supplemental Figure 3B).
MRI PET/CT Comparison at Baseline and 6 months.
There was good concordance between baseline mpMRI baseline PSMA PET/CT in detecting lesions, with all lesions being identified on both modalities. Overall, the GTVPSMA underestimated the radiographic extent of disease on MRI. At baseline, GTVMRI, however, was found to be positively correlated with GTVPSMA for gradient edge-based contouring methods independent of reconstruction technique (p<0.001) and for the 40% SUVMax threshold technique for the Q.Clear reconstruction only (p<0.05). In contrast, GTVMRI was not predictive of GTVPSMA at the 6-month timepoint owing to the disparity in the observed complete response rates between the two modalities. These findings are summarized in Supplemental Figure 4.
DISCUSSION
Primary Endpoint and Toxicity
In this prospective, phase I trial evaluating escalating dose levels of focal SBRT for the treatment of local, biopsy-proven, radiorecurrent prostate cancer, we found that the MTD was 40Gy in 5 fractions (DL1).
Two grade 3 GU toxicities were observed in patients treated on DL2 which prompted de-escalation to DL1 for expansion.
As in previous reports [9], no clear dosimetric predictors of G2+ or G3+ toxicity were identified. However, based on the trends observed in this trial, the maximum dose to bladder and urethra may merit further examination. It is conceivable that the optimal focal re-irradiation dose may be related to the size and location of the recurrence and proximity to key genitourinary structures. Prior studies of first-line SBRT have shown an association between GU toxicity and dose [10]. One reported rates of late G2+ GU toxicity of 5% and 48% in patients receiving 35Gy and 40Gy, respectively [11]. Others suggested a relationship between toxicity and urethral [12, 13] and bladder [13] exposure. An ESTRO consensus guideline for salvage SBRT recommends dose prescriptions of >35Gy in 5 fractions (65Gy1.5 EQD2) to an isodose line <80% [14]. This practice produces inhomogeneous plans which (1) may result in higher urethral dose exposure due to intra-observer differences in urethral definition and intrafraction motion [15, 16] and (2) may themselves be associated higher rates of toxicity as reported in the first-line setting [12, 17]. Our study achieved good 2-year outcomes prescribing to the 100% isodose line.
Focal, dose-escalated, salvage SBRT was found to have few GI AEs. Given significant concerns over rectal toxicity resulting from high cumulative rectal dose exposure, a previous trial [18] excluded patients if their recurrence was posterior and adjacent to the rectum unless a hydrogel spacer was employed. This may significantly limit eligibility as posterior recurrences often include a component of extraprostatic extension that precludes hydrogel spacer placement. Despite more permissive inclusion criteria than this trial and rectal constraints, we observed a similarly low rate of clinically significant GI toxicity. Similar to other studies [19, 20], GU toxicity was more common than GI toxicity in our study.
Oncologic Efficacy
The bPFS at 12- and 24-months were 100%, and during the course of the study, only one patient experienced biochemical failure at 33 months post-treatment. Although the number of treated patients is small, these rates compare favorably to published results [18, 20–25], possibly due to dose escalation, careful patient selection, and the target volume definition.
This phase I trial was designed to define the MTD dose of focal re-irradiation based upon the rationale that higher doses may improve local control. In the first line setting, tumor control probability has been shown to be proportional to the dose delivered to gland [26–29] and mpMRI-identified tumor subvolume [30]. While a similar trend has been suggested in the radiorecurrent setting [31], this trend has yet to be established with early, small, prospective reports instead focusing on feasibility as opposed to dose-response [18, 21–23, 32]. Prior retrospective series [33–35] have shown significant rates of local recurrence with lower radiation doses (38.6 – 77.1Gy1.5 EQD2) at short term follow-up providing a rationale for dose-escalation to further improve outcomes. In an interim analysis of a prospective trial [18], NCT03073278, which treated patients to doses of 36–38Gy in 6 fractions (77.1 – 85.0Gy1.5 EQD2), 5 of the 25 patients (20%) had biochemical recurrence at a median follow-up of 25 months with 4 of these patients having suspicion of local persistence/recurrence. In our series, at a dose of ≥40Gy in 5 fractions (≥108.6 Gy1.5 EQD2), no clear evidence of second local failure was observed in our cohort.
Favorable bPFS outcomes may also be explained by other factors including careful patient selection and target volume definition. Patients in our study were selected for focal treatment to ensure the highest likelihood that all tumor was within treatment volumes. This was accomplished with universal 18F-DCFPyL PET/CT screening which ruled out low-burden metastatic disease and the inclusion of patients only with biopsy-verified and imaging-concordant local recurrence. Finally, 18F-DCFPyL PET-augmented treatment planning may have improved our treatment volume definition. In our study, the GTVPSMA exceeded the MRIGTV which subsequently led to a treatment GTV larger than the MRIGTV for all lesions. Given that prior reports had no or incomplete PET utilization for GTV delineation [21, 24, 36, 37], the use of this new imaging modality may explain in part the favorable outcomes observed.
Imaging
At baseline, treatment planning MRI, CT, and 18F-DCFPyL PET were used for GTV delineation. Although all lesions were detected on both modalities, there was substantial disagreement in volume of lesions between the modalities [38]. While complete pathological assessment of the GTV was not available, mpMRI volumes were larger than the GTVPSMA in most cases, a finding independent of PET/CT reconstruction technique or automated thresholding strategy employed. Further detailed studies of salvage prostatectomy specimens with pre-operative imaging correlation will be required to better understand the comparative spatial accuracy of each modality regarding tumor volume definition. While 18 F-DCFPyL PET/CT may be helpful in staging at ruling out systemic disease, it is unlikely to replace mpMRI for target volume delineation due to both volume under-estimation and lower spatial resolution.
In contrast, PET/CT appears to outperform mpMRI at the evaluation of early treatment response. In our study, while 82% (n=9/11) of treated lesions had a radiographic CR on PET/CT at 6 months, only a single tumor was found to have a CR on mpMRI at 6 months. This suggests that PET/CT may have an increased sensitivity to detect treatment response at an early stage, although longer term outcomes are needed to compare to eventual local failure patterns. PSMA-based PET/CT imaging response was also assessed in NCT03073278 [18] using 68Ga-PSMA PET/CT at the 12-month post-treatment timepoint, although a detailed report of this endpoint is not yet available.
LIMITATIONS
While significant rates of GU toxicity occurred in patients treated on DL2, both patients had a tumor geometry which required high dose exposure to the bladder and urethra. It is possible that tumors with favorable size and location may be amenable to further dose escalation. Additionally, alternative treatment techniques such as MRI-LINAC or HDR brachytherapy may also allow for additional dose escalation. As this trial tested a focal method of re-irradiation (i.e., treatment of the GTV), these doses should not be generalized to whole gland re-irradiation. Further, while our trial did have a favorable bPFS at a median follow-up of 35 months in comparison to prior reports of salvage SBRT [20, 24, 25, 31, 39], it could not provide a high precision estimate of oncologic control given the small sample size. Additionally, while the study follow-up was limited, these results may be representative of future outcomes as prior studies have described an early temporal pattern of failure with a median time to biochemical progression of 9.4 months and a median time to clinical progression of 13.2 months for this patient population [36]. Larger studies with extended follow-up will serve to address the question of the interaction between tumor location and objective toxicity as well as high precision estimates of long-term local control.
CONCLUSIONS
The MTD for focal salvage SBRT for isolated intraprostatic radiorecurrence was 40Gy in 5 fractions producing a 100% 24-month bPFS, with one late failure observed at 33 months. The most frequent clinically significant toxicity was late grade 2+ GU toxicity. PSMA-based PET/CT appeared to improve both target volume delineation and response assessment at 6 months.
Supplementary Material
Acknowledgements:
This research was supported by the Intramural Research Program of the NIH.
Funding:
Funding was provided by grant ZIA BC 011552 awarded by the National Institutes of Health Clinical Center for Research
Footnotes
Conflict of Interest (COI):
Erich P. Huang COI: Participation on the American College of Radiology Imaging Network (ACRIN) Data Safety Monitoring Board
Peter A. Pinto COI: Philips Inc pays royalties to NIH for a licensing agreement, NIH then pays royalties to Dr. Pinto. NIH and Philips have a CRADA. NIH has intellectual property in the field, including among other patents and patent applications, Patent: “System And Method For Prostate Cancer Detection And Distribution Mapping” US Patent number: 8,447,384 and “System And Method For Computer Aided Cancer Detection Using T2- weighted And High-value Diffusion-weighted Magnetic Resonance Imaging” US Patent number: 10,215,830. NIH and Philips (In Vivo Inc) have a licensing agreement with the authors. NIH does not endorse or recommend any commercial products, processes, or services. The views and personal opinions of authors expressed herein do not necessarily state or reflect those of the US Government, nor reflect any official recommendation nor opinion of the NIH nor NCI.
Baris Turkbey COI: Royalties from the National Institutes of Health; Multiple patents in AI (details available upon request); Cooperative research and development agreements with NVIDIA and Philips.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability:
Research data are stored in an institutional repository and will be shared upon request to the senior author. This trial is registered on clinicaltrials.gov under the NCT03253744 identifier.
References
- 1.Pienta KJ, et al. , A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with (18)F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol, 2021. 206(1): p. 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith C, et al. , Patterns of Failure in Men With Radiorecurrent Prostate Cancer: A Post-Hoc Analysis of Two Prospective Ga-68-PSMA PET/CT Imaging Trials. International Journal of Radiation Oncology, Biology, Physics, 2021. 111(3): p. e294. [DOI] [PubMed] [Google Scholar]
- 3.Maitre P, et al. , Timing of Ga68-PSMA PETCT and patterns of recurrence after prostate radiotherapy: Implications for potential salvage. Radiother Oncol, 2022. 169: p. 71–76. [DOI] [PubMed] [Google Scholar]
- 4.Valle LF, et al. , A Systematic Review and Meta-analysis of Local Salvage Therapies After Radiotherapy for Prostate Cancer (MASTER). Eur Urol, 2021. 80(3): p. 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roach M, et al. , Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. International Journal of Radiation Oncology*Biology*Physics, 2006. 65(4): p. 965–974. [DOI] [PubMed] [Google Scholar]
- 6.Le Tourneau C, Lee JJ, and Siu LL, Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst, 2009. 101(10): p. 708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon WJ and Mood AM, The statistical sign test. Journal of the American Statistical Association, 1946. 41(236): p. 557–566. [DOI] [PubMed] [Google Scholar]
- 8.Storer BE, Design and analysis of phase I clinical trials. Biometrics, 1989. 45(3): p. 925–937. [PubMed] [Google Scholar]
- 9.Loi M, et al. , Robotic Stereotactic Retreatment for Biochemical Control in Previously Irradiated Patients Affected by Recurrent Prostate Cancer. Clin Oncol (R Coll Radiol), 2018. 30(2): p. 93–100. [DOI] [PubMed] [Google Scholar]
- 10.Wang K, et al. , Prostate Stereotactic Body Radiation Therapy: An Overview of Toxicity and Dose Response. Int J Radiat Oncol Biol Phys, 2021. 110(1): p. 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helou J, et al. , Stereotactic ablative radiotherapy in the treatment of low and intermediate risk prostate cancer: Is there an optimal dose? Radiotherapy and Oncology, 2017. 123(3): p. 478–482. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, et al. , Receiver operating curves and dose-volume analysis of late toxicity with stereotactic body radiation therapy for prostate cancer. Practical Radiation Oncology, 2017. 7(2): p. e109–e116. [DOI] [PubMed] [Google Scholar]
- 13.Seymour ZA, et al. , Dose-volume analysis and the temporal nature of toxicity with stereotactic body radiation therapy for prostate cancer. Practical Radiation Oncology, 2015. 5(5): p. e465–e472. [DOI] [PubMed] [Google Scholar]
- 14.Jereczek-Fossa BA, et al. , Salvage stereotactic body radiotherapy (SBRT) for intraprostatic relapse after prostate cancer radiotherapy: An ESTRO ACROP Delphi consensus. Cancer treatment reviews, 2021. 98: p. 102206. [DOI] [PubMed] [Google Scholar]
- 15.Poulsen PR, et al. , Accuracy of image-guided radiotherapy of prostate cancer based on the BeamCath® urethral catheter technique. Radiotherapy and Oncology, 2007. 83(1): p. 25–30. [DOI] [PubMed] [Google Scholar]
- 16.Pham J, et al. , Urethral Interfractional Geometric and Dosimetric Variations of Prostate Cancer Patients: A Study Using an Onboard MRI. Front Oncol, 2022. 12: p. 916254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald AM, et al. , Increased radiation dose heterogeneity within the prostate predisposes to urethral strictures in patients receiving moderately hypofractionated prostate radiation therapy. Practical Radiation Oncology, 2015. 5(5): p. 338–342. [DOI] [PubMed] [Google Scholar]
- 18.Bergamin S, et al. , Interim Results of a Prospective Prostate-Specific Membrane Antigen-Directed Focal Stereotactic Reirradiation Trial for Locally Recurrent Prostate Cancer. Int J Radiat Oncol Biol Phys, 2020. 108(5): p. 1172–1178. [DOI] [PubMed] [Google Scholar]
- 19.Pasquier D, et al. , Salvage Stereotactic Body Radiation Therapy for Local Prostate Cancer Recurrence After Radiation Therapy: A Retrospective Multicenter Study of the GETUG. Int J Radiat Oncol Biol Phys, 2019. 105(4): p. 727–734. [DOI] [PubMed] [Google Scholar]
- 20.Lewin R, et al. , Salvage re-irradiation using stereotactic body radiation therapy for locally recurrent prostate cancer: the impact of castration sensitivity on treatment outcomes. Radiation Oncology, 2021. 16(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryg U, et al. , A Prospective Study of High Dose-Rate Brachytherapy or Stereotactic Body Radiotherapy of Intra-Prostatic Recurrence: Toxicity and Long Term Clinical Outcome. Front Oncol, 2022. 12: p. 861127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasquier D, et al. , GETUG-AFU 31: a phase I/II multicentre study evaluating the safety and efficacy of salvage stereotactic radiation in patients with intraprostatic tumour recurrence after external radiation therapy-study protocol. BMJ Open, 2019. 9(8): p. e026666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuller DB, et al. , High-dose-rate stereotactic body radiation therapy for postradiation therapy locally recurrent prostatic carcinoma: Preliminary prostate-specific antigen response, disease-free survival, and toxicity assessment. Pract Radiat Oncol, 2015. 5(6): p. e615–23. [DOI] [PubMed] [Google Scholar]
- 24.Leroy T, et al. , Salvage robotic SBRT for local prostate cancer recurrence after radiotherapy: preliminary results of the Oscar Lambret Center. Radiation Oncology, 2017. 12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasquier D, et al. , Salvage Stereotactic Body Radiation Therapy for Local Prostate Cancer Recurrence After Radiation Therapy: A Retrospective Multicenter Study of the GETUG. International Journal of Radiation Oncology*Biology*Physics, 2019. 105(4): p. 727–734. [DOI] [PubMed] [Google Scholar]
- 26.Zietman AL, et al. , Randomized Trial Comparing Conventional-Dose With High-Dose Conformal Radiation Therapy in Early-Stage Adenocarcinoma of the Prostate: Long-Term Results From Proton Radiation Oncology Group/American College of Radiology 95–09. Journal of Clinical Oncology, 2010. 28(7): p. 1106–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michalski JM, et al. , Effect of Standard vs Dose-Escalated Radiation Therapy for Patients With Intermediate-Risk Prostate Cancer. JAMA Oncology, 2018. 4(6): p. e180039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zagars GK, Pollack A, and Smith LG, Conventional external-beam radiation therapy alone or with androgen ablation for clinical stage III (T3, NX/N0, M0) adenocarcinoma of the prostate. International Journal of Radiation Oncology*Biology*Physics, 1999. 44(4): p. 809–819. [DOI] [PubMed] [Google Scholar]
- 29.Dearnaley DP, et al. , Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. The Lancet Oncology, 2014. 15(4): p. 464–473. [DOI] [PubMed] [Google Scholar]
- 30.Kerkmeijer LGW, et al. , Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. Journal of Clinical Oncology, 2021. 39(7): p. 787–796. [DOI] [PubMed] [Google Scholar]
- 31.Jereczek-Fossa BA, et al. , Reirradiation for isolated local recurrence of prostate cancer: Mono-institutional series of 64 patients treated with salvage stereotactic body radiotherapy (SBRT). The British Journal of Radiology, 2019. 92(1094): p. 20180494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuller DB, et al. , Virtual HDR CyberKnife treatment for localized prostatic carcinoma: dosimetry comparison with HDR brachytherapy and preliminary clinical observations. Int J Radiat Oncol Biol Phys, 2008. 70(5): p. 1588–97. [DOI] [PubMed] [Google Scholar]
- 33.Leroy T, et al. , Salvage robotic SBRT for local prostate cancer recurrence after radiotherapy: preliminary results of the Oscar Lambret Center. Radiat Oncol, 2017. 12(1): p. 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Agostino GR, et al. , Reirradiation of Locally Recurrent Prostate Cancer With Volumetric Modulated Arc Therapy. Int J Radiat Oncol Biol Phys, 2019. 104(3): p. 614–621. [DOI] [PubMed] [Google Scholar]
- 35.Zerini D, et al. , Salvage image-guided intensity modulated or stereotactic body reirradiation of local recurrence of prostate cancer. Br J Radiol, 2015. 88(1052): p. 20150197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zerini D, et al. , Salvage image-guided intensity modulated or stereotactic body reirradiation of local recurrence of prostate cancer. The British Journal of Radiology, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuller DB, et al. , Phase 2 Multicenter Trial of Heterogeneous-dosing Stereotactic Body Radiotherapy for Low- and Intermediate-risk Prostate Cancer: 5-year Outcomes. European Urology Oncology, 2018. 1(6): p. 540–547. [DOI] [PubMed] [Google Scholar]
- 38.Liu W, et al. , Defining radio-recurrent intra-prostatic target volumes using PSMA-targeted PET/CT and multi-parametric MRI. Clin Transl Radiat Oncol, 2022. 32: p. 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergamin S, et al. , Interim Results of a Prospective Prostate-Specific Membrane Antigen-Directed Focal Stereotactic Reirradiation Trial for Locally Recurrent Prostate Cancer. International Journal of Radiation Oncology*Biology*Physics, 2020. 108(5): p. 1172–1178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the senior author. This trial is registered on clinicaltrials.gov under the NCT03253744 identifier.